Abstract
Osteopontin (OPN), a multifunctional protein, is involved in numerous pathological conditions including inflammation, immunity, angiogenesis, fibrogenesis and carcinogenesis in various tissues. Extensive studies have elucidated the critical role of OPN in cell signaling such as regulation of cell proliferation, migration, inflammation, fibrosis and tumor progression. In the liver, OPN interacts with integrins, CD44, vimentin and MyD88 signaling, thereby induces infiltration, migration, invasion and metastasis of cells. OPN is highlighted as a chemoattractant for macrophages and neutrophils during injury in inflammatory liver diseases. OPN activates hepatic stellate cells (HSCs) to exert an enhancer in fibrogenesis. The role of OPN in hepatocellular carcinoma (HCC) has also generated significant interests, especially with regards to its role as a diagnostic and prognostic factor. Interestingly, OPN acts an opposing role in liver repair under different pathological conditions. This review summarizes the current understanding of OPN in liver diseases. Further understanding of the pathophysiological role of OPN in cellular interactions and molecular mechanisms associated with hepatic inflammation, fibrosis and cancer may contribute to the development of novel strategies for clinical diagnosis, monitoring and therapy of liver diseases.
Keywords: Osteopontin, liver injury, inflammation, fibrosis, hepatocellular carcinoma.
Introduction
Osteopontin (OPN), also known as secreted phosphoprotein, was first described in 1979 1, and also independently identified as bone sialoprotein 2, early T-lymphocyte activation-1 (Eta-1) 3 later. Subsequently, numerous studies have reported that OPN plays a critical role in various organs under pathological conditions. In the liver, OPN normally exists in the bile duct epithelium physiologically 4. In 1999, liver pathological expression of OPN was first confirmed in Kupffer cells, hepatic macrophages and hepatic stellate cells (HSCs) in necrotic areas after carbon tetrachloride intoxication, where OPN was indicated to contribute to infiltration of macrophages into the necrotic areas 5. In the following years, OPN was found to be involved in many liver diseases such as acute liver failure (ALF) 6, 7, nonalcoholic fatty liver diseases (NAFLD) 8, alcoholic liver diseases (ALD) 9, liver fibrosis with chronic hepatitis B (CHB) 10, chronic hepatitis C (CHC) 11, 12. Furthermore, OPN was suggested as a better prognostic marker for early hepatocellular carcinoma (HCC) than alpha-fetoprotein (AFP) 13. In this review, we will focus on the role of OPN in liver biology and discuss potential approaches to diagnosis and clinical trials (Fig. 1).
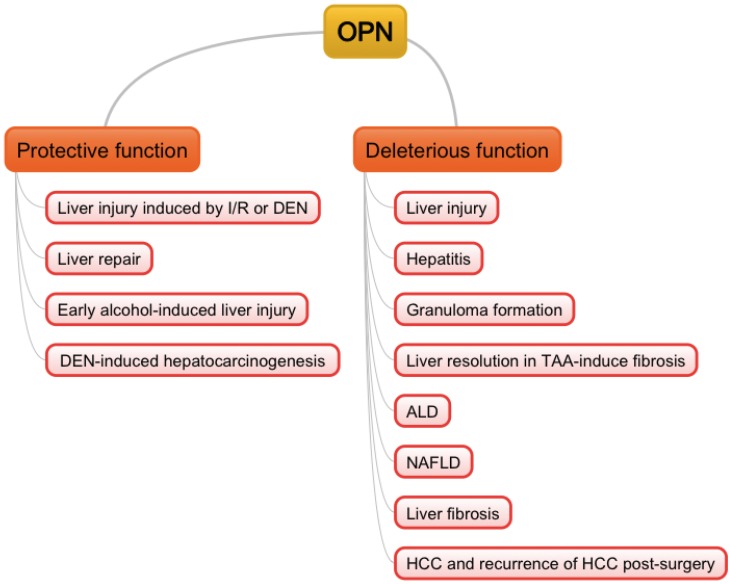
Figure 1.
Double-edged sword functions of OPN in liver pathogenesis. I/R: ischemia/reperfusion; DEN: diethylnitrosamine; TAA: thioacetamide; ALD: alcoholic liver diseases; NAFLD: non-alcoholic fatty liver diseases; HCC: hepatocellular carcinoma.
The Structure of OPN and its Receptors
OPN is located on chromosome 4 region 22 (4q22.1) in humans and is composed of about 300 amino acids (314 in human, but 297 in mouse). The multi-functionality of OPN is due to varied post-translational modifications such as phosphorylation, sulfation, glycosylation and protelytic cleavage. OPN contains an arginine-glycine-asparate (RGD) domain, which binds with high affinity to integrins such as αvβ1, αvβ3, αvβ5, αvβ6, α8β1, α5β1 14-17. Next to the RGD motif is the serine-valine-valine-typrosine-glycine-leucine-arginine (SVVYGLR) sequence in humans or serine-leucine-alanine-tyrosine-glycine-leucine-arginine (SLAYGLR) sequence in mice and rats, which is exposed upon cleavage with thrombin and interacts with α4β1, α9β1, α4β7 integrins 18-20. Additionally, another integrin αxβ2, which is neither RGD nor SVVYGLR dependent, has been reported to mediate the effects of OPN 21, 22. In addition to interacting with integrins, OPN has also been reported to bind to the spliced variant forms of CD44, notably v6 and v7, but the specific binding domain in OPN remains elusive 23. Most recently, two new proteins, vimentin and MyD88, were found to interact with OPN by coimmunoprecipitation 24, 25.
OPN in Acute Liver Injury
In humans, OPN correlates with the degree of liver necrosis during acute liver injury 6, 7. Patients with high serum OPN levels have a significantly poorer prognosis than patients with low serum OPN 6. However, very high levels of plasma OPN are associated with good outcomes 7. Whether OPN protects from disease progression in the setting of acute liver injury needs more investigation.
In animal models, OPN usually acts as a chemoattractant for macrophages and neutrophils. In carbon tetrachloride-intoxicated and Propionibacterium acnes-treated rats, OPN contributes to infiltration of monocytes and macrophages in the liver 5, 26. In addition, MMP-cleaved OPN mediates hepatic neutrophil accumulation in the early phase of liver injury during obstructive cholestasis after bile duct ligation (BDL) in mice 27. In drug-induced liver diseases, mice with both endogenous and exogenous blockade of OPN are less susceptible to acetaminophen and survive acetaminophen (APAP) overdose 28, 29. In OPN deficient mice, neutrophil infiltration and macrophage accumulation are impaired, and release of pro-inflammatory cytokines is inhibited; yet, OPN deficiency augments CYPs genes in APAP metabolism in vivo 29. Similarly, upon exposure to diethylnitrosamine (DEN), another hepatotoxic drug, OPN deficiency enhances susceptibility and abrogates estrogen-mediated hepatoprotection against DEN via augmenting oxidative stress and keeping CYP2A5 expression in mice 30. Recently, a few studies suggest that OPN acts as a protector during inflammatory liver injury. OPN have been found to inhibit activation of NF-κB and ameliorate production of IL-6 and TNFα in macrophages, thus promoting survival of hepatocytes in DEN-induced liver injury 25. Likewise, in hepatic injury induced by ischemia/reperfusion (I/R), OPN in macrophages limits IL-6, TNFα, IL-1β and toxic iNOS production; meanwhile, OPN silence in hepatocytes decreases anti-apoptotic Bcl2 and ATP levels to aggravate the injury 31.
OPN in autoimmune and viral Hepatitis
Similar to that in acute injury, plasma OPN levels are significantly elevated in patients with hepatitis including acute hepatitis, chronic hepatitis and fulminant hepatitis 32, 33. Transgenic mice expressing OPN in hepatocytes can develop mononuclear cell and CD8 positive cell infiltration in the liver after 12 weeks of age, and plasma ALT activity and focal necrosis in hepatic lobules increase after 24 weeks, which may be a useful model for autoimmune hepatitis 34. ConA-induced hepatitis mimics T cell-mediated liver diseases including autoimmune hepatitis and viral hepatitis. In 2004, Diao et al. first uncovered that OPN cleaved by thrombin enhanced NKT cell activation and neutrophil infiltration and activation to augment ConA-induced hepatitis 35. Later, OPN was found to interact with β3 integrin to induce IL-17 expression depending on p38, JNK, NF-κB pathways to aggravate ConA-induced hepatitis 36. Both silence and exogenous blockade of OPN protects ConA-treated mice from fulminant hepatitis 37, 38.
OPN production is also up-regulated in CHB and CHC patients 36, 39. OPN is shown to improve the maturation and functioning of DCs in response to HBV antigens 40. Yet, in patients infected with HCV, OPN may directly promote HCV replication 39.
OPN in Granuloma Formation
Granuloma formation is a host response against persistent irritants. In β-glucan-induced hepatic granuloma formation, OPN does not affect the size and the number of granulomas, but induces IL-12 and IFNγ at early stages. Nevertheless, at late stages, OPN increases the size and the number of hepatic granulomas due to enhanced immune cells recruitment including macrophages, CD4 T cells and DCs into the liver and increased TNFα production 41. Otherwise, OPN neutralization reduces granulomatous response and liver damage via impaired chemotactic function and inflammatory response 42, which suggested that OPN neutralizer acts as the immunosuppression therapy for liver injury with granulomatous responses.
OPN in Liver Repair
We have reported that in a model of regenerating liver after partial hepatectomy (PHx), OPN promoted inflammatory response and activated IL-6/Stat3 pathway in the early phase to facilitate hepatocyte proliferation 43. Recently, gene expression profiling after hepatectomy showed that 81genes participating in the OPN-mediated pathway were significantly changed, 65 of which were up-regulated. All of the 81 genes were mainly associated with stress response, inflammatory response, cell activation, proliferation, adhesion and migration 44. Elevation of OPN in oval cell-activated regeneration is more significant than that in PHx-induced regeneration, indicating that OPN might be specifically activated in the induction of oval cells to contribute to liver repair 45.
Under pathological conditions, OPN involves in Hh signaling and acts in a paracrine and autocrine manner to enhance liver progenitor cell response and fibrogenic liver repair during carbon tetrachloride-induced liver fibrosis and/or MCD diet-induced NASH fibrosis 46, 47. Yet in thioacetamide(TAA)-induced fibrosis, OPN delays resolution due to sustained fibrillar collagen-I deposition and decreases hepatocyte proliferation in vivo and in vitro 48, 49. Further investigation of OPN's role in pathological repair is still needed.
OPN in Alcoholic Liver Diseases
Hepatic expression and serum levels of OPN are markedly elevated in patients with alcoholic hepatitis (AH), alcoholic cirrhosis and end-stage ALD 50, 51. OPN is also progressively increased in liver fibrosis and association with the stages of fibrosis 9. Serum levels of OPN are correlated with the disease severity of AH patients 50. Otherwise, OPN deficiency mice are protected from alcohol (ethonal)-induced liver injury 50.
Although OPN levels are not elevated in hepatic alcoholic steatosis patients, expression of OPN in a rodent model of alcoholic steatohepatitis (ASH) has been confirmed 52. OPN interacts with neutrophil α4β1 and α9β1 integrins, contributing to hepatic neutrophil transmigration and activation to lead to further injury in the rat ASH model 53. Furthermore, female rats are more susceptible to ALD during ASH, which might be due to higher neutrophil infiltration mediated by OPN 54.
However, in some other established studies, OPN may play a protective role. OPN deficiency facilitates the development of AH from chronic ASH, which is a distinct spectrum of ALD with intense neutrophilic inflammation and high mortality, and promotes the intensity of neutrophil infiltration in the AH model 55, indicating that OPN is protective for AH. Ge et al. established that OPN could bind to gut-derived LPS and prevent macrophage activation, reactive oxygen, nitrogen species generation, and TNFα productionin mice fed the ethonal Lieber-DaCarli diet 56. Furthermore, by blocking LPS translocation in vivo, milk OPN was reported to diminish ethonal-mediated liver injury and protect from early alcohol-induced liver injury 56, 57. In general, further investigations are still needed to clarify the function and therapeutic values of OPN in ALD.
OPN in Non-Alcoholic Fatty Liver Diseases
In patients with NAFLD, serum levels of OPN are elevated and serve as the independent predictor of portal inflammation 8. Notably, hepatic OPN expression is also strikingly increased in obesity-induced liver steatosis with CD44 and correlates with liver triglyceride accumulation 58. Hepatic NKT-derived OPN promotes fibrogenesis during NASH via facilitation of HSC activation and neutralization of OPN with RNA aptamers attenuates these fibrogenic responses 59, 60. In addition, in OPN deficient mice, obesity-driven hepatic inflammation and macrophage accumulation are blocked. OPN deficiency also protects against obesity-induced hepatic steatosis via enhanced hepatic insulin signaling by promoting IRS-2 phosphorylation and preventing up-regulation of FOXO1 and its gluconeogenic target genes 61. Similarly, OPN neutralization decreases obesity-associated inflammation in liver and reverses transcriptional signaling associated with insulin resistance and glucose homeostasis 62. Hence, OPN may be an ideal target for treatment of NAFLD.
OPN in Fibrosis
Various studies have reported that OPN secretion or hepatic OPN expression predicts severe degrees of fibrosis in patients with HBV or HCV infection, ALD, or schistosomiasis mansoni and might serve as a prognostic index for the progression of hepatic fibrosis to cirrhosis and HCC 9, 10, 12, 63, 64. Hepatic OPN expression correlates with TGFβ expression, portal space neutrophil related inflammation, and portal hypertension in liver fibrosis in humans 9, 64. Similar to clinical data, hepatic OPN expression is significantly increased and drives fibrogenic response in mice with various animal models including BDL, carbon tetrachloride or TAA treatment, and MCD, CDE or DDC diet 49, 65-67. DNA hypomethylation of OPN enhancer during onset of early-stage liver fibrosis has been detected, which is confirmed to precede the up-regulation of OPN expression to induce the onset of fibrosis 68. Interestingly, transgenic mice overexpressing OPN in hepatocytes are able to develop spontaneous liver fibrosis over time (1 year) 69. During the pathogenesis of fibrosis, HSC-derived OPN interacts with αvβ3 on the HSCs to activate the PI3K/pAkt/NF-κB signaling cascade to up-regulate collagen-I and to modulate the HSC pro-fibrogenic phenotype 69. OPN also enhances ductular reaction and scarring in liver fibrotic mice and activates the oval cell compartment to differentiate to BECs, which then signals HSCs via OPN and TGFβ 49. In addition, OPN is up-regulated by oval cells in hepatic fibrotic patients and is confirmed to enhance viability and wound healing by modulating TGFβ signaling in cultured oval cells. OPN neutralization reverses epithelial-mesenchymal-transition (EMT) in oval cells and attenuates fibrogenesis in mouse model of liver fibrosis 47. Most recently, OPN is found to increase NADPH oxidase activity and inhibit histone deacetylases 1/2 promoting HMGB1 acetylation and translocation in HSCs, where then HMGB1 up-regulates collagen-I via RAGE and activates the PI3K-pAkt1/2/3 pathway 63. In general, the fibrogenic role of OPN in liver diseases has been confirmed via significant research over the years. However, targeting OPN for resolution of fibrosis still remains controversial due to its opposing effects on different pathological conditions of fibrosis as discussed above.
OPN in Hepatocellular Carcinoma
The research for the relationship between OPN and HCC has been popular and studied intensively. OPN is markedly elevated in the plasma of HCC patients, and has been identified as a diagnostic biomarker that could also improve AFP performance in HCC surveillance among patients with HBV or HCV-related cirrhosis 13, 70. OPN has good sensitivity in AFP-negative HCC, indicating OPN as a better marker in the early stage of HCC 13. OPN expression significantly correlates with clinicopathological features of HCC patients with HBV and/or HCV such as capsular infiltration, vascular invasion, lymph node metastasis and TNM stages 71, 72. A study of Korean HBV-related cirrhosis and HCC has showed that genetic polymorphisms in the OPN gene are associated with HBV clearance and the age of HCC occurrence 73. Accordingly, the SNPs -156 and -443 of OPN are associated to susceptibility to HCC 74. Additionally, another study agreed that allele T/T and/or T/C rather than C/C at nt -443 not only significantly enhanced metastasis, but was also associated with overall survival and time to recurrence 75. Other than in HCC with HBV or HCV, OPN is not a useful diagnostic marker for HCC in alcoholic cirrhotic patients, particularly in the early stages, which limits OPN to become a significant diagnostic marker for HCC 76.
Overexpression of OPN is found to lead to intrahepatic metastasis, early recurrence and poorer prognosis of surgically resected HCC 77, 78. Similarly, OPN positive expression facilitates recurrence and reduces patient survival after liver transplantation for HCC 79. Therefore, OPN may be a useful marker for detecting early recurrence of HCC after surgery 80, 81.
OPN signals cell proliferation, EMT, invasion and metastasis in HCC. It mediates increase in cell proliferation depending on CD44, and a form of OPN-A exerts the greatest proliferative effect 82. In addition, OPN interacts with CD44 receptor to enhance HGF-induced scattering and invasion 83 and activates c-Met to promote HCC progress 84. The binding of secreted OPN from HCV-infected cells to integrinαvβ3 and CD44 leads to elevation of reactive oxygen species and activation of Ca2+ signaling and downstream cellular kinases such as MAPK, JNK, PI3K, FAK, and Src, all of which promote EMT, cell migration and invasion to enhance tumor progression and metastasis in HCC 85, 86. OPN is also able to bind to vimentin and increase vimentin stability through inhibition of its protein degradation to improve EMT in HCC cells 24. On the other hand, OPN silence in HCC cells results in suppression of αv, β1 and β3 integrin expression, inhibition of NF-κB signaling activation, and blockade of Bcl-2/Bcl-xL and XIAP expression with increased Bax expression, thus inducing mitochondria-mediated apoptosis 87. Lentiviral-mediated miRNA against OPN induces a significant decrease in MMP-2 and uPA expression and leads to an obvious suppression of both in vitro invasion and in vivo metastasis of HCC cells 88. RNA aptamers against OPN binding markedly decrease EMT and HCC tumor growth 89. However, in a mouse model of DEN-induced hepatocarcinogenesis, intracellular OPN in macrophages interacts with the pivotal TLR signaling protein MyD88 to function as an endogenous negative regulator of TLR-mediated immune response to ameliorate production of pro-inflammatory cytokines, subsequently impeding liver carcinogenesis 25. Overall, OPN shows promise as a diagnostic marker for HCC, but further confirmation of its potential therapeutic value for HCC is urgently required.
OPN in Intrahepatic Cholangiocarcinoma
Since 2004, Terashi et al. reported that decreased expression of OPN is considered to be a reliable indicator for tumor aggressiveness in intrahepatic cholangiocarcinoma (ICC), and negative expression of OPN protein is significantly related to lymphatic permeation, perineural invasion, intrahepatic metasitasis and lymph node metastasis, leading to a lower overall survival 90. However, another study in 2013 had opposing views that stromal overexpression of OPN correlates with a poor diagnosis in patients with ICC, suggesting that OPN may be an independent prognostic marker for ICC 91.
Conclusion
OPN exists in various hepatic cells including BECs, Kupffer cells, activated HSCs, NKT cells, injured hepatocytes and HCC cells. OPN is involved in hepatic inflammation after acute or chronic liver injury, ALD, NAFLD, fibrosis and cirrhosis (Fig. 2). OPN is also linked to liver repair. Large amounts of literatures shed light on the specific diagnostic and prognostic implication of OPN in HCC, mostly with HBV and HCV. Overall, OPN exacerbates the progression of liver diseases under most pathological conditions via interacting with αv integrin, CD44, vimentin or MyD88, which suggests OPN as an appropriate and desired therapeutic target against liver diseases.
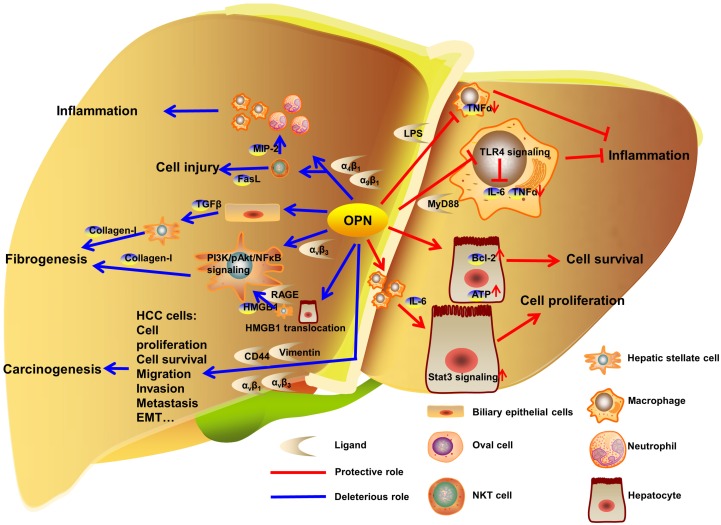
Figure 2.
OPN regulation in liver diseases. In liver, OPN interacts with integrins to deteriorate the development of liver inflammation, injury and fibrogenesis. In carcinogenesis, OPN promotes proliferation, survival, migration, invasion, metastasis and EMT in HCC cells by binding to αv integrins, CD44 and vimentin. Otherwise, OPN binds to LPS or MyD88 to inhibit the progress of inflammation. Besides, OPN protects hepatocytes from injury and facilitates hepatocytes proliferation in model of PHx.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31300742 to X Kong, 8147224 to Q Xia), and the Shanghai Education Committee (Eastern Scholar Program) and program from the Shanghai Key Laboratory of hepatocellular and cholangiocellular carcinomas (13DZ2261100) to X Kong, the Shanghai Health Bureau Key Joint Efforts Foundation (2013ZYJB001) to Q Xia.
Abbreviation
- AFP
alpha-fetoprotein
- AH
alcoholic hepatitis
- ALD
alcoholic liver disease
- ALF
acute liver failure
- ALT
alanine aminotransferase
- APAP
acetaminophen
- ASH
alcoholic steatohepatitis
- ATP
adenosine triphosphate
- Bax
Bcl-2 associated X protein
- Bcl
B-cell lymphoma
- BDL
bile duct ligation
- BEC
biliary epithelial cell
- CDE
choline-deficient ethionine-supplemented
- CHB
chronic hepatitis B
- CHC
chronic hepatitis C
- ConA
concanavalin A
- CYP2A5
cytochrome P450, family 2, subfamily A, polypeptide 1
- DC
dendritic cell
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- DEN
diethylnitrosamine
- EMT
epithelial-mesenchymal-transition
- Eta
early T-lymphocyte activation-1;FAK: focal adhesion kinase
- FOXO1
Forkhead box O1
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HGF
hepatocyte growth factor
- Hh
hedgehog
- HMGB1
high-mobility group box-1
- HSC
hepatic stellate cell
- ICC
intrahepatic cholangiocarcinoma
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IRS-2
insulin receptor substrate-2
- I/R
ischemia/reperfusion
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCD
methionine-choline deficient
- MMP
matrix metalloproteinase
- MyD88
myeloid differentiation factor 88
- NAFLD
nonalcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NF-κB
nuclear factor-κB
- NKT
natural killer T
- OPN
osteopontin
- PHx
partial hepatectomy
- PI3K
phosphoinositide 3-kinase
- RAGE
receptor for advanced glycation end-products
- RGD
arginine-glycine-asparate
- SLAYGLR
serine-leucine-alanine-tyrosine-glycine-leucine-arginine
- SNP
single nucleotide polymorphism
- Spp1
secreted phosphoprotein 1
- Stat
signal transducer and activator of transcription
- SVVYGLR
serine-valine-valine-typrosine-glycine-leucine-arginine
- TAA
thioacetamide
- TGF
transforming growth factor
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TNM
tumor-node-metastases
- uPA
urokinase plasminogen activator;XIAP: X-linked inhibitor of apoptosis protein.
References
- 1.Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16:885–93. doi: 10.1016/0092-8674(79)90103-x. [DOI] [PubMed] [Google Scholar]
- 2.Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:8819–23. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patarca R, Freeman GJ, Singh RP, Wei FY, Durfee T, Blattner F. et al. Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. The Journal of experimental medicine. 1989;170:145–61. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown LF, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ. et al. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Molecular biology of the cell. 1992;3:1169–80. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawashima R, Mochida S, Matsui A, YouLuTu ZY, Ishikawa K, Toshima K. et al. Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochemical and biophysical research communications. 1999;256:527–31. doi: 10.1006/bbrc.1999.0372. [DOI] [PubMed] [Google Scholar]
- 6.Arai M, Yokosuka O, Kanda T, Fukai K, Imazeki F, Muramatsu M. et al. Serum osteopontin levels in patients with acute liver dysfunction. Scandinavian journal of gastroenterology. 2006;41:102–10. doi: 10.1080/00365520510024061. [DOI] [PubMed] [Google Scholar]
- 7.Srungaram P, Rule JA, Yuan HJ, Reimold A, Dahl B, Sanders C. et al. Plasma osteopontin in acute liver failure. Cytokine. 2015;73:270–6. doi: 10.1016/j.cyto.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yilmaz Y, Ozturk O, Alahdab YO, Senates E, Colak Y, Doganay HL. et al. Serum osteopontin levels as a predictor of portal inflammation in patients with nonalcoholic fatty liver disease. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2013;45:58–62. doi: 10.1016/j.dld.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Patouraux S, Bonnafous S, Voican CS, Anty R, Saint-Paul MC, Rosenthal-Allieri MA. et al. The osteopontin level in liver, adipose tissue and serum is correlated with fibrosis in patients with alcoholic liver disease. PloS one. 2012;7:e35612. doi: 10.1371/journal.pone.0035612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L, Li T, Wang Y, Pan Y, Ning H, Hui X. et al. Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. International journal of clinical practice. 2008;62:1056–62. doi: 10.1111/j.1742-1241.2007.01368.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Zhu G, Huang M, Lou G, Liu Y, Wang S. Plasma osteopontin concentration correlates with the severity of hepatic fibrosis and inflammation in HCV-infected subjects. Clinica chimica acta; international journal of clinical chemistry. 2010;411:675–8. doi: 10.1016/j.cca.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Matsue Y, Tsutsumi M, Hayashi N, Saito T, Tsuchishima M, Toshikuni N. et al. Serum osteopontin predicts degree of hepatic fibrosis and serves as a biomarker in patients with hepatitis C virus infection. PloS one. 2015;10:e0118744. doi: 10.1371/journal.pone.0118744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S. et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483–90. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu DD, Lin EC, Kovach NL, Hoyer JR, Smith JW. A biochemical characterization of the binding of osteopontin to integrins alpha v beta 1 and alpha v beta 5. The Journal of biological chemistry. 1995;270:26232–8. doi: 10.1074/jbc.270.44.26232. [DOI] [PubMed] [Google Scholar]
- 15.Liaw L, Skinner MP, Raines EW, Ross R, Cheresh DA, Schwartz SM. et al. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. The Journal of clinical investigation. 1995;95:713–24. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K, Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix biology: journal of the International Society for Matrix Biology. 2005;24:418–27. doi: 10.1016/j.matbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Denda S, Reichardt LF, Muller U. Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Molecular biology of the cell. 1998;9:1425–35. doi: 10.1091/mbc.9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito K, Kon S, Nakayama Y, Kurotaki D, Saito Y, Kanayama M. et al. The differential amino acid requirement within osteopontin in alpha4 and alpha9 integrin-mediated cell binding and migration. Matrix biology: journal of the International Society for Matrix Biology. 2009;28:11–9. doi: 10.1016/j.matbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S. et al. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. The Journal of biological chemistry. 1999;274:36328–34. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 20.Green PM, Ludbrook SB, Miller DD, Horgan CM, Barry ST. Structural elements of the osteopontin SVVYGLR motif important for the interaction with alpha(4) integrins. FEBS letters. 2001;503:75–9. doi: 10.1016/s0014-5793(01)02690-4. [DOI] [PubMed] [Google Scholar]
- 21.Schack L, Stapulionis R, Christensen B, Kofod-Olsen E, Skov Sorensen UB, Vorup-Jensen T. et al. Osteopontin enhances phagocytosis through a novel osteopontin receptor, the alphaXbeta2 integrin. Journal of immunology. 2009;182:6943–50. doi: 10.4049/jimmunol.0900065. [DOI] [PubMed] [Google Scholar]
- 22.Klaning E, Christensen B, Bajic G, Hoffmann SV, Jones NC, Callesen MM. et al. Multiple low-affinity interactions support binding of human osteopontin to integrin alphaXbeta2. Biochimica et biophysica acta. 2015;1854:930–8. doi: 10.1016/j.bbapap.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K. et al. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer research. 1999;59:219–26. [PubMed] [Google Scholar]
- 24.Dong Q, Zhu X, Dai C, Zhang X, Gao X, Wei J, Osteopontin promotes epithelial-mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan X, He C, Jing W, Zhou X, Chen R, Cao L. et al. Intracellular Osteopontin inhibits toll-like receptor signaling and impedes liver carcinogenesis. Cancer research. 2015;75:86–97. doi: 10.1158/0008-5472.CAN-14-0615. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Mochida S, Kawashima R, Inao M, Matsui A, YouLuTu ZY. et al. Increased expression of osteopontin in activated Kupffer cells and hepatic macrophages during macrophage migration in Propionibacterium acnes-treated rat liver. Journal of gastroenterology. 2000;35:696–701. doi: 10.1007/s005350070049. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P. et al. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicology letters. 2014;224:186–95. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welch KD, Reilly TP, Bourdi M, Hays T, Pise-Masison CA, Radonovich MF. et al. Genomic identification of potential risk factors during acetaminophen-induced liver disease in susceptible and resistant strains of mice. Chemical research in toxicology. 2006;19:223–33. doi: 10.1021/tx050285z. [DOI] [PubMed] [Google Scholar]
- 29.He CY, Liang BB, Fan XY, Cao L, Chen R, Guo YJ. et al. The dual role of osteopontin in acetaminophen hepatotoxicity. Acta pharmacologica Sinica. 2012;33:1004–12. doi: 10.1038/aps.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He C, Fan X, Chen R, Liang B, Cao L, Guo Y. et al. Osteopontin is involved in estrogen-mediated protection against diethylnitrosamine-induced liver injury in mice. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2012;50:2878–85. doi: 10.1016/j.fct.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Patouraux S, Rousseau D, Rubio A, Bonnafous S, Lavallard VJ, Lauron J. et al. Osteopontin deficiency aggravates hepatic injury induced by ischemia-reperfusion in mice. Cell death & disease. 2014;5:e1208. doi: 10.1038/cddis.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui A, Mochida S, Ohno A, Nagoshi S, Hirose T, Fujiwara K. Plasma osteopontin levels in patients with fulminant hepatitis. Hepatology research: the official journal of the Japan Society of Hepatology. 2004;29:202–6. doi: 10.1016/j.hepres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Tajiri T, Tate G, Kunimura T, Endo Y, Inoue K, Mitsuya T. et al. Osteopontin Expression in Proliferated Bile Ductules: The Correlation with Liver Damage in Fulminant Hepatitis. Digestive diseases and sciences. 2005;50:188–95. doi: 10.1007/s10620-005-1299-4. [DOI] [PubMed] [Google Scholar]
- 34.Mochida S, Yoshimoto T, Mimura S, Inao M, Matsui A, Ohno A. et al. Transgenic mice expressing osteopontin in hepatocytes as a model of autoimmune hepatitis. Biochemical and biophysical research communications. 2004;317:114–20. doi: 10.1016/j.bbrc.2004.02.180. [DOI] [PubMed] [Google Scholar]
- 35.Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D. et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21:539–50. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Diao H, Liu X, Wu Z, Kang L, Cui G, Morimoto J. et al. Osteopontin regulates interleukin-17 production in hepatitis. Cytokine. 2012;60:129–37. doi: 10.1016/j.cyto.2012.06.287. [DOI] [PubMed] [Google Scholar]
- 37.Saito Y, Kon S, Fujiwara Y, Nakayama Y, Kurotaki D, Fukuda N. et al. Osteopontin small interfering RNA protects mice from fulminant hepatitis. Human gene therapy. 2007;18:1205–14. doi: 10.1089/hum.2007.069. [DOI] [PubMed] [Google Scholar]
- 38.Fan K, Zhang B, Yang H, Wang H, Tan M, Hou S. et al. A humanized anti-osteopontin antibody protects from Concanavalin A induced-liver injury in mice. European journal of pharmacology. 2011;657:144–51. doi: 10.1016/j.ejphar.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 39.Choi SS, Claridge LC, Jhaveri R, Swiderska-Syn M, Clark P, Suzuki A. et al. Osteopontin is up-regulated in chronic hepatitis C and is associated with cellular permissiveness for hepatitis C virus replication. Clinical science. 2014;126:845–55. doi: 10.1042/CS20130473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui G, Chen J, He J, Lu C, Wei Y, Wang L. et al. Osteopontin promotes dendritic cell maturation and function in response to HBV antigens. Drug design, development and therapy. 2015;9:3003–16. doi: 10.2147/DDDT.S81656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morimoto J, Inobe M, Kimura C, Kon S, Diao H, Aoki M. et al. Osteopontin affects the persistence of beta-glucan-induced hepatic granuloma formation and tissue injury through two distinct mechanisms. International immunology. 2004;16:477–88. doi: 10.1093/intimm/dxh044. [DOI] [PubMed] [Google Scholar]
- 42.Chen BL, Zhang GY, Wang SP, Li Q, Xu MH, Shen YM. et al. The combined treatment of praziquantel with osteopontin immunoneutralization reduces liver damage in Schistosoma japonicum-infected mice. Parasitology. 2012;139:522–9. doi: 10.1017/S0031182011002241. [DOI] [PubMed] [Google Scholar]
- 43.Wen Y, Feng D, Wu H, Liu W, Li H, Wang F. et al. Defective Initiation of Liver Regeneration in Osteopontin-Deficient Mice after Partial Hepatectomy due to Insufficient Activation of IL-6/Stat3 Pathway. International journal of biological sciences. 2015;11:1236–47. doi: 10.7150/ijbs.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang G, Chen S, Zhao C, Li X, Zhang L, Zhao W. et al. Gene expression profiles predict the possible regulatory role of OPN-mediated signaling pathways in rat liver regeneration. Gene. 2016;576:782–90. doi: 10.1016/j.gene.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Arai M, Yokosuka O, Fukai K, Imazeki F, Chiba T, Sumi H. et al. Gene expression profiles in liver regeneration with oval cell induction. Biochemical and biophysical research communications. 2004;317:370–6. doi: 10.1016/j.bbrc.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 46.Syn WK. Repair-associated inflammation in nonalcoholic fatty liver disease. Clinical medicine. 2013;13(Suppl 6):s15–9. doi: 10.7861/clinmedicine.13-6-s15. [DOI] [PubMed] [Google Scholar]
- 47.Coombes JD, Swiderska-Syn M, Dolle L, Reid D, Eksteen B, Claridge L. et al. Osteopontin neutralisation abrogates the liver progenitor cell response and fibrogenesis in mice. Gut. 2015;64:1120–31. doi: 10.1136/gutjnl-2013-306484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung TM, Wang X, Kitamura N, Fiel MI, Nieto N. Osteopontin delays resolution of liver fibrosis. Laboratory investigation; a journal of technical methods and pathology. 2013;93:1082–9. doi: 10.1038/labinvest.2013.104. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Lopategi A, Ge X, Lu Y, Kitamura N, Urtasun R. et al. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut. 2014;63:1805–18. doi: 10.1136/gutjnl-2013-306373. [DOI] [PubMed] [Google Scholar]
- 50.Morales-Ibanez O, Dominguez M, Ki SH, Marcos M, Chaves JF, Nguyen-Khac E. et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology. 2013;58:1742–56. doi: 10.1002/hep.26521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seth D, Gorrell MD, Cordoba S, McCaughan GW, Haber PS. Intrahepatic gene expression in human alcoholic hepatitis. Journal of hepatology. 2006;45:306–20. doi: 10.1016/j.jhep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee A, Burghardt RC, Johnson GA, White FJ, Ramaiah SK. The temporal expression of osteopontin (SPP-1) in the rodent model of alcoholic steatohepatitis: a potential biomarker. Toxicologic pathology. 2006;34:373–84. doi: 10.1080/01926230600806543. [DOI] [PubMed] [Google Scholar]
- 53.Banerjee A, Lee JH, Ramaiah SK. Interaction of osteopontin with neutrophil alpha(4)beta(1) and alpha(9)beta(1) integrins in a rodent model of alcoholic liver disease. Toxicology and applied pharmacology. 2008;233:238–46. doi: 10.1016/j.taap.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Banerjee A, Apte UM, Smith R, Ramaiah SK. Higher neutrophil infiltration mediated by osteopontin is a likely contributing factor to the increased susceptibility of females to alcoholic liver disease. The Journal of pathology. 2006;208:473–85. doi: 10.1002/path.1917. [DOI] [PubMed] [Google Scholar]
- 55.Lazaro R, Wu R, Lee S, Zhu NL, Chen CL, French SW. et al. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology. 2015;61:129–40. doi: 10.1002/hep.27383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge X, Leung TM, Arriazu E, Lu Y, Urtasun R, Christensen B. et al. Osteopontin binding to lipopolysaccharide lowers tumor necrosis factor-alpha and prevents early alcohol-induced liver injury in mice. Hepatology. 2014;59:1600–16. doi: 10.1002/hep.26931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ge X, Lu Y, Leung TM, Sorensen ES, Nieto N. Milk osteopontin, a nutritional approach to prevent alcohol-induced liver injury. American journal of physiology Gastrointestinal and liver physiology. 2013;304:G929–39. doi: 10.1152/ajpgi.00014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bertola A, Deveaux V, Bonnafous S, Rousseau D, Anty R, Wakkach A. et al. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes. 2009;58:125–33. doi: 10.2337/db08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH. et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–15. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H. et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323–9. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiefer FW, Neschen S, Pfau B, Legerer B, Neuhofer A, Kahle M. et al. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia. 2011;54:2132–42. doi: 10.1007/s00125-011-2170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiefer FW, Zeyda M, Gollinger K, Pfau B, Neuhofer A, Weichhart T. et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010;59:935–46. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arriazu E, Ge X, Leung TM, Magdaleno F, Lopategi A, Lu Y, Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. Gut; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pereira TA, Syn WK, Machado MV, Vidigal PV, Resende V, Voieta I. et al. Schistosome-induced cholangiocyte proliferation and osteopontin secretion correlate with fibrosis and portal hypertension in human and murine schistosomiasis mansoni. Clinical science. 2015;129:875–83. doi: 10.1042/CS20150117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lorena D, Darby IA, Gadeau AP, Leen LL, Rittling S, Porto LC. et al. Osteopontin expression in normal and fibrotic liver. altered liver healing in osteopontin-deficient mice. Journal of hepatology. 2006;44:383–90. doi: 10.1016/j.jhep.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 66.Pritchett J, Harvey E, Athwal V, Berry A, Rowe C, Oakley F. et al. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology. 2012;56:1108–16. doi: 10.1002/hep.25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espanol-Suner R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S. et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–75. doi: 10.1053/j.gastro.2012.08.024. e7. [DOI] [PubMed] [Google Scholar]
- 68.Komatsu Y, Waku T, Iwasaki N, Ono W, Yamaguchi C, Yanagisawa J. Global analysis of DNA methylation in early-stage liver fibrosis. BMC medical genomics. 2012;5:5. doi: 10.1186/1755-8794-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X. et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology. 2012;55:594–608. doi: 10.1002/hep.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH. et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. The American journal of gastroenterology. 2006;101:2051–9. doi: 10.1111/j.1572-0241.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 71.Xie H, Song J, Du R, Liu K, Wang J, Tang H. et al. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2007;39:167–72. doi: 10.1016/j.dld.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 72.Abu El Makarem MA, Abdel-Aleem A, Ali A, Saber R, Shatat M, Rahem DA. et al. Diagnostic significance of plasma osteopontin in hepatitis C virus-related hepatocellular carcinoma. Annals of hepatology. 2011;10:296–305. [PubMed] [Google Scholar]
- 73.Shin HD, Park BL, Cheong HS, Yoon JH, Kim YJ, Lee HS. SPP1 polymorphisms associated with HBV clearance and HCC occurrence. International journal of epidemiology. 2007;36:1001–8. doi: 10.1093/ije/dym093. [DOI] [PubMed] [Google Scholar]
- 74.Chimparlee N, Chuaypen N, Khlaiphuengsin A, Pinjaroen N, Payungporn S, Poovorawan Y. et al. Diagnostic and Prognostic Roles of Serum Osteopontin and Osteopontin Promoter Polymorphisms in Hepatitis B-related Hepatocellular Carcinoma. Asian Pacific Journal of Cancer Prevention. 2015;16:7211–7. doi: 10.7314/apjcp.2015.16.16.7211. [DOI] [PubMed] [Google Scholar]
- 75.Dong QZ, Zhang XF, Zhao Y, Jia HL, Zhou HJ, Dai C. et al. Osteopontin promoter polymorphisms at locus -443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. Hepatology. 2013;57:1024–34. doi: 10.1002/hep.26103. [DOI] [PubMed] [Google Scholar]
- 76.Simao A, Madaleno J, Silva N, Rodrigues F, Caseiro P, Costa JN. et al. Plasma osteopontin is a biomarker for the severity of alcoholic liver cirrhosis, not for hepatocellular carcinoma screening. BMC gastroenterology. 2015;15:73. doi: 10.1186/s12876-015-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan HW, Ou YH, Peng SY, Liu SH, Lai PL, Lee PH. et al. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98:119–27. doi: 10.1002/cncr.11487. [DOI] [PubMed] [Google Scholar]
- 78.Yu MC, Lee YS, Lin SE, Wu HY, Chen TC, Lee WC. et al. Recurrence and poor prognosis following resection of small hepatitis B-related hepatocellular carcinoma lesions are associated with aberrant tumor expression profiles of glypican 3 and osteopontin. Annals of surgical oncology. 2012;19(Suppl 3):S455–63. doi: 10.1245/s10434-011-1946-2. [DOI] [PubMed] [Google Scholar]
- 79.Sieghart W, Wang X, Schmid K, Pinter M, Konig F, Bodingbauer M. et al. Osteopontin expression predicts overall survival after liver transplantation for hepatocellular carcinoma in patients beyond the Milan criteria. Journal of hepatology. 2011;54:89–97. doi: 10.1016/j.jhep.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 80.Iso Y, Sawada T, Okada T, Kubota K. Loss of E-cadherin mRNA and gain of osteopontin mRNA are useful markers for detecting early recurrence of HCV-related hepatocellular carcinoma. Journal of surgical oncology. 2005;92:304–11. doi: 10.1002/jso.20388. [DOI] [PubMed] [Google Scholar]
- 81.Zhou C, Zhou HJ, Zhang XF, Lou LL, Ye QH, Zheng Y. et al. Postoperative serum osteopontin level is a novel monitor for treatment response and tumor recurrence after resection of hepatitis B-related hepatocellular carcinoma. Annals of surgical oncology. 2013;20:929–37. doi: 10.1245/s10434-012-2749-9. [DOI] [PubMed] [Google Scholar]
- 82.Phillips RJ, Helbig KJ, Van der Hoek KH, Seth D, Beard MR. Osteopontin increases hepatocellular carcinoma cell growth in a CD44 dependant manner. World journal of gastroenterology. 2012;18:3389–99. doi: 10.3748/wjg.v18.i26.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Medico E, Gentile A, Lo Celso C, Williams TA, Gambarotta G, Trusolino L. et al. Osteopontin is an autocrine mediator of hepatocyte growth factor-induced invasive growth. Cancer research. 2001;61:5861–8. [PubMed] [Google Scholar]
- 84.Yoo BK, Gredler R, Chen D, Santhekadur PK, Fisher PB, Sarkar D. c-Met activation through a novel pathway involving osteopontin mediates oncogenesis by the transcription factor LSF. Journal of hepatology. 2011;55:1317–24. doi: 10.1016/j.jhep.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iqbal J, McRae S, Mai T, Banaudha K, Sarkar-Dutta M, Waris G. Role of hepatitis C virus induced osteopontin in epithelial to mesenchymal transition, migration and invasion of hepatocytes. PloS one. 2014;9:e87464. doi: 10.1371/journal.pone.0087464. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Iqbal J, McRae S, Banaudha K, Mai T, Waris G. Mechanism of hepatitis C virus (HCV)-induced osteopontin and its role in epithelial to mesenchymal transition of hepatocytes. The Journal of biological chemistry. 2013;288:36994–7009. doi: 10.1074/jbc.M113.492314. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Zhao J, Dong L, Lu B, Wu G, Xu D, Chen J. et al. Down-regulation of osteopontin suppresses growth and metastasis of hepatocellular carcinoma via induction of apoptosis. Gastroenterology. 2008;135:956–68. doi: 10.1053/j.gastro.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 88.Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL, Zhu XQ. et al. Lentiviral-mediated miRNA against osteopontin suppresses tumor growth and metastasis of human hepatocellular carcinoma. Hepatology. 2008;48:1834–42. doi: 10.1002/hep.22531. [DOI] [PubMed] [Google Scholar]
- 89.Bhattacharya SD, Mi Z, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin regulates epithelial mesenchymal transition-associated growth of hepatocellular cancer in a mouse xenograft model. Annals of surgery. 2012;255:319–25. doi: 10.1097/SLA.0b013e31823e3a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terashi T, Aishima S, Taguchi K, Asayama Y, Sugimachi K, Matsuura S. et al. Decreased expression of osteopontin is related to tumor aggressiveness and clinical outcome of intrahepatic cholangiocarcinoma. Liver international: official journal of the International Association for the Study of the Liver. 2004;24:38–45. doi: 10.1111/j.1478-3231.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 91.Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, Boucher E. et al. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology. 2013;58:1992–2000. doi: 10.1002/hep.26577. [DOI] [PubMed] [Google Scholar]