Abstract
Background: Vitamin D deficiency impairs fertility in animal models, but the role of vitamin D in human fertility or treatment of infertility is less clear.
Objective: We examined the association between circulating 25-hydroxyvitamin D [25(OH)D] concentrations and the outcome in women undergoing assisted reproduction technologies (ARTs).
Design: We randomly selected 100 women undergoing infertility treatment with ART enrolled in an ongoing prospective cohort study who underwent 168 treatment cycles. Serum 25(OH)D concentrations were measured in samples collected from women between days 3 and 9 of gonadotropin treatment. Generalized linear mixed models were used to evaluate the association of 25(OH)D concentrations with ART outcomes while adjusting for potential confounders and accounting for repeated treatment cycles per woman.
Results: Median (range) serum 25(OH)D concentrations were 86.5 (33.5–155.5) nmol/L. Ninety-one percent of participants consumed multivitamins. Serum 25(OH)D concentrations were positively related to fertilization rate. The adjusted fertilization rate for women in increasing quartiles of serum 25(OH)D were 0.62 (95% CI: 0.51, 0.72), 0.53 (95% CI: 0.43, 0.63), 0.67 (95% CI: 0.56, 0.76), and 0.73 (95% CI: 0.63, 0.80), respectively (P-trend = 0.03). This association persisted when analyses were restricted to women with serum 25(OH)D between 50 and 125 nmol/L when models were further adjusted for season of blood draw and when analyses were restricted to the first treatment cycle. However, 25(OH)D concentrations were unrelated to probability of pregnancy (P-trend = 0.83) or live birth after ART (P-trend = 0.47).
Conclusion: Vitamin D may be associated with higher fertilization rates, but this apparent benefit does not translate into higher probability of pregnancy or live birth. This trial was registered at www.clinicaltrials.gov as NCT00011713.
Keywords: vitamin D, infertility, assisted reproductive technologies, epidemiology, nutrition
INTRODUCTION
Infertility is one of the most common diseases of reproductive-aged adults, affecting 15–25% of couples who try to become pregnant in Western countries (1, 2). Assisted reproductive technologies (ARTs)9 have emerged as one of the most effective treatment options to overcome this problem and are currently responsible for the birth of >50,000 children in the United States (3) and 140,000 children in Europe each year (4–6). Despite these impressive numbers, success rates per initiated cycle have remained relatively constant for >10 y (7). As a result, there is increasing interest in identifying potentially modifiable predictors of successful treatment, including diet and lifestyle factors.
Vitamin D has emerged as a potentially modifiable factor that could influence outcomes of couples undergoing infertility treatment. In rodent models, vitamin D deficiency decreases fertility as a result of uterine hypoplasia, impaired follicular development, and anovulation (8–15). Human data on the relation between vitamin D and fertility are inconclusive. Some studies have found that women with sufficient levels of vitamin D in serum were significantly more likely to achieve implantation and clinical pregnancy (10, 16) whereas others have failed to document a benefit (9, 17–19). Therefore, the role of vitamin D in assisted reproduction remains unclear. To address this question, we evaluated the association between serum levels of 25-hydroxyvitamin D [25(OH)D] and outcomes of infertility treatment with ART in women presenting to a fertility center in teaching hospital in Boston, Massachusetts.
METHODS
Participants were women enrolled in the Environment and Reproductive Health Study (NCT00011713), an ongoing prospective cohort study started in 2006 aimed at identifying determinants of fertility among couples presenting to the Massachusetts General Hospital Fertility Center (Boston, Massachusetts). All women who meet eligibility requirements (aged 18–46 y at enrollment and planned use of own gametes for treatment) are approached by research staff and invited to participate in the study. Women were eligible for this analysis if they had completed a food-frequency questionnaire and had subsequently completed at least one ART cycle by May 2013 (n = 232). From the remaining pool of 232 eligible women, we randomly selected 100 women, who underwent a total of 168 cycles by February 2014, to measure serum concentrations of 25(OH)D. Women whose 25(OH)D concentrations were measured did not systematically differ from women who did not; these groups of women did not systematically differ otherwise (Supplemental Table 1). The study was approved by the Human Subject Committees of the Harvard T.H. Chan School of Public Health and the Massachusetts General Hospital. Written, informed consent was obtained from all participants.
At enrollment, height and weight were measured by a trained research nurse to calculate BMI (in kg/m2) and a brief, nurse-administered questionnaire was used to collect data on demographic characteristics, medical history, and lifestyle factors. Participants also completed a detailed take-home questionnaire with additional questions on lifestyle factors, diet, reproductive health, and medical history. Blood samples were collected from women between day 3 and day 9 of gonadotropin treatment during their first in-study ART cycle. Samples were collected in serum separator tubes, which were processed, separated into aliquots, and stored at −80°C until sample analysis. Serum concentrations of 25(OH)D were measured at the Clinical and Epidemiologic Research Laboratory at Boston Children’s Hospital. Serum 25(OH)D was measured by an enzymeimmunoassay (Immunodiagnostic Systems Inc. Fountain Hills). The assay has a lower limit of detection of 5.0 nmol/L. The day-to-day variability of the assay at concentrations of 40.3, 72.0, and 132.0 nmol/L are 4.6%, 6.4%, and 8.7%, respectively. This assay has been standardized to the CDC Vitamin D Standardization Program isotope dilution liquid chromatrography-mass spectrometry/mass spectrometry Reference Method Procedure and received clearance by the US Food and Drug Administration for reporting actionable patient results.
Women were pretreated with oral contraceptives for a period of 2–5 wk to suppress ovulation before their ART cycle, unless contraindicated. On day 3 of induced menses, patients began controlled ovarian stimulation by using one of 3 protocols as clinically indicated: 1) luteal-phase gonadotropin-releasing hormone (GnRH) agonist protocol with the use of low-, regular-, or high-dose leuprolide with pituitary desensitization beginning in the luteal phase; 2) follicular-phase GnRH-agonist/Flare protocol, in which leuprolide started on day 2 of the follicular phase at 20 units and decreased to 5 units on day 5; or 3) GnRH-antagonist protocol, in which GnRH-antagonist began when the lead follicle reached 14 mm in size and/or estradiol concentrations were ≥1000 pg/mL. Patients were monitored during gonadotropin stimulation for serum estradiol, follicle size measurements and counts, and endometrial thickness through 2 d before oocyte retrieval. Human chorionic gonadotropin (hCG) was administered approximately 36 h before the scheduled oocyte retrieval procedure to induce oocyte maturation.
Couples underwent ART with conventional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) as clinically indicated. Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II (MII), or degenerated. Embryologists determined fertilization rate 17–20 h after insemination as the number of oocytes with 2 pronuclei divided by the number of MII oocytes inseminated or injected. The resulting embryos were monitored for cell number and morphologic quality (1 for best and up to 5 for worst) on days 2 and 3. We defined successful implantation as a serum β-hCG concentration >6 mIU/mL typically measured 17 d (range: 15–20 d) after oocyte retrieval, clinical pregnancy as the presence of an intrauterine gestational sac confirmed by ultrasound at 6 wk of gestation, and live birth as the birth of a neonate on or after 24 wk of gestation. All clinical information was abstracted from electronic medical records.
Women were assigned to quartiles depending on their serum 25(OH)D concentrations. Descriptive statistics were calculated for demographic, dietary, and reproductive characteristics according to quartiles of serum 25(OH)D concentrations. We used multivariable generalized linear mixed models to evaluate the association of serum 25(OH)D concentrations in quartiles and as continuous variable with treatment outcomes with a random intercept to account for multiple ART cycles per woman while adjusting for potential confounding factors. A normal distribution and identity link were specified for continuous outcomes (estradiol concentrations, endometrial thickness), a Poisson distribution and log link function were specified for count outcomes (number of total and MII oocytes retrieved), and binomial distribution and logit link function were specified for proportions (fertilization, dichotomized embryo quality measures, and clinical outcomes). Tests for trend were conducted across categories by using the median 25(OH)D concentrations in each category as a continuous variable in the regression models. To account for potential confounders, multivariable models included terms for age, race, BMI, infertility diagnosis, season when the sample was taken, dietary factors previously related to treatment outcomes in this population (20), and overall food choices as captured by 2 data-derived dietary patterns (21). The dietary patterns were identified via factor analysis with orthogonal transformation based on 40 predefined food groups by using the FACTOR procedure with Varimax rotation option in SAS (SAS Institute Inc.) as previously described (21). The 2 patterns identified were the Western pattern, characterized by high intakes of red and processed meat, butter, refined grains, and sweets, and the Prudent pattern, characterized by intakes of fish, fruit, vegetables, nuts, and legumes. These dietary pattern scores were used to account for potential confounding by overall diet quality in the multivariable models. We present marginal means adjusted for the covariates in the model (22) on the original scale. Marginal means were obtained by using the GLIMMIX procedure and LSMEANS statement; the most frequent category for categorical variables was used as reference for estimation of marginal means.
We evaluated whether the relation of 25(OH)D with ART outcomes was modified by type of insemination (conventional IVF compared with ICSI), BMI (<25 compared with ≥25), smoking (ever compared with never), and age (<35 compared with ≥35), by introducing cross-product terms between concentrations of 25(OH)D and the potential modifiers. All analyses were conducted with the use of the Statistical Analysis System Software package SAS 9.4 (SAS Institute Inc.).
RESULTS
The 100 women selected for this analysis underwent 168 ART cycles between January 2008 and February 2014. Of the 168 initiated ART cycles, 153 were fresh cycles of which 12 failed before oocyte retrieval (5 cancellations, 7 poor response). Of the 141 fresh cycles with oocyte retrieval, 70 were ICSI and 71 were conventional insemination cycles. Most of the women were white (82%), and their mean ± SD age was 34.8 ± 3.8 y. The median (minimum, maximum) 25(OH)D concentrations were 86.5 nmol/L (33.5–155.5 nmol/L). Table 1 summarizes the baseline characteristics of the study population according to serum 25(OH) D quartiles. Only 4 women had 25(OH)D concentrations <50 nmol/L and 5 had concentrations >125nmol/L. As expected, 25(OH)D concentrations were inversely related to BMI. In addition, 25(OH)D serum concentrations were unrelated to other baseline demographic, nutritional, or reproductive characteristics or season when the sample was taken (Table 1).
TABLE 1.
Baseline characteristics of the study participants according to serum 25(OH)D concentrations1
| Quartiles of serum 25(OH)D concentrations |
|||||
| Q1 | Q2 | Q3 | Q4 | P | |
| Serum 25(OH)D concentration, nmol/L | |||||
| Median | 65.0 | 83.1 | 92.4 | 115.0 | |
| Range | 33.5–75.0 | 75.9–86.2 | 86.8–104.6 | 107.8–155.5 | |
| Women, n | 25 | 25 | 25 | 25 | |
| Treatment cycles,2 n | 45 | 41 | 40 | 42 | |
| Age, y | 35.6 ± 3.9 | 34.5 ± 3.3 | 34.9 ± 3.9 | 34.2 ± 4.0 | 0.50 |
| Race/ethnic group, n (%) | 0.20 | ||||
| White/Caucasian | 17 (68.0) | 21 (84.0) | 23 (92.0) | 21 (84.0) | |
| Other | 8 (32.0) | 4 (16.0) | 2 (8.0) | 4 (16.0) | |
| BMI, kg/m2 | 26.2 ± 4.5 | 23.1 ± 3.2 | 24.3 ± 4.5 | 23.9 ± 3.1 | 0.04 |
| Ever smoker, n (%) | 6 (24.0) | 8 (32.0) | 4 (16.0) | 11 (44.0) | 0.18 |
| Educational level, n (%) | 0.41 | ||||
| College degree or higher | 22 (88.0) | 22 (88.0) | 23 (92.0) | 20 (80.0) | |
| Physical activity, h/wk | 3.6 ± 3.5 | 5.6 ± 4.5 | 4.3 ± 4.1 | 6.4 ± 6.0 | 0.26 |
| Dietary characteristics | |||||
| Vitamin B-12 concentration, pg/mL | 519 ± 243 | 605 ± 197 | 671 ± 392 | 612 ± 203 | 0.21 |
| Folic acid concentration, ng/mL | 26.1 ± 23.7 | 31.0 ± 30.7 | 26.0 ± 21.5 | 25.0 ± 8.0 | 0.18 |
| Vitamin D intake, IU/d | 557 ± 252 | 667 ± 360 | 582 ± 271 | 778 ± 460 | 0.10 |
| Multivitamin use, n (%) | 21 (84.0) | 23 92.0) | 24 (96.0) | 23 (92.0) | 0.62 |
| Prudent Diet Pattern score | −0.27 ± 0.6 | 0.07 ± 1.2 | −0.26 ± 0.8 | 0.12 ± 1.0 | 0.32 |
| Western Diet Pattern score | 0.07 ± 0.9 | −0.24 ± 0.8 | −0.00 ± 0.8 | 0.16 ± 0.8 | 0.41 |
| Reproductive characteristics | |||||
| Season of blood draw, n (%) | 0.40 | ||||
| Spring | 9 (36.0) | 5 (20.0) | 4 (16.0) | 7 (28.0) | |
| Summer | 4 (16.0) | 4 (16.0) | 7 (28.0) | 8 (32.0) | |
| Autumn | 4 (16.0) | 9 (36.0) | 9 (36.0) | 7 (28.0) | |
| Winter | 8 (32.0) | 7 (28.0) | 5 (20.0) | 3 (12.0) | |
| Primary infertility diagnosis, n (%) | 0.41 | ||||
| Male factor | 10 (40.0) | 9 (36.0) | 11 (44.0) | 5 (20.0) | |
| Diminished ovarian reserve | 1 (4.0) | 1 (4.0) | 0 (0.0) | 1 (4.0) | |
| Ovulatory | 2 (8.0) | 1 (4.0) | 4 (16.0) | 3 (12.0) | |
| Other female factors | 2 (8.0) | 2 (8.0) | 2 (8.0) | 5 (20.0) | |
| Unexplained | 10 (40.0) | 12 (48.0) | 8 (32.0) | 11 (44.0) | |
| Initial treatment protocol, n (%) | 0.57 | ||||
| Antagonist | 3 (12.0) | 2 (8.0) | 3 (12.0) | 2 (8.0) | |
| Flare | 3 (12.0) | 0 (0.0) | 2 (8.0) | 4 (16.0) | |
| Luteal phase agonist | 19 (76.0) | 23 (92.0) | 20 (80.0) | 19 (76.0) | |
| Day 3 follicle-stimulating hormone, IU/L | 7.3 ± 2.2 | 7.2 ± 1.9 | 6.7 ± 2.1 | 6.8 ± 2.0 | 0.83 |
| Treatment cycles per woman, n (%) | 0.73 | ||||
| One | 17 (68.0) | 15 (60.0) | 15 (60.0) | 13 (52.0) | |
| Two | 3 (12.0) | 7 (28.0) | 7 (28.0) | 7 (28.0) | |
| ≥Three | 5 (20.0) | 3 (12.0) | 3 (12.0) | 5 (20.0) | |
| Previous in vitro fertilization, n (%) | 7 (28.0) | 6 (24.0) | 6 (24.0) | 3 (12.0) | 0.57 |
| Previous intrauterine insemination, n (%) | 9 (36.0) | 12 (48.0) | 10 (40.0) | 9 (36.0) | 0.80 |
Values are means ± SDs unless otherwise indicated. Q, quartile; 25(OH)D, 25-hydroxyvitamin D.
Total number of assisted reproductive technology treatment cycles (in vitro fertilization or intracytoplasmic sperm injection) for the women included in each quartile of serum 25(OH)D concentration.
Serum 25(OH)D concentrations were not related to endometrial thickness, peak estradiol concentrations or oocyte yield (Table 2). However, 25(OH)D concentrations were positively related to fertilization rates. After adjusting for potential confounders, the adjusted fertilization rate was 62% in women in the lowest quartile of serum 25(OH)D concentrations and 73% in women in the highest quartile (P-linear trend = 0.03). This association appeared to be stronger in ICSI cycles (P-linear trend = 0.004) than in IVF cycles (P-linear trend = 0.39) (Table 3). However, these apparent differences were borderline statistically significant (P-heterogeneity = 0.05).
TABLE 2.
Serum 25(OH)D concentrations in relation to endometrial thickness and ovarian stimulation outcomes1
| Number of women | Peak estradiol concentrations,2 pmol/L | Endometrial thickness,2 mm | MII phase oocyte count,2 n | |
| Serum 25(OH)D,3 nmol/L | ||||
| Q1 65.0 (33.5–75.0) | 25 | 2051 (1659, 2443) | 9.9 (9.0, 10.8) | 11.7 (9.3, 14.8) |
| Q2 83.1 (75.9–86.2) | 25 | 2168 (1812, 2524) | 10.2 (9.4, 11.0) | 10.8 (8.8, 13.4) |
| Q3 92.4 (86.8–104.6) | 25 | 2017 (1644, 2390) | 9.8 (8.9, 10.6) | 10.6 (8.5, 13.2) |
| Q4 115.0 (107.8–155.5) | 25 | 2131 (1787, 2475) | 10.1 (9.3, 10.9) | 10.8 (8.8, 13.3) |
| P4 | 0.84 | 0.90 | 0.56 |
n = 100 women, 168 cycles. MII, metaphase II; Q, quartile; 25(OH)D, 25-hydroxyvitamin D.
Values are means (95% CIs) adjusted for age, BMI, infertility diagnosis, race, dietary patterns, folate, and vitamin B-12 serum concentrations. The mixed procedure was used for peak estradiol concentrations and endometrial thickness, and the GLIMMIX procedure was used for MII phase oocytes count.
Values are medians (ranges).
The median concentration of vitamin D in each group was used as a continuous variable in the model.
TABLE 3.
Serum 25(OH)D concentrations in relation to fertilization rate1
| 2PN2/MII3 | All cycles4 (n = 141) | 2PN2/MII3 | IVF cycles4 (n = 71) | 2PN2/MII3 | ICSI cycles4 (n = 70) | |
| Serum 25(OH)D,5 nmol/L | ||||||
| Q1 [65.0 (33.5–75.0)] | 251/386 | 0.62 (0.51, 0.72) | 158/235 | 0.63 (0.47, 0.77) | 93/151 | 0.52 (0.36, 0.68) |
| Q2 [83.1 (75.9–86.2)] | 196/349 | 0.53 (0.43, 0.63) | 119/196 | 0.53 (0.39, 0.67) | 77/153 | 0.38 (0.24, 0.55) |
| Q3 [92.4 (86.8–104.6)] | 228/327 | 0.67 (0.56, 0.76) | 52/95 | 0.50 (0.31, 0.68) | 176/232 | 0.73 (0.59, 0.83) |
| Q4 [115.0 (107.8–155.5)] | 264/356 | 0.73 (0.63, 0.80) | 137/185 | 0.71 (0.55, 0.83) | 127/171 | 0.74 (0.62, 0.83) |
| P6 | 0.03 | 0.39 | 0.004 | |||
| P-heterogeneity | 0.05 | |||||
n = 100 women, 141 cycles with attempted fertilization. ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; MII, metaphase II; Q, quartile; 2PN, 2 pronuclei; 25(OH)D, 25-hydroxyvitamin D.
Number of oocytes with 2PN after insemination.
Number of oocytes in MII.
Values are marginal means (95% CIs) adjusted for age, BMI, infertility diagnosis, race, dietary patterns, folate, and vitamin B-12 serum concentrations.
Values are medians (ranges).
The median concentration of vitamin D in each group was used as a continuous variable in the model.
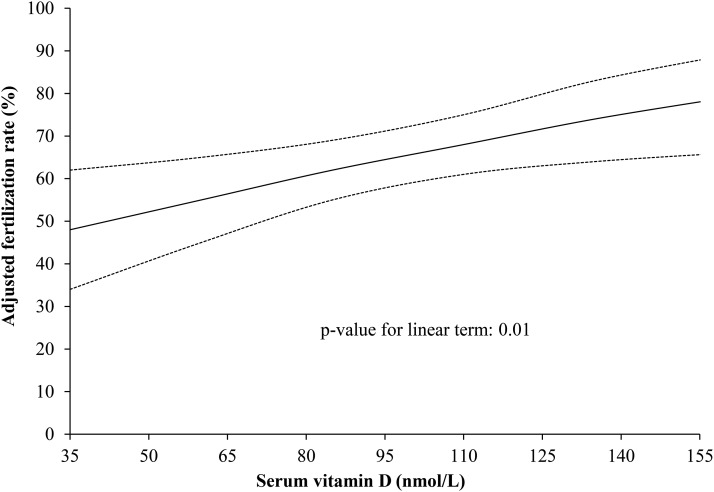
When serum 25(OH)D was modeled as a continuous variable, there was a strong linear association (Figure 1). Each 15-nmol/L increase in serum 25(OH)D was associated with an increase in the odds of fertilization of 19% (OR: 1.19; 95% CI: 1.04, 1.36). Adding a quadratic term to this model did not suggest evidence of a statistically significant nonlinear relation (P = 0.69). The association between serum 25(OH)D and fertilization rate remained unchanged when women with serum concentrations <50 nmol/L or >125 nmol/L were excluded (OR: 1.24; 95% CI: 1.04, 1.48), when additional terms for season of blood draw were added to the models (OR: 1.22; 95% CI: 1.02, 1.46), and when analyses were restricted to the first treatment cycle (OR: 1.34; 95% CI: 1.12, 1.62). The association of 25(OH)D with fertilization rate was not modified by BMI (P-interaction = 0.23), age (P-interaction = 0.72), or smoking status (P-interaction = 0.72).
FIGURE 1.
Serum 25(OH)D concentrations in relation to fertilization rate. Data are presented as predicted marginal means adjusted for age, BMI, infertility diagnosis (male factor as reference), race (white as reference), dietary patterns, folate, and vitamin B-12 serum concentrations. Dotted lines represent a 95% confidence band based on point-wise 95% CIs. Each 15-nmol/L increase in serum 25(OH)D was associated with an increase in the odds of fertilization of 19% (OR: 1.19; 95% CI: 1.04, 1.36). 25(OH)D, 25-hydroxyvitamin D.
The observed differences in fertilization rate did not translate into improvements in other outcomes of ART, however. Specifically, serum 25(OH)D concentrations were not related to markers of embryo quality (Supplemental Table 2) or the probability of implantation, clinical pregnancy, or live birth (Table 4).
TABLE 4.
Serum 25(OH)D concentrations in relation to clinical outcomes of women undergoing infertility treatment with assisted reproductive technologies1
| Implantation |
Clinical pregnancy |
Live birth |
||||
| Events/cycles, n | Adjusted P2 | Events/cycles, n | Adjusted P2 | Events/cycles, n | Adjusted P2 | |
| Serum 25(OH)D,3 nmol/L | ||||||
| Q1 [65.0 (33.5–75.0)] | 25/45 | 0.51 (0.29, 0.73) | 21/45 | 0.41 (0.22, 0.63) | 15/45 | 0.28 (0.12, 0.52) |
| Q2 [83.1 (75.9–86.2)] | 22/41 | 0.47 (0.28, 0.67) | 21/41 | 0.43 (0.25, 0.63) | 15/41 | 0.26 (0.12, 0.47) |
| Q3 [92.4 (86.8–104.6)] | 28/40 | 0.62 (0.40, 0.81) | 23/40 | 0.48 (0.28, 0.69) | 20/40 | 0.39 (0.19, 0.63) |
| Q4 [115.0 (107.8–155.5)] | 20/42 | 0.43 (0.25, 0.64) | 19/42 | 0.43 (0.25, 0.63) | 16/42 | 0.35 (0.17, 0.57) |
| P4 | 0.71 | 0.83 | 0.47 | |||
n = 100 women, 168 cycles. Q, quartile; 25(OH)D, 25-hydroxyvitamin D.
Values are predictive marginal means (95% CIs) adjusted for age, BMI, infertility diagnosis, race, dietary patterns, folate, and vitamin B-12 serum concentrations.
Values are medians (ranges).
The median concentration of vitamin D in each group was used as a continuous variable in the model.
DISCUSSION
In this prospective study of 100 women who underwent 168 ART cycles and most of whom had serum 25(OH)D concentrations between 50 and 125 nmol/L, serum 25(OH)D concentrations were positively related to fertilization rate. Women in the highest quartile of serum 25(OH)D concentrations had an 11% higher fertilization rate than women in the lowest quartile had. Despite this apparent benefit, 25(OH)D concentrations were unrelated to probability of having a clinical pregnancy or live birth after ART. These results contribute to the growing literature on the potential role of vitamin D in reproduction in general and in infertility treatment in particular. However, given the lack of association between 25(OH)D and live birth, the clinical applicability of these findings is unclear.
Our results are in agreement with previous work in animal models pointing to the importance of vitamin D in reproduction but suggest that these benefits may be obscured and not translate into greater reproductive success in the setting of assisted reproduction. Physiologic and experimental data in animal models strongly suggest that vitamin D may play an important role in reproduction. The vitamin D receptor is present in the ovary (23, 24), the endometrium (23), and the placenta (25). In addition, 25(OH)D concentrations have been correlated to the production of estradiol and progesterone in ovarian (24) and placental tissue (26), and to the regulation of the secretion hCG in the human syncytiotrophoblasts (27) in vitro. Previous work in animal models, however, has not evaluated the role of vitamin D on fertilization rates specifically.
This work adds to the emerging literature on the relation between vitamin D and treatment outcomes of couples undergoing assisted reproduction. Our finding of a positive association between serum 25(OH)D concentrations and fertilization rates contrasts with those of a previous work showing an inverse association between follicular fluid 25(OH)D and fertilization rates among women undergoing infertility treatment in Iran (16). However, follicular fluid 25(OH)D concentrations in the Iranian study were substantially lower (range: 13.4–53.1 nmol/L) than serum concentrations in our study, and all participants in the Iranian study fall within or below the lowest quartile of serum 25(OH)D in our study. Of note, serum and follicular fluid 25(OH)D are strongly related to each other (r = 0.77) (16). Interestingly, whereas the overall association between 25(OH)D and fertilization rate in our study was positive, the fertilization rate of women in the second quartile of serum 25(OH)D [mean (range): 83.1 nmol/L (75.9–86.2 nmol/L)] was lower than the fertilization rate in women in the lowest quartile of 25(OH)D [mean (range): 65.0 nmol/L (33.5–75.0 nmol/L)], although this difference was not statistically significant. It is thus possible that within the range of 25(OH)D observed in the Iranian study there is a true inverse relation with fertilization rates with a benefit only emerging for women with much higher serum and follicular fluid 25(OH)D concentrations. Our finding of no relation of serum 25(OH)D with probability of clinical pregnancy or live birth is in agreement with the majority of studies published to date that have also failed to document an association between markers of vitamin D status and infertility treatment outcomes (16, 18, 19, 28, 29). However, others have reported positive (10, 30–32) as well as inverse associations (17) between follicular fluid 25(OH)D and clinical pregnancy. Clearly, whether vitamin D plays a role in the outcomes of infertility treatment remains an open question making it necessary to conduct additional studies to clarify this issue.
Limitations of this study must be considered. First, as is the case of all observational studies, we cannot rule out the possibility of unmeasured confounding factors. However, many of the characteristics the design of the study including the prospective design with complete follow-up, the statistical adjustment of for a wide range of potential confounding factors, and the use of an objective biomarker (33–35) to characterize exposure provide reassurance of the validity of our findings. Second, the analytic sample was composed of only 100 women, raising concerns that our null findings for clinical pregnancy and live birth may result from limited statistical power. Although the current study was sufficiently powered to evaluate differences in fertilization rate, larger studies will be necessary to evaluate differences in clinical pregnancy and live birth of the magnitude suggested by our data (7% difference between the first and last quartile). Third, because all participants were women undergoing assisted reproduction, it is not possible to know to what extent these findings translate, or not, to couples without known fertility problems trying to conceive on their own.
In summary, we found that serum 25(OH)D concentrations were positively related to fertilization rate but unrelated to probability of clinical pregnancy or live birth in a prospective cohort of women undergoing infertility treatment with ART. Most of the women in this cohort had serum 25(OH)D concentrations between 50 and 125 nmol/L. Our findings do not exclude the possibility that vitamin D could have significant effects on probability of clinical pregnancy and live birth of modest magnitude. Given the expected size of the effect of vitamin D on live birth rates (∼7% difference between top and bottom quartiles) based on our data, it is important that future studies are sufficiently large to identify associations of this magnitude. These issues should be addressed in future studies, ideally in large trials.
Acknowledgments
The authors’ responsibilities were as follows—AJG, RH, and JEC: were involved in the design of the study, concept of the study, and revision of intellectual content of the manuscript; LA: analyzed data and drafted the manuscript; LA, Y-HC, and JEC: interpreted the data; Y-HC: contributed to the statistical analysis; AJG: reviewed the statistical analysis; DLW, IS, RH, and JEC: were involved in the acquisition of the data; JEC: had primary responsibility for the final content; and all authors: were involved in the critical revision of the manuscript and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ART, assisted reproductive technology; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization; MII, metaphase II; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–31.e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slama R, Hansen OK, Ducot B, Bohet A, Sorensen D, Giorgis Allemand L, Eijkemans MJ, Rosetta L, Thalabard JC, Keiding N, et al. Estimation of the frequency of involuntary infertility on a nation-wide basis. Hum Reprod 2012;27:1489–98. [DOI] [PubMed] [Google Scholar]
- 3.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Barfield WD. Assisted reproductive technology surveillance:United States, 2011. MMWR Surveill Summ 2014;63:1–28. [PubMed] [Google Scholar]
- 4.Henningsen AA, Gissler M, Skjaerven R, Bergh C, Tiitinen A, Romundstad LB, Wennerholm UB, Lidegaard O, Nyboe Andersen A, Forman JL, et al. Trends in perinatal health after assisted reproduction: a Nordic study from the CoNARTaS group. Hum Reprod 2015;30:710–6. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara O, Adamson GD, Dyer S, de Mouzon J, Nygren KG, Sullivan EA, Zegers-Hochschild F, Mansour R. International committee for monitoring assisted reproductive technologies: world report on assisted reproductive technologies, 2007. Fertil Steril 2015;103:402–13.e11. [DOI] [PubMed] [Google Scholar]
- 6.Nyboe Andersen A, Goossens V, Bhattacharya S, Ferraretti AP, Kupka MS, de Mouzon J, Nygren KG. Assisted reproductive technology and intrauterine inseminations in Europe, 2005: results generated from European registers by ESHRE: ESHRE. The European IVF Monitoring Programme (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 2009;24:1267–87. [DOI] [PubMed] [Google Scholar]
- 7.Kawwass JF, Kissin DM, Kulkarni AD, Creanga AA, Session DR, Callaghan WM, Jamieson DJ. Safety of assisted reproductive technology in the United States, 2000-2011. JAMA 2015;313:88–90. [DOI] [PubMed] [Google Scholar]
- 8.Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: a narrative review. Int J Clin Pract 2013;67:225–35. [DOI] [PubMed] [Google Scholar]
- 9.Fabris A, Pacheco A, Cruz M, Puente JM, Fatemi H, Garcia-Velasco JA. Impact of circulating levels of total and bioavailable serum vitamin D on pregnancy rate in egg donation recipients. Fertil Steril 2014;102:1608–12. [DOI] [PubMed] [Google Scholar]
- 10.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril 2010;94:1314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanni VS, Vigano P, Somigliana E, Papaleo E, Paffoni A, Pagliardini L, Candiani M. Vitamin D and assisted reproduction technologies: current concepts. Reprod Biol Endocrinol 2014;12:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickie JP, Lavigne DM, Woodward WD. Reduced fecundity of vitamin D deficient rats. Comp Biochem Physiol A Comp Physiol 1983;74:923–5. [DOI] [PubMed] [Google Scholar]
- 13.Kayser J, Sabel A, Lavollay J. [Vitamin D and reproduction in Wistar rats]. C R Acad Sci Hebd Seances Acad Sci D 1979;289:1307–9 (in French). [PubMed] [Google Scholar]
- 14.Kwiecinksi GG, Petrie GI, DeLuca HF. 1,25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am J Physiol 1989;256:E483–7. [DOI] [PubMed] [Google Scholar]
- 15.Halloran BP, DeLuca HF. Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat. J Nutr 1980;110:1573–80. [DOI] [PubMed] [Google Scholar]
- 16.Aleyasin A, Hosseini MA, Mahdavi A, Safdarian L, Fallahi P, Mohajeri MR, Abbasi M, Esfahani F. Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol 2011;159:132–7. [DOI] [PubMed] [Google Scholar]
- 17.Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, Messinis IE. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol 2010;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franasiak JM, Molinaro T, Dubell EK, Scott K, Ruiz A, Forman EJ, Werner MD, Hong KH, Scott RT Jr. Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am J Obstet Gynecol 2015;212:315.e1–6. [DOI] [PubMed] [Google Scholar]
- 19.Firouzabadi RD, Rahmani E, Rahsepar M, Firouzabadi MM. Value of follicular fluid vitamin D in predicting the pregnancy rate in an IVF program. Arch Gynecol Obstet 2014;289:201–6. [DOI] [PubMed] [Google Scholar]
- 20.Gaskins AJAM, Wright DL, Toth TL, Williams PL, Gillman MW, Hauser R, Chavarro JE. Dietary folate and reproductive success among women undergoing assisted reproduction. Obstet Gynecol 2014;124:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod 2012;27:2899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat 1980;34:216–21. [Google Scholar]
- 23.Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, Friedrich M, Taylor RN, Hornung D. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci 2007;14:486–97. [DOI] [PubMed] [Google Scholar]
- 24.Parikh G, Varadinova M, Suwandhi P, Araki T, Rosenwaks Z, Poretsky L, Seto-Young D. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res 2010;42:754–7. [DOI] [PubMed] [Google Scholar]
- 25.Tanamura A, Nomura S, Kurauchi O, Furui T, Mizutani S, Tomoda Y. Purification and characterization of 1,25(OH)2D3 receptor from human placenta. J Obstet Gynaecol (Tokyo 1995) 1995;21:631–9. [DOI] [PubMed] [Google Scholar]
- 26.Barrera D, Avila E, Hernandez G, Halhali A, Biruete B, Larrea F, Diaz L. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol 2007;103:529–32. [DOI] [PubMed] [Google Scholar]
- 27.Barrera D, Avila E, Hernandez G, Mendez I, Gonzalez L, Halhali A, Larrea F, Morales A, Diaz L. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod Biol Endocrinol 2008;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estes SJ, Ye B, Qiu W, Cramer D, Hornstein MD, Missmer SA. A proteomic analysis of IVF follicular fluid in women <or=32 years old. Fertil Steril 2009;92:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod 2012;27:3321–7. [DOI] [PubMed] [Google Scholar]
- 30.Paffoni A, Ferrari S, Vigano P, Pagliardini L, Papaleo E, Candiani M, Tirelli A, Fedele L, Somigliana E, Vitamin D. Deficiency and infertility: insights from in vitro fertilization cycles. J Clin Endocrinol Metab 2014;99:E2372–6. [DOI] [PubMed] [Google Scholar]
- 31.Garbedian K, Boggild M, Moody J, Liu KE. Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open 2013;1:E77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polyzos NP, Anckaert E, Guzman L, Schiettecatte J, Van Landuyt L, Camus M, Smitz J, Tournaye H. Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod 2014;29:2032–40. [DOI] [PubMed] [Google Scholar]
- 33.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr 2008;87:1087S–91S. [DOI] [PubMed] [Google Scholar]
- 34.Zerwekh JE. The measurement of vitamin D: analytical aspects. Ann Clin Biochem 2004;41:272–81. [DOI] [PubMed] [Google Scholar]
- 35.Hollis BW. Assessment of vitamin D status and definition of a normal circulating range of 25-hydroxyvitamin D. Curr Opin Endocrinol Diabetes Obes 2008;15:489–94. [DOI] [PubMed] [Google Scholar]