Abstract
An elevated serum IgG4 level is one of the most useful factors in the diagnosis of IgG4-related disease (IgG4-RD). In this study, we performed a meta-analysis of the published articles assessing the diagnostic accuracy of serum IgG4 concentrations for IgG4-RD. The databases of MEDLINE/PubMed, EMBASE and Web of Science were systematically searched for relevant studies. Sensitivities and specificities of serum IgG4 in each study were calculated, and the hierarchical summary receiver operating characteristic (HSROC) model with a random effects model were employed to obtain the individual and pooled estimates of sensitivities and specificities. In total, twenty-three studies comprising 6048 patients with IgG4-RD were included in the meta-analysis. The pooled sensitivity was 85% with a 95% confidence interval (CI) of 78–90%; the pooled specificity was 93% with a 95% CI of 90–95%. The HSROC curve for quantitative serum IgG4 lies closer to the upper left corner of the plot, and the area under the curve (AUC) was 0.95 (95% CI 0.93, 0.97), which suggested a high diagnostic accuracy of serum IgG4 for the entity of IgG4-RD. Our study suggests that serum IgG4 has high sensitivity and specificity in the diagnosis of IgG4-RD.
Immunoglobulin (Ig) G4-related disease (IgG4-RD) is an entity of emerging immune-mediated diseases characterised by the infiltration of IgG4-bearing plasma cells, elevated serum IgG4 concentration, and systemic disorders present in nearly all organs with the exception of cerebral parenchyma1,2,3. The role of IgG4 in autoimmune diseases was first proposed for sclerosing or autoimmune pancreatitis (AIP) in 20014. Since then, various organs in addition to the pancreas have been reported as involved in IgG4-related conditions5. Notably, many previously-recognised diseases, including AIP, Mikulicz’s disease (MD), chronic fibrosing sialadenitis, and other sclerosing disorders, are now known to belong to the clinical spectrum of IgG4-RD1.
The organ-specific diagnostic criteria for patients with IgG4-RD is likely impossible to establish due to variable organ involvements and clinical symptoms6. However, comprehensive criteria are important in clinical practice, especially in differential diagnosis from malignancies. Based on the common features of IgG4-RD, the current diagnostic criteria from Japan, Asian-Korea Consensus and Comprehensive Diagnostic Criteria generally include clinical symptoms (swelling or masses of single or multiple organs), histopathologic findings (extensive infiltration of IgG4+ plasma cells and fibrosis), haematological examination (concentrations of serum IgG4 >135 mg/dl), and imaging modalities (narrowing of ducts and/or enlargement of organs)6,7,8. An elevated serum IgG4 level is one of the most helpful of these criteria. Previous studies demonstrated that serum IgG4 concentrations significantly increased in patients with IgG4-RD4,9,10. The measurement of the serum IgG4 concentration is a useful tool in disease diagnosis, evaluating disease activity and predicting the responsiveness and clinical improvement of steroid and rituximab therapy in patients with IgG4-RD11,12.
However, many diseases other than IgG4-RD have higher levels of IgG4, and studies have suggested an unsatisfactory performance of serum IgG4 detection in the diagnosis of IgG4-RD with poor specificity and positive predictive value despite a high sensitivity and negative predictive value2,13,14. Additionally, IgG4-RD is a rare disorder with low incidence compared with other autoimmune disease, and the small sample sizes in previous studies focusing on the features of serum IgG4 level frequently led to a variable diagnostic accuracy of IgG4 in patients with IgG4-RD6,9,15. Notably, IgG4-RD is a systemic fibro-inflammatory disease that has a similar pathogenesis and cardinal features in affected organs; however, it was rarely analysed as an extensive entity but rather an individual disorder focused on a single organ, especially the pancreas and salivary or lacrimal glands2,11,16,17,18,19.
Up to now, a comprehensive overview of the accuracy and precision of the serum IgG4 concentration for the diagnosis of all IgG4-RD has not been performed. We aimed to establish the diagnostic performance of the serum IgG4 concentration for IgG4-RD involving the pancreas, bile duct, salivary gland, and lacrimal gland from non-IgG4-RD and/or healthy controls.
Methods
Search strategy
We searched the electronic databases of MEDLINE (via PubMed), EMBASE and the Web of Science from 2000 to September 2015 in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines20. The systemic search was conducted combining the terms “serum”, “immunoglobulin g4” OR “igg4”, as well as the terms “sclerosing pancreatitis” OR “autoimmune chronic pancreatitis” OR “autoimmune pancreatitis” OR “cholangitis” OR “sclerosing cholangitis” OR “Küttner tumor” OR “sialadenitis” OR “sclerosing sialadenitis” OR “sclerosing dacryoadenitis” OR “Mikulicz’s disease” OR “igg4-rd” OR “igg4-related disease” with the species restriction of Human and language restriction of English. The relevant reference lists of the review articles were also screened to identify additional eligible articles not obtained in database searches.
Data extraction and quality assessment
Prospective or retrospective case-control studies on the utility of serum IgG4 concentration in the diagnosis of IgG4-RD were deemed eligible for inclusion in the meta-analysis. The studies also met the criteria in that serum IgG4 concentration with an unambiguous cut-off value had been evaluated between IgG4-RD with a wide variety of organs involved and other diseases, as well as healthy controls. Articles with a larger sample size or more recently published articles were included when they used the same case series. Studies for which inadequate data for confirming the diagnosis of IgG4-RD and those assessing the role of IgG4 in the pathogenic mechanism were excluded. Conference or poster abstracts without sufficient clinical information or subsequent publication in full text were excluded. Studies with fewer than 10 included patients or based on animal or cell cultures were also excluded.
Risk of bias and applicability were critically assessed according to the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool, which has 4 key domains including patient selection, index text, reference standard, and flow and timing21. Risk of bias and applicability concerns were judged as “low,” “high,” or “unclear”. Data extraction and quality assessment were performed by two reviewers (Wen-long Xu and Ying-chun Ling), and disagreements were resolved by discussion.
Statistical analysis
The forest plot of individual and summarised sensitivity and specificity along with 95% confidence intervals (95% CIs) of the included studies were generated to graphically represent the diagnostic value of serum IgG4 in IgG4-RD. Subsequently, a hierarchical summary receiver operating characteristic (HSROC) model with a corresponding 95% confidence contour and 95% prediction contour was calculated. A bivariate random effects model following the DerSimonian-Laird method with a corresponding test of heterogeneity was used for data pooling. The heterogeneity across studies included in the meta-analysis was statistically detected using a Q test and I2 statistics, which ranged from 0 to 100% and were interpreted as representing low, medium and high inconsistency with the values of ≤25%, ≤50% and ≤75%, respectively, in accordance with the proposal of Higgins and Thompson22. Stratified analysis and meta-regression based on variations in features of ethnicity, spectrum of IgG4-RD and detection method were performed to explore potential sources of heterogeneity. Publication biases were tested using Egger precision weighted linear regression tests and sensitivity analysis and demonstrated graphically using funnel plots. The causes of heterogeneity were further assessed using a sensitivity analysis in which the sequential omission of individual studies was performed to analyse the influence of a single study on the overall detection rate of IgG4-RD. The meta-analysis of the data was conducted using the Stata/SE version 13.1 (StataCorp LP, Texas, USA). P < 0.05 was considered statistically significant.
Results
Study identification and selection
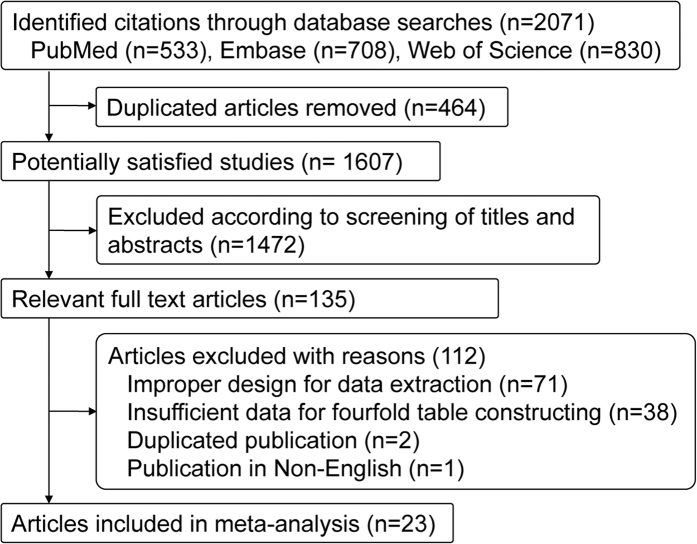
The initial keyword search yielded 2071 potentially relevant studies from the databases of PubMed (n = 533), Embase (n = 708), and the Web of Science (n = 830). After 464 duplicates were discarded, 1607 articles remained, of which the title and abstract were screened for eligibility in the meta-analysis. In accordance with the predefined inclusion criteria, 1472 articles were removed, and the remaining 135 articles were deemed potentially relevant. Following the further review of the full text articles, 111 studies were excluded due to improper design for data extraction (n = 70), insufficient data for fourfold table construction (n = 38), duplicated publication (n = 2) and published in a language other than English (n = 1). Finally, 23 articles were considered as eligible and included in this meta-analysis (Fig. 1). No disagreement occurred between the two reviewers.
Figure 1. Study flow chart.
Study characteristics and methodological quality
In total, 6048 patients in 23 studies were included in the spectrum of IgG4-RD including AIP (13 studies), MD (3 studies), IgG4-associated cholangitis (6 studies) and IgG4-RD without further classification according to the organ involved (4 studies). The study type included 10 retrospective studies, 6 prospective studies and 7 studies without reporting the study type. Ethnicity included Asian in 15 studies with 3931 patients and Caucasian in 8 studies with 2117 patients (Table 1).
Table 1. Characteristics of included studies.
| Author | Year | Study location | Ethnicity | Study design | Criteria for IgG4-RD | IgG4-RD disease (No.) | Control (No.) | Risk of bias* | Applicability concerns* |
|---|---|---|---|---|---|---|---|---|---|
| Aparisi et al. | 2005 | Spain | Caucasian | NR | Histopathological, clinical, and laboratory parameters | AIP (25) | ICP (29) | P† | No |
| Boonstra et al. | 2014 | Dutch | Caucasian | Prospective | Mayo Clinic’s HISORt criteria | IAC (73) | PSC (n = 310) | P | No |
| Carruthers et al. | 2015 | USA | Caucasian | NR | International pathology consensus guideline (2012) | IgG4-RD (72) | AID (n = 11), other disease (n = 397) | P | No |
| Choi et al. | 2007 | Korea | Asian | NR | Asan Medical Center of Korea | AIP (30) | PC (n = 76); CP (n = 67) | P | No |
| Ghazale et al. | 2007 | USA | Caucasian | Prospective | Mayo Clinic’s HISORt criteria | AIP (45) | PC (n = 135), other pancreatic diseases (n = 268); other disease (n = 62) | P | No |
| Hamano et al. | 2001 | Japan | Asian | Retrospective | Ultrasonographic, clinical, and laboratory performance | AIP (20) | PC (n = 70), CP (n = 45), PBC (n = 20), PSC (n = 8), SS (n = 11), HC (n = 20) | P | No |
| Hirano et al. | 2006 | Japan | Asian | Retrospective | Japan Pancreas Society | AIP (35) | CP (n = 24), PSC (n = 11), PC (n = 23), biliopancreatic cancer (n = 23) | P | No |
| Kaji et al. | 2012 | Japan | Asian | Prospective | AIP: Asian criteria (Japan–Korea) and ICDC | AIP (35) | PC (n = 17), CP (n = 24), PSC (n = 7), biliary cancer (n = 23) | P | No |
| Kamisawa et al. | 2008 | Japan | Asian | Retrospective | Radiological, serological histological examination | AIP (17) | PC (n = 33) | P, I# | I |
| Masaki et al. | 2012 | Japan | Asian | Retrospective | Pathological and clinical manifestations | IgG4-RD (132) | SS (n = 33), other non-AID (n = 15) | P, I | I |
| Mavragani et al. | 2014 | Greece | Caucasian | Retrospective | Comprehensive criteria | IgG4-RD (7) | SS (n = 126)* | P | No |
| Nakazawa et al. | 2012 | Japan | Asian | Retrospective | IgG4-SC: Japanese criteria 2006; AIP: HISORt criteria | IAC (47) | PC (n = 26), PSC (n = 21), CCA (n = 18) | P, I | I |
| Ohara et al. | 2013 | Japan | Asian | Retrospective | ICDC, revised Japanese criteria | IAC (344) | PC (n = 245), PSC (n = 110), CCA (n = 149) | P | No |
| Oseini et al. | 2011 | USA | Caucasian | Prospective | Mayo Clinic’s HISORt criteria | IAC (97) | CCA (n = 287) | P | No |
| Sanchez-Castanon et al. | 2012 | Spain | Caucasian | Retrospective | Mayo Clinic’s HISORt criteria | AIP (12) | CP (n = 23), ICP (n = 26), AP (n = 11), PC (n = 21), SS (n = 9), T1DM (n = 40), HC (n = 45) | P | No |
| Su et al. | 2015 | China | Asian | NR | Japan criteria | IgG4-RD (12) | AID (n = 127), other disease (n = 818) | P | No |
| Szántó et al. | 2014 | Japan | Asian | NR | Japanese Research Committee (2011) | AIP, MD (8) | SS (n = 43) | P | No |
| Tabata et al. | 2011 | Japan | Asian | Prospective | AIP: Asian criteria (Japan–Korea); MD: Clinical performance and exclusive criteria | AIP, MD (66) | Other pancreatobiliary or salivary gland diseases (n = 488) | P | No |
| Tanaka et al. | 2015 | Japan | Asian | Retrospective | Japanese Biliary Association (2012) | IAC (38) | PSC (n = 120) | P, I | I |
| Uehara et al. | 2005 | Japan | Asian | NR | Histopathological, clinical, and laboratory parameters | AIP-SC (6) | PSC (6) | P | P |
| Van Heerde et al. | 2014 | Dutch | Caucasian | Prospective | ICDC, Asian or HISORT criteria, or comprehensive criteria | AIP (33) | PC (n = 53) | P | P |
| Wu et al. | 2013 | China | Asian | Retrospective | Pathological and radiologic manifestations | AIP (15) | Non-AIP (n = 4) | P, I | No |
| Yamamoto et al. | 2012 | Japan | Asian | NR | Mayo Clinic’s HISORt criteria; Japan criteria | AIP, MD, CFSA, DA (102) | AID (n = 206), other disease (n = 72), HC (n = 21) | P | P |
IgG4-RD: IgG4-related disease; AIP: autoimmune chronic pancreatitis; MD: Mikulicz’s disease; IAC: IgG4-associated cholangitis; CFSA: Chronic fibrosing sialoadenitis; DA: IgG4-related dacryoadenitis; PC: pancreatic cancer; CP: chronic pancreatitis other than AIP; ICP: idiopathic chronic pancreatitis; PBC: primary biliary cirrhosis; SS: Sjögren’s syndrome; CCA: cholangiocarcinoma; HC: healthy control; T1DM: type 1 diabetes mellitus; AID: autoimmune diseases; ICDC: International consensus diagnostic criteria; NR: not reported. *QUADAS score; †P: patient selection; #I: index test.
Overall, the included studies were of moderate methodological quality according to the QUADAS-2 tool. The high risk of bias and concerns of applicability regarding patient selection were introduced because all included studies were of a randomised or case-control design. The risk of bias for the index test and reference standard remained either (1) unclear because the index test and/or reference standard were interpreted double-blindly in all included studies with the exception of one23 or (2) high because the index test of serum IgG4 was not reported in five studies24,25,26,27,28. There were no major concerns regarding the applicability of the reference standard for the included studies. The risk of bias on flow and timing arose from the fact that the description of the interval and interventions between index tests and the reference standard were not reported in all studies (Table 1).
Overall diagnostic accuracy
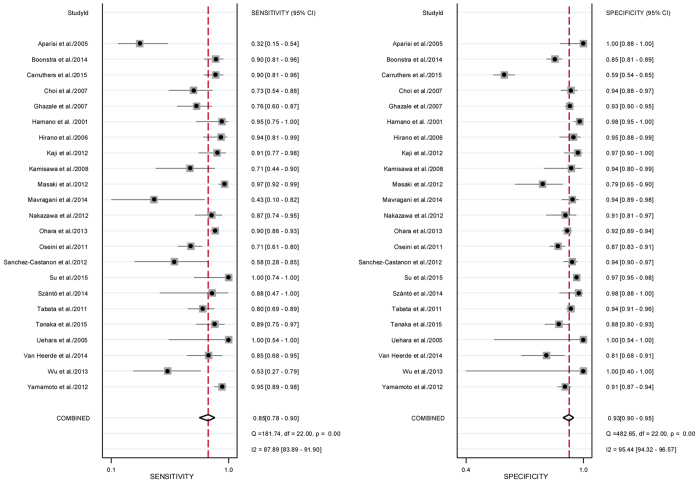
Serum IgG4 with a cut-off value ranging from 130 mg/dL to 200 mg/dL was detected using the assay methods of nephelometry (15 studies), single radial immunodiffusion (2 studies) and ELISA (1 study). Notably, the assay of serum IgG4 was not reported in 5 studies. The sensitivity and specificity of serum IgG4 concentration for the diagnosis of IgG4-RD ranged from 32% to 100% and from 59% to 100%, respectively (Table 2). The pooled sensitivity and specificity were 85% with a 95% confidence interval (CI) of 78–90% and 93% with a 95% CI of 90–95%, respectively (Fig. 2). The Q tests for pooled sensitivity and specificity were 181.74 with I2 of 87.89 (95% CI 83.89%, 91.90%) (p < 0.01) and 482.65 with I2 of 95.44 (95% CI 94.32%, 96.57%) (p < 0.01), both of which suggested significant heterogeneity (Fig. 3). The diagnostic odds ratio and positive and negative likelihood ratios were 70 (95% CI 42, 116), 11.6 (95% CI 8.1, 16.5) and 0.17 (95% CI 0.11, 0.24), respectively. These results strongly indicated that the higher rate of serum IgG4 positivity increased the chance of diagnosing IgG4-RD.
Table 2. Diagnostic accuracy of serum IgG4 concentration for individual study.
| Author | Year | Assay for IgG4 | Cut-off (mg/dL) | Participant, n | True positive | False positive | False negative | True negative | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|---|---|
| Aparisi et al. | 2005 | Nephelometry | 130 | 54 | 8 | 0 | 17 | 29 | 32% | 100% |
| Boonstra et al. | 2014 | Nephelometry | 140 | 383 | 66 | 45 | 7 | 265 | 90% | 85% |
| Carruthers et al. | 2015 | Nephelometry | 135 | 380 | 65 | 125 | 7 | 183 | 90% | 59% |
| Choi et al. | 2007 | SRI | 135 | 173 | 22 | 9 | 8 | 134 | 73% | 94% |
| Ghazale et al. | 2007 | Nephelometry | 140 | 510 | 34 | 32 | 11 | 433 | 76% | 93% |
| Hamano et al. | 2001 | SRI | 135 | 194 | 19 | 3 | 1 | 171 | 95% | 98% |
| Hirano et al. | 2006 | Nephelometry | 135 | 116 | 33 | 4 | 2 | 77 | 94% | 95% |
| Kaji et al. | 2012 | Nephelometry | 135 | 106 | 32 | 2 | 3 | 69 | 91% | 97% |
| Kamisawa et al. | 2008 | NR | 135 | 50 | 12 | 2 | 5 | 31 | 71% | 94% |
| Masaki et al. | 2012 | NR | 135 | 180 | 128 | 10 | 4 | 38 | 97% | 79% |
| Mavragani et al. | 2014 | Nephelometry | 135 | 133 | 3 | 7 | 4 | 119 | 43% | 94% |
| Nakazawa et al. | 2012 | NR | 135 | 112 | 41 | 6 | 6 | 59 | 87% | 91% |
| Ohara et al. | 2013 | Nephelometry | 135 | 848 | 309 | 41 | 35 | 463 | 90% | 92% |
| Oseini et al. | 2011 | Nephelometry | 140 | 384 | 69 | 37 | 28 | 250 | 71% | 87% |
| Sanchez-Castanon et al. | 2012 | Nephelometry | 130 | 187 | 7 | 10 | 5 | 165 | 58% | 94% |
| Su et al. | 2015 | ELISA | 135 | 957 | 12 | 32 | 0 | 913 | 100% | 97% |
| Szántó et al. | 2014 | Nephelometry | 135 | 51 | 7 | 1 | 1 | 42 | 88% | 98% |
| Tabata et al. | 2011 | Nephelometry | 135 | 554 | 53 | 31 | 13 | 457 | 80% | 94% |
| Tanaka et al. | 2015 | NR | 135 | 158 | 34 | 15 | 4 | 105 | 89% | 88% |
| Uehara et al. | 2005 | Nephelometry | 135 | 12 | 6 | 0 | 0 | 6 | 100% | 100% |
| Van Heerde et al. | 2014 | Nephelometry | 140 | 86 | 28 | 10 | 5 | 43 | 85% | 81% |
| Wu et al. | 2013 | NR | 200 | 19 | 8 | 0 | 7 | 4 | 53% | 100% |
| Yamamoto et al. | 2012 | Nephelometry | 144 | 401 | 97 | 28 | 5 | 271 | 95% | 91% |
SRI: single radial immunodiffusion; NR: not reported.
Figure 2. Forrest plot of the sensitivity and specificity of serum IgG4 demonstrating individual and summary sensitivity and specificity for the per-patient diagnosis of IgG4-RD.
The corresponding heterogeneity of Q test with p < 0.01 and I2 > 75% suggests significant heterogeneity.
Figure 3. Hierarchical summary receiver operating characteristic (HSROC), with 95% confidence contour and 95% prediction contour, illustrating the summary operating point and study estimate of the sensibility and specificity for serum IgG4.
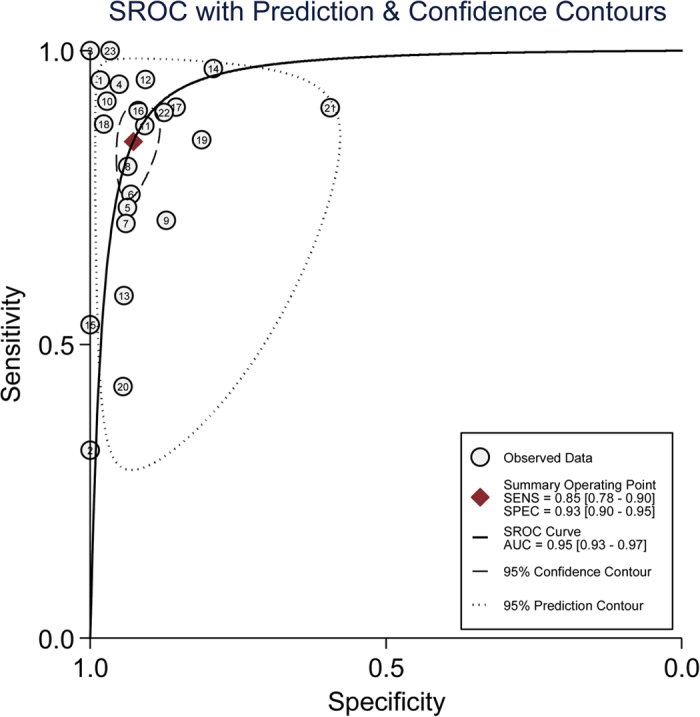
The diagnostic values of the studies were demonstrated in a HSROC graph in which the summary operating point represents the pooled sensitivity and specificity, as well as 95% confidence and the prediction region represents 95% CI of the pooled and individual sensitivity and specificity. The HSROC curve for quantitative serum IgG4 lies closer to the upper left corner of the HSROC plot, and the area under the curve (AUC) was 0.95 (95% CI 0.93, 0.97), which suggested an impressive diagnostic accuracy of serum IgG4 for the entity of IgG4-RD. Finally, the curve is symmetrical with the Z statistic of −0.58 (p = 0.564), which also indicates a high diagnostic accuracy for serum IgG4 (Fig. 4).
Figure 4. Egger tests for assessment of publication bias.
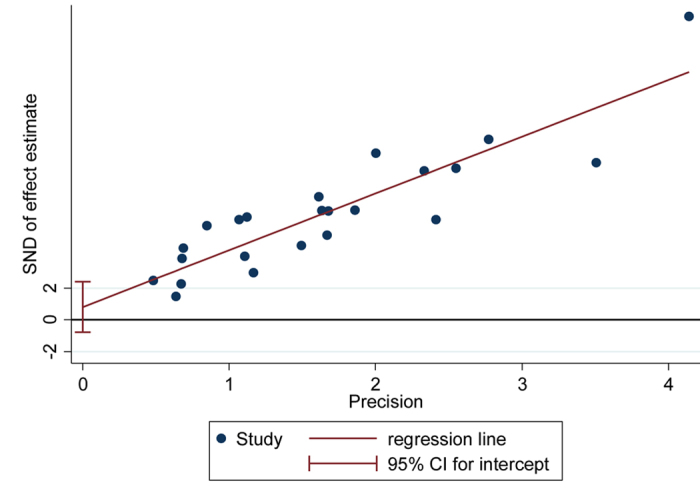
(SND: standard normal deviate).
Subgroup analysis and publication bias
The subgroup analysis was performed according to study period (published before vs. after 2011), study design (designed prospectively vs. retrospectively), sample size (less than 150 vs. more than 150), ethnicity (Asian vs. Caucasian), and serum IgG4 concentration detection assay (nephelometry vs. another method). The sensitivity of serum IgG4 was higher for (1) the studies published after 2011, (2) retrospective studies performed before 2011 that used detection methods other than assays, and (3) prospective studies using the method of nephelometry, whereas specificity was significantly different between the subgroups of all variables for the diagnosis of IgG4-RD (Table 3).
Table 3. Subgroup analysis of the sensitivity and specificity of serum IgG4 for the diagnostic performance of IgG4-RD.
| Parameter | Category | No. of study | Sensitivity | p | Specificity | p |
|---|---|---|---|---|---|---|
| Period | Before 2011 | 9 | 78% (67–90%) | 0.00 | 95% (92–98%) | 0.00 |
| After 2011 | 14 | 88% (82–94%) | 91% (87–95%) | |||
| Study design | Prospective | 6 | 83% (74–93%) | 0.04 | 90% (86–94%) | 0.00 |
| Retrospective | 10 | 85% (77–92%) | 93% (91–96%) | |||
| Sample size | Less than 150 | 13 | 88% (82–94%) | 0.35 | 91% (87–95%) | 0.00 |
| More than 150 | 10 | 79% (67–90%) | 95% (92–98%) | |||
| Ethnicity | Asian | 15 | 88% (83–93%) | 0.63 | 94% (92–97%) | 0.00 |
| Caucasian | 8 | 73% (61–86%) | 90% (84–95%) | |||
| Assay | Nephelometry | 15 | 83% (75–91%) | 0.02 | 92% (89–95%) | 0.00 |
| Other method | 8 | 87% (78–96%) | 94% (90–98%) |
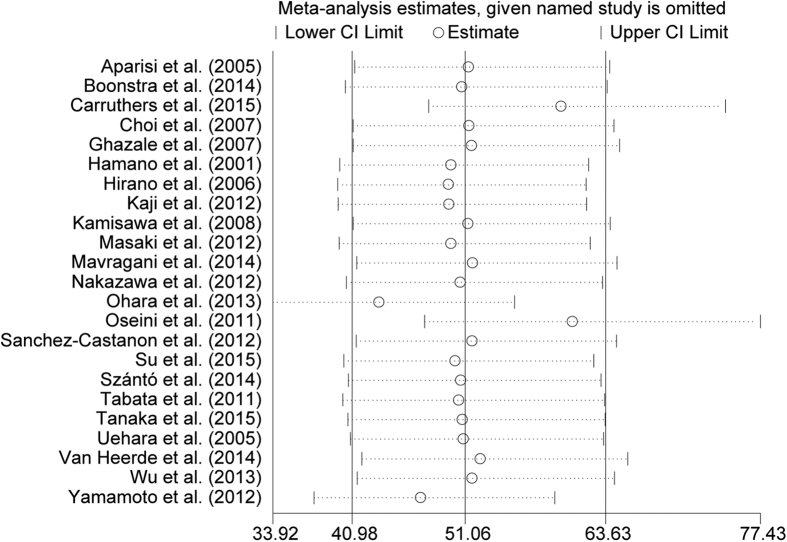
Egger’s regression test did not reveal any publication bias arising from small-study effects (p = 0.30) (Fig. 4). A sensitivity analysis suggested only a minor influence for diagnostic accuracy of omitting single-study estimates from 3 studies with larger sample sizes2,29,30; however, the estimates still fall within the indicated spread of lower and higher CI limits (Fig. 5).
Figure 5. Meta-analysis estimates, given named study is omitted.
The results shows that only little variation in summarized detection rate estimates of IgG4-RD is induced by omission of the one selected study. No systematic bias is identified although strong heterogeneity amongst studies is indicated. (Mantel-Haenzel fix model, statistic of relative risk).
Discussion
Accurately differentiating IgG4-RD from malignancies such as pancreatic cancer is very important to avoid unnecessary surgeries. Compared with the histopathological criteria, the detection of serum IgG4 is one of the most convenient and valuable non-invasive examinations in clinical practice for the diagnosis of IgG4-RD, especially in disease screening at an early stage. However, because the positive rate of serum IgG4 varied according to different studies, the diagnostic accuracy of this method is controversial. A previous meta-analysis published in 2009 demonstrated that the serum IgG4 is a good marker of a single disease of AIP with a pooled sensitivity ranging from 82.3% to 89.3% and a specificity of 94.6% to 95.8% according to different control31. Beyond that, no studies were carried out to systematically summarise the currently available data on the performance of serum IgG4 for the diagnosis of IgG4-RD as an entity.
In the last decade, numerous studies have attempted to evaluate the diagnostic value of serum IgG4 in IgG4-RD. To summarise these studies, we conducted this meta-analysis, which included 23 studies comprising a total of 6048 patients with IgG4-RD diagnosed with different criteria. The key findings of our analysis are that serum IgG4 has a very high accuracy for sensitivity (85%) and specificity (93%) in detecting IgG4-RD involving the pancreas, bile duct, salivary gland, or lacrimal gland from non-IgG4-RD involving the same organs and/or healthy controls (or both). The serum IgG4 has a higher summarised sensitivity and specificity compared with the histopathological method using the infiltration of IgG4-positive plasma cells in which the pooled sensitivity and specificity were 58.8% and 90.2%, respectively32. The diagnostic value of serum IgG4 remained significant with a sensitivity range from 78% to 88% and a specificity range from 90% to 95% when analysed separately in different subgroups. An association between the sensitivity or specificity and the study period, study, sample size, ethnicity, or detection assay of serum IgG4 were also identified using meta-regression and subgroup analysis. The findings of such an assessment are useful both in providing evidence-based patient information in clinical application and further investigation.
Our meta-analysis had several limitations. Firstly, there was inevitable heterogeneity in the meta-analysis of diagnostic accuracy due to the variability in design characteristics and the poor quality of reporting in the primary studies. The quality of the studies included in the meta-analysis was modest for our meta-analysis because ten studies were retrospective designed19,23,24,25,26,27,28,29,33,34, six studies were prospective designed11,16,18,30,35,36, and seven studies did not report the study design2,9,10,15,17,37,38. The QUADAS-2 tools for the methodological assessment indicated that other contributors to the potential heterogeneity across the studies result from the risk bias in patient selection due to the diagnosis of IgG4-RD on the basis of multiple criteria, as well as due to the detection method for serum IgG4 despite the clear cut-off value given in several studies24,25,26,27,28. Regarding procedure and timing, the interval and whether there were any interventions between the index tests and the reference standard were not described in all studies. Secondly, because differing cutoff values were used in the same primary studies2,11,18,35, widely accepted values varying from 135 mg/dL (range 130 mg/dL to 200 mg/dL) were employed in the meta-analysis. It was not possible to assess a threshold effect for an optimum serum IgG4 concentration. Finally, several studies with high quality were excluded because the results were reported in the form of means and SD, which may contribute to the heterogeneity and may have impaired the stringency of the meta-analysis.
Serum IgG4 is a more cost-effective, easy and a time-efficient assay that can be carried out to detect IgG4-RD. We conclude that this meta-analysis has achieved its primary objectives by demonstrating that the detection of serum IgG4 has high sensitivity and represents a specific investigative modality in the detection of IgG4-RD as an indicator. However, this does not necessarily indicate that the positive or negative serum IgG4 could be used to rule out (or confirm) a diagnosis of IgG4-RD. A diagnosis based on IgG4-RD should be made after comprehensive analysis that considers clinical symptoms, as well as histopathologic, haematological, and imaging findings. The histopathological assessment of biopsy specimens from the involved tissues remains the cornerstone in both the definite diagnosis of IgG4-RD and the exclusion of malignancies. In summary, appropriate considerations and cautious interpretations of these findings combined with other parameters (especially pathological examination) are highly recommended, and additional studies that evaluate the accuracy of IgG4 in a wider spectrum of IgG-RD are needed.
Additional Information
How to cite this article: Xu, W.- et al. Diagnostic performance of serum IgG4 level for IgG4-related disease: a meta-analysis. Sci. Rep. 6, 32035; doi: 10.1038/srep32035 (2016).
Footnotes
Author Contributions W.-l.X., Y.-c.L. and Z.-k.W. collected and analysed the data, as well as drafted the manuscript. F.D. provided analytical oversight and revised the manuscript. All authors have read and approved the final version to be published.
References
- Brito-Zerón P., Ramos-Casals M., Bosch X. & Stone J. H. The clinical spectrum of IgG4-related disease. Autoimmun Rev 13, 1203–1210, doi: http://dx.doi.org/10.1016/j.autrev.2014.08.013 (2014). [DOI] [PubMed] [Google Scholar]
- Carruthers M. N., Khosroshahi A., Augustin T., Deshpande V. & Stone J. H. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 74, 14–18, doi: 10.1136/annrheumdis-2013-204907 (2015). [DOI] [PubMed] [Google Scholar]
- Stone J. H., Zen Y. & Deshpande V. IgG4-Related Disease. New England Journal of Medicine 366, 539–551, doi: 10.1056/NEJMra1104650 (2012). [DOI] [PubMed] [Google Scholar]
- Hamano H. et al. High Serum IgG4 Concentrations in Patients with Sclerosing Pancreatitis. New England Journal of Medicine 344, 732–738, doi: 10.1056/NEJM200103083441005 (2001). [DOI] [PubMed] [Google Scholar]
- Kakudo K. et al. IgG4-related disease of the thyroid glands. Endocr J 59, 273–281 (2012). [DOI] [PubMed] [Google Scholar]
- Umehara H. et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 22, 21–30, doi: 10.1007/s10165-011-0571-z (2012). [DOI] [PubMed] [Google Scholar]
- Masaki Y., Sugai S. & Umehara H. IgG4-related diseases including Mikulicz’s disease and sclerosing pancreatitis: diagnostic insights. J Rheumatol 37, 1380–1385, doi: 10.3899/jrheum.091153 (2010). [DOI] [PubMed] [Google Scholar]
- Otsuki M. et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol 43, 403–408, doi: 10.1007/s00535-008-2205-6 (2008). [DOI] [PubMed] [Google Scholar]
- Szanto A. et al. Description of patients with IgG4-related disease from a Hungarian centre. Scand J Rheumatol 43, 334–337, doi: 10.3109/03009742.2013.862567 (2014). [DOI] [PubMed] [Google Scholar]
- Yamamoto M. et al. Value of serum IgG4 in the diagnosis of IgG4-related disease and in differentiation from rheumatic diseases and other diseases. Mod Rheumatol 22, 419–425, doi: 10.1007/s10165-011-0532-6 (2012). [DOI] [PubMed] [Google Scholar]
- Tabata T. et al. Serial changes of elevated serum IgG4 levels in IgG4-related systemic disease. Intern Med 50, 69–75 (2011). [DOI] [PubMed] [Google Scholar]
- Khosroshahi A., Bloch D. B., Deshpande V. & Stone J. H. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum 62, 1755–1762, doi: 10.1002/art.27435 (2010). [DOI] [PubMed] [Google Scholar]
- Ngwa T. N., Law R., Murray D. & Chari S. T. Serum immunoglobulin G4 level is a poor predictor of immunoglobulin G4-related disease. Pancreas 43, 704–707, doi: 10.1097/mpa.0000000000000118 (2014). [DOI] [PubMed] [Google Scholar]
- Yun J., Wienholt L. & Adelstein S. Poor positive predictive value of serum immunoglobulin G4 concentrations in the diagnosis of immunoglobulin G4-related sclerosing disease. Asia Pacific Allergy 4, 172–176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparisi L. et al. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut 54, 703–709, doi: 10.1136/gut.2004.047142 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji R. et al. Serum immunoglobulin G4 associated with number and distribution of extrapancreatic lesions in type 1 autoimmune pancreatitis patients. Journal of Gastroenterology and Hepatology 27, 268–272, doi: 10.1111/j.1440-1746.2011.06933.x (2012). [DOI] [PubMed] [Google Scholar]
- Choi E. K. et al. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas 35, 156–161, doi: 10.1097/MPA.0b013e318053eacc (2007). [DOI] [PubMed] [Google Scholar]
- Ghazale A. et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 102, 1646–1653, doi: 10.1111/j.1572-0241.2007.01264.x (2007). [DOI] [PubMed] [Google Scholar]
- Hirano K. et al. Serum IgG4 concentrations in pancreatic and biliary diseases. Clin Chim Acta 367, 181–184, doi: 10.1016/j.cca.2005.11.031 (2006). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. & The P. G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine 6, e1000097, doi: 10.1371/journal.pmed.1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P. F. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155, 529–536, doi: 10.7326/0003-4819-155-8-201110180-00009 (2011). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. Bmj 327, 557–560, doi: 10.1136/bmj.327.7414.557 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavragani C. P. et al. Elevated IgG4 serum levels among primary Sjögren’s syndrome patients: Do they unmask underlying IgG4-related disease? Clinical and Experimental Rheumatology 32, S48 (2014). [DOI] [PubMed] [Google Scholar]
- Tanaka A. et al. Clinical profiles of patients with primary sclerosing cholangitis in the elderly. Journal of Hepato-Biliary-Pancreatic Sciences 22, 230–236 (2015). [DOI] [PubMed] [Google Scholar]
- Wu W. C. et al. Clinical strategies for differentiating autoimmune pancreatitis from pancreatic malignancy to avoid unnecessary surgical resection. J Dig Dis 14, 500–508, doi: 10.1111/1751-2980.12075 (2013). [DOI] [PubMed] [Google Scholar]
- Nakazawa T. et al. Diagnostic criteria for IgG4-related sclerosing cholangitis based on cholangiographic classification. J Gastroenterol 47, 79–87, doi: 10.1007/s00535-011-0465-z (2012). [DOI] [PubMed] [Google Scholar]
- Masaki Y. et al. Cutoff values of serum IgG4 and histopathological IgG4+ plasma cells for diagnosis of patients with IgG4-related disease. International Journal of Rheumatology 2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisawa T. et al. Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas 37, e62–e67, doi: 10.1097/MPA.0b013e318175e3a0 (2008). [DOI] [PubMed] [Google Scholar]
- Ohara H. et al. Establishment of a serum IgG4 cut-off value for the differential diagnosis of IgG4-related sclerosing cholangitis: a Japanese cohort. J Gastroenterol Hepatol 28, 1247–1251, doi: 10.1111/jgh.12248 (2013). [DOI] [PubMed] [Google Scholar]
- Oseini A. M. et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology 54, 940–948, doi: 10.1002/hep.24487 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli-Labate A. M. & Pezzilli R. Usefulness of serum IgG4 in the diagnosis and follow up of autoimmune pancreatitis: A systematic literature review and meta-analysis. J Gastroenterol Hepatol 24, 15–36, doi: 10.1111/j.1440-1746.2008.05676.x (2009). [DOI] [PubMed] [Google Scholar]
- Deng C. et al. Histopathological Diagnostic Value of the IgG4+/IgG+ Ratio of Plasmacytic Infiltration for IgG4-Related Diseases: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 94, e579, doi: 10.1097/md.0000000000000579 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano H. et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344, 732–738, doi: 10.1056/nejm200103083441005 (2001). [DOI] [PubMed] [Google Scholar]
- Sánchez-Castañón M., Gómez C., de las Heras-Castaño G. & López-Hoyos M. Differentiation of autoimmune pancreatitis from pancreas cancer: Utility of anti-amylase and anti-carbonic anhydrase II autoantibodies. Autoimmunity Highlights 3, 11–17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra K. et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology 59, 1954–1963, doi: 10.1002/hep.26977 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heerde M. J. et al. Serum level of ca 19-9 increases ability of IgG4 test to distinguish patients with autoimmune pancreatitis from those with pancreatic carcinoma. Digestive Diseases and Sciences 59, 1322–1329 (2014). [DOI] [PubMed] [Google Scholar]
- Su Y. et al. Detection of serum IgG4 levels in patients with igG4-related disease and other disorders. PLoS One 10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T. et al. Distinct clinicopathological entity ‘autoimmune pancreatitis-associated sclerosing cholangitis’. Pathol Int 55, 405–411, doi: 10.1111/j.1440-1827.2005.01845.x (2005). [DOI] [PubMed] [Google Scholar]