Abstract
Background: The most rapid phase of brain development occurs during the neonatal period. Vitamin A (VA; retinol) is critical for many aspects of this process, including neurogenesis, synaptic plasticity, learning, and memory formation. However, the metabolism of retinol in the neonatal brain has not been extensively explored.
Objective: We examined the uptake of VA into the brain in neonatal rats raised under VA-marginal conditions (control group) and assessed the effect of VA supplementation on the uptake of VA into the brain.
Methods: Sprague-Dawley neonatal rats (n = 104) nursed by mothers fed a VA-marginal diet were randomly assigned and treated on postnatal day 4 with an oral dose of either VA (6 μg retinyl palmitate/g body weight) or canola oil as the control, both of which contained 1.8 μCi [3H]retinol. Pups (n = 4/group at a time) were killed at 13 sampling times from 30 min to 24 d after dosing. The uptake of total retinol, chylomicron-associated retinyl esters (REs), and retinol bound to retinol-binding protein (RBP) was estimated with the use of WinSAAM version 3.0.8.
Results: Total retinol mass in the brain was closely dependent on its mass in plasma over time (r = 0.91; P < 0.001). The uptake of retinol into the brain involved both postprandial chylomicrons and RBP, with RBP delivering most of the retinol in the control group [0.27 nmol/d (RBP) compared with 0.01 nmol/d (chylomicrons)]. VA supplementation increased the fractional uptake of chylomicron REs from 0.3% to 1.2% of plasma pool/d, decreased that of RBP retinol from 0.5% to 0.2% of plasma pool/d, and increased the transfer rate of chylomicron REs from nearly zero to 0.7 nmol/d, causing a day-long elevation in the brain mass of total retinol.
Conclusion: Postprandial chylomicrons may be a primary mechanism for delivering a recently ingested large dose of VA to the brain of neonatal rats raised under VA-marginal conditions.
Keywords: brain, chylomicron, neonate, rat, retinol, retinyl esters, supplementation, uptake, vitamin A
Introduction
The most rapid phase of human brain development occurs during the late fetal and early postnatal period (1). From ∼34 wk of gestation until 2 y of age in humans, ∼40,000 new neural connections are formed every second (2), and the process of myelination proceeds at its peak rate, causing a rapid accretion of white matter (3). Within 3 y after birth, the brain weight increases 3-fold (4). This process requires not only a large amount of energy, with ∼60% of total calories devoted to building new neurons and their connections (5, 6), but also specific micronutrients such as vitamin A (VA6; retinol).
VA is a fat-soluble vitamin that is essential for the proliferation and differentiation of many cell types, including those in the central nervous system. Retinoic acid, the transcriptionally active metabolite of VA, has been long recognized as a regulator of neuronal patterning in the developing embryo as well as an inducer of molecular events that lead to the development of a fully differentiated neuron (7). More evidence suggests that these processes continue into the postnatal period and even into adulthood, particularly in the hippocampus, olfactory bulb, and hypothalamus, in which neurons continue to proliferate in response to environmental factors (6, 8, 9). In these brain regions, retinoic acid has been shown to support not only neurogenesis but also neural differentiation, survival, and synaptic plasticity (9–13).
Substantial evidence also exists to support VA’s role in the functional outcomes of neuronal differentiation and plasticity, especially the formation of new memories and learning. For example, VA deficiency in rodents leads to reduced performance in memory tasks, such as maze navigation and social recognition (14–17), and these deficits can be restored in rats after VA refeeding (18). Similarly, high doses of retinoic acid given to mice cause parallel cognitive deficits, suggesting that the brain concentration of retinoic acid must be maintained within a homeostatic range to ensure optimal cognition (19, 20).
Despite growing evidence pointing to the integral role of VA in the developing brain, the metabolism of VA in neonates remains poorly defined. This knowledge gap is particularly disconcerting given the high prevalence of VA deficiency in low-income countries, especially in Africa and Southeast Asia, and the ongoing VA supplementation trials, in which high-dose (50,000 IU) VA supplements are given to infants within the first month of life (21). More mechanistic studies are needed to examine the effect of supplementation on VA metabolism in the neonatal brain as well as any potential effects on cognitive development.
In this study, our objective was to use model-based compartmental analysis to quantify the uptake of retinol into the neonatal brain, without and after VA supplementation, with distinct kinetic profiles generated for the 2 major molecular forms of VA in plasma: retinyl esters (REs) carried in postprandial chylomicrons and retinol bound to retinol-binding protein (RBP). Based on a previous study of VA kinetics in neonatal rats (22) that showed an increased uptake of both chylomicron REs and RBP retinol into a carcass composed of several peripheral tissues, including the brain, we hypothesized that VA supplementation would increase the amount of RBP retinol taken up by the brain because of its lower molecular weight and potentially greater permeability through the blood-brain barrier.
Methods
Animals and diet.
Eleven pregnant Sprague-Dawley rats (Charles River Laboratories) were fed a VA-marginal AIN-93G-purified diet (23) modified to contain 0.35 mg retinol equivalents or 1.2 IU diet/kg (Research Diets). The VA-marginal diet was provided from day 13 of pregnancy and throughout lactation to render pups in a state similar to that of low-birthweight infants in regions with a high prevalence of VA deficiency. All dams were housed individually in a room with a 12-h light/dark cycle at 22°C with free access to food and water. All animal procedures were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
Dose administration.
Over 2 consecutive days, dams gave birth to a total of 116 pups that were subsequently redistributed into 11 litters to avoid any effects resulting from litter size differences. Approximately 9–10 pups from each litter were randomly assigned to the two treatments: control (n = 52; 33 males) and VA supplementation (n = 52; 30 males). On postnatal day 4, all pups received 1.8 μCi 11,12-[3H]retinol (PerkinElmer) in an oral dose of either VA mixed with canola oil in a ratio of 1:3 or pure canola oil as the control treatment. The VA supplement consisted of all-trans-retinyl palmitate (Sigma-Aldrich) calculated to provide 50,000 IU, as is given to infants (24). The dose was scaled to the body weight of neonatal rats (∼10 g), prepared, and delivered according to a method described previously (22). The final mean dose corrected for losses incurred during administration (the amount left in the pipetting tip and on a pup’s muzzle) contained 1.4 μCi [3H]retinol.
Tissue collection.
At 13 sampling times after dosing (0.5, 1, 4, 8, and 15 h and 1, 2, 4, 8, 11, 14, 18, and 22 d), 4 pups/group were removed from cages, weighed, and killed with isoflurane or, after 14 d, with CO2. Blood was collected from the vena cava, centrifuged at 800 × g for 15 min, and stored at −20°C. Brains were excised, snap-frozen in liquid nitrogen, and stored at −80°C until analysis.
Tracer and retinol mass analyses.
To quantify the tracer, lipids were extracted from plasma and tissue homogenates with the use of a previously described procedure (22, 25). Briefly, brain samples (∼0.2 g) were homogenized and incubated in 100% ethanol for 1 h and saponified with 5% potassium hydroxide. Neutral lipids were partitioned into hexanes containing 0.1% butylated hydroxytoluene. After centrifugation, the upper-phase hexanes were removed, and the partition step was repeated to attain ∼97% extraction efficiency, as determined in prior pilot testing. The upper phases were pooled and transferred into liquid scintillation vials and evaporated, and the residue was redissolved in 4 mL scintillation fluid. A radioactivity analysis was performed with the use of the LS 6500 liquid scintillation counting system (Beckman Coulter), with each sample counted to a 1% error. The total radioactivity measured in the brain was corrected for the radioactivity of residual plasma circulating in the brain, which was calculated based on previously published estimates of the mass of plasma in the brain and the total blood volume in a rat (26, 27) (Supplemental Figure 1).
Total retinol mass (unesterified retinol + REs) in the brain was determined with the use of an Acquity ultra-performance liquid chromatography (UPLC) system (Waters) according to an adaptation of a previously described method (25). Briefly, a measured portion of the upper-phase hexanes was pooled from each extraction, and the internal standard (trimethylmethoxyphenyl-retinol) was added. The solvent was dried under nitrogen, and the residue was reconstituted in 100 μL methanol for injection onto the column. All results were corrected for the unextracted 3% of lipids.
Kinetic analysis.
VA kinetics were determined with the use of model-based compartmental analysis, which describes biological systems in terms of homogenous states called compartments connected through plasma, which serves as a medium for material exchange between compartments (28). The flow of material is quantified by fractional transfer coefficients, denoted as L(I,J)s, which represent the fraction of dose transferred from compartment (J) to compartment (I) or out of the system (0) per unit time. The number of compartments and L(I,J)s specify the structure and interconnectivities of the model. The initial model structure is based on a priori knowledge about the physiology and biochemistry of the system.
The kinetic analysis was conducted in WinSAAM version 3.0.8. The program input file contained the following components: observed data, L(I,J)s, compartment masses, and the plasma-forcing function, which specified the tracer amount in plasma available for uptake at any point in time. The observed data were expressed as the mean fraction of ingested dose (total radioactivity in the brain divided by the radioactivity in the dose) from 4 pups killed at each sampling time and weighted by the SD. The initial values of L(I,J)s were adapted from the study of VA kinetics in a neonatal rat’s lungs (22). The mean mass of chylomicron REs and RBP retinol in plasma were entered under a steady-state solution in WinSAAM to calculate the mean mass of chylomicron- and RBP-derived retinol in brain, respectively. Finally, the forcing function was obtained from the previously developed model of VA kinetics in plasma and consisted of data observed at 13 sampling times and values interpolated by the program to generate a continuous plasma tracer-response curve. The forcing function approach was used to uncouple the brain from the rest of the system and to model it individually based on the assumption that VA enters the brain directly from plasma but not from other organs. Because the transfer of VA from plasma to the brain was assumed to be unidirectional, the irreversible loss of VA included both the VA metabolized locally in the brain and the VA released back to plasma.
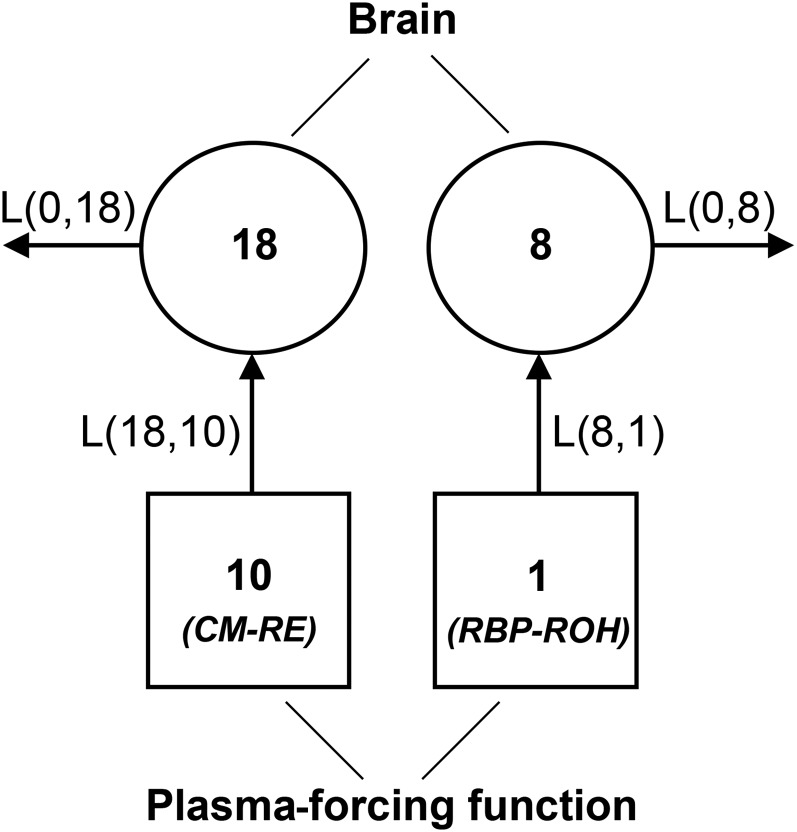
The model structure was based on the model of VA kinetics in neonatal lungs (22) and included plasma compartments 10 (chylomicron REs) and 1 (RBP retinol), both specified by the forcing function, directly exchanging with brain compartments 18 (chylomicron-derived retinol) and 8 (RBP-derived retinol), from which retinol was also lost irreversibly (Figure 1). L(18,10) and L(8,1) represented the fraction of retinol taken up each day from chylomicrons and RBP, respectively. L(0,18) and L(0,8) represented the irreversible loss of chylomicron- and RBP-derived retinol, respectively. The model parameters were adjusted in a stepwise manner to obtain the best fit of the model-calculated curve to the observed data. After finding a satisfactory fit, the final parameter values were generated through the application of weighted nonlinear regression analysis, which minimized the residuals given the weight assigned to the data, parameter constraints, and their uniqueness.
FIGURE 1.
Model of VA kinetics in the brain of neonatal rats dosed orally with [3H]ROH on postnatal day 4. Circles represent compartments; arrows represent their interconnectivities. Squares indicate compartments defined by the plasma-forcing function. Compartments 10 and 1 represent plasma CM-associated REs and RBP-bound ROH, respectively. Compartments 18 and 8 represent brain retinol derived from CMs and RBP, respectively. CM, chylomicron; L(I,J), fractional transfer coefficient, representing the fraction of dose transferred from compartment (J) to compartment (I); RBP, retinol-binding protein; RE, retinyl ester; ROH, retinol; VA, vitamin A.
Statistical analyses.
The weight of the brain and retinol mass measured at each sampling time were expressed as means ± SEMs and compared statistically by using Student’s t test with Bonferroni correction for multiple comparisons. The correlation between plasma and the brain fraction of dose was calculated with the use of the Pearson formula in GraphPad Prism version 5.0. Fractional transfer coefficients [L(I,J)s] were estimated in WinSAAM. Transfer rates [R(I,J)s] were calculated as the product of VA mass in the origin compartment, measured by UPLC or estimated in WinSAAM, and the corresponding L(I,J). The SEMs of the resulting parameters were calculated according to the error propagation formula 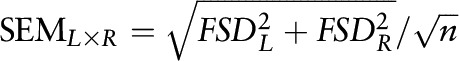 (where FSD is fractional SD). All kinetic parameters were expressed as means ± SEMs and compared statistically with the use of Student’s t test, with a t-statistic calculated for each parameter pair and evaluated for significance with the use of the table of critical values of Student’s t-distribution. P < 0.05 was considered significant.
(where FSD is fractional SD). All kinetic parameters were expressed as means ± SEMs and compared statistically with the use of Student’s t test, with a t-statistic calculated for each parameter pair and evaluated for significance with the use of the table of critical values of Student’s t-distribution. P < 0.05 was considered significant.
Results
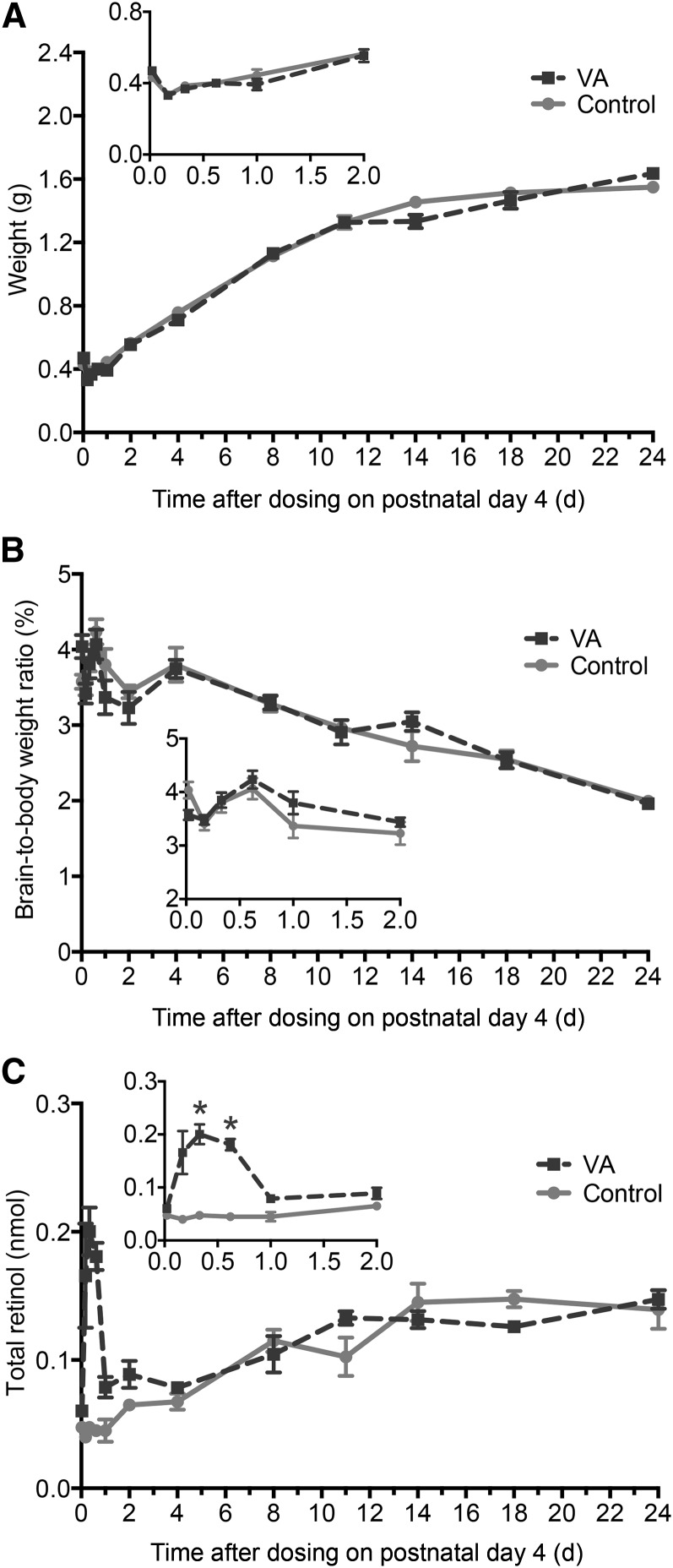
On postnatal day 4, pups had a normal body weight (10.8 ± 0.1 g) and a plasma VA concentration (0.8 ± 0.1 μmol/L) within the marginal range of 0.7–1 μM as defined for adult rats (29). During the study, the weight of the brain increased 3-fold without differences because of VA supplementation (Figure 2A), whereas the brain-to-body weight ratio decreased by 50% (Figure 2B). The mass of total retinol in the brain increased ∼2-fold in both treatment groups but was insufficient in matching the rapid increase in brain weight. VA supplementation increased the mass of total retinol ∼3-fold from the control group value at 8 and 15 h after dosing (P < 0.001 for both), with no significant differences thereafter (Figure 2C).
FIGURE 2.
Brain weight (A), brain-to-body weight ratio (B), and total retinol mass in the brain (C) in control and VA-supplemented rats from 0 to 24 d after dosing on postnatal day 4. Insets show the first 2 d after dosing. Each symbol represents the mean ± SEM of 4 rats. *P < 0.05. VA, vitamin A.
Tracer-response profiles.
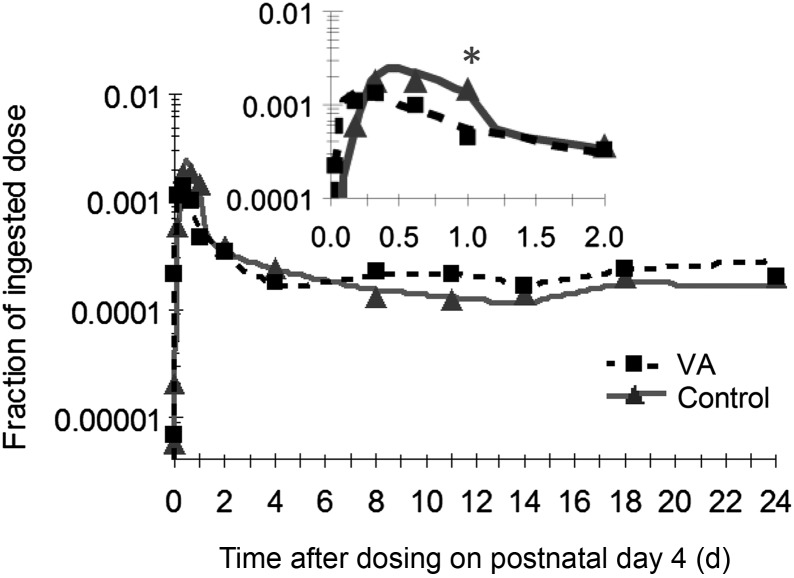
The tracer entered the brain within the first hour after dosing and peaked between 8 and 14 h (Figure 3). On a fractional basis, the uptake of retinol seemed to be more efficient in the control group, as evidenced by the higher peak value attained at 12 h (Figure 3, inset); however, the fraction of dose peaked and declined earlier in the supplemented group, with a significant difference observed at 1 d after dosing (0.2% ± 0.01% compared with 0.05% ± 0.01%; P < 0.001). After the initial peak separation, the fraction of dose decreased to ∼0.03% and remained in a steady state for the remainder of the study. There was a notably strong correlation (r = 0.91; P < 0.001) between the tracer response observed in plasma and that observed in the brain in both treatment groups (Supplemental Figure 2).
FIGURE 3.
Observed (symbols) and model-predicted (lines) fraction of ingested [3H]retinol dose in the brain in control and VA-supplemented rats from 0 to 24 d after dosing on postnatal day 4. Inset shows the first 2 d after dosing. Each symbol represents the mean of 4 rats. *P < 0.05. VA, vitamin A.
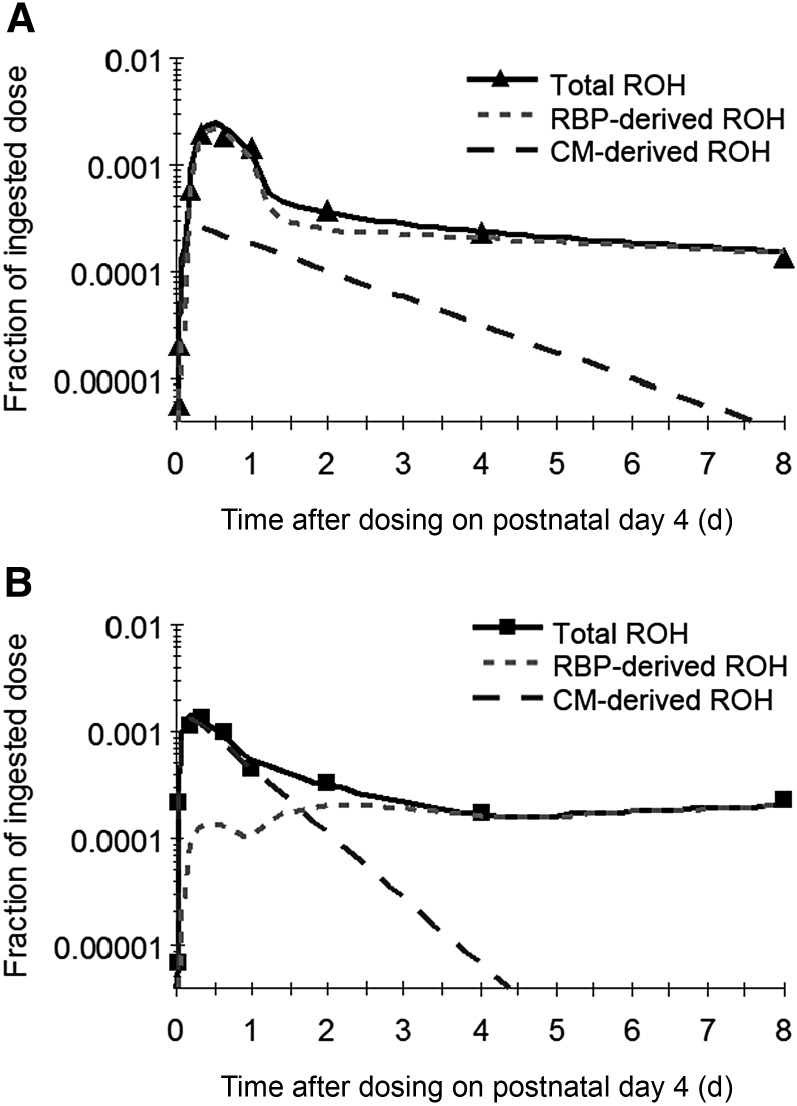
To trace the kinetics of chylomicron- and RBP-derived retinol in the brain, 2 distinct tracer-response curves were generated with the use of the XG function, which defined the observed value of total retinol as the sum of chylomicron- and RBP-derived retinol [XG(26) = F(18) + F(8)]. These curves showed that most retinol taken up by the brain in the control group was delivered by RBP, whereas in the VA-supplemented pups the uptake consisted mainly of chylomicron-derived retinol (Figure 4). The chylomicron-derived retinol was also rapidly metabolized (or released back to plasma) in the VA-supplemented group, as evidenced by the sharp decline of the curve.
FIGURE 4.
Observed (symbols) and model-predicted (lines) fraction of ingested dose as CM- and RBP-derived ROH as well as their sum (total ROH) in the brain in control (A) and VA-supplemented (B) rats from 0 to 8 d after dosing on postnatal day 4. Each symbol represents the mean of 4 rats. CM, chylomicron; RBP, retinol-binding protein; ROH, retinol; VA, vitamin A.
Model-derived kinetic parameters and compartment masses of VA.
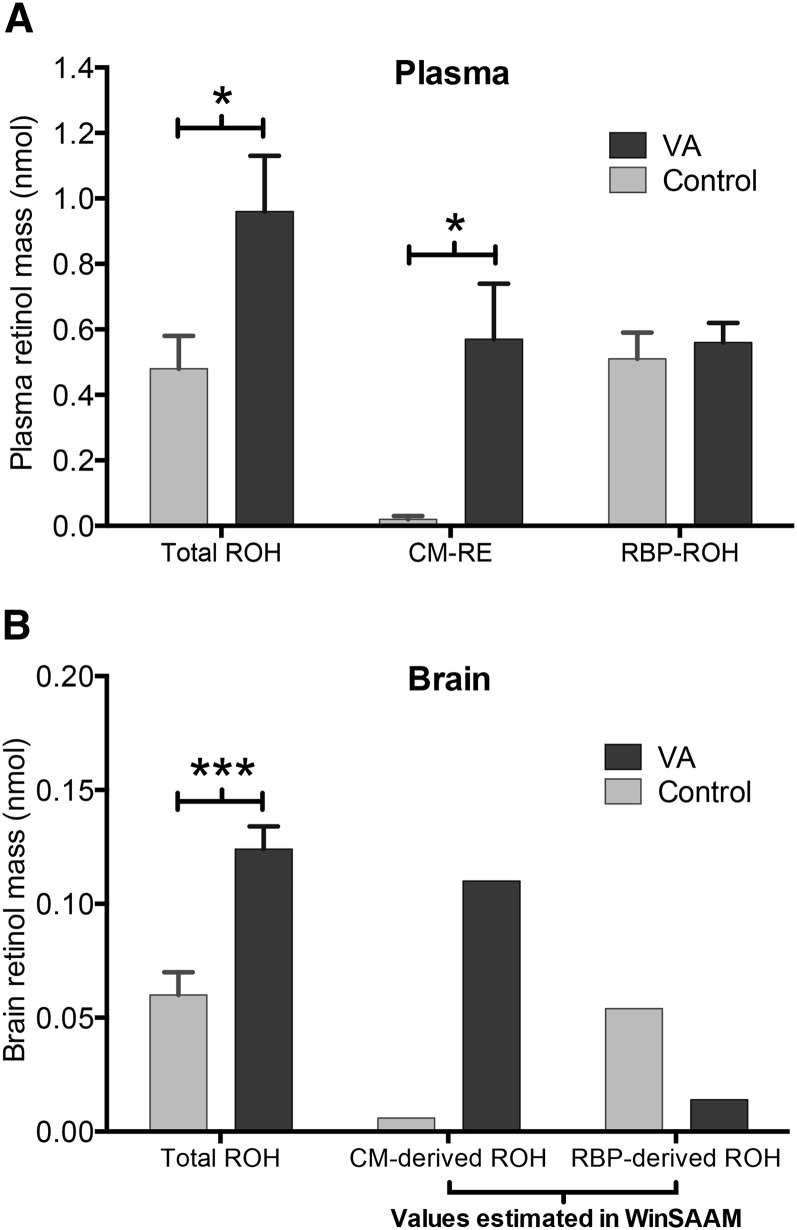
The initial model structure fit the observed data well; therefore, the number of parameters was left unchanged except for adding a time interrupt [P(0,8)] on day 1 to capture a sudden change in the rate of irreversible loss of RBP-derived retinol. Based on the statistical comparison of parameters (Table 1), VA supplementation significantly increased the fractional uptake of chylomicron REs (P < 0.001) and decreased that of RBP retinol (P < 0.05). VA supplementation also significantly increased the fraction of chylomicron-derived retinol lost irreversibly from the brain (P < 0.05) and decreased that fraction for RBP-derived retinol (P < 0.01). With respect to compartment masses, VA supplementation significantly increased the mean mass of REs (P < 0.05) and total retinol in plasma (P < 0.05), as well as the mean mass of total retinol in the brain (P < 0.001) (Figure 5). Finally, based on the comparison of transfer rates (Table 2), VA supplementation significantly increased the transfer rate of chylomicron REs from nearly zero in the control group to 0.7 nmol/d in the supplemented group (P < 0.05).
TABLE 1.
Fraction of VA in compartment J transferred to compartment I per day in neonatal rats dosed with placebo or 50,000 IU VA on postnatal day 41
| L(I,J),2 pools/d |
|||||
| Fractional transfer coefficient | Parameter description | Control group | VA group | df | P |
| L(18,10) | Uptake of CM REs into the brain | 0.31 ± 0.12 | 1.17 ± 0.05 | 7 | <0.001 |
| L(0,18) | Loss of CM-derived retinol from the brain | 0.59 ± 0.20 | 1.40 ± 0.28 | 7 | <0.05 |
| L(8,1) | Uptake of RBP retinol into the brain | 0.53 ± 0.04 | 0.17 ± 0.01 | 7 | <0.05 |
| L(0,8)3 | Loss of RBP-derived retinol from the brain | 11.43 ± 1.05 | 3.42 ± 0.47 | 7 | <0.01 |
CM, chylomicron; L(I,J), fractional transfer coefficient, representing the fraction of dose transferred from compartment (J) to compartment (I); RBP, retinol-binding protein; RE, retinyl ester; VA, vitamin A.
Values are means ± SEMs.
L(0,8) is the mean of L(0,8) and P(0,8) introduced by applying a time interrupt 1 day after dose administration.
FIGURE 5.
Plasma mass of total ROH, RE, and RBP-ROH (A) and brain mass of total ROH and CM- and RBP-derived ROH (B) in control and VA-supplemented rats. The total ROH bars represent the mean ± SEM of 20 rats killed at 5 sampling times from 0 to 8 d after dosing on postnatal day 4. The RE and RBP-ROH bars represent the mean ± SEM of 5 pooled samples from 20 rats killed at 5 sampling times from 0 to 8 d after dosing on postnatal day 4. Values for CM- and RBP-derived ROH in the brain were estimated in WinSAAM version 3.0.8 in a steady-state solution based on the input value of total ROH mass in plasma. *P < 0.05, ***P < 0.001. CM, chylomicron; RBP, retinol-binding protein; RE, retinyl ester; ROH, retinol; VA, vitamin A.
TABLE 2.
Mass of VA in compartment J transferred to compartment I in neonatal rats dosed with placebo or 50,000 IU VA on postnatal day 41
| R(I,J), nmol/d |
|||||
| Transfer rate2 | Parameter description | Control group | VA group | df | P |
| R(18,10) | Uptake rate of CM REs into the brain | 0.01 ± 0.003 | 0.67 ± 0.21 | 3 | <0.05 |
| R(8,1) | Uptake rate of RBP-derived retinol into the brain | 0.27 ± 0.05 | 0.10 ± 0.09 | 3 | 0.192 |
| R(0,18)4 | Loss rate of CM REs from the brain | 0.00 | 0.15 | — | — |
| R(0,8)4 | Loss rate of RBP-derived retinol from the brain | 0.62 | 0.05 | — | — |
| R(26,11)5 | Uptake rate of total retinol into the brain | 0.28 ± 0.04 | 0.77 ± 0.16 | 1 | 0.205 |
CM, chylomicron; RBP, retinol-binding protein; RE, retinyl ester; R(I,J), transfer rate, representing mass transferred from compartment (J) to compartment (I); VA, vitamin A.
Calculated as the product of VA mass in the origin compartment [M(J)] and the corresponding fractional transfer coefficient [L(I,J)].
Mean ± SEM (all such values).
Calculated with the use of VA mass in the brain estimated by the model without error prediction.
R(26,11) is the sum of R(18,10) and R(8,1).
Discussion
Despite the importance of VA for brain development, knowledge about its uptake and turnover in the neonatal brain is limited. We quantified the brain uptake of total retinol, chylomicron REs, and RBP retinol in neonatal rats raised under VA-marginal conditions without and after VA supplementation with 50,000 IU retinyl acetate. The transfer of retinol from plasma to the brain was estimated with the use of model-based compartmental analysis in WinSAAM with the forcing function option to model the brain separately from the rest of the system.
Our findings demonstrated a rapid gain in brain weight during the first 3 wk after birth in neonatal rats, an increase that corresponds to the brain growth spurt observed in humans during the last trimester of pregnancy and the first 3 y after birth (30). The brain-to-body weight ratio decreased from ∼4% to ∼2%, a trend that continues until the brain reaches ∼0.5% of the whole-body weight in rats (31) (Supplemental Figure 3). We also found that the mass of retinol in the brain was strongly correlated with its mass in plasma, a finding that may be explained by a relatively higher proportion of plasma volume circulating in the neonatal rat brain (∼2%) than in the adult brain (∼0.2%) (26, 27). A visual inspection of the tracer-response curves showed that retinol entered the brain within the first hour after dosing and that its uptake involved both postprandial chylomicrons and RBP, with RBP being a quantitatively more important source of retinol in the absence of supplementation. In the supplemented group, the RBP-derived retinol peaked twice (12 h and 2 d after dosing), with the first peak possibly caused by the direct transfer of retinol to RBP from enterocytes or from chylomicrons circulating in plasma, which was previously observed in humans after the ingestion of 100,000 IU labeled VA (32). The oral dose of VA significantly increased brain uptake of chylomicron REs from 0 (control) to 0.7 nmol/d. This increase resulted in a significant day-long elevation in the mass of total retinol present in the brain in the supplemented group (Figure 2C).
Our findings agree with previous reports showing that the extrahepatic tissues in neonates play an important role in clearing chylomicron REs from plasma. For example, in another kinetic study (22), a treatment with VA admixed with retinoic acid increased the fraction of REs taken up by extrahepatic organs from ∼48% in the control group to ∼75% in the VA-supplemented neonates. In adult rats, the corresponding value was 25–33% (33, 34). Similarly, a UPLC analysis of other peripheral organs collected in our experiment showed that that the VA supplementation increased retinol concentrations in several tissues, including the lungs, skin, and intestine, but the increase reached a maximum value at 1–15 h and returned to baseline within 24 h after dosing (JK Hodges, L Tan, MH Green, AC Ross, unpublished data, 2016). The transient nature of this effect suggests that chylomicrons are the main source of retinol delivered to these organs after supplementation.
In line with these findings are the results from studies conducted on RBP and Stra6 (stimulated by retinoic acid 6; cell-surface receptor for RBP) knockout mice. When fed a VA-sufficient diet (22 IU diet/g), the RBP knockout animals seemed to be healthy and displayed normal physiology apart from impaired vision at the time of weaning (35, 36). Likewise, a total absence of Stra6 in mice fed a regular unpurified diet (15 IU diet/g) did not significantly affect retinol or RE concentrations in adipose tissue, brain, heart, kidney, liver, lungs, muscle, pancreas, spleen, testis, and thymus, except those present in the eye. These results indicate that most organs can obtain sufficient VA to meet their needs from REs present in chylomicrons (37).
The exact molecular mechanism by which chylomicron REs are delivered to the neonatal brain is still unknown. It is reasonable to postulate that chylomicron REs might be more accessible to the developing brain because of the immaturity or higher penetrability of the neonatal blood-brain barrier. Nevertheless, considerable evidence exists to demonstrate that the tight junctions between cerebral endothelial cells, which make up the blood-brain barrier, are present and functionally effective even during embryonic development (38) and that the newborn and adult brain capillary endothelia do not differ with respect to lipid penetrability (39). Hence, the specific mechanism by which lipids, including REs, enter the brain is likely to be the same for all age groups.
On the other hand, some studies have suggested that neonates may differ from adults with respect to the amount of REs taken up by the brain. In adults, the main mechanism responsible for the cellular uptake of dietary lipids is lipoprotein lipase (LPL), an enzyme located on the luminal side of the capillary endothelium that binds incoming chylomicrons, hydrolyzes the core lipids and lipid-soluble vitamins, and allows for their uptake into the peripheral tissues (10). Studies show that LPL is highly expressed in the brain, especially during the suckling period (40), with the highest activity attained on postnatal day 7 (at a level 5-fold higher than in adult rat brain) and that it declines by 95% at weaning (41, 42). This timing coincides with the highest rate of nerve myelination and the most rapid phase of FA deposition in the developing brain (43, 44). Moreover, the activity of LPL is especially high in the hippocampus, the learning center of the brain, where it has been previously shown to regulate concentrations of vitamin E, another lipid-soluble vitamin (45). Collectively, these findings suggest that the increased uptake of chylomicron REs after VA supplementation may be facilitated by LPL and may accompany the large influx of lipids required to support the myelination of growing nerves.
Several limitations of our model are worth noting. The plasma concentrations of labeled REs and RBP retinol were not measured directly. The kinetics of chylomicron- and RBP-derived retinol were predicted by the model based on the assumption that the measured total labeled retinol in plasma is the sum of labeled REs and RBP retinol. Nevertheless, to ensure the accuracy of our model, we conducted a follow-up experiment that focused on the early postabsorptive phase (first 30 h after supplementation), in which the total labeled retinol in plasma was separated into labeled REs and RBP retinol before being measured. The tracer-response curves for chylomicron- and RBP-derived retinol resulting from this analysis were similar to those obtained in the current experiment, with the preponderance of brain retinol in the supplemented group delivered by chylomicrons (Supplemental Figure 4). This comparison lends further confidence to the accuracy of our model as applied to this system.
In conclusion, this study applied compartmental modeling of tracer kinetics to quantify the brain uptake of chylomicron REs and RBP retinol. Our results showed that the oral administration of VA to male and female Sprague-Dawley neonatal rats raised under VA-marginal conditions increased the brain uptake of chylomicron REs, causing a day-long elevation in the brain mass of total retinol. These findings indicate that chylomicrons may be a primary vehicle for delivering recently ingested large doses of VA to the brain and that a more frequent supplementation regimen may be necessary for maintaining a steady supply of VA to the developing brain. In view of these results and the role of VA in memory and learning, more studies are needed to examine the effect of VA supplementation on brain physiology and cognitive function.
Acknowledgments
JKH conducted the research, analyzed the data, and wrote the manuscript; LT conducted the research; MHG assisted with the kinetic analysis and interpretation; and ACR conducted the research and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: LPL, lipoprotein lipase; L(I,J), fractional transfer coefficient, representing the fraction of dose transferred from compartment (J) to compartment (I); RBP, retinol-binding protein; RE, retinyl ester; UPLC, ultra-performance liquid chromatography; VA, vitamin A.
References
- 1.Thompson RA, Nelson CA. Developmental science and the media: early brain development. Am Psychol 2001;56:5–15. [DOI] [PubMed] [Google Scholar]
- 2.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology 2010;35:147–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budday S, Steinmann P, Kuhl E. Physical biology of human brain development. Front Cell Neurosci 2015;9:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobbing J, Sands J. Head circumference, biparietal diameter and brain growth in fetal and postnatal life. Early Hum Dev 1978;2:81–7. [DOI] [PubMed] [Google Scholar]
- 5.Kuzawa CW, Chugani HT, Grossman LI, Lipovich L, Muzik O, Hof PR, Wildman DE, Sherwood CC, Leonard WR, Lange N. Metabolic costs and evolutionary implications of human brain development. Proc Natl Acad Sci USA 2014;111:13010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockburn F. Neonatal brain and dietary lipids. Arch Dis Child Fetal Neonatal Ed 1994;70:F1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCaffery P, Zhang J, Crandall JE. Retinoic acid signaling and function in the adult hippocampus. J Neurobiol 2006;66:780–91. [DOI] [PubMed] [Google Scholar]
- 8.Shearer KD, Stoney PN, Morgan PJ, McCaffery PJ. A vitamin for the brain. Trends Neurosci 2012;35:733–41. [DOI] [PubMed] [Google Scholar]
- 9.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci 2007;8:755–65. [DOI] [PubMed] [Google Scholar]
- 10.Wang TW, Zhang H, Parent JM. Retinoic acid regulates postnatal neurogenesis in the murine subventricular zone-olfactory bulb pathway. Development 2005;132:2721–32. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci USA 2006;103:3902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misner DL, Jacobs S, Shimizu Y, de Urquiza AM, Solomin L, Perlmann T, De Luca LM, Stevens CF, Evans RM. Vitamin A deprivation results in reversible loss of hippocampal long-term synaptic plasticity. Proc Natl Acad Sci USA 2001;98:11714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomoto M, Takeda Y, Uchida S, Mitsuda K, Enomoto H, Saito K, Choi T, Watabe AM, Kobayashi S, Masushige S, et al. . Dysfunction of the RAR/RXR signaling pathway in the forebrain impairs hippocampal memory and synaptic plasticity. Mol Brain 2012;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etchamendy N, Enderlin V, Marighetto A, Pallet V, Higueret P, Jaffard R. Vitamin A deficiency and relational memory deficit in adult mice: relationships with changes in brain retinoid signalling. Behav Brain Res 2003;145:37–49. [DOI] [PubMed] [Google Scholar]
- 15.Cocco S, Diaz G, Stancampiano R, Diana A, Carta M, Curreli R, Sarais L, Fadda F. Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience 2002;115:475–82. [DOI] [PubMed] [Google Scholar]
- 16.Fonzo LSN, Golini RS, Delgado SM, Ponce IT, Bonomi MR, Rezza IG, Gimenez MS, Anzulovich AC. Temporal patterns of lipoperoxidation and antioxidant enzymes are modified in the hippocampus of vitamin A-deficient rats. Hippocampus 2009;19:869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W, Yu Q, Gong M, Chen L, Wen EY, Bi Y, Zhang Y, Shi Y, Qu P, Liu YX, et al. . Vitamin A deficiency impairs postnatal cognitive function via inhibition of neuronal calcium excitability in hippocampus. J Neurochem 2012;121:932–43. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet E, Touyarot K, Alfos S, Pallet V, Higueret P, Abrous DN. Retinoic acid restores adult hippocampal neurogenesis and reverses spatial memory deficit in vitamin A deprived rats. PLoS One 2008;3:e3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai Y, Crandall JE, Brodsky J, McCaffery P. 13-Cis retinoic acid (accutane) suppresses hippocampal cell survival in mice. Ann N Y Acad Sci 2004;1021:436–40. [DOI] [PubMed] [Google Scholar]
- 20.Crandall J, Sakai Y, Zhang JH, Koul O, Mineur Y, Crusio WE, McCaffery P. 13-Cis-retinoic acid suppresses hippocampal cell division and hippocampal-dependent learning in mice. Proc Natl Acad Sci USA 2004;101:5111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald SLR, Savy M, Fulford AJC, Kendall L, Flanagan KL, Prentice AM. A double blind randomized controlled trial in neonates to determine the effect of vitamin A supplementation on immune responses: the Gambia protocol. BMC Pediatr 2014;14:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan L, Wray AE, Green MH, Ross AC. Compartmental modeling of whole-body vitamin A kinetics in unsupplemented and vitamin A-retinoic acid-supplemented neonatal rats. J Lipid Res 2014;55:1738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Neonatal vitamin A supplementation. Geneva (Switzerland): WHO; 2011. [Google Scholar]
- 25.Ross AC. Separation and quantitation of retinyl esters and retinol by high-performance liquid chromatography. Methods Enzymol 1986;123:68–74. [DOI] [PubMed] [Google Scholar]
- 26.Everett NB, Simmons B, Lasher EP. Distribution of blood (Fe-59) and plasma (I-131) volumes of rats determined by liquid nitrogen freezing. Circ Res 1956;4:419–24. [DOI] [PubMed] [Google Scholar]
- 27.Lee HB, Blaufox MD. Blood-volume in the rat. J Nucl Med 1985;26:72–6. [PubMed] [Google Scholar]
- 28.Cifelli CJ, Green JB, Green MH. Use of model-based compartmental analysis to study vitamin A kinetics and metabolism. Vitam Horm 2007;75:161–95. [DOI] [PubMed] [Google Scholar]
- 29.Ross AC, Harrison EH. Vitamin A: nutritional aspects of retinoids and carotenoids. In: Zempleni J, Rucker RB, McCormick DB, Suttie JW, editors. Handbook of vitamins. 4th ed Boca Raton (FL): Taylor & Francis; 2007. p. 1–39. [Google Scholar]
- 30.Wachs TD, Georgieff M, Cusick S, McEwen BS. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci 2014;1308:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piao Y, Liu YN, Xie XD. Change trends of organ weight background data in Sprague Dawley rats at different ages. J Toxicol Pathol 2013;26:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinersdorff DV, Bush E, Liberato DJ. Plasma kinetics of vitamin A in humans after a single oral dose of [8,9,19–13C]retinyl palmitate. J Lipid Res 1996;37:1875–85. [PubMed] [Google Scholar]
- 33.Goodman DW, Huang HS, Shiratori T. Tissue distribution and metabolism of newly absorbed vitamin A in the rat. J Lipid Res 1965;6:390–6. [PubMed] [Google Scholar]
- 34.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J Lipid Res 1999;40:565–74. [PubMed] [Google Scholar]
- 35.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J 1999;18:4633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel S, Piantedosi R, O’Byrne SM, Kako Y, Quadro L, Gottesman ME, Goldberg IJ, Blaner WS. Retinol-binding protein-deficient mice: biochemical basis for impaired vision. Biochemistry 2002;41:15360–8. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Wongsiriroj N, Blaner WS. The multifaceted nature of retinoid transport and metabolism. Hepatobiliary Surg Nutr 2014;3:126–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol 2012;3:46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornford EM, Braun LD, Oldendorf WH, Hill MA. Comparison of lipid-mediated blood-brain-barrier penetrability in neonates and adults. Am J Physiol 1982;243:C161–8. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Eckel RH. Lipoprotein lipase in the brain and nervous system. Annu Rev Nutr 2012;32:147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tavangar K, Murata Y, Patel S, Kalinyak JE, Pedersen ME, Goers JF, Hoffman AR, Kraemer FB. Developmental regulation of lipoprotein lipase in rats. Am J Physiol 1992;262:E330–7. [DOI] [PubMed] [Google Scholar]
- 42.Nuñez M, Peinado-Onsurbe J, Vilaro S, Llobera M. Lipoprotein lipase activity in developing rat brain areas. Biol Neonate 1995;68:119–27. [DOI] [PubMed] [Google Scholar]
- 43.Galan X, Llobera M, Ramírez I. Lipoprotein lipase in developing rat tissues: differences between Wistar and Sprague-Dawley rats. Biol Neonate 1993;64:295–303. [DOI] [PubMed] [Google Scholar]
- 44.Edmond J, Higa TA, Korsak RA, Bergner EA, Lee WNP. Fatty acid transport and utilization for the developing brain. J Neurochem 1998;70:1227–34. [DOI] [PubMed] [Google Scholar]
- 45.Goti D, Balazs Z, Panzenboeck U, Hrzenjak A, Reicher H, Wagner E, Zechner R, Malle E, Sattler W. Effects of lipoprotein lipase on uptake and transcytosis of low density lipoprotein (LDL) and LDL-associated alpha-tocopherol in a porcine in vitro blood-brain barrier model. J Biol Chem 2002;277:28537–44. [DOI] [PubMed] [Google Scholar]