Abstract
Sperm specific lactate dehydrogenases (LDH-C4) is a lactate dehydrogenase that catalyzes the conversion of pyruvate to lactate. In mammals, Ldh-c was originally thought to be expressed only in testes and spermatozoa. Plateau pika (Ochotona curzoniae), which belongs to the genus Ochotona of the Ochotonidea family, is a hypoxia-tolerant mammal living 3000–5000 m above sea level on the Qinghai-Tibet Plateau, an environment which is strongly hypoxic. Ldh-c is expressed not only in testes and sperm, but also in the somatic tissues of plateau pika. To reveal the effect of hypoxia on pika Ldh-c expression, we investigated the mRNA and protein level of Ldh-c as well as the biochemical index of anaerobic glycolysis in pika somatic tissues at the altitudes of 2200 m, 3200 m and 3900 m. Our results showed that mRNA and protein expression levels of Ldh-c in the tissues of pika’s heart, liver, brain and skeletal muscle were increased significantly from 2200 m to 3200 m, but had no difference from 3200 m to 3900 m; the activities of LDH and the contents of lactate showed no difference from 2200 m to 3200 m, but were increased significantly from 3200 m to 3900 m. Hypoxia up-regulated and maintained the expression levels of Ldh-c in the pika somatic cells. Under the hypoxia condition, plateau pikas increased anaerobic glycolysis in somatic cells by LDH-C4, and that may have reduced their dependence on oxygen and enhanced their adaptation to the hypoxic environment.
Keywords: Ldh-c, plateau pika (Ochotona curzoniae), hypoxia, altitude, Qinghai-Tibetan Plateau
1. Introduction
The Qinghai-Tibet Plateau, with an average elevation of over 3000 m above sea level, is the highest and largest plateau on earth and it possesses a unique environment of nature and geography. Hypoxia is the most obvious climate characteristic on the plateau, in general; the air content decreases with the elevation at a rate of 0.67 kPa for 100 m, and the average oxygen content on the Qinghai-Tibet Plateau is only 60% of that at sea level. The oxygen concentration from the atmosphere to the tissues and cells reaches the cellular level to impact the basic units of life [1]. Therefore, over long-term evolution, many plateau-native animals have developed their own unique mechanisms to adapt to the hypoxic environment on the plateau.
Plateau pika (Ochotona curzoniae), an endemic species of the Qinghai-Tibet Plateau, inhabit meadows at altitudes 3000–5000 m above sea level. The pika is a key species in that it plays an important role in the biodiversity of the ecosystem on the Qinghai-Tibetan Plateau [2,3]. So far, pika fossil samples found on the north edge of the Qinghai-Tibet Plateau are about 37 million years old [4]. During evolution, the pika underwent a series of changes to adapt to the harsh environment. First, the pika obtains oxygen sufficiently by a special pulmonary structure and erythrocyte characteristics to get a high oxygen uptake capacity [5,6,7,8,9]. Secondly, the pika transports oxygen effectively by a large heart volume and a special cardiac structure to get a strong cardiac pumping function [10]. Thirdly, the pika has a high ratio of oxygen utilization by increasing the capillary and mitochondrial densities [11] and myoglobin (Mb) content [7,10] in tissues to get higher oxygen saturation in the arterial blood. Besides these physiological mechanisms, the pika reduces its dependence on oxygen by increasing anaerobic glycolysis in the skeletal muscle [12] and gluconeogenesis in the liver [13]. The molecular basis of these adaptations in pika has occurred with a series changes of genetic evolutionary and with genes related to hypoxia, such as HIF-1α [4,10,13,14,15,16,17,18,19,20,21]. We also found that Ldh-c (s testes-specific lactate dehydrogenase gene) is expressed not only in testes and sperm, but also in the somatic tissues of plateau pika [22].
The lactate dehydrogenase (LDH) family enzymes catalyze the inter-conversion of pyruvate to lactate with the concomitant oxidation/reduction of nicotinamide adenine dinucleotide hydrogen (NADH) to nicotinamide adenine dinucleotide (NAD+) [23]. Different forms of LDH are the products of three different genes: Ldh-a, Ldh-b, and Ldh-c which encode A, B and C subunits, respectively [24,25]. LDH consists of A and B subunits that assemble into homo- or hetero-tetramers that are distributed in the body in various combinations, reflecting the metabolic requirements of different tissues, and are consistent with the catalytic properties of the isozymes [26,27]. However, the homo-tetramer LDH-C4 was only detected in testes and spermatozoa and not in any other tissues or cells, and it was irreplaceable in the male reproductive capacity [28,29,30,31]. Ldh-c expressed in the somatic cells of plateau pika may play a crucial role in anaerobic glycolysis and ATP rapid generation; the pika has a reduced dependence on oxygen and an enhanced adaptation to the hypoxic environment due to increased anaerobic glycolysis by LDH-C4 in the somatic tissues [32].
Under hypoxia conditions, many organisms switch from aerobic metabolism to anaerobic metabolism in order to maintain their function. Since LDH is one of the main enzymes used under anaerobic conditions, studies have showed that hypoxic conditions resulted in the alteration of the LDH activity [33,34,35,36]. LDH activities in animals would increase after being exposed to hypoxic conditions [35,36]. LDH-A isozyme activity and its protein expression are increased most significantly at 24 h in cells under hypoxia. However, the LDH-B protein is unchanged while its mRNA is decreased [37]. The LDH activities in pika tissues had been proved to respond to the hypoxic environment, and the results showed the activities of LDH-A were up-regulated at a higher elevation [38]. However, up until now, the relationship between Ldh-c and hypoxia has not precisely been known.
In the current study, we investigated the effect of hypoxia on Ldh-c expression in somatic cells of plateau pika. The mRNA and protein level of pika Ldh-c was measured and the biochemical indexes of anaerobic glycolysis were assayed in different altitude conditions.
2. Material and Methods
2.1. Animals and Sample Collection
Plateau pikas were live-trapped from Qinghai Province in China. Pikas were divided into three groups: (1) high altitude (3900 m Group), pikas collected from Laji Mountain in Guide County at an altitude of 3900 m, the oxygen content is 174.2 g/m3; (2) medium altitude (3200 m Group), pikas collected from Haibei Alpine Meadow Ecosystem Research Station in Menyuan County at an altitude of 3200 m, the oxygen content is 196.6 g/m3; (3) low altitude (2200 m Group), pikas collected from Haibei Alpine Meadow Ecosystem Research Station, and raised in Xining City for 16 months, the oxygen content is 228.6 g/m3. The sample size is eight for each group above. In order to detect the mRNA levels of Ldh-a and Ldh-b of plateau pikas at different altitudes, the plateau pikas were live-trapped from Menyuan Country at an altitude of 3200 m, the oxygen content is 196.6 g/m³, and Guoluo Country at an altitude of 4200 m, the oxygen content is 164.6 g/m³, respectively, in Qinghai Province, China. The sample size is 10 for each group above. All animals were anesthetized with sodium pentobarbital (5%) and then sacrificed by cervical dislocation immediately before dissection. Heart, liver, brain and skeletal muscle were removed and stored in liquid nitrogen rapidly. All procedures involved in the handling and care of animals were in accordance with the China Practice for the Care and Use of Laboratory Animals and were approved by the China Zoological Society (permit number: GB 14923-2010).
2.2. RNA Extraction and Quantification of Ldh-c mRNA Level by qRT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen Corp., Carlsbad, CA, USA). RNA concentration and purity were assessed by Ultra Violet (UV) spectrophotometry (1.8 < A260/A280 < 2.0). RNA integrity was checked using electrophoresis. Reverse transcription reaction was carried out starting from 4 μg of total RNA using the First Strand complementary DNA (cDNA) Synthesis kit (Thermo Scientific, Boston, MA, USA).
To make standard curves, 1 μL of first-strand cDNA were amplified with Premix Ex Taq Version Kit (TaKaRa BIO INC., Kusatsu, Japan), and quantification of Polymerase Chain Reaction (PCR) products were used for plotting standard curves. The initial product concentration was set at 1 and standard curves were generated using a 10-fold serial dilution ranging from 1 to 10−7.
Quantitative Real-time Polymerase Chain Reaction (qRT-PCR) was performed using the SYBR® Premix Ex Taq™ II (TaKaRa BIO INC., Kusatsu, Japan) protocol on BIO-RAD Connect real-time PCR detection system with cycling conditions of 95 °C for 3 min, followed by 40 cycles of 95 °C for 30 s and 60 °C for 30 s. β-actin was used as an internal control. The PCR primers for Ldh-c, Ldh-a, Ldh-b, GAPDH and β-actin were designed as follows: Ldh-c: forward, 5′-TATCGAGAATCTGATCGCAGAAGAC-3′ and reverse, 5′-GGGCAAGTTCATCAGCCAAATCC-3′, the amplicon length was 130 bp. Ldh-a: forward, 5′-TTGGTCCAGCGGAATGTA-3′ and reverse, 5′-GGTGAACTCCCAGCCTTT-3′, the amplicon length was 220 bp. Ldh-b: forward, 5′-TGTTGGACAAGTCGGAATG-3′ and reverse, 5′-CTGAAGAAACAGGCTCCC-3′, the amplicon length was 139 bp. GAPDH: forward, 5′-GGGAAATCGTGCGTGACTT-3′ and reverse, 5′-GCGGCAGTGGCCATCTC-3′, the amplicon length was 203 bp. β-actin: forward, 5′-CTCTTCCAGCCCTCCTTCTT-3′ and reverse, 5′-AGGTCCTTACGGATCTCCAC-3′, the amplicon length was 98 bp. Ldh-c mRNA level was normalized with β-actin mRNA to compensate for variations in initial RNA amounts. Normalization was carried out by dividing the logarithmic value of Ldh-c by the logarithmic value of β-actin. Ldh-a and Ldh-b mRNA level was normalized with GAPDH mRNA to compensate for variations in initial RNA amounts. Normalization was carried out by dividing the logarithmic value of Ldh-a and Ldh-b by the logarithmic value of GAPDH.
2.3. Western Blot Analysis
Total cellular proteins were extracted by RIPA buffer containing protein inhibitors, BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA) was used to assess protein concentration. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE), and transferred onto a 0.22 mmol/L polyvinylidene difluoride (PVDF) membrane. After blocking the non-specific binding sites for 120 min with 5% non-fat milk, the membranes were incubated with a rabbit polyclonal antibody against LDH-C (SIGMA-ALDRICH, Saint Louis, MO, USA, at 1:4000 dilution) or GAPDH (Genetex, San Antonio, TX, USA, at 1:5000 dilution) at 4 °C overnight. The membranes were then washed with TBST (Tris-Buffered Saline with Tween-20) six times at room temperature for 10 min. After washing, the target protein was probed with the horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (Santa Cruz, Santa Cruz, CA, USA, at 1:6000 dilution) at 37 °C for 2 h. After 10 washes, the bound antibody was detected by chemiluminescence with the ECL Detection Reagent (Pierce Biotechnology, Rockford, IL, USA).
2.4. LDH Activities and LD Contents Assessment
The tissues were homogenized on ice as a 1:9 (w/v) dilution in 0.9% physiological saline. The homogenate was centrifuged at 2500 rpm/min at 4 °C for 10 min, and the supernatant was collected. Protein level was measured by Bradford assay. The LD assay kit and the LDH activity assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing China) were used to assess the LD content and LDH activity in tissues.
2.5. Data Analysis
Data were expressed as mean ± SD. Statistical analysis was performed by one-way analysis of ANOVA and Duncan’s test using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. qRT-PCR Analysis of Ldh-a and Ldh-b mRNA Expression
The mRNA levels of Ldh-a and Ldh-b of plateau pikas at different altitudes were examined in the heart, liver, brain and skeletal muscle by qRT-PCR assays. As the statistics results showed in Table 1, the relative expression levels of Ldh-a and Ldh-b mRNA in the heart, liver, brain and skeletal muscle have no increase in plateau pika tissues with increasing of elevations from 3200 m to 4200 m.
Table 1.
Quantification mRNA levels of Ldh-a, Ldh-b in plateau pika tissues at 3200 m and 4200 m altitudes.
| Tissues | Ldh-a | Ldh-b | ||
|---|---|---|---|---|
| 3200 m | 4200 m | 3200 m | 4200 m | |
| heart | 0.955 ± 0.029 ** | 0.811 ± 0.045 | 0.973 ± 0.022 ** | 0.880 ± 0.024 |
| liver | 0.959 ± 0.087 | 0.895 ± 0.095 | 0.909 ± 0.037 | 0.894 ± 0.035 |
| brain | 0.919 ± 0.032 ** | 0.785 ± 0.033 | 0.870 ± 0.048 ** | 0.785 ± 0.033 |
| skeletal muscle | 0.935 ± 0.037 | 0.847 ± 0.170 | 0.735 ± 0.046 * | 0.641 ± 0.118 |
* p < 0.05, ** p < 0.01; The sample size is 10 for each group.
3.2. qRT-PCR Analysis of Ldh-c mRNA Expression
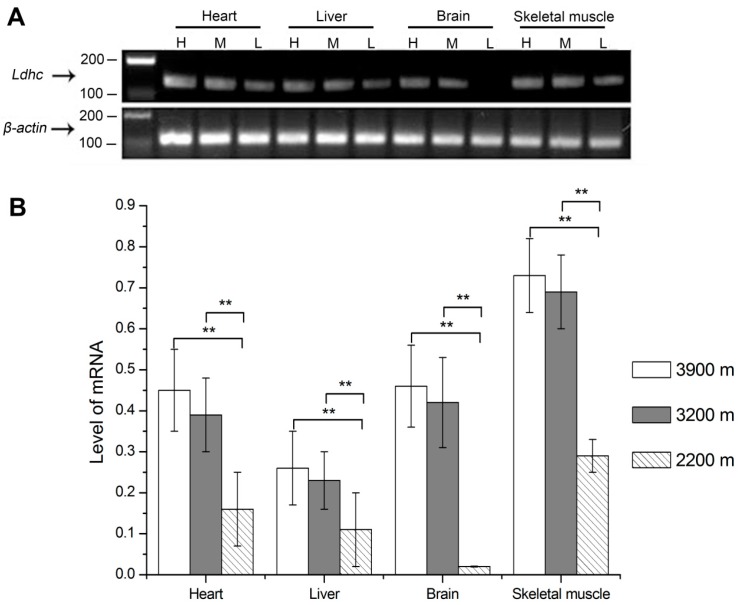
The mRNA levels of Ldh-c of plateau pikas at different altitudes were examined in the heart, liver, brain and skeletal muscle by qRT-PCR assays. As the statistics results showed in Figure 1, the relative expression levels of Ldh-c at 3900 m, 3200 m and 2200 m altitude were 0.45 ± 0.10, 0.39 ± 0.09 and 0.16 ± 0.09 in the heart; 0.26 ± 0.09, 0.23 ± 0.07 and 0.11 ± 0.09 in the liver; 0.46 ± 0.10, 0.42 ± 0.11, and 0.02 ± 0.00 in the brain; and 0.73 ± 0.09, 0.69 ± 0.09 and 0.29 ± 0.04 in the skeletal muscle, respectively. For all tissues, the Ldh-c expression levels were significantly higher in the 3900 m group than in the 2200 m group; they were also significantly higher in the 3200 m group than in the 2200 m group (p < 0.01); however, there was no difference between the 3200 m and 3900 m groups.
Figure 1.
Quantification of Ldh-c mRNA levels in plateau pikas tissues at different altitudes. (A) The electrophoresis results of real-time PCR of Ldh-c and β-actin in plateau pika tissues; (B) Quantification of Ldh-c mRNA levels in plateau pikas tissues at different altitudes. H: high altitude group, 3900 m; M: medium altitude group, 3200 m; L: low altitude group, collected from Haibei Station, raised in Xining (2200 m) for 16 months. ** p < 0.01. The sample size is eight for each group.
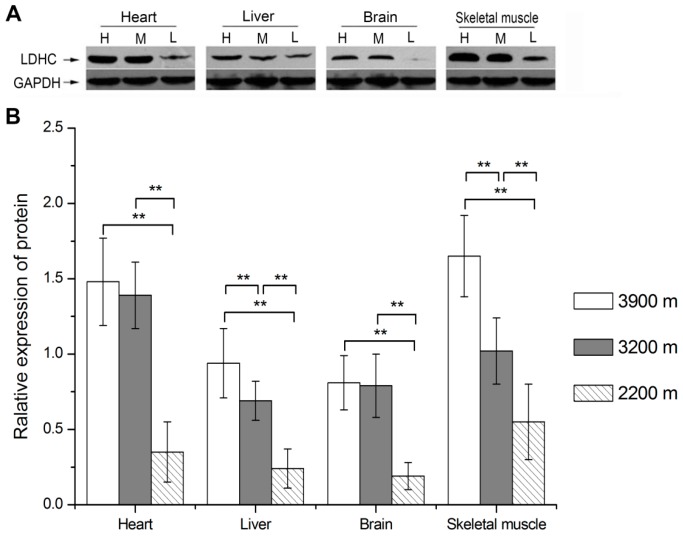
3.3. Western Blotting Analysis of LDH-C Protein Expression
The protein levels of LDH-C of plateau pikas at different altitudes were examined in the heart, liver, brain and skeletal muscle by Western blotting. The relative expression levels of LDH-C protein at the elevations of 3900 m, 3200 m and 2200 m were 1.48 ± 0.29, 1.39 ± 0.22 and 0.35 ± 0.20 in the heart; 0.94 ± 0.23, 0.69 ± 0.13 and 0.24 ± 0.13 in the liver; 0.81 ± 0.18, 0.79 ± 0.21 and 0.19 ± 0.09 in the brain; and 1.65 ± 0.27, 1.02 ± 0.22 and 0.55 ± 0.25 in the skeletal muscle, respectively. Statistics results showed that LDH-C expression levels increased with the altitude. It was significantly higher in the 3900 m group and the 3200 m group compared with the 2200 m group in the heart, liver, brain and skeletal muscle (p < 0.01). It was also significantly higher in the 3900 m group than in the 3200 m group in the liver (p < 0.05) and skeletal muscle (p < 0.01) (Figure 2).
Figure 2.
Quantification of LDH-C protein levels in plateau pika tissues at different altitudes. (A) Western blot result of LDH-C and GAPDH protein in plateau pika tissues; (B) Quantification of LDH-C protein levels in plateau pika tissues at different altitudes. H: high altitude group, 3900 m; M: medium altitude group, 3200 m; L: low altitude group, collected from Haibei Station, raised in Xining (2200 m) for 16 months. * p < 0.05, ** p < 0.01. The sample size is eight for each group.
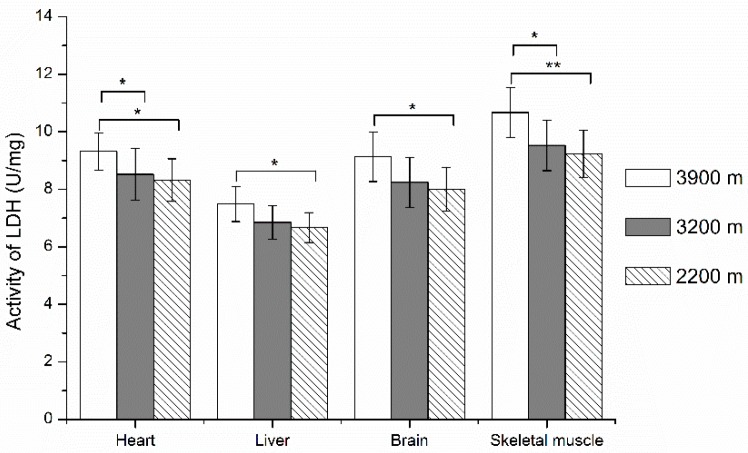
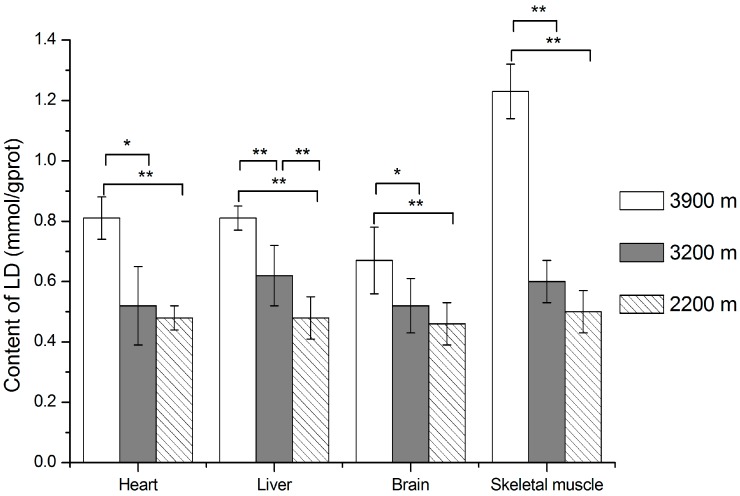
3.4. LDH Activities and LD Contents in Plateau Pika Tissues at Different Altitudes
As shown in Figure 3 and Figure 4, LDH activities and LD contents increased with the altitude ascended from 2200 m to 3900 m. LDH activities were significantly higher in the 3900 m group than in the 2200 m group in pika heart, liver and brain (p < 0.05), and skeletal muscle (p < 0.01). They were also significantly higher in the 3900 m group than in the 3200 m group in heart and skeletal muscle (p < 0.05). For LD contents in tissues, it was significantly higher in the 3900 m group compared with the 3200 m group and 2200 m group in all tissues (p < 0.01); there was no difference between the 3200 m group and 2200 m group, except for the liver.
Figure 3.
LDH activities in plateau pika tissues at different altitudes. For LDH activities at 3900 m, 3200 m, 2200 m group pikas, they were 9.32 ± 0.65, 8.52 ± 0.91 and 8.32 ± 0.74 in heart; 7.49 ± 0.61, 6.85 ± 0.59 and 6.67 ± 0.52 in liver; 9.13 ± 0.86, 8.24 ± 0.87 and 8.01 ± 0.76 in brain; 10.67 ± 0.87, 9.53 ± 0.88 and 9.24 ± 0.82 in skeletal muscle. * p < 0.05, ** p < 0.01. The sample size is eight for each group.
Figure 4.
LD contents in plateau pika tissues at different altitudes. For LD contents of 3900 m, 3200 m, 2200 m group pikas, they were 0.81 ± 0.07, 0.52 ± 0.13 and 0.48 ± 0.04 in heart; 0.81 ± 0.04, 0.62 ± 0.10 and 0.48 ± 0.07 in liver; 0.67 ± 0.11, 0.52 ± 0.09 and 0.46 ± 0.07 in brain; 1.23 ± 0.09, 0.60 ± 0.07 and 0.50 ± 0.07 in skeletal muscle. * p < 0.05, ** p < 0.01. The sample size is eight for each group.
4. Discussion
The LDH family is encoded by three genes, Ldh-a, Ldh-b, and Ldh-c, for the expression of the LDH subunits LDH-A, LDH-B, and LDH-C. LDH-A is the most abundant in the liver and skeletal muscle and LDH-B is the most abundant in the heart and red blood cells [39]. The isoform LDH-A preferentially converts pyruvate to lactate and it is found predominantly in poorly vascularized tissues with low oxygen [39]. However, the isoform LDH-B is more active in aerobic conditions, converting lactate to pyruvate in well-oxygenated tissues [39]. Besides, Ldh-c was previously only detected being expressed in the testes and spermatozoa of mammals and birds, supporting its role in energy production in spermatids that favor lactate as a substrate with a characteristic aerobic glycolytic path to generate ATP [40]. LDH-C4 is the main LDH isozyme in sperm that accounts for 80%–100% of the total LDH activity in mammal spermatozoa [41].
Studies had demonstrated that the disruption of Ldh-c or the inhibition of LDH-C4 in sperm led to a rapid decline in sperm ATP levels [42,43], a decrease in progressive motility, and a failure to develop hyperactivated motility. Metabolic tracing experiments revealed that all consumed 13C-labeled pyruvate added in sperm culture medium was converted to lactate rather than oxidized in the tricarboxylic acid cycle. The ATP concentration was increased by more than 50% in the presence of exogenous pyruvate [44]. When carbonyl cyanide mchlorophenylhydrazone (CCCP) and NaCN were applied to suppress the oxidative phosphorylation in mitochondria, the vigorous motility of sperm was maintained and the amount of ATP was kept at the level equivalent to that without CCCP [44,45]. These results had proved that LDH-C4 is the key factor of sperm glycolysis, which has an important role in providing the ATP required for sperm motility and capacitation [42,45,46].
Surprisingly, we found that Ldh-c is expressed not only in testes and sperm, but it was also expressed generally in somatic tissues of plateau pika in our previous study [22]. Compared with LDH-A4 and LDH-B4, LDH-C4 had a lower Km for pyruvate (~0.052 mmol/L) and a higher Km for lactate (~4.934 mmol/L); thus, LDH-C4 was less sensitive to lactate inhibition than LDH-A4 and LDH-B4 [47]. These properties of pika LDH-C4 were beneficial for catalyzing the conversion of pyruvate to lactate even at a high concentration of lactate. Furthermore, both the activity of pika LDH-C4 inhibited by the specific inhibitor and the content of LDH-C4 reduced by silencing Ldh-c can also decrease the level of anaerobic glycolysis, ultimately leading to a decline in their exercise tolerance in hypoxic environments [32]. Ldh-c expressed in the somatic cells of plateau pikas enhances their anaerobic glycolysis, and generates ATP and lactate more rapidly. Lactate accumulation in the body can cause acidosis [48]. The liver is the main organ responsible for metabolizing excess lactate; in the liver, lactate is converted into pyruvate by LDH, and some of the pyruvic acid is oxidized through Kreb’s cycle, most of which is then converted into glucose and glycogen through gluconeogenesis [49]. LDH and pyruvate carboxylase (PC) are key enzymes in the process of gluconeogenesis; indeed, the rate of gluconeogenesis in the mammalian liver is related to the relative constituents of LDH isoenzymes and its enzymatic activities, as well as the enzymatic activity of PC, which is a rate-limiting enzyme gluconeogenesis [50]. During long-term evolution, the pikas evolved a series of physiological adaptations that allow them to convert lactate promptly in the liver. Our previous results indicated that, in the liver of plateau pikas, the mRNA levels of LDH-A, LDH-B and PC, the concentration of lactate, as well as the enzymatic activities of LDH and PC were significantly higher than those of plateau zokors [13]. Furthermore, the isoenzymatic spectrum of LDH in the livers of these two species showed that the main LDH isoenzymes in the liver of plateau pikas were LDH-A3B, LDH-A2B2, LDH-AB3 and LDH-B4, while LDH-A4 was the only isoenzyme found in the liver of mouse, rats and plateau zokors [13,51]. These results suggest that plateau pikas can rapidly convert the lactate produced from their skeletal muscle and other tissues by increasing the hepatic enzymatic activity of PC and their LDH, which contains a higher ratio of LDH-B subunits. Therefore, higher levels gluconeogenesis in the liver is one of the important mechanisms of plateau pikas’ adaptation to the hypoxic environment.
To reveal the role and physiological mechanism of LDH-C4 in the skeletal muscle of plateau pikas, we investigated the effect of silencing Ldh-c by RNAi on exercise tolerance as well as the physiological mechanism. We found that the expression levels of Ldh-a and Ldh-b have no statistical difference in skeletal muscle. Compared with the control group, Ldh-c mRNA and protein in the siRNA-Ldh-c group decreased by 82.18% and 82.29%, respectively; the LDH activity, LD content, and ATP level in the siRNA-Ldh-c group was reduced by 28.21%, 48.38% and 27.88%, respectively. Our results suggested that at least 27.88% of the ATP in pika skeletal muscle is catalyzed by LDH-C4 by anaerobic glycolysis; thus, pika has a reduced dependence on oxygen and enhanced adaptation to hypoxic environments due to increased anaerobic glycolysis by LDH-C4 in the skeletal muscle (unpublished). In order to shed light on the effect of LDH-C4 on the anaerobic glycolysis in plateau pika heart, liver, brain and skeletal muscle, we used a specific LDH-C4 inhibitor (N-isopropyl oxamate), and injected 1 mL 1 mol/L N-isopropyl oxamate into the biceps femoris muscle of the hind legs of plateau pika; each leg received 500 μL. The pikas of the control group were injected with the same volume of normal saline (0.9% NaCl). The results showed, compared to the control group, that the inhibition rates of N-isopropyl oxamate to LDH, LD and ATP were 31.98%, 20.90% and 28.70% in the heart [52]; 30.19% ± 3.90%, 32.22% ± 8.70% and 24.94% ± 7.80% in the liver [53]; 30.78%, 46.47% and 21.04% in the brain [54]; 37.12%, 66.27% and 32.42% in the skeletal muscle, respectively [32]. The above results suggested that pikas adapted to hypoxic environments by increasing anaerobic glycolysis by LDH-C4 in the somatic tissues.
Under hypoxia conditions, LDH-A and LDH-B subunits in the tissues of plain animals were increased significantly with the decrease of PO2 [38]. However, the contents of LDH-A and LDH-B in the heart, liver, kidney and skeletal muscle of plateau pikas had no differences at the same area at altitudes of 3400 m to 5000 m [38]. When the pikas inhabiting the spot at an altitude of 3400 m were transferred to 2300 m, the contents of LDH-A and LDH-B in the heart, liver, and kidney had no differences, while those of LDH-A and LDH-B in the skeletal muscle were down-regulated about 14%, and the activities of LDH showed no differences [38]. These results indicated that the LDH-A and LDH-B expression patterns and levels in plateau pikas had obvious differences from the plain animals; the pikas’ LDH-A was less sensitive to the changes of PO2, except for in the skeletal muscle. Our present study results showed that in the heart, liver, brain and skeletal muscle of plateau pikas, the mRNA expression levels of Ldh-a and Ldh-b had no increase in plateau pika tissues with increasing of elevations from 3200 m to 4200 m; the mRNA and protein expression levels of Ldh-c had no difference from the spot at 3900 m elevation to 3200 m elevation; and when the pikas inhabiting their original environment at an elevation of 3200 m were transferred to 2200 m, the mRNA and protein expression levels of Ldh-c decreased by more than 50%, but the activities of LDH showed no difference. These results suggested that, in their original inhabiting environment from the elevation of 3200 m to 3900 m, Ldh-c was less sensitive to PO2 changes, and its expression levels showed no difference in the mRNA and protein, but the higher PO2 in the spot where the pika is a non-indigenous animal had a significant inhibition on the Ldh-c expression.
5. Conclusions
In conclusion, the expression of Ldh-c in the somatic tissues of plateau pikas is an important adaptation mechanism. In spite of lowering the PO2 in their original inhabiting environment with increasing altitudes, the expression levels of Ldh-c in the pikas were not up-regulated by the lower PO2, but it was thought to be one of the important factors that maintained the expression levels of Ldh-c in the somatic tissues of plateau pikas. Under hypoxia conditions, plateau pikas increased the anaerobic glycolysis in somatic cells by LDH-C4, and that may reduce their dependence on oxygen and enhance their adaptation to the hypoxic environment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 31260512, 30960054, and 31040011), the scientific research key project fund of the Ministry of Education of China (209132) and the Natural Science Foundation of Qinghai Province (No. 2012-Z-905).
Author Contributions
Dengbang Wei and Linna Wei planned the experiments; Dengbang Wei, Linna Wei and Yang Wang performed the experiments; Xiao Li, Linna Wei and Lian Wei analyzed the data; Dengbang Wei and Linna Wei wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wu T. Life on the high Tibetan Plateau. High. Alt. Med. Biol. 2004;5:1–2. doi: 10.1089/152702904322963609. [DOI] [PubMed] [Google Scholar]
- 2.Smith A.T., Foggin J.M. The plateau pika (Ochotona curzoniae) is a keystone species for biodiversity on the Tibetan Plateau. Anim. Conserv. 1999;2:235–240. doi: 10.1111/j.1469-1795.1999.tb00069.x. [DOI] [Google Scholar]
- 3.Lai C.H., Smith A.T. Keystone status of plateau pikas (Ochotona curzoniae): Effect of control on biodiversity of native birds. Biodivers. Conserv. 2003;12:1901–1912. doi: 10.1023/A:1024161409110. [DOI] [Google Scholar]
- 4.Yang Y.Z., Cao Y., Jin G.E., Bai Z.Z., Lan M.L., Yun H.X., Ge R.L. Molecular cloning and characterization of hemoglobin α and β chains from plateau pika (Ochotona curzoniae) living at high altitude. Gene. 2007;403:118–124. doi: 10.1016/j.gene.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Wang X.J., Wei D.B., Wei L., Qi X.Z., Zhu S.H. Characteristics of pulmonary acinus structure in the plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniae) J. Zool. 2008;54:531–539. [Google Scholar]
- 6.Ge R.L., Kubo K., Kobayashi T., Sekiguchi M., Honda T. Blunted hypoxic pulmonary vasoconstrictive response in the rodent Ochotona curzoniae (pika) at high altitude. Am. J. Physiol. Heart Circ. Physiol. 1998;274:1792–1799. doi: 10.1152/ajpheart.1998.274.5.H1792. [DOI] [PubMed] [Google Scholar]
- 7.Wang X.J., Wei D.B., Wei L., Zhang J.M., Yu H.Y. Physiological character of erythrocyte adapting to hypoxia in plateau zokor and plateau pika. Sichuan J. Zool. 2008;27:1100–1103. (In Chinese) [Google Scholar]
- 8.Ye R.R., Cao Y.F., Bai Q.H. Blood indices of plateau pika and relationship with hypoxia adaptation. J. Zool. 1994;2:114–119. [Google Scholar]
- 9.He J., Xu C., Meng X., Li H., Wang Y. Comparative analysis in transport and intake of oxygen between pikas (Ochotona curzoniae) and rats. J. Prev. Med. Chin. People’s Libr. 1994;12:431–435. (In Chinese) [Google Scholar]
- 10.Qi X.Z., Wang X.J., Zhu S.H., Rao X.F., Wei L., Wei D.B. Hypoxic adaptation of the hearts of plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniae) J. Zool. 2008;60:348–354. [PubMed] [Google Scholar]
- 11.Wei D.B., Wei L., Zhang J.M., Yu H.Y. Blood-gas properties of plateau zokor (Myospalax baileyi) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006;145:372–375. doi: 10.1016/j.cbpa.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhu S.H., Qi X.Z., Wang X.J., Rao X.F., Wei L., Wei D.B. Difference in oxygen uptake in skeletal muscles between plateau zokor (Myospalax rufescens baileyi) and plateau pika (Ochotona curzoniac) Acta Physiol. Sin. 2009;61:373–378. (In Chinese) [PubMed] [Google Scholar]
- 13.Sun S.Z., Wei L., Wei D.B., Wang D.W., Ma B.Y. Differences of glycolysis in skeletal muscle and lactate metabolism in liver between plateau zokor (Myospalax baileyi) and plateau pika (Ochotona curzoniae) Acta Physiol. Sin. 2013;65:276–284. (In Chinese) [PubMed] [Google Scholar]
- 14.Li H.G., Ren Y.M., Guo S.C., Cheng L., Wang D.P., Yang J., Chang Z.J., Zhao X.Q. The protein level of hypoxia-inducible factor-1α is increased in the plateau pika (Ochotona curzoniae) inhabiting high altitudes. J. Exp. Zool. Part. A Ecol. Genet. Physiol. 2009;311:134–141. doi: 10.1002/jez.510. [DOI] [PubMed] [Google Scholar]
- 15.Zhao T.B., Ning H.X., Zhu S.S., Sun P., Xu S.X., Chang Z.J., Zhao X.Q. Cloning of hypoxia-inducible factor-1α cDNA from a high hypoxia tolerant mammal-plateau pika (Ochotona curzoniae) Biochem. Biophys. Res. Commun. 2004;316:565–572. doi: 10.1016/j.bbrc.2004.02.087. [DOI] [PubMed] [Google Scholar]
- 16.Li H.G., Guo S.C., Ren Y.M., Wang D.P., Yu H.H., Li W.J., Zhao X.Q., Chang Z.J. VEGF189 expression is highly related to adaptation of the plateau pika (Ochotona curzoniae) Inhabiting High Altitudes. High Alt. Med. Biol. 2013;14:395–404. doi: 10.1089/ham.2013.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y.N., Zhu R.J., Wang D.W., Wei L., Wei D.B. Gene coding and mRNA expression of vascular endothelial growth factor as well as microvessel density in brain of plateau zokor: Comparison with other rodents. Acta Physiol. Sin. 2011;63:155–163. (In Chinese) [PubMed] [Google Scholar]
- 18.Luo Y.J., Gao W.X., Gao Y.Q., Tang S., Huang Q.Y., Tan X.L., Chen J., Huang T.S. Mitochondrial genome analysis of Ochotona curzoniae and implication of cytochrome c oxidase in hypoxic adaptation. Mitochondrion. 2008;8:352–357. doi: 10.1016/j.mito.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Pichon A., Zhenzhong B., Favret F., Jin G., Shufeng H., Marchant D., Richalet J.P., Ge R.L. Long-term ventilatory adaptation and ventilatory response to hypoxia in plateau pika (Ochotona curzoniae): Role of nNOS and dopamine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:978–987. doi: 10.1152/ajpregu.00108.2009. [DOI] [PubMed] [Google Scholar]
- 20.Yang J., Wang Z.L., Zhao X.Q., Xu B.H., Ren Y.H., Tian H.F. Natural selection and adaptive evolution of leptin in the ochotona family driven by the cold environmental stress. PLoS ONE. 2008 doi: 10.1371/journal.pone.0001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J., Zhao X.Q., Guo S.C., Li H.G., Qi D.L., Wang D.P., Cao J.H. Leptin cDNA cloning and its mRNA expression in plateau pikas (Ochotona curzoniae) from different altitudes on Qinghai-Tibet Plateau. Biochem. Biophys. Res. Commun. 2006;345:1405–1413. doi: 10.1016/j.bbrc.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 22.Wang D.W., Wei L., Wei D.B., Rao X.F., Qi X.Z., Wang X.J., Ma B.Y. Testis-specific lactate dehydrogenase is expressed in somatic tissues of plateau pikas. FEBS Open Biol. 2013;3:118–123. doi: 10.1016/j.fob.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everse J., Kaplan N.O. Lactate dehydrogenases: Structure and function. Adv. Enzymol. Relat. Areas Mol. Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- 24.Li S. Lactate dehydrogenase isoenzymes A (muscle), B (heart) and C (testis) of mammals and the genes coding for these enzymes. Biochem. Soc. Trans. 1989;17:304. doi: 10.1042/bst0170304. [DOI] [PubMed] [Google Scholar]
- 25.Li S., O’brien D., Hou E., Versola J., Rockett D., Eddy E. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol. Reprod. 1989;40:173–180. doi: 10.1095/biolreprod40.1.173. [DOI] [PubMed] [Google Scholar]
- 26.Cahn R.D., Zwilling E., Kaplan N.O., Levine L. Nature and development of lactic dehydrogenases. Science. 1962;136:962–969. doi: 10.1126/science.136.3520.962. [DOI] [PubMed] [Google Scholar]
- 27.Fine I., Kaplan N., Kuftinec D. Developmental changes of mammalian lactic dehydrogenases. Biochemistry. 1963;2:116–121. doi: 10.1021/bi00901a020. [DOI] [Google Scholar]
- 28.Goldberg E. Reproductive implications of LDH-C4 and other testis-specific isozymes. Exp. Clin. Immunogenet. 1984;2:120–124. [PubMed] [Google Scholar]
- 29.Coonrod S., Vitale A., Duan C., Bristol-Gould S., Herr J., Goldberg E. Testis-specific lactate dehydrogenase (LDH-C4; Ldh3) in murine oocytes and preimplantation embryos. J. Androl. 2006;27:502–509. doi: 10.2164/jandrol.05185. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg E. Lactate dehydrogenase-X from mouse testes and spermatozoa. Methods Enzymol. 1975;41:318. doi: 10.1016/s0076-6879(75)41072-2. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg E. Lactate dehydrogenases and malate dehydrogenases in sperm: Studied by polyacrylamide gel electrophoresis. Ann. N. Y. Acad. Sci. 1964;121:560–570. doi: 10.1111/j.1749-6632.1964.tb14226.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Wei L., Wei D.B., Xiao L., Xu L.N., Wei L.N. Testis-specific lactate dehydrogenase (LDH-C4) in skeletal muscle enhances a pika’s sprint-running capacity in hypoxic environment. Int. J. Environ. Res. Public Health. 2015;12:9218–9236. doi: 10.3390/ijerph120809218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marti H.H., Jung H.H., Pfeilschifter J., Bauer C. Hypoxia and cobalt stimulate lactate dehydrogenase (LDH) activity in vascular smooth muscle cells. Pflugers. Arch. 1995;429:216–222. doi: 10.1007/BF00374315. [DOI] [PubMed] [Google Scholar]
- 34.Cooper R.U., Clough L.M., Farwell M.A., West T.L. Hypoxia-induced metabolic and antioxidant enzymatic activities in the estuarine fish Leiostomus xanthurus. J. Exp. Mar. Biol. Ecol. 2002;279:1–20. doi: 10.1016/S0022-0981(02)00329-5. [DOI] [Google Scholar]
- 35.Webster K.A. Regulation of glycolytic enzyme RNA transcriptional rates by oxygen availability in skeletal musclecells. Mol. Cell. Biochem. 1987;77:19–28. doi: 10.1007/BF00230147. [DOI] [PubMed] [Google Scholar]
- 36.Wenger R.H. Mammalian oxygen sensing, signalling and gene regulation. J. Exp. Biol. 2000;203:1253–1263. doi: 10.1242/jeb.203.8.1253. [DOI] [PubMed] [Google Scholar]
- 37.Kay H.H., Zhu S., Tsoi S. Hypoxia and lactate production in trophoblast cells. Placenta. 2007;28:854–860. doi: 10.1016/j.placenta.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Liu G.F., Wen D.Q., Han S.G., Hu X.M. Lactate dehydrogenase isoenzyme responds to hypoxia in pika. Acta Theriol. Sin. 1988;8:60–64. (In Chinese) [Google Scholar]
- 39.Markert C.L. Lactate dehydrogenase. Biochemistry and function of lactate dehydrogenase. Cell. Biochem. Funct. 1984;2:131–134. doi: 10.1002/cbf.290020302. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg D.E., Eddy E.M., Duan C., Odet F. LDHC: The ultimate testis-specific gene. Int. J. Androl. 2010;31:86–94. doi: 10.2164/jandrol.109.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinkham W.H., Holtzman N.A., Isensee H. The molecular size of lactate dehydrogenase isozymes in mature testes. Biochim. Biophys. Acta. 1968;160:172–177. doi: 10.1016/0005-2795(68)90084-6. [DOI] [PubMed] [Google Scholar]
- 42.Odet F., Gabel S.A., Williams J., London R.E., Goldberg E., Eddy E.M. Lactate dehydrogenase C and energy metabolism in mouse sperm. Biol. Reprod. 2011;85:556–564. doi: 10.1095/biolreprod.111.091546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odet F., Duan C., Willis W.D., Goulding E.H., Kung A., Eddy E.M., Goldberg E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol. Reprod. 2008;79:26–34. doi: 10.1095/biolreprod.108.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hereng T.H., Elgstøen K.B., Cederkvist F.H., Eide L., Jahnsen T., Skålhegg B.S., Rosendal K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011;26:3249–3263. doi: 10.1093/humrep/der317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukai C., Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol. Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 46.Williams A.C., Ford W.C.L. The role of glucose in supporting motility and capacitation in human spermatozoa. J. Androl. 2001;22:680–695. [PubMed] [Google Scholar]
- 47.Wang Y., Wei L., Wei D.B., Li X., Xu L.N., Wei L.N. Enzymatic kinetic properties of the lactate dehydrogenase isoenzyme C4 of the plateau pika (Ochotona curzoniae) Int. J. Mol. Sci. 2016;17:39. doi: 10.3390/ijms17010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haworth J.C., Robinson B.H., Perry T.L. Lactic acidosis due to pyruvate carboxylase deficiency. J. Inherit. Metab. Dis. 1981;4:57–58. doi: 10.1007/BF02263589. [DOI] [PubMed] [Google Scholar]
- 49.Ma Y.C., Gao S.S., Zhang Z.F. Analysis of the mechanism of muscle lactate production in sport. J. Shengyang Inst. Phys. Edu. 2004;23:312–314. (In Chinese) [Google Scholar]
- 50.Utter M.F., Keech D.B. Formation of oxaloacetate from pyruvate and carbon dioxide. J. Biol. Chem. 1960;235:17–18. (In Chinese) [PubMed] [Google Scholar]
- 51.Liu G.F., Wen D.Q., Hu X.M. Lactate dehydrogenase isoenzymes of the pika and the plateau zokor. Acta Theriol. Sin. 1985;5:223–228. (In Chinese) [Google Scholar]
- 52.Li X., Wei L., Wang Y., Xu L.N., Wei L.N., Wei D.B. The expression of the sperm-specific lactate dehydrogenase gene Ldh-c in plateau pika (Ochotona curzoniae) cardiac muscle and its effect on the anaerobic glycolysis. Acta Physiol. Sin. 2015;67:312–318. (In Chinese) [PubMed] [Google Scholar]
- 53.Wei L.N., Wei L., Wang Y., Li X., Xu L.N., Wei D.B. Expression of the sperm-specific lactate dehydrogenase gene (Ldh-c) in plateau pika (Ochotona curzoniae) liver and its influence on the anaerobic glycolysis. Chin. J. Zool. 2015;6:846–854. (In Chinese) [PubMed] [Google Scholar]
- 54.Xu L.N., Wei L., Wang Y., Li X., Wei L.N., Wei D.B. The role of the sperm-specific lactate dehydrogenase (LDH-C4) in plateau pika brain. Acta Theriol. Sin. 2015;35:431–437. (In Chinese) [Google Scholar]