Abstract
Importance
Treatment of hepatitis C virus (HCV) infection in HIV-1 co-infected patients has been limited due to the use of interferon and drug interactions with antiretroviral therapies (ART).
Objective
To determine the rates of HCV eradication (sustained virologic response, SVR) and adverse events in HCV/HIV-1 co-infected patients receiving sofosbuvir and ribavirin treatment.
Design
Multicenter, open-label, non-randomized, uncontrolled phase 3 trial (PHOTON-1) conducted in the United States and Puerto Rico from August 2012 until November 2013 evaluating treatment with sofosbuvir and ribavirin.
Setting
Thirty-four academic, private practice, and community health centers with a median of 6 patients (range 1–17) enrolled at each site.
Participants
Patients with HCV genotypes 1, 2, or 3 and concurrent HIV-1 were eligible. Patients were required to be receiving antiretroviral treatment with an HIV-1 RNA <50 copies/mL and a CD4 T-cell count of >200 cells/mm3 or have untreated HIV-1infection with a CD4 T-cell count of >500 cells/mm3. In total, 223 participants (114 treatment-naïve participants with HCV genotype 1, 68 treatment-naïve participants with HCV genotype 2 or 3, and 41 peginterferon/ribavirin treatment-experienced participants with HCV genotype 2 or 3) were enrolled.
Interventions
Participants received sofosbuvir (400 mg) and weight-based ribavirin for 12 weeks (for treatment-naïve patients with HCV genotype 2 or 3) or for 24 weeks (for treatment-naïve patients with HCV genotype 1 or treatment-experienced patients with HCV genotype 2 or 3).
Main Outcome and Measure
The primary study outcome was proportion of patients with SVR (serum HCV <25 copies/mL) 12 weeks after cessation of HCV therapy (SVR12).
Results
Among treatment-naïve participants, SVR12 rates were 76% (95% confidence interval [CI], 67–84) for genotype 1 infection, 88% (95% CI, 70–98) for genotype 2 infection, and 67% (95% CI, 51–80) for genotype 3 infection. Among treatment-experienced participants, SVR12 was 92% (95% CI, 73–99) for genotype 2 infection and 94% (95% CI, 71–100) for genotype 3 infection. The most common adverse events were fatigue, insomnia, headache, and nausea. Seven patients (3%) discontinued HCV treatment due to adverse events. No adverse effect on HIV disease or its treatment was observed.
Conclusions and Relevance
In this open-label, nonrandomized, uncontrolled phase 3 study, HIV-1-infected patients coinfected with HCV genotype 1, 2, or 3 who received the oral, interferon-free combination of sofosbuvir and ribavirin for 12 or 24 weeks had high rates of sustained virologic response 12 weeks after cessation of therapy. Further studies of this oral regimen in diverse populations of coinfected patients are warranted.
Trial Registration
clinicaltrials.gov Identifier: NCT01667731
INTRODUCTION
Up to 7 million persons worldwide are infected with both human immunodeficiency virus (HIV-1) and hepatitis C virus (HCV).1 Coincident HIV-1 infection is associated with increased rates of fibrosis, cirrhosis, hepatocellular carcinoma (HCC), and overall mortality in HCV-infected patients.2 Coinfected patients who are cured of HCV have improved clinical outcomes and survival.3
In treatment-naïve patients coinfected with HCV and HIV-1, treatment for HCV genotypes 1, 2 or 3 infection has required 24 to 48 weeks of interferon/ribavirin with or without an HCV NS3/4A protease inhibitor, telaprevir or boceprevir, resulting in sustained virologic response (SVR) rates of 62–74%.4,5 However, the use of these regimens is limited due to complex dosing of the HCV NS3/4A protease inhibitors, poor tolerability, and drug interactions between HCV and antiretroviral drugs; moreover, up to 70% of HIV-1/HCV co-infected patients are not eligible for HCV treatment regimens that include interferon.6,7 Sofosbuvir is an oral nucleotide analog HCV NS5B polymerase inhibitor recently approved for the treatment of HCV genotypes 1–4.8,9 Sofosbuvir has minimal or no drug interactions with a wide range of antiretroviral (ARV) drugs.10 Phase 3 trials of sofosbuvir in HCV-monoinfected patients have demonstrated high rates of SVR when used in combination with ribavirin for 12 weeks for HCV genotype 2, or 12 to 24 weeks for HCV genotype 3, and in combination with peginterferon and ribavirin for 12 weeks for HCV genotypes 1, 4, 5, and 6.11–13 The combination of sofosbuvir plus ribavirin for 24 weeks has also shown promise in patients with HCV genotype 1.14
We evaluated the rates of SVR and adverse events in HIV-1 infected patients coinfected with HCV genotype 1, 2, or 3 treated with the oral regimen of sofosbuvir and ribavirin for 12 or 24 weeks.
METHODS
Patients
We enrolled patients chronically infected with HCV at 34 academic, private practice, and community health centers in the United States and Puerto Rico from August 2012 through March 2013. Written consent was obtained from all patients prior to screening. Eligible patients were aged ≥18 years with infection with HCV genotype 1, 2, or 3 and HIV-1, and a body mass index (BMI) ≥18 kg/m2. Patients were HCV treatment-naïve (genotype 1, 2, and 3) or HCV treatment-experienced (genotype 2 and 3 only), and had documentation of the presence or absence of cirrhosis. Persons who inject drugs (PWIDs) were not excluded by medical history, however, individuals actively using drugs were excluded if they had a positive urine toxicology at the screening visit. Patients on antiretroviral treatment were required to have been on a stable regimen for at least 8 weeks prior to screening, have an HIV-1 RNA <50 copies/mL, and a CD4 T-lymphocyte count >200 cells/mm3. Antiretroviral regimens (ART) containing emtricitabine/tenofovir in combination with either atazanavir/ritonavir, darunavir/ritonavir, efavirenz, raltegravir, or rilpivirine were permitted based on drug interaction studies with sofosbuvir. Patients not on ART were required to have a CD4 T-lymphocyte count >500 cells/mm3 at screening. No more than 20% of the entire study population were permitted to have evidence of cirrhosis at screening as assessed by a liver biopsy within 2 years of screening or by FibroTest®.
Study Design
In this multicenter, open-label, non-randomized, uncontrolled phase 3 trial,15 all patients received 400 mg of sofosbuvir (Gilead Sciences) administered orally once daily along with ribavirin (Ribasphere, Kadmon) administered orally twice daily, with doses determined according to body weight (1000 mg daily in patients with a body weight of <75 kg, and 1200 mg daily in patients with a body weight of ≥75 kg). The dose of ribavirin could be decreased or discontinued to manage hemoglobin reductions according to the product label. The treatment duration was 12 weeks for treatment-naive patients with HCV genotype 2 or 3, and 24 weeks for treatment-naive patients with HCV genotype 1 and treatment-experienced patients with HCV genotype 2 or 3.
Study Assessments
Serum HCV RNA was measured at screening and every subsequent visit with the COBAS® TaqMan® HCV Test, version 2.0 for Use with the High Pure System (Roche Molecular Systems, West Sussex, United Kingdom) with a lower limit of quantification (LLOQ) of 25 IU/mL. HCV viral genotyping was determined using the Siemens Versant HCV Genotype 2.0 Assay. HIV RNA was measured at screening and every subsequent visit using the AmpliPrep/COBAS® TaqMan® HIV-1 Test, version 2.0.
HCV viral relapse was defined as HCV RNA >LLOQ at post-treatment Week 4 or 12. HCV viral breakthrough was defined as HCV RNA ≥LLOQ during treatment after having previously had HCV RNA <LLOQ while on treatment, confirmed with 2 consecutive values.
Other on-treatment assessments were performed at 1–4 week intervals and included physical exam, review of medications and safety assessments, including evaluations of renal function, hemoglobin levels, and liver function. Post-treatment assessments included safety assessments during post-treatment Week 4.
Plasma samples for HCV viral sequencing and possible phenotypic monitoring were collected at baseline (pre-treatment) and at every visit thereafter. We evaluated HCV NS5B gene nucleotide changes that may confer resistance to sofosbuvir on baseline and at the time of virologic relapse or breakthrough.
Statistical Analysis
The primary efficacy endpoint was sustained virologic response (SVR), defined as HCV RNA <LLOQ 12 weeks after discontinuation of study drugs in all patients who were enrolled and received study drugs.
We planned to enroll approximately 115 HCV treatment-naïve patients with HCV genotype 1, 55 HCV treatment-naïve patients HCV genotype 2/3, and 55 patients with HCV genotype 2/3 who had been previously treated for HCV. No inferential statistics or statistical comparison were planned for efficacy endpoints. Point estimates and 95% confidence interval were provided for the primary efficacy endpoint of SVR12.
The point estimates for the SVR12 rate along with their 2-sided 95% exact confidence intervals (based on the Clopper-Pearson method) were provided for each treatment group. Exploratory multivariable logistic-regression analyses characterizing the relationship between SVR12 and various pre-specified demographic and baseline clinical characteristics were performed for each genotype group. A stepwise selection procedure was used to identify independent predictors of SVR12.
Study Oversight
This study was approved by the institutional review board or independent ethics committees at all participating sites and was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements. The study was designed and conducted according to protocol by the sponsor (Gilead) in collaboration with the principal investigators. The sponsor collected the data, monitored study conduct, and performed the statistical analyses. An independent data monitoring committee reviewed the progress of the study.
RESULTS
Baseline Characteristics
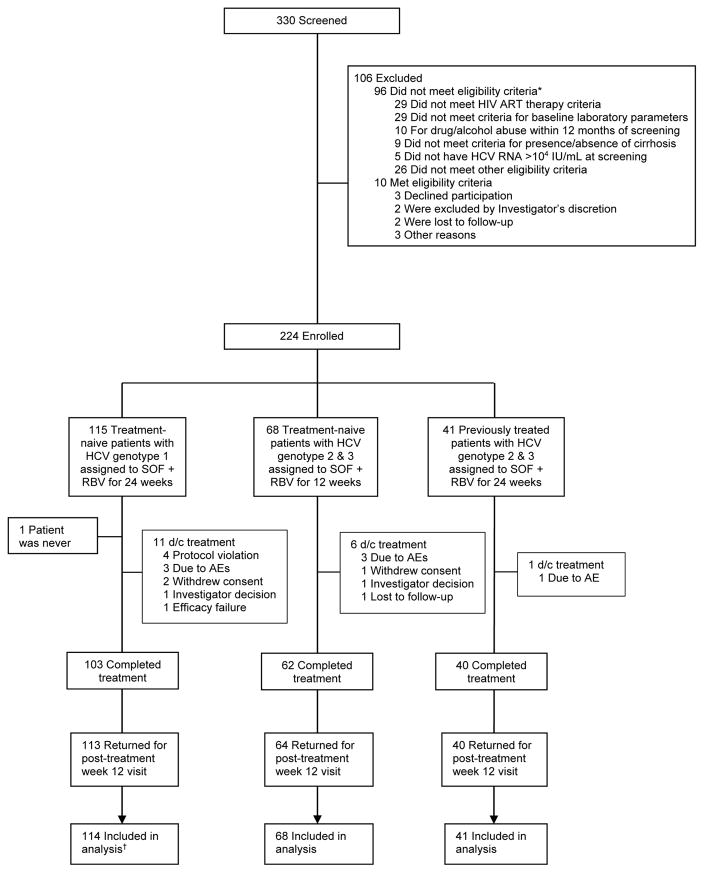
Overall, 330 patients coinfected with HIV-1 and HCV were screened for the study, of which 224 were eligible for the study and 223 began treatment (Figure 1 and eTable 10 in the supplement). The median number of patients at the 34 sites was six, with a range of one to 17. The demographic and baseline clinical characteristics of the patients are shown in Table 1. Cirrhosis was more common in treatment-experienced patients and the mean CD4 cell count was 585 to 658 cells/μL. In each treatment group, 90% to 98% of patients were taking antiretroviral therapy. Of 11 patients not taking antiretroviral treatment, five had HIV-1 RNA <50 copies/mL at baseline. Among treatment-naïve patients with genotype 1, the majority had subtype 1a and 32% were black.
Figure 1. Study Flow Diagram.
*Patients could be excluded for more than one criterion. See eTable 12 in the supplement for further details.
†Patient who did not receive study treatment was not included in the efficacy analysis per protocol.
Table 1.
Baseline Demographic Characteristics
| Characteristic | Genotype 1 Treatment Naïve (n = 114) | Genotype 2/3 Treatment Naïve (n = 68) | Genotype 2/3 Treatment Experienced (n = 41) |
|---|---|---|---|
| Mean age, years (range) | 48 (25–70) | 49 (24–71) | 54 (34–68) |
| Mean BMI, kg/m2 (range) | 27.3 (18.5–46.2) | 27.4 (19.8–43.5) | 27.3 (18.8–39.7) |
| Male, n (%) | 93 (81.6) | 55 (80.9) | 37 (90.2) |
| Race, n (%)† | |||
| White | 70 (61.4) | 54 (79.4) | 32 (78.0) |
| Black | 37 (32.5) | 8 (11.8) | 7 (17.1) |
| Other | 7 (6.1) | 6 (8.8) | 2 (4.9) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 25 (21.9) | 19 (27.9) | 10 (24.4) |
| Not Hispanic or Latino | 89 (78.1) | 49 (72.1) | 31 (75.6) |
| HCV genotype, n (%) | |||
| 1a | 90 (78.9) | NA | NA |
| 1b | 24 (21.1) | NA | NA |
| 2 | NA | 26 (38.2) | 24 (58.5) |
| 3 | NA | 42 (61.8) | 17 (41.5) |
| Mean HCV RNA, log10 IU/mL (SD) | 6.6 (0.83) | 6.3 (0.60) | 6.5 (0.69) |
| HCV RNA ≥6 log10 IU/mL, n (%) | 92 (80.7) | 47 (69.1) | 34 (82.9) |
| IL28B genotype, n (%)* | |||
| CC | 30 (26.5) | 25 (36.8) | 20 (48.8) |
| CT | 57 (50.4) | 37 (54.4) | 17 (41.5) |
| TT | 26 (23.0) | 6 (8.8) | 4 (9.8) |
| Cirrhosis, n (%)‡ | 5 (4.4) | 7 (10.3) | 10 (24.4) |
| Interferon ineligible, n (%)§ | 29 (25.4) | 19 (27.9) | NA |
| Due to psychiatric illness | 25 (86.2) | 17 (89.5) | NA |
| Autoimmune disorder | 3 (10.3) | 2 (10.5) | NA |
| Seizure disorder | 1 (3.4) | 2 (10.5) | NA |
| Poorly controlled diabetes | 1 (3.4) | 0 | NA |
| Other | 1 (3.4) | 1 (5.3) | NA |
| Baseline ALT >1.5 × ULN | 50 (43.9) | 45 (66.2) | 22 (53.7) |
| Mean eGFR, mL/min (SD) | 106.2 (27.7) | 110.4 (27.7) | 104.9 (29.7) |
| Median CD4 T-cell count (cells/μL), (Q1, Q3) | 581 (455, 781) | 562 (395, 723) | 579 (482, 744) |
| On ART, n (%) | 112 (98.2) | 61 (89.7) | 39 (95.1) |
| Tenofovir DF/Emtricitabine PLUS | |||
| Efavirenz | 42 (37.5) | 20 (32.8) | 16 (41.0) |
| Atazanavir/ritonavir | 24 (21.4) | 7 (11.5) | 8 (20.5) |
| Darunavir/ritonavir | 15 (13.4) | 17 (27.9) | 2 (5.1) |
| Raltegravir | 21 (18.8) | 8 (13.1) | 7 (17.9) |
| Rilpilvirine | 7 (6.3) | 5 (8.2) | 2 (5.1) |
| Other | 3 (2.7) | 4 (6.6) | 4 (10.3) |
| Ribavirin dose assignment according to body weight, n (%) | |||
| 1000 mg/day | 40 (35) | 18 (26) | 15 (37) |
| 1200 mg/day | 74 (65) | 50 (74) | 26 (63) |
Missing information on 1 patient out of the 114 patients with GT 1.
Race was self-reported.
Of the 22 patients with cirrhosis, 12 were determined by biopsy and 10 by FibroTest.
Some patients were ineligible to receive interferon for more than one reason.
On-treatment and sustained virologic response
Sofosbuvir and ribavirin treatment resulted in rapid decline in levels of serum HCV. By treatment week 2, 75% of patients with HCV genotype 1, 91% of treatment-naïve patients with HCV genotype 2 or 3, and 98% of treatment-experienced patients with HCV genotype 2 or 3 had HCV RNA <LLOQ; by the fourth week of treatment, those proportions were 97%, 99%, and 100%, respectively (Table 2). Two patients (one each with genotype 1 and 2) experienced HCV virologic breakthrough; both had undetectable serum levels of sofosbuvir and its metabolite, GS-331007, at the time of HCV breakthrough, suggesting non-adherence to sofosbuvir.
Table 2.
HCV Virologic Response during and after Treatment
| Response | Genotype 1 Treatment Naïve (n = 114) | Genotype 2/3 Treatment Naïve (n = 68) | Genotype 2/3 Treatment Experienced (n = 41) |
|---|---|---|---|
| HCV RNA <LLOQ during treatment period, n/n (%)a | |||
| At week 2 | 85/114 (74.6%) | 62/68 (91.2%) | 40/41 (97.6%) |
| 95% CI | 65.6% to 82.3% | 81.8% to 96.7% | 87.1% to 99.9% |
| At week 4 | 110/114 (96.5%) | 66/67 (98.5%) | 41/41 (100%) |
| 95% CI | 91.3% to 99% | 92% to 100% | 91.4% to 100% |
| At week 12 | 111/111 (100%) | 61/63 (96.8%)b | 40/40 (100%) |
| 95% CI | 96.7% to 100% | 89.0% to 99.6% | 91.2% to 100% |
| At week 24 | 103/103 (100%) | -- | 40/40 (100%) |
| 95% CI | 96.5% to 100% | -- | 91.2% to 100% |
| HCV RNA <LLOQ after end of treatment, n (%) | |||
| At week 4 (SVR4) | 92/114 (80.7%) | 53/68 (77.9%) | 39/41 (95.1%) |
| 95% CI | 72.3% to 87.5% | 66.2% to 87.1% | 83.5% to 99.4% |
| At week 12 (SVR12) | 87/114 (76.3%) | 51/68 (75.0%)c | 38/41 (92.7%)d |
| 95% CI | 67.4% to 83.8% | 63% to 84.7% | 80.1% to 98.5% |
| On-treatment virologic breakthrough | 1/114 (0.9%) | 1/68 (1.5%) | 0 |
| Relapse in patients with HCV RNA <LLOQ at EOT, n/n (%) | |||
| Patients who completed treatment | 19/103 (18.4%) | 11/61 (18.0%) | 1/40 (2.5%) |
| Patients who did not complete treatment | 6/10 (60.0%) | 1/6 (16.7%) | 1/1 (100%) |
HCV RNA <LLOQ response during treatment is in patients for whom HCV RNA results are available. LLOQ denotes lower limit of quantification, which is 25 IU/mL. SVR denotes sustained virologic response.
One patient with HCV RNA >LLOQ had virologic breakthrough during treatment and the other completed treatment but did not have a week 12 assessment.
SVR12 rate was 88% (95% CI, 70 to 98) for treatment-naïve patients with HCV genotype 2, and 67% (95% CI, 51 to 80) for treatment-naïve patients with HCV genotype 3 infection.
SVR12 rate was 92% (95% CI, 73 to 99) for treatment-experienced patients with HCV genotype 2, and 94% (95% CI, 71 to 100) for treatment-experienced patients with HCV genotype 3.
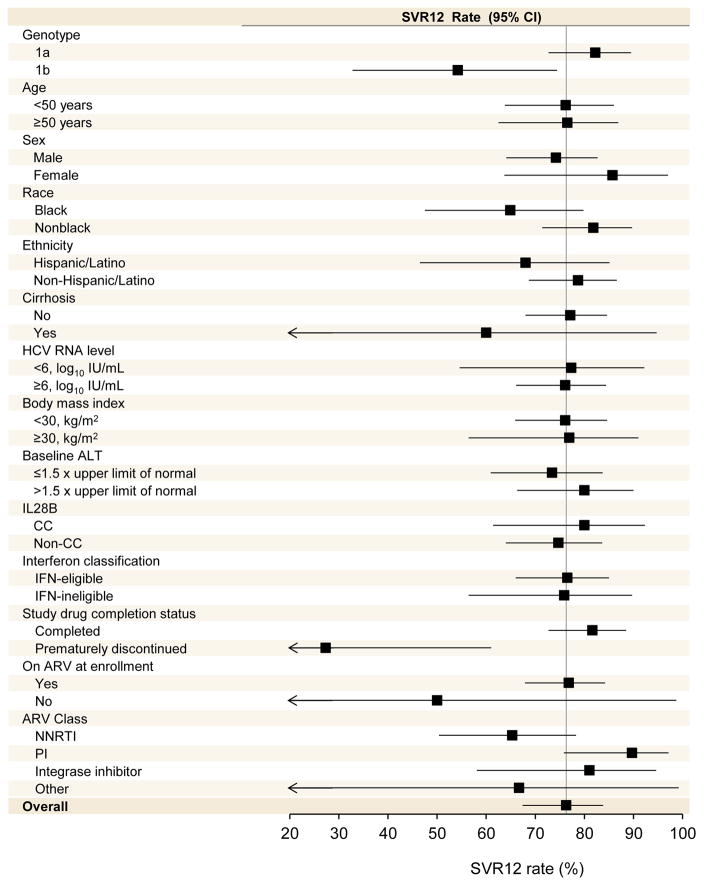
Of the 114 treatment-naïve patients with HCV genotype 1, 87 (76%; 95% CI, 67%-84%) achieved SVR12 (Table 2). Of the 27 patients who did not achieve SVR12, 25 had virologic relapse after stopping study medications, one had on-treatment breakthrough as described above, and one was not assessable for SVR12 (patient withdrew consent prior to treatment week 20). Rates of SVR12 for treatment-naïve patients with HCV genotype 1 by patient subgroups, including sex, age, HCV subtype, presence of cirrhosis, and type of coadministered antiretrovirals, are shown in Figure 2. For subgroup rates in patients with HCV genotype 2, see eTable 3 in the Supplement, and in patients with HCV genotype 3, see eTable 5 in the Supplement. Notably, SVR12 rates were 82% (95% CI, 73%–89%) among the 103 HCV genotype 1 patients who completed study treatment and 27% (95% CI, 6%–61%) among the 11 patients who discontinued study treatment early. Among genotype 1 infected patients, exploratory multivariable analysis indicated that non-black race (OR 2.87; 95% CI, 1.01–8.20; p=0.049), HCV genotype 1a (OR: 3.42; 95% CI, 1.15–10.16; p=0.027) and completing 24 weeks of study treatment (OR 17.54; 95% CI, 3.77–83.33; p=0.0003) were independently associated with achieving SVR12 (eTable 2 in the Supplement).
Figure 2. Rates of SVR12 by subgroup in treatment-naïve patients with HCV genotype 1 receiving 24 weeks of sofosbuvir and ribavirin.
The position of the solid squares indicates the rate of virologic response 12 weeks after the end of treatment for each subgroup; the horizontal lines indicate 95% confidence intervals. The vertical lines represent the overall rates of sustained virologic response for the treatment groups. Arrows indicate confidence intervals that exceed the x-axis scale.
Of the 26 treatment-naïve patients with HCV genotype 2 receiving 12 weeks of treatment, 23 (88%; 95% CI, 70%–98%) achieved an SVR12. Of the 3 patients who did not achieve SVR12, one patient had on-treatment virologic breakthrough (non-adherence) and 2 patients could not be assessed for SVR12 (one patient withdrew consent and another was lost to follow-up). Of the 42 treatment-naive patients with HCV genotype 3 receiving 12 weeks of treatment, 28 (67%; 95% CI, 51%–80%) achieved an SVR12. Among the 14 patients who did not achieve SVR12, twelve experienced virologic relapse and two could not be assessed for SVR12 (one patient was lost to follow-up and another died after completing treatment).
Of the 24 treatment-experienced patients with HCV genotype 2 receiving 24 weeks of treatment, 22 (92%; 95% CI, 73%–99%) had an SVR12. One patient from this group could not be assessed for SVR12 (withdrew consent) and another had virologic relapse. Notably, the latter patient only received 8 weeks of study treatment and was found to have discordant NS5B sequencing from screening HCV genotyping (genotype 1 by sequencing and genotype 2 by the screening INNO-LiPA), which may represent a chimeric virus. Two other patients with discordant NS5B sequencing and INNO-LiPA analysis in this arm completed 24 weeks of treatment and achieved SVR12. Of the 17 treatment-experienced patients with HCV genotype 3, 16 (94%; 95% CI, 71%–100%) achieved an SVR12, with one patient having viral relapse at SVR12 assessment. Among patients with HCV genotype 2 (treatment naïve and experienced), an exploratory multivariable analysis demonstrated only study drug completion to be associated with SVR (OR: 200; 95% CI 4 to >1000; p=0.0022: eTable 4 in the Supplement). Among patients with HCV genotype 3 (treatment naïve and experienced), no baseline factors were predictive of SVR12 by exploratory multivariable analysis, though treatment-experienced patients assigned to a 24-week treatment arm had numerically higher SVR12 rates (OR: 8; CI 0.96, 66.6; p=0.055: eTables 5 and 6 in the Supplement).
Viral resistance testing
Baseline population sequencing was performed in 219 patients. No S282T was detected in any patient at baseline. L159F was detected in two patients with one patient achieving SVR12 and the other experiencing viral relapse. No baseline V321A mutations were identified. NS5B deep sequencing (1% detection threshold) was performed for 30 patients at baseline and at the time of virologic breakthrough (n=2), or relapse (n=28).
In the 2 patients with HCV virologic breakthrough, deep sequencing did not demonstrate S282T, L159F, or V321A NS5B mutations. Deep sequencing of the 28 patients with viral relapse with a 1% threshold of detection demonstrated no evidence of the S282T or V321A mutations. The L159F mutation emerged in 4 patients (n=2 genotype 3a at 1.1% and 1.2%, n=1 genotype 1a patient at >99%, and n=1 genotype 1b patient at >99%), but did not confer phenotypic shift to sofosbuvir resistance in vitro.
Safety
Of the 223 patients who received at least 1 dose of study drug, 7 (3%) discontinued treatment due to an adverse event (Table 3 and eTable 7 in the Supplement). Serious adverse events were experienced by 14 (6%) patients (eTable 8 in the Supplement). The incidence of serious adverse events and adverse events leading to discontinuation was similar in the 12- and 24-week groups. One death occurred; a patient with HCV genotype 3 committed suicide 9 days after completing 12 weeks of treatment per protocol.
Table 3.
Discontinuations, Adverse Events, and Hematologic Abnormalities
| Parameter | Genotype 1 Treatment Naïve (n = 114) | Genotype 2/3 Treatment Naïve (n = 68) | Genotype 2/3 Treatment Experienced (n = 41) |
|---|---|---|---|
| Mean duration of treatment, wks (SD) | 23.0 (4.04) | 11.7 (1.52) | 23.8 (2.44) |
| Discontinuation of treatment due to AE | 3 (2.6%) | 3 (4.4%) | 1 (2.4%) |
| Deaths | 0 | 1 (1.5%) | 0 |
| Serious adverse events* | 8 (7.0%) | 5 (7.4%) | 1 (2.4%) |
| Common AEs† | |||
| Fatigue | 41 (36.0%) | 24 (35.3%) | 19 (46.3%) |
| Insomnia | 15 (13.2%) | 14 (20.6%) | 8 (19.5%) |
| Nausea | 18 (15.8%) | 12 (17.6%) | 6 (14.6%) |
| Headache | 16 (14.0%) | 9 (13.2%) | 5 (12.2%) |
| Irritability | 14 (12.3%) | 7 (10.3%) | 2 (4.9%) |
| Cough | 14 (12.3%) | 4 (5.9%) | 4 (9.8%) |
| Upper Respiratory Tract Infection | 13 (11.4%) | 8 (11.8%) | 5 (12.2%) |
| Diarrhea | 12 (10.5%) | 6 (8.8%) | 5 (12.2%) |
| Dizziness | 7 (6.1%) | 1 (1.5%) | 5 (12.2%) |
| Anemia | 13 (11.4%) | 6 (8.8%) | 3 (7.3%) |
| Laboratory events, n (%) | |||
| Decreased Hb concentration | |||
| <10 g/dL | 22 (19.3%) | 7 (10.3%) | 5 (12.2%) |
| <8.5 g/dL | 2 (1.8%) | 1 (1.5%) | 0 |
| Total Bilirubin | |||
| 3 to <6 mg/dL | 13 (11.4%) | 3 (4.4%) | 4 (9.8%) |
| Taking atazanavir | 12 (92.3%) | 3 (100%) | 4 (100%) |
| Not taking atazanavir | 1 (7.7%) | 0 | 0 |
| >6 mg/dL | 9 (7.9%) | 1 (1.5%) | 2 (4.9%) |
| Taking atazanavir | 8 (88.9%) | 1 (100%) | 2 (100%) |
| Not taking atazanavir | 1 (11.1%) | 0 | 0 |
None of the serious adverse events was deemed related to study drugs.
Adverse events occurring in at least 10% of patients in any arm.
Most adverse events were grade 1 or 2 in severity with the most common adverse events in all treatment groups being fatigue, insomnia, nausea, and headache. The most common laboratory abnormalities included anemia and elevations in indirect bilirubin. Thirty-four (15%) had declines in hemoglobin to below 10 mg/dL with 3 patients experiencing declines in hemoglobin to below 8.5 mg/dL. Forty-three patients (19% of total) required dose reduction of ribavirin; the use of erythropoietin was prohibited in this study (Table 3). Overall, 32 (14%) patients experienced elevations of total bilirubin to >3.0 mg/dL. Consistent with ribavirin associated hemolysis, the maximum increase was observed during the first 2 weeks of sofosbuvir plus ribavirin therapy and no patient had a grade 3 or 4 increase in direct bilirubin. The majority (30 of 32, 94%) of these patients were taking ritonavir-boosted atazanavir as part of the baseline ART regimen (Table 3). Four patients changed ART regimens from atazanavir to darunavir due to increased indirect bilirubin during the study with all patients having normalization of total bilirubin levels following the change in ART (eTable 9 in the Supplement). All patients with elevations in total bilirubin had a return to baseline levels by the post-treatment week 12 evaluation.
There were no clinically significant changes in HIV-1 RNA levels in those patients not receiving ART at baseline (eTable 11). Of patients taking ART with suppressed HIV viremia upon entering the study, 2 experienced HIV viral breakthrough. One of these patients had increases in HIV RNA due to non-adherence and the other re-suppressed the HIV RNA without changes to the ART regimen.
Consistent with the known lymphopenic effect of ribavirin, there was a decrease in absolute lymphocyte counts and absolute CD4 T-cell counts during study treatment.16 The CD4 T-cell percentage did not change throughout study treatment (eTable 10 in the Supplement). Absolute CD4 T-cell counts returned to baseline by post-treatment week 12 assessments (eTable 10 in the Supplement).
DISCUSSION
In this open-label, non-randomized, uncontrolled phase 3 study, HIV-1-infected patients coinfected with HCV genotype 1, 2, or 3 who received the oral, interferon-free combination of sofosbuvir and ribavirin for 12 or 24 weeks had high rates of sustained virologic response 12 weeks after cessation of therapy.
For patients coinfected with HCV genotype 1, the sustained virologic response rate of 76% following 24 weeks of sofosbuvir plus ribavirin was similar to results observed in coinfected patients in smaller, phase 2 studies of 48 weeks of treatment with an HCV protease inhibitor plus peginterferon and ribavirin and to results observed in a smaller study of 24 weeks of sofosbuvir plus ribavirin in HCV monoinfected patients.14 In this coinfected patient population, patients with HCV genotype 1 and characteristics that have historically been considered difficult to cure had high rates of SVR12 following receipt of the 24-week treatment regimen of sofosbuvir and ribavirin. By exploratory multivariable analysis, the strongest factor associated with SVR12 was completion of the treatment regimen. Patients infected with HCV genotype 1/subtype a had a higher SVR12 rate compared to those infected with HCV genotype 1/subtype b. This finding may be explained by the small number of patient with genotype 1b infection or by confounding factors such as black race, that were more common in persons infected with genotype 1b compared to those with 1a. Overall, the observed HCV responses in patients with HIV-1/HCV coinfection were consistent with those previously reported in patients with HCV monoinfection. Further, because sofosbuvir and its metabolites do not have significant interactions with the cytochrome P450 system, sofosbuvir plus ribavirin could be co-administered with a wide range of antiretroviral drugs including those that induce (e.g., efavirenz) or inhibit (e.g., ritonavir) or are metabolized (e.g., darunavir; atazanavir) by these enzymes. Importantly, more than one quarter of the HIV-1/HCV coinfected patients treated with sofosbuvir plus ribavirin were not eligible for current HCV treatment regimens due to contraindications to interferon.
Among treatment-naïve and previously treated patients with HCV genotype 2, high rates of SVR12 were observed with both the 12- and 24-week regimens. Among patients with HCV genotype 3, there was a substantially higher rate of SVR12 in treatment-experienced patients treated with 24 weeks of sofosbuvir and ribavirin (94%) than those treated with 12 weeks of sofosbuvir and ribavirin (67%). This observation is consistent with the results of the VALENCE study which demonstrated higher rates of SVR12 in HCV genotype 3 mono-infected patients treated for 24 weeks compared to those treated for 12 or 16 weeks in other studies.13 These data suggest that the sofosbuvir and ribavirin treatment durations for HIV-infected patients with HCV genotype 2 or 3 infection should mirror those established for HCV mono-infected patients, namely, 12 and 24 weeks for HCV genotypes 2 and 3, respectively.
No S282T mutations were detected in patients with viral relapse or breakthrough, confirming the high barrier to resistance demonstrated in other studies of sofosbuvir.11,12 In vitro analyses identified no other mutations that conferred phenotypic resistance to sofosbuvir.
Rates of premature discontinuation of study drug were low in both 12- and 24-week regimens (3–4%), rates lower than those observed in the phase 2 studies of HCV protease inhibitors (8–20%), but were somewhat higher than those observed in phase 3 studies of sofosbuvir and ribavirin in HCV monoinfected patients.4,5,11,12 The most common adverse events were mild to moderate in severity and included fatigue, insomnia, nausea, and headache; no serious adverse events related to the study drugs were observed. Laboratory abnormalities were consistent with known effects from ribavirin therapy, including decrease in hemoglobin and absolute CD4 T-cell counts. Among patients taking ritonavir-boosted atazanavir, increases in indirect bilirubin were observed, typically early in the course of therapy. This finding is consistent with the ribavirin-induced hemolysis in the setting of UGT1A1 inhibition by atazanavir which has been previously reported.5,17,18 While this increase was not associated with elevations in serum liver enzyme levels or other markers of liver injury, four patients elected to switch to another boosted HIV-1 protease inhibitor with normalization of total bilirubin. For all patients with elevations in indirect bilirubin, levels returned to baseline after ribavirin treatment was completed.
No other untoward effects of sofosbuvir and ribavirin on HIV disease and/or its treatment with antiretrovirals were detected. Among patients not taking antiretroviral therapy at entry, no HIV-1-specific antiviral effect of sofosbuvir was observed, consistent with in vitro data.8 Among those taking antiretroviral therapy, two patients experienced HIV-1 virologic rebound, but both were documented to be poorly adherent to ART.
This study has several limitations. First, patients with cirrhosis (10%) and women (17%) were underrepresented. In addition, relatively few patients with advanced HIV disease (AIDS or low CD4 cell count) were enrolled; as such, the safety, tolerability, and efficacy of sofosbuvir plus ribavirin among such patients is not known and additional studies are warranted. Further, the absence of a control group limits the ability to derive definitive conclusions regarding the safety and efficacy of this regimen. Lastly, sofosbuvir was not studied in combination with other anti-HCV therapies such as peginterferon alfa or other HCV direct-acting antivirals. The combination of sofosbuvir, ribavirin, and peginterferon has been studied in 23 HIV/HCV coinfected patients receiving concurrent antiretroviral therapy, of whom, 21 achieved SVR12.19 In addition, studies of sofosbuvir in combination with ledipasivir, an inhibitor of HCV NS5A are underway (Clinicaltrials.gov NCT02073656).
Conclusions
In this open-label, nonrandomized, uncontrolled study, HIV-1-infected patients with HCV genotypes 1, 2, or 3 co-infection who received an oral combination of sofosbuvir plus ribavirin for 12 or 24 weeks had high rates of sustained HCV virologic response 12 weeks after cessation of therapy. Further studies of this regimen in more diverse populations of coinfected patients are needed.
Supplementary Material
Acknowledgments
Funding/Support: This trial was funded by Gilead Sciences, which was responsible for the design and conduct of the study as well as data collection and interpretation, management, and analysis.
PHOTON-1 Principal Investigators
David Asmuth, M.D., University of California Davis Medical Center, Sacramento, CA
Meena Bansal, M.D., Mount Sinai School of Medicine, New York, NY
Maurizio Bonacini, M.D., Saint Luke’s Hospital, San Francisco, CA
Eric Daar, M.D., Harbor-UCLA Medical Center, Torrance, CA
Douglas Dieterich, M.D., Mount Sinai School of Medicine, New York, NY
Craig Dietz, D.O., Kansas City Care Clinic, Kansas City, MO
Richard Elion, M.D., Whitman-Walker Clinic, Washington, DC
W. Jeffrey Fessel, M.D., Kaiser Permanente, San Francisco, CA
Timothy Friel, M.D., Lehigh Valley Hospital and Health Network, Allentown, PA
Joseph Gathe, Jr., M.D., Therapeutic Concepts, Houston, TX
David Hardy, M.D., Cedars-Sinai Health Systems, Los Angeles, CA
Tarek Hassanein, M.D., SCTI Research Foundation, Coronado, CA
Trevor Hawkins, M.D., Southwest Care Center, Santa Fe, NM
Federico Hinestrosa, M.D., Orlando Immunology Center, Orlando, FL
Gregory Huhn, M.D., The Ruth M. Rothstein CORE Center, Chicago, IL
Mamta Jain, M.D., UT Southwestern Medical Center, Dallas, TX
Dushyantha Jayaweera, M.D., University of Miami School of Medicine, Miami, FL
Donald Kotler, M.D., St. Luke’s Roosevelt Hospital Center, New York, NY
Jacob Lalezari, M.D., Quest Clinical Research, San Francisco, CA
Anne Luetkemeyer, M.D., San Francisco General Hospital, San Francisco, CA
Claudia Martorell, M.D., The Research Institute, Springfield, MA
Cynthia Mayer, D.O., St. Joseph Comprehensive Research Institute, Tampa, FL
Anthony Mills, M.D., Private Practice, Los Angeles, CA
Karam Mounzer, M.D., Philadelphia FIGHT, Philadelphia, PA
Susanna Naggie, M.D., Duke University Medical Center, Durham, NC
Ronald Nahass, M.D., I.D. Care, Inc., Hillsborough, NJ
Edgar Overton, M.D., University of Alabama – Birmingham, Birmingham, AL
Maribel Rodriguez-Torres, M.D., Fundacion De Investigacion, San Juan, PR
Peter Ruane, M.D., Private Practice, Los Angeles, CA
Margaret Shuhart, M.D., Harborview Medical Center, Seattle, WA
Mark Sulkowski, M.D., Johns Hopkins University, Lutherville, MD
Diana Sylvestre, M.D., Oasis Clinic, Oakland, CA
Karen Tashima, M.D., The Miriam Hospital, Providence, RI
Pablo Tebas, M.D., University of Pennsylvania, Philadelphia, PA
David Wohl, M.D., University of North Carolina at Chapel Hill, Chapel Hill, NC
Footnotes
Author Contributions: Dr. Sulkowski had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study: Sulkowski, Symonds, McHutchison, Subramanian.
Collection, management, analysis, or interpretation of the data: Sulkowski, Naggie, Lalezari, Fessel, Mounzer, Shuhart, Luetkemeyer, Asmuth, Gaggar, Ni, Svarovskaia, Brainard, McHutchison, Rodriguez-Torres, Dieterich.
Preparation, review, or approval of the manuscript: Sulkowski, Naggie, Lalezari, Fessel, Mounzer, Shuhart, Luetkemeyer, Asmuth, Gaggar, Ni, Svarovskaia, Brainard, Subramanian, McHutchison, Rodriguez-Torres, Dieterich
Decision to submit the manuscript for publication: Sulkowski, McHutchison.
Previous Presentation
Presented in part at the 64th annual meeting of the American Association for the Study of Liver Diseases; November 1–5, 2013; Washington, DC; and at the Conference on Retroviruses and Opportunistic Infections (CROI); March 3–6, 2014; Boston, MA.
Additional Contributions: We acknowledge Maryanne Lenoci and Sherri Paxton of Gilead Sciences for providing clinical operations support and David McNeel, MA, and Kellie Chu of Gilead Sciences for providing editorial support. These persons received no compensation apart from usual salary for their contributions.
Contributor Information
Mark S. Sulkowski, Email: msulkowski@jhmi.edu.
Susanna Naggie, Email: susanna.naggie@duke.edu.
Jacob Lalezari, Email: drjay@questclinical.com.
Walford Jeffrey Fessel, Email: jeffrey.fessel@kp.org.
Karam Mounzer, Email: mounzerk@fight.org.
Margaret Shuhart, Email: margarets@medicine.washington.edu.
Anne F. Luetkemeyer, Email: aluetkemeyer@php.ucsf.edu.
David Asmuth, Email: david.asmuth@ucdmc.ucdavis.edu.
Anuj Gaggar, Email: Anuj.Gaggar@gilead.com.
Liyun Ni, Email: liyun.ni@gilead.com.
Evguenia Svarovskaia, Email: jenny.svarovskaia@gilead.com.
Diana M. Brainard, Email: Diana.brainard@gilead.com.
William T. Symonds, Email: bill.symonds@gilead.com.
G. Mani Subramanian, Email: mani.subramanian@gilead.com.
John G. McHutchison, Email: john.mchutchison@gilead.com.
Maribel Rodriguez-Torres, Email: mrodrigueztorres@fdipr.com.
Douglas Dieterich, Email: douglas.dieterich@mountsinai.org.
References
- 1.Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res. 2010;85(1):303–315. doi: 10.1016/j.antiviral.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2013;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 3.Limketkai BN, Mehta SH, Sutcliffe CG, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308(4):370–378. doi: 10.1001/jama.2012.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulkowski M, Pol S, Mallolas J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13(7):597–605. doi: 10.1016/S1473-3099(13)70149-X. [DOI] [PubMed] [Google Scholar]
- 5.Sulkowski MS, Sherman KE, Dieterich DT, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159(2):86–96. doi: 10.7326/0003-4819-159-2-201307160-00654. [DOI] [PubMed] [Google Scholar]
- 6.Hulskotte EGJ, Feng H-P, Xuan F, et al. Pharmacokinetic interactions between the hepatitis C virus protease inhibitor boceprevir and ritonavir-boosted HIV-1 protease inhibitors atazanavir, darunavir, and lopinavir. Clin Infect Dis. 2013;56(5):718–726. doi: 10.1093/cid/cis968. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS. Current management of hepatitis C virus infection in patients with HIV co-infection. J Infect Dis. 2013;207(Suppl 1):S26–S32. doi: 10.1093/infdis/jis764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam AM, Murakami E, Espiritu C, et al. PSI-7851, a pronucleotide of beta-D-2′-deoxy-2′-fluoro-2′-C-methyluridine monophosphate, is a potent and pan-genotype inhibitor of hepatitis C virus replication. Antimicrob Agents Chemother. 2010;54(8):3187–3196. doi: 10.1128/AAC.00399-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. doi: 10.1002/hep.31060. Available at: www.hcvguidelines.org. [DOI] [PMC free article] [PubMed]
- 10.Kirby B, Mathias A, Rossi S, et al. No clinically significant pharmacokinetic drug interactions between sofosbuvir (GS-7977) and HIV antiretrovirals Atripla®, rilpivirine, darunavir/ritonavir, or raltegravir in healthy volunteers. Hepatology. 2012;56(Suppl):1067A. abstract. [Google Scholar]
- 11.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 13.Zeuzem S, Dusheiko G, Salupere R, et al. Sofosbuvir + ribavirin for 12 or 24 weeks for patients with HCV genotype 2 or 3: the VALENCE trial. Hepatology. 2013;58(Suppl):733A. abstract. [Google Scholar]
- 14.Osinusi A, Meissner EG, Lee Y-J, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310(8):804–811. doi: 10.1001/jama.2013.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Code of Federal Regulations. Phases of an investigation. 21 CFR 312.21. 2013.
- 16.US prescribing information. South San Francisco, CA: Genentech USA; 2013. Pegasys (peginterferon alfa-2a) injection for subcutaneous use. Available at: http://www.gene.com/download/pdf/pegasys_prescribing.pdf. [Google Scholar]
- 17.Rodríguez-Nóvoa S, Morello J, González M, et al. Increase in serum bilirubin in HIV/hepatitis-C virus-coinfected patients on atazanavir therapy following initiation of pegylated-interferon and ribavirin. AIDS. 2008;22(18):2535–2548. doi: 10.1097/QAD.0b013e3283177f38. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33(11):1729–1739. doi: 10.1124/dmd.105.005447. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Torres M, Rodriguez-Orengo J, Gaggar A, et al. Sofosbuvir and peginterferon alfa-2a/ribavirin for treatment-naive genotype 1–4 HCV infected patients who are HIV coinfected with HIV. Infectious Diseases Week; October 8–12, 2013; Philadelphia, PA. Abstract 714. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.