Abstract
The Biomarkers of Nutrition for Development (BOND) project is designed to provide evidence-informed advice to anyone with an interest in the role of nutrition in health. The BOND program provides information with regard to selection, use, and interpretation of biomarkers of nutrient exposure, status, function, and effect, which will be especially useful for readers who want to assess nutrient status. To accomplish this objective, expert panels are recruited to evaluate the literature and to draft comprehensive reports on the current state of the art with regard to specific nutrient biology and available biomarkers for assessing nutritional status at the individual and population levels. Phase I of the BOND project includes the evaluation of biomarkers for 6 nutrients: iodine, folate, zinc, iron, vitamin A, and vitamin B-12. This review of vitamin A is the current article in this series. Although the vitamin was discovered >100 y ago, vitamin A status assessment is not trivial. Serum retinol concentrations are under homeostatic control due in part to vitamin A’s use in the body for growth and cellular differentiation and because of its toxic properties at high concentrations. Furthermore, serum retinol concentrations are depressed during infection and inflammation because retinol-binding protein (RBP) is a negative acute-phase reactant, which makes status assessment challenging. Thus, this review describes the clinical and functional indicators related to eye health and biochemical biomarkers of vitamin A status (i.e., serum retinol, RBP, breast-milk retinol, dose-response tests, isotope dilution methodology, and serum retinyl esters). These biomarkers are then related to liver vitamin A concentrations, which are usually considered the gold standard for vitamin A status. With regard to biomarkers, future research questions and gaps in our current understanding as well as limitations of the methods are described.
Keywords: BOND, vitamin A biomarkers, vitamin A review, breast milk, dose response tests, dried blood spot, isotope dilution, retinol-binding protein, serum retinol, xerophthalmia
Introduction
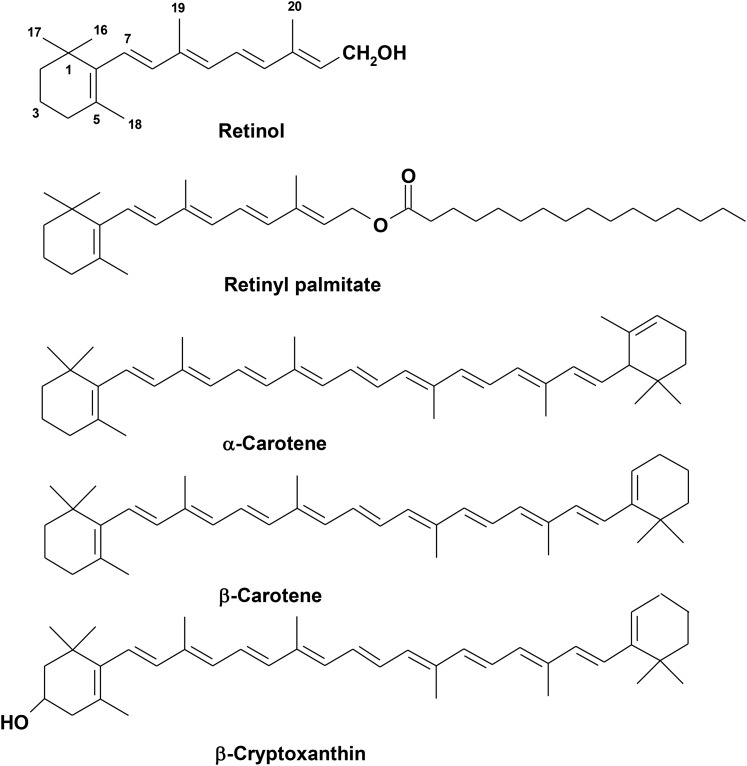
Vitamin A, a term encompassing a group of unsaturated organic compounds, which include retinol, retinal, and retinoic acid (Figure 1), is an essential nutrient because it cannot be produced by humans and must be provided as part of the diet. Provitamin A carotenoids, which are produced in plants, are also a primary dietary source of the vitamin after enzymatic cleavage. Vitamin A deficiency continues to contribute significantly to the global burden of disease, particularly affecting resource-constrained countries (1). Vitamin A deficiency disorders include xerophthalmia and increased risk of death from infectious diseases, especially among preschool children. The current state of the science and challenges for assessing vitamin A status at the individual and population levels are the focus of this review.
FIGURE 1.
The structures of the major dietary forms of vitamin A. Preformed retinol is primarily found as retinyl palmitate in most animal livers, fortificants, and supplements. From ∼50 provitamin A carotenoids in plants, the common ones include α-carotene, β-carotene, and β-cryptoxanthin. These forms of provitamin A carotenoids are often quantified in human serum.
Historical overview.
The history of our knowledge of vitamin A spans centuries, although its chemical properties, biochemical functions, and global significance have come to light over the past century (2–46) (Table 1). In addition to the review article by Wolf (2), other comprehensive historical overviews have been published (21, 47, 48). Xerophthalmia, a spectrum of ocular manifestations resulting from vitamin A deficiency, has been known for ≥3500 y and was treated in ancient Assyria, Egypt, and Greece with foods now known to be rich in preformed vitamin A esters (2). The earliest association between nightblindness and a corneal defect was made by von Hubbenet in 1860, followed by the characterization of conjunctival xerosis with foamy white spots on the cornea by Bitot in 1862 (2, 3), now known as “Bitot’s spots” and regarded as a strong clinical indicator of vitamin A deficiency, which is usually reversible with vitamin A supplementation.
TABLE 1.
Historical accounts in the discovery and history of vitamin A research and biomarker development1
| Year | Discovery |
| 460–325 bc | Ancient Egyptians and Greeks cured nightblindness with roasted oxen liver (2) |
| 1860–1863 | von Hubbenet associated nightblindness with eye defects and Bitot described conjunctival xerosis by identifying “Bitot’s spots” (reviewed in references 2 and 3) |
| 1861 | Schwarz diagnosed nightblindness as a nutritional disease during a naval expedition (reviewed in reference 2) |
| 1904 | Mori reported “Hikan” in Japanese children, which responded to cod liver oil and liver (reviewed in reference 2) |
| 1913 | McCollum and Davis (4) and Osborne and Mendel (5) discovered “fat-soluble A” in rat feeding studies |
| 1919 | Bloch (6) found xerophthalmia in Danish orphans subsisting on a fat-free milk, oatmeal, and barley diet |
| 1919–1920 | Steenbock and Gross (7, 8) identified a yellow pigment (β-carotene) that was converted to active colorless vitamin A |
| 1928 | Green and Melanby (9) first coined the term “anti-infective” for vitamin A |
| 1930 | Moore (10) purified yellow pigment from plants, butter fat, and egg yolk as carotene and showed that it was converted to vitamin A |
| 1931 | Karrer et al. (11) isolated and described the chemical structures of retinol and β-carotene (Figure 1) |
| 1931 | Green et al. (12) showed that cod liver oil reduces puerperal fever |
| 1932 | Ellison (13) reported that vitamin A reduces measles fatality |
| 1935 | Wald (14) described “the visual cycle” |
| 1937 | Holmes and Corbet (15) isolated and crystallized pure vitamin A from fish liver oil |
| 1947 | Isler et al. (16) synthesized retinol |
| 1960 | Gopalan et al. (17) drew global attention to endemic vitamin A deficiency in India |
| 1965 | Olson and Hayaishi (18) discovered β-carotene 15,15′-dioxygenase as the core enzyme in the conversion of provitamin A carotenoids to vitamin A in the intestine |
| 1966 | McLaren et al. (19) published detailed photo accounts of xerophthalmia |
| 1967 | Wald (20) received the Nobel Prize in physiology for describing the visual cycle |
| 1974 | The International Vitamin A Consultative Group was established (21) |
| 1978 | Loerch et al. (22) proposed the principle of the relative-dose-response test in rats |
| 1986 | Sommer et al. (23) reported that vitamin A can reduce child mortality in Indonesia followed by recommendations by UNICEF and WHO for the use of high-dose vitamin A supplements (21) |
| 1987–1988 | Petkovich et al. (24), Giguere et al. (25), and Benbrooke et al. (26) simultaneously discovered the retinoic acid receptors in cell nuclei |
| 1988 | Tanumihardjo and Olson (27) proposed vitamin A2 in a modified-relative-dose-response test |
| 1989 | Furr et al. (28) published the use of deuterated retinyl acetate for vitamin A assessment of humans |
| 1990 | Beaton et al. (29) summarized 16 studies using vitamin A supplementation, concluding an average childhood mortality reduction of 23% |
| 1992 | At the International Conference on Nutrition in Rome, countries committed to preventing vitamin A deficiency (21) |
| 1995 | Bioavailability of provitamin A carotenoids in green leafy vegetables challenged by de Pee et al. (30) in Indonesia |
| 1998 | Christian et al. (31, 32) in Nepal revealed maternal nightblindness as an indicator of maternal vitamin A deficiency, poor health, and survival |
| 1999 | West et al. (33) in Nepal reported that vitamin A or β-carotene supplementation can lower maternal mortality |
| 2001 | The Institute of Medicine (34) in the United States revised the β-carotene:retinol bioconversion ratio from 6 μg β-carotene:1 μg retinol to 12:1 and the ratio for other provitamin A carotenoids from 12:1 to 24:1 |
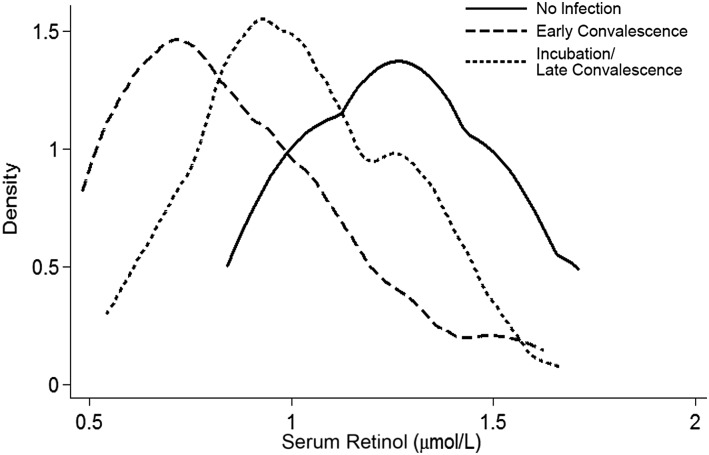
| 2003 | Thurnham et al. (35) systematically showed the influence of inflammation on serum retinol concentrations |
| 2003 | Ramathullah et al. (36) reported from India a 23% reduction in infant mortality by giving newborns a single ∼50,000 IU oral dose of vitamin A, affirming earlier work by Humphrey et al. (37) in Indonesia |
| 2011 | The WHO (38–42) revised vitamin A supplementation recommendations for women and children on the basis of the current evidence base |
| 2013 | Awasthi et al. (43) found a nonsignificant 4% decrease in mortality of Indian children given periodic 200,000-IU supplements |
| 2014 | Large randomized, double-blind, placebo-controlled trials in Ghana (44) and Tanzania (45) showed no impact, whereas India showed a 10% reduction (46) in infant mortality after 50,000 IU vitamin A was administered at birth |
Although comprehensive, the table does not include all of the important discoveries for applications of vitamin A.
Although deficiency is the primary public health focus and the WHO advocates high-dose vitamin A supplements to select groups (38–42) (Table 2), it is well recognized that vitamin A excess is also a consideration. Bone abnormalities in fossilized skeletal remains of early humans may have been caused by excessive preformed vitamin A intakes (49, 50), suggesting that vitamin A toxicity may also have been an ancient phenomenon (51). Vitamin A has been referred to as the “luxus” vitamin in the past, suggesting that excessive intake habitually exists among some individuals (52), reinforcing the point that maintaining balance is important for overall health (53).
TABLE 2.
The WHO’s vitamin A supplementation recommendations based on evidence to reduce infant and maternal morbidity and mortality as of 20111
| Age group and vitamin A dosage2 | Frequency |
| Neonatal | Not recommended |
| Age 1–5 mo | Not recommended |
| Age 6–11 mo | |
| 100,000 IU | One-time dose |
| Age 12–59 mo | |
| 200,000 IU | Every 4–6 mo |
| Postpartum women | Not recommended |
| Pregnant women | Routinely not recommended |
| 10,000 IU | Daily in at-risk areas for nightblindness |
| 25,000 IU | Weekly in at-risk areas for nightblindness |
As highlighted in Table 1, over the past 100 y, vitamin A was chemically identified, purified, and synthesized and its clinical relevance and molecular importance were discovered. These findings have had important implications for global efforts to prevent blindness and mitigate mortality risk through large-scale supplementation efforts. However, our understanding of its role in fat metabolism, immune function, and epigenetic gene regulation is still evolving (54). The simultaneous discovery of retinoic acid receptors (RARs)13 by Chambon’s group in Strasbourg, France (24), Evans’ group in San Diego, California (25), and Pfahl’s group in La Jolla, California (26), in the 1980s opened up new research areas that helped to expand our understanding of the molecular actions and functions of vitamin A (2).
Exposure: food sources.
Sources of vitamin A are required throughout the life cycle. During infancy, colostrum and mature human milk are rich sources of both preformed vitamin A and provitamin A carotenoids, especially when the mother has adequate dietary intake (55) as recommended by dietary guidelines (56). Beyond infancy, vitamin A is consumed in the diet as preformed retinyl esters, predominantly retinyl palmitate (Figure 1), from animal sources. It is also provided by provitamin A carotenoids, such as β-carotene and β-cryptoxanthin (Figure 1), primarily from plant sources (57, 58).
Fortified foods in both developed and developing countries can serve as important dietary sources of preformed vitamin A. In developed countries, these foods include ready-to-eat cereals, snack foods, beverages, margarine, and processed dairy products (59). In developing countries, vitamin A–fortified foods, although limited, include sugar, cereal flours, edible oils (59, 60), margarine (61), and noodles (62). The WHO has developed guidelines for the fortification of foods with micronutrients (63).
In addition to these food-based sources, vitamin A may be provided alone or as a component of multivitamin dietary supplements. Both preformed vitamin A (predominantly as either retinyl acetate or palmitate) and provitamin A (predominantly as β-carotene) are used in commercial dietary supplements (64). Dietary and supplemental sources of preformed vitamin A and provitamin A carotenoids are highlighted in Text Box 1.
Text Box 1. Food and Supplemental Sources High in Vitamin A.
Preformed vitamin A in foods
liver
fish liver oils
dairy products: butter, cheese, milk fat, other dairy products
egg yolk
Provitamin A carotenoids in plant foods
dark-green leafy vegetables
deeply colored yellow and orange vegetables and fruit
Supplemental vitamin A
preformed retinyl acetate or palmitate
β-carotene in some multivitamins
β-carotene
Intakes in developed countries of preformed retinol tend to be higher than in developing countries where provitamin A carotenoids represent the major source of dietary vitamin A. Children and teenagers in the United States derive >80% of their vitamin A as preformed vitamin A and average adult estimates are >65% (65). In comparison, Ugandan preschool children consume 5–25% and adults consume 5–20% as preformed vitamin A (66). China appears to lie in the middle with a reference intake of ∼50% from preformed retinol sources (after recalculating a bioconversion factor of 6- to 12-μg β-carotene equivalents to 1 μg retinol for comparison purposes) (67). In addition to β-carotene, β-cryptoxanthin, found predominantly in orange citrus fruit, some pumpkins, and yellow/orange maize, is another common provitamin A carotenoid and has similar bioefficacy to β-carotene in both animals (68) and humans (69). Another common provitamin A carotenoid often quantified in human serum is α-carotene, predominantly found in orange carrots and some pumpkins (58). Due to its chemical structure (Figure 1), α-carotene theoretically yields half the retinol that β-carotene does, which was demonstrated in a gerbil model (70).
Absorption and bioavailability of provitamin A carotenoids.
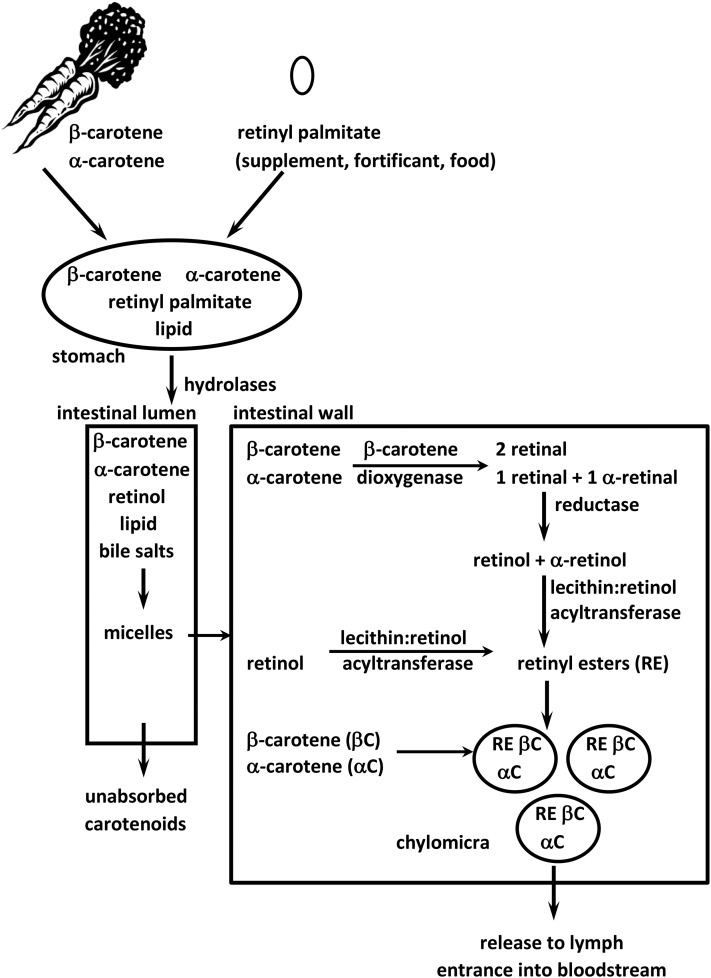
Approximately 70–90% of preformed vitamin A esters are absorbed and utilized or stored (71) (Figure 2), but the range for absorption of dietary provitamin A carotenoids is much wider. Provitamin A carotenoids can be found in either the cis- or trans-configuration. Between 35% and 88% of absorbed all-trans β-carotene is oxidatively cleaved by β-carotene 15,15'-dioxygenase 1 (BCO1) into 2 molecules of all-trans retinal in the enterocyte (18, 72), which can be oxidized irreversibly to retinoic acid by 1 of 3 retinal dehydrogenases or reduced reversibly to retinol by a number of retinal reductases (73, 74). The cis isomers must be isomerized to the all-trans configuration before BCO1 can cleave them, resulting in lower bioconversion efficiency (56, 75). Retinoic acid can also be formed by an excentric cleavage pathway mediated by the enzyme β-carotene 9,10-oxygenase (BCO2). This, however, represents a minor pathway for β-carotene cleavage (76).
FIGURE 2.
Vitamin A and provitamin A carotenoids are fat-soluble. Therefore, lipid is needed for the best absorption. After consumption, retinyl esters are hydrolyzed by nonspecific hydrolases and mixed with lipid and bile salts to form micelles along with the provitamin A carotenoids. After the micelles are absorbed into the enterocyte, provitamin A carotenoids can be cleaved to retinol by β-carotene 15,15′-dioxygenase. Retinol is then esterifed to FAs and incorporated into chylomicra along with carotenoids that were not cleaved. The chylomicra travel through the lymph into the bloodstream and lose some of the retinyl esters and carotenoids to tissues while in circulation. Ultimately, the remaining retinyl esters and carotenoids are taken up by the liver as chylomicron remnants.
A number of studies in developing countries examined the bioavailability of β-carotene from individual vegetables and fruit. The bioavailability and bioconversion were low from vegetables (30, 77) and better from orange fruit (78). The determining bioaccessibility factor (i.e., release from the plant matrix) influencing the bioavailability appears to be the location of carotenoids within the chromoplast as opposed to the chloroplasts. In addition, the crystalline form, such as carotene crystals in carrots, may also negatively affect overall bioavailability (79). Cooking and heat processing also disrupt the plant matrix (80), and these processes usually result in greater bioavailability (79).
As a consequence of this complex and variable absorption process, concerns have been raised with regard to populations who are largely dependent on vegetables and fruit to meet dietary vitamin A requirements (77). Current studies have raised questions about this premise based largely on methods used to assess conversion and subsequent storage of vitamin A from plant sources. For example, by using a sensitive stable isotope dilution methodology, green and yellow vegetables were able to maintain total body vitamin A stores in Chinese children during a 10-wk intervention (81). Furthermore, stable isotope methodologies have shown a wide range for β-carotene conversion from different plant sources (82–85). The bioconversion mass ratio of β-carotene equivalents in various food matrices to yield 1 μg vitamin A ranged from high values of 21:1 in spinach and 15:1 in carrots (82), moderate values of 13:1 for sweet potatoes, and 10:1 for pureed spinach (83) and low values of ∼2:1 in genetically engineered Golden rice (84). In part, these ranges may be due to differences in initial vitamin A status among study participants such that vitamin A–deficient individuals may cleave provitamin A carotenoids at a higher rate, resulting in greater bioefficacy (79).
Given the evidence of poor provitamin A carotenoid bioavailability from green leafy and some other vegetables, a range of approaches to improve vitamin A status through diet have been proposed (79, 86–88) including the following:
promotion of food sources of preformed vitamin A (e.g., animal and fortified foods),
promotion of a wider variety of high provitamin A carotenoid–containing foods, including biofortified staples, and
food preparation methods that enhance carotenoid absorption.
Individually or in combination, these approaches offer practical tactics for improving vitamin A status (89).
Fortification efforts with preformed vitamin A.
Food fortification with preformed vitamin A is a viable option that can be 2–4 times more cost-effective in providing vitamin A than either capsule distribution with preformed retinyl palmitate or dietary diversification efforts (63). Fortification with vitamin A has been used successfully in the developed world for >80 y, and its potential was recognized in the developing world 40 y ago (59). In general, food fortification with vitamin A has the advantages of being socially acceptable and requiring minimal changes in food habits.
The implementation of fortification in some areas has been slow due to lack of centralized processing of potential food vehicles (59). Nonetheless, there have been notable successes [e.g., vitamin A fortification of sugar in Guatemala and other Central American countries (59, 90)]. Fortification needs to satisfy certain specifications to be effective at the population level (Table 3). Each country should evaluate which approach or approaches (i.e., dietary diversification, biofortification, fortification, and/or supplementation) provide the greatest benefit for investment with available resources and whether the most vulnerable population groups are reached by the proposed approach or approaches, such as was shown in Zambia (91).
TABLE 3.
Fortification specifications for effective implementation1
| • A regularly consumed food, produced by a few centralized factories, is required |
| • The fortificant must not change sensory attributes and must contain a highly bioavailable form of the nutrient |
| • Fortified foods should provide ≥15% of the recommended daily intakes for the target group |
| • Voluntary fortification of processed foods should be regulated to prevent excessive consumption of vitamin A |
| • Neighboring countries should harmonize technical standards, facilitate compliance, and minimize conflicts over global trade laws |
| • A practical monitoring system should be instituted |
| • Social marketing activities should be permanent and aimed at industry, government, and consumers |
| • Food fortification might be combined with other strategies (e.g., supplementation) to reach those not adequately covered by fortification alone |
Summarized from reference 59.
Public health significance.
In 2009, the WHO estimated that 5.2 million preschool children and 9.8 million pregnant women were affected with nightblindness, which corresponds to 0.9% and 7.8% prevalence of vitamin A deficiency, respectively (1). It has also been estimated that based on the currently accepted cutoff for low serum retinol concentrations (<0.7 μmol/L), 190 million preschool children and 19.1 million pregnant women globally are affected. These estimates correspond to 33.3% of the preschool population and 15.3% of pregnant women in populations at risk of vitamin A deficiency. Africa and Southeast Asia are the most affected by vitamin A deficiency for both population groups (1) (Table 4).
TABLE 4.
Global prevalence of serum retinol concentrations <0.7 μmol/L and number of individuals affected in populations of countries at risk of vitamin A deficiency, 1995–20051
| Preschool children |
Pregnant women |
|||
| WHO region | Prevalence,2 % | Number affected, in millions | Prevalence, % | Number affected, in millions |
| Africa | 44.4 (41.3, 47.5)3 | 56.4 (52.4–60.3) | 13.5 (8.9, 18.2) | 4.18 (2.73–5.63) |
| Americas | 15.6 (6.6, 24.5) | 8.68 (3.70–13.7) | 2 (0.4, 3.6) | 0.23 (0.04–0.41) |
| Southeast Asia | 49.9 (45.1, 54.8) | 91.5 (82.6–100) | 17.3 (0.0, 36.2) | 6.69 (0.00–14.0) |
| Europe | 19.7 (9.7, 29.6) | 5.81 (2.87–8.75) | 11.6 (2.6, 20.6) | 0.72 (0.16–1.29) |
| Eastern Mediterranean | 20.4 (13.2, 27.6) | 13.2 (8.54–17.9) | 16.1 (9.2, 23.1) | 2.42 (1.38–3.47) |
| Western Pacific | 12.9 (12.3, 13.5) | 14.3 (13.6–14.9) | 21.5 (0.0, 49.2) | 4.9 (1.00–11.2) |
| Global | 33.3 (31.3, 35.4) | 190 (178–202) | 15.3 (7.4, 23.2) | 19.1 (9.30–29.0) |
Data are from the WHO (1). Population subgroups: preschool children (<5 y old) and pregnant women (no age range defined).
Excludes countries with a 2005 gross domestic product ≥$15,000.
Estimated prevalence; 95% CI in parentheses (all such values).
Although body vitamin A stores increase toward normal values during infancy in well-nourished societies (92), the status of infants reared under vitamin A–deprived and infection-exposed conditions tends to remain depressed (93). It is common for vitamin A deficiency to extend into the adolescent years. For example, in Southeast Asia, 23% of children aged 5–15 y had serum retinol concentrations <0.7 μmol/L, although inflammation markers were not measured, and nearly 3% presented with nonblinding, mild xerophthalmia (94).
Women of child-bearing age who live in low-resource, food-insecure settings are vulnerable to vitamin A deficiency. Of particular concern are those in the latter half of pregnancy when nutritional demands are high and circulating vitamin A concentrations are low in part due to hemodilution, which complicates evaluation. Consequently, the risk of developing nightblindness and other adverse health outcomes is greatest during this period (31, 32).
Vitamin A deficiency tends to cluster within countries. Common features of areas of endemic vitamin A deficiency include poverty, a high incidence of infectious diseases, limited infrastructure, and food insecurity resulting in poor availability and accessibility to vitamin A–containing foods. In such settings, a vicious cycle often exists of vitamin A deficiency leading to increased susceptibility to and severity of infection, which, in turn, can reduce intake and accelerate body losses of vitamin A (95).
The global response: vitamin A supplements.
Global progress has been made to reduce child deaths from vitamin A deficiency, with estimates decreasing from 1.3 to 2.5 million preventable deaths to ∼650,000 deaths annually from 1992 (96) to 2003 (97). This trend can be explained, in part, by reduced fatality from measles [by ∼50% (89)] and severe diarrhea and dysentery [by ∼40% (98–101)] as a result of improved vitamin A status. Between 1986 and 1992, 5 population-based intervention trials in Southeast Asia (23), South Asia (98, 102), and Africa (103, 104) found that high-dose vitamin A supplementation (200,000 IU retinyl palmitate every 6 mo) could reduce child mortality among children aged 6–59 mo by 6–54%. Meta-analyses of vitamin A supplementation trials concluded that supplementation reduced mortality by 23% (29) and 30% (105). These findings are in agreement with a more recent meta-analysis that investigated 17 trials and reported a similar reduction of 24% (106). Nonetheless, population data from India reported a modest 4% reduction and the 95% CI included 1.0 (43). Thus, the effectiveness of vitamin A supplementation in early childhood is well-established but may be population dependent. Further placebo-controlled trials of vitamin A supplementation in children between 6 and 59 mo of age are not needed, as recommended by the WHO (38), but monitoring is important to determine when supplementation is no longer needed.
Although the WHO continues to recommend high-dose supplements for infants and young children aged 6–59 mo (38) (Table 2), the most effective dose and frequency of delivery, especially among neonates, have not been determined because of equivocal findings. This lack of consensus is reinforced by several conflicting reports. In 3 studies in neonates, oral supplementation with ∼50,000 IU vitamin A in oil shortly after birth was reported to reduce mortality by 15% in Bangladesh (107), by 23% in India (36), and by 64% in Indonesia (37). Another study showed a beneficial impact of vitamin A in boys, but not girls, in response to infant supplementation in Guinea-Bissau (108).
These findings indicate that reductions in infant mortality may be population dependent. Recent large, randomized, double-blind, placebo-controlled trials in Ghana (44) and Tanzania (45) showed no impact on child survival. In another study in India (46), a non–statistically significant reduction in mortality of 10% was observed in infants followed up to 6 mo of age. In the latter findings, the 95% CI of the estimate (0–19%) included the possibility of “no effect.” At the current time, the WHO does not recommend infant dosing for a reduction in morbidity and mortality (39).
Progress in reducing maternal mortality has also been achieved under high-risk conditions. For example, in southern Nepal, maternal mortality rates exceeded 600/100,000 live births in 1994 (33). Once-weekly supplementation with either 7 mg vitamin A or 42 mg β-carotene reduced all-cause pregnancy-related mortality by 40% and 49%, respectively (33). However, a trial in Bangladesh with similar supplementation amounts failed to improve maternal or infant survival in a population in whom vitamin A status was marginal to normal and in whom maternal mortality was lower than that observed in Nepal (109).
In summary, periodic vitamin A supplementation is beneficial, especially in preschool children living in the developing world when dietary sources of vitamin A are scarce and not commonly consumed. However, some population groups may not respond to supplementation, and this is likely due to the underlying adequate vitamin A status of the intervened groups.
Current guidelines for use.
As will be covered in greater detail below, the storage of vitamin A in the liver tends to mitigate the development of intoxication due to intakes in excess of physiologic needs by esterifying it to FAs and storing it in stellate cells for future use during times of low dietary intake. Thus, vitamin A can be administered in relatively large, infrequent doses; however, frequent vitamin A supplementation or fortification may accumulate in stores over time. As discussed above, this approach has shown efficacy in the prevention of mortality but does not necessarily increase group serum retinol concentrations (110) or result in a shift in the distribution of serum retinol concentrations at the population level (111). The most common delivery regimens and response times for women and infants with vitamin A deficiency are highlighted in Text Box 2. Table 2 lists current WHO recommendations for vitamin A supplementation.
Text Box 2. Recommended Dosing Regimens to Treat Vitamin A Deficiency.
In children with clear or suspected xerophthalmia, vitamin A should be administered orally in age-specific large doses according to WHO treatment guidelines, followed by an additional dose the next day, and 2–4 wk later (112).
Xerophthalmia cases should be treated with age-specific dosages on days 1, 2, and 14; measles cases on days 1 and 2; and severe undernutrition cases (i.e., kwashiorkor or weight-for-height < –3 z scores below the international referent median) on day 1 (113, 114).
For women of reproductive age (13–49 y) with active corneal lesions, 200,000 IU should be given on days 1, 2, and 14.
Women with milder eye signs (nightblindness or Bitot’s spots) should be treated with 10,000 IU/d or 25,000 IU/wk for >3 mo (113–115).
Expected responses
Nightblindness responds within hours to days of high-potency vitamin A treatment, leading to a return to normal scotopic (nighttime) vision (115, 116), although full recovery of visual function may take weeks.
Bitot’s spots in preschool children generally respond rapidly to high-potency vitamin A treatment within 2–5 d, becoming smaller in size and disappearing within 2–3 wk (116, 117).
The recommended dosing regimens outlined are safe, although symptoms, such as headache, nausea, or vomiting and diarrhea, indicative of acute hypervitaminosis immediately after the dose, have been reported at a frequency of 3–7% (114). Importantly, these short-term side effects are transient, with the large majority (more than two-thirds) starting and disappearing within 24 h of dosing (114). Furthermore, metabolic studies have suggested that the recommended dosing regimen for children at risk of vitamin A deficiency will not cause any important risk of longer-term toxic side effects (114, 118).
High doses of vitamin A (>10,000 IU/d) should not be given to women who could be pregnant because of the risk of teratogenic effects in the fetus (119). There is strong evidence, however, that a vitamin A dose of up to 10,000 IU given daily, or a dose of up to 25,000 IU given weekly, is safe (119). Indeed, once-weekly supplementation with ∼23,000 IU vitamin A reduced all-cause pregnancy-related mortality by 40% in a vitamin A–deficient population (33).
At the current time, the WHO does not recommend that pregnant or postpartum women receive vitamin A supplements due to a lack of evidence that they improve infant and maternal mortality rates (40, 41) or for the prevention of mother-to-child transmission of HIV (42). However, in areas in which vitamin A deficiency is a public health problem determined by using biochemical and clinical indicators, supplements should be given to prevent nightblindness (Table 2) (40).
Periodic high-dose vitamin A supplementation remains the most widely practiced method to prevent deficiency (38). The efficacy of high-dose vitamin A prophylaxis appears to be ∼90% in preventing any stage of xerophthalmia for 6 mo in children (89), despite a likely dosage absorption and retention of only 30–50% under conditions of morbidity and undernutrition found in developing countries when the original supplementation studies were performed (89). Program effectiveness was expected to be ∼75% effective in preventing children from developing xerophthalmia in high-risk populations (89). Maternal supplementation after delivery, which is usually administered within 8 wk of birth, has improved nightblindness and low serum retinol concentrations in mothers after delivery (31, 32, 120) but is currently not recommended by the WHO (41).
Intake recommendations and Tolerable Upper Intake Levels.
The 2 most widely cited sources of dietary vitamin A intake recommendations are those of the Institute of Medicine (IOM) in the United States (34) and the FAO/WHO (57). As highlighted in Table 5, recommendations are presented by age and life-stage group to reflect physiologic needs. In general, the FAO/WHO recommended intakes are lower or similar to those of the IOM when comparing different ages and sexes. The main difference between these recommendations is that those by the IOM are based on preformed vitamin A and retinol activity equivalents (reflecting an estimate of 12 μg β-carotene or 24 μg other provitamin A carotenoids to supply 1 μg retinol), whereas the FAO/WHO uses retinol equivalents (reflecting an estimate of 6 μg β-carotene or 12 μg other provitamin A carotenoids to supply 1 μg retinol).
TABLE 5.
Recommendations for vitamin A intake by age or population group by the Institute of Medicine (34) and the FAO (57)1
| US Institute of Medicine |
FAO |
||||
| Life-stage group | EAR, μg RAEs/d | AI or RDA, μg RAEs/d | UL,2 μg REs/d | Mean requirement, μg REs/d | Recommended safe intake, μg REs/d |
| Infants | |||||
| 0–6 mo | — | 400 | 600 | 180 | 375 |
| 7–12 mo | — | 500 | 600 | 190 | 400 |
| Children | |||||
| 1–3 y | 210 | 300 | 600 | 200 | 400 |
| 4–6 y | — | — | — | 200 | 450 |
| 4–8 y | 275 | 400 | 900 | ||
| 7–9 y | — | — | — | 250 | 500 |
| 9–13 y | — | — | — | — | — |
| Male | 445 | 600 | 1700 | — | — |
| Female | 420 | 600 | 1700 | — | — |
| Adolescents aged 10–18 y | 330–400 | 600 | |||
| Adults | |||||
| Females | |||||
| 14–18 y | 485 | 700 | 2800 | — | — |
| ≥19 y | 500 | 700 | 3000 | 270–300 | 500–600 |
| Males | |||||
| 14–18 y | 630 | 900 | 2800 | 300 | 600 |
| ≥19 y | 625 | 900 | 3000 | 300 | 600 |
| Pregnancy | |||||
| 14–18 y | 530 | 750 | 2800 | 370 | 800 |
| 19–50 y | 550 | 770 | 3000 | 370 | 800 |
| Lactation | |||||
| 14–18 y | 885 | 1200 | 2800 | 450 | 850 |
| 19–50 y | 900 | 1300 | 3000 | 450 | 850 |
RAEs are based on preformed retinol and a bioconversion factor of 12 μg β-carotene to 1 μg retinol (34), and REs are based on preformed retinol and a bioconversion factor of 6 μg β-carotene to 1 μg retinol (57). AI, Adequate Intake; EAR, Estimated Average Requirement; RAE, retinol activity equivalent; RE, retinol equivalent; UL, Tolerable Upper Intake Level.
The UL is based on preformed retinol alone.
The IOM has defined a Tolerable Upper Intake Level (UL) for vitamin A, which is extrapolated from the lowest-observed-adverse-effect level of intake from a small number of case reports (34). The UL is set at the no-observed-adverse-effect level of intake, which is derived with uncertainty factors. The UL for vitamin A is based only on preformed retinol and is 600 μg/d for infants and children aged <3 y and increases to 3000 μg/d for adults (Table 5). The WHO recommends that women who are or might become pregnant limit their total daily vitamin A intake to a maximum of 3000 μg retinol equivalents/d (10,000 IU) or weekly intakes of <7500 μg to minimize the risk of fetal toxicity (40). The lowest reported daily supplement associated with liver cirrhosis is 7500 μg/d taken for 6 y (121, 122). Implications of vitamin A toxicity have been reviewed (51, 121).
Biology of Vitamin A
To support efforts to 1) discover, develop, and implement new biomarkers and 2) most effectively utilize and interpret existing ones, it is essential to understand the metabolism and roles of vitamin A within biological systems. The following sections cover the role of vitamin A to provide context to the subsequent discussions on specific existing and new biomarkers.
Functional roles of vitamin A.
Vitamin A is required for the regulation of numerous key biological processes including roles in the following:
vision,
maintenance of epithelial surfaces,
immune competence,
reproduction, and
embryonic growth and development.
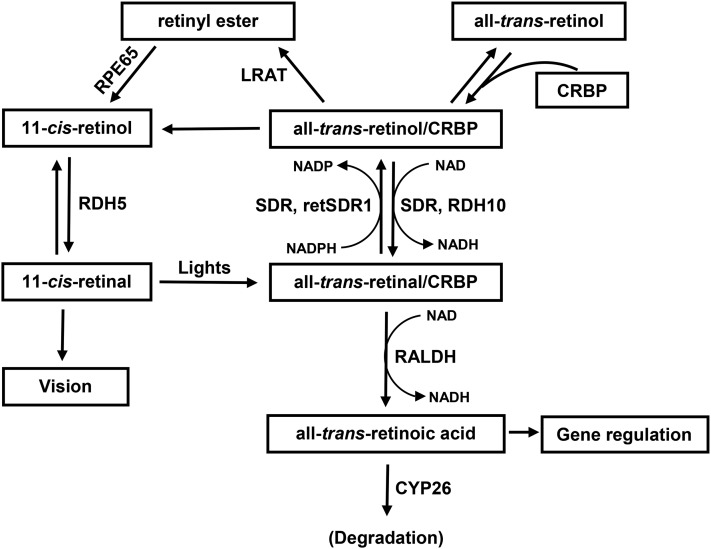
Retinol is the precursor for ≥2 essential biologically active molecules: all-trans retinoic acid as the ligand of nuclear receptors, such as RARs (71, 123), and 11-cis-retinal required in the visual cycle (124, 125). The details of the vitamin A pathways involved in these 2 functions are shown in Figure 3. The steps involved in vitamin A’s role in vision and nucleic receptors are described in Text Box 3.
FIGURE 3.
Schematic diagram showing the metabolic pathways of vitamin A metabolism. CRBP, cellular retinol-binding protein; CYP26, cytochrome P450 26; LRAT, lecithin:retinol acyltransferase; RALDH, retinaldehyde dehydrogenase; RDH, retinol dehydrogenase; retSDR1, short-chain retinol dehydrogenase/reductase; RPE65, retinal pigment epithelium-specific protein 65kDa; SDR, short-chain dehydrogenase/reductases. Adapted from reference 124 with permission.
Text Box 3. Critical Steps in the Functions of Vitamin A.
Vision [reviewed in (125)]
11-cis Retinal binds to opsin to form rhodopsin, which can absorb light within the visible spectrum.
When 11-cis retinal absorbs a photon, it isomerizes to all-trans retinal through several intermediate species and is rapidly released from opsin.
The isomerization of 11-cis retinal is the initial step in vision. An excited intermediate of rhodopsin greatly amplifies light-induced hyperpolarization of the rod membrane, resulting in generation of the nerve impulses for vision.
Nuclear receptors/gene regulation
All-trans retinoic acid is the most biologically relevant metabolite of vitamin A.
Retinoic acid binds and activates several nuclear receptors, i.e., RARs, retinoid X receptor, and PPARs (126).
Upon ligand binding, RAR dimerizes with a retinoid X receptor to form a heterodimer, which then initiates gene transcription by binding to the retinoic acid response element in the promoter region of >500 target genes (71).
Vitamin A absorption.
Figure 2 presents the current understanding of the events and components associated with absorption of preformed vitamin A and provitamin A carotenoids. Most dietary preformed vitamin A is composed of long-chain FA esters of retinol, which must be hydrolyzed in the proximal small intestinal lumen before absorption by the intestinal epithelial cells (127). Some fortificants and supplements are in the acetate form, which can sometimes be found in the circulation, suggesting that retinyl acetate, which is not found endogenously, can be absorbed intact without previous hydrolysis in the gut (128).
After hydrolysis in the gut and absorption by the mucosal cells, retinol is re-esterified with long-chain, mainly saturated, FAs, such as palmitate, by the enzyme lecithin:retinol acyltransferase (LRAT) (127). The resulting retinyl esters are incorporated with other neutral lipid esters into chylomicra and secreted via the lymph duct into the general circulation. Much of the chylomicron TG is hydrolyzed by lipoprotein lipase in extrahepatic tissues. Some retinyl esters are released to extrahepatic tissues from the chylomicra (129), particularly in the lung and spleen (130), whereas the majority is deposited into the liver (129, 130).
Vitamin A in the liver.
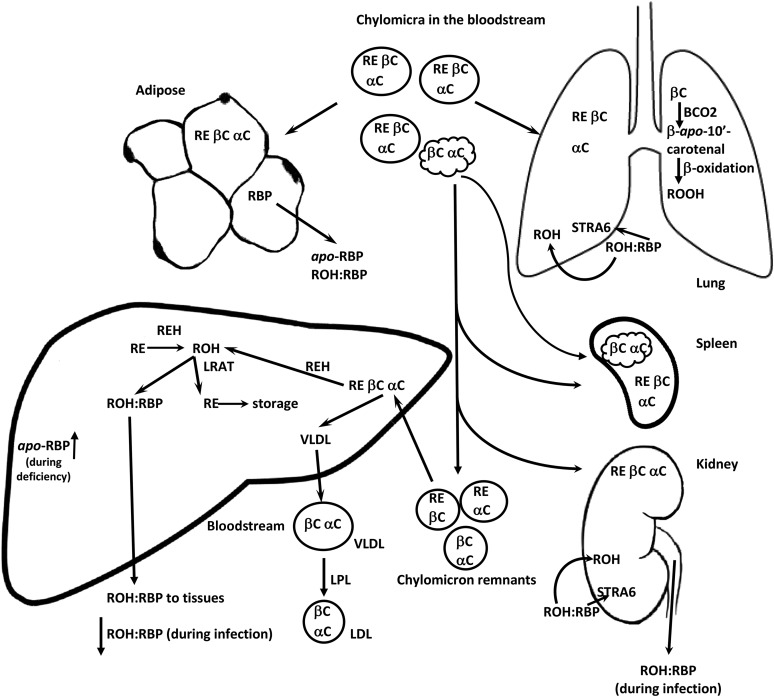
The key components of vitamin A metabolism in the liver are highlighted in Figure 4. The majority of the chylomicron remnants, containing most of the newly absorbed retinyl esters, are taken up by the liver where the retinyl esters are hydrolyzed to retinol, which can then be bound to retinol-binding protein (RBP) or re-esterified to FAs by LRAT (127). Under conditions of adequate vitamin A nutriture, the liver is the main site of vitamin A storage, with >95% of the total retinol esterified to FAs, predominantly retinyl palmitate and stearate in vitamin A–adequate humans. Small amounts of retinyl esters can be stored in the intestine and kidney (130, 131). Adipose tissue likely represents a large underappreciated storage site (132), especially in vitamin A–adequate individuals. Before mobilization from the liver, the retinyl esters are hydrolyzed and free retinol is complexed to RBP for secretion from the liver into blood (127). The retinol:RBP complex is further stabilized by binding to transthyretin in plasma (133).
FIGURE 4.
After absorption, retinyl esters and carotenoids can be released from chylomicra into tissues. Malformed chylomicra can be scavenged by the spleen. However, the bulk of retinyl esters and carotenoids make their way to the liver in chylomicron remnants. In the liver, the retinyl esters can be hydrolyzed by retinyl ester hydrolase. Retinol can then be either complexed with RBP and released into the plasma or re-esterifed by LRAT for long-term storage. The carotenoids can be cleaved to retinol (although not a major pathway in the liver), stored, or packaged into VLDLs and released into the circulation. Retinol uptake from RBP by cells can be facilitated by STRA6. BCO2, β-carotene 9,10-oxygenase; LPL, lipoprotein lipase; LRAT, lecithin:retinol acyltransferase; RBP, retinol-binding protein; RE, retinyl esters; REH, retinyl ester hydrolase; ROH, retinol; ROOH, retinoic acid; STRA6, stimulated by retinoic acid 6 receptor; αC, α-carotene; βC, β-carotene.
In 2007, stimulated by retinoic acid 6 receptor (STRA6) was identified as a cell surface receptor for RBP to release retinol (134). STRA6, however, is not expressed in all cell types, and studies in Stra6-deficient mice suggest that there are other pathways that facilitate retinol uptake into cells (135). These pathways, which include distribution from within chylomicra or other lipoproteins, are likely those that distribute α-retinol, which does not bind to RBP (130, 136), and retinol in RBP-knockout mice tissues (137, 138). α-Retinol is formed from the symmetric cleavage of α-carotene and supports growth in vitamin A–deficient rats (139).
Vitamin A homeostasis and storage.
Total body vitamin A stores regulate vitamin A homeostasis (140). Vitamin A status also indirectly regulates bioconversion of provitamin A carotenoids to retinol (79, 141, 142). Bioconversion in the intestine is regulated through a diet-responsive regulatory network (74). The expression of intestine-specific homeobox transcription factor (ISX) is activated by retinoic acid via RARs that bind to a specific retinoic acid response element within the ISX promoter. Once activated, ISX represses intestinal scavenger receptor class B type 1 and BCO1 expression, indicating that intestinal provitamin A uptake and vitamin A production are under negative feedback control via induction of ISX expression (143).
It is currently not known to what extent provitamin A carotenoid absorption and/or bioconversion are affected by liver vitamin A reserves (74). However, Zambian children with hypervitaminosis A, diagnosed by using stable isotope dilution (85), also had high serum carotenoid concentrations (144) and many of them experienced hypercarotenodermia during mango season, a period of high carotenoid intake (145). This would indicate that even at mean estimated liver vitamin A concentrations, at or above the current cutoff for hypervitaminosis A (>1 μmol/g liver), carotenoid absorption is not severely impaired.
Studies that described the influence of vitamin A status on utilization and provitamin A carotenoid bioconversion come predominantly from animal models. In rats with low to marginal vitamin A status, vitamin A utilization decreases to maintain balance (140). In a gerbil model, bioconversion of provitamin A carotenoids slows at a liver reserve concentration of 0.4 μmol/g (79, 142), suggesting that between 0.1 and 0.4 μmol/g the body is in balance (53). Above 0.4 μmol/g liver, decreased provitamin A carotenoid bioconversion likely causes serum carotenoid concentrations to increase in humans who consume high amounts of plant-source foods, as observed in Zambian children (85, 144, 145).
Vitamin A interactions with other nutrients.
Nutrient-nutrient interactions have been observed between preformed vitamin A/provitamin A carotenoids and both macronutrients and other micronutrients. Text Box 4 contains a summary of some human studies that investigated interactions with various micronutrients.
Text Box 4. Examples of Vitamin A–Micronutrient Interactions in Humans.
Iodine
Vitamin A status modulates thyroid gland and hormone metabolism and the production of thyrotropin by the pituitary gland. Vitamin A deficiency is associated with a decrease in vitamin A–mediated suppression of the pituitary thyroid-stimulating hormone β (TSHB) gene and an associated increase in thyroid-stimulating hormone stimulation and goiter (146).
In Moroccan children with concurrent deficiencies of vitamin A and iodine, those who received both iodized salt and vitamin A supplementation had enhanced efficacy in iodine deficiency disorder outcomes (147).
Hypothyroidism does not reduce the efficacy of high oral doses of vitamin A, vitamin A deficiency does not reduce the efficacy of dietary iodine to correct iodine deficiency disorders, and high-dose vitamin A supplementation may reduce thyroid hyperstimulation and the risk of goiter (146).
Iron
Administering vitamin A enhanced hemoglobin response to iron supplementation during adolescence and pregnancy (148–150).
In a subgroup of pregnant Indonesian women (148), vitamin A status when assessed by the modified-relative-dose-response (MRDR) test, improved more markedly with the combination of vitamin A and iron than with either nutrient alone (151).
In human infants, iron supplementation lowered plasma retinol concentrations but led to greater liver storage of vitamin A (152).
Ethiopian children given a single high-dose vitamin A supplement had improved hemoglobin concentrations (153).
Zinc
Zinc and vitamin A work in synergy for many functions in the body. Therefore, poor zinc status may negatively affect vitamin A status biomarkers.
In a human study, zinc was suggested as a cofactor for the action of BCO1 (154), but the exact role of zinc has not been elucidated.
Obviously, as a “fat-soluble” vitamin, dietary fat has long been understood to affect absorption of both preformed vitamin A and provitamin A carotenoids (34). Studies of the impact of fat on provitamin A carotenoid absorption and metabolism are illustrative of this relation and include the following observations:
Numerous studies have shown that intestinal absorption of carotenoids can be increased by dietary fat intake through increased incorporation into mixed micelles (74).
In animals fed graded amounts of fat, the bioefficacy of sweet potato β-carotene was enhanced with the highest fat amount (155).
Only 3–5 g fat/meal ensures efficient absorption of β-carotene in humans (156, 157).
Higher bioconversion of provitamin A carotenoids has been observed in animals fed a diet rich in MUFAs and n–3 PUFAs compared with an n–6 PUFA–rich diet (74).
Another macronutrient shown to influence vitamin A metabolism is protein. Conversion efficiency is influenced by an Adequate Intake of high-quality protein, which is crucial for BCO1 protein biosynthesis (74).
Implications for public health.
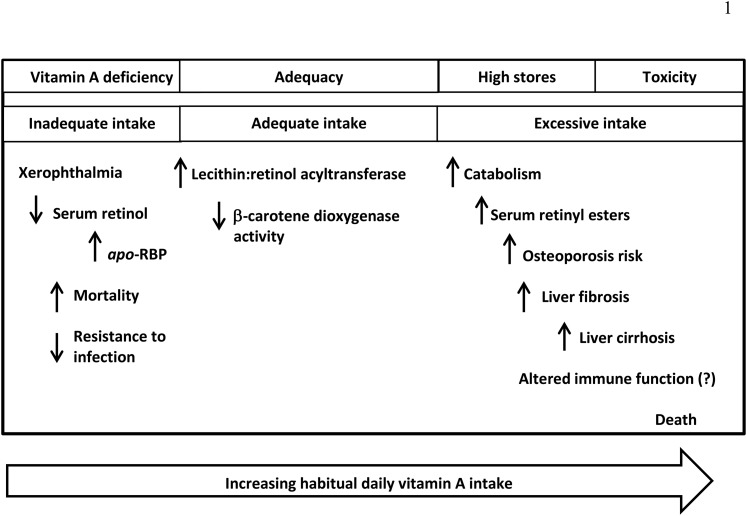
Among the uses of biomarkers of nutrients, biological function is critical both in terms of understanding the role of nutrients within biological systems but also for identifying relevant clinical or biological outcomes of a given nutrient status. Vitamin A serves a number of functional roles in human biology, and a clearer understanding of those roles will not only improve our appreciation of vitamin A’s role in health and disease but will also help identify current and new biomarkers that might be used clinically and in population surveys. Some groups of individuals are more at risk than others of vitamin A deficiency and excess and these are described in Text Box 5. Ramifications of deficiency and excess are shown in Figure 5.
Text Box 5. Risks Groups for Deficiency and Excess.
Risk of vitamin A deficiency
Preterm infants, who have low stores as a result of interrupted accretion during gestation
Infants and children, particularly those whose mothers are vitamin A deficient during lactation
People with malabsorption conditions including biliary atresia and cystic fibrosis
People living in food-insecure environments without access to fortified foods
Individuals suffering from alcoholism
Risk of vitamin A excess
People with access to multiple fortified foods or who consume liver on a regular basis
Chronic preformed vitamin A supplement users
FIGURE 5.
Dietary intake of vitamin A is in 2 major forms: preformed vitamin A from animal-source foods, supplements, and fortificants and provitamin A carotenoids from plant foods and some supplements. The body can regulate how much vitamin A is formed from provitamin A carotenoids, but preformed vitamin A is highly bioavailable. Thus, as dietary intake increases beyond requirements so do liver retinol reserves. The manifestations of vitamin A deficiency are severe, from blindness to death. The manifestations of vitamin A excess are not entirely known, but when severe, can also lead to ill health and death. RBP, retinol-binding protein.
One of vitamin A’s best characterized functions is its role in the immune system. Text Box 6 highlights the known regulatory roles.
Text Box 6. Regulatory Roles of Vitamin A in Immune Function.
The regulatory roles of vitamin A in mammals include the maintenance of epithelial cell differentiation and immune competence (95, 158).
Vitamin A supports innate immune function by supporting regeneration of mucosal barriers damaged by infection in children and by enhancing the function of neutrophils, macrophages, and NK cells, as described primarily in rodents (95).
Vitamin A is required for adaptive immunity and plays a role in the development of T-helper (Th), T-regulatory, and B cells, but more in vivo work is needed (95).
All-trans retinoic acid is a potent regulator of gene expression, and it controls leukocyte homing and T-regulatory function (reviewed in 159). Its production by cells of the immune system is regulated during an immune response in a manner that is still being elucidated (160).
In human T lymphocytes, all-trans retinoic acid inhibited the production of cytokines that favor the generation of Th1-type T cells and enhanced the production of cytokines favoring the Th2-type T cells (161).
Vitamin A may play a role in numerous communicable and noncommunicable diseases of public health concern. Some of these are highlighted in Text Box 7.
Text Box 7. Functional Roles of Vitamin A: Implications for Public Health.
Anemia
Vitamin A deficiency negatively affects hemoglobin concentrations. Supplementing women with vitamin A and iron enhanced hemoglobin concentrations more than either nutrient alone (148).
Cancer
Meta-analyses of human studies have shown an inverse relation between dietary amounts of vitamin A and various cancers, for example, bladder (162), breast (163), cervical (164), and gastric (165).
Synthetic retinoids and some naturally occurring retinoids (e.g., all-trans retinoic acid, 9-cis retinoic acid, 13-cis retinoic acid) have been used in clinical studies (reviewed in 166).
Certain retinoids inhibit the growth of various tumors (e.g., lung, gastrointestinal, breast) and may have chemopreventive and/or chemotherapeutic properties (167, 168).
All-trans retinoic acid is used as a chemotherapeutic agent to treat acute promyelocytic leukemia, and in the vast majority of these patients this treatment leads to a complete remission (169).
Diabetes
Type 1 diabetes has been associated with lower concentrations of serum retinol and its carrier proteins (RBP and transthyretin) in patients (170, 171).
Type 2 diabetes has a less-clear relation with serum retinol and carrier proteins, with some studies showing no change and others showing reductions in type 2 diabetes (172).
Vitamin A deficiency and excess have varying and discordant effects on macronutrient metabolism in various tissues and cell types (173–175).
Vitamin A is involved in pancreatic development and function: deficiency caused reduced β cell mass in fetal islets (173, 174) and reduced glucagon and insulin secretion from pancreatic α and β cells, respectively (173–175).
RBP secreted by adipose tissue has been implicated as a link between obesity and insulin resistance by interrupting insulin signaling in muscle and increasing hepatic glucose output (176).
In human macrophages, RBP may cause insulin resistance by contributing to adipocyte inflammation through proinflammatory cytokine activation, and the mechanism is retinol- and STRA6-independent (177).
The public health link of vitamin A status to diabetes needs further investigation.
Energy metabolism and obesity
Evidence in animals exists for a role of vitamin A in maintaining energy metabolism; however, more research is needed.
Retinoic acid exerts its broad range of biological effects in large part by controlling gene expression. Early in adipogenesis, retinoic acid blocks differentiation, whereas after 48 h of differentiation, it promotes fat cell formation (178).
Mice lacking retinaldehyde dehydrogenase 1 (Raldh1) resisted diet-induced obesity and insulin resistance. Administration of retinal or an Raldh1 inhibitor to obesity-susceptible mice reduced fat accumulation and increased insulin sensitivity (178).
In mice with active protein kinase C, retinol supplementation showed that retinol is a metabolic cofactor involved in the regulation of mitochondrial fuel utilization (179).
HIV and pregnancy
Currently, no conclusive evidence of vitamin A supplementation on vertical HIV transmission exists; therefore, the WHO does not recommend supplementation in HIV-positive pregnant women to reduce the risk of mother-to-child transmission (40, 42).
Cochrane reviews indicated that vitamin A alone (180) and micronutrient supplementation (181) should not be used as a substitute for recommended antiretroviral medication.
In infants with mannose-binding lectin-2 variants, vitamin A supplementation to mothers at delivery was associated with a decreased risk of HIV transmission (182).
Future work is needed on the effect of vitamin A supplementation on HIV transmission from mother to child that accounts for the potential effect of an innate immune deficiency.
Measles
On the basis of a randomized, placebo-controlled clinical trial in children with measles, along with other clinical research, the WHO recommends that age-appropriate doses of vitamin A be given twice 24 h apart to infants and children with measles in populations in whom vitamin A deficiency may be present to decrease the risk of death from measles (183).
Prevention of vitamin A deficiency by using periodic, high-dose supplements in communities in which vitamin A deficiency is a public health problem decreases the risk of developing measles in children 6–59 mo of age (184).
Currently Available Vitamin A Biomarkers: Overview
The currently available biomarkers of vitamin A considered by the Biomarkers of Nutrition for Development (BOND) initiative are summarized in Table 6 (185), which includes a concise overview of the biomarker and the utility for different purposes/user groups (i.e., research, clinical, program policy). To provide some additional clarity, Table 7 includes a rating system for the usefulness of each indicator for a specific use, including the advantages and disadvantages, and a brief coverage of analytical considerations, which are further covered in Table 8.
TABLE 6.
Summary of biomarkers for vitamin A status from the BOND initiative1
| Biomarkers | Type | Use | Utility |
| Serum RBP | Status | Population | Not released from the liver when retinol is limiting. Used as a proxy for serum retinol in identification of vitamin A deficiency. |
| Serum/plasma retinol | Status | Population | Most commonly used biomarker. Correlates with the prevalence and severity of xerophthalmia and may change in response to interventions. |
| Dried blood spot retinol | Status | Population | Surrogate measure of serum retinol. Correlates with serum retinol measured by HPLC. |
| Relative dose response | Status | Population, individual | Based on hepatic accumulation of RBP during vitamin A depletion. Requires blood sample before and after an oral retinyl ester dose. |
| Modified relative dose response | Status | Population, individual | More responsive than serum retinol. Qualitatively identifies low or adequate liver vitamin A reserves. |
| Retinol isotope dilution | Status, marker of excess | Population | Although technically challenging, it is the most sensitive test to measure vitamin A status and intervention impact on vitamin A reserves. Minimally invasive and accurate. |
| Breast-milk retinol | Status, exposure | Population | Good indicator of vitamin A status in areas where breastfeeding is common until ≥6 mo of age. Milk retinol varies with milk fat; measurement of milk fat is recommended. |
| Retinyl esters | Status, marker of excess | Population, individual | Validated qualitative measure of hypervitaminosis A. May be confounded by liver disease at the individual level. |
| Dark adaptation | Function | Population (small scale), individual | Dark-adapted final threshold is inversely and sensitively correlated with serum vitamin A concentrations in the low-to-deficient ranges. |
| Electroretinography | Function | Population, individual | Measures the bioelectrical response of the retina to a flash of light. Invasive and not suitable for children. |
| Pupillary threshold testing | Function | Population, groups of individuals | Inversely correlates with serum vitamin A values in the low-deficient range and the concentration of vitamin A in the retina. Noninvasive and can be used in field conditions |
| Dietary assessment | Exposure | Population, individual with repeated testing | Qualitative measure of exposure. Provides useful information to support biochemical biomarkers. Seasonality of fruit and vegetables must be included. |
Data are from reference 185. BOND, Biomarkers of Nutrition for Development; RBP, retinol-binding protein.
TABLE 7.
Overview of currently available biomarkers for the assessment of vitamin A nutrition1
| Usefulness assessment2 |
||||||||||||
| Exposure |
Status |
Function |
Effect |
|||||||||
| Biomarker name | Research | Clinical | Program | Research | Clinical | Program | Research | Clinical | Program | Research | Clinical | Program |
| Dietary assessment | ++ | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + |
| Functional tests | + | + | + | + | + | + | ++ | ++ | + | + | + | + |
| Dark adaptation | ||||||||||||
| Electroretinography | ||||||||||||
| Pupillary threshold | ||||||||||||
| Biochemical tests | ||||||||||||
| Serum retinol | + | + | + | ++ | ++ | + | ++ | ++ | 0 | + | 0 | + |
| RBP | ||||||||||||
| Dried blood spots | ++ | ++ | 0 | +++ | ++ | ++ | ++ | + | 0 | 0 | 0 | + |
| Breast-milk retinol | + | + | + | + | + | + | 0 | 0 | 0 | + | 0 | + |
| RDR | 0 | 0 | 0 | + | 0 | 0 | + | + | 0 | + | 0 | + |
| MRDR | 0 | 0 | 0 | ++ | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Isotope dilution | ++ | 0 | 0 | ++ | 0 | + | 0 | 0 | 0 | ++ | 0 | + |
| Serum retinyl esters | + | + | + | + | + | + | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver samples | + | + | 0 | + | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Radio-isotopes | + | 0 | 0 | + | 0 | 0 | + | 0 | 0 | + | 0 | 0 |
| Proteomics or nutri-genomics3 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
Usefulness assessment uses the following grading system: 0, Not useful for the specific purpose; +, Useful to some extent and in certain population groups, but either not commonly used or has important disadvantages (e.g., no reference values); ++, Useful in certain population groups, often used with some limitations (e.g., lack of specificity or sensitivity); +++, Useful, often used in relevant population groups, with no or only minor limitations; ?, Unknown utility for vitamin A status assessment at this time. MRDR, modified relative dose response; RBP, retinol-binding protein; RDR, relative dose response.
Exposure means that the biomarker responds to dietary vitamin A intake, status means that the biomarker measures vitamin A status, function means that the biomarker measures a shift in function, and effect means that the biomarker is affected by deficiency of vitamin A. Research means that the biomarker can be used in research settings for evaluation and intervention outcomes, clinical means that the biomarker can be used in clinical settings at the individual level, and program means that the biomarker is applicable to program evaluations that usually include population assessments with large numbers of individuals.
Not discussed systematically in this article.
TABLE 8.
Comparison of sample processing among common biochemical biomarkers of vitamin A status1
| Difficulty of sample collection | Difficulty of sample transportation | Technical analysis | Relative analysis costs | Analytical accuracy | |
| Serum retinol | ++ | ++ | ++ | $ | ** |
| Serum RBP | ++ | ++ | + | $ | * |
| DBS retinol | + | + | ++ | $ | * |
| DBS RBP | + | + | + | $ | * |
| Breast-milk retinol2 | + | ++ | +++ | $$ | ** |
| Dose-response tests3 | |||||
| RDR | ++ | ++ | ++ | $$ | *** |
| MRDR | ++ | ++ | ++ | $ | **** |
| Isotope dilution | +++ | ++ | ++++ | $$$ | ***** |
| Liver analysis2 | +++++ | ++ | +++ | $$ | ***** |
The greater the number of “+” the greater the difficulty; the greater the number of “*” the more sensitive the analysis and accuracy to determine vitamin A status; the greater the number of “$” the “greater the relative costs of the assay in regards to sample number and the instrumentation involved.” DBS, dried blood spot; MRDR, modified relative dose response; RBP, retinol-binding protein; RDR, relative dose response.
Breast-milk retinol requires saponification. Liver analysis either requires saponification or lengthy analysis times to add together the variety of retinyl esters found in humans.
The RDR test requires 2 blood samples for analysis, making it logistically more expensive than the MRDR, but the MRDR requires procurement of 3,4-didehydroretinyl acetate.
Biomarker-Specific Issues
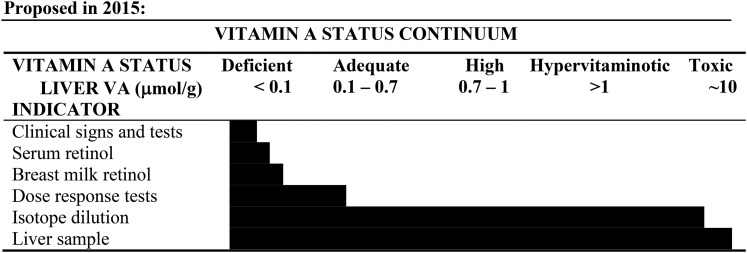
Liver reserves of vitamin A are considered the gold standard for vitamin A assessment. The previous cutoff of 0.07 μmol/g liver was based on an estimated protection from clinical signs for 4 mo in average-weight individuals (34). In 2010, commonly used biomarkers were plotted against liver reserves of vitamin A to define the range of liver reserves associated with specific biomarkers (186) (Figure 6). Research in animals suggests that the cutoff for vitamin A deficiency should be set at <0.1 μmol/g liver, as follows:
FIGURE 6.
The definition of vitamin A status related to liver vitamin A concentrations and the range of liver reserves in which the vitamin A biomarker has utility in predicting vitamin A status. In the past, 0.7–1 μmol/g was considered excessive, but until more biologically meaningful data are generated this range is considered high. Updated in 2015. VA, vitamin A. Reproduced from reference 186 with permission.
Data obtained in rats showed downregulation of LRAT, which is the enzyme responsible for retinol esterification leading to storage (187).
Studies in rats and piglets showed that apo-RBP begins to accumulate below this liver concentration (186, 188), eliciting a response with the MRDR test (186).
In rats, biliary excretion of metabolites of vitamin A significantly increased at liver reserves >0.1 μmol/g liver (189).
The evidence for setting the cutoff for hypervitaminosis A is limited. Nonetheless, >1 μmol/g is likely associated with hypervitaminosis A for most individuals (145). In 2010, the term subtoxic was used to define this cutoff; however, the consensus of the BOND Vitamin A Expert Panel is that until more data exist with regard to adverse effects at either higher or lower liver concentrations, the terms hypervitaminosis and toxic are more appropriately used for liver concentrations >1 μmol/g liver and 10 μmol/g liver with 1 significant digit, respectively (Figure 6). In the past, a more conservative cutoff of 0.7–1 μmol/g was considered excessive (190). Considering the widespread use of fortified foods in both developed and developing countries, future research needs to determine if there are deleterious effects at liver retinol concentrations <1 μmol/g.
After briefly discussing dietary assessment of vitamin A, biomarkers that are in use will be discussed under 2 main subheadings: “Physiologic (functional) measures of vitamin A status” and “Biochemical indicators of vitamin A status.” The emphasis of the following sections is primarily on biomarkers used to assess vitamin A dietary insufficiency, although markers to assess excessive vitamin A status are also briefly reviewed.
Dietary assessment
Although currently no sensitive or specific biomarkers of short- or long-term exposure exist, dietary assessment is an essential component of the vitamin A assessment toolkit. Dietary assessment methods include the following: dietary records, 24-h dietary recall, FFQs, brief dietary assessment instruments, and diet history. The dietary assessment methodologies and the issues around them have been extensively reviewed (191).
Researchers need to be aware of which foods in the country being evaluated are fortified, especially with preformed vitamin A. An awareness of seasonality of different fruit and vegetable sources of provitamin A carotenoids is also important when determining which times of the year to complete dietary assessments. Supplement coverage or individual usage can also support dietary record data.
Vitamin A deficiency occurs when dietary intake is not enough to meet demands (Figure 5). The body can downregulate bioconversion of provitamin A carotenoids, and therefore high intakes of fruit and vegetables will usually not cause concern for hypervitaminosis A. However, if the dietary intake of preformed vitamin A becomes high through foods, such as liver, fortified foods, or supplements, the body will store the excess in the liver and the concentration will continue to increase (142).
Physiologic (functional) measures of vitamin A status
As highlighted throughout this report, vitamin A plays a number of key roles in human biology. Many tools exist to assess particular biological functions. However, it is essential to recognize that many functional tests are actually “bioindicators,” reflecting perturbations in specific biological systems rather than sensitive and specific biomarkers of nutrient exposure, status, or effect (192). In the context of evaluating the functional impact of vitamin A, a number of measures exist that, in combination with specific biomarkers, can be used to assess functional implications of vitamin A status. The following sections highlight the most commonly used of these bioindicators.
Dark adaptation.
Dark adaptation, which is the ability to see under dim lighting conditions with time, was first described in the mid-19th century (193) and first measured under standardized conditions at the beginning of the 20th century (194). Once the importance of vitamin A was recognized for the visual cycle, it was only a matter of time before dark adaptation testing (vision at low light intensity) began to be used for the detection of vitamin A deficiency. In individuals who do not have adequate vitamin A nutrition, the ability of the rod cells to adapt in the dark, and for pupils to properly meter light in and out of the eye, may be impaired. This may result in a condition called nightblindness.
The retina consists of 2 types of photoreceptors: rods and cones. The rods are located in the peripheral retina and the cones are located in the central macula region of the retina. The rods enable vague, colorless vision at low light-intensity levels (e.g., moonlight), whereas the cones enable clear vision with color at higher light intensities. This important rod function is linked to the size of the pupil, which opens in the dark to allow light to reach the back of the eye and becomes smaller in bright light. When an individual first moves from a brightly lit space into relative darkness, he or she initially can see practically nothing. However, as the retina gradually shifts from cone to rod vision, the individual can begin to see dimly lit objects take form. The ability to accurately measure the speed at which this adaptation takes place and the absolute lowest level of light intensity while still being able to see allowed for the physiologic demonstration of the importance of vitamin A in the dark adaptation process and for using dark adaptation to detect vitamin A deficiency. Eventually, dark adaptation testing under highly controlled experimental conditions was used to help define the dietary requirements for vitamin A and the efficiency of the conversion of β-carotene to vitamin A (195). Initially, the time to perform dark adaptation testing was lengthy, and the equipment for classical dark adaptation testing was cumbersome. However, newer equipment and techniques for measuring dark adaptation in field settings are now available, and some of these are described below.
Classical testing.
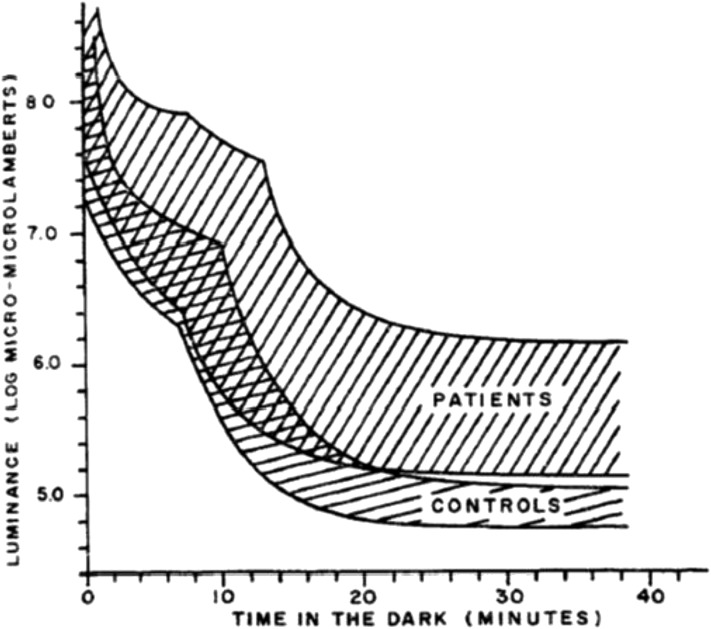
In classical dark adaptation testing, a cumbersome and expensive adaptometer is used, which consists of 3 main elements: a globe that is open on 1 side, a chin rest, and a light-intensity adjustment knob, which is operated by a trained technician. The test subject must first have his or her eye dilated, and then is light-adapted at a bright standard intensity for ∼10 min. In a darkened room, the seated subject rests his or her chin on the chin rest and fixates on a red light that is 15° above the center of a test (white) light (both lights are located at the back of the open globe). The test white light consist of flashes lasting 1 s, the intensity of which can be increased or decreased by the technician depending on the subject’s responses. When the subject says that he or she can see the test flashes, the technician can lower the light intensity until the subject can no longer detect the flashes. Then, the technician can slowly increase the light level until the subject says that he or she can now see the flashing light. A tracking method is used such that the light intensity detected by the subject [measured in log candela (log cd)/m2] is plotted against time (Figure 7). The dark adaptation curve follows a characteristic pattern: there is an initial decrease in the luminance threshold due to cone adaptation, followed by a short plateau. Then, at the rod-cone break, rod vision kicks in and a slower, second luminance threshold descent begins, becoming stabilized in ∼35–40 min. A learning effect can occur, so each subject should be given a trial run before a true dark adaptation test is begun. In one study, the final dark-adapted threshold among healthy adult subjects was −5.0 (±0.3 log cd/m2).
FIGURE 7.
The rod-cone break period measured in luminance (log micro-microlamberts) occurs in <10 min in normal individuals when placed in a dark room. Vitamin A–deficient subjects have a higher luminance need to see in the dark as depicted by patients (n = 13) with chronic small intestinal disease compared with the range of 7 controls. Reproduced from reference 196 with permission.
In vitamin A deficiency, the subject’s rod-cone break takes a longer time to achieve and the final dark-adapted threshold is elevated by 1–2 log cd/m2. Classical dark adaptation will be abnormal in subclinical vitamin A deficiency before symptoms of nightblindness occur (196). Classical dark adaptation is a direct test of a vitamin A–dependent biological system; thus, no surrogate biomarkers are needed. However, the precise delicate equipment can only be used in controlled clinical or experimental conditions. Due to the high degree of attention that is required on the part of the test subject, children should not be tested with this technique. Moreover, there are a number of confounding factors, which, if present, might yield an abnormal final threshold result that is unrelated to vitamin A deficiency. Among the confounders are eye diseases and zinc (197) or protein (198) deficiency. Subjects to be tested must be free of these deficiencies, because these nutrients are needed for RBP synthesis and for transport of vitamin A to the eye. Furthermore, aging can affect dark adaptation in an inconsistent manner due to varying degrees of age-related cataracts, retinal degeneration, and, in some older people, the inability to concentrate (199, 200).
Rapid dark adaptation testing.
Rapid dark adaptation testing is dependent on the shift from cone to rod vision when dark adaptation is occurring. When one shifts to rod vision under dim-light settings, a shift in wavelength sensitivity of the retina takes place (Purkinje phenomena), such that blue appears brighter than red as the rods become activated (201). The rod-cone break (described above and in Figure 7) normally occurs in <10 min in the dark; therefore, the ability to see blue as brighter than red occurs well within this time interval.
The equipment needed for rapid dark adaptation is much simpler than that needed for classical dark adaptation: a dark room; a very dim light source (7.5 watts filtered to allow 1% transmission); a dark, nonreflective work surface; a stop watch; a bright light-adapting source; and colored chips or discs (6 each of a blue-dominant wavelength of 475 nm, a red-dominant wavelength of 605 nm, and white). After an initial period of bright light adaptation of 1 min, the subject sits in darkness in front of the nonreflective table surface on which the colored discs have been placed. He or she can quickly separate the white chips away from the rest, but the separation of the blue chips from the red takes longer. If a mistake is made, a trained technician returns the disc to the work surface until the subject has accurately separated 100% of the blue from the red discs. At this point, the time it took for the subject to complete the task is noted and the test is over. The rapid test time in control subjects aged 20–39 y was mean ± SD 3.03 ± 1.00 min and in control subjects aged 40–60 y was 4.41 ± 0.83 min (202). The colored discs have been used with children ≥4 y of age (203).
The advantages of this test are as follows: 1) it can be performed under most field conditions, 2) it requires only simple and inexpensive equipment, 3) it is relatively fast to conduct, and 4) it can be performed in children. The disadvantages of this test are that it 1) has only been performed in a relatively small number of subjects and is in need of further validation, 2) still requires a trained technician and a totally darkened room, and 3) has the same confounders as classical dark adaptation.
Electroretinography.
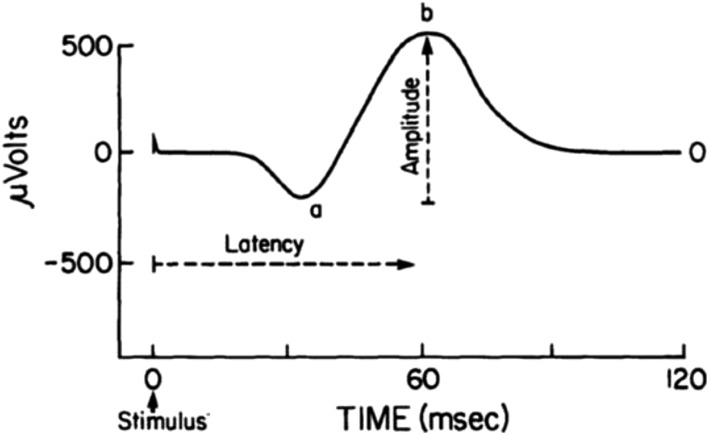
The use of electroretinography (or variants thereof) is even more restricted than for classical dark adaptation due to the fact that the test is invasive and requires not only eye dilation but also direct eye contact with a measuring electrode (204). Thus, this test is only used in highly structured clinical or research settings to measure the bioelectrical response of the retina to a flash of light. There are 2 basic components of the electroretinogram: a negative “a” wave, followed by a positive “b” wave, the absolute heights of which are measured in microvolts (Figure 8). Latencies of the “a” and “b” waves can also be measured (i.e., the time from the light flash to the peak amplitudes of the “a” or “b” waves). In addition to the “a” and “b” wave amplitudes and latencies, the electroretinogram can be used to evaluate dark adaptation time (i.e., the time for recovery from a bright light exposure of the electroretinogram to a maximal electroretinographic dark-adapted response). The maximal electroretinogram response reflects the number of photoreceptors, whereas the dark adaptation recovery time reflects the ability of the photoreceptors to regenerate rhodopsin after a bleaching, bright light exposure.
FIGURE 8.
The electroretinogram response in the μvolts is generated by light stimulation of the retina and occurs in milliseconds. The electroretinogram is characterized by a negative “a” wave originating from the photoreceptor inner segments followed by a positive “b” wave, which originates from the retinal bipolar cell layer. The electroretinogram amplitude is proportional to the intensity of light, the degree of dark adaptation, the number of photoreceptors, and the rhodopsin visual pigment concentration. Reproduced from reference 204 with permission.
This technique has not been used extensively to evaluate vitamin A deficiency. The test is not appropriate for field settings or for use in young children, except under strict medical supervision. The test has the same disadvantages and confounders as classical dark adaptation and in addition has the disadvantage of being invasive. Its advantage is that the test is wholly objective.
Pupillary threshold testing.
Pupillary threshold testing reflects the dark-adapted pupillary reflex (pupillary restriction on light exposure) at low-intensity light exposures that are near the visual threshold (205). This test has the advantage over classical and rapid dark adaptation in that it is entirely objective. Moreover, the test can be carried out in children as young as 3 y old and under field conditions (206). Although the test has not been widely used to date, it may provide an objective, noninvasive, rapid, portable, physiologic field test for the assessment of vitamin A deficiency.
The absolute pupillary threshold is known to be higher than the visual threshold. Special pairs of goggles were invented to measure pupillary response to light stimuli (207). One of the earlier goggle versions required a dark room, a camera with flash, a goggle apparatus to fit over 1 eye in order to illuminate the entire retina of that eye, and an oblique red-light–emitting diode source. After exposure of both eyes to the camera flash, the test subject is dark-adapted for 10 min. The illuminating goggle is then placed over the subject’s left eye, while the pupil of the right eye is observed (while under red illumination from a side-mounted light) by a trained technician. The brightness of the light stimulus delivered by the goggle is then increased at 10-s intervals (range: −4.16 to −0.575 log cd/m2], while the right eye is observed for a clearly visible restrictive response. A version of this apparatus has been used in nightblind Nepalese women to evaluate various vitamin A interventions (208). The newer versions of these goggles do not require a dark room, but measurements are challenging for children under the age of 5 y (207).
A pupillary threshold of < −0.575 log cd/m2 is presently considered normal for children aged 3–5 y, although more thorough testing is needed before arriving upon validated normal values. The same confounders as for classical dark adaptation are expected to be operative here as well.
Conjunctival impression cytology.
In the mid-1980s, it was recognized that the conjunctiva of the eye could be sampled and the cells stained to determine abnormalities (209). This method was termed conjunctival impression cytology and abnormality was defined by the absence of goblet cells and hyperplasia of epithelial cells. Although the method compared well with positive MRDR tests in Indonesian children (210), it did not work well in children in Africa due to arid conditions and it has not been further refined (211, 212).
Biochemical indicators of vitamin A status
Serum retinol concentrations.
Assessing vitamin A deficiency in prevalence surveys at the population level.
Serum retinol concentration measurements by HPLC are a common method used to assess vitamin A status of populations (111). The current cutoff used to define a severe public health problem for vitamin A deficiency is when 20% of children aged 6–71 mo have a serum retinol concentration <0.7 μmol/L (213). Although currently recommended for use by the WHO, serum retinol concentrations should not be used alone to define the degree of public health significance but should be used in conjunction with another biological indicator or when ≥4 of the following risk factors are found in the population being evaluated (213):
infant mortality rate >75 of 1000 live births and under-5-y mortality rate of >100 of 1000 live births;
full immunization coverage in <50% of children at 12–23 mo of age;
<50% prevalence of breastfeeding in 6-mo-old infants;
median dietary intakes <50% of recommended safe levels of intake among 75% of children 1–6 y of age;
2-wk period prevalence of diarrhea of ≥20%;
measles case fatality rate of ≥1%;
no formal schooling for ≥50% of women 15–44 y of age; and
<50% of households with a safe water source (e.g., boiled, treated, filtered, properly stored).
Serum retinol and RBP tend to be lower in infants and young children than in adults, even in vitamin A–adequate populations. Therefore, the same cutoff cannot be used for infants <6 mo of age (214). However, the relative concentration to define vitamin A deficiency in infants has not been determined and is a research question that needs to be addressed. Serum retinol concentration tends to increase through middle-age in the US population (particularly in men but also in women >60 y of age) and may represent a continuing, although less pronounced, association of liver vitamin A stores (215).
RBP concentrations do not vary by sex, generally speaking, and the lower serum retinol concentrations in women than in men in middle-age may be due to differences in liver stores or to the higher prevalence of inflammation in women (215). The cutoff used for deficiency in adults varies, and some researchers choose to use 0.7 μmol/L whereas others suggest 1.05 μmol/L (214). The hepatic synthesis of RBP is depressed during zinc deficiency (216), which results in lower plasma retinol concentrations that confound measurements in zinc-deficient individuals. Pregnancy lowers serum retinol concentrations through hemodilution. In Indonesian women, serum retinol concentrations <0.7 μmol/L were associated with positive MRDR tests during lactation, reflecting low liver retinol stores (217), whereas during pregnancy a lower cutoff of <0.53 μmol/L was associated with positive MRDR values (218). This likely reflects hemodilution from the increase in intravascular volume during this physiologic state.
Use in program evaluation.
Serum retinol distribution curves are used to evaluate program impact (111). However, the lack of change in serum retinol distribution over time in several countries that have sustained >70% coverage with vitamin A supplementation has raised the concern about the appropriate indicator (219). For this reason, the impact of supplementation programs is not measured by a change in the prevalence of low serum retinol concentrations but may be better served by evaluating coverage rates. Retinol concentrations may respond to sustained, improved dietary intakes and therefore can guide programmatic decisions about whether to maintain or change interventions (219). Thus, the use of serum retinol distributions among preschool children from cross-sectional surveys to assess the need for vitamin A interventions is still recommended. Furthermore, it is important to determine whether serum retinol concentrations are being affected by the acute-phase response to evaluate its influence on the prevalence of low concentrations (35, 220).
With respect to monitoring the impact of widespread fortification and interventions that increase dietary intake, serum retinol distribution may be expected to shift; thus, serum retinol is an appropriate indicator of response to these programs (111). The Global Alliance for Vitamin A outlined a framework for shifting from universal high-dose supplementation toward other interventions to improve dietary intake. The Global Alliance recommended that scaling back supplementation could occur in vulnerable populations when the government can verify Adequate Intakes from diet and other interventions. By using WHO prevalence cutoffs as a guide, a threshold of documented and verified prevalence of <5% for serum retinol concentrations <0.7 μmol/L was proposed as a basis for scaling back or withdrawing universal supplementation to children <5 y of age (219). To date, this recommendation has not been evaluated by the WHO.
Use in intervention research.
The response of serum retinol concentrations to interventions will rely heavily on the underlying vitamin A status. If serum retinol concentrations are initially low, and the population is not experiencing a high degree of inflammation, serum retinol concentrations may increase. Serum retinol concentrations in Indonesian children improved significantly when they were fed retinol-rich foods and provitamin A–containing orange fruit or leafy vegetables and carrots compared with a low vitamin A–fed group (78). Nonetheless, in Zambian children of a similar age with adequate to hypervitaminotic vitamin A stores, serum retinol did not respond to an intervention when given as either when given the RDA as supplemental retinyl palmitate or when fed orange biofortified maize 6 d/wk (85).
Use in clinical application.
Serum retinol concentrations are affected by infection status. RBP is a negative acute-phase protein, and thus retinol and RBP concentrations decrease during the acute-phase response (35, 220). Therefore, serum retinol has very little utility to diagnose vitamin A deficiency when individuals have an infection. In addition, serum retinol concentrations are homeostatically controlled over a broad range of liver reserves and thus are best used at the population level as detailed above.
Use of serum retinol concentrations in epidemiologic studies.
Serum retinol concentrations are determined and have been evaluated in epidemiologic surveys. A recent evaluation of NHANES III data in adults ≥50 y of age determined that serum retinol concentrations that were either <1 μmol/L or >2.8 μmol/L were associated with increased risk of all-cause and cardiovascular or coronary artery disease–related mortality (221). The significance of these findings is not entirely known. In otherwise well-nourished individuals who are not experiencing inflammation, serum retinol concentrations <1 μmol/L may reflect vitamin A deficiency (214). It appears that serum retinol concentrations are increasing over time in well-nourished Americans, which may be associated with increased catabolism (140) due to the fortification of a variety of foods and multivitamin supplement use that increase total body stores over time (222).
Effect of infection or inflammation on serum retinol concentrations.
The acute-phase response to either infection or inflammation affects retinol homeostasis. The acute-phase response on micronutrient status markers has been reviewed (223, 224). Currently, concentrations of C-reactive protein (CRP) and α1-acid glycoprotein (AGP) have been used to identify infection stage according to a proposed published paradigm: elevated CRP alone indicates the incubation or early phase of an acute infection, elevated CRP and AGP indicate early convalescence, and elevated AGP only indicates late convalescence. These inflammation stages have been used to correct serum retinol concentrations or to choose lower cutoffs for serum retinol as a marker of deficiency (225). During early convalescence, serum retinol concentrations are shifted further to the left than during late convalescence or incubation (Figure 9) (220). In Zambian (226) and Indonesian (227) children, lower serum retinol concentrations were associated with elevated CRP, whereas the MRDR test response was not. In contrast, Thai children who had low rates of inflammation were able to maintain serum retinol concentrations >0.7 μmol/L, even though mean liver reserves were 0.09 ± 0.05 μmol/g liver, which was determined by retinol isotope dilution (220, 228).
FIGURE 9.
Kernel density estimation visually depicts smoothed distribution trends in serum retinol concentration shifts (μmol/L) by infection stage. The acute-phase response lowers circulating concentrations of retinol. Adapted from reference 220 with permission.
RBP.
Plasma RBP, sometimes referred to as RBP4, is the transport protein for retinol in the blood and is found in many vertebrate species. Rodents and pigs are particularly useful models for translation to human studies (229). RBP is synthesized primarily in hepatocytes as the apo-form and secreted bound to retinol as the holo-RBP complex to provide vitamin A to peripheral tissues. holo-RBP binds 1 molecule of retinol (230). RBP is also synthesized in other cells (e.g., adipocytes) (231), although the magnitude of the contribution of such sites to plasma RBP is not clear. RBP is not secreted from the liver when retinol is limiting (i.e., when vitamin A stores are low) and RBP accumulates (186, 188). This is the biology by which the dose-response tests work (described below). The release of the holo-RBP complex is interrupted during inflammation, resulting in lower plasma retinol and RBP concentrations than would be seen without inflammation (95). To account for this phenomenon, which can lead to false identification of someone as being vitamin A deficient, serum markers of inflammation (e.g., CRP, AGP) can be used to identify individuals with inflammation. Currently, a consensus on how to deal with this problem has not been reached, although some suggest adjusting serum retinol or RBP concentrations with the use of acute-phase proteins (35) or adjusting cutoffs to modify prevalence (220). It is possible that this transient decrease in serum holo-RBP during infection has a significant effect on transport and may cause a functional deficiency in peripheral tissues, but this has never been convincingly shown.
Plasma RBP is normally ∼85% saturated with retinol (i.e., 85% holo, 15% apo), although this differs on the basis of the degree of adiposity (232). holo-RBP forms a noncovalent complex with transthyretin, the transport protein for thyroid hormones. The association between holo-RBP and transthyretin strengthens and stabilizes the binding between retinol and RBP (133, 233) and prevents holo-RBP from loss to glomerular filtration in the kidney (234). In addition, the transthyretin-RBP complex is sufficiently large that it remains in the blood rather than being filtered into the kidney tubules by passing through the fenestrations of the glomerulus. apo-RBP, which is a small protein with a molecular weight of 21 kDa, does pass into the tubular filtrate and is usually reabsorbed by the proximal tubular epithelium via the transport protein megalin. Under some conditions, this reabsorption process is disrupted (e.g., high fever, use of aminoglycoside antibiotics) and low-molecular-weight serum proteins, including RBP, appear in the urine. Substantial vitamin A can be lost in the urine during illness accompanied by high fever (235) or in some genetic diseases that affect tubular function (236). This may increase the risk of vitamin A deficiency in individuals who do not have adequate liver stores to draw from. In subjects with kidney disease, the glomerular filtration rate decreases, resulting in an increase in the serum concentration of apo-RBP (237) and a lower percentage of saturation with retinol than the typical 85%. This altered ratio would decrease the utility of RBP as a surrogate indicator of serum retinol concentration.
In obese individuals, serum RBP concentrations appear to be affected by adipose tissue, and some consider RBP to be an adipokine, secreted by adipocytes and affecting other tissues, perhaps directly affecting insulin resistance (176). In studies in adults and adolescents, serum RBP was positively correlated with BMI, visceral adiposity, and insulin resistance, which are components of metabolic syndrome, and even inflammatory markers (although inflammatory markers have a negative association with RBP outside of obesity). Thus, serum RBP may be particularly unsuitable for extrapolation to serum retinol concentrations during obesity, because the concentration of apo-RBP not bound to retinol may increase relative to holo-RBP (232, 238). Note that in high-income countries, where most of these obesity studies were conducted, the risk of vitamin A deficiency is low. However, in countries undergoing economic and nutritional transitions, obesity may coexist with vitamin A deficiency, and it is unclear how RBP would perform as an indicator of serum retinol concentrations in these settings. For example, if adipocyte-derived RBP tends to be apo-RBP not bound to retinol, this phenomenon would produce false-negative results, indicating adequate serum retinol in obese subjects when, in fact, vitamin A–deficient concentrations are present.
Retinol from holo-RBP is transported into cells via the RBP receptor protein STRA6 (239), and this process may be inhibited by transthyretin binding to RBP (240). The implications of this for vitamin A transport to tissues are not yet clear. STRA6 may also initiate intracellular signaling when RBP is bound (241), a newly suggested role for this receptor, and may thus affect cellular function in novel ways that may not be directly related to vitamin A transport. It is not known if STRA6 contributes to the decrease in holo-RBP that occurs during inflammation.
Assessment of deficiency.
Serum RBP is used as a proxy for serum retinol concentrations in the identification of vitamin A deficiency. As discussed above, serum retinol correlates with liver vitamin A stores only when liver stores are very low. When stores are replete, serum retinol concentrations are homeostatically regulated and do not correlate with liver stores. Because the RBP-retinol complex is released by the liver as part of this homeostatic process, serum RBP correlates closely with serum retinol concentrations, at least in subjects with normal kidney function who are not obese. Quantitative assessment of RBP concentration is cheaper and easier than the standard method for measuring serum retinol concentrations (HPLC analysis). Serum RBP can be measured by immune-detection methods because highly specific antibodies are available. The most common methods include ELISA, which is useful for small laboratories, and other methods (e.g., nephelometry) that are more commonly used in automated clinical analyzers found in hospital or reference laboratories. Many of the commercially available reagents and kits are intended for serum, but kits are also manufactured for measuring urinary RBP as an indicator of kidney proximal tubular function.
RBP is not always 100% saturated with retinol; therefore, a 1:1 molecular equivalence between retinol and RBP in the blood does not usually occur. Thus, one cannot generally use the retinol cutoffs for RBP unless liver stores are hypervitaminotic, as discovered in Zambian preschool children (85) in whom the ratio was 1.0 (144). In addition, the added variability in differences in kidney function (e.g., low glomerular filtration rate can cause an increase in RBP but not retinol) among subjects as well as the apparent contribution of adipose tissue to serum RBP (which may not reflect tissue stores in the same way as liver-derived RBP) make it unlikely that the same retinol-RBP correlation that might be observed in 1 population would be appropriate for another. It is possible that certain populations, such as young children not at risk of obesity and with normal kidney function, may be relatively homogeneous with regard to this association, but such associations have not been systematically examined. Thus, the best approach for RBP is to take a subset of samples of interest and measure serum retinol concentrations by HPLC and then use these data to construct a standard curve to predict serum retinol from serum RBP in the population of interest. Of course, the sample should have a wide range of serum retinol concentrations in order to produce a reasonable correlation (i.e., enough high and low values to make a standard curve). This could be done by first measuring RBP in a large number of samples and then selecting low, middle, and high values for serum retinol analysis. Serum RBP is not a useful marker of recent vitamin A intake (exposure) because of its homeostatic regulation by the liver.
Prevalence surveys.
Prevalence surveys in nearly all settings will probably be large enough to benefit from the economy of the RBP method and should also be large enough to select a subset for retinol analysis for construction of a standard curve. Most populations will contain individuals with current or recent acute infections or chronic inflammation associated with obesity or chronic disease, which all depress serum retinol. Thus, analysis of acute-phase proteins, such as CRP and AGP, is recommended.
Surveillance.
Surveillance raises the issue of time-sensitive analysis of data that may preclude the approach used in surveys of analyzing a subset for retinol analysis that has been selected over a wide range of serum RBP concentrations. One approach may be to perform both assays on early samples from the surveillance (if the surveillance is going on for months to years and data are needed periodically). To determine if RBP measurements are within the diagnostic range of the assay used and to produce an initial standard curve for predicting serum retinol from RBP, some of the samples with the highest RBP concentrations could be diluted by 2- to 4-fold with physiologic saline and reanalyzed by both the RBP and retinol assays and used as standards until completion of the survey.
Program evaluation.
The issues for program evaluation are quite similar to those discussed above for surveys.
Intervention research.
Intervention research in communities raises many of the same issues as discussed for surveys but also allows one to consider the use of only RBP to compare 2 interventions—for example, in cases in which external validity (i.e., correlation with actual retinol concentration) may not be necessary (although it is probably always desirable). Thus, one could use RBP by itself in such a situation to compare multiple intervention groups, assuming the subjects in the intervention groups are relatively homogenous with regard to other characteristics that may affect serum RBP (e.g., age, BMI).
Clinical applications.
Serum retinol concentrations should probably be used in clinical research given that clinical populations may have underlying medical issues that could affect the relation between retinol and RBP, such as differences in kidney function, which may invalidate the use of RBP.
Epidemiologic studies.
The population under study will affect the decision to use RBP as an indicator of vitamin A status. For example, in obesity research, serum RBP is considered to act, perhaps independently of retinol, as a mediatory in the development of insulin resistance (176); and the retinol-to-RBP ratio may be lowered due to RBP secreted from adipose tissue unbound to retinol (232), as discussed above. RBP measurements may not be useful in populations with a high prevalence of obesity because some of the RBP would be derived from adipose and not bound to retinol. However, in most instances, these associations are seen at serum RBP concentrations that are much higher than those used for the diagnosis of deficiency.
Dried blood spots as a surrogate of serum retinol concentration
Historically, serum/plasma retinol was a common indicator of vitamin A status (242). It correlates with the prevalence and severity of xerophthalmia and may change in response to vitamin A interventions if the population being studied has underlying vitamin A deficiency. Serum retinol concentrations do have limitations as described above. With current analytical techniques, it is not necessary to draw venous blood to measure retinol because <20 μL serum is obtainable from a finger-prick, which is preferable in some nonresearch settings. The primary restrictions to the use of serum are maintaining a cold-chain, transporting and storing samples frozen until analysis, and requiring separation from whole blood, which can be challenging in field settings in which electricity may not be available.
The use of dried whole blood as a sample can circumvent the issues of cold-chain and centrifugation. The measurement of retinol in dried blood spots (DBSs) makes sample collection more practical and convenient. The collection of DBSs obtained from a finger-prick is usually less invasive and more acceptable than the collection of venous blood. The volume of blood required for retinol or RBP analysis can also be obtained from neonates via heel-pricks. The measurement of retinol in the serum component of the dried blood on the collection card is associated with serum retinol concentration by HPLC, but a correction factor is often needed. Its use and interpretation as an indicator of vitamin A status are subject to the same considerations as serum retinol.
The measurement of retinol on filter paper was first reported for dried serum samples by Oliver et al. (243). This study showed the potential for transporting samples without a cold-chain but still required centrifugation to obtain serum and used a 200-μL serum volume. The measurement of vitamin A in microsamples of whole dried blood as holo-RBP was first shown by Shi et al. (244) who used capillary electrophoresis and laser-excited fluorescence detection. Although an impractical method for routine use, it showed that the RBP complex protects retinol in whole dried blood. The analysis of retinol in DBSs was transferred to HPLC and further refined at Craft Technologies (245–248). Houzé et al. (249) reported analyzing DBSs with electrochemical detection, which may increase sensitivity for other DBS applications. DBS retinol is measured in several laboratories internationally. Manuals are available that provide instructions for DBS collection (250, 251).
Validation studies.
Several studies have been performed to validate the performance of DBS retinol compared with serum retinol. The first was conducted with matching DBSs and serum samples collected from 17 adult volunteers (245). The coefficient of determination (r2) was 0.90 between plasma and DBS retinol (245). Another small study performed in 20 healthy Guatemalan adults also compared capillary with venous blood (246). The coefficient of determination (r2) was 0.88 between plasma retinol and capillary DBS retinol, and there was no significant difference between serum retinol and capillary or venous DBS retinol (246).
For application in the field, DBS retinol was included in a group of 146 pregnant Nepali women (33). Blood spot retinol predicted low serum retinol concentrations with a sensitivity of 89.5% and a specificity of 98.4% compared with serum retinol determined by HPLC (247). In 54 Nepali children, the sensitivity of DBS retinol was 92.9% and the specificity was 90% compared with serum retinol concentrations.
DBS limitations.
Although DBS retinol is essentially equivalent to serum retinol under ideal conditions, it carries some limitations that are unique to the DBS matrix, particularly in a field setting. Liquid serum volumes can be accurately measured and estimates of serum volume in DBSs are reasonably accurate in a laboratory setting in which known volumes can be spotted reproducibly. However, obtaining DBSs directly from the finger of a child is less than ideal. DBS samples that are not saturated, have layers of blood, or form “halos" will prevent an accurate estimation of the volume on the basis of a circular punched hole of the blood spot. This variability can be corrected by estimating volume on the basis of sodium content in the punch (248).
Furthermore, there is an initial decline of ∼20% in retinol during 7–10 d after spots are collected (245, 248). Adjustment of the DBS values by 20% brings the average retinol value into agreement with the average serum retinol concentration; however, this apparent loss will vary somewhat from sample to sample. It is recommended to calibrate the DBS retinol analysis with standard blood spots of known serum retinol content, thereby adjusting for the decline in recovery with the use of the same sample matrix.
Just as environmental factors can influence the stability of serum retinol, there are factors that alter DBS retinol. The collection manuals cited above discuss these factors. Samples should be kept away from sunlight, maintained in a low-humidity environment by including desiccant in sealed plastic bags (e.g., Ziploc; SC Johnson & Son, Inc.) (248), protected from exposure to elevated temperatures (>35°C), and maintained at refrigerated temperatures to prolong retinol stability.
Breast-milk retinol concentration
Breast-milk retinol concentration is a biochemical indicator of maternal vitamin A status, and it also provides indirect information on the risk of inadequate vitamin A intake in predominantly breastfed infants. In many poor settings, breast milk provides the sole reliable source of vitamin A for the first 6 mo of life or longer and can provide a breastfed infant with 435–500 μg dietary vitamin A (34), which is regarded as an adequate amount of vitamin A during infancy (57). Even if breast milk contains half this amount in undernourished settings (34, 252), it can still provide clinically protective amounts to infants and toddlers (253, 254).
Breast-milk retinol concentrations can be used to assess vitamin A deficiency at the population level, to assess the efficacy of vitamin A interventions, and to monitor and evaluate vitamin A intervention programs. Breast-milk retinol concentrations ≤1.05 μmol/L or ≤8 μg/g milk fat are considered inadequate, and vitamin A deficiency is considered a moderate public health problem when the prevalence of inadequate milk retinol concentrations is ≥10% to <25% (242). Breast-milk samples are relatively easy to obtain, and milk vitamin A can be measured in community settings by using a portable fluorometer (255) or in more sophisticated laboratory settings by using HPLC (255, 256).
It is important to carefully define the study population when breast-milk retinol is used as an indicator of vitamin A status in areas in which breastfeeding is common until ≥6 mo of age (242). Methods for selecting participants and estimating sample size will vary depending on the purpose of the study. Briefly, factors that should be considered in selecting a study population include age, stage of lactation, geographic location, season, and pregnancy status. The collection of information on other factors that may affect milk vitamin A is also useful, such as socioeconomic status, anemia, morbidity, exposure to postpartum high-dose or daily vitamin A supplements, and maternal dietary vitamin A intake.
Stage of lactation, time of day, and “fullness” of the breast can affect milk retinol concentration; thus, the method for collecting milk samples should be carefully considered when planning a study (257). Because concentrations of vitamin A are high in colostrum and early milk (≤1 mo postpartum), it is important to collect milk samples at >1 mo postpartum when milk retinol has stabilized. Vitamin A is found in milk fat, and milk fat varies throughout the day and tends to be highest later in day. In addition, because milk fat increases during a feeding episode, the concentration of vitamin A is higher in hind-milk. Thus, the collection of milk samples is often standardized in relation to time of day and time since last feeding episode, as described below. Milk fat is also often measured so that milk vitamin A concentration can be expressed per gram of milk fat; this can be accomplished by determining the creamatocrit, and commercial centrifuges are available specifically for this purpose (258).
The sampling method for collecting breast milk should be chosen carefully and will depend on the purpose of the study and the resources available (257). In general, “casual” milk samples (∼5–10 mL) can be collected by hand-expression, and the vitamin A concentration of milk can be expressed per gram of fat to account for fat variability. This method of milk collection is more feasible for large-scale surveys and surveillance programs. “Full” milk samples can be collected by emptying all of the milk from a breast that has not been used to feed an infant for ≥1 h. The full milk sample is considered to be more representative of what the infant consumes in a feeding episode. A sample of well-mixed milk (∼5–10 mL) is taken from the full milk sample for analysis of vitamin A concentration and the remaining milk is returned to the mother to spoon-feed to her infant. The collection of full milk samples is usually standardized to time of day and to time since the last feeding episode (≥1 h since last feeding) to minimize variability in milk fat, and the vitamin A concentration is expressed per unit volume and/or per gram of fat. Full milk samples are generally recommended for longitudinal studies that aim to determine the efficacy or effectiveness of interventions for increasing vitamin A status. However, the milk sampling method (full compared with casual) may affect the sensitivity of the indicator for detecting a change in vitamin A status in response to an intervention. In lactating Bangladeshi women, the milk retinol concentration of casual milk samples, when expressed per gram of fat, was more sensitive for detecting a response to a vitamin A intervention than the milk retinol concentration of full milk samples, expressed per gram of fat or per unit volume (259).
Uncertainty exists about whether the expression of milk retinol concentration on the basis of volume or fat affects the sensitivity of the indicator to detect an intervention response. In Indonesian women, milk retinol, expressed per gram of fat, was more sensitive for detecting a change in vitamin A status in response to an intervention than was milk vitamin A, expressed per unit volume, in full milk samples (260). In contrast, milk vitamin A, expressed per unit volume, was more responsive than milk vitamin A, expressed per gram of fat, in full milk samples in a later study in Indonesian women (261). Theoretically, a full milk sample, when standardized to time of day and time since last feeding, should be a more sensitive indicator for detecting a change in vitamin A status in response to an intervention. However, until the potential effect of the milk sampling method on the responsiveness of the indicator is better understood, it may be best to use both casual and full milk sampling methods and to measure milk fat when assessing efficacy or effectiveness of an intervention in longitudinal studies.
Milk should be processed carefully to ensure that representative aliquots of milk are taken for the vitamin A and fat analyses. Milk should be processed away from direct light to prevent photodegradation of vitamin A. It is very important to mix the fresh milk well so that the cream layer is evenly distributed throughout the aliquot that is taken for measurement of the vitamin A concentration. Because the vitamin A is found in the milk fat, the distribution of fat in the aliquot of milk that is taken for analysis can affect the measurement. In general, milk samples (∼1 mL) are stored in cryovials at −20°C (or colder) until analyzed for vitamin A content. Because it can be difficult to fully homogenize milk samples that have been previously frozen, the precise aliquots of milk that will be used for measuring the vitamin A content can be prepared and stored to avoid the need to homogenize previously frozen milk samples (256). The vitamin A content of fresh milk samples can also be analyzed, and this is feasible with the use of a portable fluorometer.
Relative-dose-response tests
Liver reserves are frequently defined as the “gold standard” for defining vitamin A status (34, 186). The liver has a large storage capacity for vitamin A, and in humans with adequate status ∼90% of vitamin A is stored in the liver in specialized fat-storing cells (Ito cells) (121). The MRDR test and its precursor, the relative-dose-response (RDR) test, are based on hepatic accumulation of apo-RBP during vitamin A inadequacy. Hepatic RBP synthesis is independent of vitamin A status, but its release from the liver is dependent on vitamin A status (188, 262–264). When vitamin A intake is inadequate and liver reserves are low, apo-RBP accumulates (188). In rats fed a vitamin A–deficient diet, apo-RBP accumulated in the liver well before serum retinol concentrations decreased and the liver was depleted (188). In vitamin A–deficient rats, apo-RBP accumulation reached a steady state concentration (188, 262). In response to newly ingested vitamin A, the accumulated apo-RBP binds retinol (or 3,4-didehydroretinol in the MRDR test) and becomes rapidly mobilized from the liver to the serum as the holo-complex (188). The accumulation of apo-RBP, the rapid release of holo-RBP after ingestion of vitamin A, and the subsequent increase in the plasma during deficiency provide the biological framework for both of the dose-response tests.
RDR test, precursor to the MRDR test.
The RDR test involves administering an oral dose of retinyl ester (22) and collecting a serum sample just before dosing and again 5 h after dosing. The RDR value, expressed as a percentage, is calculated as [(A5 – A0)/A5] × 100, where A5 is the serum retinol concentration at 5 h postdosing and A0 is the concentration just before dosing (22). An RDR value of 20% is often considered a vitamin A–deficient response (210). An oral dose of 1 mg retinyl acetate is recommended in children (265). The RDR test was validated against liver vitamin A reserves in children with liver disease by using an intravenous injection (266). Although the RDR test is a useful indicator, it requires 2 blood samples/subject, and an accurate RDR value is dependent on the correct analysis of both serum samples, usually by using HPLC. Often, negative values are obtained and these are difficult to interpret (267). One study showed that the RDR test is not reliable in children who are recovering from pneumonia who still have an active acute-phase response (CRP >10 mg/L) (268).
MRDR test.
The MRDR test uses 3,4-didehydroretinyl acetate (DRA), or vitamin A2 acetate, for the challenge dose, followed by a high-fat snack to ensure adequate absorption; and a single blood sample is taken between 4 and 7 h postdosing (27, 265, 269, 270). As little as 200 μL serum can be analyzed for 3,4-didehydroretinol (DR) and retinol by using HPLC on the same serum sample (271), and 100–150 μL has been used with young infants. The structure of DR is similar to retinol, and the only difference is a double bond located in the 3–4 position on the β-ionone ring of DR. This structural difference between DR and retinol provides enough difference in polarity to allow them to separate from one another with the use of HPLC. In parallel to retinyl esters, DRA is hydrolyzed in the small intestine to DR, which is taken up by enterocytes and esterified to form various didehydroretinyl esters that are incorporated into chylomicra, enter the lymph, and then enter the general circulation. Once at the liver, the esters are de-esterified to form DR, which can bind to apo-RBP and be released into the serum or can be re-esterified and stored in stellate cells. Standard doses of 5.3 μmol DRA are recommended for children <6 y old, 7.0 μmol DRA for children aged between 6 and 12 y, and 8.8 μmol DRA for adults (265).
The ratio of DR to retinol in serum is called the MRDR value and is used to indicate liver reserves. Values ≥0.060 at the individual level usually indicate insufficient liver reserves (≤0.1 μmol retinol/g), whereas values <0.060 are indicative of sufficient liver reserves (≥0.1 μmol retinol/g) (186). Group mean ratios of <0.030 appear to correlate with adequate status. The lower ratio (0.030) that indicates better liver reserves was established by performing the MRDR test on several groups of well-nourished, healthy American children and adults with presumably adequate liver reserves (269, 270). In a study in 3 adults and 2 children (269), the highest MRDR value obtained was 0.023. In a time-course study involving 12 American children whose blood samples were collected between 4 and 7 h postdosing (270), only 2 children had ratios >0.030. Nine healthy American adults and 1 child all had MRDR values <0.030 and normal conjunctival impression cytology, which measures abnormal pathology of the eye due to poor vitamin A status (209–212, 272). The highest MRDR value of this group was 0.021, and the ratio was reproducible. Cumulatively, these data led to the recommendation that healthy, well-nourished individuals with sufficient liver reserves should invariably have MRDR values <0.030 (265, 270). The choice of an MRDR value ≥0.060, indicating poor liver reserves, was based on a comparison of MRDR values obtained from Indonesian children before and after the administration of a high dose of retinyl ester (200,000 IU retinyl palmitate) (110, 210).
In a study in 13 lactating Indonesian women whose serum retinol concentrations were <0.70 μmol/L, all but one had an MRDR value ≥0.060 (218). Another study that used the cutoff of ≥0.060 examined 19 lactating Indonesian women before and after vitamin A supplementation (8.4 μmol daily in capsule form for 35 d) (273). The women were tested by using the MRDR test 3 times before supplementation; the prevalence of vitamin A deficiency was between 70% and 83%, and the mean MRDR value did not differ at the 3 assessments. After supplementation, the prevalence of vitamin A deficiency decreased to 13% with the use of the MRDR test. No woman in the entire group had a preintervention MRDR value <0.030, the cutoff value established for a well-nourished and healthy population, but 10 had MRDR values <0.030 postintervention, indicating that these women showed a dramatic improvement in liver stores.
The MRDR test has been used to assess the vitamin A status in many population groups, including Senegalese infants (274), Zambian (226, 275) and American (270, 276) children, Indonesian (218) and American (277) pregnant women, and Indonesian (217, 273) and Bangladeshi (278) lactating women, among others. Although useful, it does not give a quantitative estimate of liver reserves but does offer a semiquantitative range in groups who have marginal to adequate vitamin A status (186). The MRDR test, as currently applied, is not useful in defining vitamin A status above adequacy (Figure 6). Nonetheless, it is more sensitive to changes in vitamin A status than serum retinol concentrations alone and will respond better in interventions when liver reserves change from marginal to adequate (110). Furthermore, it can be used in a randomly selected subset of individuals in population surveys to qualify the underlying vitamin A status (242).
Retinol isotope dilution
Isotope dilution is a quantitative method for determining total body stores of vitamin A (279, 280). It can be used to determine total body stores of vitamin A for population monitoring or program evaluation by using a randomly selected subsample who are not experiencing fever. In research settings, isotope dilution testing can be paired to evaluate the efficacy of interventions (279, 281). Although technically sophisticated and requiring specialized mass spectrometric equipment, isotope dilution has the advantage of being the most sensitive test to measure vitamin A status and intervention impact on vitamin A reserves while being minimally invasive because it only requires blood samples (279–281). Furthermore, because of increased sensitivity, lower numbers of subjects can be assessed in intervention studies (279).
In brief, the technique involves administering a dose of deuterium or 13C-labeled retinyl acetate. For some 13C applications (or for repeated assessment with the use of deuterium), a baseline blood sample is taken to determine background enrichment. After the dose is allowed to mix fully with the body stores of vitamin A, another blood sample is taken. Various types of MS in conjunction with separation by GC or LC are used to measure isotopic enrichment in the serum (279–282). The serum (or plasma) is then analyzed to determine the ratio of the labeled to native retinol (tracer-to-tracee ratio) (283). The higher the total body stores, the more the isotope is diluted, resulting in a lower tracer-to-tracee ratio. The total body stores of vitamin A can then be calculated from these data (283). Isotope dilution has been validated against liver reserves of humans in the United States (28) and Bangladesh (284), in rats (285), and in rhesus monkeys (286). The test is able to work over a wide range of physiologic human conditions, from deficient (<0.1 μmol/g liver) through hypervitaminotic concentrations (>1 μmol/g liver) (85), and in some cases for toxic concentrations (>10 μmol/g liver) (286) (Figure 6).
The duration of assessment for total body stores is dependent mainly on the equilibration period. Current applied periods of equilibration are 14–21 d for adults and 14 d for children. This allows the vitamin A to fully mix and therefore provides a quantitative reading of the vitamin A in the whole body (279–281). Accurate qualitative assessment can also be made with 3-d equilibration (287), and researchers have attempted to further refine the shorter 3-d equilibration period to make this method more field-friendly (288).
To measure efficacy, the paired isotope dilution technique is used to compare the change that occurred in total amount of vitamin A in the body during the intervention (281). Each subject serves as his or her own control, which maximizes the power of studies. Isotope dilution can be used to detect even small differences in treatment groups, making it a powerful technique to evaluate interventions (281, 289). A main consideration that needs to be taken into account during the test is the utilization rate of vitamin A in the body. The individual’s utilization rate changes with age, and this is reflected in the calculations depending on the subject population (280, 281). Furthermore, in some studies, subjects are placed on a low vitamin A diet before the dose and during the equilibration to minimize interference from incoming dietary vitamin A on the isotope ratio (85, 290).
Some possible confounders of the test include food ingested before the blood sample, co-micronutrient deficiencies, and inflammation. If large amounts of vitamin A are ingested within a few hours before the blood draw, this vitamin A will dilute the isotopically labeled vitamin A and affect the results. To prevent interference, blood draws are preferably done while the subject is fasting, although sometimes this is not practical in infants and children. Inflammation causes more rapid removal of vitamin A from the body and altered vitamin A metabolism (291), which may confound the results obtained from the test. Inflammation can be monitored by taking temperatures and/or measuring CRP and AGP concentrations in subjects (85, 281).
The strength of isotope dilution over other vitamin A assessment techniques is that it is both quantitative and sensitive. Although other vitamin A status indicators may only be able to qualitatively diagnose a subject as deficient or not deficient, isotope dilution can quantitatively reveal what the total body stores of vitamin A are from deficiency through hypervitaminosis (186, 279) (Figure 6). This makes isotope dilution an ideal technique for measuring intervention impact or obtaining accurate measures of liver reserves. It must be noted, however, that the interpretation is best used at the group level and not at the individual level (280, 281). This becomes especially critical if a researcher is investigating excessive concentrations of vitamin A, because these are outside the range of other vitamin A tests (186). Because vitamin A fortificants are preformed vitamin A, monitoring excess vitamin A status is important in the near future (222).
For isotope studies, a sample size of 30 subjects/treatment group may yield enough power for supplement studies (279). However, the sample size should be increased for studies that involve provitamin A carotenoids and should be based on a power analysis (85, 281).
Analysis of the samples can be intensive and can be a major drawback. For example, some isotope dilution techniques require the retinol to be extracted from serum and purified by HPLC to remove impurities before injection on the GC (85, 286). Furthermore, most deuterium-labeled vitamin A techniques require derivatization before analysis (284, 292). All methods derive the tracer-to-tracee ratio, and the calculations used to estimate total body stores or total liver reserves are similar (283).
This test can be carried out as easily as any other test that requires venous blood samples that can be drawn in the field. The blood should be kept cold (not frozen) until centrifuged to prepare serum, which should be frozen and maintained at −70° to −80°C until analysis (293). For transport, liquid nitrogen tanks or dry ice may be used. The isotopically labeled dose should be kept frozen until administration. A positive displacement pipette will be required to administer an accurate dose to each subject or capsules can be prepared ahead of time. The dose should preferably remain at a constant temperature throughout dosing to ensure that the volume stays constant for all subjects.
If there is a large prevalence of disease or other infections, care must be taken when interpreting the data. The test relies on the assumption that absorption and metabolism of vitamin A, which are both affected by infection, are constant throughout the equilibration period; and major differences between subjects could cause error in calculations. Monitoring infections and inflammation is critical for ensuring accurate data (281, 291). The concurrent use of CRP and AGP can determine the degree of inflammation in the participant (223), and adjustments can be made to account for the presence of inflammation (85, 283).
Retinyl esters as a measure of vitamin A excess
Although many biomarkers are available to assess whether or not vitamin A deficiency is present in an individual or a population, only a few biomarkers are presently available to assess vitamin A toxicity. In addition to isotope dilution techniques, the use of fasting serum or plasma retinyl esters is a qualitative measure of hypervitaminosis A. Retinyl esters as a biomarker of vitamin A excess was first described in 3 patients with clinically apparent, chronic hypervitaminosis A in whom up to 67% of circulating vitamin A was in the form of retinyl esters (294). Although, retinyl esters are the principal form of dietary preformed vitamin A, they only appear at high concentrations in the chylomicra in plasma shortly after a high vitamin A- and fat-containing meal unless liver reserves are excessive.
The liver normally clears the retinyl esters within chylomicron remnants from the general circulation, a process that may take several hours after ingesting a meal or a vitamin A-containing supplement. However, the liver storage capacity for vitamin A is not infinite and can become overwhelmed by ingesting, either acutely or chronically, too much vitamin A (51). In this situation, the liver can no longer clear the retinyl esters from the circulation, and retinyl esters begin to spill out from (or are not sequestered by) the liver into the circulation. Some of the circulating retinyl esters become converted to free retinol (unbound to RBP). Free retinol in the circulation is highly toxic to cell membranes, and vitamin A toxicity can thus be manifested by a myriad of signs and symptoms: liver damage, hepatomegaly, bone demineralization, skin rashes and desquamation, anemia, alopecia, and increased cerebral spinal fluid pressure, among others (121). The lowest amount of chronic vitamin A ingestion reported to cause hepatotoxicity is 14,000 μg/d. Thus, by using an uncertainty factor of ∼5, the IOM has set the UL for vitamin A at 3000 μg/d (10,000 IU). The same UL is applied to women of child-bearing age (i.e., 2800–3000 μg/d) (34).
Plasma or serum retinyl esters can be measured by either normal- or reverse-phase HPLC. The same procedures and precautions must be used in collecting and handling the blood taken for retinyl ester determination as for serum retinol determination (295, 296). Both retinol and retinyl ester concentrations can be obtained in the same HPLC analysis. However, blood drawn for retinyl ester determination must be taken when the subject is fasting in order to correctly interpret the result. Patients with hypertriglyceridemia are at increased risk of high circulating concentrations of retinyl esters associated with the chylomicron VLDL, which may predispose them to vitamin A toxicity (297). Furthermore, individuals with RBP gene mutations may also have higher circulating retinyl esters to compensate for low serum retinol concentrations (298).
In one study, the normal percentage of retinyl ester in fasting blood plasma was reported to be 11% of the total circulating vitamin A in people aged 19–59 y and 13% in people >60 y old. These percentages corresponded to a normal reference range of ≤130 nmol/L for young adults and ≤170 nmol/L for elderly subjects (299). In NHANES III (1988–1994), a normal fasting retinyl ester concentration in serum was <244 nmol/L (<7 μg/dL), which is a higher cutoff for defining vitamin A excess than suggested by the Krasinski et al. study (299). However, the normal percentage of total serum vitamin A as retinyl ester in HANES III was 10%, which is almost the same as in the Krasinski et al. study (11%) (299). A lower percentage of 5% of total circulating retinol as retinyl ester was suggested as a potential cutoff for children on the basis of a high percentage of hypervitaminosis A in the community determined with isotope dilution and should be further investigated (144).
The use of retinyl esters as a biomarker for vitamin A toxicity has several confounding issues. As stated above, the blood must be drawn in the fasting state for proper interpretation. Recent dietary and/or supplemental intakes can cause an increase in circulating retinyl esters, which can last for 3–5 h after a meal (depending on the nature of the meal that was ingested). Protein malnutrition (300), liver disease (301), and hypertriglyceridemia (297) can all result in high circulating retinyl ester values even when vitamin A status is normal. Finally, old age alone can result in impaired clearance of retinyl esters from chylomicron circulation after a meal, and thus result in higher blood retinyl ester concentrations for longer periods of time after eating than in young adults (302). Nonetheless, retinyl esters were not found to be elevated in a group of postmenopausal women with intakes of total vitamin A (preformed plus provitamin A carotenoids) that were twice the US RDA (128).
A fasting retinyl ester measurement is currently the most widely used indicator of vitamin A intoxication. However, in geographic areas with a high prevalence of hepatitis, it is not known how reliable an indicator of intoxication retinyl ester concentrations are because high circulating concentrations may reflect vitamin spillage out of an already diseased and inflamed liver or impaired and delayed uptake of newly absorbed vitamin A by a diseased liver.
After a meal, even in geographic areas without a high prevalence of liver disease, it is not known how early an indicator of vitamin A intoxication serum retinyl ester measurement is: that is, by the time high concentrations of circulating retinyl esters appear, has liver disease from vitamin A overload already occurred from the high vitamin A intakes? Thus, there is great interest in developing additional and possibly more reliable indicators of vitamin A overload.
Liver biopsy or autopsy samples
Liver biopsies to assess retinol concentrations of humans are only justifiable in special cases (28, 266, 284, 303). Autopsy samples, on the other hand, should be considered for population monitoring. For example, the addition of vitamin A to milk in the United States became mandatory in 1978 because of skim and lower-fat milks, which remove the naturally occurring retinyl esters during processing (304). Thus, there have been ∼4 decades of fortification. Furthermore, dietary supplements and other fortified foods have become popular in the United States (305). Perhaps autopsy samples could shed light on current vitamin A status in the United States. This would also be true for other countries that have introduced successful vitamin A fortification programs, such as sugar fortification in Guatemala and in Zambia.
Assay-Specific Queries
The previous section covered the biological aspects of the important biomarkers of vitamin A status. This section briefly reviews the analytical methods, quality-control measures, and sample collection, processing, and storage. Comparisons between the biochemical methods are outlined in Table 8.
Sample collection for serum, plasma, or blood spot assays
Most vitamin A assessment techniques (retinol, RBP, RDR, MRDR, and isotope dilution studies) require the collection of blood to be processed into serum or plasma, saved as DBSs, or, with anticipated techniques such as iCheck (BioAnalyt), used as whole blood at the time of collection. Collecting blood can be a challenge in many populations and may require community sensitization or appropriate (but never coercive) incentives. An excellent manual of blood collection procedures and precautions is available from the WHO (306). Blood volume for vitamin A assessment depends on the test used. Serum retinol concentration can be measured in venous or capillary blood, which can be separated into serum or plasma, depending on whether or not it is mixed with an anticoagulant. Blood can be spotted onto paper cards as DBSs.
Venous blood collection is preferable when relatively large volumes of serum or plasma are desired, especially for higher volume assays or multiple assays. Venous collection produces a higher-quality sample than does capillary collection in that the potential mixing of interstitial fluid is not a concern. Blood can be collected in a clinic setting, central site, or in a subject’s home. Regardless of setting, care should be taken to ensure the safety and health of the subject and phlebotomist through the provision of a hygienic environment and procedures. Care should also be taken to ensure the integrity of the blood sample, an issue in particular in field settings—blood collection and samples should be protected from excessive heat, light, and oxygen (air). Blood drawing requires specially trained phlebotomists. Processing of blood samples requires laboratory staff that can run a centrifuge, pipet, and maintain accurate records of sample identifiers.
Supplies include items to keep the blood collection area clean (gloves for phlebotomist, alcohol swabs, sterile cotton gauze, bandages) and safe (biohazardous waste disposal and sharps containers). Venous blood involves the insertion of a needle into a vein, typically in the forearm, with the upper arm secured with a tourniquet. Needles should have an appropriate bore size and length for the subject and may connect directly to a blood collection container or be connected by tubing (i.e., “butterfly” needles). Blood can be drawn manually into a syringe (and transferred to a blood collection tube) or may automatically be drawn into evacuated tubes. Blood collection tubes may contain additives, such as clot activator for serum or anticoagulant for plasma. Cold packs and coolers may be required to maintain the sample until it is processed by centrifugation to separate the serum/plasma from RBCs. Transfer pipettes with proper tips are required to remove the serum/plasma to cryovials for frozen storage in an electric freezer, under dry ice, or in liquid nitrogen. Proper labels should be used to identify samples.
Capillary blood collection is often preferred when venous blood collections may be perceived as unacceptable, when subjects are infants or small children, when only small amounts of blood are required, or when highly skilled phlebotomists are unavailable. Capillary blood can be collected from either the heel (infants) or fingertip by using a lancet and small blood collection tubes (e.g., Microtainer brand by Becton Dickinson or Safe-T-Fill by Ram Scientific). Then it is carefully separated into serum or plasma by using a microcentrifuge or it can be spotted onto cards designed to store DBSs for future use. The latter means of sample collection is often considered more convenient, because it eliminates the need for laboratory processing of whole blood to serum/plasma because volumes are usually <200 μL. However, collecting blood as DBSs also requires careful handling and may require cold packs and coolers to maintain the samples until processed. Care should be taken to ensure the integrity of the blood samples, an issue in particular in field settings, by protecting them from excessive heat and light. When capillary blood collection is used to collect blood as DBSs, care must be taken to ensure that blood spots are thoroughly dried and protected from humidity. Proper blood collection cards are required (e.g., Protein Saver 903, Whatman; GE Healthcare). Desiccant and pouches for packaging the blood spot cards are required and available from the manufacturer. Proper labels should be used to identify all samples.
Sample processing and storage
Care must be taken in the proper processing of blood samples to ensure the integrity of the analytes of interest during collection, initial processing, storage, and use. In particular, retinol and carotenoids are sensitive to degradation in the presence of exposure to UV light and oxygen. There are some processing and storage requirements that are specific to the collection of serum, plasma, and DBSs. Centrifugation for serum or plasma preparation should ideally take place within hours of blood collection, although limited data suggest that retinol remains stable in uncentrifuged samples even up to 24 h postcollection at room or refrigerator temperatures (307). Exposure to excessive shaking, vibrations, or cold temperatures before centrifugation may result in hemolysis of the blood. Stability data show that retinol is stable at −20°C or below for up to 15 y (307, 308), whereas provitamin A carotenoids may be subject to degradation unless they are kept at temperatures below −40°C (307, 308).
Serum.
Serum is the supernatant that results when blood is collected without an anticoagulant and allowed to clot naturally. Serum is often the preferred matrix for long-term storage of biospecimens. After venous or capillary blood collection into anticoagulant-free blood collection containers, blood should be allowed to clot at room temperature or refrigerated, but not frozen, unexposed to light. After allowing the blood to clot, the blood collection tube should be centrifuged to concentrate the clot. After centrifugation, the supernatant should immediately be pipetted or poured (if a serum separator tube is used) into a cryovial and frozen at −20°C or below.
Plasma.
Plasma is the supernatant that results when blood is collected with an anticoagulant, such as sodium heparin or EDTA placed within the blood collection tube. Blood collected in such tubes must be mixed thoroughly immediately after collection by gently inverting the tube several times. An advantage of collecting blood with anticoagulants is that the whole blood is preserved to allow for other laboratory tests that require it or for the collection of white blood cells during processing. A disadvantage is that fibrin clots may form during long-term storage. The blood sample should be kept at room or refrigerated temperatures (not freezing), unexposed to light, until it is centrifuged to separate the plasma (supernatant) from cellular material. After centrifugation, the plasma supernatant should immediately be pipetted into a cryovial and frozen at −20°C or below.
DBSs.
Capillary blood should be thoroughly spotted into the circles of a DBS protein saver card after wiping away the first drop of blood with sterile gauze. The card should never touch the skin of the participant; rather, there should be sufficient flow of blood to soak each circle. The cards must be completely dried after they are spotted before storage; this may be accomplished by using a customized slotted storage box that allows airflow around the front and back of the card. Once dried, cards can be stored in air-tight pouches with desiccant. Although retinol may be stable when complexed with its binding proteins, the retinol measured on DBS cards tends to decline by ∼20% in the first week after spotting (248). Freezing DBSs as a precautionary measure to eliminate potential influences of temperature and humidity changes should be considered.
Analytical approaches for retinol
HPLC.
Reverse- and normal-phase HPLC methods for serum or plasma retinol determination have been available for decades (295, 309, 310). By using the reverse-phase approach with multi-wavelength detection, it is also possible to analyze carotenoids and tocopherols in the same run. Sample preparation typically requires protein precipitation, the extraction of the fat-soluble components by using a liquid:liquid extraction with hexanes preferably 2–3 times, drying, and reconstituting in appropriate solvent. Recovery of the analytes of interest through the extraction process is estimated by using a known amount of internal standard (e.g., retinyl acetate as a proxy for retinol). The rate of throughput is highly variable and is limited by multiple analytes. Processed samples are typically injected by using an autosampler. Retinol is visible at 325 nm and carotenoids at 450 nm with the use of a photodiode array detector. Costs of the assay include those required for HPLC maintenance, columns, HPLC-grade reagents, consumable supplies, and salaries for relatively skilled laboratory technicians who can run and maintain the equipment, process the samples, and monitor the quality of the assays over time.
Methods under development.
Efforts are being made to reduce the time required to assess serum retinol by making field-ready devices. Limited data are currently available with respect to their comparison to serum retinol with the use of traditional HPLC. The CRAFTi is a portable device produced by Craft Technologies that can assess retinol with 25 μL serum on the basis of fluorescent properties and compared well with serum retinol by HPLC (255). The iCheck is also a portable device based on fluorescent properties of retinol. It uses 500 μL whole blood, which eliminates sample processing, although it was recently compared with HPLC with the use of 500 μL serum (311).
Quality assurance and control.
Serum standard reference materials to ensure interlaboratory agreement with respect to retinol and other related compounds are available through the National Institute of Standards and Technology (312), and a quality assurance testing program is available through the CDC (313).
New Directions and Technologies
Biomarkers that are relatively noninvasive, respond to interventions, and work along the entire continuum of vitamin A status are needed. Aside from liver biopsy, isotope dilution methods are the only indirect measure of vitamin A status that work along the continuum. However, even isotope dilution may be limited in groups of individuals who have high infection rates or co-micronutrient deficiencies, and research gaps have been identified (314) (Table 9). Identifying what factors affect each of the biomarkers is important to understanding their limitations in clinical and population applications.
TABLE 9.
Summary of recommendations to improve retinol isotope dilution techniques1
| Further development of isotope dilution techniques: |
| • Make the method more field-friendly |
| • Increase accessibility and further verify usefulness of different tracers and doses to optimize costs |
| • Further establish guidelines for interpreting retinol isotope dilution techniques |
| • Further validate the method for different population groups, especially children and pregnant and lactating women |
| • Design studies with sufficient statistical power for specific conditions |
| • Determine how vitamin A status and other population characteristics influence vitamin A absorption, distribution, and metabolism |
| • Consider designs with paired comparisons and both positive and negative controls |
| Further application of retinol isotope dilution techniques to benefit public health: |
| • Monitor the safety and effectiveness of high-dose supplementation to young children in developing countries |
| • Assess alternative vitamin A interventions |
| • Determine the effectiveness of routinely supplementing with or feeding β-carotene, rather than retinol |
| • Investigate the usefulness of retinol isotope dilution techniques to assess vitamin A status in populations affected by inflammation |
| • Improve understanding of the influence of iron or zinc deficiencies on vitamin A metabolism and status evaluation |
Adapted from reference 314 with permission.
Research Gaps and Needs
Although serum retinol concentrations have limitations, there are still groups of individuals for whom serum retinol concentrations have not been well-defined. One of these groups is young infants, where the reference range has not been defined for an adequate population. RBP continues to increase as children age; therefore, it may be that the cutoff for serum retinol concentrations in young infants should be lower than in older children. This should be determined against more sensitive indicators of vitamin A status, such as isotope dilution testing. Furthermore, after this reference range is determined, setting the prevalence to define a public health problem is also necessary. Although it is well known that inflammation and infection affect serum retinol concentrations, questions still remain on how to use this information when acute-phase proteins are measured (35, 220, 225). Are there functional indicators that can be applied to young infants to help to define this reference range? An additional research need related to retinol and RBP involves better defining the role of the apparent receptor protein for plasma RBP, STRA6, with regard to its role in vitamin A uptake by tissues at different levels of status, in different cell types, and during the acute-phase response when serum retinol and RBP decrease (315). For example, is transport to target tissues somehow modulated via differential STRA6 expression?
Another research need is to help countries decide which combination of biomarkers to use to inform programs. The selection of these biomarkers needs to have reference values for different age subgroups of the population and the prevalence of deficiency that triggers the need for an intervention.
The association of serum retinol to breast-milk retinol concentrations needs to be better understood. Although it appears that breast-milk concentrations may be less affected by infection and inflammation, retinol delivery to the mammary gland on RBP contributes to breast-milk concentration. Because serum retinol is negatively affected by the acute-phase response, breast milk is likely affected during severe serum retinol depression.
Vitamin A deficiency is defined by the risk of xerophthalmia and the increased risk of death from infectious disease. The latter risk is due, we presume, to impaired immune function in deficient individuals. Some data suggest that increasing intake may affect immune function through the range of vitamin A stores found in healthy adults (316). It is thus plausible that immune markers may vary across a continuum of vitamin A statuses from the adequate range and into the range of chronic toxicity. The evaluation of such effects may provide additional methods to determine the impact of excess vitamin A intake.
Acknowledgments
We thank Ramkripa Raghavan for her assistance in coordinating the early drafts of sections on this manuscript and Devika Suri for adapting Figure 9 for this manuscript. We also thank Jenica K Abram for her perseverance and support in preparing and coordinating the successful preparation of this manuscript for submission. This review is the result of deliberations and contributions of the Vitamin A Expert Panel [SAT, RMR (Chair), CBS, and GL] and scientific consultants (BMG, NEC, MJH, and KS). SAT and RMR wrote major sections of the manuscript; CBS and BMG wrote a manuscript section and acted as readers of the manuscript during development; NEC, MJH, GL, and KS contributed a section of the manuscript; and SAT and DJR edited all sections of the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGP, α1-acid glycoprotein; BCO1, β-carotene 15,15′ dioxygenase; BCO2, β-carotene 9,10-oxygenase; BOND, Biomarkers of Nutrition for Development; CRP, C-reactive protein; DBS, dried blood spot; DR, 3,4-didehydroretinol; DRA, 3,4-didehydroretinyl acetate; IOM, Institute of Medicine; ISX, intestine-specific homeobox transcription factor; log cd, log candela; LRAT, lecithin:retinol acyltransferase; MRDR, modified relative dose response; Raldh1, retinaldehyde dehydrogenase 1; RAR, retinoic acid receptor; RBP, retinol-binding protein; RDR, relative dose response; STRA6, stimulated by retinoic acid 6 receptor; Th, T-helper; TSHB, pituitary thyroid-stimulating hormone β gene; UL, Tolerable Upper Intake Level.
References
- 1.WHO. Global prevalence of vitamin A deficiency in populations at risk: 1995–2005. WHO Global Database on Vitamin A Deficiency. Geneva (Switzerland): WHO; 2009. [Google Scholar]
- 2.Wolf G. A history of vitamin A and retinoids. FASEB J 1996;10:1102–7. [DOI] [PubMed] [Google Scholar]
- 3.Guggenheim KY. Nutrition and nutritional diseases. Lexington (MA): Collamore Press; 1981. [Google Scholar]
- 4.McCollum EV, Davis M. The necessity of certain lipins in the diet during growth. J Biol Chem 1913;15:167–75. [Google Scholar]
- 5.Osborne TB, Mendel LB. The influence of butter-fat on growth. J Biol Chem 1913;16:423–37. [Google Scholar]
- 6.Bloch CE. Clinical investigation of xerophthalmia and dystrophy in infants and young children (xerophthalmia et dystropia alipogenetica). J Hyg (Lond) 1921;19:283–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steenbock H. White corn vs. yellow corn and a probable relation between the fat-soluble vitamine and yellow plant pigments. Science 1919;50:352–3. [DOI] [PubMed] [Google Scholar]
- 8.Steenbock H, Gross EG. Fat-soluble vitamine. J Biol Chem 1920;41:149–62. [Google Scholar]
- 9.Green HN, Mellanby E. Vitamin A as an anti-infective agent. BMJ 1928;2:691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore T. Vitamin A and carotene. Biochem J 1930;24:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karrer P, Morf R, Schoepp K. Zur kenntnis des vitamins-A aus fischtranen. [For knowledge of vitamin A from fish oil.] Helv Chim Acta 1931;14:1431–6 (in German). [Google Scholar]
- 12.Green HN, Pindar D, Davis G, Mellanby E. Diet as a prophylactic agent against puerperal sepsis. BMJ 1931;2:595–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison JB. Intensive vitamin A therapy in measles. BMJ 1932;2:708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wald G. Carotenoids and the visual cycle. J Gen Physiol 1935;19:351–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes HN, Corbet RE. The isolation of crystalline vitamin A. J Am Chem Soc 1937;59:2042–7. [Google Scholar]
- 16.Isler O, Huber W, Ronco A, Kofler M. Synthese des vitamin A. [Synthesis of vitamin A.] Helv Chim Acta 1947;30:1911–27 (in German). [DOI] [PubMed] [Google Scholar]
- 17.Gopalan C, Venkatachalam PS, Bhavani B. Studies of vitamin A deficiency in children. Am J Clin Nutr 1960;8:833–40. [DOI] [PubMed] [Google Scholar]
- 18.Olson JA, Hayaishi O. The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc Natl Acad Sci USA 1965;54:1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaren DS, Oomen HAPC, Escapini H. Ocular manifestations of vitamin A deficiency in man. Bull World Health Organ 1966;34:357–61. [PMC free article] [PubMed] [Google Scholar]
- 20.Wald G. Molecular basis of visual excitation. Science 1968;162:230–9. [DOI] [PubMed] [Google Scholar]
- 21.Sommer A. Vitamin A deficiency and clinical disease: an historical overview. J Nutr 2008;138:1835–9. [DOI] [PubMed] [Google Scholar]
- 22.Loerch JD, Underwood BA, Lewis KC. Response of plasma levels of vitamin A to a dose of vitamin A as an indicator of hepatic vitamin A reserves in rats. J Nutr 1979;109:778–86. [DOI] [PubMed] [Google Scholar]
- 23.Sommer A, Tarwotjo I, Djunaedi E, West KP Jr, Loedin AA, Tilden R, Mele L. Impact of vitamin A supplementation on childhood mortality: a randomized controlled community trial. Lancet 1986;1:1169–73. [DOI] [PubMed] [Google Scholar]
- 24.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature 1987;330:444–50. [DOI] [PubMed] [Google Scholar]
- 25.Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature 1987;330:624–9. [DOI] [PubMed] [Google Scholar]
- 26.Benbrook D, Lernhardt E, Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature 1988;333:669–72. [DOI] [PubMed] [Google Scholar]
- 27.Tanumihardjo SA, Olson JA. A modified relative dose-response assay employing 3,4-didehydroretinol (vitamin A2) in rats. J Nutr 1988;118:598–603. [DOI] [PubMed] [Google Scholar]
- 28.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen H III, Jones A, Anderson D, Olson JA. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr 1989;49:713–6. [DOI] [PubMed] [Google Scholar]
- 29.Beaton GH, Martorell R, Aronson KJ, Edmonston B, McCabe G, Ross AC, Harvey B. Effectiveness of vitamin A supplementation in control of young child morbidity and mortality in developing countries - nutrition policy discussion paper No. 13. Geneva (Switzerland): UN; 1993.
- 30.de Pee S, West CE, Muhilal, Karyadi D, Hautvast JGAJ. Lack of improvement in vitamin A status with increased consumption of dark green leafy vegetables. Lancet 1995;346:75–81. [DOI] [PubMed] [Google Scholar]
- 31.Christian P, West KP Jr, Khatry SK, Katz J, LeClerq S, Pradhan EK, Shrestha SR. Vitamin A or β-carotene supplementation reduces but does not eliminate maternal night blindness in Nepal. J Nutr 1998;128:1458–63. [DOI] [PubMed] [Google Scholar]
- 32.Christian P, West KP Jr, Khatry SK, Katz J, Shrestha SR, Pradhan EK, LeClerq SC, Pokhrel RP. Night blindness of pregnancy in rural Nepal—nutritional and health risks. Int J Epidemiol 1998;27:231–7. [DOI] [PubMed] [Google Scholar]
- 33.West KP Jr, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, Connor PB, Dali SM, Christian P, Pokhrel RP, et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or β-carotene on mortality related to pregnancy in Nepal. BMJ 1999;318:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 35.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003;362:2052–8. [DOI] [PubMed] [Google Scholar]
- 36.Rahmathullah L, Tielsch JM, Thulasiraj RD, Katz J, Coles C, Devi S, John R, Prakash K, Sadanand AV, Edwin N, et al. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in southern India. BMJ 2003;327:254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, Hidayat S, Tielsch J, West KP Jr, Sommer A. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr 1996;128:489–96. [DOI] [PubMed] [Google Scholar]
- 38.WHO. Guideline: vitamin A supplementation for infants and children 6–59 months of age. 2011 [cited 2014 Mar 14]. Available from: http://www.who.int/nutrition/publications/micronutrients/guidelines/vas_6to59_months/en/.
- 39.WHO. Guideline: vitamin A supplementation for infants 1–5 months of age. 2011 [cited 2014 Mar 14]. Available from: http://www.who.int/nutrition/publications/micronutrients/guidelines/vas_infants_1–5/en/.
- 40.WHO. Guideline: vitamin A supplementation in pregnant women. Geneva (Switzerland): WHO; 2011. [Google Scholar]
- 41.WHO. Guideline: vitamin A supplementation in postpartum women. Geneva (Switzerland): WHO; 2011. [PubMed] [Google Scholar]
- 42.WHO. Guideline: vitamin A supplementation in pregnancy for reducing the risk of mother-to-child transmission of HIV. Geneva (Switzerland): WHO; 2011. [PubMed] [Google Scholar]
- 43.Awasthi S, Peto R, Read S, Clark S, Pande V, Bundy D; DEVTA (Deworming and Enhanced Vitamin A) Team. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet 2013;381:1469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edmond KM, Newton S, Shannon C, O'Leary M, Hurt L, Thomas G, Amenga-Etego S, Tawiah-Agyemang C, Gram L, Hurt CN, et al. Effect of early neonatal vitamin A supplementation on mortality during infancy in Ghana (Neovita): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:1315–23. [DOI] [PubMed] [Google Scholar]
- 45.Masanja H, Smith ER, Muhihi A, Briegleb C, Mshamu S, Ruben J, Noor RA, Khudyakov P, Yoshida S, Martines J, et al. ; Neovita Tanzania Study Group. Effect of neonatal vitamin A supplementation on mortality in infants in Tanzania (Neovita): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazumder S, Taneja S, Bhatia K, Yoshida S, Kaur J, Dube B, Toteja GS, Bahl R, Fontaine O, Martines J, et al. ; Neovita India Study Group. Efficacy of early neonatal supplementation with vitamin A to reduce mortality in infancy in Haryana, India (Neovita): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:1333–42. [DOI] [PubMed] [Google Scholar]
- 47.Semba RD. The vitamin A story: lifting the shadow of death. Basel (Switzerland): Karger Medical and Scientific Publishers; 2012. [Google Scholar]
- 48.Semba RD. On the ‘discovery’ of vitamin A. Ann Nutr Metab 2012;61:192–8. [DOI] [PubMed] [Google Scholar]
- 49.Walker A, Zimmerman MR, Leakey RE. A possible case of hypervitaminosis A in Homo erectus. Nature 1982;296:248–50. [DOI] [PubMed] [Google Scholar]
- 50.Zimmerman MR. The paleopathology of the liver. Ann Clin Lab Sci 1990;20:301–6. [PubMed] [Google Scholar]
- 51.Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr 2006;83:191–201. [DOI] [PubMed] [Google Scholar]
- 52.McLaren DS. The luxus vitamins—A and B12. Am J Clin Nutr 1981;34:1611–6. [DOI] [PubMed] [Google Scholar]
- 53.Tanumihardjo SA. Vitamin A and bone health: the balancing act. J Clin Densitom 2013;16:414–9. [DOI] [PubMed] [Google Scholar]
- 54.Yasmeen R, Jeyakumar SM, Reichert B, Yang F, Ziouzenkova O. The contribution of vitamin A to autocrine regulation of fat depots. Biochim Biophys Acta 2012;1821:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haskell MJ, Brown KH. Maternal vitamin A nutriture and the vitamin A content of human milk. J Mammary Gland Biol Neoplasia 1999;4:243–57. [DOI] [PubMed] [Google Scholar]
- 56.Institute of Medicine. Dietary Reference Intakes: the essential guide to nutrient requirements. In: Otten J, Hellwig J, Meyers L, editors. Vitamin A. Washington (DC): National Academies Press; 2006. p. 170–81. [Google Scholar]
- 57.Joint FAO/WHO Expert Consultation. Vitamin A. In: Vitamin and mineral requirements in human nutrition. 2nd ed. Geneva (Switzerland):WHO/FAO; 2004. p. 17–44.
- 58.Arscott SA. Carotenoids and human health. In: Tanumihardjo S, editor. Food sources of carotenoids. New York: Springer Science and Business Media; 2013. p. 3–19.
- 59.Dary O, Mora JO. Food fortification to reduce vitamin A deficiency: International Vitamin A Consultative Group recommendations. J Nutr 2002;132(9, Suppl):2927S–33S. [DOI] [PubMed] [Google Scholar]
- 60.Solon FS, Klemm RD, Sanchez L, Darnton-Hill I, Craft NE, Christian P, West KP Jr. Efficacy of a vitamin A-fortified wheat-flour bun on the vitamin A status of Filipino schoolchildren. Am J Clin Nutr 2000;72:738–44. [DOI] [PubMed] [Google Scholar]
- 61.Solon FS, Solon MS, Mehansho H, West KP, Sarol J, Perfecto C, Nano T, Sanchez L, Isleta M, Wasantwisut E, et al. Evaluation of the effect of vitamin A-fortified margarine on the vitamin A status of preschool Filipino children. Eur J Clin Nutr 1996;50:720–3. [PubMed] [Google Scholar]
- 62.Melse-Boonstra A, Pee S, Martini E, Halati S, Sari M, Kosen S, Muhilal, Bloem M. The potential of various foods to serve as a carrier for micronutrient fortification, data from remote areas in Indonesia. Eur J Clin Nutr 2000;54:822–7. [DOI] [PubMed] [Google Scholar]
- 63.WHO/FAO. Guidelines on food fortification with micronutrients. In: Allen L, de Benoist B, Dary O, Hurrell R, editors. Geneva (Switzerland) and Rome (Italy): WHO/FAO; 2006. [Google Scholar]
- 64.Ross C. Encyclopedia of dietary supplements. In: Coates P, Betz J, Blackman M, Al E, editors. 2nd ed London and New York: Informa Healthcare; 2010. p. 778–91. [Google Scholar]
- 65.USDA. What we eat in America. NHANES 2011–2012, individuals 2 years and over (excluding breast-fed children), day 1. Nutrient Intakes from Food and Beverages [cited 2016 Apr 18]. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/1112/Table_1_NIN_GEN_11.pdf.
- 66.Hotz C, Loechl C, Lubowa A, Tumwine JK, Ndeezi G, Nandutu Masawi A, Baingana R, Carriquiry A, de Brauw A, Meenakshi JV, et al. Introduction of β-carotene-rich orange sweet potato in rural Uganda resulted in increased vitamin A intakes among children and women and improved vitamin A status among children. J Nutr 2012;142:1871–80. [DOI] [PubMed] [Google Scholar]
- 67.Ministries of Health and Science and Technology and the National Bureau of Statistics of the Peoples Republic of China. The nutrition and health status of the Chinese people. Beijing (China): State Information Office; 2004. [cited 2016 Apr 18]. Available from: http://www.goldenrice.org/PDFs/China_nutr_rep_2004_en.pdf.
- 68.Davis C, Jing H, Howe JA, Rocheford T, Tanumihardjo SA. Beta-cryptoxanthin from supplements or carotenoid-enhanced maize maintains liver vitamin A in Mongolian gerbils (Meriones unguiculatus) better than or equal to beta-carotene supplements. Br J Nutr 2008;100:786–93. [DOI] [PubMed] [Google Scholar]
- 69.Burri BJ, Chang JST, Neidlinger TR. β-Cryptoxanthin and α-carotene-rich foods have greater apparent bioavailability than β-carotene-rich foods in Western diets. Br J Nutr 2011;105:212–9. [DOI] [PubMed] [Google Scholar]
- 70.Tanumihardjo SA, Howe JA. Twice the amount of alpha-carotene isolated from carrots is as effective as beta-carotene in maintaining the vitamin A status of Mongolian gerbils. J Nutr 2005;135:2622–6. [DOI] [PubMed] [Google Scholar]
- 71.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol 2006;66:606–30. [DOI] [PubMed] [Google Scholar]
- 72.dela Seña C, Riedl KM, Narayanasamy S, Curley RW, Schwartz SJ, Harrison EH. The human enzyme that converts dietary provitamin A carotenoids to vitamin A is a dioxygenase. J Biol Chem 2014;289:13661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duester G, Mic FA, Molotkov A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem Biol Interact 2003;143–144:201–10. [DOI] [PubMed] [Google Scholar]
- 74.Lietz G, Lange J, Rimbach G. Molecular and dietary regulation of β,β-carotene 15,15′-monooxygenase 1 (BCMO1). Arch Biochem Biophys 2010;502:8–16. [DOI] [PubMed] [Google Scholar]
- 75.Bresnahan KA, Davis CR, Tanumihardjo SA. Relative vitamin A values of 9-cis- and 13-cis-β-carotene do not differ when fed at physiological levels during vitamin A-depletion in Mongolian gerbils (Meriones unguiculatus). Br J Nutr 2014;112:162–9. [DOI] [PubMed] [Google Scholar]
- 76.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 2001;276:14110–6. [DOI] [PubMed] [Google Scholar]
- 77.West CE, Eilander A, van Lieshout M. Consequences of revised estimates of carotenoid bioefficacy for dietary control of vitamin A deficiency in developing countries. J Nutr 2002;132(9, Suppl):2920S–6S. [DOI] [PubMed] [Google Scholar]
- 78.de Pee S, West CE, Permaesih D, Martuti S, Muhilal, Hautvast JG. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am J Clin Nutr 1998;68:1058–67. [DOI] [PubMed] [Google Scholar]
- 79.Tanumihardjo SA, Palacios N, Pixley KV. Provitamin A carotenoid bioavailability: what really matters? Int J Vitam Nutr Res 2010;80:336–50. [DOI] [PubMed] [Google Scholar]
- 80.Rodriguez-Amaya DB. Changes in carotenoids during processing and storage of foods. Arch Latinoam Nutr 1999;49(3, Suppl 1):38S–47S. [PubMed] [Google Scholar]
- 81.Tang G, Gu X, Hu S, Xu Q, Qin J, Dolnikowski GG, Fjeld CR, Gao X, Russell RM, Yin S. Green and yellow vegetables can maintain body stores of vitamin A in Chinese children. Am J Clin Nutr 1999;70:1069–76. [DOI] [PubMed] [Google Scholar]
- 82.Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Spinach or carrots can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterated vegetables. Am J Clin Nutr 2005;82:821–8. [DOI] [PubMed] [Google Scholar]
- 83.Haskell MJ, Jamil KM, Hassan F, Peerson JM, Hossain MI, Fuchs GJ, Brown KH. Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. Am J Clin Nutr 2004;80:705–14. [DOI] [PubMed] [Google Scholar]
- 84.Tang G, Hu Y, Yin S, Wang Y, Dallal GE, Grusak MA, Russell RM. β-Carotene in Golden Rice is as good as β-carotene in oil at providing vitamin A to children. Am J Clin Nutr 2012;96:658–64. Retraction in: Am J Clin Nutr 2015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Gannon B, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, Kalungwana N, Mosonda M, Pixley K, Masi C, Tanumihardjo SA. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial. Am J Clin Nutr 2014;100:1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bloem MW, Huq N, Gorstein J, Burger S, Kahn T, Islam N, Baker S, Davidson F. Production of fruits and vegetables at the homestead is an important source of vitamin A among women in rural Bangladesh. Eur J Clin Nutr 1996;50(Suppl 3):S62–7. [PubMed] [Google Scholar]
- 87.Shankar AV, West KP Jr, Gittelsohn J, Katz J, Pradhan R. Chronic low intakes of vitamin A-rich foods in households with xerophthalmic children: a case-control study in Nepal. Am J Clin Nutr 1996;64:242–8. [DOI] [PubMed] [Google Scholar]
- 88.Devadas RP, Saroja S, Murthy NK. Availability of β-carotene from papaya fruit and amaranth in preschool children. Indian J Nutr Diet 1980;17:41–4. [Google Scholar]
- 89.Sommer A, West K Jr. Vitamin A deficiency: health, survival, and vision. New York: Oxford University Press; 1996. [Google Scholar]
- 90.Phillips M, Sanghvi T, Suárez R, McKigney J, Fiedler J. The costs and effectiveness of three vitamin A interventions in Guatemala. Soc Sci Med 1996;42:1661–8. [DOI] [PubMed] [Google Scholar]
- 91.Fiedler JL, Lividini K. Managing the vitamin A program portfolio: a case study of Zambia, 2013–2042. Food Nutr Bull 2014;35:105–25. [DOI] [PubMed] [Google Scholar]
- 92.Lindblad BS, Patel M, Hamadeh M, Helmy N, Ahmad I, Dawodu A, Zaman S. Age and sex are important factors in determining normal retinol levels. J Trop Pediatr 1998;44:96–9. [DOI] [PubMed] [Google Scholar]
- 93.West KJ., Jr Public health impact of preventing vitamin A deficiency in the first 6 months of life. In: Delange FM, West KP Jr., editors. Micronutrient deficiencies in the first 6 months of life. Nestle Nutrition Workshop Series Pediatric Program. Basel (Switzerland): Karger AG; 2002. p. 103–27.
- 94.Singh V, West KP Jr. Vitamin A deficiency and xerophthalmia among school-aged children in Southeastern Asia. Eur J Clin Nutr 2004;58:1342–9. [DOI] [PubMed] [Google Scholar]
- 95.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 2001;21:167–92. [DOI] [PubMed] [Google Scholar]
- 96.Humphrey JH, West KP, Sommer A. Vitamin A deficiency and attributable mortality among under-5-year-olds. Bull World Health Organ 1992;70:225–32. [PMC free article] [PubMed] [Google Scholar]
- 97.Rice A, West KP Jr, Black R. Vitamin A deficiency. In: Ezzati M, Lopez A, Rodgers A, Murray C, editors. Global and regional burden of disease attributable to selected major risk factors. Vol. 1, 4th ed Geneva (Switzerland): WHO; 2004. p. 211–56. [Google Scholar]
- 98.Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC, Ramaswamy K, Rahmathullah R, Babu G. Reduced mortality among children in southern India receiving a small weekly dose of vitamin A. N Engl J Med 1990;323:929–35. [DOI] [PubMed] [Google Scholar]
- 99.West KP Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, Pradhan EK, Tielsch JM, Pandey MR, Sommer A. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 1991;338:67–71. [DOI] [PubMed] [Google Scholar]
- 100.Daulaire NM, Starbuck ES, Houston RM, Church MS, Stukel TA, Pandey MR. Childhood mortality after a high dose of vitamin A in a high risk population. BMJ 1992;304:207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arthur P, Kirkwood B, Ross D, Morris S, Gyapong J, Tomkins A, Addy H. Impact of vitamin A supplementation on childhood morbidity in northern Ghana. Lancet 1992;339:361–2. [DOI] [PubMed] [Google Scholar]
- 102.Vijayaraghavan K, Radhaiah G, Prakasam BS, Sarma KV, Reddy V. Effect of massive dose vitamin A on morbidity and mortality in Indian children. Lancet 1990;336:1342–5. [DOI] [PubMed] [Google Scholar]
- 103.Herrera MG, Nestel P, el Amin A, Fawzi WW, Mohamed KA, Weld L. Vitamin A supplementation and child survival. Lancet 1992;340:267–71. [DOI] [PubMed] [Google Scholar]
- 104.Ghana VAST Study Team. Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet 1993;342:7–12. [PubMed] [Google Scholar]
- 105.Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality: a meta-analysis. JAMA 1993;269:898–903. [PubMed] [Google Scholar]
- 106.Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev 2010;12:CD008524. [DOI] [PubMed] [Google Scholar]
- 107.Klemm RDW, Labrique AB, Christian P, Rashid M, Shamim AA, Katz J, Sommer A, West KP Jr. Newborn vitamin A supplementation reduced infant mortality in rural Bangladesh. Pediatrics 2008;122:e242–50. [DOI] [PubMed] [Google Scholar]
- 108.Benn CS, Aaby P. Sex-differential responses to preventive health interventions. Maybe we treat boys and girls differently when we treat them equally? Secondary publication. Dan Med Bull 2007;54:153–4. [PubMed] [Google Scholar]
- 109.West KP Jr, Christian P, Labrique AB, Rashid M, Shamim AA, Klemm RD, Massie AB, Mehra S, Schulze KJ, Ali H, et al. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: a cluster randomized trial. JAMA 2011;305:1986–95. [DOI] [PubMed] [Google Scholar]
- 110.Tanumihardjo SA, Permaesih D, Muherdiyantiningsih, Rustan E, Rusmil K, Fatah AC, Wilbur S, Muhilal, Karyadi D, Olson JA. Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. J Nutr 1996;126:451–7. [DOI] [PubMed] [Google Scholar]
- 111.Palmer AC, West KP, Dalmiya N, Schultink W. The use and interpretation of serum retinol distributions in evaluating the public health impact of vitamin A programmes. Public Health Nutr 2012;15:1201–15. [DOI] [PubMed] [Google Scholar]
- 112.Control of vitamin A deficiency and xerophthalmia. Report of a Joint WHO/UNICEF/USAID/Helen Keller International/IVACG meeting. World Health Organ Tech Rep Ser 1982;672:1–70. [PubMed]
- 113.West KP Jr, Darnton-Hill I. Nutrition and health in developing countries. 2nd ed Totowa (NJ): Humana Press; 2008. [Google Scholar]
- 114.Ross DA. Recommendations for vitamin A supplementation. J Nutr 2002;132(9, Suppl):2902S–6S. [DOI] [PubMed] [Google Scholar]
- 115.Sommer A, Hussaini G, Muhilal, Tarwotjo I, Susanto D, Saroso JS. History of nightblindness: a simple tool for xerophthalmia screening. Am J Clin Nutr 1980;33:887–91. [DOI] [PubMed] [Google Scholar]
- 116.Sommer A. Nutritional blindness: xerophthalmia and keratomalacia. Oxford (United Kingdom): Oxford University Press; 1981. [Google Scholar]
- 117.Sommer A. Vitamin A deficiency and its consequences: a field guide to their detection and control. 3rd ed Geneva (Switzerland):WHO; 1995. [Google Scholar]
- 118.Allen LH, Haskell M. Estimating the potential for vitamin A toxicity in women and young children. J Nutr 2002;132(9, Suppl):2907S–19S. [DOI] [PubMed] [Google Scholar]
- 119.WHO/Micronutrient Initiative. Safe vitamin A dosage during pregnancy and lactation. Recommendations and report of a consultation. WHO/NUT/98.4. Geneva (Switzerland): WHO; 1998. [cited 2016 Jul 25]. Available from: http://apps.who.int/iris/bitstream/10665/63838/1/WHO_NUT_98.4_eng.pdf?ua=1. [Google Scholar]
- 120.Stoltzfus RJ, Hakimi M, Miller KW, Rasmussen KM, Dawiesah S, Habicht JP, Dibley MJ. High dose vitamin A supplementation of breast-feeding Indonesian mothers: effects on the vitamin A status of mother and infant. J Nutr 1993;123:666–75. [DOI] [PubMed] [Google Scholar]
- 121.Hathcock JN, Hattan DG, Jenkins MY, McDonald JT, Sundaresan PR, Wilkening VL. Evaluation of vitamin A toxicity. Am J Clin Nutr 1990;52:183–202. [DOI] [PubMed] [Google Scholar]
- 122.Hathcock JN. Vitamins and minerals: efficacy and safety. Am J Clin Nutr 1997;66:427–37. [DOI] [PubMed] [Google Scholar]
- 123.von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr 2010;30:35–56. [DOI] [PubMed] [Google Scholar]
- 124.Kam RKT, Deng Y, Chen Y, Zhao H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci 2012;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kiser PD, Golczak M, Palczewski K. Chemistry of the retinoid (visual) cycle. Chem Rev 2014;114:194–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res 2013;54:1761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harrison EH. Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr 2005;25:87–103. [DOI] [PubMed] [Google Scholar]
- 128.Penniston KL, Weng N, Binkley N, Tanumihardjo SA. Serum retinyl esters are not elevated in postmenopausal women with and without osteoporosis whose preformed vitamin A intakes are high. Am J Clin Nutr 2006;84:1350–6. [DOI] [PubMed] [Google Scholar]
- 129.Goodman DW, Huang HS, Shiratori T. Tissue distribution and metabolism of newly absorbed vitamin A in the rat. J Lipid Res 1965;6:390–6. [PubMed] [Google Scholar]
- 130.Riabroy N, Tanumihardjo SA. Oral doses of α-retinyl ester track chylomicron uptake and distribution of vitamin A in a male piglet model for newborn infants. J Nutr 2014;144:1188–95. [DOI] [PubMed] [Google Scholar]
- 131.Senoo H, Mezaki Y, Morii M, Hebiguchi T, Miura M, Imai K. Uptake and storage of vitamin A as lipid droplets in the cytoplasm of cells in the lamina propria mucosae of the rat intestine. Cell Biol Int 2013;37:1171–80. [DOI] [PubMed] [Google Scholar]
- 132.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, Blaner WS. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem 1992;267:1805–10. [PubMed] [Google Scholar]
- 133.Zanotti G, Berni R. Plasma retinol-binding protein: structure and interactions with retinol, retinoids, and transthyretin. Vitam Horm 2004;69:271–95. [DOI] [PubMed] [Google Scholar]
- 134.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007;315:820–5. [DOI] [PubMed] [Google Scholar]
- 135.Ruiz A, Mark M, Jacobs H, Klopfenstein M, Hu J, Lloyd M, Habib S, Tosha C, Radu RA, Ghyselinck NB, et al. Retinoid content, visual responses, and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci 2012;53:3027–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dever JT, Surles RL, Davis CR, Tanumihardjo SA. α-Retinol is distributed through serum retinol-binding protein-independent mechanisms in the lactating sow-nursing piglet dyad. J Nutr 2011;141:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 1999;18:4633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paik J, Vogel S, Quadro L, Piantedosi R, Gottesman M, Lai K, Hamberger L, Vieira Mde M, Blaner WS. Vitamin A: overlapping delivery pathways to tissues from the circulation. J Nutr 2004;134(Suppl):276S–80S. [DOI] [PubMed] [Google Scholar]
- 139.Riabroy N, Dever JT, Tanumihardjo SA. α-Retinol and 3,4-didehydroretinol support growth in rats when fed at equimolar amounts and α-retinol is not toxic after repeated administration of large doses. Br J Nutr 2014;111:1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Green MH, Green JB. Vitamin A intake and status influence retinol balance, utilization and dynamics in rats. J Nutr 1994;124:2477–85. [DOI] [PubMed] [Google Scholar]
- 141.Ribaya-Mercado JD, Solon FS, Solon MA, Cabal-Barza MA, Perfecto CS, Tang G, Solon JA, Fjeld CR, Russell RM. Bioconversion of plant carotenoids to vitamin A in Filipino school-aged children varies inversely with vitamin A status. Am J Clin Nutr 2000;72:455–65. [DOI] [PubMed] [Google Scholar]
- 142.Tanumihardjo SA. Food-based approaches for ensuring adequate vitamin A nutrition. Compr Rev Food Sci Food Saf 2008;7:373–81. [Google Scholar]
- 143.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J 2010;24:1656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mondloch S, Gannon BM, Davis CR, Chileshe J, Kaliwile C, Masi C, Rios-Avila L, Gregory JF III, Tanumihardjo SA. High provitamin A carotenoid serum concentrations, elevated retinyl esters, and saturated retinol-binding protein in Zambian preschool children are consistent with the presence of high liver vitamin A stores. Am J Clin Nutr 2015;102:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tanumihardjo SA, Gannon BM, Kaliwile C, Chileshe J. Hypercarotenodermia in Zambia: which children turned orange during mango season? Eur J Clin Nutr 2015;69:1346–9. [DOI] [PubMed] [Google Scholar]
- 146.Zimmermann MB. Interactions of vitamin A and iodine deficiencies: effects on the pituitary-thyroid axis. Int J Vitam Nutr Res 2007;77:236–40. [DOI] [PubMed] [Google Scholar]
- 147.Zimmermann MB, Wegmüller R, Zeder C, Chaouki N, Torresani T. The effects of vitamin A deficiency and vitamin A supplementation on thyroid function in goitrous children. J Clin Endocrinol Metab 2004;89:5441–7. [DOI] [PubMed] [Google Scholar]
- 148.Suharno D, West CE, Muhilal, Karyadi D, Hautvast JG. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet 1993;342:1325–8. [DOI] [PubMed] [Google Scholar]
- 149.Ahmed F, Khan MR, Jackson AA. Concomitant supplemental vitamin A enhances the response to weekly supplemental iron and folic acid in anemic teenagers in urban Bangladesh. Am J Clin Nutr 2001;74:108–15. [DOI] [PubMed] [Google Scholar]
- 150.Muslimatun S, Schmidt MK, Schultink W, West CE, Hautvast JA, Gross R, Muhilal. Weekly supplementation with iron and vitamin A during pregnancy increases hemoglobin concentration but decreases serum ferritin concentration in Indonesian pregnant women. J Nutr 2001;131:85–90. [DOI] [PubMed] [Google Scholar]
- 151.Tanumihardjo SA. Vitamin A and iron status are improved by vitamin A and iron supplementation in pregnant Indonesian women. J Nutr 2002;132:1909–12. [DOI] [PubMed] [Google Scholar]
- 152.Wieringa FT, Dijkhuizen MA, West CE, Thurnham DI, Muhilal, Van der Meer JWM. Redistribution of vitamin A after iron supplementation in Indonesian infants. Am J Clin Nutr 2003;77:651–7. [DOI] [PubMed] [Google Scholar]
- 153.Gebremedhin S. Effect of a single high dose vitamin A supplementation on the hemoglobin status of children aged 6–59 months: propensity score matched retrospective cohort study based on the data of Ethiopian Demographic and Health Survey 2011. BMC Pediatr 2014;14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dijkhuizen MA, Wieringa FT, West CE, Muhilal. Zinc plus beta-carotene supplementation of pregnant women is superior to beta-carotene supplementation alone in improving vitamin A status in both mothers and infants. Am J Clin Nutr 2004;80:1299–307. [DOI] [PubMed] [Google Scholar]
- 155.Mills JP, Tumuhimbise GA, Jamil KM, Thakkar SK, Failla ML, Tanumihardjo SA. Sweet potato beta-carotene bioefficacy is enhanced by dietary fat and not reduced by soluble fiber intake in Mongolian gerbils. J Nutr 2009;139:44–50. [DOI] [PubMed] [Google Scholar]
- 156.Ribaya-Mercado JD. Influence of dietary fat on beta-carotene absorption and bioconversion into vitamin A. Nutr Rev 2002;60:104–10. [DOI] [PubMed] [Google Scholar]
- 157.Ribaya-Mercado JD, Maramag CC, Tengco LW, Dolnikowski GG, Blumberg JB, Solon FS. Carotene-rich plant foods ingested with minimal dietary fat enhance the total-body vitamin A pool size in Filipino schoolchildren as assessed by stable-isotope-dilution methodology. Am J Clin Nutr 2007;85:1041–9. [DOI] [PubMed] [Google Scholar]
- 158.Semba RD. Vitamin A, immunity, and infection. Clin Infect Dis 1994;19:489–99. [DOI] [PubMed] [Google Scholar]
- 159.Pino-Lagos K, Benson MJ, Noelle RJ. Retinoic acid in the immune system. Ann N Y Acad Sci 2008;1143:170–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Raverdeau M, Mills KHG. Modulation of T cell and innate immune responses by retinoic acid. J Immunol 2014;192:2953–8. [DOI] [PubMed] [Google Scholar]
- 161.Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, Deep-Dixit V, Nadal CN, Taub DD. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol 2006;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tang JE, Wang RJ, Zhong H, Yu B, Chen Y. Vitamin A and risk of bladder cancer: a meta-analysis of epidemiological studies. World J Surg Oncol 2014;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fulan H, Changxing J, Baina WY, Wencui Z, Chunqing L, Fan W, Dandan L, Dianjun S, Tong W, Da P, et al. Retinol, vitamins A, C, and E and breast cancer risk: a meta-analysis and meta-regression. Cancer Causes Control 2011;22:1383–96. [DOI] [PubMed] [Google Scholar]
- 164.Zhang X, Dai B, Zhang B, Wang Z. Vitamin A and risk of cervical cancer: a meta-analysis. Gynecol Oncol 2012;124:366–73. [DOI] [PubMed] [Google Scholar]
- 165.Wu Y, Ye Y, Shi Y, Li P, Xu J, Chen K, Xu E, Yang J. Association between vitamin A, retinol intake and blood retinol level and gastric cancer risk: a meta-analysis. Clin Nutr 2015;34:620–6. [DOI] [PubMed] [Google Scholar]
- 166.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer 2001;1:181–93. [DOI] [PubMed] [Google Scholar]
- 167.Niles RM. Recent advances in the use of vitamin A (retinoids) in the prevention and treatment of cancer. Nutrition 2000;16:1084–9. [DOI] [PubMed] [Google Scholar]
- 168.Siddikuzzaman GC, Berlin Grace VM. All trans retinoic acid and cancer. Immunopharmacol Immunotoxicol 2011;33:241–9. [DOI] [PubMed] [Google Scholar]
- 169.Wang Z-Y, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 2008;111:2505–15. [DOI] [PubMed] [Google Scholar]
- 170.Basu TK, Tze WJ, Leichter J. Serum vitamin A and retinol-binding protein in patients with insulin-dependent diabetes mellitus. Am J Clin Nutr 1989;50:329–31. [DOI] [PubMed] [Google Scholar]
- 171.Martinoli L, Di Felice M, Seghieri G, Ciuti M, De Giorgio LA, Fazzini A, Gori R, Anichini R, Franconi F. Plasma retinol and alpha-tocopherol concentrations in insulin-dependent diabetes mellitus: their relationship to microvascular complications. Int J Vitam Nutr Res 1993;63:87–92. [PubMed] [Google Scholar]
- 172.Iqbal S, Naseem I. Role of vitamin A in type 2 diabetes mellitus biology: effects of intervention therapy in a deficient state. Nutrition 2015;31:901–7. [DOI] [PubMed] [Google Scholar]
- 173.Chen W, Chen G. The roles of vitamin A in the regulation of carbohydrate, lipid, and protein metabolism. J Clin Med 2014;3:453–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao S, Li R, Li Y, Chen W, Zhang Y, Chen G. Roles of vitamin A status and retinoids in glucose and fatty acid metabolism. Biochem Cell Biol 2012;90:142–52. [DOI] [PubMed] [Google Scholar]
- 175.Brun P-J, Yang KJ, Lee S-A, Yuen JJ, Blaner WS. Retinoids: potent regulators of metabolism. Biofactors 2013;39:151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Graham TE, Kahn BB. Tissue-specific alterations of glucose transport and molecular mechanisms of intertissue communication in obesity and type 2 diabetes. Horm Metab Res 2007;39:717–21. [DOI] [PubMed] [Google Scholar]
- 177.Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and Toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol 2012;32:2010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med 2007;13:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shabrova E, Hoyos B, Vinogradov V, Kim YK, Wassef L, Leitges M, Quadro L, Hammerling U. Retinol as a cofactor for PKCδ-mediated impairment of insulin sensitivity in a mouse model of diet-induced obesity. FASEB J 2016;30:1339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wiysonge CS, Shey M, Kongnyuy EJ, Sterne JA, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev 2011;1:CD003648. [DOI] [PubMed] [Google Scholar]
- 181.Siegfried N, Irlam JH, Visser ME, Rollins NN. Micronutrient supplementation in pregnant women with HIV infection. Cochrane Database Syst Rev 2012;3:CD009755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kuhn L, Coutsoudis A, Trabattoni D, Archary D, Rossi T, Segat L, Clerici M, Crovella S. Synergy between mannose-binding lectin gene polymorphisms and supplementation with vitamin A influences susceptibility to HIV infection in infants born to HIV-positive mothers. Am J Clin Nutr 2006;84:610–5. [DOI] [PubMed] [Google Scholar]
- 183.WHO. Measles Factsheet, 2016. [cited 2016 Jul 25]. Available from: http://www.who.int/mediacentre/factsheets/fs286/en/.
- 184.Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 2011;343:d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Biomarkers of Nutrition for Development (BOND) Program [cited 2016 Apr 22]. Available from: https://www.nichd.nih.gov/global_nutrition/programs/bond/Pages/index.aspx.
- 186.Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 2011;94(Suppl):658S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ross AC, Zolfaghari R. Regulation of hepatic retinol metabolism: perspectives from studies on vitamin A status. J Nutr 2004;134(Suppl):269S–75S. [DOI] [PubMed] [Google Scholar]
- 188.Muto Y, Smith JE, Milch PO, Goodman DS. Regulation of retinol-binding protein metabolism by vitamin A status in the rat. J Biol Chem 1972;247:2542–50. [PubMed] [Google Scholar]
- 189.Hicks VA, Gunning DB, Olson JA. Metabolism, plasma transport and biliary excretion of radioactive vitamin A and its metabolites as a function of liver reserves of vitamin A in the rat. J Nutr 1984;114:1327–33. [DOI] [PubMed] [Google Scholar]
- 190.Olson J. Vitamin A. In: Brown ML, editor. Present knowledge in nutrition. 6th ed Washington (DC): International Life Sciences Institute; 1990. p. 96–107. [Google Scholar]
- 191.Coulston A, Boushey CJ, Ferruzzi M, editors. Nutrition in the prevention and treatment of disease. 3rd ed Waltham (MA): Academic Press; 2013. [Google Scholar]
- 192.Raiten D, Combs GJ. Directions in nutritional assessment—biomarkers and bio-indicators: providing clarity in the face of complexity. Sight Life 2015;29:39–44. [Google Scholar]
- 193.Aubert H. Physiologie der Netzhaut. [Retinal physiology.] Breslau (Germany): E Morgenstern; 1865. (in German). [Google Scholar]
- 194.Piper H. Ueber Dunkeladaptation. [Concerning dark adaptation.] Ztschr f Psychol n Physiol d Sinnesorg. (Journal of the Psychology and Physiology of the Sensory Organs.) 1903;31:161–7 (in German).
- 195.Hume EM, Krebs HA. Vitamin A requirements of human adults. Medical Research Council Special Report No.: 264. London:HM Stationery Office; 1949. [Google Scholar]
- 196.Russell RM, Smith VC, Multack R, Krill AE, Rosenberg IH. Dark-adaptation testing for diagnosis of subclinical vitamin-A deficiency and evaluation of therapy. Lancet 1973;2:1161–4. [DOI] [PubMed] [Google Scholar]
- 197.Morrison SA, Russell RM, Carney EA, Oaks EV. Zinc deficiency: a cause of abnormal dark adaptation in cirrhotics. Am J Clin Nutr 1978;31:276–81. [DOI] [PubMed] [Google Scholar]
- 198.Dutta S, Russell R, Lakhanpal V. Abnormal dark adaptation in adult patients with protein-energy malnutrition: correction by protein-energy repletion. Nutr Res 1981;1:443–8. [Google Scholar]
- 199.McFarland R, Domey R, Warren A, Ward D. Dark adaptation as a function of age: I. A statistical analysis. J Gerontol 1960;15:149–54. [DOI] [PubMed] [Google Scholar]
- 200.Domey RG, McFarland R, Chadwick E. Dark adaptation as a function of age and time: II. A derivation. J Gerontol 1960;15:267–79. [DOI] [PubMed] [Google Scholar]
- 201.Thornton SP. A rapid test for dark adaptation. Ann Ophthalmol 1977;9:731–4. [PubMed] [Google Scholar]
- 202.Vinton NE, Russell RM. Evaluation of a rapid test of dark adaptation. Am J Clin Nutr 1981;34:1961–6. [DOI] [PubMed] [Google Scholar]
- 203.Solomons NW, Russell RM, Vinton E, Guerrero AM, Mejia L. Application of a rapid dark adaptation test in children. J Pediatr Gastroenterol Nutr 1982;1:571–4. [DOI] [PubMed] [Google Scholar]
- 204.Bankson DD, Ellis JK, Russell RM. Effects of a vitamin-A-free diet on tissue vitamin A concentration and dark adaptation of aging rats. Exp Gerontol 1989;24:127–36. [DOI] [PubMed] [Google Scholar]
- 205.Stewart BE, Young RS. Pupillary response: an index of visual threshold. Appl Opt 1989;28:1122–7. [DOI] [PubMed] [Google Scholar]
- 206.Congdon N, Sommer A, Severns M, Humphrey J, Friedman D, Clement L, Wu LS, Natadisastra G. Pupillary and visual thresholds in young children as an index of population vitamin A status. Am J Clin Nutr 1995;61:1076–82. [DOI] [PubMed] [Google Scholar]
- 207.Labrique AB, Palmer AC, Healy K, Mehra S, Sauer TC, West KP Jr, Sommer A. A novel device for assessing dark adaptation in field settings. BMC Ophthalmol 2015;15:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Haskell MJ, Pandey P, Graham JM, Peerson JM, Shrestha RK, Brown KH. Recovery from impaired dark adaptation in nightblind pregnant Nepali women who receive small daily doses of vitamin A as amaranth leaves, carrots, goat liver, vitamin A-fortified rice, or retinyl palmitate. Am J Clin Nutr 2005;81:461–71. [DOI] [PubMed] [Google Scholar]
- 209.Wittpenn JR, Tseng SC, Sommer A. Detection of early xerophthalmia by impression cytology. Arch Ophthalmol 1986;104:237–9. [DOI] [PubMed] [Google Scholar]
- 210.Tanumihardjo SA, Permaesih D, Dahro AM, Rustan E, Muhilal, Karyadi D, Olson JA. Comparison of vitamin A status assessment techniques in children from two Indonesian villages. Am J Clin Nutr 1994;60:136–41. [DOI] [PubMed] [Google Scholar]
- 211.Filteau SM, Morris SS, Tomkins AM, Arthur P, Kirkwood BR, Ross DA, Abbott RA, Gyapong JO. Lack of association between vitamin A status and measures of conjunctival epithelial integrity in young children in northern Ghana. Eur J Clin Nutr 1994;48:669–77. [PubMed] [Google Scholar]
- 212.Ajaiyeoba AI, Samaila E, Adeyefa AI. Conjunctival impression cytology and biochemical assessment of vitamin A status in Nigerian children. Niger J Med 2002;11:63–6. [PubMed] [Google Scholar]
- 213.WHO. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations [Internet]. Geneva (Switzerland); WHO; 2011. [cited 2016 Jul 25]. Available from: http://apps.who.int/iris/bitstream/10665/85859/4/WHO_NMH_NHD_MNM_11.3_eng.pdf?ua=1. [Google Scholar]
- 214.de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr 2002;132(9, Suppl):2895S–901S. [DOI] [PubMed] [Google Scholar]
- 215.Stephensen CB, Gildengorin G. Serum retinol, the acute phase response, and the apparent misclassification of vitamin A status in the third National Health and Nutrition Examination Survey. Am J Clin Nutr 2000;72:1170–8. [DOI] [PubMed] [Google Scholar]
- 216.Smith JE, Brown ED, Smith JC Jr. The effect of zinc deficiency on the metabolism of retinol binding protein in the rat. J Lab Clin Med 1974;84:692–7. [PubMed] [Google Scholar]
- 217.Tanumihardjo SA, Muherdiyantiningsih, Permaesih D, Dahro AM, Muhilal, Karyadi D, Olson JA. Assessment of the vitamin A status in lactating and nonlactating, nonpregnant Indonesian women by use of the modified-relative-dose-response (MRDR) test. Am J Clin Nutr 1994;60:142–7. [DOI] [PubMed] [Google Scholar]
- 218.Tanumihardjo SA, Suharno D, Permaesih D, Muherdiyantiningsih, Permaesih D, Dahro AM, Muhilal, Karyadi D, Olson JA. Application of the modified relative dose response test to pregnant Indonesian women for assessing vitamin A status. Eur J Clin Nutr 1995;49:897–903. [PubMed] [Google Scholar]
- 219.Klemm RD, Palmer A, Greig A, Engle-Stone R, Dalmiya N. A changing landscape for vitamin A programs: implications for optimal intervention packages, program monitoring, and safety. Food Nutr Bull 2016;37(2 Suppl):S75–86. [DOI] [PubMed] [Google Scholar]
- 220.Suri DJ, Tanumihardjo JP, Gannon BM, Pinkaew S, Kaliwile C, Chileshe J, Tanumihardjo SA. Serum retinol concentrations demonstrate high specificity after correcting for inflammation but questionable sensitivity compared with liver stores calculated from isotope dilution in determining vitamin A deficiency in Thai and Zambian children. Am J Clin Nutr 2015;102:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Min K-B, Min J-Y. Relation of serum vitamin A levels to all-cause and cause-specific mortality among older adults in the NHANES III population. Nutr Metab Cardiovasc Dis 2014;24:1197–203. [DOI] [PubMed] [Google Scholar]
- 222.Tanumihardjo SA. Vitamin A fortification efforts require accurate monitoring of population vitamin A status to prevent excessive intakes. Procedia Chem 2015;14:398–407. [Google Scholar]
- 223.Bresnahan KA, Tanumihardjo SA. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv Nutr 2014;5:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev P, van Ommen B; INSPIRE Consultative Group. Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE). J Nutr 2015;145(Suppl):1039S–108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Thurnham D, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. WHO report: priorities in the assessment of vitamin A and iron status in populations. Geneva (Switzerland): WHO;2012. [cited 2016 Jul 25]. Available from: http://www.who.int/nutrition/publications/micronutrients/background_paper4_report_assessment_vitAandIron_status.pdf. [Google Scholar]
- 226.Bresnahan KA, Chileshe J, Arscott S, Nuss E, Surles R, Masi C, Kafwembe E, Tanumihardjo SA. The acute phase response affected traditional measures of micronutrient status in rural Zambian children during a randomized, controlled feeding trial. J Nutr 2014;144:972–8. [DOI] [PubMed] [Google Scholar]
- 227.Wieringa FT, Dijkhuizen MA, West CE, Northrop-Clewes CA, Muhilal. Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J Nutr 2002;132:3061–6. [DOI] [PubMed] [Google Scholar]
- 228.Pinkaew S, Wegmuller R, Wasantwisut E, Winichagoon P, Hurrell RF, Tanumihardjo SA. Triple-fortified rice containing vitamin A reduced marginal vitamin A deficiency and increased vitamin A liver stores in school-aged Thai children. J Nutr 2014;144:519–24. [DOI] [PubMed] [Google Scholar]
- 229.Tanumihardjo SA. Mammalian models for understanding mechanisms of retinol and retinoid actions. In: WHO Technical Consultation on Vitamin A in Newborn Health: Mechanistic Studies. Geneva (Switzerland):WHO; 2012; p. 93–108. [cited 2016 Jul 25]. Available from: http://apps.who.int/iris/bitstream/10665/44806/1/9789241503167_eng.pdf. [Google Scholar]
- 230.Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest 1968;47:2025–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010;316:129–39. [DOI] [PubMed] [Google Scholar]
- 232.Mills JP, Furr HC, Tanumihardjo SA. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp Biol Med (Maywood) 2008;233:1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Peterson PA. Studies on the interaction between prealbumin, retinol-binding protein, and vitamin A. J Biol Chem 1971;246:44–9. [PubMed] [Google Scholar]
- 234.Peterson PA. Demonstration in serum of two physiological forms of the human retinol binding protein. Eur J Clin Invest 1971;1:437–44. [DOI] [PubMed] [Google Scholar]
- 235.Stephensen CB, Alvarez JO, Kohatsu J, Hardmeier R, Kennedy JI, Gammon RB. Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr 1994;60:388–92. [DOI] [PubMed] [Google Scholar]
- 236.Becker-Cohen R, Rinat C, Ben-Shalom E, Feinstein S, Ivgi H, Frishberg Y. Vitamin A deficiency associated with urinary retinol binding protein wasting in Dent’s disease. Pediatr Nephrol 2012;27:1097–102. [DOI] [PubMed] [Google Scholar]
- 237.Park H, Green MH, Shaffer ML. Association between serum retinol-binding protein 4 concentrations and clinical indices in subjects with type 2 diabetes: a meta-analysis. J Hum Nutr Diet 2012;25:300–10. [DOI] [PubMed] [Google Scholar]
- 238.Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol 2011;165:703–11. [DOI] [PubMed] [Google Scholar]
- 239.Sun H, Kawaguchi R. The membrane receptor for plasma retinol-binding protein, a new type of cell-surface receptor. Int Rev Cell Mol Biol 2011;288:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Berry DC, Croniger CM, Ghyselinck NB, Noy N. Transthyretin blocks retinol uptake and cell signaling by the holo-retinol-binding protein receptor STRA6. Mol Cell Biol 2012;32:3851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Berry DC, Noy N. Signaling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochim Biophys Acta 2012;1821:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.WHO. Indicators for assessing vitamin a deficiency and their application in monitoring and evaluating intervention programmes: report of a Joint WHO/UNICEF Consultation. Geneva (Switzerland); WHO; 1996. [Google Scholar]
- 243.Oliver RW, Kafwembe EM, Mwandu D. Stability of vitamin A circulating complex in spots of dried serum samples absorbed onto filter paper. Clin Chem 1993;39:1744–5. [PubMed] [Google Scholar]
- 244.Shi H, Ma Y, Humphrey JH, Craft NE. Determination of vitamin A in dried human blood spots by high-performance capillary electrophoresis with laser-excited fluorescence detection. J Chromatogr B Biomed Appl 1995;665:89–96. [DOI] [PubMed] [Google Scholar]
- 245.Craft NE, Haitema T, Brindle LK, Yamini S, Humphrey JH, West KP. Retinol analysis in dried blood spots by HPLC. J Nutr 2000;130:882–5. [DOI] [PubMed] [Google Scholar]
- 246.Craft NE, Bulux J, Valdez C, Li Y, Solomons NW. Retinol concentrations in capillary dried blood spots from healthy volunteers: method validation. Am J Clin Nutr 2000;72:450–4. [DOI] [PubMed] [Google Scholar]
- 247.Craft NE. Innovative approaches to vitamin A assessment. J Nutr 2001;131:1626S–30S. [DOI] [PubMed] [Google Scholar]
- 248.Erhardt JG, Craft NE, Heinrich F, Biesalski HK. Rapid and simple measurement of retinol in human dried whole blood spots. J Nutr 2002;132:318–21. [DOI] [PubMed] [Google Scholar]
- 249.Houzé P, Beltz S, Samba C, Malvy D, Bousquet B, Gourmel B. [Dried blood spot vitamin A determination by high pressure liquid chromatography with electrochemical detection.] Ann Biol Clin (Paris) 2004;62:539–46 (in French). [PubMed] [Google Scholar]
- 250.WHO. Module 14. Blood collection and handling—dried blood spot (DBS) [cited 2016 Apr 22]. Available from: http://cdrwww.who.int/diagnostics_laboratory/documents/guidance/pm_module14.pdf.
- 251.UNICEF. Taking blood from infants for the HIV DNA PCR test [cited 2016 Apr 22]. Available from: http://www.unicef.org/southafrica/SAF_resources_pcrtesting.pdf.
- 252.Wallingford J, Underwood B. Vitamin A deficiency in pregnancy, lactation and the nursing child. In: Bauernfeind J, editor. Vitamin A deficiency and its control. Orlando (FL): Academic Press; 1986. p. 101–52. [Google Scholar]
- 253.West KP, Chirambo M, Katz J, Sommer A. Breast-feeding, weaning patterns, and the risk of xerophthalmia in southern Malawi. Am J Clin Nutr 1986;44:690–7. [DOI] [PubMed] [Google Scholar]
- 254.Khatry SK, West KP Jr, Katz J, LeClerq SC, Pradhan EK, Wu LS-F, Thapa MD, Pokhrel RP. Epidemiology of xerophthalmia in Nepal: a pattern of household poverty, childhood illness, and mortality. The Sarlahi Study Group. Arch Ophthalmol 1995;113:425–9. [DOI] [PubMed] [Google Scholar]
- 255.Chaimongkol L, Pinkaew S, Furr HC, Estes J, Craft NE, Wasantwisut E, Winichagoon P. Performance of the CRAFTi portable fluorometer comparing with the HPLC method for determining serum retinol. Clin Biochem 2011;44:1030–2. [DOI] [PubMed] [Google Scholar]
- 256.Tanumihardjo SA, Penniston KL. Simplified methodology to determine breast milk retinol concentrations. J Lipid Res 2002;43:350–5. [PubMed] [Google Scholar]
- 257.Stoltzfus RJ, Underwood BA. Breast-milk vitamin A as an indicator of the vitamin A status of women and infants. Bull World Health Organ 1995;73:703–11. [PMC free article] [PubMed] [Google Scholar]
- 258.Lucas A, Gibbs JA, Lyster RL, Baum JD. Creamatocrit: simple clinical technique for estimating fat concentration and energy value of human milk. BMJ 1978;1:1018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rice AL, Stoltzfus RJ, de Francisco A, Kjolhede CL. Evaluation of serum retinol, the modified-relative-dose-response ratio, and breast-milk vitamin A as indicators of response to postpartum maternal vitamin A supplementation. Am J Clin Nutr 2000;71:799–806. [DOI] [PubMed] [Google Scholar]
- 260.Stoltzfus RJ, Habicht JP, Rasmussen KM, Hakimi M. Evaluation of indicators for use in vitamin A intervention trials targeted at women. Int J Epidemiol 1993;22:1111–8. [DOI] [PubMed] [Google Scholar]
- 261.de Pee S, Yuniar Y, West CE, Muhilal. Evaluation of biochemical indicators of vitamin A status in breast-feeding and non-breast-feeding Indonesian women. Am J Clin Nutr 1997;66:160–7. [DOI] [PubMed] [Google Scholar]
- 262.Soprano DR, Smith JE, Goodman DS. Effect of retinol status on retinol-binding protein biosynthesis rate and translatable messenger RNA level in rat liver. J Biol Chem 1982;257:7693–7. [PubMed] [Google Scholar]
- 263.Soprano DR, Soprano KJ, Goodman DS. Retinol-binding protein messenger RNA levels in the liver and in extrahepatic tissues of the rat. J Lipid Res 1986;27:166–71. [PubMed] [Google Scholar]
- 264.Dixon JL, Goodman DS. Studies on the metabolism of retinol-binding protein by primary hepatocytes from retinol-deficient rats. J Cell Physiol 1987;130:14–20. [DOI] [PubMed] [Google Scholar]
- 265.Tanumihardjo SA, Cheng JC, Permaesih D, Muherdiyantiningsih, Rustan E, Muhilal, Karyadi D, Olson JA. Refinement of the modified-relative-dose-response test as a method for assessing vitamin A status in a field setting: experience with Indonesian children. Am J Clin Nutr 1996;64:966–71. [DOI] [PubMed] [Google Scholar]
- 266.Amedee-Manesme O, Mourey MS, Hanck A, Therasse J. Vitamin A relative dose response test: validation by intravenous injection in children with liver disease. Am J Clin Nutr 1987;46:286–9. [DOI] [PubMed] [Google Scholar]
- 267.Ribaya-Mercado JD, Mazariegos M, Tang G, Romero-Abal ME, Mena I, Solomons NW, Russell RM. Assessment of total body stores of vitamin A in Guatemalan elderly by the deuterated-retinol-dilution method. Am J Clin Nutr 1999;69:278–84. [DOI] [PubMed] [Google Scholar]
- 268.Stephensen CB, Franchi LM, Hernandez H, Campos M, Colarossi A, Gilman RH, Alvarez JO. Assessment of vitamin A status with the relative-dose-response test in Peruvian children recovering from pneumonia. Am J Clin Nutr 2002;76:1351–7. [DOI] [PubMed] [Google Scholar]
- 269.Tanumihardjo SA, Furr HC, Erdman JW Jr, Olson JA. Use of the modified relative dose response (MRDR) assay in rats and its application to humans for the measurement of vitamin A status. Eur J Clin Nutr 1990;44:219–24. [PubMed] [Google Scholar]
- 270.Tanumihardjo SA, Koellner PG, Olson JA. The modified relative-dose-response assay as an indicator of vitamin A status in a population of well-nourished American children. Am J Clin Nutr 1990;52:1064–7. [DOI] [PubMed] [Google Scholar]
- 271.Valentine AR, Tanumihardjo SA. Adjustments to the modified relative dose response (MRDR) test for assessment of vitamin A status minimize the blood volume used in piglets. J Nutr 2004;134:1186–92. [DOI] [PubMed] [Google Scholar]
- 272.Tanumihardjo SA, Olson JA. The reproducibility of the modified relative dose response (MRDR) assay in healthy individuals over time and its comparison with conjunctival impression cytology (CIC). Eur J Clin Nutr 1991;45:407–11. [PubMed] [Google Scholar]
- 273.Tanumihardjo SA, Muherdiyantiningsih, Permaesih D, Komala, Muhilal, Karyadi D, Olson JA. Daily supplements of vitamin A (8.4 μmol, 8000 IU) improve the vitamin A status of lactating Indonesian women. Am J Clin Nutr 1996;63:32–5. [DOI] [PubMed] [Google Scholar]
- 274.Agne-Djigo A, Idohou-Dossou N, Kwadjode KM, Tanumihardjo SA, Wade S. High prevalence of vitamin A deficiency is detected by the modified relative dose-response test in six-month-old Senegalese breast-fed infants. J Nutr 2012;142:1991–6. [DOI] [PubMed] [Google Scholar]
- 275.Kafwembe EM, Sukwa TY, Manyando C, Mwandu D, Chipipa J, Chipaila P. The vitamin A status of Zambian children attending an under five clinic as evaluated by the modified relative dose response (MRDR) test. Int J Vitam Nutr Res 1996;66:190–6. [PubMed] [Google Scholar]
- 276.Spannaus-Martin DJ, Cook LR, Tanumihardjo SA, Duitsman PK, Olson JA. Vitamin A and vitamin E statuses of preschool children of socioeconomically disadvantaged families living in the midwestern United States. Eur J Clin Nutr 1997;51:864–9. [DOI] [PubMed] [Google Scholar]
- 277.Duitsman PK, Cook LR, Tanumihardjo SA, Olson JA. Vitamin A inadequacy in socioeconomically disadvantaged pregnant Iowan women as assessed by the modified relative dose response (MRDR) test. Nutr Res 1995;15:1263–76. [Google Scholar]
- 278.Rice AL, Stoltzfus RJ, de Francisco A, Chakraborty J, Kjolhede CL, Wahed MA. Maternal vitamin A or beta-carotene supplementation in lactating Bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J Nutr 1999;129:356–65. [DOI] [PubMed] [Google Scholar]
- 279.Vitamin A Tracer Task Force. Appropriate uses of vitamin A tracer (stable isotope) methodology. Washington (DC): ILSI Human Nutrition Institute; 2004. [Google Scholar]
- 280.Furr HC, Green M, Haskell M, Mokhtar N, Nestel P, Newton S, Ribaya-Mercado JD, Tang G, Tanumihardjo S, Wasantwisut E. Stable isotope dilution techniques for assessing vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutr 2005;8:596–607. [DOI] [PubMed] [Google Scholar]
- 281.Haskell M, Ribaya-Mercado JD; Vitamin A Tracer Task Force. Handbook on vitamin A tracer dilution methods to assess status and evaluate intervention programs. Technical Monograph 5. Washington (DC):HarvestPlus; 2005. [Google Scholar]
- 282.Oxley A, Berry P, Taylor GA, Cowell J, Hall MJ, Hesketh J, Lietz G, Boddy AV. An LC/MS/MS method for stable isotope dilution studies of β-carotene bioavailability, bioconversion, and vitamin A status in humans. J Lipid Res 2014;55:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gannon BM, Tanumihardjo SA. Comparisons among equations used for retinol isotope dilution in the assessment of total body stores and total liver reserves. J Nutr 2015;145:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Haskell MJ, Handelman GJ, Peerson JM, Jones AD, Rabbi MA, Awal MA, Wahed MA, Mahalanabis D, Brown KH. Assessment of vitamin A status by the deuterated-retinol-dilution technique and comparison with hepatic vitamin A concentration in Bangladeshi surgical patients. Am J Clin Nutr 1997;66:67–74. [DOI] [PubMed] [Google Scholar]
- 285.Tanumihardjo SA. Vitamin A status assessment in rats with 13C4-retinyl acetate and gas chromatography/combustion/isotope ratio mass spectrometry. J Nutr 2000;130:2844–9. [DOI] [PubMed] [Google Scholar]
- 286.Escaron AL, Green MH, Howe JA, Tanumihardjo SA. Mathematical modeling of serum 13C-retinol in captive rhesus monkeys provides new insights on hypervitaminosis A. J Nutr 2009;139:2000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Tang G, Qin J, Hao L, Yin S, Russell RM. Use of a short-term isotope-dilution method for determining the vitamin A status of children. Am J Clin Nutr 2002;76:413–8. [DOI] [PubMed] [Google Scholar]
- 288.Green MH. Evaluation of the “Olson equation”, an isotope dilution method for estimating vitamin A stores. Int J Vitam Nutr Res 2014;84(Suppl 1):9–15. [DOI] [PubMed] [Google Scholar]
- 289.Haskell MJ, Jamil KM, Peerson JM, Wahed MA, Brown KH. The paired deuterated retinol dilution technique can be used to estimate the daily vitamin A intake required to maintain a targeted whole body vitamin A pool size in men. J Nutr 2011;141:428–32. [DOI] [PubMed] [Google Scholar]
- 290.Valentine AR, Davis CR, Tanumihardjo SA. Vitamin A isotope dilution predicts liver stores in line with long-term vitamin A intake above the current Recommended Dietary Allowance for young adult women. Am J Clin Nutr 2013;98:1192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Gieng SH, Green MH, Green JB, Rosales FJ. Model-based compartmental analysis indicates a reduced mobilization of hepatic vitamin A during inflammation in rats. J Lipid Res 2007;48:904–13. [DOI] [PubMed] [Google Scholar]
- 292.Tang G, Qin J, Dolnikowski GG. Deuterium enrichment of retinol in humans determined by gas chromatography electron capture negative chemical ionization mass spectrometry. J Nutr Biochem 1998;9:408–14. [Google Scholar]
- 293.Drammeh BS, Schleicher RL, Pfeiffer CM, Jain RB, Zhang M, Nguyen PH. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clin Chem 2008;54:1883–91. [DOI] [PubMed] [Google Scholar]
- 294.Smith FR, Goodman DS. Vitamin A transport in human vitamin A toxicity. N Engl J Med 1976;294:805–8. [DOI] [PubMed] [Google Scholar]
- 295.Gunter E, Lewis B, Koncikowski S. Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Hyattsville (MD):National Center for Environmental Health and National Center for Health Statistics;1996. [cited 2016 Jul 25]. Available from: https://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf.
- 296.Bankson DD, Russell RM, Sadowski JA. Determination of retinyl esters and retinol in serum or plasma by normal-phase liquid chromatography: method and applications. Clin Chem 1986;32:35–40. [PubMed] [Google Scholar]
- 297.Ellis JK, Russell RM, Makrauer FL, Schaefer EJ. Increased risk for vitamin A toxicity in severe hypertriglyceridemia. Ann Intern Med 1986;105:877–9. [DOI] [PubMed] [Google Scholar]
- 298.Biesalski HK, Frank J, Beck SC, Heinrich F, Illek B, Reifen R, Gollnick H, Seeliger MW, Wissinger B, Zrenner E. Biochemical but not clinical vitamin A deficiency results from mutations in the gene for retinol binding protein. Am J Clin Nutr 1999;69:931–6. [DOI] [PubMed] [Google Scholar]
- 299.Krasinski SD, Russell RM, Otradovec CL, Sadowski JA, Hartz SC, Jacob RA, McGandy RB. Relationship of vitamin A and vitamin E intake to fasting plasma retinol, retinol-binding protein, retinyl esters, carotene, alpha-tocopherol, and cholesterol among elderly people and young adults: increased plasma retinyl esters among vitamin A-supplement users. Am J Clin Nutr 1989;49:112–20. [DOI] [PubMed] [Google Scholar]
- 300.Weber FL, Mitchell GE, Powell DE, Reiser BJ, Banwell JG. Reversible hepatotoxicity associated with hepatic vitamin A accumulation in a protein-deficient patient. Gastroenterology 1982;82:118–23. [PubMed] [Google Scholar]
- 301.Hatoff DE, Gertler SL, Miyai K, Parker BA, Weiss JB. Hypervitaminosis A unmasked by acute viral hepatitis. Gastroenterology 1982;82:124–8. [PubMed] [Google Scholar]
- 302.Krasinski SD, Cohn JS, Russell RM, Schaefer EJ. Postprandial plasma vitamin A metabolism in humans: a reassessment of the use of plasma retinyl esters as markers for intestinally derived chylomicrons and their remnants. Metabolism 1990;39:357–65. [DOI] [PubMed] [Google Scholar]
- 303.Tanumihardjo SA, Furr HC, Amedée-Manesme O, Olson JA. Retinyl ester (vitamin A ester) and carotenoid composition in human liver. Int J Vitam Nutr Res 1990;60:307–13. [PubMed] [Google Scholar]
- 304.Newcomer C, Murphy S. Guideline for vitamin A amp D fortification of fluid milk. Bulletin 53. Keyport:The Dairy Practices Council; 2001.
- 305.Penniston KL, Tanumihardjo SA. Vitamin A in dietary supplements and fortified foods: too much of a good thing? J Am Diet Assoc 2003;103:1185–7. [DOI] [PubMed] [Google Scholar]
- 306.WHO. WHO guidelines on drawing blood: best practices in phlebotomy. Geneva (Switzerland): WHO; 2010. [PubMed] [Google Scholar]
- 307.Craft NE, Brown ED, Smith JC. Effects of storage and handling conditions on concentrations of individual carotenoids, retinol, and tocopherol in plasma. Clin Chem 1988;34:44–8. [PubMed] [Google Scholar]
- 308.Comstock GW, Alberg AJ, Helzlsouer KJ. Reported effects of long-term freezer storage on concentrations of retinol, beta-carotene, and alpha-tocopherol in serum or plasma summarized. Clin Chem 1993;39:1075–8. [PubMed] [Google Scholar]
- 309.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr 1979;32:2143–9. [DOI] [PubMed] [Google Scholar]
- 310.De Leenheer AP, Nelis HJ, Lambert WE, Bauwens RM. Chromatography of fat-soluble vitamins in clinical chemistry. J Chromatogr 1988;429:3–58. [DOI] [PubMed] [Google Scholar]
- 311.Elom AK, el Imane M, Kaoutar B, el Khalid K, el Asmaa H, Mehdi A, el Noureddine H, Hassan A. Comparison of a fluorometric assay kit with high-performance liquid chromatography for the assessment of serum retinol concentration. Afr Health Sci 2015;15:641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.National Institute of Standards and Technology. Development of reference methods and reference materials for clinical diagnostic markers. Washington (DC): National Institute of Standards and Technology;2009. [cited 2016 Jul 25]. Available from: http://www.nist.gov/mml/csd/organic/clindiagnosticmarkers.cfm.
- 313.CDC. Laboratory Quality Assurance and Standardization Programs. Vitamin A laboratory—external quality assurance [Internet] [cited 2015 Jul 7]. Available from: http://www.cdc.gov/labstandards/vitaleqa.html.
- 314.Tanumihardjo SA, Kurpad AV, Hunt JR. Research recommendations for applying vitamin A-labelled isotope dilution techniques to improve human vitamin A nutrition. Int J Vitam Nutr Res 2014;84(Suppl 1):52–9. [DOI] [PubMed] [Google Scholar]
- 315.Sun H. Membrane receptors and transporters involved in the function and transport of vitamin A and its derivatives. Biochim Biophys Acta 2012;1821:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ahmad SM, Haskell MJ, Raqib R, Stephensen CB. Men with low vitamin A stores respond adequately to primary yellow fever and secondary tetanus toxoid vaccination. J Nutr 2008;138:2276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]