Abstract
Background: Prolonged high intakes of dietary selenium have been shown to induce gestational diabetes in rats and hyperinsulinemia in pigs.
Objective: Two experiments were conducted to explore metabolic and molecular mechanisms for the diabetogenic potential of high dietary selenium intakes in pigs.
Methods: In Expt. 1, 16 Yorkshire-Landrace-Hampshire crossbred pigs (3 wk old, body weight = 7.5 ± 0.81 kg, 50% males and 50% females) were fed a corn-soybean meal basal diet supplemented with 0.3 or 1.0 mg Se/kg (as selenium-enriched yeast for 6 wk). In Expt. 2, 12 pigs of the same crossbreed (6 wk old, body weight = 16.0 ± 1.8 kg) were fed a similar basal diet supplemented with 0.3 or 3.0 mg Se/kg for 11 wk. Biochemical and gene and protein expression profiles of lipid and protein metabolism and selenoproteins in plasma, liver, muscle, and adipose tissues were analyzed.
Results: In Expt. 1, the 1-mg-Se/kg diet did not affect body weight or plasma concentrations of glucose and nonesterified fatty acids. In Expt. 2, the 3-mg-Se/kg diet, compared with the 0.3-mg-Se/kg diet, increased (P < 0.05) concentrations of plasma insulin (0.2 compared with 0.4 ng/mL), liver and adipose lipids (41% to 2.4-fold), and liver and muscle protein (10–14%). In liver, the 3-mg-Se/kg diet upregulated (P < 0.05) the expression, activity, or both of key factors related to gluconeogenesis [phosphoenolpyruvate carboxykinase (PEPCK); 13%], lipogenesis [sterol regulatory element binding protein 1 (SREBP1), acetyl-coenzyme A carboxylase (ACC), and fatty acid synthase (FASN); 46–90%], protein synthesis [insulin receptor (INSR), P70 ribosomal protein S6 kinase (P70), and phosphorylated ribosomal protein S6 (P-S6); 88–105%], energy metabolism [AMP-activated protein kinase (AMPK); up to 2.8-fold], and selenoprotein glutathione peroxidase 3 (GPX3; 1.4-fold) and suppressed (P < 0.05) mRNA levels of lipolysis gene cytochrome P450, family 7, subfamily A, polypeptide 1 (CYP7A1; 88%) and selenoprotein gene selenoprotein W1 (SEPW1; 46%). In muscle, the 3-mg-Se/kg diet exerted no effect on the lipid profiles but enhanced (P < 0.05) expression of P-S6 and mammalian target of rapamycin (mTOR; 42–176%; protein synthesis); selenoprotein P (SELP; 40-fold); and tumor suppressor protein 53 (P53) and peroxisome proliferator–activated receptor γ (PPARG; 52–58%; lipogenesis) and suppressed (P < 0.05) expression of INSR (59%; insulin signaling); selenoprotein S (SELS); deiodinases, iodothyronine, type I (DIO1); and thioredoxin reductase 1 (TXNRD1; 50%; selenoproteins); and ACC1 and FASN (35–51%; lipogenesis).
Conclusion: Our research showed novel roles, to our best knowledge, and mechanisms of high selenium intakes in regulating the metabolism of protein, along with that of lipid, in a tissue-specific fashion in pigs.
Keywords: AMPK, lipid, protein, Se, selenoprotein
Introduction
Our laboratory previously showed that the overproduction of glutathione peroxidase (GPX4) 1, a selenoprotein, induced type 2 diabetes–like phenotypes in mice at the age of 6 mo (1). Although dietary selenium restriction in mice ameliorated most of the phenotypes (2), prolonged feeding of a high-selenium diet (3 mg Se/kg) caused mild gestational diabetes in pregnant rats and insulin resistance in their offspring (3). Likewise, 2 pig studies (4, 5) showed the potential of high selenium intakes in causing hyperinsulinemia. Furthermore, several large human studies showed an alarming association between a high selenium intake and/or body selenium status and increased risk of hyperglycemia, hyperlipidemia, or type 2 diabetes (6–8). However, the molecular mechanism for the diabetogenic potential of a high selenium intake remains unclear.
Dietary selenium and/or GPX1 production (9) affected the functional expression of important proteins involved in gluconeogenesis [phosphoenolpyruvate carboxykinase (PEPCK)] and glycolysis [glucokinase (GK)] (10), insulin signaling [insulin receptor (INSR), insulin receptor substrate 1 (IRS1), and v-akt murine thymoma viral oncogene homolog 2 (AKT2)], and lipid metabolism (11) [sterol regulatory element binding protein (SREBP) 1 and SREBP2, acetyl-CoA carboxylase 1 (ACC1); cholesterol 7α-hydroxylase (CYP7A1), PPARG, fatty acid synthase (FASN), and tumor suppressor p53 (P53)] (12–17). Alterations of these factors were associated with expressions of 12 selenoproteins (1, 3, 5, 9, 18, 19). However, to the best of our knowledge, there was no information on effects of dietary selenium, in particular high selenium intake, on body protein metabolism, although protein synthesis was reported to negatively regulate insulin sensitivity (20, 21) and a high protein diet inhibited the development of type 2 diabetes (22). Mammalian target of rapamycin (mTOR), ribosomal protein S6 kinase (P70), ribosomal protein S6 (S6), factor 4E binding protein 1 (4EBP1), and eukaryotic translation initiation factor 4E (EIF4E) are key factors involved in the protein synthesis pathway (23–27). Furthermore, AMP-activated protein kinase (AMPK) plays a key role in regulating energy metabolism (28) and showed a negative association with selenium intake in rats (29). The expression or function of AMPK seems to be affected by superoxide (30, 31) or a high-fat diet (32). However, the role of AMPK in the high-selenium-intake–induced phenotype is unknown.
Pigs represent an excellent model to study human nutrition and medicine because of their great similarities in digestive systems (33), nutrient metabolism, and responses to high selenium intakes (4, 5). Furthermore, pigs are an important food-producing species worldwide, and selenium supplementation is required in diets for pigs. Therefore, 2 pig experiments were conducted, with the use of a control diet of 0.3 mg Se/kg, to determine the following: 1) whether a moderately high selenium intake (1.0 mg Se/kg diet) produced diabetogenic or hyperlipidemic effects similar to those of a 3.0-mg-Se/kg diet; 2) whether the 3.0-mg-Se/kg diet was able to affect protein metabolism and amino acid and FA profiles in liver, muscle, and adipose tissue; and 3) how the 3.0-mg-Se/kg diet exerted its metabolic effects in those tissues.
Methods
Animals, diets, and management.
Our animal protocols were approved by the Institutional Animal Care and Use Committee of Cornell University. All pigs used in both experiments were weanling crossbreeds (Yorkshire-Landrace-Hampshire) selected from the Cornell University Swine Farm, with half males and half females. Expt. 1 was conducted to determine if a moderately high dietary selenium intake affected plasma glucose and lipid concentrations. A total of 16 weanling pigs (3 wk old, body weight = 7.5 ± 0.81 kg) were divided into 2 groups (n = 8/group) and fed a corn-soybean meal basal diet (BD1; Supplemental Table 1) supplemented with 0.3 or 1.0 mg Se/kg (as selenium-enriched yeast; courtesy of ADM Alliance Nutrition) for 6 wk. Expt. 2 was conducted, on the basis of the results of Expt. 1, to determine if and how a high-selenium diet (3.0 mg Se/kg) altered lipid and protein metabolism and the underlying mechanism in liver, muscle, and adipose tissue of pigs. A total of 12 pigs (6 wk old, body weight = 16 ± 1.8 kg) were fed a similar corn-soybean meal basal diet (BD2; Supplemental Table 1) supplemented with 0.3 or 3.0 mg Se/kg (selenium supplemented as selenium-enriched yeast (n = 6/group) for 11 wk. Pigs in both experiments were allotted to treatment groups on the basis of body weight, litter, and sex and were housed in pens with a concrete floor in a temperature-controlled barn (22–25°C) with a light-dark cycle of 12:12 h. Pigs were given free access to feed and water, and orts and feed intakes were recorded daily. Individual body weights of pigs were recorded biweekly.
Plasma biochemical analyses.
Whole-blood samples of individual pigs were collected at week 0 and then biweekly after overnight food deprivation (8 h) to prepare plasma samples as previously described (3). Plasma concentrations of total TGs, total cholesterol (TC), nonesterified fatty acids (NEFAs), glucose, and insulin (at weeks 6 and 11) were analyzed as previously described (9).
Liver, muscle, and adipose biochemical analyses.
At the end of Expt. 2, all pigs were killed after overnight (8 h) food deprivation (5) to collect samples of liver, muscle (longissimus dorsi), and adipose tissue (retroperitoneal fat) (5). The samples were snap-frozen in liquid nitrogen and stored at −80°C until analyses. Total lipids were extracted from liver (50 mg) and adipose tissue (50 mg) for analyses of TG, TC, and NEFA concentrations (34). Total FAs were extracted from the liver (250 mg) and adipose tissue (50 mg) (35) for the subsequent analyses by using a GC system (HP 6890; Hewlett Packard). Protein concentrations in the liver and muscle were determined following a previously described method (36). Total amino acids were extracted from the liver (500 mg) and muscle (500 mg) (37), and the subsequent analyses of amino acid profiles were performed on an HPLC system (Nexera X2; Shimadzu).
Real-time qPCR, immunoblotting, and enzyme activity analyses.
Total mRNA was isolated from liver (20 mg) and muscle (20 mg), and the subsequent quantification was carried out by real-time qPCR (7900 HT; Applied Biosystems) of key genes involved in insulin signaling and lipid metabolism and selected selenoprotein genes as previously described (9). The primer sequences used for all of the assayed genes are shown in Supplemental Table 2. The 2−delta delta Ct (ddCt) method was used for the quantification with β-actin (ACTB) as a reference gene, and the relative abundance was normalized to the control (as “1”). The validity of ACTB as the reference was verified by its amplification plots and dissociation curves of all samples from the liver and muscle samples (Supplemental Figure 1). GPX, GK, and PEPCK activities were measured in the liver and/or muscle (38), and FASN activity was measured in the liver and muscle (39, 40). Western blot analyses of key factors in the pathways of protein synthesis, insulin signaling, energy metabolism, and selected selenoproteins were performed as previously described (9); and the relative density of the protein bands was quantified by using ImageJ software (NIH) and normalized to ACTB as the loading control. The relative density of the phosphorylated protein bands was quantified by using ImageJ software and normalized first to ACTB as the loading control and then to the relative density of their corresponding total protein bands. The primary antibodies used for the Western blot analyses are presented in Supplemental Table 3.
Statistical analyses.
Data were analyzed by using the general linear model procedure of SAS (SAS Institute). The main effects of dietary treatments on body weight and plasma biochemical measures were subjected to 1-factor ANOVA with time-repeated measurements (3). Data generated from qPCR, Western blot, and biochemical assays of tissue samples were analyzed by using SAS with Student’s t test. For all of the analyses, pooled SEs were listed and the significance level for differences was P < 0.05.
Results
Expt. 1.
The 1-mg-Se/kg diet exerted no significant effect on body weight or plasma concentrations of glucose and NEFAs at any of the time points. However, pigs fed 1.0 mg Se/kg had 23–28% lower (P < 0.05) plasma TG concentrations than did those fed 0.3 mg Se/kg. Pigs fed the high-selenium diet also had lower (P < 0.05) plasma TC concentrations than those fed the control diet at week 2 (Table 1).
TABLE 1.
Body weight and plasma glucose and lipid concentrations of pigs fed 0.3 or 1.0 mg Se/kg for 6 wk (Expt. 1)1
| Diet |
||||
| 0.3 mg Se/kg | 1.0 mg Se/kg | SE, mg Se/kg | P (time × Se) | |
| Body weight, kg | 0.7 | |||
| 0 wk | 7.23d | 7.24 | 0.43 | |
| 2 wk | 14.3c | 14.3 | 0.91 | |
| 4 wk | 22.8b | 23.3 | 1.74 | |
| 6 wk | 34.8a | 35.3 | 2.02 | |
| Plasma glucose, mg/dL | 0.1 | |||
| 0 wk | 134a | 189 | 16.8 | |
| 2 wk | 101b | 106 | 5.64 | |
| 4 wk | 87c | 93 | 3.3 | |
| 6 wk | 89c | 90 | 3.2 | |
| Plasma cholesterol, mg/dL | 0.3 | |||
| 0 wk | 84.5a | 79.8 | 3.49 | |
| 2 wk | 59.5c | 42.2* | 4.17 | |
| 4 wk | 65.3bc | 64.4 | 3.55 | |
| 6 wk | 70.1b | 70.2 | 3.62 | |
| Plasma TGs, mg/dL | 0.9 | |||
| 0 wk | 58.2a | 46.3 | 5.41 | |
| 2 wk | 58.7a | 42.2* | 4.11 | |
| 4 wk | 40.6b | 31.2* | 1.68 | |
| 6 wk | 42.8b | 31.1* | 2.13 | |
| Plasma nonesterified fatty acids, μmol/L | 0.8 | |||
| 0 wk | 137d | 175 | 23.0 | |
| 2 wk | 459c | 413 | 44.4 | |
| 4 wk | 533b | 512 | 74.6 | |
| 6 wk | 655a | 766 | 110 | |
Values are means, n = 6. Within a column, labeled means without a common superscript letter differ, P < 0.05. *Different from 0.3 mg Se/kg at that time, P < 0.05.
Expt. 2.
Pigs fed the high-selenium diet (3 mg Se/kg) had lower (P < 0.05) body weight at weeks 4 and 8 than pigs fed 0.3 mg Se/kg, but the difference between the 2 groups at week 11 was not significant (P = 0.098; Table 2). Thus, there was no difference (P = 0.30) in the overall average daily body weight gain (0.76 ± 0.042 compared with 0.76 ± 0.039 kg), average daily feed intake (1.9 ± 0.13 compared with 1.6 ± 0.12 kg), or gain-to-feed ratio (0.44 ± 0.021 compared with 0.47 ± 0.024) between the 2 groups. The high-selenium–fed pigs had a 1-fold higher (P < 0.05) plasma insulin concentration at the end of experiment (week 11) than pigs fed 0.3 mg Se/kg. Although pigs fed the high-selenium diet showed 23% lower (P < 0.05) plasma glucose concentrations and higher (P < 0.05) plasma NEFA concentrations than those fed 0.3 mg Se/kg at week 2, the high selenium supplementation produced no effect on either of the measures at other time points (midinterval data not shown) or on plasma TGs or TC at any time point.
TABLE 2.
Body weight and plasma biochemical indicators of pigs fed 0.3 or 3.0 mg Se/kg for 11 wk (Expt. 2)1
| Diet |
||||
| 0.3 mg Se/kg | 3.0 mg Se/kg | SE, mg Se/kg | P (time × Se) | |
| Body weight, kg | 0.1 | |||
| 0 wk | 16.0d | 15.8 | 0.518 | |
| 4 wk | 42.4c | 38.3* | 1.03 | |
| 8 wk | 68.9b | 61.7* | 1.62 | |
| 11 wk | 82.7a | 78.5 | 2.05 | |
| Plasma glucose, mg/dL | 0.003 | |||
| 2 wk | 123a | 95* | 4.97 | |
| 11 wk | 121b | 119 | 2.04 | |
| Plasma cholesterol, mg/dL | 0.2 | |||
| 2 wk | 77.0b | 78.5 | 2.18 | |
| 11 wk | 81.1a | 81.5 | 2.01 | |
| Plasma TGs, mg/dL | 0.7 | |||
| 2 wk | 46.1a | 38.5 | 3.00 | |
| 11 wk | 25.8b | 27.8 | 2.49 | |
| Plasma nonesterified fatty acids, μmol/L | 0.1 | |||
| 2 wk | 287a | 619* | 56.6 | |
| 11 wk | 195b | 245 | 34.2 | |
| Plasma insulin, ng/mL | 0.2 | |||
| 6 wk | 0.3 | 0.2 | 0.04 | |
| 11 wk | 0.2 | 0.4* | 0.03 | |
Values are means, n = 6. Within a column, labeled means without a common superscript letter differ, P < 0.05. *Different from 0.3 mg Se/kg at that time, P < 0.05. Only the data of the final time points, those with the diet effect and initial body weight of pigs, are shown in the table.
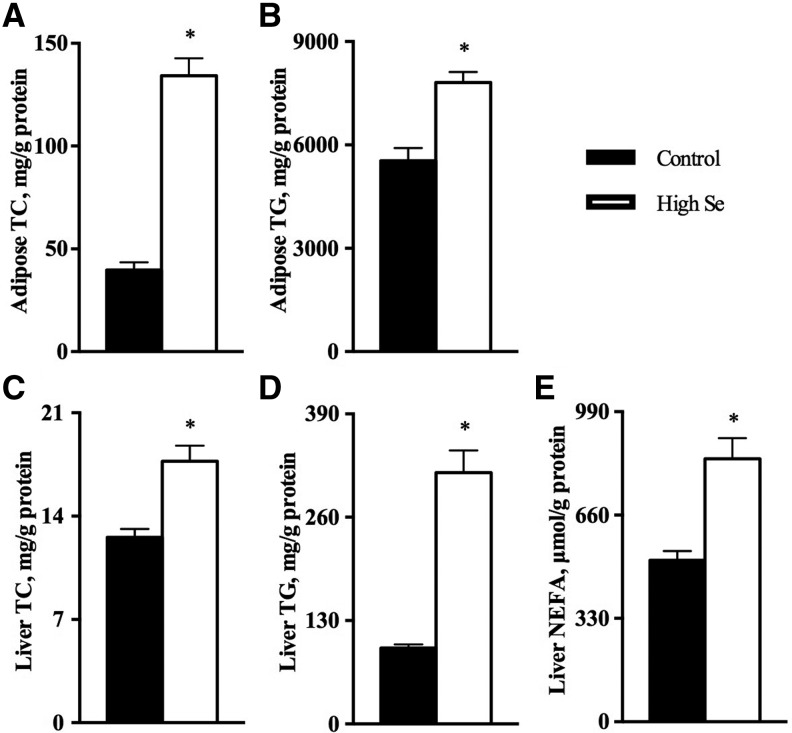
In contrast, TC and TG concentrations in the adipose tissue of pigs fed the high-selenium diet were 2.4-fold and 41% greater (P < 0.05), respectively, than those fed 0.3 mg Se/kg (Figure 1A, B), whereas NEFA concentrations in adipose tissue were unaffected by the high-selenium diet (Supplemental Figure 2A). Likewise, hepatic concentrations of TC, TGs, and NEFAs in pigs fed the high-selenium diet were 40%, 2.3-fold, and 63% greater (P < 0.05), respectively, than those in pigs fed 0.3 mg Se/kg ( ). However, the high-selenium supplementation exerted no effect on these measures of lipid profiles in muscle tissue (Supplemental Figure 2B–D). Compared with those fed 0.3 mg Se/kg, the high-selenium–fed pigs showed greater concentrations (P < 0.05) of myristic acid, palmitic acid, stearic acid, behenic acid, oleic acid, linoleic acid, and palmitoleic acid and lower concentrations (P < 0.05) of dihomo-γ-linolenic acid in liver and lower concentrations (P < 0.05) of stearic acid, oleic acid, α-linolenic acid, eicosatrienoic acid, and behenic acid in adipose tissue (Table 3, Supplemental Table 4). Total protein concentrations in the liver (10.1 ± 0.253 compared with 11.5 ± 0.253 g/g dry tissue) and muscle (12.3 ± 0.121 compared with 13.6 ± 0.237 g/g dry tissue) were 14% and 10% greater (P < 0.05), respectively, in pigs fed the high-selenium diet than those fed 0.3 mg Se/kg. The high-selenium diet increased (P < 0.05) concentrations of aspartic acid, glutamine, and alanine in muscle but not in liver (Supplemental Table 5).
FIGURE 1.
Adipose tissue concentrations of TC (A) and TGs (B) and liver concentrations of TC (C), TGs (D), and NEFAs (E) in pigs fed 0.3 or 3.0 mg Se/kg for 11 wk (Expt. 2). Values are means ± SEs, n = 6. *Different from control, P < 0.05. Control = 0.3 mg Se/kg; high Se = 3.0 mg Se/kg. NEFA, nonesterified fatty acid; TC, total cholesterol.
TABLE 3.
FA profiles in liver and adipose tissue of pigs fed 0.3 or 3.0 mg Se/kg for 11 wk (Expt. 2)1
| Diet |
|||
| 0.3 mg Se/kg | 3.0 mg Se/kg | SE, mg Se/kg | |
| Liver, mg/g tissue | |||
| Myristic acid (14:0) | 0.1b | 0.2a | 0.01 |
| Palmitic acid (16:0) | 4.5b | 6.9a | 0.67 |
| Palmitoleic acid (16:1) | 0.1b | 0.3a | 0.02 |
| Stearic acid (18:0) | 6.9b | 9.3a | 0.40 |
| Oleic acid (18:1n-9c) | 3.6b | 5.4a | 0.29 |
| Linoleic acid (18:2n-6c) | 3.9b | 4.9a | 0.13 |
| Dihomo-γ-linolenic acid (20:3n–6) | 0.2a | 0.0b | 0.01 |
| Behenic acid (22:0) | 3.7b | 4.7a | 0.21 |
| Adipose tissue, mg/g tissue | |||
| Stearic acid (18:0) | 29a | 21b | 1.9 |
| Oleic acid (18:1n–9c) | 56a | 42b | 3.1 |
| α-Linolenic acid (18:3n–3) | 1.0a | 0.7b | 0.049 |
| Eicosatrienoic acid (20:3n–3) | 0.6a | 0.0b | 0.012 |
| Behenic acid (22:0) | 0.2a | 0.0b | 0.011 |
Values are means, n = 6. Means in a row without a common superscript letter differ, P < 0.05.
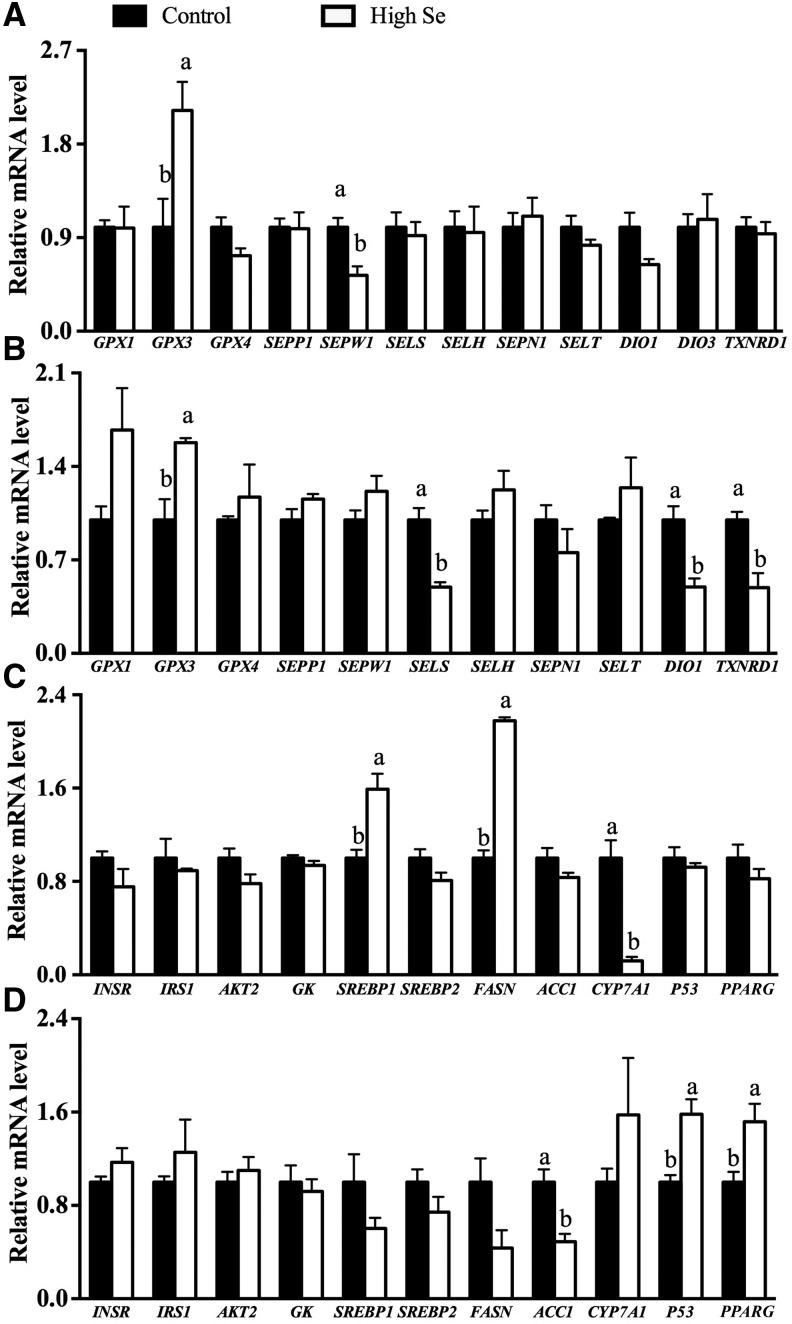
Compared with the 0.3-mg-Se/kg diet, the high-selenium diet enhanced (P < 0.05) mRNA levels of GPX3 in both the liver and muscle but decreased (P < 0.05) mRNA levels of selenoprotein W (SEPW1) in the liver and selenoprotein S (SELS), deiodinases, iodothyronine, type 1 (DIO1), and thioredoxin reductase 1 (TXNRD1) in the muscle (Figure 2A, B). The mRNA levels of the other 7 selenoprotein genes [GPX1, GPX4, selenoprotein P (SEPP1), selenoprotein H (SELH), selenoprotein N (SEPN1), selenoprotein T (SELT), and deiodinases, iodothyronine, type 3 (DIO3)] were not affected by the high-selenium diet. Compared with those fed the 0.3-mg-Se/kg diet, pigs fed the high-selenium diet showed 59% greater (P < 0.05) SREBP1 and 88% lower (P < 0.01) CYP7A1 mRNA levels in the liver (Figure 2C) as well as 42–48% greater (P < 0.05) peroxisome proliferator–activated receptor γ (PPARG) and P53 and 51% lower (P < 0.05) ACC1 mRNA levels in the muscle (Figure 2D). The high-selenium diet doubled (P < 0.05) the mRNA levels of FASN in the liver but resulted in a 57% decrease (P = 0.12) in the muscle compared with the 0.3-mg-Se/kg diet. The high-selenium diet showed no effect on mRNA levels of INSR, IRS1, AKT2, GK, or SREBP2 in either liver or muscle.
FIGURE 2.
Relative mRNA levels of selenoproteins in the liver (A) and muscle (B) and molecules of insulin signaling, lipid synthesis, and lipid hydrolysis pathways in the liver (C) and muscle (D) of pigs fed 0.3 or 3.0 mg Se/kg for 11 wk (Expt. 2). Values are means ± SEs, n = 6. Means without a common letter differ, P < 0.05. Control = 0.3 mg Se/kg; high Se = 3.0 mg Se/kg. ACC1, acetyl-CoA carboxylase 1; AKT2, v-akt murine thymoma viral oncogene homolog 2; CYP7A1, cytochrome P450, family 7, subfamily A, polypeptide 1; DIO1, deiodinases, iodothyronine, type I; DIO3, deiodinases, iodothyronine, type III; FASN, fatty acid synthase; GK, glucokinase; GPX, glutathione peroxidase; INSR, insulin receptor; IRS1, insulin receptor substrate 1; P53, tumor suppressor protein 53; PPARG, peroxisome proliferator–activated receptor γ; SELH, selenoprotein H; SELS, selenoprotein S; SELT, selenoprotein T; SEPN1, selenoprotein N; SEPP1, selenoprotein P; SEPW1, selenoprotein W; SREBP, sterol regulatory element binding protein; TXNRD1, thioredoxin reductase 1.
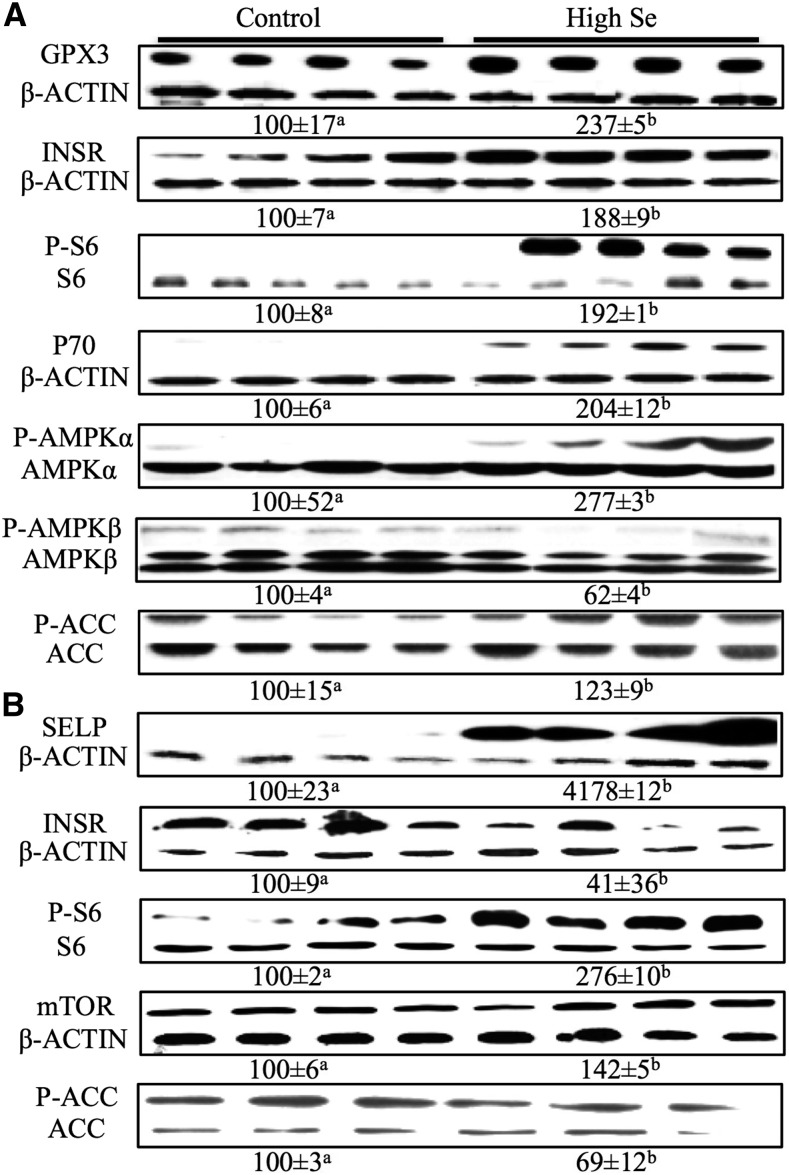
The high-selenium diet elevated (P < 0.05) GPX3 (1.4-fold) and INSR (88%) protein amounts but showed no effect on selenoprotein P (SELP) protein in the liver (Figure 3A, Supplemental Figure 3A). Meanwhile, the high-selenium diet resulted in 41-fold higher (P < 0.01) SELP, 59% lower (P < 0.05) INSR, and no change in GPX3 protein in the muscle (Figure 3B, Supplemental Figure 3B). Whereas total AKT protein in either liver or muscle was affected by the high-selenium diet (Supplemental Figure 3A, B), hepatic amounts of phosphor-AMPKα to total AMPKα protein were 2.8-fold greater (P < 0.05), whereas hepatic amounts of phosphor-AMPKβ to total AMPKβ were 38% lower (P < 0.05) in the high-selenium–fed pigs than in controls (Figure 3A). The high-selenium diet also enhanced (P < 0.05) hepatic phosphor-ACC to total ACC (23%) (Figure 3A) but decreased (P < 0.05) muscle phosphor-ACC to total ACC (31%) (Figure 3B). The high-selenium diet enhanced (P < 0.05) phosphor-S6 to S6 and total P70 in the liver (1-fold) (Figure 3A) and phosphor-S6 to S6 and mTOR protein in the muscle (42% to 1.8-fold) (Figure 3B).
FIGURE 3.
Relative protein concentrations of liver GPX3, INSR, P-S6, P70, P-AMPKα, AMPKα, P-AMPKβ, P-ACC, and ACC (A) and muscle SELP, INSR, P-S6, P-ACC, and mTOR (B) in pigs fed 0.3 or 3.0 mg Se/kg for 11 wk (Expt. 2). Values below the protein band were relative densities and are expressed as means ± SEs, n = 4–6. Means without a common letter differ, P < 0.05. Control = 0.3 mg Se/kg; high Se = 3.0 mg Se/kg. ACC, acetyl-CoA carboxylase; AMPKα, AMP-activated protein kinase α; AMPKβ, AMP-activated protein kinase β; GPX3, glutathione peroxidase 3; INSR, insulin receptor; mTOR, mammalian target of rapamycin; P70, P70 ribosomal protein S6 kinase; P-ACC, phosphorylated acetyl-CoA carboxylase; P-AMPKα, phosphorylated AMP-activated protein kinase α; P-AMPKβ, phosphorylated AMP-activated protein kinase β; P-S6, phosphorylated ribosomal protein S6; SELP, selenoprotein P; S6, ribosomal protein S6.
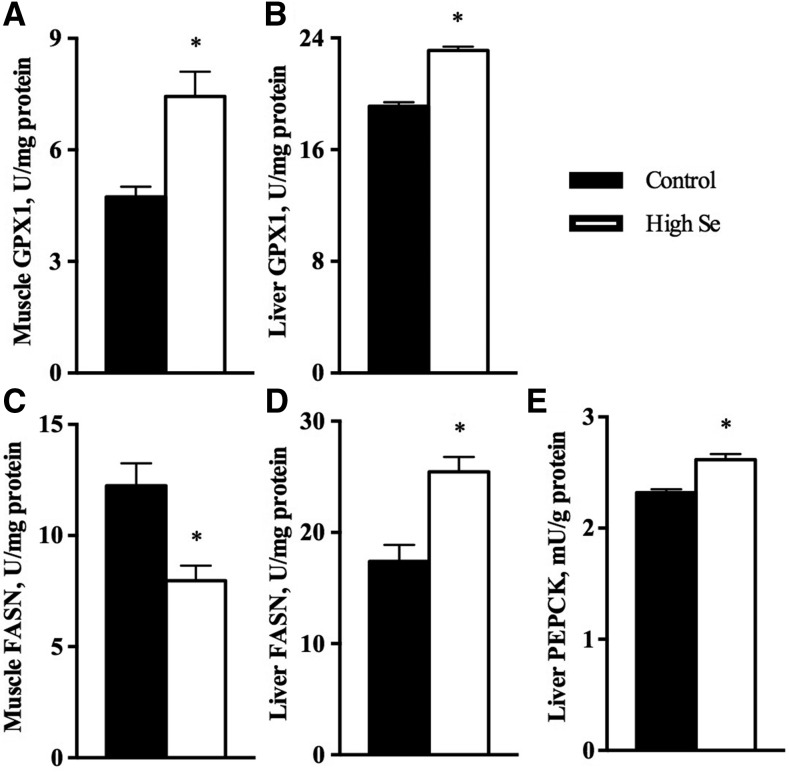
The high-selenium diet upregulated (P < 0.05) GPX activity in the liver and muscle by 21% and 57%, respectively (Figure 4A, B). The high-selenium diet increased (P < 0.05) hepatic FASN activity by 46% but decreased the enzyme activity (P < 0.05) in muscle by 34% (Figure 4C, D). The high-selenium diet increased (P < 0.05) hepatic PEPCK activity by 13% (Figure 4E). There was no significant effect of the high-selenium diet on activities of GK in liver (133 ± 13.2 compared with 162 ± 8.19 U/g protein) or of both GK (14 ± 1.9 compared with 14 ± 1.1 U/g protein) and PEPCK (14 ± 0.95 compared with 13 ± 0.87 U/g protein) in muscle.
FIGURE 4.
GPX activities in muscle (A) and liver (B), FASN activities in muscle (C) and liver (D), and PEPCK activities in liver (E) of pigs fed 0.3 or 3.0 mg Se/kg for 11 wk (Expt. 2). The GPX activity unit is defined as 1 μmol NAD(P)H used/min. The FASN activity unit is defined as 1 mmol NAD(P)H used/min. Values are means ± SEs, n = 6. *Different from control, P < 0.05. Control = 0.3 mg Se/kg; high Se = 3.0 mg Se/kg. FASN, fatty acid synthase; GPX, glutathione peroxidase; PEPCK, phosphoenolpyruvate carboxykinase.
Discussion
The present study showed 3 unique metabolic responses in growing pigs to high dietary selenium intakes. First, although plasma lipid profiles were not affected by either the 1- or 3-mg-Se/kg diet, the 3-mg-Se/kg (high-selenium) diet elevated TGs, TC, and NEFAs in liver and/or adipose tissue up to 3-fold compared with control pigs. In contrast, there was no such effect of the high-selenium diet on muscle lipid profiles. Second, the high-selenium diet enhanced concentrations of 3 SFAs and 5 unsaturated FAs in the liver but decreased concentrations of 5 FAs, including 3 unsaturated FAs in muscle. Third, the high-selenium diet elevated total protein concentrations in both liver and muscle and concentrations of aspartic acid, glutamine, and alanine in muscle. It appears that the high-selenium diet altered lipid and protein metabolism in a tissue-specific fashion. The lack of a consistent effect of the high-selenium diets on plasma lipid profiles indicates that measuring these changes only in plasma without examining internal organs may not be a reliable method to assess the true metabolic effects of high dietary selenium intakes (41). However, several human studies showed a positive association between serum/plasma selenium concentrations and lipid concentrations (42–44). The high-selenium diet did not elevate fasting plasma concentrations of glucose at any time point but increased insulin at week 11 in Expt. 2. Although we measured plasma insulin at only 2 time points (weeks 6 and 11) and were unable to perform insulin tolerance tests in the growing pigs, the high-selenium-intake–induced hyperinsulinemia appeared only at the late stage of the feeding, which was consistent with the findings from 2 previous studies (4, 5) and a downregulation of INSR in the muscle. Although pigs fed the high-selenium diet had lower body weight than those fed the control diet at weeks 4 and 8, the difference was not significant (P = 0.10) at week 11 in Expt. 2. The lack of a striking overt phenotype in response to the high-selenium intake might reflect a progressive adaption of the body to the metabolic effect of selenium supranutrition, and our observed biochemical changes were early events instead of endpoints. Therefore, a longer feeding period may be needed to define the full effect of a high-selenium intake on body nutrient metabolism, including activity in pigs.
The high-selenium diet–induced elevations of hepatic TG, TC, and NEFA concentrations were associated with upregulation of gene expression of SREBP1 and FASN (9), protein production of total ACC, and activities of FASN and PEPCK, along with a downregulation of CYP7A1 mRNA. Because the liver is a main organ of lipogenesis in pigs, an elevated functional expression of SREBP1 and FASN could stimulate the pathway (45). The enhanced PEPCK activity, as the rate-limiting step of gluconeogenesis (46, 47), might help generate glucose as the carbon source for FA synthesis. The increased total ACC should also support lipogenesis, although the increased phosphor-ACC might inhibit ACC complex activity (48). Because the CYP7A1 enzyme is involved in the cholesterol hydrolysis pathway (49), the decrease of 90% in its mRNA level by the high-selenium diet might contribute to the accumulation of hepatic TC. It is intriguing to find effects of a high-selenium diet on gene expression or on the activities of FASN and ACC in the muscle that are opposite to those in the liver because the muscle lipid profile was not affected by the high-selenium diet. Although adipose tissue showed lipid accumulation similar to that in the liver in response to the high-selenium diet, we did not determine whether that accumulation was a direct effect of the high-selenium diet in the adipose tissue or a secondary response to the accumulation in the liver (as the main organ for storing lipids) (50).
Likewise, the opposite effects of the high-selenium diet on FA profiles between the liver (increase) and adipose tissue (decrease) remain intriguing. Notably, 4 SFAs [14:0 (myristic acid), 16:0 (palmitic acid), 18:0 (stearic acid), and 22:0 (behenic acid)] and 2 MUFAs [16:1 (palmitoleic acid), 18:1n–9c (oleic acid)] were elevated by the high selenium intake. A previous report showed a positive correlation between SFAs and obesity (51), whereas MUFAs could reduce TGs with high glucose and low fat intakes (52). However, previous research has suggested that long-chain MUFAs actually contributed to the development of obesity (53). Seemingly, the SFA elongation pathway in the liver of pigs was upregulated by the high selenium intake. In contrast, 2 PUFAs (18:3n–3 and 20:3n–3) in adipose tissue were greatly suppressed by the high selenium intake. Because PUFAs may help induce FA β-oxidation (54), the decreased PUFAs in the adipose tissue, combined with the increased SFAs and MUFAs in the liver, in pigs fed the high-selenium diet might also suppress lipolysis, contributing to the lipid accumulation in these tissues.
The high-selenium diet led to increases of 10–14% in concentrations of liver and muscle proteins, which was associated with stimulated signaling for protein synthesis of mTOR, P70, and phosphor-S6 (55). Although it is unclear to us how 3 specific amino acids were elevated in the muscle by the high selenium intake, our results showed a novel function of dietary selenium in regulating metabolism of not only glucose (56) and FAs (9) but also of amino acids. This finding implies a common role of dietary selenium in the overall fuel nutrient metabolism and is reinforced by the high-selenium diet–induced increases in AMPKα and phosphor-AMPKα (activation of the kinase) and decreases in phosphor-AMPKβ (inactivation of the kinase) in the liver (48). Because AMPK is a key upstream regulator of PEPCK, SREBP1, p53, ACC, PPARG, CYP7A1, and mTOR (57–59), the stimulation of this enzyme could help explain the accelerated lipogenesis and protein synthesis in the liver. However, AMPK was not affected by the high-selenium diet in muscle. It is also difficult to understand the mechanism and implication of high dietary selenium intake in increasing hepatic INSR but decreasing the protein in the muscle. Because muscle represents the largest insulin-responsive tissue in the body (60), downregulation of INSR and upregulation of the mTOR-S6 pathway (49) might impair insulin signaling, contributing to the hyperinsulinemia that is an early event of insulin resistance. Similar to the responses of several FFAs in the liver and adipose tissue to the high selenium intake, the physiologic relevance or molecular mechanism for the rather minor elevations of 3 amino acids in the muscle by the 3-mg-Se/kg diet remains unclear.
Our fundamental interest in the present study was to elucidate if the metabolic effects of the high dietary selenium intake were ultimately mediated by specific selenoproteins. As shown in our previous studies (3, 18), the high-selenium diet resulted in a moderate (20–50%) increase in GPX activities in both liver and muscle. Overproduction of GPX activity by Gpx1 overexpression in mice led to type 2 diabetes–like phenotypes, including hyperlipidemia and fatty liver (1, 9, 19), and the selenium deficiency partially rescued those disorders (9). The high-selenium diet induced gene expression of GPX3 in both liver and muscle and elevated the GPX3 protein in the liver. Although it is unclear why the high-selenium diet induced the gene expression but not the protein production of GPX3 in the muscle, an increase in GPX3 has been shown to be involved in the development of type 2 diabetes (61). Another very striking tissue-specific response was the 41-fold increase in SELP in the muscle, but no change in the liver, with the high-selenium diet. Because previous human and animal studies have suggested that SELP acts as a “hepatokine” and a negative regulator of insulin signaling (62–65), its substantial increase in the muscle might also contribute to the development of insulin resistance and hyperinsulinemia. In addition, the high-selenium diet downregulated the gene expression of SEPW1 in the liver and of SELS, DIO1, and TRXND1 in muscle. In one of our previous pig studies (5), gene expressions of 8 selenoproteins in 6 tissues were decreased by both dietary selenium deficiency and excess (3 mg Se/kg). The regulatory mechanism and the physiologic implications for these types of paradoxical responses remain a question for further research.
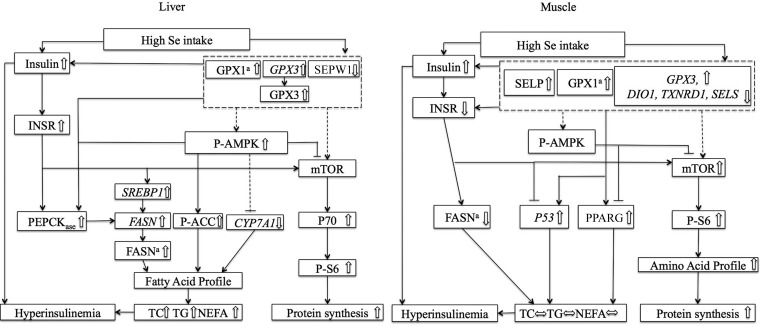
In summary, our study shows a new role for selenium in regulating protein and lipid metabolism through the activation of AMPK and the differential responses of selenoproteins between different tissues. As shown in Figure 5, the induced hepatic lipid accumulation by a high selenium intake was associated with stimulation of lipogenesis and gluconeogenesis and the suppression of lipolysis, along with an increase in 4 SFAs and 2 MUFAs in the liver and a decrease in 2 PUFAs in adipose tissue. The high-selenium diet–induced slight increases in liver and muscle protein concentrations were associated with an elevated protein synthesis pathway and 3 amino acids in the muscle. The unique activation of hepatic AMPK reinforced the role of a high selenium intake in regulating overall nutrient metabolism. The metabolic differential effects of a high dietary selenium intake in different tissues might be mediated by specific selenoproteins. The stimulated hepatic GPX3 might be involved in regulating hepatic lipid deposition and the substantially elevated SELP in muscle might contribute to the development of insulin resistance and hyperinsulinemia.
FIGURE 5.
Scheme of postulated regulatory pathways and mechanisms for the effects of feeding a high-selenium diet on lipid and protein metabolism in tissues of pigs. The solid lines represent data-supported pathways, whereas the dashed lines indicate potential, unapproved pathways. CYP7A1, cytochrome P450, family 7, subfamily A, polypeptide 1; DIO1, deiodinases, iodothyronine, type I; FASN, fatty acid synthase; GPX, glutathione peroxidase; INSR, insulin receptor; mTOR, mammalian target of rapamycin; NEFA, nonesterified fatty acid; P53, tumor suppressor protein 53; P70, P70 ribosomal protein S6 kinase; P-ACC, phosphorylated acetyl-CoA carboxylase; P-AMPK, phosphorylated AMP-activated protein kinase; P-S6, phosphorylated ribosomal protein S6; PEPCKase, phosphoenolpyruvate carboxykinase activity; PPARG, peroxisome proliferator–activated receptor γ; SELP, selenoprotein P; SELS, selenoprotein S; SEPW1, selenoprotein W; SREBP1, sterol regulatory element binding protein 1; TC, total cholesterol; TXNRD1, thioredoxin reductase 1.
Acknowledgments
XGL designed the research and had primary responsibility for the final content; ZZ, MB, JK, KLL, CM, and XGL conducted the experiments and analyzed the data; and ZZ, KLL, and XGL wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ACC, acetyl-CoA carboxylase; ACTB, β-actin; AKT2, v-akt murine thymoma viral oncogene homolog 2; AMPK, AMP-activated protein kinase; CYP7A1, cytochrome P450, family 7, subfamily A, polypeptide 1; DIO1, deiodinases, iodothyronine, type I; DIO3, deiodinases, iodothyronine, type III; EIF4E, eukaryotic initiation factor 4E; FASN, fatty acid synthase; GK, glucokinase; GPX, glutathione peroxidase; INSR, insulin receptor; IRS1, insulin receptor substrate 1; mTOR, mammalian target of rapamycin; NEFA, nonesterified fatty acid; PEPCK, phosphoenolpyruvate carboxykinase; PPARG, peroxisome proliferator–activated receptor γ P-S6, phosphorylated ribosomal protein S6; P53, tumor suppressor protein 53; P70, P70 ribosomal protein S6 kinase; S6, ribosomal protein S6; SELH, selenoprotein H; SELS, selenoprotein S; SELT, selenoprotein T; SEPN1, selenoprotein N; SEPP1 (SELP), selenoprotein P; SEPW1, selenoprotein W; SREBP, sterol regulatory element binding protein; TC, total cholesterol; TXNRD1, thioredoxin reductase 1.
References
- 1.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA 2004;101:8852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia 2008;51:1515–24. [DOI] [PubMed] [Google Scholar]
- 3.Zeng MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, Huang JQ, Sun LH, Tang JY, Xia XJ, et al. . A high-selenium diet induces insulin resistance in gestating rats and their offspring. Free Radic Biol Med 2012;52:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto A, Juniper DT, Sanil M, Morgan L, Clark L, Sies H, Rayman MP, Steinbrenner H. Supranutritional selenium induces alterations in molecular targets related to energy metabolism in skeletal muscle and visceral adipose tissue of pigs. J Inorg Biochem 2012;114:47–54. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Zhao H, Zhang Q, Tang J, Li K, Xia XJ, Wang KN, Li K, Lei XG. Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J Nutr 2012;142:1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care 2007;30:829–34. [DOI] [PubMed] [Google Scholar]
- 7.Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and diabetes in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect 2009;117:1409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E, Laclaustra M, Muti P, Berrino F, Krogh V. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health 2010;10:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan X, Pepper MP, Vatamaniuk MZ, Roneker CA, Li L, Lei XG. Dietary selenium deficiency partially rescues type 2 diabetes-like phenotypes of glutathione peroxidase-1-overexpressing male mice. J Nutr 2012;142:1975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan D, Zhang D, Wu J, Chen C, Xu Z, Yang H, Zhou P. Antidiabetic, antihyperlipidemic and antioxidant activities of a novel proteoglycan from ganoderma lucidum fruiting bodies on db/db mice and the possible mechanism. PLoS One 2013;8:e68332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nocito L, Kleckner AS, Yoo EJ, Jones Iv AR, Liesa M, Corkey BE. The extracellular redox state modulates mitochondrial function, gluconeogenesis, and glycogen synthesis in murine hepatocytes. PLoS One 2015;10:e0122818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimano H. Sterol regulatory element-binding protein family as global regulators of lipid synthetic genes in energy metabolism. Vitam Horm 2002;65:167–94. [DOI] [PubMed] [Google Scholar]
- 13.Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci USA 2006;103:8552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulauskis JD, Sul HS. Cloning and expression of mouse fatty acid synthase and other specific mRNAs: developmental and hormonal regulation in 3T3–L1 cells. J Biol Chem 1988;263:7049–54. [PubMed] [Google Scholar]
- 15.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA 2005;102:6207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yahagi N, Shimano H, Matsuzaka T, Sekiya M, Najima Y, Okazaki S, Okazaki H, Tamura Y, Iizuka Y, Inoue N, et al. . p53 Involvement in the pathogenesis of fatty liver disease. J Biol Chem 2004;279:20571–5. [DOI] [PubMed] [Google Scholar]
- 17.Chen Q, Wang E, Ma L, Zhai P. Dietary resveratrol increases the expression of hepatic 7alpha-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis 2012;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou JC, Zhao H, Li JG, Xia XJ, Wang KN, Zhang YJ, Liu Y, Zhao Y, Lei XG. Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J Nutr 2009;139:1061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepper MP, Vatamaniuk MZ, Yan X, Roneker CA, Lei XG. Impacts of dietary selenium deficiency on metabolic phenotypes of diet-restricted GPX1-overexpressing mice. Antioxid Redox Signal 2011;14:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SY, Baik KH, Baek KH, Chah KH, Kim KA, Moon G, Jung E, Kim ST, Shim JH, Greenblatt MB, et al. . S6K1 negatively regulates TAK1 activity in the toll-like receptor signaling pathway. Mol Cell Biol 2014;34:510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Völkers M, Doroudgar S, Nguyen N, Konstandin MH, Quijada P, Din S, Ornelas L, Thuerauf DJ, Gude N, Friedrich K, et al. . PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol Med 2014;6:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gannon MC, Nuttall FQ. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 2004;53:2375–82. [DOI] [PubMed] [Google Scholar]
- 23.Laufenberg LJ, Kazi AA, Lang CH. Salutary effect of aurintricarboxylic acid on endotoxin- and sepsis-induced changes in muscle protein synthesis and inflammation. Shock 2014;41:420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner AL, Urschel KL, Betancourt A, Adams AA, Horohov DW. Effects of advanced age on whole-body protein synthesis and skeletal muscle mechanistic target of rapamycin signaling in horses. Am J Vet Res 2013;74:1433–42. [DOI] [PubMed] [Google Scholar]
- 25.Urschel KL, Escobar J, McCutcheon LJ, Geor RJ. Insulin infusion stimulates whole-body protein synthesis and activates the upstream and downstream effectors of mechanistic target of rapamycin signaling in the gluteus medius muscle of mature horses. Domest Anim Endocrinol 2014;47:92–100. [DOI] [PubMed] [Google Scholar]
- 26.Appuhamy JA, Nayananjalie WA, England EM, Gerrard DE, Akers RM, Hanigan MD. Effects of AMP-activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. J Dairy Sci 2014;97:419–29. [DOI] [PubMed] [Google Scholar]
- 27.Issur M, Bougie I, Despins S, Bisaillon M. Enzymatic synthesis of RNAs capped with nucleotide analogues reveals the molecular basis for substrate selectivity of RNA capping enzyme: impacts on RNA metabolism. PLoS One 2013;8:e75310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg GR. AMPK and the endocrine control of energy metabolism. Mol Cell Endocrinol 2013;366:125–6. [DOI] [PubMed] [Google Scholar]
- 29.He S, Guo X, Tan W, Su X, Li J, Pan W, Qiu H. Effect of selenium deficiency on phosphorylation of the AMPK pathway in rats. Biol Trace Elem Res 2016;169;254–60. [DOI] [PubMed] [Google Scholar]
- 30.Domitrović R, Jakovac H, Romic Z, Rahelic D, Tadic Z. Antifibrotic activity of Taraxacum officinale root in carbon tetrachloride-induced liver damage in mice. J Ethnopharmacol 2010;130:569–77. [DOI] [PubMed] [Google Scholar]
- 31.Davaatseren M, Hur HJ, Yang HJ, Hwang JT, Park JH, Kim HJ, Kim MS, Kim MJ, Kwon DY, Sung MJ. Dandelion leaf extract protects against liver injury induced by methionine- and choline-deficient diet in mice. J Med Food 2013;16:26–33. [DOI] [PubMed] [Google Scholar]
- 32.Lindholm CR, Ertel RL, Bauwens JD, Schmuck EG, Mulligan JD, Saupe KW. A high-fat diet decreases AMPK activity in multiple tissues in the absence of hyperglycemia or systemic inflammation in rats. J Physiol Biochem 2013;69:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller ER, Ullrey DE. The pig as a model for human-nutrition. Annu Rev Nutr 1987;7:361–82. [DOI] [PubMed] [Google Scholar]
- 34.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 35.Fritsche KL, Johnston PV. Effect of dietary alpha-linolenic acid on growth, metastasis, fatty acid profile and prostaglandin production of two murine mammary adenocarcinomas. J Nutr 1990;120:1601–9. [DOI] [PubMed] [Google Scholar]
- 36.Barber BJ, Schultz TJ, Randlett DL. Comparative analysis of protein content in rat mesenteric tissue, peritoneal fluid, and plasma. Am J Physiol 1990;258:G714–8. [DOI] [PubMed] [Google Scholar]
- 37.Goel A, Eckhardt TH, Puri P, de Jong A, Branco Dos Santos F, Giera M, Fusetti F, de Vos WM, Kok J, Poolman B, et al. . Protein costs do not explain evolution of metabolic strategies and regulation of ribosomal content: does protein investment explain an anaerobic bacterial Crabtree effect? Mol Microbiol 2015;97:77–92. [DOI] [PubMed] [Google Scholar]
- 38.Lei XG, Evenson JK, Thompson KM, Sunde RA. Glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase are differentially regulated in rats by dietary selenium. J Nutr 1995;125:1438–46. [DOI] [PubMed] [Google Scholar]
- 39.Hillyard LA, Lin CY, Abraham S. Lipogenic enzyme activities in primary cultures of adult mouse hepatocytes. Lipids 1988;23:242–7. [DOI] [PubMed] [Google Scholar]
- 40.Martin DB, Horning MG, Vagelos PR. Fatty acid synthesis in adipose tissue. I. Purification and properties of a long chain fatty acid-synthesizing system. J Biol Chem 1961;236:663–8. [PubMed] [Google Scholar]
- 41.Jonkers RA, van Loon LJ, Nicolay K, Prompers JJ. In vivo postprandial lipid partitioning in liver and skeletal muscle in prediabetic and diabetic rats. Diabetologia 2013;56:618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Huang K, Lei XG. Selenium and diabetes—evidence from animal studies. Free Radic Biol Med 2013;65:1548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laclaustra M, Stranges S, Navas-Acien A, Ordovas JM, Guallar E. Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis 2010;210:643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bleys J, Navas-Acien A, Stranges S, Menke A, Miller ER III, Guallar E. Serum selenium and serum lipids in US adults. Am J Clin Nutr 2008;88:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gauger KJ, Bassa LM, Henchey EM, Wyman J, Bentley B, Brown M, Shimono A, Schneider SS. Mice deficient in Sfrp1 exhibit increased adiposity, dysregulated glucose metabolism, and enhanced macrophage infiltration. PLoS One 2013;8:e78320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Granner D, Pilkis S. The genes of hepatic glucose metabolism. J Biol Chem 1990;265:10173–6. [PubMed] [Google Scholar]
- 47.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 1996;45:223–41. [DOI] [PubMed] [Google Scholar]
- 48.Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol 2002;92:2475–82. [DOI] [PubMed] [Google Scholar]
- 49.Chow EC, Magomedova L, Quach HP, Patel R, Durk MR, Fan J, Maeng HJ, Irondi K, Anakk S, Moore DD, et al. . Vitamin D receptor activation down-regulates the small heterodimer partner and increases CYP7A1 to lower cholesterol. Gastroenterology 2014;146:1048–59. [DOI] [PubMed] [Google Scholar]
- 50.Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab 2012;15:805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryzhenkov VE. [Features of the effect of saturated and unsaturated fatty acids on lipid metabolism, lipoproteins, and development of ischemic heart disease.] Vopr Pitan 2002;71:40–5 (in Russian). [PubMed]
- 52.Bos MB, de Vries JH, Feskens EJ, van Dijk SJ, Hoelen DW, Siebelink E, Heijligenberg R, de Groot LC. Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr Metab Cardiovasc Dis 2010;20:591–8. [DOI] [PubMed] [Google Scholar]
- 53.van Dam RM, Stampfer M, Willett WC, Hu FB, Rimm EB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–24. [DOI] [PubMed] [Google Scholar]
- 54.Bargut TC, Frantz ED, Mandarim-de-Lacerda CA, Aguila MB. Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids 2014;49:431–44. [DOI] [PubMed] [Google Scholar]
- 55.Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 1999;253:100–9. [DOI] [PubMed] [Google Scholar]
- 56.Steinbrenner H, Speckmann B, Pinto A, Sies H. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr 2011;48:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis CA, Griffiths B, Santos CR, Pende M, Schulze A. Regulation of the SREBP transcription factors by mTORC1. Biochem Soc Trans 2011;39:495–9. [DOI] [PubMed] [Google Scholar]
- 58.Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene 2002;21:1299–303. [DOI] [PubMed] [Google Scholar]
- 59.Ono H, Shimano H, Katagiri H, Yahagi N, Sakoda H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Viana AY, et al. . Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes 2003;52:2905–13. [DOI] [PubMed] [Google Scholar]
- 60.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009;32(Suppl 2):S157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mutakin MA, Wijaya A, Kobayashi K, Yamazaki C, Kameo S, Nakazawa M, Koyama H. Association between selenium nutritional status and metabolic risk factors in men with visceral obesity. J Trace Elem Med Biol 2013;27:112–6. [DOI] [PubMed] [Google Scholar]
- 62.Hellwege JN, Palmer ND, Ziegler JT, Langefeld CD, Lorenzo C, Norris JM, Takamura T, Bowden DW. Genetic variants in selenoprotein P plasma 1 gene (SEPP1) are associated with fasting insulin and first phase insulin response in Hispanics. Gene 2014;534:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, et al. . A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab 2010;12:483–95. [DOI] [PubMed] [Google Scholar]
- 64.Steinbrenner H. Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic Biol Med 2013;65:1538–47. [DOI] [PubMed] [Google Scholar]
- 65.Rayman MP, Stranges S. Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radic Biol Med 2013;65:1557–64. [DOI] [PubMed] [Google Scholar]