Abstract
Background: To our knowledge the efficacy of soy-dairy protein blend (PB) supplementation with resistance exercise training (RET) has not been evaluated in a longitudinal study.
Objective: Our aim was to determine the effect of PB supplementation during RET on muscle adaptation.
Methods: In this double-blind randomized clinical trial, healthy young men [18–30 y; BMI (in kg/m2): 25 ± 0.5] participated in supervised whole-body RET at 60–80% 1-repetition maximum (1-RM) for 3 d/wk for 12 wk with random assignment to daily receive 22 g PB (n = 23), whey protein (WP) isolate (n = 22), or an isocaloric maltodextrin (carbohydrate) placebo [(MDP) n = 23]. Serum testosterone, muscle strength, thigh muscle thickness (MT), myofiber cross-sectional area (mCSA), and lean body mass (LBM) were assessed before and after 6 and 12 wk of RET.
Results: All treatments increased LBM (P < 0.001). ANCOVA did not identify an overall treatment effect at 12 wk (P = 0.11). There tended to be a greater change in LBM from baseline to 12 wk in the PB group than in the MDP group (0.92 kg; 95% CI: −0.12, 1.95 kg; P = 0.09); however, changes in the WP and MDP groups did not differ. Pooling data from combined PB and WP treatments showed a trend for greater change in LBM from baseline to 12 wk compared with MDP treatment (0.69 kg; 95% CI: −0.08, 1.46 kg; P = 0.08). Muscle strength, mCSA, and MT increased (P < 0.05) similarly for all treatments and were not different (P > 0.10) between treatments. Testosterone was not altered.
Conclusions: PB supplementation during 3 mo of RET tended to slightly enhance gains in whole-body and arm LBM, but not leg muscle mass, compared with RET without protein supplementation. Although protein supplementation minimally enhanced gains in LBM of healthy young men, there was no enhancement of gains in strength. This trial was registered at clinicaltrials.gov as NCT01749189.
Keywords: plant protein, strength training, milk proteins, muscle mass, animal proteins, weight training, function
Introduction
Increased muscle size and strength are 2 of the many benefits of resistance exercise training (RET)14. Many acute molecular and metabolic investigations claimed an additive anabolic effect of protein/amino acid supplementation after an acute resistance exercise session (1–7), yet there is less certainty whether chronic protein supplementation during RET enhances muscle growth compared with RET without protein supplementation (8–10). Although meta-analysis has determined the existence of an additive effect of protein supplements for independently enhancing muscle size and strength (8), this effect is not universal (9, 10). This incongruity may stem from dissimilarities in the following: study design, choice, and measurement of outcomes; target populations; exercise training protocols; and timing, source, and amount of the protein and/or placebo supplement (8–11).
Investigation of the most effective protein source for this enhancement has prompted acute (5, 12–18) and chronic (19–25) clinical trials. Isotopic tracer studies suggested that the rapid digestion rate and high leucine content of whey protein (WP) are 2 primary factors driving the protein anabolic response after postexercise ingestion (26). Furthermore, some studies (14, 15, 27–29), but not all (5, 16, 17, 30), suggested that WP represents the gold standard compared with other high-quality protein sources such as soy or casein. We recently found that, when compared with whey, a soy-dairy protein blend (PB) containing 25% soy protein, 25% WP, and 50% casein protein (matching leucine content to WP) induced similar acute increases in mammalian target of rapamycin complex 1 (mTORC1) signaling and mixed-muscle/myofibrillar protein synthesis when ingested after resistance exercise (12, 13). Interestingly, these similar effects were observed despite temporal differences in hyper-aminoacidemia and amino acid transport between treatments. We hypothesized that a high-quality protein supplement consisting of a PB, with adequate leucine, would enhance lean mass and strength compared with an isocaloric placebo during 12 wk of RET but show effects equivalent to WP treatment.
Methods
Screening of participants.
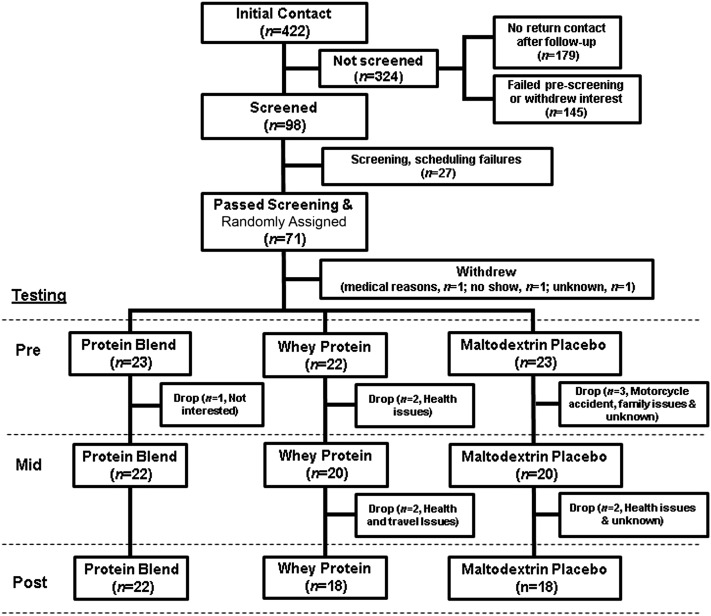
We recruited healthy male participants for this double-blind randomized clinical trial (Table 1). Participants were recruited through locally posted flyers, through newspaper advertisements, and by word of mouth. After initial contact, prospective participants completed a baseline screening questionnaire to determine eligibility and availability to participate. Eligible participants were screened in the morning after an overnight fast at the Institute for Translational Sciences–Clinical Research Center (ITS-CRC) at the University of Texas Medical Branch. The screening day included the following: 3-d food diary analysis, muscle strength testing, a clinical history, physical examination, resting electrocardiogram, and laboratory tests (complete blood count with differential, liver and kidney function tests, coagulation profile, fasting blood glucose, hepatitis B and C screenings, HIV test, thyroid-stimulating hormone, lipid profile, urinalysis, and drug screening). Participants with clinical signs of malnutrition; with anabolic steroid or corticosteroid use in the past 6 mo; current tobacco users; admitted vegan or vegetarians; adhering to a high-protein diet, a high-soy diet (>2 servings soy/d, ∼500 mL soy milk), or a high-dairy diet (>6 servings dairy/d, ∼1400 mL milk); currently using protein supplements; or with food allergies were excluded. The participants were healthy and recreationally active but were not engaged in any regular exercise-training program (<2 sessions high-intensity aerobic or resistance exercise/wk) at the time of enrollment. All of the participants gave written informed consent before enrollment in the study. The study was approved by the Institutional Review Board of the University of Texas Medical Branch and is in compliance with the Declaration of Helsinki as revised in 1983. The CONSORT (Consolidated Standards of Reporting Trials) diagram is shown in Figure 1.
TABLE 1.
Baseline characteristics1
| Treatment group |
|||
| PB (n = 23) | WP (n = 22) | MDP (n = 23) | |
| Age, y | 24 ± 1 | 25 ± 1 | 25 ± 1 |
| Height, cm | 179 ± 2 | 178 ± 2 | 176 ± 2 |
| Weight, kg | 78.0 ± 2.5 | 81.8 ± 2.5 | 76.6 ± 2.5 |
| BMI, kg/m | 24.4 ± 0.6 | 25.8 ± 0.7 | 24.6 ± 0.6 |
| Strength 1-RM, kg | |||
| Squat | 109 ± 10 | 120 ± 11 | 120 ± 10 |
| Knee extension | 107 ± 6 | 120 ± 6 | 110 ± 6 |
| Chest press | 83 ± 7 | 90 ± 7 | 84 ± 7 |
| Mean | 78 ± 4 | 83 ± 4 | 78 ± 4 |
Values are means ± SEMs. MDP, maltodextrin placebo; PB, soy-dairy protein blend; WP, whey protein; 1-RM, 1-repetition maximum.
FIGURE 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram of study recruitment, enrollment, randomization follow-up, and analysis. Eligible participants were randomly assigned to receive the protein blend, whey protein, or maltodextrin placebo treatment during 3 mo of progressive resistance training. Participants withdrew for personal or medical non–study-related reasons or were dropped if they met exclusion criteria.
Study design.
After enrollment, participants completed a 10- to 14-d baseline run-in period that consisted of the baseline study day at the University of Texas Medical Branch and then 3 nonconsecutive days of exercise familiarization and baseline 1-repetition-maximum (1-RM) strength testing at the University of Texas Medical Branch Alumni Fieldhouse. During the run-in period, participants were given a study binder containing study information, food diary record instructions, and supplement logs. Power analyses, based on previous studies (19, 31), were conducted to determine required sample size. By using a power of 0.8 and an α of 0.0167, it was determined that ∼20 subjects/treatment group were needed to detect significant differences between groups for whole-body lean mass.
The baseline study day included assessment of body composition, thigh muscle thickness, blood and serum collection, and isokinetic and isometric strength testing. Two to three days later, participants reported to the University of Texas Medical Branch Alumni Fieldhouse for familiarization/testing before beginning 12 wk of training. At 6 wk of training, participants were retested for all baseline measures, after an overnight fast, the morning after an exercise training day. After 12 wk of training, participants were retested 3 d after the final exercise session. For the 12-wk testing, participants reported to the ITS-CRC at the same time in the morning as the baseline study day to repeat laboratory tests and sample collection. Recruitment began in June 2012 and was conducted by starting 5 phases of 10–15 participants until final testing was completed in May 2014, with the goal of completing 20 participants/treatment. The resistance training protocol was fully supervised by highly qualified personnel and is described in the Supplemental Methods and in Supplemental Figure 1. This trial was registered at clinicaltrials.gov as NCT01749189.
Clinical testing.
Participants reported to the ITS-CRC at the University of Texas Medical Branch in the morning after an overnight fast. They were instructed to refrain from any medication that affects muscle metabolism as well as caffeine, fish-oil supplementation, and alcohol for several days before testing. They were instructed to avoid strenuous or long-duration exercise for 3 d before arrival and to drink 1 L water the night before testing. After arrival on the unit, participants were instructed to void to ensure an empty bladder and bowel and to then lie supine for 30 min before assessment of body composition by DXA scan (Hologic ADR 4500W). To maintain a supine position, participants were transported to and from the DXA unit in a stretcher to avoid regional fluid shifts. The same technician set the regions-of-interest for all of the DXA scans. A catheter was placed in the antecubital vein for blood sampling.
After the DXA scan, ultrasound (Phillips HDI 5000) of the vastus lateralis (VL) and vastus intermedius was conducted with the participant in a supine position as previously described (32). Briefly, several B-mode real-time images of the VL and vastus intermedius were taken in the midsagittal position at 50% and 75% of the femur length (from the the anterior superior iliac spine to the superior border of the patella). The mean of both sites, with 3 images at each site, was used to assess muscle thickness.
A percutaneous biopsy sample of the VL muscle was performed at baseline and again in the morning after an overnight fast at 12 wk by using a 5-mm Bergström biopsy needle (33), with suction, under sterile procedure and local anesthesia (1% lidocaine). Longitudinal muscle cross-sections were carefully laid on Tissue Tek Optimal Cutting Temperature (Thermo Fisher Scientific) affixed to cork, submerged in liquid nitrogen–cooled isopentane, and then placed on dry ice until they could be stored at −80◦C until subsequent immunohistochemical analysis. Peak torque of the knee extensors and knee flexors of the nonbiopsied leg were subsequently determined by dynamometry (Biodex Medical) as previously described (32).
After the strength test, participants were fed a meal before leaving the unit. All of the testing was repeated on the post-testing day in the same order as the baseline testing day.
Supplementation.
Participants were randomly assigned to maltodextrin placebo (MDP), WP, or PB treatments. A block-randomization method used to generate the random allocation sequence, with random block size generated in nQuery Advisor 3.0 (Statistical Solutions). Immediately after each workout, under direct observation of the study personnel, the participants ingested their assigned supplement. Thus, all treatments had 100% postexercise supplement compliance. On each of the 4 resting (nonexercise) days during the week, the participants ingested the placebo or supplement once between meals. Participants were instructed to refrain from any other food or macronutrient-containing beverage for 2 h before exercise and after supplementation.
All of the treatments were provided by DuPont Nutrition & Health, and the composition of each was independently tested (Supplemental Table 1). The PB was composed of 25% protein from soy protein isolate, 25% protein from WP isolate, and 50% protein from sodium caseinate. The WP treatment consisted of 100% protein from WP isolate, and the MDP was an isocaloric maltodextrin powder. To assess the overall effect of protein supplementation, the PB and WP groups were combined and tested in a separate analysis. The dose for the 2 protein supplements was ∼22 g protein/d. The dose was chosen on the basis of our preliminary data showing that it contained an amount of leucine sufficient to acutely maximize protein synthesis for all protein supplements (i.e., ≥2 g leucine) in young men (12, 13, 34). Therefore, the leucine content was 2.00 g for the PB and 2.31 g for the WP. Supplements were separated into individual ready-made treatment-coded packets for daily consumption, and participants were given a 2-wk supply. The personal trainer collected the empty supplement packets from each participant every 2 wk. The supplements and placebo were given in powder form and dispersed into 300 mL water to ensure rapid and predictable absorption. Participants who received different treatments were instructed not to discuss their treatments and were separated at the time of ingestion. All of the authors were blinded to the treatment assignments. Once analysis was complete, a DuPont representative not involved in the analysis released the code for the treatments.
Nutritional intake.
Participants were instructed to maintain their habitual diet and to complete a 3-d food diary on 3 occasions: at baseline testing and at the 6- and 12-wk testing periods. On each occasion, participants were given detailed instructions and were told to record their normal diet during the week before the testing day on 2 weekdays and 1 weekend day. Participants were instructed to also include the day before testing. Dietary intake data were collected and estimated by using Nutrition Data System for Research software, version 2012, developed by the Nutrition Coordinating Center, University of Minnesota.
Serum testosterone.
Serum testosterone was assayed in duplicate on an Immulite 2000 Immunoassay System (Siemens) at the ITS-CRC core laboratory per the manufacturer’s instructions.
Mean myofiber cross-sectional area.
Myofiber cross-sectional area (mCSA) was determined by a method similar to Fry et al. (35), with modification. Images for fiber typing were captured at 100× magnification by using a fluorescence microscope (Axio Imager.M1m; Carl Zeiss) and an AxioCam MRm camera (Carl Zeiss). Image processing and analysis were performed by using AxioVision 4.8.2 software. Approximately 200 myofibers were analyzed for mean mCSA in each sample. (See Supplemental Methods for additional methods regarding the resistance exercise protocol and treatment and nutritional intake log compliance.)
Statistical analysis.
Values are raw values or change scores expressed as mean ± SEMs or means (95% CIs). Primary outcome data were evaluated for equal variances and normality, and no major violations of model assumptions were found. For each outcome, a mixed-model ANOVA with fixed effects of treatment, time, and a treatment-by-time interaction was conducted. Subject was a random effect, and all available time points (e.g., baseline, 6 wk, and 12 wk) were incorporated into each outcome’s mixed model. In addition, outcome change scores between time points were calculated and analyzed by ANOVA with treatment as the main effect. Each change score (12 wk − baseline, 12 wk − 6 wk, and 6 wk − baseline) was analyzed separately. When multiple time points were measured for an outcome, ANCOVA was conducted on 12-wk randomization time points, with treatment as the main effect and baseline as the covariate. To test the effect of protein supplementation, we pooled the protein treatments WP and PB. An additional model was conducted with both the ANCOVA and mixed model, with treatment effects of pooled WP and PB and MDP only. To further test the effectiveness of protein supplementation on whole-body lean mass, the values of responses above or below an a priori threshold of an expected 1.5-kg placebo response were tabulated for each treatment. The difference in these proportions between treatments was tested with Fisher’s exact test. An α level of 0.05 was set for significance, but P values between 0.05 and 0.10 were considered indicative of a trend. When interactions and/or main effects were found to be significant (P < 0.05) or as indicating a trend (P < 0.10), Tukey’s honestly significant difference pairwise comparisons were conducted to compare time points. All of the analyses were conducted with R version 3.1.1 (R Foundation for Statistical Computing), with the exception of Pearson correlations, which were calculated by using Graph Pad Prism 6.0f (GraphPad Software) for Mac. All figures were generated with the same program.
Results
Participant characteristics.
Descriptive characteristics at baseline for all participants are shown in Table 1. There were no differences between treatment groups at baseline for any variable (P > 0.10).
Treatment and nutritional intake log compliance.
Because participants ingested their assigned supplement under the direct observation of the study personnel immediately after each workout, all treatments had 100% postexercise compliance. Participants were instructed to log their non–exercise day compliance; thus, compliance on these days was likely underestimated. Treatment compliance was similar for all treatment groups, with 92.3% (range: 80.5–100%, median: 93.0%), 87.2% (range: 56.5–100%, median: 91.7%), and 88.1% (range: 64–100%, median: 91.8%) for PB, WP, and MDP treatment groups, respectively. There were no differences between treatments for treatment compliance (P > 0.10). Most of the low compliance values were from participants who dropped out of the study. One WP participant had lower compliance (56.5%), but his values for the primary outcomes were similar or greater than the mean values. We conducted correlation analyses between treatment compliance and the primary outcomes of whole-body lean mass, leg lean mass, and muscle thickness changes for all treatments pooled as well as for WP only, and these analyses indicated no relation (R2 = 0.002–0.01, P = 0.4–0.9). Dietary log compliance was not as influenced by dropout participants, but a treatment difference was present (P = 0.037). Dietary log compliance was higher (P < 0.05) for the PB group (98.5% ± 4.3%) than for the WP (85.2% ± 4.8%) and MDP (83.3% ± 4.7%) groups.
Nutritional intake.
Mean habitual energy and macronutrient intakes were not significantly different between treatment groups at baseline. Protein supplementation (Supplemental Table 2, Table 2) showed significant time (P = 0.032), treatment (P = 0.003), and treatment-by-time (P = 0.025) interactions. Protein intake trended toward being significantly greater for the PB group than for the MDP group at 6 wk (P = 0.06), but no significant differences were found between WP and MDP groups. Carbohydrate and fat intakes were not affected by time or treatment (P > 0.10), and there was no significant interaction (P = 0.90 and P = 0.48 respectively).
TABLE 2.
Dietary intake (with supplementation) in healthy young men who received MDP, PB, or WP supplements for 12 wk of resistance exercise training1
| Time period |
|||
| Treatment | Baseline | 6 wk | 12 wk |
| Total energy nonsupplemented, kcal/d | |||
| PB | 2460 ± 158 | 2460 ± 158 | 2270 ± 161 |
| WP | 2490 ± 179 | 2500 ± 189 | 2670 ± 194 |
| MDP | 2220 ± 189 | 2190 ± 195 | 2200 ± 195 |
| Protein intake | |||
| g/d | |||
| PB | 101 ± 7 | 129 ± 7a* | 122 ± 7a* |
| WP | 102 ± 7 | 126 ± 8a* | 135 ± 8a* |
| MDP | 95 ± 7 | 95 ± 8b | 93 ± 8b |
| g ⋅ kg−1 ⋅ d−1 | |||
| PB | 1.33 ± 0.10 | 1.68 ± 0.10a* | 1.54 ± 0.10a* |
| WP | 1.29 ± 0.10 | 1.54 ± 0.11a* | 1.64 ± 0.11a* |
| MDP | 1.27 ± 0.10 | 1.22 ± 0.11b | 1.23 ± 0.11b |
| Carbohydrate intake | |||
| g/d | |||
| PB | 274 ± 18 | 290 ± 18 | 272 ± 19 |
| WP | 280 ± 21 | 291 ± 22 | 284 ± 23 |
| MDP | 246 ± 21 | 272 ± 23 | 274 ± 23 |
| g ⋅ kg−1 ⋅ d−1 | |||
| PB | 3.58 ± 0.24 | 3.71 ± 0.24 | 3.42 ± 0.24 |
| WP | 3.54 ± 0.27 | 3.52 ± 0.28 | 3.46 ± 0.29 |
| MDP | 3.27 ± 0.27 | 3.16 ± 0.29 | 3.31 ± 0.29 |
| Fat intake | |||
| g/d | |||
| PB | 91 ± 8 | 97 ± 8 | 87 ± 8 |
| WP | 100 ± 9 | 97 ± 9 | 110 ± 10 |
| MDP | 92 ± 9 | 87 ± 10 | 83 ± 10 |
| g ⋅ kg−1 ⋅ d−1 | |||
| PB | 1.19 ± 0.11 | 1.26 ± 0.11 | 1.08 ± 0.11 |
| WP | 1.26 ± 0.12 | 1.18 ± 0.13 | 1.29 ± 0.13 |
| MDP | 1.23 ± 0.12 | 1.13 ± 0.13 | 1.11 ± 0.13 |
Values are means ± SEMs. Sample sizes/group at baseline, 6 wk, and 12 wk, respectively—MDP: n = 17, 14, and 14; PB: n = 22, 22, and 21; and WP: n = 17, 15, and 14. Labeled means (at that time point) without a common superscript letter differ, P < 0.05. *Different from baseline, P < 0.05. MDP, maltodextrin placebo; PB, soy-dairy protein blend; WP, whey protein.
Serum testosterone.
Serum testosterone (Supplemental Table 3) was similar at baseline between treatments (P = 0.57). Serum testosterone indicated a significant time effect (P = 0.012) but no significant treatment effect (P = 0.48) or treatment-by-time interaction (P = 0.67). Analysis indicated that serum testosterone at 6 wk was significantly greater than at baseline (P = 0.008).
Weight lifted and 1-RM strength.
The total mean weights lifted per participant for WP (569,600 ± 57,130 kg), PB (579,500 ± 61,400 kg), and MDP (513,700 ± 58,440 kg) treatments did not differ between groups (data not shown) (P = 0.22). At baseline, 1-RM strength was not different between treatments for any exercise. Mean gym strength (strength assessed by the exercises used for training) changed during the study (P < 0.001), but there were no significant treatment × time interactions (P = 0.68) or effects of treatment (P = 0.32). Mean gym strength increased (P < 0.05). Pairwise comparisons for the overall time effect showed a significant increase (P < 0.001) between each time point (Table 3).
TABLE 3.
Changes in 1-RM strength, every 3 wk, on select exercises in healthy young men who received MDP, PB, or WP supplements for 12 wk of resistance exercise training1
| Time period |
||||
| Treatment | 3 wk | 6 wk | 9 wk | 12 wk |
| ∆ Squat 1-RM, kg | ||||
| PB | 20 (–0.3, 40) | 42 (21, 62) | 61 (41, 82) | 90 (69, 110) |
| WP | 26 (5, 47) | 41 (20, 62) | 60 (38, 82) | 100 (80, 120) |
| MDP | 19 (–2, 39) | 41 (20, 62) | 62 (41, 83) | 94.0 (72, 120) |
| ∆ Knee extension, kg | ||||
| PB | 17 (6, 29) | 32 (20, 43) | 46 (34, 57) | 62 (50, 74) |
| WP | 21 (9, 33) | 39 (27, 51) | 51 (39, 63) | 64 (51, 77) |
| MDP | 19 (7, 30) | 31 (19, 43) | 42 (29, 54) | 64 (52, 77) |
| ∆ Chest press, kg | ||||
| PB | 13 (6, 19) | 21 (14, 27) | 27 (20, 33) | 36 (30, 43) |
| WP | 14 (7, 20) | 22 (16, 29) | 28 (21, 35) | 34 (27, 41) |
| MDP | 12 (6, 19) | 21 (14, 28) | 29 (22, 36) | 38 (31, 45) |
| ∆ Mean of all exercises,2 kg | ||||
| PB | 13 (6, 19) | 22 (15, 28) | 31 (25, 37) | 41 (34, 47) |
| WP | 14 (8, 21) | 23 (17, 30) | 33 (26, 40) | 47 (40, 54) |
| MDP | 13 (6, 19) | 22 (16, 28) | 31 (24, 37) | 43 (37, 50) |
Values are means (95% CIs). Sample sizes/group at 3, 6, 9, and 12 wk, respectively—MDP: n = 20, 17, 16, and 16; PB: n = 23, 23, 23, and 22; and WP: n = 20, 20, 19, and 18. MDP, maltodextrin placebo; PB, soy-dairy protein blend; WP, whey protein; 1-RM, 1 repetition maximum; ∆, change.
The mean represents the mean 1-RM increase from all of the exercises used in training.
Individual chest press, squat, and knee extension 1-RM strength (Table 3) exercises as well as other exercises (calf raise, incline press, knee curl, and seated row) showed similar results (data not shown). For knee extension strength, ANCOVA adjusted for baseline showed a trend (P = 0.06) for a treatment effect at 6 wk, and comparisons indicated a trend for greater knee extension strength in the WP group compared with the MDP group (P = 0.07).
Dynamometry.
At baseline, isometric and isokinetic peak torques for flexion and extension were similar between treatment groups (P > 0.10). Absolute values are shown in Supplemental Table 4 with the main effects from the mixed model.
Isometric knee extension (Table 4, Supplemental Table 4) changed during the study (P < 0.001), but there were no significant treatment × time interactions (P = 0.50) or effects of treatment (P = 0.44). Significantly greater strength was shown at 12 wk than at 6 wk and baseline, and at 6 wk than at baseline, for all treatments (P < 0.005 for all). Isometric knee extension change scores from baseline to 6 wk, 6 to 12 wk, and baseline to 12 wk did not show any treatment effect (P = 0.23, 0.27, and 0.86, respectively).
TABLE 4.
Changes in values of isometric and isokinetic torque in healthy young men who received MDP, PB, or WP supplements for 12 wk of resistance exercise training1
| Change values, Nm |
||
| Treatment | Baseline to 6 wk | Baseline to 12 wk |
| Isometric KE | ||
| PB | 13 (−9, 36) | 32 (9, 55) |
| WP | 29 (5, 52) | 36 (11, 60) |
| MDP | 9 (−15, 33) | 29 (4, 53) |
| Isometric KF | ||
| PB | 13 (2, 25) | 12 (1, 24) |
| WP | 5 (−7, 17) | 11 (−1, 24) |
| MDP | 2 (−11, 14) | 13 (−1, 25) |
| Isokinetic KE | ||
| PB | 4 (−9, 18) | 17 (3, 31)a,b |
| WP | 15 (1, 29) | 19 (4, 34)a |
| MDP | 5 (−9, 20) | 4 (−11, 19)b |
| Isokinetic KF | ||
| PB | 7 (−5, 19) | 6 (−6, 19) |
| WP | 3 (−9, 16) | 13 (−1, 26) |
| MDP | 1 (−11, 14) | 3 (−10, 17) |
Values are means (95% CIs). Sample sizes/group at baseline, 6 wk, and 12 wk, respectively—MDP: n = 23, 19, and 18; PB: n = 23, 23, and 21; and WP: n = 21, 20, and 18. Labeled means (at that time point) without a common superscript letter differ, P < 0.05. KE, knee extension; KF, knee flexion; MDP, maltodextrin placebo; Nm, Newton meters; PB, soy-dairy protein blend; WP, whey protein.
Isometric knee flexion (Table 4, Supplemental Table 4) changed during the study (P <0.001), but there were no significant treatment × time interactions (P = 0.20) or effects of treatment (P = 0.15). Significantly greater strength was shown at 12 wk compared with 6 wk (P = 0.017) and baseline (P < 0.001), and a trend for greater strength at 6 wk was shown compared with baseline (P = 0.07) for all treatments. Isometric knee flexion change scores from baseline to 6 wk and from baseline to 12 wk did not show any treatment effect (P = 0.14 and 0.97, respectively), but there was a trend for a treatment effect for 12-wk to 6-wk change scores (P = 0.06), with a trend for a higher change score for MDP treatment than for PB treatment (P = 0.05).
Isokinetic knee extension (Table 4, Supplemental Table 4) changed during the study (P < 0.001), but there were no significant effects of treatment (P = 0.39); however, there was a trend for a treatment × time interaction (P = 0.09). Greater strength was shown at 12 wk than at baseline in WP (P = 0.007) and PB (P = 0.012) treatment groups, but not in the MDP group (P = 0.99). Isokinetic knee extension change scores from baseline to 6 wk and from 6 wk to 12 wk did not show a significant treatment effect (P = 0.26 and 0.24, respectively); however, there was a significant treatment effect from baseline to 12 wk (P = 0.030). There was a significantly greater 12 wk–baseline change score for the WP group than for the MDP group (P = 0.040), and a trend for a greater change score in the PB group compared with the MDP group (P = 0.07).
Isokinetic knee flexion peak torque (Table 4, Supplemental Table 4) changed during the study (P = 0.014), but there were no significant treatment × time interactions (P = 0.37) or effects of treatment (P = 0.36). Significantly greater strength was shown at 12 wk than at baseline for all treatments (P = 0.010). Isokinetic knee flexion change scores from baseline to 6 wk and from baseline to 12 wk did not show any treatment effect (P = 0.69 and 0.52, respectively).
Knee extensor muscle thickness and mCSA.
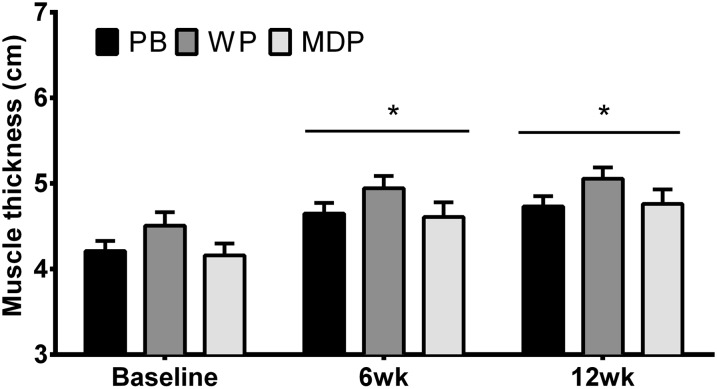
Knee extensor muscle thickness (Figure 2, Supplemental Table 5) changed during the study (P < 0.001), but there were no significant treatment × time interactions (P = 0.86) or effects of treatment (P = 0.16). Mean mCSA (Supplemental Figure 2, with representative images) increased in all groups similarly (∼20%), with no effect of treatment (P = 0.99). This indicated that all treatment groups experienced similar leg muscle hypertrophy.
FIGURE 2.
Vastus lateralis and vastus intermedius muscle thickness in healthy young men who received MDP, PB, or WP supplements during 12 wk of resistance exercise training. Values are means ± SEMs; n = 22 (MDP), n = 22 (WP), and n = 21 (PB). *Different from baseline, P < 0.05. MDP, maltodextrin placebo; PB, soy-dairy protein blend; WP, whey protein.
Body weight.
Body weight (data not shown) changed during the study (P < 0.001), but there were no significant treatment × time interactions (P = 0.12) or effects of treatment (P = 0.33). Body weight change scores from baseline to 6 wk and from 6 wk to 12 wk did not show any treatment effect (P = 0.57 and 0.16, respectively). There was a trend toward a treatment effect on changes from baseline to 12 wk (P = 0.08), with a trend for greater body weight change in the PB group compared with the MDP group (P = 0.08).
Lean body mass.
The absolute values of lean mass were not different (P > 0.10) between treatments at baseline. The change values of lean mass and their corresponding CIs as calculated and analyzed by ANCOVA are shown in Table 5. The absolute values of lean mass, including the main effects from the mixed model, are shown in Supplemental Table 6.
TABLE 5.
Whole-body and regional lean mass changes in healthy young men who received MDP, PB, or WP supplements for 12 wk of resistance exercise training1
| Change over time |
|||
| Treatment | Baseline to 6 wk | 6 wk to 12 wk | Baseline to 12 wk |
| Whole-body LM, g | |||
| PB | 1930 (1129, 2750) | 945 (132, 1760) | 2880 (2060, 3690) |
| WP | 1670 (819, 2520) | 623 (−265, 1510) | 2290 (1410, 3180) |
| MDP | 1720 (874, 2580) | 318 (−570, 1210) | 2040 (1160, 2930) |
| Arm LM, g | |||
| PB | 393 (249, 538) | 180 (38, 330) | 576 (431, 720) |
| WP | 321 (170, 473) | 130 (−32, 280) | 447 (290, 605) |
| MDP | 341 (190, 493) | 65 (−93, 220) | 406 (249, 564) |
| Leg LM, g | |||
| PB | 1000 (600, 1400) | 94 (−310, 500) | 1100 (694, 1500) |
| WP | 772 (352, 1190) | 93 (−350, 530) | 865 (428, 1300) |
| MDP | 770 (350, 1190) | 78 (−360, 520) | 849 (411, 1290) |
| Appendicular LM, g | |||
| PB | 1390 (943, 1840) | 219 (−172, 730) | 1670 (1220, 2120) |
| WP | 1090 (618, 1560) | 219 (−273, 711) | 1310 (817, 1800) |
| MDP | 1120 (642, 1590) | 142 (−351, 634) | 1260 (764, 1750) |
| Trunk LM, g | |||
| PB | 475 (−147, 1100) | 806 (172, 1440) | 1280 (647, 1910) |
| WP | 634 (−17, 1290) | 352 (−327, 1030) | 986 (308, 1660) |
| MDP | 491 (−161, 1140) | 252 (−442, 946) | 742 (49, 1440) |
Values are means (95% CIs). Sample sizes/group at baseline, 6 wk, and 12 wk, respectively—MDP: n = 23, 20, and 18; PB: n = 23, 22, and 22; and WP: n = 22, 20, and 18. LM, lean mass; MDP, maltodextrin placebo; PB, soy-dairy protein blend; WP, whey protein.
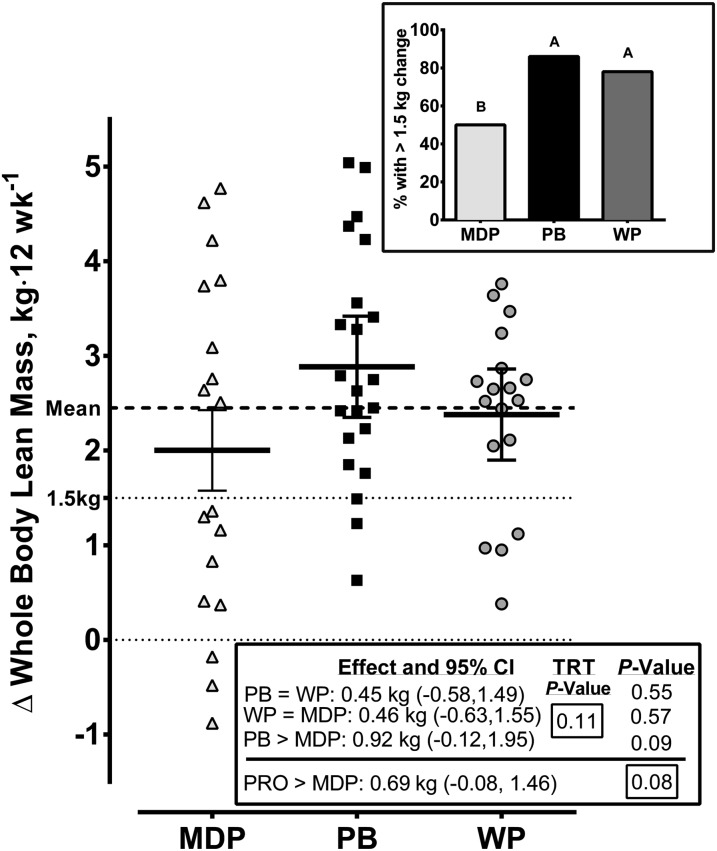
Whole-body lean mass changed during the study (P < 0.001), but there were no significant treatment × time interactions (P = 0.33) or effects of treatment (P = 0.50). Whole-body lean mass showed significant (P < 0.001) increases between each time point over time. Changes in whole-body lean mass scores from baseline to 6 wk, from 6 wk to 12 wk, and from baseline to 12 wk did not show treatment effects (P = 0.76, 0.36, and 0.13, respectively). Whole-body lean mass, by using ANCOVA and covarying for baseline values, indicated no significant treatment effects at 6 wk (P = 0.71) or at 12 wk (P = 0.11). ANCOVA at 12 wk revealed some patterns that approached significance. The data at 12 wk, covarying for baseline, indicated that the PB group showed a trend for a greater change than the MDP group (0.92 kg; 95% CI: −0.12, 1.95 kg; P = 0.09). This was not shown in the WP group compared with the MDP group (0.46 kg; 95% CI: −0.63, 1.55 kg; P = 0.57), and the PB group was not different from the WP group (0.45 kg; 95% CI: −0.58, 1.49 kg; P = 0.54). As such, there was a trend for the pooled PB and WP groups to show a greater increase in whole-body lean mass than in the MDP group from baseline to 12 wk (0.69 kg; 95% CI: −0.08, 1.46 kg; P = 0.08). Supplemental Table 7 presents the overall protein effects (pooled WP and MPD treatments) on lean mass at 6 and 12 wk as calculated from ANCOVA as means and 95% CIs.
Arm, leg, appendicular, and trunk lean masses changed during the study (P < 0.05), but there were no significant treatment × time interactions or effects of treatment. Arm, leg, appendicular, and trunk lean mass across time showed significant (P < 0.001) increases between each time point. The value at 12 wk was greater than at both baseline and 6 wk for arm and trunk lean mass (P < 0.001). Leg and appendicular lean mass at 6 and 12 wk was greater than at baseline (P < 0.001); however, there were no differences between 6 and 12 wk (P > 0.05).
Arm lean mass, covarying for baseline, indicated no treatment effects at 6 wk (P = 0.57) and a trend for a treatment effect at 12 wk (P = 0.09). This treatment difference (Supplemental Figure 3) showed as a trend for a greater arm lean mass in the PB group compared with the MDP group at 12 wk (171 g; 95% CI: −20, 358 g; P = 0.08).
When examining the percentage frequency of responses of whole-body lean mass above the a priori 1.5-kg change threshold expected for a carbohydrate placebo response to RET (inset in Figure 3), both WP (78%) and PB (86%) groups showed a greater frequency of responses than the MDP group (50%) (P < 0.05). This threshold was set a priori on the basis of the mean placebo response across 34 exercise training studies that compared placebo and protein supplementation in young adults.
FIGURE 3.
Absolute changes in whole-body lean mass in healthy young men who received MDP, PB, or WP supplements during 12 wk of resistance exercise training. Values are individual responses. Horizontal lines represent means ± SEMs; n = 18 (MDP), n = 22 (PB), and n = 18 (WP). The inset shows the percentage frequency of responses above the a priori 1.5-kg change threshold expected for a placebo response to resistance exercise training. In the inset, bars without a common letter differ (P < 0.05). MDP, maltodextrin placebo; PB, soy-dairy protein blend; PRO, pooled whey protein and protein blend; TRT, treatment; WP, whey protein.
Changes in lean mass did not correlate with changes in strength; however, absolute values of lean mass correlated with changes in strength (data not shown). Changes from baseline to 12 wk in lean mass were not associated with changes in protein intake (data not shown). The absolute protein intake at all time points was significantly associated with absolute amounts of lean mass, with low model fit to absolute values of lean mass at all time points (r = 0.30–0.35, P < 0.030). As an internal validation of our methods (Supplemental Figure 4), changes from baseline to 12 wk in whole-body lean mass were positively correlated with change in muscle thickness (r = 0.47, P < 0.001).
Fat mass and bone.
The absolute values of fat mass and relative body fat were not different between treatments at baseline (Table 6, Supplemental Table 6). Relative body fat changed during the study (P < 0.001), but there were no significant treatment × time interactions (P = 0.77) or effects of treatment (P = 0.47). Universal decreases (P < 0.05) from baseline to 6 wk and from baseline to 12 wk drove the effect for a decrease in relative body fat (Table 6).
TABLE 6.
Changes in relative and absolute total and trunk body fat in healthy young men who received MDP, PB, or WP supplements for 12 wk of resistance exercise training1
| Change over time |
|||
| Treatment | Baseline to 6 wk | 6 wk to 12 wk | Baseline to 12 wk |
| Relative fat, % | |||
| PB | −0.9 (−1.8, −0.1) | −0.4 (−1.3, 0.5) | −1.3 (−2.3, −0.4) |
| WP | −1.2 (−2.1, −0.2) | −0.8 (−1.8, 0.2) | −1.9 (−2.9, −0.9) |
| MDP | −1.0 (−2.0, −0.1) | −0.7 (−1.7, 0.3) | −1.7 (−2.7, −0.7) |
| Fat mass, g | |||
| PB | −360 (−1200, 510) | −120 (−1000, 770) | −480 (−1360, 410) |
| WP | −590 (−1500, −320) | −570 (−1500, 380) | −1162 (−2100, −210) |
| MDP | −560 (−1500, 350) | −590 (−1600, 380) | −1156 (−2100, −190) |
| Fat mass trunk, g | |||
| PB | −290 (−880, 300) | 17 (−580, 620) | −270 (−870, 330) |
| WP | −340 (−960, 280) | −359 (−1000, 290) | −700 (−1300, −53) |
| MDP | −360 (−980, 260) | −437 (−1100, 220) | −790 (−1500, −130) |
Values are means (95% CIs). Sample sizes/group at baseline, 6 wk, and 12 wk, respectively—MDP: n = 23, 20 and 18; PB: n = 23, 22, and 22; and WP: n = 22, 20, and 18. MDP, maltodextrin placebo; PB, soy-dairy protein blend; WP, whey protein.
Trunk fat mass changed during the study (P < 0.001), but there were no significant treatment × time interactions (P = 0.42) or effects of treatment (P = 0.49). Bone mineral content (BMC) did not differ between treatments at baseline (data not shown). Although there was no significant effect of treatment on BMC, there were effects over time. Specifically, there were increases in BMC with WP and PB treatments from baseline to 6 wk and from baseline to 12 wk (P < 0.05 in all cases). There was a trend for a difference between pooled WP and PB treatments and the MDP treatments at 12 wk (P = 0.07).
Discussion
To our knowledge, clinical trials have yet to show a consistent effect of protein/amino acid supplementation on enhancing RET outcomes compared with placebo (8–10, 36). Current theories posit that protein type may be a modulating factor behind this inconsistency. Almost all of the selected protein types investigated were single-protein sources and types, with few comparisons of a blended protein supplement against single proteins (i.e., whey) or an isocaloric placebo. We previously showed the effectiveness of a PB in promoting muscle protein synthesis in response to an acute bout of high-intensity resistance exercise (12, 13). We thereby tested this novel and promising protein combination against WP and isocaloric MDP supplementation during 3 mo of RET in young healthy men. All of the treatments improved lean mass, muscle thickness, and muscle mCSA and strength, as would be expected during a progressive resistance training program. However, although we did not find treatment differences in lean mass changes, after 3 mo of RET the PB group had a marginally higher whole-body lean mass than the MDP group by 0.92 kg (95% CI: −0.12, 1.95 kg; P = 0.09). This was not shown in the WP compared with MDP group (0.46 kg; 95% CI: −0.63, 1.55 kg; P = 0.57), and the PB group was not different from the WP group (0.45 kg; 95% CI: −0.58, 1.49 kg; P = 0.55) (Figure 3). Interestingly, all of the treatments improved all regions of lean mass to a similar extent during the first 6 wk, but further analysis of the data suggests that the PB continued to increase whole-body, arm, and trunk lean mass over the remaining 6 wk, although there were no prominent treatment differences in the 6- to 12-wk change. Further comparisons at 12 wk showed that the PB group tended (P = 0.08) to have greater arm lean mass than the MDP group. This was not shown in the WP compared with MDP treatment, and PB treatment did not differ from the WP treatment. This suggests that most of the effect of PB supplementation on lean mass occurred in the upper body. The PB supplement enhanced lean mass in the whole body and arms to values that were 3–5% outside the standard 95% CIs, suggesting a very weak, but probable, ability of PB supplementation to enhance lean mass. However, because there were no treatment effects on more direct measures of muscle mass/size, these changes in whole-body and arm lean mass are likely to be irrelevant with regard to muscle function. Others might speculate that effects between treatments would be resolved over a longer (>3 mo) exercise training duration; yet, as we discussed elsewhere (36), muscle hypertrophy would likely have reached a plateau by 3 mo.
Although the group mean for the change in lean mass in our WP group, ∼2.3 kg, is almost identical to the amount shown via meta-analysis of RET and WP supplementation (37), our WP treatment did not differ significantly from placebo (0.46 kg; 95% CI: −0.63, 1.55 kg; P = 0.57), which, in itself, is not an uncommon finding (21, 38, 39). These findings are not the result of low compliance to the WP treatment; correlation analysis between treatment compliance and primary outcomes in this group indicated no relation (see Results). In this case, we believe this lack of an effect was largely due to the heterogeneity of responses in the MDP group (Figure 3). This observation in placebo participants supports the concept that some individuals are high responders to RET regardless of nutritional intervention (40, 41). The fact that some individuals do not respond to RET regardless of added nutritional variance (41) may be the most likely reason for the inconsistency for an effect of protein supplements in the literature. To test the consistency of changes in lean mass, we determined the percentage frequency of responses for each treatment above an a priori 1.5-kg change threshold expected for a placebo response to RET (inset in Figure 3). This analysis revealed that both the PB and WP groups exhibited consistently more responses (86% and 78%, respectively) above this threshold than the MDP group (50%), suggesting that these protein supplements were more reliably effective in enhancing whole-body lean mass gain. In addition, after combining the protein treatments, a strong trend for an effect of the pooled PB and WP treatments compared with MDP treatment only was observed (0.69 kg; 95% CI: −0.08, 1.46 kg; P = 0.08) that was similar in magnitude to that shown for untrained young adults via an unadjusted meta-analysis (8). However, the absolute change in daily protein intake did not correlate with changes in lean mass. These data add further support to the concept that increasing absolute protein intake above habitual intakes of 1.2–1.3 g ⋅ kg−1 ⋅ d−1 may not further enhance lean mass during RET (42, 43). Rather, the timing or distribution of protein throughout the day may play a more pivotal role in the regulation of lean mass (44), but this commonly promoted theory has yet to be proven (36). Indeed, optimal changes in muscle size and strength after RET may not be required if additional protein in a well-balanced diet is consumed (36).
The enhancement in lean mass with protein supplementation did not translate to improved strength at the time of our assessment. Enhancement in strength during RET is not a universal finding with protein supplementation (10). As discussed elsewhere (36), the accrual of lean/muscle mass is not well coupled to changes in strength early on in a strength-training program regardless of supplementation.
The observed increases in lean mass shown in young subjects during RET are always assumed to be increases in muscle, yet the effect of protein supplementation on lean mass measured by DXA is primarily tested at the whole-body level (8), and appendicular lean mass is rarely reported. For example, only a few studies, to our knowledge, have included data describing regional changes in lean mass after RET and pooled WP and PB/amino acid supplementation (45, 46). To fill these gaps in the literature, we provided a table (Supplemental Table 7) that highlights the overall effect of protein (WP and PB groups pooled) at 6 and 12 wk of training. It is possible that changes in lean mass may not reflect contractile protein accrual and may partially explain why changes in lean mass are infrequently coupled to changes in muscle strength.
Meta-analysis has shown that soy protein does not alter testosterone profile (47, 48); this idea still remains a common myth. As further support of this evidence from these meta-analyses, we found no changes in serum testosterone with a soy-dairy PB or WP during RET.
Limitations.
Treatment compliance was likely underestimated in this intention-to-treat analysis due to inflation from the inclusion of data from dropout subjects and poor non–exercise day treatment record-keeping by the research participants. However, we conducted correlation analysis between treatment compliance and primary outcomes of changes in whole-body lean mass, leg lean mass, and muscle thickness and these analyses indicated no relations (R2 = 0.002–0.01, P = 0.40–0.90). Thus, our findings are not a result of compliance issues. The findings from this clinical trial are limited to young, healthy, recreationally active men undergoing this particular type of RET. Further research should examine other exercise modes and subject populations.
Conclusions.
We previously showed that PB supplementation prolongs muscle protein synthesis and muscle protein net balance (12, 13). The current study showed that PB supplementation may provide a marginal, but probable, effect on enhancing lean mass growth during RET in young men, which appears to be limited to the upper body. A post hoc power analysis of whole-body lean mass indicated that with a 0.30 treatment effect size, the power to detect this effect was 0.50, and a total of 70 completed participants would have been needed to obtain a significant treatment effect at 95% confidence; however, we only completed 59 participants. In addition, our 2 independent and more direct measures of muscle hypertrophy (thigh muscle thickness and mCSA) showed that hypertrophy will occur similarly regardless of supplementation, which suggests that changes in lean mass by using DXA may be somewhat misleading. In conclusion, PB supplementation during 3 mo of RET may marginally enhance lean body mass, but not leg muscle mass, compared with RET without protein supplementation. Furthermore, the effect of protein supplementation on enhancing outcomes during RET is minimal at best and does not improve muscle function or muscle size in this young, healthy, active population. These findings are intriguing and point to the limited effectiveness of protein supplementation beyond “normal” intake amounts of protein during RET.
Acknowledgments
We thank Samantha Dillon, Matthew Nguyen, Benjamin Brightwell, Camille Brightwell, and Jennifer Thedinga for their assistance in supervising the exercise training of research participants. PTR, MMM, JMD, RRD, SHH, DKW, SI, MBC, RM, EV, and BBR designed the research; PTR, MSB, JMD, RRD, SHH, DKW, SI, and SMR conducted the research; PTR, MSB, JMD, DKW, MBC, RM, KJ, EV, and BBR reviewed the manuscript; PTR, MSB, JMD, RRD, SMR, JMH-P, KJ, and BBR analyzed the data; PTR and BBR wrote the manuscript and had primary responsibility for final content; KJ generated the random allocation sequence; PTR, MSB, SHH, and EV enrolled participants; and PTR assigned participants to coded interventions. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BMC, bone mineral content; ITS-CRC, Institute for Translational Sciences–Clinical Research Center; MDP, maltodextrin placebo; mCSA, myofiber cross-sectional area; mTORC1, mammalian target of rapamycin complex 1; PB, soy-dairy protein blend; RET, resistance exercise training; VL, vastus lateralis; WP, whey protein; 1-RM, 1 repetition maximum.
References
- 1.Rahbek SK, Farup J, Moller AB, Vendelbo MH, Holm L, Jessen N, Vissing K. Effects of divergent resistance exercise contraction mode and dietary supplementation type on anabolic signalling, muscle protein synthesis and muscle hypertrophy. Amino Acids 2014;46:2377–92. [DOI] [PubMed] [Google Scholar]
- 2.Farnfield MM, Breen L, Carey KA, Garnham A, Cameron-Smith D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl Physiol Nutr Metab 2012;37:21–30. [DOI] [PubMed] [Google Scholar]
- 3.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 2008;294:E392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camera DM, West DW, Burd NA, Phillips SM, Garnham AP, Hawley JA, Coffey VG. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. J Appl Physiol 2012;113:206–14. [DOI] [PubMed] [Google Scholar]
- 5.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, Frystyk J, Flyvbjerg A, Schjerling P, van Hall G, et al. Whey and casein labeled with L-[1-13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am J Physiol Endocrinol Metab 2011;300:E231–42. [DOI] [PubMed] [Google Scholar]
- 6.Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab 2007;32:1132–8. [DOI] [PubMed] [Google Scholar]
- 7.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 1997;273:E122–9. [DOI] [PubMed] [Google Scholar]
- 8.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 9.Pasiakos SM, McLellan TM, Lieberman HR. The effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: a systematic review. Sports Med 2015;45:111–31. [DOI] [PubMed] [Google Scholar]
- 10.Schoenfeld BJ, Aragon AA, Krieger JW. The effect of protein timing on muscle strength and hypertrophy: a meta-analysis. J Int Soc Sports Nutr 2013;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosse JD, Dixon BM. Dietary protein to maximize resistance training: a review and examination of protein spread and change theories. J Int Soc Sports Nutr 2012;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Cope MB, Mukherjea R, Jennings K, Volpi E, et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol 2014;116:1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Fry CS, Borack MS, Cope MB, Mukherjea R, et al. Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. J Nutr 2013;143:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 2012;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr 2012;108:958–62. [DOI] [PubMed] [Google Scholar]
- 16.Dideriksen KJ, Reitelseder S, Petersen SG, Hjort M, Helmark IC, Kjaer M, Holm L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand J Med Sci Sports 2011. [DOI] [PubMed] [Google Scholar]
- 17.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 2009;107:987–92. [DOI] [PubMed] [Google Scholar]
- 18.Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc 2004;36:2073–81. [DOI] [PubMed] [Google Scholar]
- 19.Volek JS, Volk BM, Gomez AL, Kunces LJ, Kupchak BR, Freidenreich DJ, Aristizabal JC, Saenz C, Dunn-Lewis C, Ballard KD, et al. Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr 2013;32:122–35. [DOI] [PubMed] [Google Scholar]
- 20.Joy JM, Lowery RP, Wilson JM, Purpura M, De Souza EO, Wilson SM, Kalman DS, Dudeck JE, Jager R. The effects of 8 weeks of whey or rice protein supplementation on body composition and exercise performance. Nutr J 2013;12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herda AA, Herda TJ, Costa PB, Ryan ED, Stout JR, Cramer JT. Muscle performance, size, and safety responses after eight weeks of resistance training and protein supplementation: a randomized, double-blinded, placebo-controlled clinical trial. J Strength Cond Res 2013;27:3091–100. [DOI] [PubMed] [Google Scholar]
- 22.Candow DG, Burke NC, Smith-Palmer T, Burke DG. Effect of whey and soy protein supplementation combined with resistance training in young adults. Int J Sport Nutr Exerc Metab 2006;16:233–44. [DOI] [PubMed] [Google Scholar]
- 23.Brown EC, DiSilvestro RA, Babaknia A, Devor ST. Soy versus whey protein bars: effects on exercise training impact on lean body mass and antioxidant status. Nutr J 2004;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demling RH, DeSanti L. Effect of a hypocaloric diet, increased protein intake and resistance training on lean mass gains and fat mass loss in overweight police officers. Ann Nutr Metab 2000;44:21–9. [DOI] [PubMed] [Google Scholar]
- 25.Babault N, Deley G, Le Ruyet P, Morgan F, Allaert FA. Effects of soluble milk protein or casein supplementation on muscle fatigue following resistance training program: a randomized, double-blind, and placebo-controlled study. J Int Soc Sports Nutr 2014;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci 2015;80(Suppl 1):A8–15. [DOI] [PubMed] [Google Scholar]
- 27.Churchward-Venne TA, Breen L, Di Donato DM, Hector AJ, Mitchell CJ, Moore DR, Stellingwerff T, Breuille D, Offord EA, Baker SK, et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: a double-blind, randomized trial. Am J Clin Nutr 2014;99:276–86. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 2012;108:1780–8. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr 2007;85:1031–40. [DOI] [PubMed] [Google Scholar]
- 30.Reitelseder S, Agergaard J, Doessing S, Helmark IC, Schjerling P, van Hall G, Kjaer M, Holm L. Positive muscle protein net balance and differential regulation of atrogene expression after resistance exercise and milk protein supplementation. Eur J Nutr 2014;53:321–33. [DOI] [PubMed] [Google Scholar]
- 31.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 2007;86:373–81. [DOI] [PubMed] [Google Scholar]
- 32.Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 2015;47:1922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergström J. Muscle electrolytes in man. Vol. 14, Suppl 68. Oslo (Norway): Scandinavian Journal of Clinical and Laboratory Investigation; 1962. [Google Scholar]
- 34.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 2010;140:1970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA, Peterson CA. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol 2014;592:2625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reidy PT, Rasmussen BB. Role of ingested amino acids and protein in the promotion of resistance exercise-induced muscle protein anabolism. J Nutr 2016;146:155–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller PE, Alexander DD, Perez V. Effects of whey protein and resistance exercise on body composition: a meta-analysis of randomized controlled trials. J Am Coll Nutr 2014;33:163–75. [DOI] [PubMed] [Google Scholar]
- 38.Weisgarber KD, Candow DG, Vogt ES. Whey protein before and during resistance exercise has no effect on muscle mass and strength in untrained young adults. Int J Sport Nutr Exerc Metab 2012;22:463–9. [DOI] [PubMed] [Google Scholar]
- 39.Erskine RM, Fletcher G, Hanson B, Folland JP. Whey protein does not enhance the adaptations to elbow flexor resistance training. Med Sci Sports Exerc 2012;44:1791–800. [DOI] [PubMed] [Google Scholar]
- 40.Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol 2011;110:309–17. [DOI] [PubMed] [Google Scholar]
- 41.Thalacker-Mercer AE, Petrella JK, Bamman MM. Does habitual dietary intake influence myofiber hypertrophy in response to resistance training? A cluster analysis. Appl Physiol Nutr Metab 2009;34:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonio J, Peacock CA, Ellerbroek A, Fromhoff B, Silver T. The effects of consuming a high protein diet (4.4 g/kg/d) on body composition in resistance-trained individuals. J Int Soc Sports Nutr 2014;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman JR, Ratamess NA, Kang J, Falvo MJ, Faigenbaum AD. Effect of protein intake on strength, body composition and endocrine changes in strength/power athletes. J Int Soc Sports Nutr 2006;3:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 2014;144:876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ispoglou T, King RF, Polman RC, Zanker C. Daily L-leucine supplementation in novice trainees during a 12-week weight training program. Int J Sports Physiol Perform 2011;6:38–50. [DOI] [PubMed] [Google Scholar]
- 46.Rankin JW, Goldman LP, Puglisi MJ, Nickols-Richardson SM, Earthman CP, Gwazdauskas FC. Effect of post-exercise supplement consumption on adaptations to resistance training. J Am Coll Nutr 2004;23:322–30. [DOI] [PubMed] [Google Scholar]
- 47.Niederberger C. Re: clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: results of a meta-analysis. J Urol 2011;185:638. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton-Reeves JM, Vazquez G, Duval SJ, Phipps WR, Kurzer MS, Messina MJ. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: results of a meta-analysis. Fertil Steril 2010;94:997–1007. [DOI] [PubMed] [Google Scholar]