Abstract
Background: The Power of Food Scale (PFS) seeks to identify individuals who experience high appetitive drive in response to food cues, which is a construct termed “hedonic hunger.”
Objective: The purpose of this study was to assess cross-sectional correlates and predictive power of PFS scores to probe the construct of hedonic hunger.
Methods: Separate data from 3 studies (study 1, n = 44; study 2, n = 398; study 3, n = 100) were used to evaluate the construct of hedonic hunger. We examined the correlations between the PFS and neural responsivity during intake and anticipated intake of palatable foods, behavioral food reinforcement, perceptual hedonic ratings of food images, and change in body mass index (BMI) and binge eating over time.
Results: Hedonic hunger was strongly related to bilateral brain response in regions implicated in oral somatosensory processing during cue-elicited anticipation of food intake (study 1; right postcentral gyrus: r = 0.67, P < 0.001; left postcentral gyrus: r = 0.64, P < 0.001), and was correlated with behavioral food reinforcement (study 2; r = 0.31, P = 0.03) and perceptual hedonic ratings (study 3; r = 0.24, P = 0.02). Hedonic hunger was not associated with baseline BMI (studies 1–3: P = 0.14, 0.21, and 0.37, respectively) or change in BMI over the 2-y follow-up (studies 1 and 2: P = 0.14 and 0.37, respectively) but was significantly correlated with baseline binge eating in 2 samples (study 1: r = 0.58, P = 0.001; study 2: r = 0.31, P = 0.02; and study 3: P = 0.02).
Conclusions: Hedonic hunger was not predictive of weight regulation. However, individuals who report high hedonic hunger are likely to show increased neural and perceptual responses to cues of palatable foods, increased motivation to consume such foods, and a greater likelihood of current binge eating.
Keywords: Power of Food Scale, binge eating, fMRI, obesity, food reinforcement, food reward
Introduction
The dramatic rise in obesity seen in the United States and in other industrialized nations over the past 40 y has been frequently credited to increases in the availability of highly palatable, energy-dense foods and food cues that encourage increased consumption (1). Despite the ubiquity of eating-related cues, approximately one-third of Americans are able to maintain a healthy body weight (1), suggesting that individuals are differentially susceptible to cues that encourage excess food consumption. An individual’s tendency to experience appetitive thoughts, feelings, and urges about food in response to palatable food cues has been termed “hedonic hunger” (2). The Power of Food Scale (PFS)5 was developed to measure individual differences in hedonic hunger (3). Although other measures, such as the external eating subscale of the Dutch Eating Behavior Questionnaire and the Food Craving Inventory, assess individual differences in drive to consume palatable food (4), the PFS was designed specifically to assess susceptibility to environmental food cues, and compared with other external eating measures, explains unique variations in measures of aberrant eating behavior (3, 5).
The PFS is shown to predict a number of behavioral and brain responses to food cues. Studies that use neuroimaging tools showed that high PFS scores relate to increased neural response in the insula, a region thought to house the primary taste cortex (6). In addition, studies showed that PFS scores positively relate to binge eating behavior in healthy and eating-disordered samples (7, 8). Interestingly, whereas models of obesity suggest that increased susceptibility to environmental food cues leads to increased consumption of palatable foods, studies reporting that PFS scores do predict ad libitum consumption of a palatable food in the absence of hunger are inconsistent (9, 10). Furthermore, reports present mixed results comparing PFS and current weight status (3, 5, 11), and the scale’s ability to predict future weight change has not been examined. Together, these data suggest that hedonic hunger may not be directly related to food consumption, as theorized. In addition, there is a lack of evidence that shows a connection between PFS scores and food reward, because, theoretically, increased consummatory motivation is congruent with increased food reward and valuation. In light of these inconsistent reports, further characterization of hedonic hunger, the PFS, and associated neurobehavioral responses is warranted.
Thus, in this report, we provide secondary analyses from 3 studies (12–14) that provide 3 approaches to examine correlates of hedonic hunger as measured by the PFS. This investigation used functional neuroimaging to provide an objective measurement of neural responses to palatable food intake and cue-elicited anticipation of food intake from study 1 (13), behavioral assessment of food reinforcement with the use of a progressive reinforcement button-pressing task from study 2 (12), perceptual hedonic food ratings with the use of visual analog scales from study 3 (14), and longitudinal changes in measured body mass and binge eating collected in all 3 studies. Together, the 3 studies used different, unique measures to give a more coherent characterization of hedonic hunger and the PFS. Given the theoretical aim of the PFS, we hypothesized that PFS scores (i.e., greater hedonic hunger) would be positively correlated with neural responsivity in reward-, gustatory-, and somatosensory-related brain regions during intake and anticipated intake of palatable food, greater behavioral food reinforcement, and higher perceptual hedonic food ratings. Moreover, we hypothesized that hedonic hunger would be associated with, and predict increases in, body mass and binge eating over time. We tested the correlation between hedonic hunger and brain activity with the use of data from study 1. Data from study 2 were used to examine the correlation between hedonic hunger and food reinforcement, and study 3 was used to test the correlation between hedonic hunger and food ratings. The relation between hedonic hunger and weight change and binge eating was assessed by using data from studies 1 and 2.
Methods
Participants
Study 1 participants.
Forty-four young women (mean ± SD age: 20.8 ± 1.3 y) were recruited from a large college campus in Oregon to participate in a weight-gain-prevention intervention study. Two-thirds of participants were in the normal-weight range [BMI (in kg/m2): 23.8 ± 2.9]. Seventy-nine percent of participants reported being white, 8% Asian/Pacific Islander, 8% multiethnic, 2% Native American, and 2% Hispanic. Individuals who reported binge eating or compensatory behaviors in the previous 3 mo, use of psychotropic medications or illicit drugs, head injury with a loss of consciousness, or current Axis I psychiatric disorder were excluded. The weight-control intervention had no effect on the measures reported and was included as a covariate in the analyses.
Study 2 participants.
Participants were 398 college freshman women (mean ± SD age: 18.4 ± 0.6 y) recruited from a large state university to participate in a study evaluating body acceptance interventions. Participants had a mean BMI of 23.8 ± 4.3 at baseline. The sample was 7% Asian/Pacific Islander, 1% African American, 8% Hispanic, 83% European American, and 2% other or mixed racial heritage. Informed written consent was obtained before data collection. The sole exclusion criterion was a current diagnosis of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, anorexia nervosa, bulimia nervosa, or binge eating disorder.
Study 3 participants.
Participants were 100 individuals (mean ± SD age: 32.7 ± 11.3; 42 men and 58 women) recruited via flyers, e-mail distribution lists, and website message boards in the Denver Metro and Northern Colorado areas. Most participants were in the nonobese weight range (BMI: 25.9 ± 7.3); 85% reported being white, 9% black, 2% Asian/Pacific Islander, and 1% Native American. Individuals were excluded if they reported having a visual disability that would affect the ability to differentiate colors, impaired night vision, or any developmental impairment that could affect the ability to complete the measures. Each participant provided written consent and completed all procedures and measures. The local institutional review boards approved studies 1, 2, and 3.
Study 1 measures
Hedonic hunger.
The PFS is an 18-item self-report measure of responsivity to appetitive cues, which aims to assess responsivity to omnipresent food cues in our environment (3). Respondents are instructed to indicate the extent to which each statement describes their personal experience. Examples of PFS items include the following: “If I see or smell a food I like, I get a powerful urge to have some” “It seems like I have food on my mind a lot” and “I think I enjoy eating a lot more than most other people.” Response options are on a 5-point Likert scale, anchored with 1 “don’t agree at all” to 5 “strongly agree.” PFS total scores range from 18 to 90, with higher scores indicating greater appetitive responsivity. Participants in all 3 studies completed the PFS at baseline. In study 1, participants completed the PFS again at the 3- and 6-mo follow-ups. In study 2, the PFS was implemented at the 6-wk and 6-mo follow-ups.
Body mass.
BMI was used to reflect adiposity (15) at baseline and at the 6-wk, 6-mo, 1-y, and 2-y follow-ups for participants in study 1; at baseline and at the 3-mo, 6-mo, 1-y, and 2-y follow-ups for participants in study 2; and at baseline only in study 3. For all participants at each assessment, after removal of shoes and coats, height was measured to the nearest millimeter by using a standard stadiometer, and weight was assessed to the nearest 0.1 kg by using a digital scale. Two measures of both height and weight were obtained and averaged per assessment.
Binge eating.
The Eating Disorder Diagnostic Interview (EDDI), a semistructured interview adapted from the Eating Disorder Examination (16), assessed Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, eating disorder symptoms, including binge eating. Female assessors attended 24 h of training, wherein they received instruction in interview skills, reviewed diagnostic criteria for eating disorders, observed simulated interviews, and role-played interviews. Assessors showed high interrater agreement (κ > 0.80) with supervisors using 12 tape-recorded interviews conducted with individuals with and without eating disorders before collecting data. Weekly consensus meetings were used to resolve diagnostic ambiguities. EDDI eating disorder diagnoses have shown test-retest reliability (κ = 0.96) and interrater agreement [κ = 0.86 (17)].
fMRI acquisition, procedures and paradigm.
Scanning for study 1 participants was performed by using a Siemens Allegra 3 Tesla head-only MRI scanner to acquire BOLD responses from the entire brain as a measure of neural activation. For the fMRI assessment, study 1 participants were asked to consume regular meals leading up to the assessment and to refrain from eating or drinking (including caffeinated beverages) for 4–6 h preceding their imaging session for standardization. The fMRI paradigm was designed to examine activation in response to receipt and a cue preceding receipt of palatable food. Stimuli consisted of an image (glass containing a milkshake or a glass of water) that signaled the delivery of 0.5 mL of either of the tastants (chocolate milkshake or tasteless solution). The visual stimulus was presented for 2 s by using a digital projector/reverse screen display. A manifold was fit into the participants’ mouths, which delivered the tastant to a consistent segment of the tongue. The tasteless solution, which was designed to mimic the natural taste of saliva, was noncaloric and consisted of 25 mM KCl and 2.5 mM NaHCO3 (18). During 40% of the milkshake and tasteless-solution trials, the taste was not delivered as expected in order to allow the investigation of the neural response to the cue preceding receipt not confounded by actual receipt of the tastant. Fluids were delivered by using programmable syringe pumps controlled by Matlab (Mathworks, Inc.) to ensure consistent volume, rate, and timing of taste delivery.
Study 2 measures
Participants in study 2 completed measures of hedonic hunger, body mass, and binge eating as described above. Although binge eating was used as an exclusion criterion in study 2, the EDDI is a more sensitive measure than that used during screening. Therefore, there were binge-eating episodes present at baseline in study 2.
Participants in study 2 completed the Epstein Task progressive reinforcement paradigm (19). In this task, participants worked to earn points toward a snack food reward of their choice (e.g., small servings of salted peanuts, chips, peanut butter cups, Mars M&M’s, or cookies). First, participants performed a taste test of 1 g of each food and then selected the snack food they wanted to earn in the progressive reinforcement task. In the second phase, 3 boxes varying in shape and color are displayed on a computer screen. The boxes flip, rotate, and change in color each time the participant presses the mouse button. Points can be earned each time the shapes match in color and shape after the participant presses a button. A total of 5 points are worth 1 standard portion of the food (per the nutritional information for the snack). The task starts at a variable ratio (VR) 1:4 schedule, meaning that, on average, 1 point is awarded for every 4 button presses. The progressive ratio schedule for the food item doubles (VR8, VR16, VR32, etc.) each time the participant earns 5 points for 1 snack portion. Participants played for as long as they liked and were allowed to consume earned food upon task completion. This food reinforcement paradigm shows 2- to 7-d test-retest reliability [r = 0.80 (14)]. Previous data suggest that food reinforcement is positively related to ad libitum intake, higher hedonic ratings, and BMI (19–21).
Study 3 measures
Participants in study 3 completed measures of hedonic hunger and body mass as described in study 1 methods. Binge eating was not assessed in this study. This study did not include follow-up assessment.
In study 3, participants used the ImageRate protocol developed by our laboratory to assess the hedonic ratings of foods (14). The computer program ImageRate presented food images, one at a time, in random order, and was previously reported to be a reliable instrument to assess hedonic ratings of food images (14). Perceptual hedonic ratings were assessed by a measure of food appeal, which queried “How appealing is this food?” anchored by 0 (“Not appealing at all”) to 100 (“Extremely appealing”). Food categories (energy-dense entrées, meats, desserts, fruit, light entrées, seafood, and breads) were determined by using independent component analyses of perceptual hedonic ratings as described in reference 22.
Neuroimaging processing and statistical analyses
Neuroimaging data collected in study 1 were preprocessed and analyzed primarily by using SPM12 (Functional Imaging Laboratory, University College of London) in Matlab for Mac OSX. All images were manually realigned to the AC-PC line in SPM and skull-stripped by using the Brain Extraction Tool in FSL (FMRIB Analysis Group). Anatomic data were segmented and normalized by using DARTEL, resulting in a sample-specific template and individual-level deformation fields for application to the normalization step during functional data preprocessing. Functional data were as follows: 1) slice-timing-corrected because these methods can successfully compensate for the temporal offset between slice acquisition and can therefore increase the robustness of the data analysis (23), 2) adjusted for variation in magnetic field distortion by using field maps, 3) realigned to the mean functional and coregistered with the anatomical image, and 4) normalized to Montreal Neurological Institute (24) space by using the DARTEL template and deformation field output, which allows more precise alignment (25). Last, functional data were smoothed to 6 mm Gaussian full width at half maximum function. Functional data were then assessed for detected spikes in global mean response and motion outliers in the functional data by using the Artifact Detection Toolbox (Gabrieli Lab, McGovern Institute for Brain Research). Motion variables were included as regressors in the design matrix at individual-level analysis. Image volumes where the z-normalized global brain activation exceeded 3 SDs from the mean of the run or showed >1 mm of composite (linear plus rotational) movement were flagged as outliers and deweighted during individual-level model estimation.
At the individual level, T-maps were constructed for comparison of activation within each participant for contrasts on the individual level (e.g., milkshake receipt > tasteless-solution receipt). These individual contrasts were entered into a second-level regression model with PFS scores. Whole-brain analyses were conducted after the biranized sample-specific gray matter mask was applied. An overall significance level of cluster-level q-false discovery rate <0.05 was considered significant and corrected for multiple comparisons across the gray matter–masked whole brain. Effect sizes (r) for neuroimaging data were calculated as (Z/√n).
Analyses of non-fMRI data collected from all studies were considered significant with 2-sided hypotheses at P < 0.05. All data were first checked for assumptions of normality and overly influential data points. Cronbach’s α was used to assess internal consistency, and Pearson’s r was used in correlational analyses and test-retest reliability of the PFS. In the 2 prospective studies (studies 1 and 2), BMI and binge eating were used from all available points in mixed-effects growth curve analyses [version 9.3; SAS Institute (26)] to model weight change (or change in binge eating). After this, we 1) examined empirical growth plots, 2) fit an unconditional means model, 3) fit an unconditional linear growth model, and 4) fit unconditional nonlinear models. Data are presented as means ± SDs unless otherwise noted.
Results
Neural and behavioral correlates of hedonic hunger.
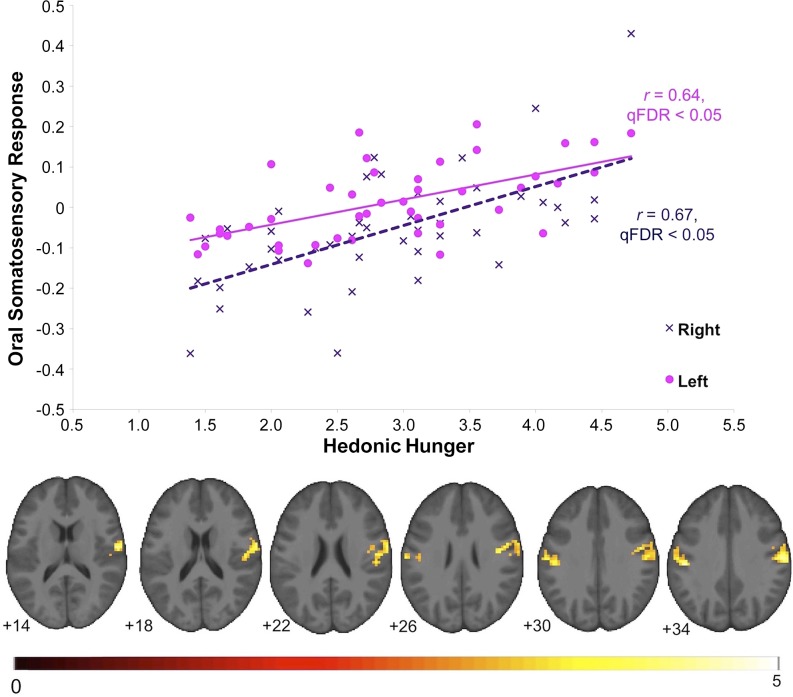
From data collected in study 1, when assessing the BOLD response to the anticipatory food cue (> anticipatory tasteless-solution cue) contrast as a function of hedonic hunger (study 1, n = 44), we observed robust bilateral activity in the oral somatosensory region of the postcentral gyrus, extending into the central operculum (Table 1, Figure 1). No significant relation was observed between hedonic hunger and responsivity to the palatable food receipt (> tasteless-solution receipt). From this receipt contrast, we observed a peak in the premotor area of the precentral gyrus that could be considered trending (Montreal Neurological Institute: 51, 6, 24; k = 19; Z = 4.8, r = 0.72; voxel-level family-wise error rate P = 0.04). This peak voxel met a common voxel-level threshold of significance (whole-brain family-wise error rate P < 0.05) but failed to meet the cluster-level significance threshold of q-false discovery rate <0.05 used here. There was no significant BOLD response negatively associated with hedonic hunger to either cue-elicited anticipation or receipt, meaning that all BOLD responses were related to the cue presented. Measured in study 2, “food reinforcement,” as defined by the breakpoint at which the participant stopped the food reinforcement behavioral task, was positively related to hedonic hunger (r = 0.31, P = 0.03).
TABLE 1.
Regional brain response to cue-elicited anticipated receipt of a palatable food as a function of hedonic hunger in a sample of nonobese young women (study 1)1
| Postcentral gyrus | x, y, z2 | k3 | Peak Z value | r4 | Peak P5 |
| Right | 60, −18, 36 | 1016 | 4.40 | 0.67 | 5.5 × 10−6 |
| 60, −9, 15 | 4.08 | 0.62 | 2.9 × 10−5 | ||
| 63, −6, 18 | 3.86 | 0.58 | 5.6 × 10−5 | ||
| 54, −12, 21 | 4.08 | 0.62 | 1.1 × 10−4 | ||
| Left | −54, −18, 45 | 506 | 4.23 | 0.64 | 1.1 × 10−5 |
| −48, −21, 33 | 4.09 | 0.62 | 2.2 × 10−5 | ||
| −63, −15, 27 | 3.54 | 0.54 | 2.0 × 10−4 | ||
| −51, −9, 39 | 3.33 | 0.50 | 4.3 × 10−4 |
n = 44.
Stereotactic coordinates in Montreal Neurological Institute space (24).
k = Number of contiguous voxels.
Effect sizes calculated as (Z/√n).
Cluster significant q-false discovery rate < 0.05 corrected across the whole brain.
FIGURE 1.
Positive relation between oral somatosensory brain response and hedonic hunger ratings in a sample of 44 nonobese women. Shown are BOLD responses during cue-elicited anticipated palatable food intake (> anticipated tasteless intake) as a function of hedonic hunger in the bilateral postcentral gyrus and scatterplot of the parameter estimates from those peaks (qFDR < 0.05). The color bar indicates the T value of strength of activity. Additional details can be seen Table 1 (study 1; n = 44). qFDR, q-false discovery rate.
Hedonic hunger, body mass, and binge eating.
In the 3 studies, participants reported mean ± SD PFS scores of 2.9 ± 0.8 (study 1; range: 1.4–4.7; n = 44), 2.5 ± 0.8 (study 2; range: 1.0–4.9; n = 389), and 2.6 ± 0.8 (study 3; range: 1.0–4.8; n = 100). Hedonic hunger was not related to baseline BMI in any of the 3 studies (study 1: r = −0.22, P = 0.14; study 2: r = 0.05, P = 0.37; study 3: r = 0.13, P = 0.21). Hedonic hunger did not predict future weight change (study 1: r = −0.22, P = 0.14; study 2: r = 0.05, P = 0.37). Hedonic hunger was positively related to current binge eating episodes in both study 1 (r = 0.58, P < 0.001) and study 2 (r = 0.31, P = 0.02). Conversely, hedonic hunger predicted future decreases in binge eating (study 1: r = −0.41, P < 0.05) and showed no relation with change in binge eating in the large sample (study 2: r = 0.02, P = 0.69). Binge eating was not assessed in study 3.
Perceptual food ratings and hedonic hunger.
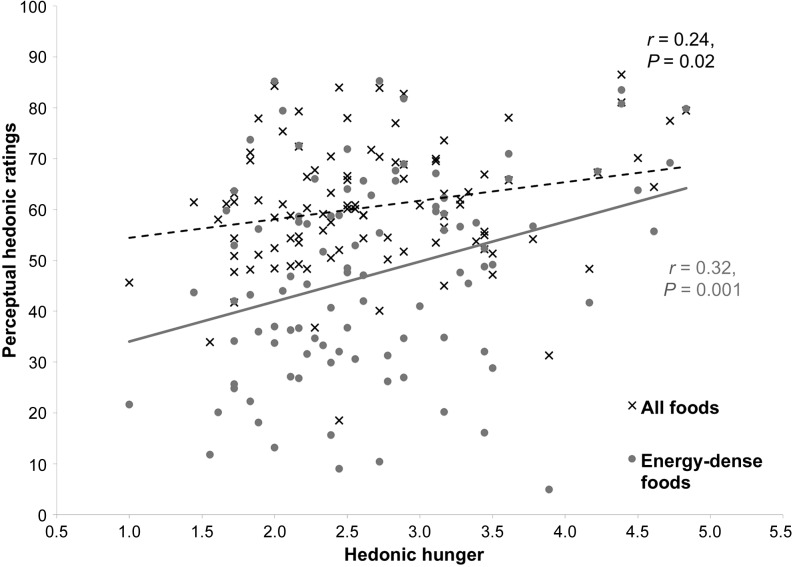
In study 3, participants’ (n = 100) overall perceptual ratings of food appeal were positively related to hedonic hunger (r = 0.24, P = 0.02; Figure 2). Interestingly, this effect was driven by the ratings for the energy-dense entrée (r = 0.32, P = 0.001) and meat (r = 0.21, P = 0.033) categories. Hedonic hunger was not related to ratings of the dessert (r = 0.13, P = 0.19), fruit (r = −0.09, P = 0.38), light entrée (r = 0.07, P = 0.49), seafood (r = 0.18, P = 0.08), or bread (r = 0.14, P = 0.18) categories.
FIGURE 2.
Positive relation between perceptual hedonic ratings of food images and hedonic hunger in a sample of 100 adults (study 3). Participants showed a positive relation between perceptual ratings of all foods and hedonic hunger (r = 0.24, P = 0.02). This relation appears to be stronger in energy-dense foods (r = 0.32, P = 0.001).
Internal consistency and temporal reliability of the PFS.
The PFS showed internal consistency in both study 1 (Cronbach’s α = 0.95, n = 44) and study 2 (Cronbach’s α = 0.94, n = 389). The PFS showed test-retest reliability in study 1 from baseline to the 3-mo (r = 0.84, P < 0.001) and 6-mo (r = 0.73, P < 0.001) follow-ups. This was also seen in study 2 from baseline to the 6-wk (r = 0.78, P < 0.001) and 6-mo (r = 0.72, P < 0.001) follow-ups.
Discussion
Data indicate that hedonic hunger as measured by the PFS is positively related to the following: 1) BOLD response in brain regions commonly associated with oral somatosensory processing during cue-elicited anticipation of palatable food (study 1); 2) food reinforcement (study 2); 3) perceived appeal of foods, particularly energy-dense foods (study 3); and 4) current binge eating (studies 1 and 2). These data indicate that self-reported hedonic hunger is related to an elevated response to food stimuli independent of modality (i.e., brain response, observed behavior, or explicit ratings). In addition, the present results support previous reports of internal consistency and temporal reliability (3, 5), supporting that hedonic hunger is a stable construct. In summary, the heightened responses to external food stimuli reported suggest that that an individual reporting greater hedonic hunger is hyperresponsive to the external food environment. In line with this notion, hedonic hunger was related to increased binge eating. However, hedonic hunger was not related to BMI or change in BMI, suggesting that the relation between hedonic hunger, habitual energy intake, and weight regulation is less clear.
Data from the fMRI study showed that hedonic hunger is positively correlated with the BOLD response in brain regions previously associated with oral somatosensory processing during the anticipatory cue preceding the intake of palatable food. Activity in the postcentral gyrus is thought to encode the somatosensory aspects of stimuli (e.g., touch, taste, and properties of food such as viscosity and fat). Differential response patterns in the oral somatosensory regions have also been shown between obese and lean individuals when exposed to anticipatory food cues, showing that differential neural responses can lead to BMI change (27, 28). Moreover, greater basal somatosensory functioning in this area has been posited as a risk factor for obesity (29). Although our findings did not support a direct association between PFS scores and weight gain, PFS scores do correlate with neural activity that confers risk for weight gain.
Food reinforcement has previously been associated with obesity as well as increased acute food intake (20), indicating that individuals who are more willing to work for palatable food are more likely to consume greater amounts of food in an ad libitum assessment. These individuals are also more likely to be obese. This supports our findings in the neuroimaging data, in which we also observed a positive relation between hedonic hunger and food reinforcement via an operant behavioral task. Collectively, the above findings support the notion that individuals reporting high hedonic hunger have greater anticipatory response to food stimuli and are more willing to work to receive this food.
Hedonic hunger was positively related to binge eating behavior in both of the samples in which it was assessed, but it was not related to body mass in any of the 3 studies. This phenomenon of finding a correlation between binge eating and hedonic hunger but not BMI was also found in previous studies (7, 30). Previous reports related hedonic hunger to the loss of control while eating in studies in overweight and obese women (31) and women with eating disorder pathology (7). Collectively, these data suggest that hedonic hunger may be related to binge eating behavior independent of weight status or disordered eating symptoms. The fact that hedonic hunger was not related to BMI in all 3 studies echoes previous reports in the initial validation study and others (3, 32, 33). However, some studies found a relation between hedonic hunger and BMI (5, 11). Of note, the 2 studies reported relatively small effect sizes between BMI and hedonic hunger [mean r’s = 0.12–0.13 (5, 11)], which suggests that this relation is weak at best. If there were a large effect, one would expect that results from study 2 (n = 398) would be able to show such a relation. Other studies have suggested that hedonic hunger is more likely to predict palatable food intake when paired with a measure for inhibitory control (30, 31). This warrants further exploration, because our findings suggest that an additional explanatory variable may be present to explain the lack of correlation between PFS scores and weight gain. Furthermore, hedonic hunger was not predictive of BMI change or escalation in binge eating over time, suggesting that the PFS is not a tool that identifies risk factors for future weight gain or increases in disordered eating.
Certain limitations in the studies being analyzed should be acknowledged. First, the interpretation of the present results in the context of the validity of hedonic hunger and the PFS should be made with caution. Specifically, we do not suggest that the current results provide criterion validity for the PFS, but they do support that an individual indicating greater hedonic hunger may also show greater responses in neural, behavioral, and perceptive measures of food reward and/or reinforcement. However, given our findings, this does not directly indicate that these neural and behavioral responses are driving factors in the assessment of hedonic hunger. Considering the methodologic limitations of these studies, the samples in studies 1 and 2 consisted of women recruited for a body-acceptance and weight-management intervention, which limits generalizability. Of note, the presented data did not differ as a function of intervention group. Second, the sample size in study 1 is relatively small to detect significant changes in weight over follow-up, but it is relatively large for an fMRI study. Moreover, in the anticipatory cue portion of the fMRI paradigm, participants were exposed to a cartoon drawing of a milkshake and received only 2 tastants (milkshake and tasteless control solution), which may also decrease the generalizability of results. In addition, the participants in study 1 were in a moderately fasted state, which may increase perceived hunger and cravings, and therefore influence neural response. Despite these limitations, the collection of results here presents the first attempt, to our knowledge, to relate a widely used self-report measure of hedonic hunger to methodologies thought to capture various aspects of susceptibility to palatable food stimuli outside of homeostatic needs.
In sum, the results indicate that hedonic hunger, although not predicative of weight gain, does relate to increased neural and perceptive responses to cues of palatable foods and to an increased motivation to consume such foods and is associated with current binge eating. Given the consistent findings with current binge eating, hedonic hunger may be a useful tool in eating disorder research and treatment settings. Accurately predicting individual differences in longitudinal weight regulation is extremely challenging given the multitude of internal and external factors that influence food intake along with the variability in individual biology and physiologic determinates of metabolism. The PFS provides valuable information with regard to acute response to food stimuli, which might be more effective in combination with additional measures that assess other known determinists of habitual food intake (e.g., impulsivity, stress, socioeconomic status, or dieting).
Acknowledgments
We thank Eric Stice for supporting this manuscript and for data sharing. KSB, AJS, and JRG were responsible for writing and revising the manuscript; and KSB assisted in the data collection and performed the data analyses. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: EDDI, Eating Disorder Diagnostic Interview; PFS, Power of Food Scale; VR, variable ratio.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010 [Internet]. JAMA 2012;307:491–7. [cited 2015 Mar 25]. Available from: http://jama.jamanetwork.com/article.aspx?articleID=1104933. [DOI] [PubMed]
- 2.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav 2007;91:432–9. [DOI] [PubMed] [Google Scholar]
- 3.Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Ochner CN, Coletta MC, Bellace D, Wallaert M, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite [Internet]. 2009;53:114–8. [cited 2015 Mar 25]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19500623. [DOI] [PubMed]
- 4.Van Strien T, Frijters JER, Bergers G, Defares PB, Van Strien T, Frijters JER. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord 1986;5:295–315. [Google Scholar]
- 5.Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Karlsson J, Lowe MR. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. Int J Obes (Lond) 2009;33:913–22. [DOI] [PubMed] [Google Scholar]
- 6.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain [Internet]. J Neurophysiol 2001;85:1315–21. [cited 2015 Apr 29]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11248000. [DOI] [PubMed]
- 7.Witt AA, Lowe MR. Hedonic hunger and binge eating among women with eating disorders. Int J Eat Disord 2014;47:273–80. [DOI] [PubMed] [Google Scholar]
- 8.Ochner CN, Green D, van Steenburgh JJ, Kounios J, Lowe MR. Asymmetric prefrontal cortex activation in relation to markers of overeating in obese humans. Appetite 2009;53:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan-Poupart S, Veldhuizen MG, Geha P, Small DM. Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger [Internet]. Appetite 2013;60:168–74. [cited 2015 Mar 25]. Available from: http://www.sciencedirect.com/science/article/pii/S0195666312004035. [DOI] [PMC free article] [PubMed]
- 10.Levitsky DA, Shen X. Food Power Scale predicts dessert eating, but not meal eating or portion size effect. Appetite 2008;51:381. [Google Scholar]
- 11.Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr 2010;92:277–83. [DOI] [PubMed] [Google Scholar]
- 12.Stice E, Shaw H, Burton E, Wade E. Dissonance and healthy weight eating disorder prevention programs: a randomized efficacy trial [Internet]. J Consult Clin Psychol 2006;74:263–75. [cited 2015 Apr 1]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1479305&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed]
- 13.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food [Internet]. J Neurosci 2010;30:13105–9. [cited 2015 Mar 25]. Available from: http://www.jneurosci.org/content/30/39/13105.short. [DOI] [PMC free article] [PubMed]
- 14.Burger KS, Cornier MA, Ingebrigtsen J, Johnson SL. Assessing food appeal and desire to eat: the effects of portion size & energy density [Internet]. Int J Behav Nutr Phys Act 2011;8:101. [cited 2015 Mar 25]. Available from: http://www.ijbnpa.org/content/8/1/101. [DOI] [PMC free article] [PubMed]
- 15.Dietz WH, Robinson TN. Use of the body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr 1998;132:191–3. [DOI] [PubMed] [Google Scholar]
- 16.Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self‐report questionnaire? Int J Eat Disord 1994;16:363–70. [PubMed] [Google Scholar]
- 17.Stice E, Marti CN, Shaw H, Jaconis M. An 8-year longitudinal study of the natural history of threshold, subthreshold, and partial eating disorders from a community sample of adolescents [Internet]. J Abnorm Psychol 2009;118:587–97. [cited 2015 Mar 25]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2849679&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed]
- 18.Zald DH, Pardo JV. Cortical activation induced by intraoral stimulation with water in humans [Internet]. Chem Senses 2000;25:267–75. [cited 2015 Mar 25]. Available from: http://chemse.oxfordjournals.org/content/25/3/267.abstract. [DOI] [PubMed]
- 19.Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food [Internet]. Physiol Behav 2003;78:221–7. [cited 2015 Mar 25]. Available from: http://www.sciencedirect.com/science/article/pii/S0031938402009782. [DOI] [PubMed]
- 20.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D-2 receptor genotype, and energy intake in obese and nonobese humans [Internet]. Behav Neurosci 2007;121:877–86. [cited 2015 Mar 25]. Available from: http://psycnet.apa.org/journals/bne/121/5/877/. [DOI] [PMC free article] [PubMed]
- 21.Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LW, Jaroni JL, Saad FG, Crystal-Mansour S, Shields PG, Lerman C. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers [Internet]. Am J Clin Nutr 2004;80:82–8. [cited 2015 Mar 25]. Available from: http://ajcn.nutrition.org/content/80/1/82.abstract. [DOI] [PubMed]
- 22.Johnson SL, Boles RE, Burger KS. Using participant hedonic ratings of food images to construct data driven food groupings [Internet]. Appetite 2014;79:189–96. [cited 2015 Mar 25]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4104662&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed]
- 23.Sladky R, Friston KJ, Tröstl J, Cunnington R, Moser E, Windischberger C. Slice-timing effects and their correction in functional MRI [Internet]. Neuroimage 2011;58:588–94. [cited 2015 Feb 25]. Available from: http://www.sciencedirect.com/science/article/pii/S1053811911007245. [DOI] [PMC free article] [PubMed]
- 24.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development the international consortium for brain mapping (ICBM). Neuroimage 1995;2:89–101. [DOI] [PubMed] [Google Scholar]
- 25.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage [Internet]. Elsevier B.V.; 2009;46:786–802. [cited 2015 Mar 25]. Available from: http://dx.doi.org/10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed]
- 26.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat 1998;23:323–55. [Google Scholar]
- 27.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele [Internet]. Science. 2008;322:449–52. [cited 2015 Mar 25]. Available from: http://science.sciencemag.org/content/322/5900/449. [DOI] [PMC free article] [PubMed]
- 28.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study [Internet]. J Abnorm Psychol. 2008;117:924–35. [cited 2015 Mar 25]. Available from: http://psycnet.apa.org/journals/abn/117/4/924/. [DOI] [PMC free article] [PubMed]
- 29.Wang G-J, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Galanti K, Selig PA, Han H, Zhu W, et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring) [Internet]. Nature Publishing Group 2011;19:1601–8. [cited 2015 Mar 25]. Available from: http://onlinelibrary.wiley.com/doi/10.1038/oby.2011.27/abstract;jsessionid=FEB207D2D1BF57263F091C30365667BA.f04t02. [DOI] [PMC free article] [PubMed]
- 30.Appelhans BM. Neurobehavioral inhibition of reward‐driven feeding: implications for dieting and obesity. Obesity (Silver Spring) 2009;17:640–7. [DOI] [PubMed] [Google Scholar]
- 31.Manasse SM, Espel HM, Forman EM, Ruocco AC, Juarascio AS, Butryn ML, Zhang F, Lowe MR. The independent and interacting effects of hedonic hunger and executive function on binge eating [Internet]. Appetite 2015;89:16–21. [cited 2015 Mar 25]. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0195666315000227. [DOI] [PMC free article] [PubMed]
- 32.Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, King N, Curtis C, Kennedy JL. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 2009;17:1220–5. [DOI] [PubMed] [Google Scholar]
- 33.Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity (Silver Spring) 2011;19:2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]