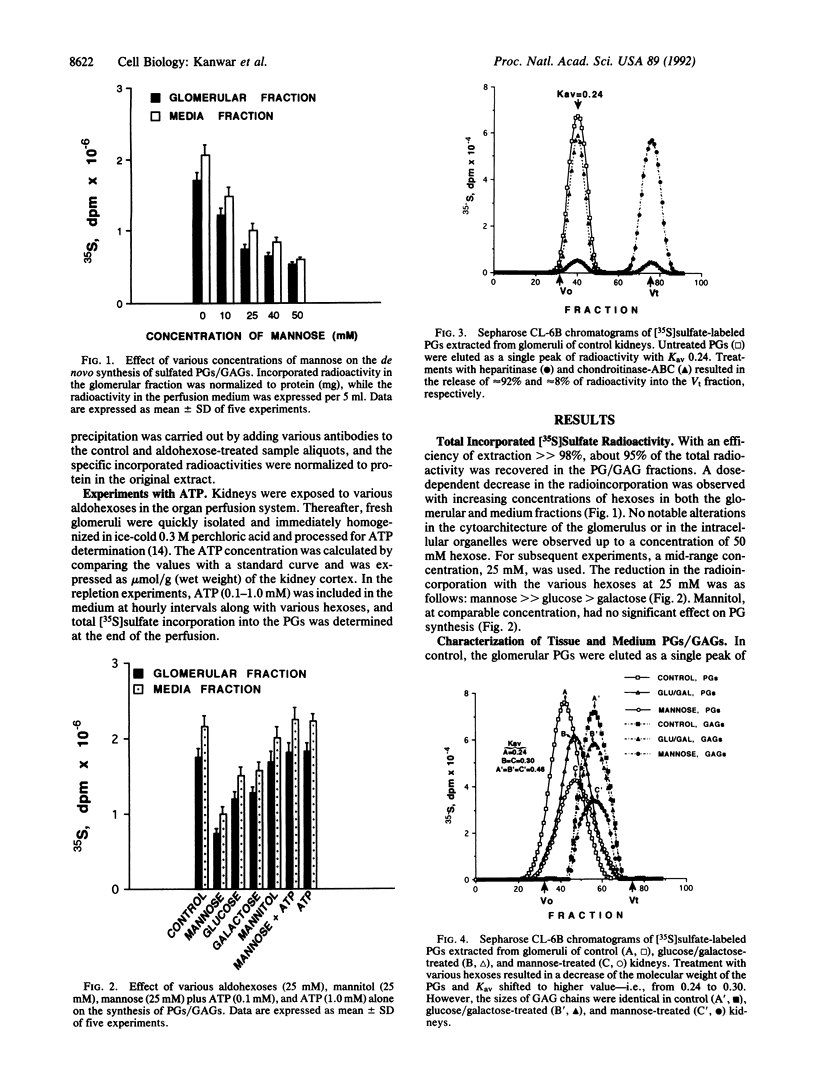
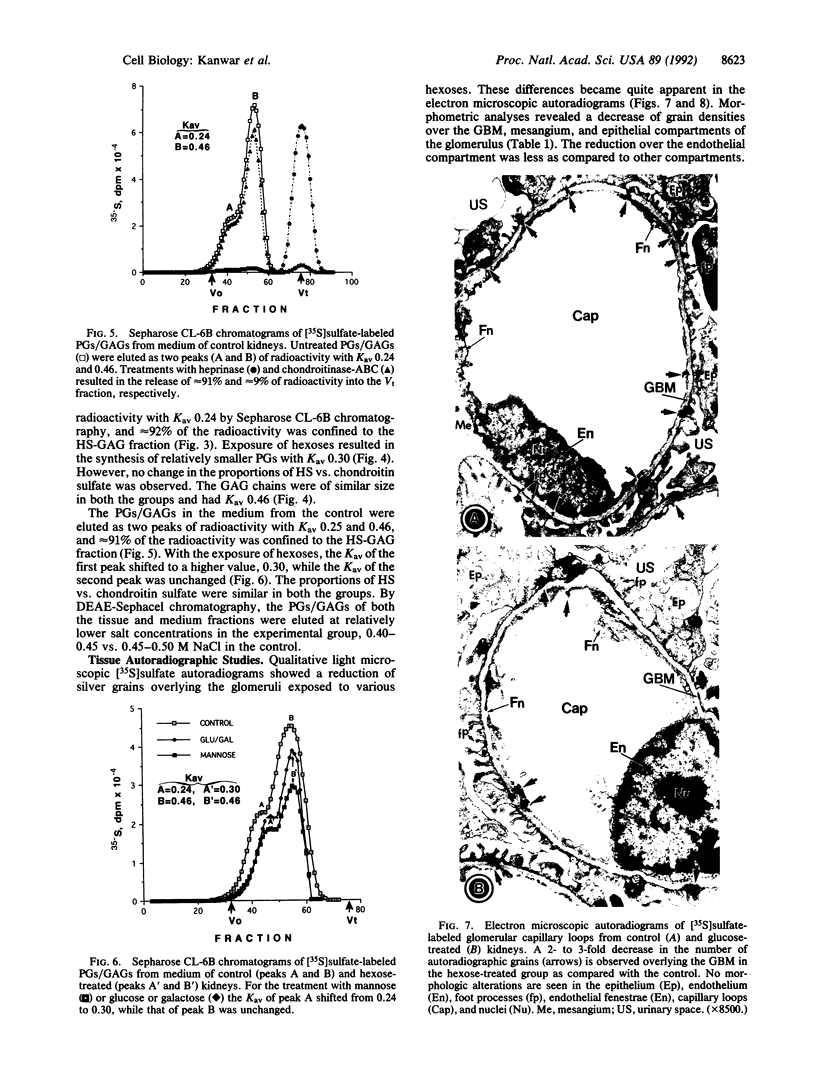
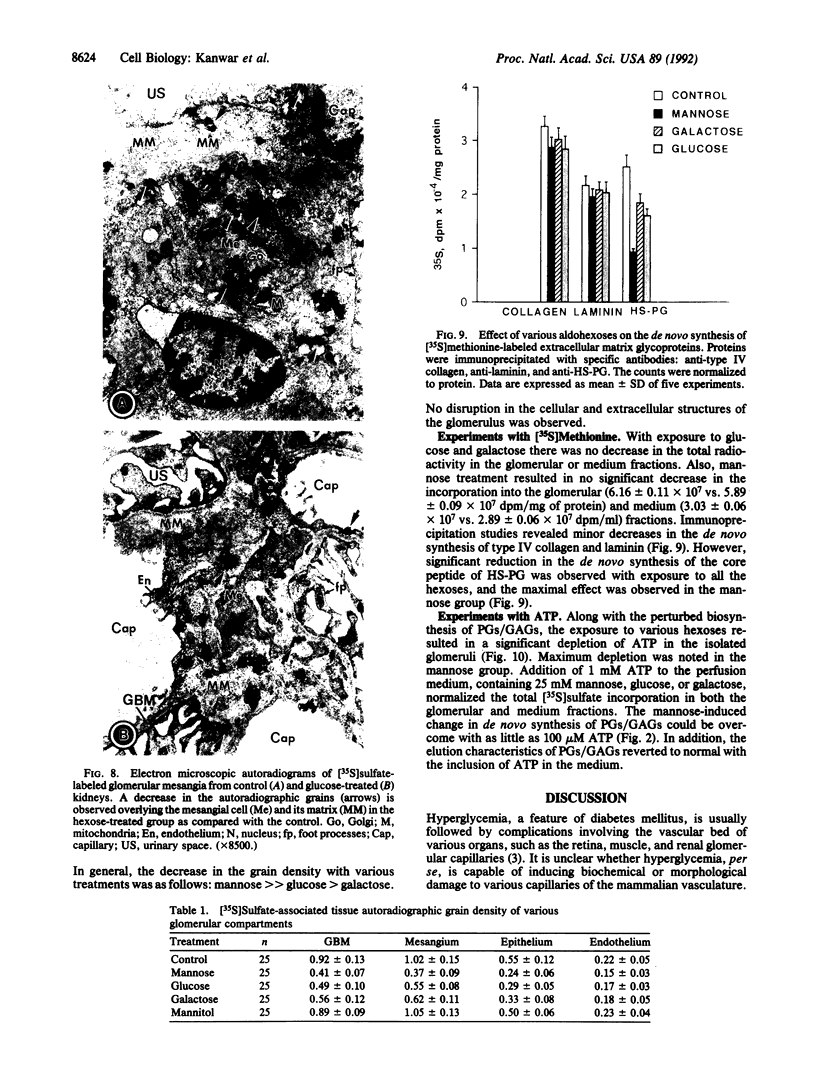
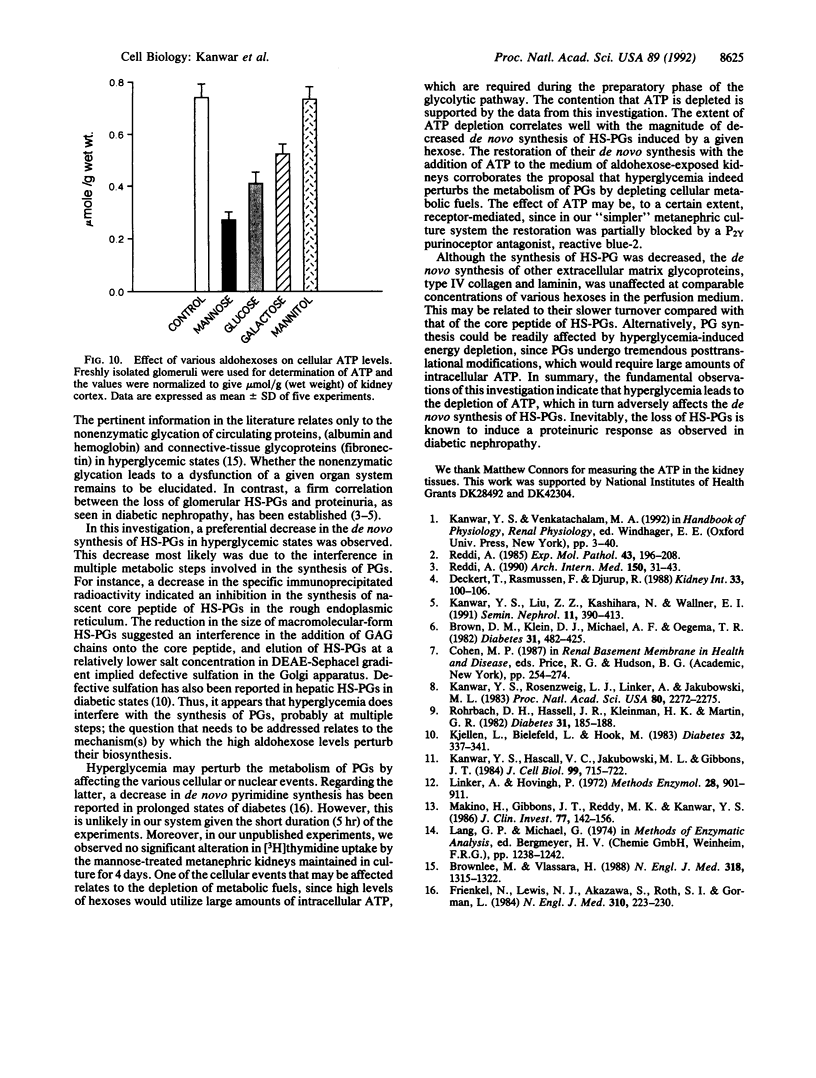
Abstract
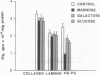
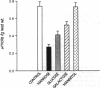
Biosynthetic regulation of renal glomerular heparan sulfate-proteoglycans by various aldohexoses (mannose, glucose, and galactose) was investigated. Isolated kidneys were perfused for 5 hr with medium containing [35S]sulfate, to label sulfated proteoglycans, or [35S]methionine, to label total glomerular proteins. All the hexoses, above 10 mM concentration, caused a significant decrease in the de novo synthesis of [35S]sulfate-labeled proteoglycans. The relative effectiveness of the hexoses was as follows: mannose much greater than glucose greater than galactose. The proteoglycans were of relatively lower molecular weights and exhibited reduced charge-density characteristics. Autoradiographic studies revealed a 2- to 3-fold decrease of grain density over the glomerular basement membrane and mesangial compartments, and immunoprecipitable heparan sulfate-proteoglycans were similarly decreased 2- to 3-fold. There was no significant decrease in the total [35S]methionine-labeled glomerular proteins or immunoprecipitable type IV collagen and laminin. Cellular ATP levels were dramatically reduced in all groups, and the maximal depletion was caused by mannose. Addition of ATP (0.1-1.0 mM) to the perfusion medium resulted in the normalization of the de novo synthesis and of the biochemical characteristics of heparan sulfate-proteoglycans. The relevance of decreased de novo synthesis of proteoglycans due to the depletion of ATP in hyperglycemic states is discussed in terms of increased glomerular permeability to plasma proteins, as seen in diabetes mellitus.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. M., Klein D. J., Michael A. F., Oegema T. R. 35S-glycosaminoglycan and 35S-glycopeptide metabolism by diabetic glomeruli and aorta. Diabetes. 1982 May;31(5 Pt 1):418–425. doi: 10.2337/diab.31.5.418. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Deckert T., Feldt-Rasmussen B., Djurup R., Deckert M. Glomerular size and charge selectivity in insulin-dependent diabetes mellitus. Kidney Int. 1988 Jan;33(1):100–106. doi: 10.1038/ki.1988.16. [DOI] [PubMed] [Google Scholar]
- Freinkel N., Lewis N. J., Akazawa S., Roth S. I., Gorman L. The honeybee syndrome - implications of the teratogenicity of mannose in rat-embryo culture. N Engl J Med. 1984 Jan 26;310(4):223–230. doi: 10.1056/NEJM198401263100404. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Hascall V. C., Jakubowski M. L., Gibbons J. T. Effect of beta-D-xyloside on the glomerular proteoglycans. I. Biochemical studies. J Cell Biol. 1984 Aug;99(2):715–722. doi: 10.1083/jcb.99.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Liu Z. Z., Kashihara N., Wallner E. I. Current status of the structural and functional basis of glomerular filtration and proteinuria. Semin Nephrol. 1991 Jul;11(4):390–413. [PubMed] [Google Scholar]
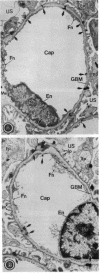
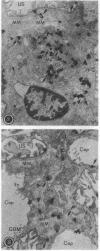
- Kanwar Y. S., Rosenzweig L. J., Linker A., Jakubowski M. L. Decreased de novo synthesis of glomerular proteoglycans in diabetes: biochemical and autoradiographic evidence. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2272–2275. doi: 10.1073/pnas.80.8.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Bielefeld D., Hook M. Reduced sulfation of liver heparan sulfate in experimentally diabetic rats. Diabetes. 1983 Apr;32(4):337–342. doi: 10.2337/diab.32.4.337. [DOI] [PubMed] [Google Scholar]
- Makino H., Gibbons J. T., Reddy M. K., Kanwar Y. S. Nephritogenicity of antibodies to proteoglycans of the glomerular basement membrane--I. J Clin Invest. 1986 Jan;77(1):142–156. doi: 10.1172/JCI112269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. S., Camerini-Davalos R. A. Diabetic nephropathy. An update. Arch Intern Med. 1990 Jan;150(1):31–43. [PubMed] [Google Scholar]
- Reddi A. S. Metabolism of glomerular basement membrane in normal, hypophysectomized, and growth-hormone-treated diabetic rats. Exp Mol Pathol. 1985 Oct;43(2):196–208. doi: 10.1016/0014-4800(85)90040-1. [DOI] [PubMed] [Google Scholar]
- Rohrbach D. H., Hassell J. R., Kleinman H. K., Martin G. R. Alterations in the basement membrane (heparan sulfate) proteoglycan in diabetic mice. Diabetes. 1982 Feb;31(2):185–188. doi: 10.2337/diab.31.2.185. [DOI] [PubMed] [Google Scholar]