Abstract
Idiopathic choroidal neovascularization (ICNV) is a disorder that primarily affecting patients younger than 50 years and can cause severe loss of vision. Choroidal abnormalities, especially choroidal inflammation, have been thought to be involved in the pathophysiology of ICNV. However, the exact pathogenesis of ICNV remains unclear. The aim of our study was investigate the levels of 27 inflammatory cytokines in the aqueous humor of eyes with ICNV, and to determine the effect of intravitreal injection of ranibizumab (IVR) on cytokine levels. Significantly higher levels of IL-2, IL-10, IL-15, IL-17, basic FGF, and GM-CSF were observed in patients with ICNV compared with controls. However, only IL-17 levels were significantly higher in patients with ICNV compared with controls after adjusting for axial length. Furthermore, there were significant correlations between the levels of IL-10, IL-17, GM-CSF, and VEGF and the lesion area. Significant changes in visual acuity and central retinal thickness were observed after IVR. Besides VEGF, IVR also significantly reduced the levels of IL-2, IL-10, basic FGF, and IL-12, however, the IL-6 levels were significantly increased. Our results suggest that there may be an involvement of IL-17-related inflammatory processes in the etiology of ICNV.
Choroidal neovascularization (CNV), which often causes severe vision loss and eventually blindness, is a common pathologic change that may occur in more than 30 ocular diseases1. Age-related macular degeneration (AMD) is the most common cause of CNV in the elderly2. In patients aged 50 years or younger, CNV may also develop secondary to some underlying conditions such as pathologic myopia (PM), angioid streak, trauma and other inflammatory or hereditary disorders3. However, in some young patients with CNV, no apparent cause can be detected, and the CNV is generally categorized as idiopathic CNV (ICNV)4.
Choroidal abnormalities, such as focal choroiditis, compensation of choroidal vessels, choroidal ischemia, myopia, or impaired functional activities of circulating hematopoietic stem cells have been thought to be involved in the pathophysiology of ICNV; however, the exact pathogenesis of ICNV is not yet fully understood3,5,6,7.
Although the natural progression and the final visual outcomes of ICNV are generally considered to be more favorable than CNV attributable to AMD or PM, severe and irreversible visual loss can occur in some untreated eyes4. Nowadays, various ICNV treatments have been reported, such as intravitreal anti-vascular endothelial growth factor (VEGF) therapy or photodynamic therapy (PDT) with verteporfin8,9,10. However, the optimal treatment for ICNV is not well established.
In order to improve ICNV treatment results, the complex molecular mechanisms involved in the pathogenesis of ICNV must be understood more completely. Although inflammatory cytokines such as VEGF and immunoglobulin E were found to be significantly elevated in the serum of patients with ICNV, studies of intraocular inflammatory cytokines in patients with ICNV are sparse11. Additionally, little is known about the biologic response of the intraocular milieu to anti-VEGF therapy. Levels of cytokines in the aqueous humor are supposed to reflect their levels in the vitreous, and have been shown to correlate with the corresponding vitreous levels; however, no correlation between cytokine levels in the aqueous humor and plasma or serum has been detected12,13. Moreover, aqueous humor samples are more easily and safely obtained than vitreous samples.
The purpose of this study was to obtain the profiles of inflammatory cytokines in the aqueous humor of patients with ICNV, and to investigate changes in the levels of various cytokines during anti-VEGF therapy. Furthermore, we also evaluated correlations between cytokines and clinical presentations.
Results
In this prospective study, 39 eyes of 39 patients with ICNV were included and compared with 28 congenital or age-related cataract control eyes. The clinical characteristics of the 2 groups are summarized in Table 1. There were no significant differences in sex and mean age between the two groups. However, mean axial lengths of the ICNV group were significantly longer than those of the control group. Of the 28 control eyes, 15 eyes were congenital cataract, 13 eyes were age-related cataract. All of the ICNV eyes were phakic. The location of ICNV was subfoveal in 19 eyes (48.72%), juxtafoveal in 10 eyes (25.64%), and extrafoveal in 10 eyes (25.64%). Mean spherical equivalent refractive error was −2.41 ± 1.88 diopters. At baseline, mean best corrected visual acuity (BCVA) was 0.67 ± 0.45 logarithm of the minimum angle of resolution (logMAR), mean central retinal thickness (CRT) was 354.05 ± 86.52 μm in the ICNV patients, and mean lesion area was 2.45 ± 1.75 mm2 in the ICNV patients. The mean duration before intravitreal injection of ranibizumab (IVR) in the ICNV group was 56.6 ± 56.2 days (median 30 days, range 7–180 days).
Table 1. Clinical characteristics of the patients. Data are presented as mean ± SD or n (%).
| Characteristic | ICNV Group (n = 39) | Control Group (n = 28) | P value |
|---|---|---|---|
| Mean age of patients, yrs | 35.1 ± 7.8 | 35.6 ± 12.1 | 0.533 |
| Sex, n (%) | 0.400 | ||
| Male | 15 (38.5) | 8 (28.6) | |
| Female | 24 (61.5) | 20 (71.4) | |
| Mean axial length, mm | 24.18 ± 1.15 | 23.06 ± 1.08 | <0.001 |
Cytokine Levels in Aqueous Humor at Baseline Presentation
The levels of 27 types of aqueous humor cytokines in the ICNV and control groups at baseline are listed in Table 2. Compared to the control group, the ICNV group showed significantly higher levels of IL-2, IL-10, IL-15, IL-17, basic FGF, and GM-CSF. However, VEGF levels were not significantly different between the ICNV and control groups. Logistic regression analysis demonstrated that only IL-17 levels were significantly higher in the ICNV group compared with the control group after adjusting for axial length (Table 3).
Table 2. Aqueous humor levels of 27 types of cytokines in the ICNV and control groups.
| ICNV Group (n = 39) | Control Group (n = 28) | P Value | |
|---|---|---|---|
| IL-2 | 1.8 (0–11.2) | <0.001 | |
| IL-10 | 4.3 (2.2–15.2) | 3.6 (0–16.1) | 0.039 |
| IL-15 | 7.6 (0–27.8) | 5.9 (0–39.6) | 0.049 |
| IL-17 | 9.5 (0–55.7) | 0 (0–45.7) | <0.001 |
| basic FGF | 13.2 (5.0–88.4) | 7.2 (0–79.6) | <0.001 |
| GM-CSF | 169.0 (35.6–1450.1) | 131.0 (0–1533.1) | 0.001 |
| IL-1b | 0 (0–3.2) | 0 | 0.397 |
| IL-1ra | 0 (0–131.3) | 0 (0–26.1) | 0.847 |
| IL-4 | 0 | 0 (0–0.4) | 0.238 |
| IL-5 | 0 | 0 | — |
| IL-6 | 3.2 (0–120.9) | 5.1 (0–15.6) | 0.346 |
| IL-7 | 1.9 (0–3.6) | 2.0 (0–11.4) | 0.418 |
| IL-8 | 0 (0–4.5) | 0 (0–6.9) | 0.668 |
| IL-9 | 0 (0–5.2) | 0 (0–3.1) | 0.157 |
| IL-12 | 10.3 (0–25.0) | 9.5 (0–29.9) | 0.559 |
| IL-13 | 2.0 (0–6.5) | 1.6 (0–8.4) | 0.220 |
| Eotaxin | 0 | 0 (0–2.3) | 0.238 |
| G-CSF | 0 | 0 | — |
| IFN-γ | 0 (0–9.6) | 0 | 0.397 |
| IP-10 | 47.7 (0–407.4) | 69.5 (0–184.9) | 0.162 |
| MCP-1 | 338.9 (180.7–596.7) | 334.8 (15.9–544.6) | 0.575 |
| MIP-1a | 0.7 (0–3.9) | 0 (0–4.1) | 0.152 |
| MIP-1b | 7.1 (1.9–22.0) | 8.2 (1.1–19.8) | 0.100 |
| PDGF-bb | 0 | 0 (0–1.4) | 0.238 |
| RANTES | 0 | 0 (0–25.8) | 0.093 |
| TNF-α | 0 | 0 | — |
| VEGF | 40.9 (17.6–108.8) | 38.9 (0–105.9) | 0.869 |
Values in boldface type indicate statistical significance. Levels are expressed as the median (range) pg/ml.
Table 3. Association of ICNV with cytokines in aqueous humor by logistic regression analysis.
| OR | 95% CI | P Value | |
|---|---|---|---|
| IL-2 | 1.259 | (0.967–1.638) | 0.087 |
| IL-10 | 1.252 | (1.000–1.569) | 0.052 |
| IL-15 | 1.046 | (0.958–1.141) | 0.361 |
| IL-17 | 1.131 | (1.019–1.256) | 0.021 |
| basic FGF | 1.068 | (0.997–1.144) | 0.059 |
| GM-CSF | 1.002 | (0.999–1.004) | 0.183 |
| VEGF | 1.010 | (0.987–1.034) | 0.388 |
P values are adjusted for axial length.
Correlations between Aqueous Cytokines and Clinical Parameters
In the ICNV group, no significant correlation was noted between baseline visual acuity (VA) and CRT (r = 0.061, P = 0.712). The aqueous humor levels of IL-2, IL-10, IL-15, IL-17, basic FGF, GM-CSF, and VEGF did not correlate significantly with baseline VA or CRT (Table 4). However, there were significant correlations between the levels of IL-10, IL-17, GM-CSF, and VEGF and the lesion area (Table 4).
Table 4. Correlations between aqueous humor cytokines and visual acuity, central retinal thickness, or lesion area.
| Variables | Visual Acuity |
Central Retinal Thickness |
Lesion Area |
|||
|---|---|---|---|---|---|---|
| r | P Value | r | P Value | r | P Value | |
| IL-2 | 0.272 | 0.095 | −0.147 | 0.372 | 0.372 | 0.051 |
| IL-10 | 0.282 | 0.082 | −0.118 | 0.475 | 0.538 | 0.003 |
| IL-15 | 0.165 | 0.316 | −0.120 | 0.468 | 0.316 | 0.101 |
| IL-17 | 0.304 | 0.060 | −0.280 | 0.084 | 0.520 | 0.005 |
| basic FGF | 0.108 | 0.514 | −0.287 | 0.077 | 0.286 | 0.140 |
| GM-CSF | 0.293 | 0.070 | −0.255 | 0.117 | 0.454 | 0.015 |
| VEGF | 0.164 | 0.318 | 0.050 | 0.763 | 0.465 | 0.013 |
Correlation coefficient (r) and P values are calculated by Spearman’s correlation.
Changes in Aqueous Cytokines and Clinical Parameters during Treatment
All patients received an initial IVR at their baseline visit. Twenty-four of the 39 patients (61.5%) were retreated according to protocol criteria during a 12-month follow-up period, of which 11, 8, and 5 patients required 2, 3, and 4 injections, respectively. In total, the mean number of ranibizumab injections was 2.1 ± 1.1 during the 12-month follow-up period. We obtained a second aqueous sample from 11 patients who received the second injection 1 month after the first injection.
The mean BCVA was 0.25 ± 0.28 logMAR and 0.16 ± 0.21 logMAR at 1 month and 12 months, respectively (Fig. 1). There was a statistically significant difference in BCVA at month 1 (P < 0.001) and at month 12 (P < 0.001) compared with baseline. CRT as measured by OCT demonstrated a statistically significant difference at baseline versus month 1 (P < 0.001) and month 12 (P < 0.001). The mean CRT was 237.26 ± 30.03 μm and 219.15 ± 19.11 μm at 1 month and 12 months, respectively (Fig. 2). One month after IVR, in the 11 patients, the levels of IL-2, IL-10, and basic FGF were significantly decreased. Furthermore, the VEGF level was significantly decreased from a median of 40.3 pg/ml to 5.7 pg/ml. Moreover, the IL-12 level was significantly decreased. In contrast, the IL-6 levels were significantly increased. Levels of IL-15, IL-17, GM-CSF, and other cytokines were not significantly altered by IVR in the 11 patients (Table 5).
Figure 1. Changes of mean best-corrected visual acuity (BCVA) during 12 months after IVR for ICNV.
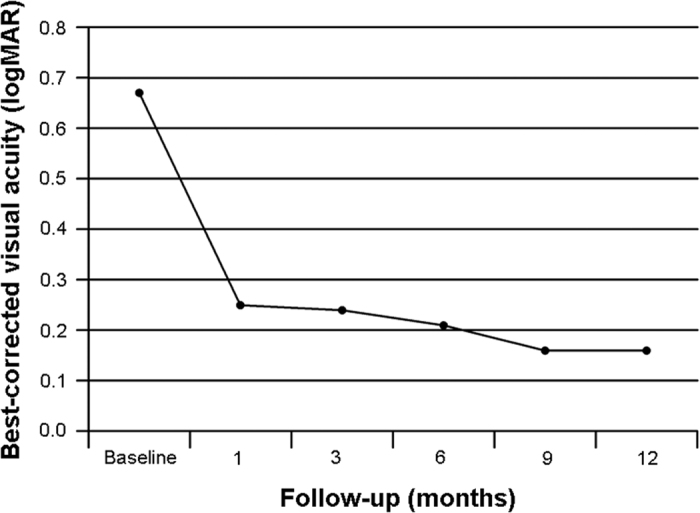
Figure 2. Changes of mean central retinal thickness during 12 months after IVR for ICNV.
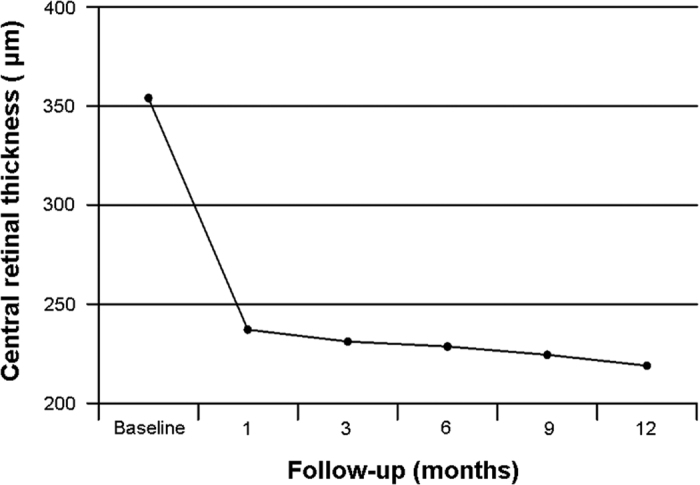
Table 5. Aqueous humor levels of 27 types of cytokines in subgroup A and subgroup B and changes after IVR.
| Subgroup A (n = 15) | Subgroup B (n = 24) | Preinjection (n = 11) | Postinjection (n = 11) | P Value | ||
|---|---|---|---|---|---|---|
| Subgroup A vs Subgroup B | Preinjection vs Postinjection | |||||
| IL-2 | 1.3 (0–3.3) | 1.8 (0–11.2) | 2.0 (0–11.2) | 1.8 (0–2.8) | 0.187 | 0.028 |
| IL-10 | 4.1 (2.8–6.1) | 5.0 (2.2–15.2) | 5.3 (2.2–15.2) | 2.4 (0–4.3) | 0.043 | 0.004 |
| IL-15 | 6.6 (3.5–10.5) | 7.9 (0–27.8) | 8.1 (3.8–18.1) | 7.8 (3.1–10.3) | 0.112 | 0.175 |
| IL-17 | 9.5 (0–15.1) | 9.8 (0–55.7) | 9.5 (0–55.7) | 9.2 (0–11.2) | 0.675 | 0.221 |
| basic FGF | 13.4 (7.5–28.3) | 12.9 (5.0–88.4) | 12.5 (5.0–88.4) | 10. 0 (0–42.4) | 0.544 | 0.041 |
| GM-CSF | 169.0 (114.2–291.8) | 171.8 (35.6–1450.1) | 207.6 (71.5–1450.1) | 165.4 (39.6–259.9) | 0.624 | 0.075 |
| IL-1b | 0 (0–3.2) | 0 | 0 | 0 | 0.206 | — |
| IL-1ra | 0 (0–12.0) | 5.0 (0–131.3) | 0 (0–131.3) | 0 (0–29.4) | 0.122 | 0.612 |
| IL-4 | 0 | 0 | 0 | 0 (0–0.3) | — | 0.317 |
| IL-5 | 0 | 0 | 0 | 0 | — | — |
| IL-6 | 3.4 (0–7.5) | 3.0 (0–120.9) | 2.7 (0–120.9) | 4.5 (1.9–698.2) | 0.897 | 0.016 |
| IL-7 | 1.6 (0–3.2) | 2.0 (0–3.6) | 2.1 (0–3.6) | 1.5 (0–3.4) | 0.056 | 0.663 |
| IL-8 | 0 | 0 (0–4.5) | 0 (0–4.1) | 0 (0–16.5) | 0.100 | 0.317 |
| IL-9 | 0 (0–2.0) | 0 (0–5.2) | 0 (0–1.4) | 0 (0–1.4) | 1.000 | 1.000 |
| IL-12 | 10.0 (4.2–15.3) | 11.5 (0–25.0) | 10.3 (0–25.0) | 0 (0–9.1) | 0.238 | 0.008 |
| IL-13 | 1.8 (0.6–6.5) | 2.0 (0–5.4) | 2.1 (0–5.4) | 1.7 (0.5–3.4) | 0.462 | 0.386 |
| Eotaxin | 0 | 0 | 0 | 0 | — | — |
| G-CSF | 0 | 0 | 0 | 0 (0–12.8) | — | 0.317 |
| IFN-γ | 0 | 0 (0–9.6) | 0 | 0 (0–9.3) | 0.429 | 0.317 |
| IP-10 | 59.4 (0–202.7) | 39.4 (0–407.4) | 32.2 (0–407.4) | 46.2 (1.6–1375.8) | 0.231 | 0.110 |
| MCP-1 | 331.5 (180.7–443.7) | 346.4 (185.5–596.7) | 343.2 (206.1–476.8) | 311.2 (171.6–625.9) | 0.335 | 0.648 |
| MIP-1a | 0.7 (0–1.1) | 0 (0.3–3.9) | 0 (0–3.9) | 0.7 (0–0.8) | 0.616 | 0.674 |
| MIP-1b | 5.1 (1.9–13.3) | 7.9 (3.2–22.0) | 7.1 (3.2–13.5) | 7.0 (4.0–15.4) | 0.030 | 0.407 |
| PDGF-bb | 0 | 0 | 0 | 0 | — | — |
| RANTES | 0 | 0 | 0 | 0 | — | — |
| TNF-α | 0 | 0 | 0 | 0 (0–5.4) | — | 0.317 |
| VEGF | 37.5(18.5–69.1) | 44.8(17.6–108.8) | 40.3 (17.6–108.8) | 5.7 (0–59.9) | 0.097 | 0.003 |
Values in boldface type indicate statistical significance. Levels are expressed as the median (range) pg/ml.
Although our findings showed that IVR therapy was effective for CNV and retinal edema, 24 of the 39 eyes (61.5%) needed reinjections (refractory group). We sought to identify the exacerbating cytokine or cytokines by comparing refractory group (subgroup B) with improved group (subgroup A). There was no significant difference between the two groups in terms of sex, age, axial length, duration before IVR, spherical equivalent, CNV location, baseline BCVA, CRT, or lesion area (see Supplementary Table S1). When the baseline cytokine levels of both groups were compared, we found that the levels of IL-10 and MIP-1b were significantly higher in subgroup B than in subgroup A (Table 5).
Discussion
Using the multiplex bead immunoassays, we found significantly higher levels of IL-17 in the aqueous humor of ICNV patients. To the best of our knowledge, our study was the first to analyze the distribution of various inflammatory cytokines in the aqueous humor of ICNV patients and to assess their changes after IVR treatment.
It is now quite evident that the development and progression of CNV is associated with alterations in various pro- and anti-angiogenic factors14. The collective evidence suggests that VEGF is a critical angiogenic factor for promoting ocular angiogenesis15. Ocular anti-VEGF therapy is highly effective for treating CNV, however, it is important to note that not all patients respond to such therapy, which suggests that VEGF-driven pathways are only part of the complex processes regulating angiogenesis, and that other molecules besides VEGF may play a crucial role in aberrant angiogenesis15. Increasing evidence suggests that inflammation and the immune system may be involved in the development of CNV15,16,17.
Interestingly, we found significantly elevated aqueous humor levels of IL-17 in the ICNV eyes. IL-17, the signature cytokine of T-helper 17 (Th17) cells, is an inflammatory cytokine that plays a crucial role in the pathogenesis of autoimmune and inflammatory diseases, including rheumatoid arthritis, psoriasis, uveitis, and scleritis, by inducing the expression of inflammatory cytokines and chemokines18,19. Additionally, IL-17 also promotes the angiogenesis effect of VEGF, basic FGF, and hepatocyte growth factor20. Compelling evidence suggests that IL-17 could be involved in the pathogenesis of neovascular AMD (nAMD) by promoting CNV21. Elevated serum levels of IL-17 and other inflammatory cytokines were recently shown in nAMD patients21. Moreover, Wei et al. have demonstrated the elevated expression of IL-17A and its receptor IL-17RC in the macular tissues of nAMD patients22. Importantly, Hasegawa et al. have demonstrated that IL-17 has a strong potential for stimulating neovascularization in a VEGF-independent manner in laser-induced experimental CNV model23. Furthermore, the suppressive effect of anti-IL-17 therapy on CNV volume and area was similar to that of anti-VEGF therapy, and the combination of anti-VEGF and anti-IL-17 therapy was more effective23. The observed presence of elevated intraocular IL-17 in our study indicates that IL-17 may be involved in the pathogenesis of ICNV. Furthermore, IL-17 was not remarkably inhibited by the preceding anti-VEGF therapy. Considering the effect of anti-IL-17 treatment in laser-induced experimental CNV, we speculate that IL-17 could potentially be an additional target molecule in therapy for ICNV. Indeed, anti-IL-17 therapy inhibits multiple inflammatory cytokines and appears to be effective for both rheumatoid arthritis and psoriasis in clinical trials24,25.
In our study, the aqueous VEGF levels measured in the eyes of control patients with cataract were aligned with study by Funk et al., in which multiplex bead immunoassays were also used26. However, no significant difference in the VEGF levels was noted between the ICNV group and the control group. This finding was well supported by various previous studies that VEGF levels in the aqueous humor of patients with nAMD were not significantly different from patients with cataract although there were some contradictory results26,27,28,29,30,31. It was also interesting to note that anti-VEGF treatment significantly decreased VEGF levels although VEGF levels were not necessarily elevated in aqueous humour, which was consistent with the results of the previous studies28,30. The total levels of cytokines in the ocular fluid may be affected by the area of CNV lesions30,32. Correspondingly, the aqueous humor levels of IL-10, IL-17, GM-CSF, and VEGF were positively correlated with the lesion area in our study. Because the relatively small lesion area, we speculate that the levels of some inflammatory cytokines, including VEGF, may not be high enough to show statistical difference from the control in the aqueous humor although they are elevated in the lesion site and vitreous fluid in ICNV29,30.
Our study demonstrated that inflammatory cytokine levels other than VEGF, including IL-2, IL-10, IL-12, and basic FGF were also decreased after IVR in the refractory group. Although aqueous humor levels of IL-2, IL-10, and basic FGF were not significantly different between the ICNV group and the control group after adjusting for axial length, these cytokines appear to be involved in the mechanism of CNV as well. IL-2, a T-helper, lymphocyte-derived cytokine, upregulates VEGF expression in vitro33,34. Although the precise mechanism through which IL-10 promotes angiogenesis in the eye remains unclear, recent studies have demonstrated that it promotes angiogenesis either by preventing the infiltration of anti-angiogenic macrophages into the choroid or by directly polarizing macrophages towards a proangiogenic phenotype35,36. Basic FGF is a potent angiogenic molecule that occupied the central stage in the angiogenesis field before the discovery of the VEGF family37. Previous studies have demonstrated that basic FGF is involved in the pathogenesis of proliferative diabetic retinopathy38. However, aqueous IL-6 concentration was significantly increased after IVR. IL-6 is an important inflammatory cytokine, which are increased in patients with ischemic retinal disorders39,40. It induces numerous angiogenic and inflammatory cytokines directly or indirectly, including VEGF, which may explain the suboptimal response to IVR in the refractory group of our study41. However, further investigations are necessary to determine the exact underlying effect of anti-VEGF treatment in other intraocular inflammatory cytokines.
Previous studies have reported promising results with anti-VEGF therapy in ICNV patients8,9,10,42. Zhang et al. reported that the mean number of bevacizumab injections was 2 during the 12-month follow-up, and 40.0% and 70.0% of eyes had complete resolution of fluid after a single or an additional injection, respectively9. Similarly, the mean number of ranibizumab injections was 2.1 during the 12-month follow-up in our study. It suggests that the number of injections does not differ significantly between ranibizumab and bevacizumab in treatment of ICNV. Another important result of this study was the significantly elevated aqueous humor levels of IL-10 and MIP-1b in the refractory group of our study. These cytokines may play a role in the inflammation-induced angiogenesis by regulating macrophages35,36,43. Some more studies are needed to allow a better understanding of the pathogenesis of ICNV.
The present study has several limitations. First, we were unable to measure the cytokine levels in the aqueous humor of all the ICNV patients 1 month later after the first injection. Since we collected aqueous humor samples just before IVR, patients without recurrent macular edema did not receive IVR. Second, although our study identified key inflammatory cytokines in ICNV patients and investigated their changes during anti-VEGF therapy, we did not reveal any potential mechanisms. Further animal experiments and in vitro experiments are required to determine the mechanism by which these cytokines are correlated with ICNV.
In conclusion, this study investigated a profile of inflammatory cytokines in the aqueous humor of patients with ICNV. We found that IL-17 levels were increased significantly in the aqueous humor of patients with ICNV. This suggests that there may be an involvement of IL-17-related inflammatory processes in the etiology of ICNV and that may facilitate new treatments for this vision-threatening disease.
Methods
Study Subjects
This study was performed at the Eye Center, Second Affiliated Hospital of Zhejiang University School of Medicine. The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine, and the procedures used conformed to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients before study inclusion. Thirty-nine consecutive ICNV patients who were scheduled to undergo intravitreal injection of 0.5 mg ranibizumab (Lucentis; Novartis Pharma AG, Basel, Switzerland) were studied.
Inclusion criteria for ICNV in this study were: (1) patients younger than 50 years; (2) previous untreated CNV; (3) OCT showing intraretinal edema, subretinal fluid (SRF), or pigment epithelial detachment (PED); and (4) absence of concurrent ocular diseases in the study eyes that compromised or could have compromised vision and ocular condition. Exclusion criteria were: (1) clinical features suggesting that CNV was secondary to other causes such as AMD, PM, trauma, or hereditary diseases; (2) axial length >26.0 mm or myopia> −6 diopter; (3) history of prior treatment for ICNV, including intravitreal drug injection, PDT, systemic or focal steroids; (4) previous intraocular surgery.
Diagnostic Procedures and Follow-up
All eyes underwent a full ophthalmological examination at baseline, including BCVA testing using a Snellen chart, slit-lamp biomicroscopy, dilated fundus examination, intraocular pressure (IOP) measurement, color fundus photography, axial length (IOLMaster; Carl Zeiss Meditec, Dublin, CA, USA), fundus fluorescein angiography (FFA, Heidelberg Engineering HRA Spectralis, Heidelberg, Germany), and OCT (Cirrus OCT; Carl Zeiss Meditec, Dublin, CA, USA). CRT (thickness of the 1-mm central retina) was measured by the fast macular thickness map scan modes. Lesion area, including dye leakage area, PED, subretinal hemorrhage, and hyperfluorescent staining of fibrous tissue was measured as described previously44. For statistical analysis, BCVA were converted to logMAR units. Follow-up examinations were performed on 1 day, 1 week, 1 month, 2 months, 3 months, and every 3 months thereafter over a period of 12 months. However, patients were asked to come earlier in cases of visual loss and/or recurrence of metamorphopsia. Slit-lamp biomicroscopy, dilated fundus examination, IOP measurement, and the measurements of BCVA and CRT were performed at each study visit. Additional FFA and color fundus photography was performed if physicians suspected vision loss had occurred without associated signs of recurrent macular edema by OCT. IVR was repeated if OCT showed persistent intraretinal edema, SRF, or PED. Patients were defined as refractory to IVR treatment if they needed reinjections. Therefore, all patients were divided into 2 subgroups: the improved group (subgroup A) included patients who received 1 injection during the 12-month follow-up period, and the refractory group (subgroup B) included patients who received 2 or more injections during the 12-month follow-up period.
Intravitreal Injection of Ranibizumab and Sample Collection
All patients received an initial IVR as described previously45. Immediately before intravitreal injection, approximately 0.05–0.1 mL of undiluted aqueous humor was collected by performing an anterior chamber limbal paracentesis, and the aqueous samples were stored at −80 °C until processing. We obtained aqueous samples from all patients before the first injection. We also obtained a second aqueous sample from 11 patients who received the second injection 1 month after the first injection.
Control Group
Control aqueous samples were obtained from 28 eyes of 28 congenital or age-related cataract patients who underwent cataract surgery by limbal paracentesis, and the samples were stored at −80 °C until processing. Exclusion criteria were the following: (1) any type of retinal disease, uveitis, or glaucoma; (2) previous intraocular surgery; (3) diabetes mellitus, use of immunosuppressive drugs, or malignant tumors any location; and (4) axial length >26.0.
Multiplex Bead Analyses
A Bio-Plex multiplex assay (Bio-Plex Human Cytokine 27-plex panel; Bio-Rad, Hercules, CA, USA) and a multiplex bead analysis system (Bio-Plex Suspension Array System; Bio-Rad) were used simultaneously to measure the levels of 27 types of cytokines in the aqueous humor, as previously described46,47,48. The following were analyzed: IL-1b, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, Eotaxin, basic FGF, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1a, MIP-1b, PDGF-bb, RANTES, TNF-α, and VEGF. The levels of aqueous humor cytokines were set to 0 if the levels were below the detectable levels.
Statistical Analysis
Statistical analyses were performed using SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA). Data were recorded as the means ± standard deviation (SD) or the median and range. To evaluate the differences between groups, unpaired t test or Mann-Whitney U test was used to determine their significance. The χ2 test or Fisher exact test was used to compare categorical variables. Paired t test or Wilcoxon signed rank test was used to compare within-group categorical variable changes from baseline. Spearman’s correlation analysis was used to evaluate the relationship between numerical data. Logistic regression analysis was carried out to confirm the association of elevated cytokines with ICNV. P < 0.05 was considered to be statistically significant.
Additional Information
How to cite this article: Yin, H. et al. Idiopathic Choroidal Neovascularization: Intraocular Inflammatory Cytokines and the Effect of Intravitreal Ranibizumab Treatment. Sci. Rep. 6, 31880; doi: 10.1038/srep31880 (2016).
Supplementary Material
Acknowledgments
This work was supported by the grant from the National Natural Science Foundation of China (81571819 and 81300757); and by the grant from the Natural Science Foundation of Zhejiang Province (LY14H120004 and LY16H120002). The funders had no role in study design, data collection and analysis, decision on publish, or preparation of the manuscript.
Footnotes
Author Contributions H.Y. and X.F. designed the study and prepared the manuscript. J.M., Y.Y., Z.C., Z.S. and L.F. collected the clinical data. H.Y. and S.G. performed the experiments. M.C., P.Y., F.W. and J.Y. carried out statistical analysis and revised manuscript. All authors have reviewed the manuscript.
References
- Green W. R. & Wilson D. J. Choroidal neovascularization. Ophthalmology 93, 1169–1176 (1986). [DOI] [PubMed] [Google Scholar]
- Grossniklaus H. E. & Green W. R. Choroidal neovascularization. Am J Ophthalmol 137, 496–503 (2004). [DOI] [PubMed] [Google Scholar]
- Cohen S. Y., Laroche A., Leguen Y., Soubrane G. & Coscas G. J. Etiology of choroidal neovascularization in young patients. Ophthalmology 103, 1241–1244 (1996). [DOI] [PubMed] [Google Scholar]
- Ho A. C., Yannuzzi L. A., Pisicano K. & DeRosa J. The natural history of idiopathic subfoveal choroidal neovascularization. Ophthalmology 102, 782–789 (1995). [DOI] [PubMed] [Google Scholar]
- Sasahara M., Otani A., Yodoi Y. & Yoshimura N. Circulating hematopoietic stem cells in patients with idiopathic choroidal neovascularization. Invest Ophthalmol Vis Sci 50, 1575–1579 (2009). [DOI] [PubMed] [Google Scholar]
- Lee J. E. et al. Topographical relationship between the choroidal watershed zone and submacular idiopathic choroidal neovascularisation. Br J Ophthalmol (2015). [DOI] [PubMed] [Google Scholar]
- Inagaki M., Harada T., Kiribuchi T., Ohashi T. & Majima J. Subfoveal choroidal neovascularization in uveitis. Ophthalmologica 210, 229–233 (1996). [DOI] [PubMed] [Google Scholar]
- Sudhalkar A., Yogi R. & Chhablani J. Anti-Vascular Endothelial Growth Factor Therapy for Naive Idiopathic Choroidal Neovascularization: A Comparative Study. Retina 35, 1368–1374 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang H., Liu Z. L., Sun P. & Gu F. Intravitreal bevacizumab for treatment of subfoveal idiopathic choroidal neovascularization: results of a 1-year prospective trial. Am J Ophthalmol 153, 300–306, e301 (2012). [DOI] [PubMed] [Google Scholar]
- Kang H. M. & Koh H. J. Intravitreal anti-vascular endothelial growth factor therapy versus photodynamic therapy for idiopathic choroidal neovascularization. Am J Ophthalmol 155, 713–719, 719 e711 (2013). [DOI] [PubMed] [Google Scholar]
- Yang F. et al. Serum inflammatory factors in patients with idiopathic choroidal neovascularization. Ocul Immunol Inflamm 18, 390–394 (2010). [DOI] [PubMed] [Google Scholar]
- Noma H. et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond) 22, 42–48 (2008). [DOI] [PubMed] [Google Scholar]
- Funatsu H. et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol 243, 3–8 (2005). [DOI] [PubMed] [Google Scholar]
- Nowak J. Z. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 58, 353–363 (2006). [PubMed] [Google Scholar]
- Sene A., Chin-Yee D. & Apte R. S. Seeing through VEGF: innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol Med 21, 43–51 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin B. et al. Connecting the innate and adaptive immune responses in mouse choroidal neovascularization via the anaphylatoxin C5a and gammadeltaT-cells. Sci Rep 6, 23794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X. et al. Choroidal neovascularization is inhibited via an intraocular decrease of inflammatory cells in mice lacking complement component C3. Sci Rep 5, 15702 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi-Obi A. et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med 13, 711–718 (2007). [DOI] [PubMed] [Google Scholar]
- Patel D. D., Lee D. M., Kolbinger F. & Antoni C. Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis 72 Suppl 2, ii116–ii123 (2013). [DOI] [PubMed] [Google Scholar]
- Takahashi H., Numasaki M., Lotze M. T. & Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol Lett 98, 189–193 (2005). [DOI] [PubMed] [Google Scholar]
- Liu B. et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J Transl Med 9, 1–12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. et al. Hypomethylation of the IL17RC promoter associates with age-related macular degeneration. Cell Rep 2, 1151–1158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa E. et al. IL-23-independent induction of IL-17 from gammadeltaT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J Immunol 190, 1778–1787 (2013). [DOI] [PubMed] [Google Scholar]
- Genovese M. C. et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum 62, 929–939 (2010). [DOI] [PubMed] [Google Scholar]
- Russell C. B. et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol 192, 3828–3836 (2014). [DOI] [PubMed] [Google Scholar]
- Funk M. et al. Neovascular age-related macular degeneration: intraocular cytokines and growth factors and the influence of therapy with ranibizumab. Ophthalmology 116, 2393–2399 (2009). [DOI] [PubMed] [Google Scholar]
- Fauser S., Viebahn U. & Muether P. S. Intraocular and systemic inflammation-related cytokines during one year of ranibizumab treatment for neovascular age-related macular degeneration. Acta Ophthalmol 93, 734–738 (2015). [DOI] [PubMed] [Google Scholar]
- Jonas J. B., Tao Y., Neumaier M. & Findeisen P. Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age-related macular degeneration. Arch Ophthalmol 128, 1281–1286 (2010). [DOI] [PubMed] [Google Scholar]
- Muether P. S. et al. Intraocular growth factors and cytokines in patients with dry and neovascular age-related macular degeneration. Retina 33, 1809–1814 (2013). [DOI] [PubMed] [Google Scholar]
- Roh M. I. et al. Concentration of cytokines in the aqueous humor of patients with naive, recurrent and regressed CNV associated with amd after bevacizumab treatment. Retina 29, 523–529 (2009). [DOI] [PubMed] [Google Scholar]
- Sakurada Y. et al. Aqueous humor cytokine levels in patients with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Ophthalmic Res 53, 2–7 (2015). [DOI] [PubMed] [Google Scholar]
- Kvanta A., Algvere P. V., Berglin L. & Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 37, 1929–1934 (1996). [PubMed] [Google Scholar]
- Mor F., Quintana F. J. & Cohen I. R. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol 172, 4618–4623 (2004). [DOI] [PubMed] [Google Scholar]
- Ohtani K. et al. High expression of GADD-45alpha and VEGF induced tumor recurrence via upregulation of IL-2 after photodynamic therapy using NPe6. Int J Oncol 32, 397–403 (2008). [PubMed] [Google Scholar]
- Dace D. S., Khan A. A., Kelly J. & Apte R. S. Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. Plos One 3, e3381 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte R. S., Richter J., Herndon J. & Ferguson T. A. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med 3, e310 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu C., Heymach J., Overman M., Tran H. & Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res 17, 6130–6139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo R., Carrasco E., Garcia-Ramirez M. & Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev 2, 71–98 (2006). [DOI] [PubMed] [Google Scholar]
- Shimura M. et al. Visual prognosis and vitreous cytokine levels after arteriovenous sheathotomy in branch retinal vein occlusion associated with macular oedema. Acta Ophthalmol 86, 377–384 (2008). [DOI] [PubMed] [Google Scholar]
- Shimura M. et al. Panretinal photocoagulation induces pro-inflammatory cytokines and macular thickening in high-risk proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 247, 1617–1624 (2009). [DOI] [PubMed] [Google Scholar]
- Holzinger C. et al. Effects of interleukin-1, -2, -4, -6, interferon-gamma and granulocyte/macrophage colony stimulating factor on human vascular endothelial cells. Immunol Lett 35, 109–117 (1993). [DOI] [PubMed] [Google Scholar]
- Carreno E. et al. Phase IIb clinical trial of ranibizumab for the treatment of uveitic and idiopathic choroidal neovascular membranes. Br J Ophthalmol (2015). [DOI] [PubMed] [Google Scholar]
- Yang F., Bai Y. & Jiang Y. Effects of Apelin on RAW264.7 cells under both normal and hypoxic conditions. Peptides 69, 133–143 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang M. et al. A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology 118, 672–678 (2011). [DOI] [PubMed] [Google Scholar]
- Wickremasinghe S. S., Busija L., Guymer R. H., Wong T. Y. & Qureshi S. Retinal venular caliber predicts visual outcome after intravitreal ranibizumab injection treatments for neovascular AMD. Invest Ophthalmol Vis Sci 53, 37–41 (2012). [DOI] [PubMed] [Google Scholar]
- Nagarkatti-Gude N., Bronkhorst I. H., van Duinen S. G., Luyten G. P. & Jager M. J. Cytokines and chemokines in the vitreous fluid of eyes with uveal melanoma. Invest Ophthalmol Vis Sci 53, 6748–6755 (2012). [DOI] [PubMed] [Google Scholar]
- Nagata K. et al. Simultaneous analysis of multiple cytokines in the vitreous of patients with sarcoid uveitis. Invest Ophthalmol Vis Sci 53, 3827–3833 (2012). [DOI] [PubMed] [Google Scholar]
- Sato T., Kusaka S., Shimojo H. & Fujikado T. Simultaneous analyses of vitreous levels of 27 cytokines in eyes with retinopathy of prematurity. Ophthalmology 116, 2165–2169 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


