Abstract
Background:The nonessential amino acid cysteine is known to be involved in many antioxidant and anticarcinogenic pathways. Cysteinylglycine is a pro-oxidant metabolite of glutathione and a precursor of cysteine.
Objective: To examine the relation between serum cysteine and cysteinylglycine and risk of gastric adenocarcinomas, esophageal squamous cell carcinomas, and head and neck squamous cell carcinomas, we conducted a nested case-control study within the Alpha-Tocopherol, Beta-Carotene Cancer Prevention study of male Finnish smokers aged 50–69 y at baseline.
Design: In total, 170 gastric adenocarcinomas, 68 esophageal squamous cell carcinomas, and 270 head and neck squamous cell carcinomas (identified from the Finnish Cancer Registry) were matched one-to-one with cancer-free control subjects on age and the date of serum collection. We calculated ORs and 95% CIs with the use of a multivariate-adjusted conditional logistic regression.
Results: Cysteine had a U-shaped association with gastric adenocarcinomas; a model that included a linear and a squared term had a significant global P-test (P = 0.036). Serum cysteinylglycine was inversely associated with adenocarcinomas of the gastric cardia (OR for above the median compared with below the median: 0.07; 95% CI: 0.01, 0.70; n = 38 cases) but not for other sites. Both cysteine and cysteinylglycine were not associated with esophageal squamous cell carcinoma or head and neck squamous cell carcinoma.
Conclusions: We observed associations between serum cysteine and cysteinylglycine with upper gastrointestinal cancer risk. Future studies are needed to replicate these findings. This trial was registered at clininicaltrials.gov as NCT00342992.
Keywords: cysteine, esophageal neoplasms, head and neck neoplasms, stomach neoplasms, upper gastrointestinal tract cancers
INTRODUCTION
Cancers of the head and neck and upper gastrointestinal (UGI)7 tract cause a major disease burden worldwide; gastric cancer is the third most-common cause of cancer death worldwide, and cancers of the head, neck, and esophagus collectively cause ∼700,000 deaths/y (1). Certain risk factors for these cancers have been well established including smoking for gastric adenocarcinomas, esophageal squamous cell carcinomas (ESCCs), and head and neck squamous cell carcinomas (HNSCCs), alcohol for ESCCs and HNSCCs, and Helicobacter pylori infection for gastric adenocarcinoma (2–4). Mechanisms by which these risk factors result in cancer remain an active area of investigation.
Cysteine could play an etiological role in head and neck or UGI cancer risk through several different mechanisms. The nonessential amino acid cysteine can be synthesized from methionine and serine (5) as well as obtained via the diet from high-protein foods. Cysteine serum concentrations are thought to be tightly regulated (5) and lie at the intersection of a wide range of important biologic pathways. For example, cysteine plays an important role in the regulation of the extracellular redox environment (6) and is a rate-limiting precursor of glutathione (7), which is an important intracellular antioxidant (8). Cysteine is also a metabolite of homocysteine (9), which is part of the one-carbon metabolism pathway, that is required for accurate DNA replication and appropriate gene expression (10). However, at high serum concentrations, cysteine may be cytotoxic (6) and may act as a pro-oxidant (15, 16).
Relatively few studies have examined the association of serum cysteine with cancer risk. One publication from the Nutritional Intervention Trial, which was conducted in Linxian, China, observed inverse associations between serum cysteine and incident ESCCs and gastric cardia adenocarcinomas (11). However, studies that have investigated the association of serum cysteine with other cancer sites have shown inconsistent results (12–20).
Cysteinylglycine is a metabolite of glutathione and also serves as a precursor to cysteine (7). The pro-oxidant cysteinylglycine has been suggested to promote the reduction of Fe3+ to Fe2+ (21) and also to lead to lipid peroxidation (22). Only a few studies have investigated associations of cysteinylglycine with chronic disease and have provided some evidence for increased risk of heart disease (23) and breast cancer (24) with increased serum cysteinylglycine. Because little is known about the association between serologic cysteine or cysteinylglycine and risk of head and neck or UGI cancer, we investigated these associations in a nested case-control study within the ATBC (Alpha-Tocopherol, Beta-Carotene Cancer Prevention) study cohort (25).
METHODS
Study cohort
The ATBC study was a randomized, double-blind, placebo-controlled, primary prevention trial that was conducted to determine whether daily supplementation with α-tocopherol, β-carotene, or both would reduce the incidence of lung or other cancers in male smokers. Between 1985 and 1988, 29,133 southwestern Finnish men between the ages of 50 and 69 y who smoked ≥5 cigarettes/d were recruited and randomly assigned to receive either α-tocopherol (50 mg/d), β-carotene (20 mg/d), both supplements, or placebo capsules with the use of a 2 × 2-factorial design for 5–8 y (median: 6.1 y). Participants were excluded from the trial if they had a history of a malignancy other than nonmelanoma skin cancer or carcinoma in situ, severe angina on exertion, chronic renal insufficiency, liver cirrhosis, alcoholism, or other medical conditions that could have limited long-term participation. After the end of the trial, participants were followed as a cohort. The ATBC Study was approved by the institutional review boards of the National Cancer Institute and the National Public Health Institute, and written informed consent was obtained from each participant before random assignment.
Data collection
During 2 clinic visits before random assignment, participants completed questionnaires on general background characteristics, educational attainment, medical histories, smoking intensity and duration, and occupational histories. Height and weight were also measured at this first baseline visit; participants who had been fasting for 12 h before their first baseline clinic visit provided a fasting blood sample. Serum samples were stored at −70°C and later analyzed. The food-frequency questionnaire assessed usual food consumption over the previous year and included 276 common foods, mixed dishes, and beverages including alcohol with the use of a picture booklet to aid in the estimation of portion size (26). The food-frequency questionnaire was completed satisfactorily by 93% of study participants. Participants who were missing dietary data were included in the analyses with an indicator variable for missing. The exclusion of these participants from the study did not affect risk estimates (data not shown).
Case identification and control selection
Cases were defined as incident gastric adenocarcinomas (International Classification of Diseases (ICD)-9 code 151), ESCCs (ICD-9 code 150), or HNSCCs (ICD-9 codes 140–149 and 161) that were diagnosed by 30 April 2002 with adequate serum for measurements. ICD for Oncology codes were further used to classify histology as an adenocarcinoma or squamous cell carcinoma. Cancer cases were identified through the Finnish Cancer Registry. For cases that were diagnosed through April 1999, medical records were reviewed centrally by 1 or 2 physicians for diagnostic confirmation and staging. Information on cases that were diagnosed since May 1999 was derived from the Finnish Cancer Registry, which provided almost 100% case ascertainment (27). Controls were alive and cancer free at the time of case diagnosis and matched to cases (1:1) for the age at random assignment (±5 y) and date of blood draw (±30 d).
Serum nutrient determination
Fasting blood samples were collected from participants at the time of their first baseline visit and stored at −70°C. Cysteine and cysteinylglycine were determined with the use of a modification of an HPLC method (28, 29). Each batch contained blinded case and matched controls that were placed consecutively within each batch as well as 6 blinded quality-control (QC) samples that were derived from a pool of serum. Within-batch CVs were 7.6% for cysteine and 5.3% for cysteinylglycine, whereas between-batch CVs were 10.8% for cysteine and 16.8% for cysteinylglycine. For cysteine, the QC samples were in a different range from that of the case and control samples. Because of the substantial batch-to-batch variation in the mean cysteine concentration of controls, we used locally weighted regression models (PROC LOESS) to describe the deviation in each batch from the mean of the controls and to calculate residuals from this regression (29). We added the mean value of measured cysteine to these residuals and assigned quartiles on the basis of the distribution of these values in controls.
H. pylori seropositivity was measured with the use of commercially available ELISAs (Biohit ELISA kit; Biohit Oyj) for serum antibodies to H. pylori cell surface antigen according to the manufacturer’s instructions. Each batch included 2 QC samples that were provided in the kit (a negative control and a positive control) and 3 blinded QC samples from a single serum pool from the ATBC study. Cases, controls, and QC samples were all measured in duplicate. A cutoff was used to define H. pylori positivity. The concordance between QC samples was 100%.
Statistical analysis
Statistical analyses were performed with the use of Stata version 13.0 software (StataCorp LP) and SAS version 9.1.3 software (SAS Institute Inc.), and all P values were 2 sided. We compared the distribution of selected characteristics for case and 1:1-matched control subjects with the use of Wilcoxon’s rank-sum tests for continuous variables and chi-square tests for categorical variables. In controls, the associations between serum cysteine and cysteinylglycine (in quartiles) and selected characteristics were determined with the use of Cuzik’s nonparametric tests for trend.
OR and 95% CIs for the association between serum cysteine or cysteinylglycine concentrations and incident cancer were determined with the use of conditional logistic regression. For continuous estimates of cysteine and cysteinylglycine, ORs were scaled to one-half the IQR, i.e.,
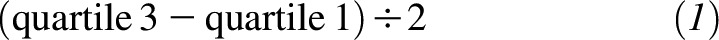 |
and were equivalent to 27 and 41 nmol/mL, respectively. We also fit continuous models with the addition of a square term to investigate possible nonlinear associations.
The multivariate-adjusted conditional logistic regression models were adjusted for age at random assignment, alcohol consumption (ethanol; g/d), BMI (in kg/m2) calculated as weight in divided by the square of height, energy intake (kcal/d), cigarettes smoked per day, years of cigarette smoking, pack-years of cigarette smoking, education, fruit intake (g/d), and vegetable intake (g/d). For gastric adenocarcinomas only, we also present models that were further adjusted for H. pylori seropositivity because H. pylori infection is a strong risk factor for gastric cancer risk (30) but is not thought to increase ESCC or HNSCC risk (31, 32). The intervention group and gastric atrophy as measured by a low pepsinogen I status (33) were not confounders of the associations in our study (data not shown); therefore, they were not included in the multivariable models.
We examined a possible effect modification by age, smoking dose (<20 compared with ≥20 cigarettes/d), years of smoking (<37 compared with ≥37 y), alcohol use (<13, 13–26, and ≥26 g/d), study intervention, baseline serum α-tocopherol (<11.9 and ≥11.9 mg/L) or β-carotene (<174.5 and ≥174.5 μg/L), intake of total vitamin C (<96.3 and ≥96.3 mg/d) or vitamin E (<10.8 and ≥10.8 mg/d), and H. pylori by stratification with the use of a visual inspection of the data and formally tested for an effect modification with the use of unconditional logistic regression.
RESULTS
This investigation included 170 gastric adenocarcinoma cases, 68 ESCC cases, 270 HNSCC cases, and 508 1:1-matched controls. H. pylori seropositivity was more common in gastric adenocarcinoma cases than in matched controls (P < 0.001) (Table 1). Concentrations of serum cysteine were significantly lower in gastric adenocarcinoma cases than in matched controls (P = 0.04), whereas concentrations of cysteinylglycine along with other examined covariates were similar in gastric adenocarcinoma cases and in controls. ESCC cases had lower BMI (P = 0.01) than controls. Other examined covariates were similar in both cases and controls. Concentrations of serum cysteine (P = 0.13) and cysteinylglycine (P = 0.81) were not significantly different between ESCC cases and controls. HNSCC cases smoked more cigarettes per day, drank more alcohol, had higher energy intake, and ate less fruit and vegetables than matched controls did (P < 0.05 for all). Concentrations of serum cysteine and cysteinylglycine were not significantly different between HNSCC cases and controls (P = 0.29 and P = 0.82, respectively).
TABLE 1.
Study characteristics in cases and controls1
| Gastric adenocarcinoma |
Esophageal squamous cell carcinoma |
Head and neck squamous cell carcinoma |
|||||
| Controls | Value | P | Value | P | Value | P | |
| Participants, n | 508 | 170 | — | 68 | — | 270 | — |
| Age at random assignment | 57 (54, 61)2 | 58 (54, 62) | 0.12 | 57 (54, 62) | 0.76 | 57 (54, 61) | 0.72 |
| BMI, kg/m2 | 25.9 (23.9, 28.6) | 25.6 (23.3, 28.3) | 0.26 | 24.3 (22.6, 28.0) | 0.01 | 25.4 (23.1, 28.1) | 0.08 |
| Age started smoking, y | 19 (17, 20) | 19 (17, 21) | 0.56 | 18 (17, 21) | 0.98 | 18 (16, 20) | 0.30 |
| Smoking, y | 37 (32, 41) | 37 (31, 43) | 0.79 | 39 (34, 43) | 0.49 | 38 (32, 43) | 0.09 |
| Cigarettes, n/d | 20 (15, 20) | 20 (15, 25) | 0.10 | 20 (15, 25) | 0.23 | 20 (17, 30) | <0.0001 |
| Alcohol use, g/d | 11.0 (2.8, 25.2) | 10.7 (1.5, 22.9) | 0.95 | 19.2 (7.6, 38.7) | 0.06 | 15.6 (4.7, 30.1) | 0.02 |
| Energy intake, kcal/d | 2693 (2205, 3244) | 2493 (1996, 3246) | 0.17 | 2510 (2152, 3286) | 0.63 | 2535 (1873, 3224) | 0.03 |
| Vegetable intake, g/d | 92.0 (52.2, 139.7) | 80.5 (42.8, 132.1) | 0.19 | 84.0 (35.1, 136.4) | 0.43 | 73.7 (36.7, 126.8) | 0.004 |
| Fruit intake, g/d | 102.3 (47.1, 160.5) | 91.7 (33.8, 155.3) | 0.26 | 74.5 (16.7, 137.7) | 0.06 | 74.4 (29.5, 141.3) | 0.002 |
| Elementary education, % | 78 | 86 | 0.09 | 87 | 0.12 | 81 | 0.52 |
| α-Tocopherol intervention group, % | 49 | 56 | 0.23 | 59 | 0.23 | 51 | 0.67 |
| β-Carotene intervention group, % | 46 | 51 | 0.33 | 47 | 0.73 | 50 | 0.26 |
| Serum measures | |||||||
| Helicobacter pylori seropositivity,3 % | 68 | 86 | <0.001 | — | — | — | — |
| Cysteine, nmol/mL | 310.1 (282.5, 337.0) | 298.0 (267.6, 329.5) | 0.04 | 308.5 (279.6, 333.1) | 0.13 | 305.1 (278.0, 333.8) | 0.29 |
| Cysteinylglycine, nmol/mL | 223.0 (190.9, 279.0) | 231.0 (186.4, 273.3) | 0.51 | 230.9 (185.6, 274.9) | 0.81 | 221.9 (184.8, 268.9) | 0.82 |
P values are for each cancer type and their 1:1-matched controls (matched for age and date of blood draw). Wilcoxon’s rank-sum test was used for continuous variables, and the chi-square test was used for categorical variables.
Median; IQR in parentheses (all such values).
Restricted to adenocarcinoma cases and controls because H. pylori is a strong risk factor for gastric adenocarcinoma but is not generally considered a risk factor for esophageal squamous cell carcinoma or head and neck squamous cell carcinoma.
In controls, serum cysteine was positively associated with BMI and inversely associated with energy intake and H. pylori seropositivity (Table 2), whereas serum cysteinylglycine was positively associated with education (data not shown).
TABLE 2.
Study characteristics by quartile of serum cysteine in controls1
| Cutoffs for quartiles of serum cysteine, nmol/mL |
|||||
| 1 (≤283) | 2 (284–310) | 3 (311–337) | 4 (≥338) | P | |
| Age at random assignment, y | 57 (53, 59.5)2 | 57 (54, 59) | 59 (55, 62) | 57 (54, 61) | 0.061 |
| BMI, kg/m2 | 25.1 (23.5, 26.8) | 26.0 (23.7, 28.9) | 25.5 (23.6, 28.0) | 27.5 (25.3, 30.4) | <0.001 |
| Age started smoking, y | 20 (17, 21) | 19 (16, 20) | 18 (17, 20) | 19 (17, 20) | 0.23 |
| Smoking, y | 35 (31, 41) | 37 (32, 41) | 38 (34, 43) | 37 (32, 43) | 0.10 |
| Cigarettes, n/d | 20 (12, 20) | 20 (15, 20) | 20 (15, 24) | 20 (15, 25) | 0.45 |
| Alcohol use, g/d | 9.9 (2.5, 19.3) | 13.0 (5.4, 26.7) | 10.3 (2.5, 25.0) | 11.8 (3.0, 26.5) | 0.47 |
| Energy intake, kcal/d | 2878 (2383, 3292) | 2768 (2172, 3229) | 2986 (2315, 3470) | 2597 (1905, 3144) | 0.017 |
| Vegetables, g/d | 94.9 (54.8, 135.8) | 93.9 (44.6, 136.7) | 101.7 (51.3, 151.7) | 95.9 (56.1, 129.3) | 0.97 |
| Fruit, g/d | 124.1 (44.7, 170.6) | 101.8 (42.8, 148.2) | 115.6 (52.3, 155.5) | 100.5 (50.7, 161.9) | 0.96 |
| Elementary education, % | 84 | 70 | 77 | 81 | 0.84 |
| Helicobacter pylori seropositivity,3 % | 78 | 76 | 56 | 64 | 0.017 |
P values were calculated from Cuzik’s nonparametric test for trend.
Median; IQR in parentheses (all such values).
Restricted to controls for adenocarcinoma cases because H. pylori is a strong risk factor for gastric adenocarcinoma but is not generally considered a risk factor for esophageal squamous cell carcinoma or head and neck squamous cell carcinoma.
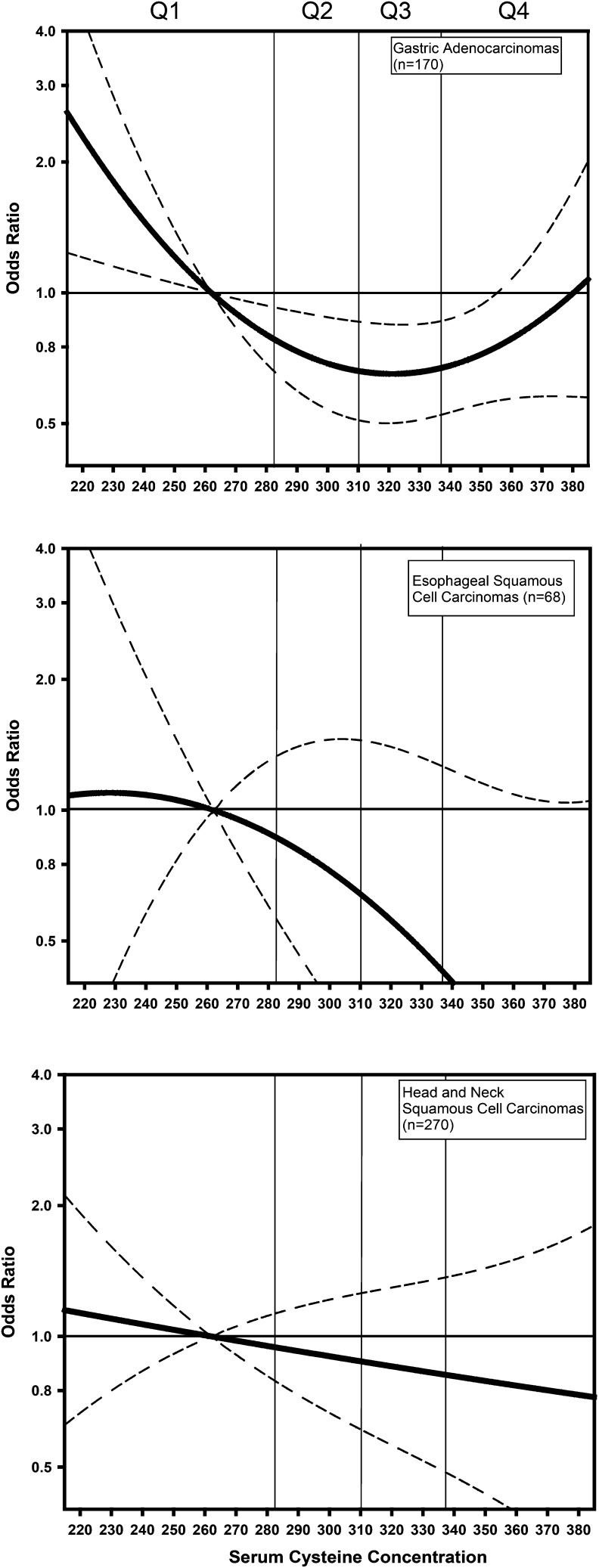
Table 3 presents ORs for the association between serum cysteine and the following 3 cancer types: gastric adenocarcinomas, ESCCs, and HNSCCs, in analyses that divided serum cysteine concentration into quartiles. Although NS, we noted that, for gastric adenocarcinoma models that were adjusted for H. pylori and other known and suspected risk factors, the point estimate for ORs for quartiles 2 and 3 (compared with quartile 1) were <1.0, whereas the point estimate for the OR for quartile4 (compared with quartile 1) was >1.0. These results prompted us to consider the possibility of a nonlinear association between serum cysteine and gastric adenocarcinomas. We investigated the possibility of a nonlinear association between serum cysteine concentration and gastric adenocarcinomas by including a square term in the models (Figure 1). Tests for nonlinearity reached significance (Wald’s P values for the linear term, square term, and the global 2-df test for a model including the linear and the square term were as follows: P = 0.029, P = 0.037, and P = 0.036, respectively). The gastric cardia adenocarcinoma (n = 38) and noncardia adenocarcinoma (n = 132) stratified analyses showed patterns of association that were similar in each analysis (Supplemental Table 1). We again investigated the possibility of a nonlinear association for each of the 2 gastric adenocarcinoma subsites by including a square term in the models. For gastric adenocarcinomas of the noncardia region, Wald’s P values f for the linear term, square term, and the global 2-df test for a model including the linear and the square term were all borderline (P = 0.07, P = 0.09, and P = 0.05, respectively). For cardia gastric adenocarcinomas, the Wald’s P values for the linear term, square term, and the global 2-df test for a model including the linear and the square term were also all borderline (P = 0.06, P = 0.07, and P = 0.13, respectively)
TABLE 3.
Association of serum cysteine with risks of gastric adenocarcinoma, esophageal squamous cell carcinoma, and head and neck squamous cell carcinoma1
| Gastric adenocarcinomas (n = 170) |
Esophageal squamous cell carcinomas (n = 68) |
Head and neck squamous cell carcinomas (n = 270) |
|||||||
| Serum cysteine quartile (cutoff) | Controls/cases, n | Crude | Multivariable | Controls/cases, n | Crude | Multivariable | Controls/cases, n | Crude | Multivariable |
| 1 (≤283 nmol/mL) | 47/61 | 1.00 (reference)2 | 1.00 (reference) | 12/18 | 1.00 (reference) | 1.00 (reference) | 69/82 | 1.00 (reference) | 1.00 (reference) |
| 2 (284–310 nmol/mL) | 37/40 | 0.80 (0.43, 1.50) | 0.77 (0.37, 1.61) | 18/18 | 0.72 (0.27, 1.94) | 1.08 (0.29, 4.06) | 71/69 | 0.77 (0.46, 1.28) | 0.81 (0.46, 1.42) |
| 3 (311–337 nmol/mL) | 46/31 | 0.44 (0.22, 0.87) | 0.59 (0.26, 1.31) | 15/16 | 0.75 (0.25, 2.28) | 1.19 (0.32, 4.49) | 64/57 | 0.67 (0.38, 1.17) | 0.78 (0.42, 1.45) |
| 4 (≥338 nmol/mL) | 40/38 | 0.64 (0.31, 1.30) | 1.10 (0.46, 2.63) | 23/16 | 0.45 (0.16, 1.23) | 0.36 (0.08, 1.55) | 66/62 | 0.70 (0.40, 1.24) | 0.73 (0.38, 1.40) |
| P | — | 0.113 | 0.493 | — | 0.144 | 0.244 | — | 0.194 | 0.344 |
Cutoffs for quartiles were defined by all of the controls used in the study. Crude ORs were calculated from conditional logistic regression models. Multivariable-adjusted ORs for gastric adenocarcinomas were from conditional logistic regression models that were adjusted for age, alcohol intake, BMI, energy intake, cigarettes smoked per day, years of cigarette smoking, education, fruit intake, vegetable intake, and Helicobacter pylori seropositivity. H. pylori was included in these models because it is a strong risk factor for gastric adenocarcinoma. Multivariable-adjusted ORs for head and neck squamous cell carcinomas and esophageal squamous cell carcinomas were from conditional logistic regression models that were adjusted for age, alcohol intake, BMI, energy intake, cigarettes smoked per day, years of cigarette smoking, education, fruit intake, and vegetable intake. H. pylori was not included these models because as H. pylori is not a risk factor for esophageal or head and neck squamous cell carcinomas.
OR; 95% CIs in parentheses (all such values).
Calculated from a 3-df likelihood ratio test. P-trend test was not used for gastric adenocarcinomas because of evidence of a nonlinear association.
P-trend.
FIGURE 1.
OR (95% CIs) for the association between serum cysteine and cancer risk relative to the median of the first quartile. We calculated estimates from models with both linear and quadratic terms for serum cysteine with adjustment for age, alcohol intake, BMI, energy intake, cigarettes smoked per day, years of cigarette smoking, education, fruit intake, vegetable intake, gastric adenocarcinomas, and Helicobacter pylori seropositivity. For gastric adenocarcinomas, we observed evidence of a nonlinear association with a P value for the quadratic term of 0.037 and the overall 2-df test for an association of 0.036. For esophageal squamous tumors, we showed no evidence of a deviation from linearity (for the quadratic term: P value= 0.46; for the 2-df test: P = 0.19). For head and neck squamous tumors, we showed no evidence of a deviation from linearity (for the quadratic term: P = 0.19; for the 2-df test: P = 0.34). Q, quartile.
Table 3 presents results for the association between serum cysteine and ESCCs and the association between serum cysteine and HNSCCs. There was no association between serum cysteine and either ESCCs or HNSCCs. In contrast to the results for gastric adenocarcinomas, we showed no evidence for a nonlinear association between serum cysteine and either ESCCs or HNSCCs (Figure 1).
Table 4 displays associations between serum cysteinylglycine and gastric adenocarcinomas, ESCCs, and HNSCCs. In contrast to the results for cysteine, we showed no significant associations between serum cysteinylglycine and risk of gastric adenocarcinoma, ESCC, or HNSCC. In Supplemental Table 2, we stratified gastric adenocarcinoma cases by anatomic subsite (cardia or noncardia). We showed a borderline inverse association of cysteinylglycine with adenocarcinomas of the gastric cardia (P-trend from multivariate model = 0.05). Because case numbers for this outcome were small (n = 38), and risk estimates from the quartiles suggested a possible threshold effect, we collapsed categories. The OR for greater than the median serum cysteinylglycine concentration (>223 nmol/mL) relative to that below the median was 0.07 (95% CI: 0.01, 0.70). We observed no evidence of an association between cysteinylglycine and adenocarcinomas of the noncardia (P-trend from multivariate model = 0.46).
TABLE 4.
Association of serum cysteinylglycine with risk of gastric adenocarcinoma, esophageal squamous cell carcinoma, and head and neck squamous cell carcinoma1
| Gastric adenocarcinomas (n = 170) |
Esophageal squamous cell carcinomas (n = 68) |
Head and neck squamous cell carcinomas (n = 270) |
|||||||
| Serum cysteinylglycine quartile (cutoff) | Controls/cases, n | Crude | Multivariate | Controls/cases, n | Crude | Multivariate | Controls/cases, n | Crude | Multivariate |
| 1 (<192 nmol/mL) | 41/49 | 1.00 (reference)2 | 1.00 (reference) | 19/19 | 1.00 (reference) | 1.00 (reference) | 71/79 | 1.00 (reference) | 1.00 (reference) |
| 2 (192–223 nmol/mL) | 34/29 | 0.72 (0.38, 1.38) | 0.68 (0.32, 1.46) | 18/14 | 0.79 (0.28, 2.20) | 1.31 (0.34, 5.09) | 73/58 | 0.68 (0.41, 1.14) | 0.75 (0.43, 1.30) |
| 3 (224–273 nmol/mL) | 44/50 | 0.92 (0.49, 1.72) | 0.83 (0.39, 1.76) | 15/16 | 1.13 (0.40, 3.19) | 2.26 (0.53, 9.67) | 62/75 | 1.03 (0.63, 1.70) | 1.11 (0.65, 1.91) |
| 4 (>273 nmol/mL) | 51/42 | 0.64 (0.33, 1.24) | 0.45 (0.20, 1.00) | 16/19 | 1.47 (0.42, 5.20) | 2.05 (0.41, 10.33) | 64/58 | 0.74 (0.42, 1.32) | 0.70 (0.37, 1.30) |
| P-trend | — | 0.28 | 0.08 | — | 0.76 | 0.65 | — | 0.49 | 0.28 |
Cutoffs for quartiles were defined by all of the controls used in the study. Crude ORs were calculated from conditional logistic regression models. Multivariate-adjusted ORs for gastric adenocarcinomas were from conditional logistic regression models that were adjusted for age, alcohol intake, BMI, energy intake, cigarettes smoked per day, years of cigarette smoking, education, fruit intake, vegetable intake, and Helicobacter pylori seropositivity. H. pylori was included in these models because it is a strong risk factor for gastric adenocarcinoma. Multivariate-adjusted ORs for head and neck squamous cell carcinomas and esophageal squamous cell carcinomas were from conditional logistic regression models that were adjusted for age, alcohol intake, BMI, energy intake, cigarettes smoked per day, years of cigarette smoking, education, fruit intake, and vegetable intake. H. pylori was not included these models because H. pylori is not a risk factor for esophageal or head and neck squamous cell carcinomas.
OR; 95% CIs in parentheses (all such values).
We showed no evidence of an effect modification of any association overall or for the cancer subtypes by alcohol use, smoking history, α-tocopherol or β-carotene intervention group, baseline serum α-tocopherol or β-carotene concentrations, vitamin C or vitamin E intake, or H. pylori seropositivity by inspection or by formal hypothesis testing (data not shown).
DISCUSSION
In this nested case-control study within the ATBC study, we observed a significant (P = 0.036) U-shaped association between serum cysteine and gastric adenocarcinomas. Although case numbers were low, serum cysteinylglycine was inversely associated with adenocarcinomas of the gastric cardia. No associations were observed between serum cysteine or cysteinylglycine and ESCCs or HNSCCs.
We observed a significant U-shaped association between serum cysteine and gastric adenocarcinomas. Our findings differed from the results of the only previous publication to our knowledge to have investigated the association of cysteine with cancers of the UGI tract, which was a study within the Nutritional Intervention Trial in Linxian, China. Murphy et al. (11) showed evidence of an inverse linear association between serum cysteine and gastric cardia adenocarcinomas. Several differences between the studies should be noted. Although the previous publication was restricted to cancers of the gastric cardia, the current publication included cases of both the cardia and noncardia, with a majority of cases in the noncardia group. In addition, the range of serum cysteine differed between the 2 studies. Cysteine serum concentrations were substantially lower in the previous publication (median: 196 nmol/mL; IQR: 178–216 nmol/mL) than in the current one (median: 310.1 nmol/mL; IQR: 282.5–337.0 nmol/mL). In the current study, the nadir of risk was seen at a serum concentration of 320 nmol/mL, with risk trending toward the null at higher concentrations. In the Linxian study, few participants had cysteine serum concentrations in this higher range. In the Linxian study, few participants had cysteine serum concentrations in this higher range. The subjects of the previous study also had lower BMI (mean ± SE: 21.8 ± 2.6) than in the current study (median: 25.8; IQR: 23.9–28.6). Several studies have shown an association between serum cysteine and obesity and metabolic syndrome (34–37). Furthermore, the Linxian population consisted mostly of subsistence farmers, whereas members of the ATBC study population had a more-ample and varied diet. Finally, the previous publication lacked adjustment for H. pylori. In the current study, H. pylori seropositivity was associated with lower serum cysteine concentrations in controls, and the U-shape became somewhat more pronounced after adjustment for H. pylori (data not shown).
The U-shaped association between serum cysteine and UGI adenocarcinomas is mechanistically plausible. Cysteine concentrations are thought to be tightly regulated, and high concentrations of cysteine have been shown to be cytotoxic in laboratory studies (5). These findings suggest a detrimental effect of either excessively high or excessively low cysteine serum concentrations. Cysteine also may be able to act as both a pro-oxidant and antioxidant depending on the physiological context. In its antioxidant context, cysteine serves as the rate-limiting precursor of the crucial antioxidant glutathione, and supplementation of cysteine has been shown to increase glutathione concentrations and decrease inflammatory markers in elderly subjects (38). However, N-acetyl-cysteine supplements have also been shown to increase serum concentrations of markers of oxidative stress in humans after an acute muscle injury (39), thereby suggesting a function of cysteine as a pro-oxidant. U-shaped associations with serum cysteine have been reported for one other disease outcome; in a previous study of vascular disease, there was evidence of a U-shape association with serum cysteine (40).
To our knowledge, only one previous study has investigated the mechanistic link between N-acetyl-cysteine and gastric cancer in vitro. The study reported that N-acetyl-cysteine inhibited human gastric cancer SJ-89 cell growth by inducing DNA synthesis arrest and apoptosis (41). The authors postulated that this process may have functioned through the regulation of the transcriptional checkpoint nuclear transcription factor κB; however, to our knowledge, additional mechanistic details have not been elucidated. N-acetylcysteine has also been investigated as a chemopreventive agent in a rat model of esophageal adenocarcinoma in which tumors were induced by esophagogastroduodenal anastomosis. N-acetylcysteine alone had no effect on tumor incidence, but N-acetylcysteine in combination with α-tocopherol significantly reduced tumor incidence (42).
Alternatively, as in all observational studies, serum cysteine could be a surrogate for another exposure. In our analysis, estimates were only slightly attenuated by adjustment for other risk factors including alcohol use, BMI, energy intake, education, and fruit and vegetable intake. Adjustment for H. pylori attenuated the association of serum cysteine with gastric adenocarcinomas overall, and adjustment for smoking attenuated associations for serum cysteine and gastric adenocarcinomas, ESCCs, and HNSCCs. Residual confounding may have been present.
In contrast with cysteine, only one study, to our knowledge, has investigated the association of cysteinylglycine with risk of any cancer type and showed null results for breast cancer overall but elevated risks in certain subgroups (24). Cysteinylglycine is a pro-oxidant such that it could be hypothesized that there is a positive association with increased cancer risk particularly in individuals with poor antioxidant status. Results in a previous study of breast cancer followed this pattern (24). However, we observed no association of cysteinylglycine with any cancer site overall and a borderline inverse association with adenocarcinomas of gastric cardia. We attempted to examine associations in individuals with poor antioxidant status by examining results in subjects with low dietary vitamin C and vitamin E intakes and low serum α-tocopherol or low serum β-carotene concentrations; however, the results in these strata were similar to those overall (data not shown). With only 38 cases, the observed inverse association of cysteinylglycine with gastric cardia adenocarcinomas could have been due to chance. Future studies are needed to replicate this result.
This study has several notable strengths including the prospective collection of cancer cases, a carefully matched study design, the identification of cases and controls from the same pool of ATBC trial participants (which limited the possibility of selection bias), and adjustment for possible confounders including H. pylori, alcohol, cigarette smoking, and education. However, this study also has several limitations. Because the ATBC study was a trial of male smokers, our results may not be generalizable to nonsmokers. In contrast, smokers may be exposed to higher levels of oxidative stress than other populations and, thus, are a group well suited for a study of cysteine and cysteinylglycine. Also, we had only a single measurement of cysteine and cysteinylglycine, which may or may not have reflected long-term serum concentrations. Finally, the power was low for some subsites and for examining a possible effect modification.
In conclusion, we observed evidence of a U-shaped association between serum cysteine and gastric adenocarcinoma. This association is biologically plausible and should be investigated in subsequent studies.
Acknowledgments
The authors’ responsibilities were as follows—EHM and NDF: drafted the manuscript; EHM, NDF, CCA, GM, and RZS-S: analyzed and interpreted the data; SJW and RZS-S: designed the study; SM, PRT, and DA: acquired the data; GM: provided the H. pylori data; JS and LD: provided the laboratory measurements of cysteine and cysteinylglycine metabolites; SJW, CCA, GM, SM, PRT, and DA: critically revised the manuscript for important intellectual content; RZS-S: created the study concept, provided the cysteine and cysteinylglycine metabolite measures, and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. The authors had no conflicts of interest to disclose.
Footnotes
Abbreviations used: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention; ESCC, esophageal squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; ICD, International Classification of Diseases; QC, quality control; UGI, upper gastrointestinal.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol 2004;31:450–64. [DOI] [PubMed] [Google Scholar]
- 3.Enzinger PC, Mayer RJ. Medical progress - esophageal cancer. N Engl J Med 2003;349:2241–52. [DOI] [PubMed] [Google Scholar]
- 4.Sturgis EM, Wei QY, Spitz MR. Descriptive epidemiology and risk factors for head and neck cancer. Semin Oncol 2004;31:726–33. [DOI] [PubMed] [Google Scholar]
- 5.Stipanuk MH, Dominy JE, Lee JI, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr 2006;136:1652S–9S. [DOI] [PubMed] [Google Scholar]
- 6.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr 2004;24:481–509. [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr 2004;134:489–92. [DOI] [PubMed] [Google Scholar]
- 8.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006;160:1–40. [DOI] [PubMed] [Google Scholar]
- 9.Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr 2006;136:1636S–40S. [DOI] [PubMed] [Google Scholar]
- 10.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr 2000;130:129–32. [DOI] [PubMed] [Google Scholar]
- 11.Murphy G, Fan JH, Mark SD, Dawsey SM, Selhub J, Wang J, Taylor PR, Qiao YL, Abnet CC. Prospective study of serum cysteine levels and oesophageal and gastric cancers in China. Gut 2011;60:618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JW, Beresford SA, Neuhouser ML, Cheng TY, Song X, Brown EC, Zheng Y, Rodriguez B, Green R, Ulrich CM. Homocysteine, cysteine, and risk of incident colorectal cancer in the Women’s Health Initiative observational cohort. Am J Clin Nutr 2013;97:827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang FF, Wang HM, Lan YC, Yang MH, Huang SC, Huang YC. High homocysteine is associated with increased risk of colorectal cancer independently of oxidative stress and antioxidant capacities. Clin Nutr 2014;33:1054–60. [DOI] [PubMed] [Google Scholar]
- 14.Le Marchand L, White KK, Nomura AM, Wilkens LR, Selhub JS, Tiirikainen M, Goodman MT, Murphy SP, Henderson BE, Kolonel LN. Plasma levels of b vitamins and colorectal cancer risk: the Multiethnic Cohort study. Cancer Epidemiol Biomarkers Prev 2009;18:2195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stabler S, Koyama T, Zhao ZG, Martinez-Ferrer M, Allen RH, Luka Z, Loukachevitch LV, Clark PE, Wagner C, Bhowmick NA. Serum methionine metabolites are risk factors for metastatic prostate cancer progression. PLoS One 2011;6:e22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson TM, Weinstein SJ, Mayne ST, Pfeiffer RM, Selhub J, Taylor PR, Virtamo J, Albanes D, Stolzenberg-Solomon R. A prospective study of one-carbon metabolism biomarkers and risk of renal cell carcinoma. Cancer Causes Control 2010;21:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman MT, McDuffie K, Hernandez B, Wilkens LR, Selhub J. Case-control study of plasma folate, homocysteine, vitamin B-12, and cysteine as markers of cervical dysplasia. Cancer 2000;89:376–82. [DOI] [PubMed] [Google Scholar]
- 18.Goodman JE, Lavigne JA, Wu K, Helzlsouer KJ, Strickland PT, Selhub J, Yager JD. COMT genotype, micronutrients in the folate metabolic pathway and breast cancer risk. Carcinogenesis 2001;22:1661–5. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Lee IM, Song YQ, Cook NR, Selhub J, Manson JE, Buring JE, Zhang SM. Plasma homocysteine and cysteine and risk of breast cancer in women. Cancer Res 2010;70:2397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang SMM, Willett WC, Selhub J, Manson JAE, Colditz GA, Hankinson SE. A prospective study of plasma total cysteine and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2003;12:1188–93. [PubMed] [Google Scholar]
- 21.Spear N, Aust SD. Thiol-mediated NTA-Fe(III) reduction and lipid-peroxidation. Arch Biochem Biophys 1994;312:198–202. [DOI] [PubMed] [Google Scholar]
- 22.Dominici S, Paolicchi A, Lorenzini E, Maellaro E, Comporti M, Pieri L, Minotti G, Pompella A. gamma-Glutamyltransferase-dependent prooxidant reactions: a factor in multiple processes. Biofactors 2003;17:187–98. [DOI] [PubMed] [Google Scholar]
- 23.Mendis S, Athauda SBP, Kenji T. Association between hyperhomocysteinemia and ischemic heart disease in Sri Lankans. Int J Cardiol 1997;62:221–5. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Manson JE, Selhub J, Buring JE, Zhang SMM. Plasma cysteinylglycine levels. and breast cancer risk in women. Cancer Res 2007;67:11123–7. [DOI] [PubMed] [Google Scholar]
- 25.The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol 1994;4:1–10. [DOI] [PubMed] [Google Scholar]
- 26.Pietinen P, Hartman AM, Haapa E, Rasanen L, Haapakoski J, Palmgren J, Albanes D, Virtamo J, Huttunen JK. Reproducibility and validity of dietary assessment instruments. I. A self-administered food use questionnaire with a portion size picture booklet. Am J Epidemiol 1988;128:655–66. [DOI] [PubMed] [Google Scholar]
- 27.Korhonen P, Malila N, Pukkala E, Teppo L, Albanes D, Virtamo J. The Finnish Cancer Registry as follow-up source of a large trial cohort–accuracy and delay. Acta Oncol 2002;41:381–8. [DOI] [PubMed] [Google Scholar]
- 28.Araki A, Sako Y. Determination of free and total homocysteine in human-plasma by high-performance liquid-chromatography with fluorescence detection. J Chromatogr 1987;422:43–52. [DOI] [PubMed] [Google Scholar]
- 29.Borkowf CB, Albert PS, Abnet CC. Using lowess to remove systematic trends over time in predictor variables prior to logistic regression with quantile categories. Stat Med 2003;22:1477–93. [DOI] [PubMed] [Google Scholar]
- 30.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001;49:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila) 2008;1:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandis JR, Perez-Perez GI, Yu VL, Johnson JT, Blaser MJ. Lack of serologic evidence for Helicobacter pylori infection in head and neck cancer. Head Neck 1997;19:216–8. [DOI] [PubMed] [Google Scholar]
- 33.Varis K, Sipponen P, Laxen F, Samloff IM, Huttunen JK, Taylor PR, Heinonen OP, Albanes D, Sande N, Virtamo J, et al. . Implications of serum pepsinogen I in early endoscopic diagnosis of gastric cancer and dysplasia. Scand J Gastroenterol 2000;35:950–6. [DOI] [PubMed] [Google Scholar]
- 34.Elshorbagy AK, Nurk E, Gjesdal CG, Tell GS, Ueland PM, Nygard O, Tverdal A, Vollset SE, Refsum H. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: does cysteine link amino acid and lipid metabolism? Am J Clin Nutr 2008;88:738–46. [DOI] [PubMed] [Google Scholar]
- 35.Elshorbagy AK, Valdivia-Garcia M, Refsum H, Butte N. The association of cysteine with obesity, inflammatory cytokines and insulin resistance in Hispanic children and adolescents. PLoS One 2012;7;e44166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohorko N, Petelin A, Jurdana M, Biolo G, Jenko-Praznikar Z. Elevated serum levels of cysteine and tyrosine: early biomarkers in asymptomatic adults at increased risk of developing metabolic syndrome. Biomed Res Int 2015;2015:418681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.da Silva NP, de Souza FIS, Pendezza AI, Fonseca FLA, Hix S, Oliveira AC, Sarni ROS, D’Almeida V. Homocysteine and cysteine levels in prepubertal children: association with waist circumference and lipid profile. Nutrition 2013;29:166–71. [DOI] [PubMed] [Google Scholar]
- 38.Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, Jahoor F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am J Clin Nutr 2011;94:847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med 2001;31:745–53. [DOI] [PubMed] [Google Scholar]
- 40.El-Khairy L, Ueland PM, Refsum H, Graham IM, Vollset SE. Plasma total cysteine as a risk factor for vascular disease - The European Concerted Action project. Circulation 2001;103:2544–9. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Tu HJ, Li J, Dai G, Dai YC, Wu Q, Shi QZ, Cao Q, Li ZJ. N-acetyl cysteine inhibits human signet ring cell gastric cancer cell line (SJ-89) cell growth by inducing apoptosis and DNA synthesis arrest. Eur J Gastroenterol Hepatol 2007;19:769–74. [DOI] [PubMed] [Google Scholar]
- 42.Hao J, Zhang B, Liu B, Lee M, Hao X, Reuhl KR, Chen X, Yang CS. Effect of alpha-tocopherol, N-acetylcysteine and omeprazole on esophageal adenocarcinoma formation in a rat surgical model. Int J Cancer 2009;124:1270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]