Abstract
Ultraviolet (UV) filters are used widely in cosmetics, plastics, adhesives and other industrial products to protect human skin or products against direct exposure to deleterious UV radiation. With growing usage and mis-disposition of UV filters, they currently represent a new class of contaminants of emerging concern with increasingly reported adverse effects to humans and other organisms. Exposure to UV filters induce various endocrine disrupting effects, as revealed by increasing number of toxicological studies performed in recent years. It is necessary to compile a systematic review on the current research status on endocrine disrupting effects of UV filters toward different organisms. We therefore summarized the recent advances on the evaluation of the potential endocrine disruptors and the mechanism of toxicity for many kinds of UV filters such as benzophenones, camphor derivatives and cinnamate derivatives.
Keywords: ultraviolet filters, cosmetics, endocrine disrupting effects, nuclear receptor
1. Introduction
Ultraviolet (UV) filters are a class of chemicals that can absorb or reflect UV light in the ultraviolet A (UVA) range and ultraviolet B (UVB) range with specific wavelengths between 320 and 400 nm, 290 and 320 nm, respectively [1]. They can protect human skin against direct exposure to deleterious UV radiation [2,3]. Many kinds of organic UV filters were incorporated into cosmetics, plastics, adhesives and other industrial products to avoid potential UV-induced damage. There are 16 UV filters permitted to be used in cosmetics by the US Food and Drug Administration and 27 components permitted to be used in cosmetics by the EU Scientific Committee on Consumer Products [4].
Many UV filters were produced in large quantities and used widely [5]. Residues of UV filters have been detected in multiple environmental matrices including wastewater treatment plants, surface water, sewage sludge, river sediments, fish, human milk and placenta [6,7,8,9]. UV filters can be bioaccumulated in organisms due to their persistence, stability and lipophilicity [10,11]. They are now becoming contaminants of emerging concern [12,13,14]. UV filters were reported to induce acute toxicities, developmental toxicities and reproductive toxicities to different organisms [15,16,17,18,19,20,21].
Many kinds of UV filters (Table 1) have been identified as potential environmental endocrine disruptors [22]. There are an increasing number of studies on endocrine disrupting effects of UV filters. Exposure to UV filters was increasingly reported to cause the disruption of the endocrine systems in many organisms such as rat, frog Xenopus laevis, Japanese quail (Coturnix japonica), Chironomus riparius (Meigen) and fish [22,23,24,25,26,27,28,29,30,31]. Considering increasing toxicological studies performed in recent years, a systematic compilation of current research status on their endocrine disrupting effects is necessary. We therefore compiled a state-of-the-art review on the endocrine disrupting effects of many type of UV filters (Table 2).
Table 1.
The commonly used ultraviolet (UV) filters.
| Compound | CAS No. | Chemical Structure | Kp (cm/h) * |
|---|---|---|---|
| BP-1 | 131-56-6 | 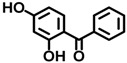 |
0.00917 |
| BP-2 | 131-55-5 | 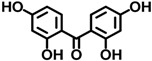 |
0.00458 |
| BP-3 | 131-57-7 | 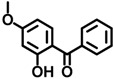 |
0.0271 |
| BP-4 | 4065-45-6 | 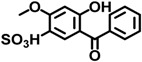 |
0.0000511 |
| 4-MBC | 36861-47-9 | 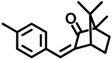 |
0.504 |
| 3-BC | 15087-24-8 | 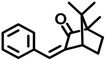 |
0.261 |
| OMC | 5466-77-3 | 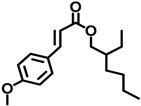 |
0.264 |
| IMC | 71617-10-2 | 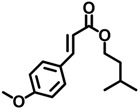 |
0.0477 |
| OC | 6197-30-4 | 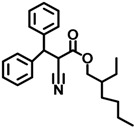 |
0.549 |
BP: benzophenone; 4-MBC: 4-methyl benzylidene camphor; 3-BC: 3-benzylidene camphor; OMC: octyl methoxycinnamate; IMC: isopentyl-4-methoxycinnamate; OC: octocrylene. * Kp, the dermal permeability coefficient, calculated by Program (DERMWIN) v2.0, was calculated following the equation: Log Kp = −2.80 + 0.66 Log Kow − 0.0056 MW.
Table 2.
Endocrine disrupting effects of the commonly used UV filters.
| UV Filters | Endocrine Disrupting Effects | References | |
|---|---|---|---|
| Benzophenones | Estrogenic disrupting effects | Activation of ERα, ERβ; Inhibition of the activity of 17β-Estradiol; Induction of proliferation of MCF-7 cell; Induction of VTG in fathead minnows; Reduce of the uterine weight in immature Long-Evans rats | [22,26,32,33,34,35,36,37] |
| Androgenic disrupting effects | Antagonists of human AR transactivation; Repression of 4,5-dihydrotestosterone-induced transactivational activity; Inhibition of testosterone formation in mice and rats | [34,36,37,38,39] | |
| Disrupting effects toward other nuclear receptors | Inhibition of human recombinant TPO; Interference with THR; Inhibition of TPO activity in rats; Antagonists of PR | [38,40,41,42] | |
| Camphor derivatives | Disrupting effects toward estrogen receptor | Activation of ERα, ERβ; Inhibition of the activity of 17β-Estradiol; Induction of proliferation of MCF-7 cell; Induction of pS2 protein in MCF-7 cells; Reduce of the uterine weight in rats; Induction of VTG in fish | [22,26,43,44,45,46,47,48,49] |
| Disrupting effects toward androgen receptor | Repression of 4,5-dihydrotestosterone-induced transactivational activity; Inhibition of testosterone formation in HEK-293 cells; Antagonists of Human AR | [36,38,39,50] | |
| Disrupting effects toward progesterone receptor | Antagonists of PR; Increase of PR mRNA levels in rats; Inhibition of the expression of PR protein in rats; Disturbance of the expression of membrane-associate PR in insects | [38,47,51,52] | |
| Cinnamate derivatives | Disrupting effects toward estrogen receptor | Activation of ERα; Inhibition of the activity of 17β-Estradiol; Induction of proliferation of MCF-7 cell; Reduce of the uterine weight in rats; Induction of VTG in fish | [22,36,43,45,48,49] |
| Disrupting effects toward thyroid hormone receptor | Decrease of T4 level; Inhibition of the conversion of T4 to triiodothyronine in rats | [16,53,54] | |
| Disrupting effects toward other nuclear receptors | Antagonists of PR and AR; Inhibition of 4,5-dihydrotestosterone activity; Reduce of the prostate and testicular weight in rats | [16,36,38] | |
AR: androgen receptor; ER: estrogen receptor alpha; PR: progesterone receptor; T4: thyroxine; THR: thyroid hormone receptor; TPO: thyroid peroxidase; VTG: vitellogenin.
2. Endocrine Disrupting Effects of Typical UV Filters
2.1. Benzophenones
Benzophenone (BP)-type UV filters were used widely in many cosmetics for the protection of skin from UVA and UVB light [12]. Their molecular structures have a diarylketone scaffold with different substitute groups. Residues of BPs were detected in wastewater, surface water, soil, sediment, human urine and breast milk [55,56,57,58]. Many BPs were identified as endocrine disruptors and were involved in the disruption of the hypothalamic–pituitary–gonadal system [13,59]. As revealed by various in vivo, in vitro bioassays and in silico methods, BPs showed multiple endocrine disrupting effects toward estrogen receptor (ER), androgen receptor (AR), progesterone receptor (PR) and other nuclear receptors [22,32,38,39,60].
2.1.1. Estrogenic Disrupting Effects
BP-type UV filters could cause multiple estrogenic effects, developmental and reproductive toxicity as revealed by cell-based bioassay [26,33,34,43,61]. BP-3 was reported to cause a dose-dependent increase of uterine weight of immature Long-Evans rats by the activation of ERα and ERβ [22,43]. BP-2 caused estrogenic effects such as vitellogenin (VTG) induction in fish [35]. BPs such as BP-1, BP-2, BP-3 and BP-4 caused estrogenic activity, developmental and reproductive toxicity to fish and rat [26,35,36,43]. BPs showed moderate potency to activate the proliferation of MCF-7 breast cancer cell and estrogen-responsive CHO cells at concentrations of the order of micromolar and lower [37,62]. This potency ranks as 4-hydroxy-benzophenone > 4,4′-dihydroxy-benzophenone > BP-8 > 2,3,4,4′-tetrahydroxy-benzophenone > BP-2 > 2,4, 4′-trihydroxy-benzophenone. However, there was no significant induction of proliferation induced by BP, BP-1, BP-3 and 2,3,4-trihydroxy-benzophenone. Molecular modeling can provide atomic-level information and has been well used to probe interactions of chemicals with biomacromolecules [39,63,64,65,66]. BPs interacted with residues Glu353, Arg394 or Phe404 of ERα ligand binding domain and such binding mode enhanced binding stability, contributing partly to their estrogenic activities [62].
Biotransformation or chemical transformation of BPs may have influence on their endocrine disrupting effects. BP-3 can be metabolized within human body and can be metabolized to various metabolites including BP-1 and BP-3. BP-1 was detected in human urine [37] and BP-1 and BP-8 were detected in rats [67,68]. BP-1 possessed higher estrogenic activity than that of BP-3 [32,33,34]. BPs were revealed to be converted to 4-hydroxybenzophenone after exposure to sunlight, indicating the potential estrogenic risk of BP-containing sunscreen in direct contact with the skin [62,69].
2.1.2. Androgenic Disrupting Effects
BP-1, BP-2 and BP-3 showed no agonistic activity toward AR [50]; however, they exhibited anti-androgenic activity in various cells-based bioassays [33,34,36,38]. BP-1, BP-2 and BP-3 showed complete inhibition of dihydrotestosterone activity in concentration-dependent mode. BPs disturbed the normal hormonal level of testosterone during male development of mouse and rat by inhibiting the conversion of androstenedione to testosterone [39,70]. BP-2 displayed antagonistic activity with non-monotonic dose–response curves. There were very weak or no inhibitory effects for BP-4, BP-7, BP-8 and BP-12 and weak effects for BP-2, BP-3 and BP-6 [39]. The androgenic disrupting effects of BPs were also affected by the biotransformation of BPs. BP-3 showed decreased androgenic activity after the metabolism mediated by rat and human liver microsomes [37]. BP-1 was the most potent anti-androgenic UV filter and concentration dependently inhibited 17β-HSD3.
2.1.3. Disrupting Effects toward Other Nuclear Receptors
BPs also exhibited disrupting effects towards PR and thyroid hormone receptor (THR). BP-3 exhibited antagonistic effects to PR and BP-2 interferes with the thyroid hormone (TH) axis in rats [38,40]. Although BP-3 did not activate PR, it is the antagonist of PR as revealed by PR CALUX1 bioassay [38]. BPs can also affect the TH axis by inhibiting the activity of thyroid peroxidase (TPO) or inactivate it, disturbing the biosynthesis of TH [71]. BP-2 was demonstrated as a very potent inhibitor of TPO activity [41]. The study revealed that BP-2-treated rats exhibited decreased thyroxine (T4) and increased thyroid-stimulating hormone (TSH) serum levels. BP-2 disturbed TH homeostasis by inhibiting or inactivating TPO as revealed by the stably transfected human recombinant TPO [40]. As evaluated by an in vitro reporter system containing a duplicated thyroid hormone response element of the HLA-DR4 serotype, BP-2 and BP-3 can induce luciferase activity, showing agonistic activity toward THR [42].
2.2. Camphor Derivatives
Camphor derivatives are highly effective UVB-absorbers incorporated in many kinds of cosmetics. These chemicals have high bioconcentration factors and can be bioaccumulated in tissues of organisms after prolonged exposure [72,73]. The camphor derivatives such as 4-methylbenzylidene camphor (4-MBC) and 3-benzylidene camphor (3-BC) are very lipophilic and can be easily absorbed after direct contact with the skin. 4-MBC and 3-BC were revealed as potential endocrine disruptors, adversely affecting the reproduction and development of many organisms [74,75].
2.2.1. Disrupting Effects toward Estrogen Receptor
4-MBC and 3-BC showed anti-estrogenic activity in fish, mammals and cell-based bioassays [22,44,76]. Exposure to 3-BC during the early development and postnatal life of rat, could lead to significant changes of the expression of ERs and estrogen target genes [77]. 4-MBC and 3-BC also showed specific estrogenic activity in the HELN ERα cell and MCF-7 cell line proliferation assay [22,45]. 3-BC exhibited estrogenic potency in immature rats [76] and also showed high estrogenic potency of inducing VTG in juvenile fathead minnow [26]. 4-MBC and 3-BC negatively affected the sex ratio of frog Xenopus laevis at environmental concentrations [23]. They were selective ER ligands [46] and bound preferentially to ERβ [76]. 4-MBC activated ERα weakly and showed higher potency toward ERβ mediated transactivation in Ishikawa cells [47]. 4-MBC could activate human ERα in concentration-dependent mode and also significantly activate transcription through human ERβ [43]. 4-MBC caused an increase of mRNA expression level of ERα and VTG in male Japanese medaka [48].
2.2.2. Disrupting Effects toward Androgen Receptor
4-MBC and 3-BC showed no agonistic activation toward AR [50]. They exhibited anti-androgenic activity toward AR in AR CALUX® cell line as revealed by the transcriptional-activation assay [38,50]. They could inhibit the activity of AR in a concentration-dependent mode as revealed by the recombinant yeast assays [36]. 4-MBC was proved to be a potent human AR antagonist and significantly inhibited luciferase activity [78]. 4-MBC and 3-BC also concentration-dependently prevented testosterone formation by inhibiting androgen-metabolizing 17β-HSD3 in HEK-293 cells [39].
2.2.3. Disrupting Effects toward Progesterone Receptor
The developmental 4-MBC exposure could cause an increase of PR mRNA levels in male medial preoptic area, but this change was not detected in female rat [52]. 4-MBC at very low dose can down-regulate the expression level of PR protein. With the increasing doses, the expression of PR protein returned to normal or slightly supranormal levels [77]. 4-MBC disturbed the expression of membrane-associate PR, measured by changes in mRNA levels at different developmental stages [51]. 4-MBC and 3-BC showed no PR transactivation in U2-OS cells, and these two UV filters at low concentrations were antagonists of PR [38].
2.3. Cinnamate Derivatives
Cinnamate derivatives are the most frequently used cosmetic UV filters with the high efficiency to absorb UVA or UVB light. Their molecular structures have a special unsaturated bond between the aromatic ring and the carboxyl group, allowing the molecule to better absorb the 305 nm wavelength UV [79]. Their residues were detected in wastewater, surface water, sewage sludge, fish and marine mammals [11,80,81]. Octyl methoxycinnamate (OMC) is one of the most commonly-used UV filters. It was listed as one of 27 UV filters approved for use in cosmetics formulations in the EU and US [82]. OMC has disrupting activities toward ER, AR, PR and THR as reported by multiple in vitro and in vivo studies [16,38,78].
2.3.1. Disrupting Effects toward Estrogen Receptor
The potential estrogenic activities of cinnamate derivatives have been reported by various in vivo and in vitro experiments [16,22,43]. OMC, isopentyl-4-methoxycinnamate (IMC) and octocrylene (OC) could completely inhibit the activity of E2 by yeast human assays [36]. OMC was found to moderately activate ERα but no obvious effect on ERβ by using reporter cell lines including HELN, HELN ERα, and HELN ERβ [45] was observed, in line with the transactivation bioassay using HEK293 cells in which OMC dose-dependently activated ERα, but did not activate transcription of ERβ [43]. Exposure to OMC induced weak estrogenic effect to the uterus and the vagina of female Sprague–Dawley rats [49]. OMC could cause an increase of plasma concentration of VTG and up-regulate the mRNA expression levels of ERα in medaka fish [48].
2.3.2. Disrupting Effects toward Thyroid Hormone Receptor
Cinnamate derivatives also interfered with the TH axis in rats. The perinatal OMC-exposure could induce adverse effects on the reproductive and neurological development of rat offspring [16,53]. The treatment with OMC for 12 weeks caused a decrease of T4 level in the blood of ovariectomised female rats and inhibited the activity of 5′-deiodinase that converts T4 to T3 in the liver [53]. Exposure to OMC caused a dose-dependent decrease of serum concentrations of TSH, T4 or T3 in rats [54]. TPO activity was unaltered but T3-responsive hepatic type I 5′ deiodinase activity was reduced by OMC. OMC was found exerting effects on the TH axis by using female ovariectomized rats [83]. OMC affected TH via inhibition of type I 5′-Deiodinase activity and gene expression.
2.3.3. Disrupting Effects toward Other Nuclear Receptors
Cinnamate derivatives also induced disrupting effects toward other NRs such as PR and AR. OMC, IMC and OC showed obvious antagonistic effects toward PR and AR as revealed by cell-based bioassays and in vivo experiments using rats [16,38]. OMC showed antagonistic activity toward PR as determined by sensitive and specific reporter gene cell lines [38]. OMC, IMC and OC showed anti-androgenic activities as revealed by the yeast assay [36]. They inhibited 4,5-dihydrotestosterone activity in concentration-dependent mode.
3. Perspectives
With the continuous demand of cosmetics, plastics and various industrial products containing UV filters, the production and application of UV filters, especially new type of UV filters will be increased. For better risk assessment of these chemicals and their metabolites, the investigation of endocrine disrupting effects caused by direct and indirect exposure to UV filters is currently becoming a research hotspot. Adverse outcome pathways of emerging UV filters should be well characterized for their potential disruption of the hypothalamus-pituitary-gonadal endocrine axis. Although multiple in vivo and in vitro studies have investigated the adverse effects of UV filters, the underlying mechanism of endocrine disruption should be further explored at the atomic level. Considering the versatile role of computational toxicology for the study of physiochemical properties of organic contaminants and their interactions with various biomacromolecules [39,63,84,85], more in silico studies should be performed, primarily for UV filters to probe the molecular initiating event toward target receptors.
4. Conclusions
We reviewed the potential endocrine disruptors of typical UV filters including benzophenones, camphor derivatives and cinnamate derivatives. These UV filters are generally involved in the disruption of the hypothalamic–pituitary–gonadal system. As revealed by in vivo and in vitro assays, exposure to these chemicals induced various endocrine disrupting effects such as estrogenic disrupting effects, androgenic disrupting effects as well as the disrupting effects towards TR, PR. The underlying mechanism of endocrine disruption was summarized (Table 2). The minor structural changes of these kinds of UV filters have influence on the potency of their endocrine disrupting effects.
Acknowledgments
This project was sponsored by the Major Science and Technology Projects of Zhejiang Province (2014C03026), the National Natural Science Foundation of China (No. 21477113, 21277122), the Guangzhou Key Laboratory of Environmental Exposure and Health (No. GZKLEEH201605) and the Fundamental Research Funds for the Central Universities.
Author Contributions
Jiaying Wang, Min Guo and Shulin Zhuang designed the review; Jiaying Wang, Liping Lu, Shenggan Wu. Yiwen Xu and Yanye Zhu compiled the references; Jiaying Wang, Liumeng Pan and Shulin Zhuang wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Morabito K., Shapley N.C., Steeley K.G., Tripathi A. Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection. Int. J. Cosmet. Sci. 2011;33:385–390. doi: 10.1111/j.1468-2494.2011.00654.x. [DOI] [PubMed] [Google Scholar]
- 2.Diffey B.L. Sources and measurement of ultraviolet radiation. Methods. 2002;28:4–13. doi: 10.1016/S1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- 3.Marie C., Cabut S., Vendittelli F., Sauvant-Rochat M.P. Changes in cosmetics use during pregnancy and risk perception by women. Int. J. Environ. Res. Public Health. 2016;13:383. doi: 10.3390/ijerph13040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahie S., Lloyd J.J., Farr P.M. Sunscreen ingredients and labelling: A survey of products available in the UK. Clin. Exp. Dermatol. 2007;32:359–364. doi: 10.1111/j.1365-2230.2007.02404.x. [DOI] [PubMed] [Google Scholar]
- 5.Díaz-Cruz M.S., Barceló D. Chemical analysis and ecotoxicological effects of organic UV-absorbing compounds in aquatic ecosystems. TrAC Trends Anal. Chem. 2009;28:708–717. doi: 10.1016/j.trac.2009.03.010. [DOI] [Google Scholar]
- 6.Balmer M.E., Buser H.R., Müller M.D., Poiger T. Agroscope. Swiss Federal Research Station for Horticulture, Plant Protecting Chemistry; Wädenswil, Switzerland: 2004. Occurrence of the organic UV-filter compounds BP-3,4-MBC, EHMC, and OC in wastewater, surface waters, and in fish from Swiss lakes. [Google Scholar]
- 7.Zhang N.S., Liu Y.S., Van den Brink P.J., Price O.R., Ying G.G. Ecological risks of home and personal care products in the riverine environment of a rural region in South China without domestic wastewater treatment facilities. Ecotoxicol. Environ. Saf. 2015;122:417–425. doi: 10.1016/j.ecoenv.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Schlumpf M., Kypke K., Wittassek M., Angerer J., Mascher H., Mascher D., Vokt C., Birchler M., Lichtensteiger W. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: Correlation of UV filters with use of cosmetics. Chemosphere. 2010;81:1171–1183. doi: 10.1016/j.chemosphere.2010.09.079. [DOI] [PubMed] [Google Scholar]
- 9.Valle-Sistac J., Molins-Delgado D., Díaz M., Ibáñez L., Barceló D., Silvia Díaz-Cruz M. Determination of parabens and benzophenone-type UV filters in human placenta. First description of the existence of benzyl paraben and benzophenone-4. Environ. Int. 2016;88:243–249. doi: 10.1016/j.envint.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Groz M.P., Bueno M.M., Rosain D., Fenet H., Casellas C., Pereira C., Maria V., Bebianno M.J., Gomez E. Detection of emerging contaminants (UV filters, UV stabilizers and musks) in marine mussels from Portuguese coast by QuEChERS extraction and GC–MS/MS. Sci. Total Environ. 2014;493:162–169. doi: 10.1016/j.scitotenv.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 11.Gago-Ferrero P., Alonso M.B., Bertozzi C.P., Marigo J., Barbosa L., Cremer M., Secchi E.R., Azevedo A., Lailson-Brito J., Jr., Torres J.P.M., et al. First determination of UV Filters in marine mammals. Octocrylene levels in Franciscana dolphins. Environ. Sci. Technol. 2013;47:5619–5625. doi: 10.1021/es400675y. [DOI] [PubMed] [Google Scholar]
- 12.Kunisue T., Chen Z., Buck Louis G.M., Sundaram R., Hediger M.L., Sun L., Kannan K. Urinary concentrations of benzophenone-type UV Filters in U.S. women and their association with endometriosis. Environ. Sci. Technol. 2012;46:4624–4632. doi: 10.1021/es204415a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause M., Klit A., Jensen M.B., Søeborg T., Frederiksen H., Schlumpf M., Lichtensteiger W., Skakkebaek N.E., Drzewiecki K.T. Sunscreens: Are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int. J. Androl. 2012;35:424–436. doi: 10.1111/j.1365-2605.2012.01280.x. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert E., Pirot F., Bertholle V., Roussel L., Falson F., Padois K. Commonly used UV filter toxicity on biological functions: Review of last decade studies. Int. J. Cosmet. Sci. 2013;35:208–219. doi: 10.1111/ics.12030. [DOI] [PubMed] [Google Scholar]
- 15.French J.E. NTP technical report on the toxicity studies of 2-Hydroxy-4-methoxybenzophenone (CAS No. 131-57-7) Administered topically and in dosed feed to F344/N rats and B6C3F1 mice. Toxic. Rep. Ser. 1992;21:1-E14. [PubMed] [Google Scholar]
- 16.Axelstad M., Boberg J., Hougaard K.S., Christiansen S., Jacobsen P.R., Mandrup K.R., Nellemann C., Lund S.P., Hass U. Effects of pre- and postnatal exposure to the UV-filter octyl methoxycinnamate (OMC) on the reproductive, auditory and neurological development of rat offspring. Toxicol. Appl. Pharmacol. 2011;250:278–290. doi: 10.1016/j.taap.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Faass O., Schlumpf M., Reolon S., Henseler M., Maerkel K., Durrer S., Lichtensteiger W. Female sexual behavior, estrous cycle and gene expression in sexually dimorphic brain regions after pre- and postnatal exposure to endocrine active UV filters. Neurotoxicology. 2009;30:249–260. doi: 10.1016/j.neuro.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Kinnberg K.L., Petersen G.I., Albrektsen M., Minghlani M., Awad S.M., Holbech B.F., Green J.W., Bjerregaard P., Holbech H. Endocrine-disrupting effect of the ultraviolet filter benzophenone-3 in zebrafish, Danio rerio. Environ. Toxicol. Chem. 2015;34:2833–2840. doi: 10.1002/etc.3129. [DOI] [PubMed] [Google Scholar]
- 19.Paredes E., Perez S., Rodil R., Quintana J.B., Beiras R. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44–50. doi: 10.1016/j.chemosphere.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 20.Molins-Delgado D., Gago-Ferrero P., Díaz-Cruz M.S., Barceló D. Single and joint ecotoxicity data estimation of organic UV filters and nanomaterials toward selected aquatic organisms. Urban groundwater risk assessment. Environ. Res. 2016;145:126–134. doi: 10.1016/j.envres.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Hui L., Ping S., Liu H., Yang S., Wang L., Wang Z. Acute toxicity of benzophenone-type UV filters for Photobacterium phosphoreum and Daphnia magna: QSAR analysis, interspecies relationship and integrated assessment. Chemosphere. 2015;135:182–188. doi: 10.1016/j.chemosphere.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Schlumpf M., Cotton B., Conscience M., Haller V., Steinmann B., Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001;109:239–244. doi: 10.1289/ehp.01109239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunz P.Y., Galicia H.F., Fent K. Assessment of hormonal activity of UV filters in tadpoles of frog Xenopus laevis at environmental concentrations. Mar. Environ. Res. 2004;58:431–435. doi: 10.1016/j.marenvres.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Axelsson J. Master’s Thesis. Uppsala University; Uppsala, Sweden: 2008. Differentiation of Brain and Reproductive Organs in Birds: Effects of Environmental Contaminants. [Google Scholar]
- 25.Ozáez I., Morcillo G., Martínez-Guitarte J.L. Ultraviolet filters differentially impact the expression of key endocrine and stress genes in embryos and larvae of Chironomus riparius. Sci. Total Environ. 2016;557–558:240–247. doi: 10.1016/j.scitotenv.2016.03.078. [DOI] [PubMed] [Google Scholar]
- 26.Kunz P.Y., Galicia H.F., Fent K. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol. Sci. 2006;90:349–361. doi: 10.1093/toxsci/kfj082. [DOI] [PubMed] [Google Scholar]
- 27.Coronado M., de Haro H., Deng X., Rempel M.A., Lavado R., Schlenk D. Estrogenic activity and reproductive effects of the UV-filter oxybenzone (2-hydroxy-4-methoxyphenyl-methanone) in fish. Aquat. Toxicol. 2008;90:182–187. doi: 10.1016/j.aquatox.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser D., Sieratowicz A., Zielke H., Oetken M., Hollert H., Oehlmann J. Ecotoxicological effect characterisation of widely used organic UV filters. Environ. Pollut. 2012;163:84–90. doi: 10.1016/j.envpol.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Maerkel K., Durrer S., Henseler M., Schlumpf M., Lichtensteiger W. Sexually dimorphic gene regulation in brain as a target for endocrine disrupters: Developmental exposure of rats to 4-methylbenzylidene camphor. Toxicol. Appl. Pharmacol. 2007;218:152–165. doi: 10.1016/j.taap.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Balázs A., Krifaton C., Orosz I., Szoboszlay S., Kovács R., Csenki Z., Urbányi B., Kriszt B. Hormonal activity, cytotoxicity and developmental toxicity of UV filters. Ecotoxicol. Environ. Saf. 2016;131:45–53. doi: 10.1016/j.ecoenv.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 31.Soto L. Evaluation of the estrogenic effects of UV filters on the sergeant major damselfish, Abudefduf saxatilis. Cienc. Mar. 2014;49:187–196. doi: 10.7773/cm.v40i3.2390. [DOI] [Google Scholar]
- 32.Morohoshi K., Yamamoto H., Kamata R., Shiraishi F., Koda T., Morita M. Estrogenic activity of 37 components of commercial sunscreen lotions evaluated by in vitro assays. Toxicol. in Vitro. 2005;19:457–469. doi: 10.1016/j.tiv.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T., Kitamura S., Khota R., Sugihara K., Fujimoto N., Ohta S. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol. Appl. Pharmacol. 2005;203:9–17. doi: 10.1016/j.taap.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Molina-Molina J.M., Escande A., Pillon A., Gomez E., Pakdel F., Cavailles V., Olea N., Ait-Aissa S., Balaguer P. Profiling of benzophenone derivatives using fish and human estrogen receptor-specific in vitro bioassays. Toxicol. Appl. Pharmacol. 2008;232:384–395. doi: 10.1016/j.taap.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Weisbrod C.J., Kunz P.Y., Zenker A.K., Fent K. Effects of the UV filter benzophenone-2 on reproduction in fish. Toxicol. Appl. Pharmacol. 2007;225:255–266. doi: 10.1016/j.taap.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Kunz P.Y., Fent K. Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat. Toxicol. 2006;79:305–324. doi: 10.1016/j.aquatox.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe Y., Kojima H., Takeuchi S., Uramaru N., Sanoh S., Sugihara K., Kitamura S., Ohta S. Metabolism of UV-filter benzophenone-3 by rat and human liver microsomes and its effect on endocrine-disrupting activity. Toxicol. Appl. Pharmacol. 2015;282:119–128. doi: 10.1016/j.taap.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Schreurs R.H.M.M., Sonneveld E., Jansen J.H.J., Seinen W., Burg B.V.D. Interaction of polycyclic musks and UV Filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays. Toxicol. Sci. 2004;83:264–272. doi: 10.1093/toxsci/kfi035. [DOI] [PubMed] [Google Scholar]
- 39.Nashev L.G., Schuster D., Laggner C., Sodha S., Langer T., Wolber G., Odermatt A. The UV-filter benzophenone-1 inhibits 17β-hydroxysteroid dehydrogenase type 3: Virtual screening as a strategy to identify potential endocrine disrupting chemicals. Biochem. Pharmacol. 2010;79:1189–1199. doi: 10.1016/j.bcp.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Schmutzler C., Bacinski A., Gotthardt I., Huhne K., Ambrugger P., Klammer H., Schlecht C., Hoang-Vu C., Grüters A., Wuttke W., et al. The ultraviolet filter benzophenone 2 interferes with the thyroid hormone axis in rats and is a potent in vitro Inhibitor of human recombinant thyroid peroxidase. Endocrinology. 2007;148:2835–2844. doi: 10.1210/en.2006-1280. [DOI] [PubMed] [Google Scholar]
- 41.Jarry H., Christoffel J., Rimoldi G., Koch L., Wuttke W. Multi-organic endocrine disrupting activity of the UV screen benzophenone 2 (BP2) in ovariectomized adult rats after 5 days treatment. Toxicology. 2004;205:87–93. doi: 10.1016/j.tox.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 42.Schmutzler C., Gotthardt I., Hofmann P.J., Radovic B., Kovacs G., Stemmler L., Nobis I., Bacinski A., Mentrup B., Ambrugger P., et al. Endocrine disruptors and the thyroid gland—A combined in vitro and in vivo analysis of potential new biomarkers. Environ. Health Perspect. 2007;115:77–83. doi: 10.1289/ehp.9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreurs R., Lanser P., Seinen W., Van der Burg B. Estrogenic activity of UV filters determined by an in vitro reporter gene assay and an in vivo transgenic zebrafish assay. Arch. Toxicol. 2002;76:257–261. doi: 10.1007/s00204-002-0348-4. [DOI] [PubMed] [Google Scholar]
- 44.Tinwell H., Lefevre P.A., Moffat G.J., Burns A., Odum J., Spurway T.D., Orphanides G., Ashby J. Confirmation of uterotrophic activity of 3-(4-methylbenzylidine)camphor in the immature rat. Environ. Health. Perspect. 2002;110:533–536. doi: 10.1289/ehp.02110533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez E., Pillon A., Fenet H., Rosain D., Duchesne M.J., Nicolas J.C., Balaguer P., Casellas C. Estrogenic activity of cosmetic components in reporter cell lines: Parabens, UV Screens, and musks. J. Toxicol. Environ. Health A. 2005;68:239–251. doi: 10.1080/15287390590895054. [DOI] [PubMed] [Google Scholar]
- 46.Holbech H., Norum U., Korsgaard B., Poul B. The chemical UV-filter 3-benzylidene camphor causes an oestrogenic effect in an in vivo fish assay. Pharmacol. Toxicol. 2002;91:204–208. doi: 10.1034/j.1600-0773.2002.t01-3-910403.x. [DOI] [PubMed] [Google Scholar]
- 47.Mueller S.O., Kling M., Firzani P.A., Mecky A., Duranti E., Shields-Botella J., Delansorne R., Broschard T., Kramer P.J. Activation of estrogen receptor α and ERβ by 4-methylbenzylidene-camphor in human and rat cells: Comparison with phyto- and xenoestrogens. Toxicol. Lett. 2003;142:89–101. doi: 10.1016/S0378-4274(03)00016-X. [DOI] [PubMed] [Google Scholar]
- 48.Inui M., Adachi T., Takenaka S., Inui H., Nakazawa M., Ueda M., Watanabe H., Mori C., Lguchi T., Miyatake K. Effect of UV screens and preservatives on vitellogenin and choriogenin production in male medaka (Oryzias latipes) Toxicology. 2003;194:43–50. doi: 10.1016/S0300-483X(03)00340-8. [DOI] [PubMed] [Google Scholar]
- 49.Seidlovawuttke D., Jarry H., Christoffel J., Rimoldi G., Wuttke W. Comparison of effects of estradiol (E2) with those of octylmethoxycinnamate (OMC) and 4-methylbenzylidene camphor (4MBC)—2 filters of UV light—On several uterine, vaginal and bone parameters. Toxicol. Appl. Pharmacol. 2006;210:246–254. doi: 10.1016/j.taap.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Ma R., Cotton B., Lichtensteiger W., Schlumpf M. UV Filters with antagonistic action at androgen receptors in the MDA-kb2 Cell transcriptional-activation assay. Toxicol. Sci. 2003;74:43–50. doi: 10.1093/toxsci/kfg102. [DOI] [PubMed] [Google Scholar]
- 51.Ozáez I., Aquilino M., Morcillo G., Martínez-Guitarte J.L. UV filters induce transcriptional changes of different hormonal receptors in Chironomus riparius embryos and larvae. Environ. Pollut. 2016;214:239–247. doi: 10.1016/j.envpol.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 52.Maerkel K., Lichtensteiger W., Durrer S., Conscience M., Schlumpf M. Sex- and region-specific alterations of progesterone receptor mRNA levels and estrogen sensitivity in rat brain following developmental exposure to the estrogenic UV filter 4-methylbenzylidene camphor. Environ. Toxicol. Pharmacol. 2005;19:761–765. doi: 10.1016/j.etap.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 53.Schmutzler C., Hamann I., Hofmann P.J., Kovacs G., Stemmler L., Mentrup B., Schomburg L., Ambrugger P., Grüters A., Seidlova-Wuttke D., et al. Endocrine active compounds affect thyrotropin and thyroid hormone levels in serum as well as endpoints of thyroid hormone action in liver, heart and kidney. Toxicology. 2004;205:95–102. doi: 10.1016/j.tox.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 54.Klammer H., Schlecht C., Wuttke W., Schmutzler C., Gotthardt I., Kohrle J., Jarry H. Effects of a 5-day treatment with the UV-filter octyl-methoxycinnamate (OMC) on the function of the hypothalamo-pituitary–thyroid function in rats. Toxicology. 2007;238:192–199. doi: 10.1016/j.tox.2007.06.088. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z., Ren N., Li Y.F., Kunisue T., Gao D., Kannan K. Determination of benzotriazole and benzophenone UV Filters in sediment and sewage sludge. Environ. Sci. Technol. 2011;45:3909–3916. doi: 10.1021/es2004057. [DOI] [PubMed] [Google Scholar]
- 56.Tsui M.M.P., Leung H.W., Wai T.C., Yamashita N., Taniyasu S., Liu W., Lam P.K.S., Murphy M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014;67:55–65. doi: 10.1016/j.watres.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Wang L., Kannan K. Characteristic profiles of benzonphenone-3 and its derivatives in urine of children and adults from the United States and China. Environ. Sci. Technol. 2013;47:12532–12538. doi: 10.1021/es4032908. [DOI] [PubMed] [Google Scholar]
- 58.Loraine G.A., Pettigrove M.E. Seasonal variations in concentrations of pharmaceuticals and personal care products in drinking water and reclaimed wastewater in Southern California. Environ. Sci. Technol. 2006;40:687–695. doi: 10.1021/es051380x. [DOI] [PubMed] [Google Scholar]
- 59.Schlumpf M., Schmid P., Durrer S., Conscience M., Maerkel K., Henseler M., Gruetter M., Herzog I., Reolon S., Ceccatelli R., et al. Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology. 2004;205:113–122. doi: 10.1016/j.tox.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 60.Ozáez I., Martínez-Guitarte J.L., Morcillo G. Effects of in vivo exposure to UV filters (4-MBC, OMC, BP-3, 4-HB, OC, OD-PABA) on endocrine signaling genes in the insect Chironomus riparius. Sci. Total Environ. 2013;456–457:120–126. doi: 10.1016/j.scitotenv.2013.03.081. [DOI] [PubMed] [Google Scholar]
- 61.Rodríguez-Fuentes G., Sandoval-Gío J.J., Arroyo-Silva A., Noreña-Barroso E., Escalante-Herrera K.S., Olvera-Espinosa F. Evaluation of the estrogenic and oxidative stress effects of the UV filter 3-benzophenone in zebrafish (Danio rerio) eleuthero-embryos. Ecotoxicol. Environ. Saf. 2015;115:14–18. doi: 10.1016/j.ecoenv.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 62.Kerdivel G., Le Guevel R., Habauzit D., Brion F., Ait-Aissa S., Pakdel F. Estrogenic potency of benzophenone UV filters in breast cancer cells: Proliferative and transcriptional activity substantiated by docking analysis. PLoS ONE. 2013;8:782. doi: 10.1371/journal.pone.0060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Correa B.A., Goncalves A.S., de Souza A.M., Freitas C.A., Cabral L.M., Albuquerque M.G., Castro H.C., dos Santos E.P., Rodrigues C.R. Molecular modeling studies of the structural, electronic, and UV absorption properties of benzophenone derivatives. J. Phys. Chem. A. 2012;116:10927–10933. doi: 10.1021/jp306130y. [DOI] [PubMed] [Google Scholar]
- 64.Zhuang S., Zhang C., Liu W. Atomic insights into distinct hormonal activities of bisphenol A analogues toward PPARγ and ERα receptors. Chem. Res. Toxicol. 2014;27:1769–1779. doi: 10.1021/tx500232b. [DOI] [PubMed] [Google Scholar]
- 65.Zhang F., Zhang J., Tong C., Chen Y., Zhuang S., Liu W. Molecular interactions of benzophenone UV filters with human serum albumin revealed by spectroscopic techniques and molecular modeling. J. Hazard. Mater. 2013;263:618–626. doi: 10.1016/j.jhazmat.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 66.Zhuang S., Zhang J., Wen Y., Zhang C., Liu W. Distinct mechanisms of endocrine disruption of DDT-related pesticides toward estrogen receptor α and estrogen-related receptor γ. Environ. Toxicol. Chem. 2012;31:2597–2605. doi: 10.1002/etc.1986. [DOI] [PubMed] [Google Scholar]
- 67.Jeon H.K., Sarma S.N., Kim Y.J., Ryu J.C. Toxicokinetics and metabolisms of benzophenone-type UV filters in rats. Toxicology. 2008;248:89–95. doi: 10.1016/j.tox.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Okereke C.S., Abdel-Rhaman M.S., Friedman M.A. Disposition of benzophenone-3 after dermal administration in male rats. Toxicol. Lett. 1994;73:113–122. doi: 10.1016/0378-4274(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi T., Okamoto Y., Ueda K., Kojima N. Formation of estrogenic products from benzophenone after exposure to sunlight. Toxicol. Lett. 2006;167:1–7. doi: 10.1016/j.toxlet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Geissler W.M., Davis D.L., Wu L., Bradshaw K.D., Patel S., Mendonca B.B., Elliston K.O., Wilson J.D., Russell D.W., Andersson S. Male pseudohermaphroditism caused by mutations of testicular 17 beta-hydroxysteroid dehydrogenase 3. Nat. Genet. 1994;7:34–39. doi: 10.1038/ng0594-34. [DOI] [PubMed] [Google Scholar]
- 71.Taurog A., Dorris M.L., Doerge D.R. Mechanism of simultaneous iodination and coupling catalyzed by thyroid peroxidase. Arch. Biochem. Biophys. 1996;330:24–32. doi: 10.1006/abbi.1996.0222. [DOI] [PubMed] [Google Scholar]
- 72.Søeborg T., Ganderup N.C., Kristensen J.H., Bjerregaard P., Pedersen K.L., Bollen P., Hansen S.H., Halling-Sørensen B. Distribution of the UV filter 3-benzylidene camphor in rat following topical application. J. Chromatogr. B. 2006;834:117–121. doi: 10.1016/j.jchromb.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 73.Buser H.R., Balmer M.E., Schmid P., Kohler M. Occurrence of UV filters 4-methylbenzylidene camphor and octocrylene in fish from various Swiss rivers with inputs from wastewater treatment plants. Environ. Sci. Technol. 2006;40:1427–1431. doi: 10.1021/es052088s. [DOI] [PubMed] [Google Scholar]
- 74.Schlumpf M., Kypke K., Vökt C.C., Birchler M., Durrer S., Faass O., Ehnes C., Fuetsch M., Gaille C., Henseler M. Endocrine active UV filters: Developmental toxicity and exposure through breast milk. Chimia. 2008;62:345–351. doi: 10.2533/chimia.2008.345. [DOI] [Google Scholar]
- 75.Hofkamp L., Bradley S., Tresguerres J., Lichtensteiger W., Schlumpf M., Timms B. Region-specific growth effects in the developing rat prostate following fetal exposure to estrogenic ultraviolet filters. Environ. Health. Perspect. 2008;116:867–872. doi: 10.1289/ehp.10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlumpf M., Jarry H., Wuttke W., Ma R., Lichtensteiger W. Estrogenic activity and estrogen receptor beta binding of the UV filter 3-benzylidene camphor. Comparison with 4-methylbenzylidene camphor. Toxicology. 2004;199:109–120. doi: 10.1016/j.tox.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 77.Durrer S., Maerkel K., Schlumpf M., Lichtensteiger W. Estrogen target gene regulation and coactivator expression in rat uterus after developmental exposure to the ultraviolet filter 4-methylbenzylidene camphor. Endocrinology. 2005;146:2130–2139. doi: 10.1210/en.2004-1272. [DOI] [PubMed] [Google Scholar]
- 78.Jiménez-Díaz I., Molina-Molina J.M., Zafra-Gómez A., Ballesteros O., Navalón A., Real M., Sáenz J.M., Fernández M.F., Olea N. Simultaneous determination of the UV-filters benzyl salicylate, phenyl salicylate, octyl salicylate, homosalate, 3-(4-methylbenzylidene) camphor and 3-benzylidene camphor in human placental tissue by LC–MS/MS. Assessment of their in vitro endocrine activity. J. Chromatogr. B. 2013;936:80–87. doi: 10.1016/j.jchromb.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Giokas D.L., Salvador A., Chisvert A. UV filters: From sunscreens to human body and the environment. TrAC Trends Anal. Chem. 2007;26:360–374. doi: 10.1016/j.trac.2007.02.012. [DOI] [Google Scholar]
- 80.Balmer M.E., Buser H.R., Müller M.D., Poiger T. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss lakes. Environ. Sci. Technol. 2005;39:953–962. doi: 10.1021/es040055r. [DOI] [PubMed] [Google Scholar]
- 81.Kupper T., Plagellat C., Brandli R.C., de Alencastro L.F., Grandjean D., Tarradellas J. Fate and removal of polycyclic musks, UV filters and biocides during wastewater treatment. Water Res. 2006;40:2603–2612. doi: 10.1016/j.watres.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 82.Janjua N.R., Kongshoj B., Andersson A.M., Wulf H.C. Sunscreens in human plasma and urine after repeated whole-body topical application. J. Eur. Acad. Dermatol. Venereol. 2008;22:456–461. doi: 10.1111/j.1468-3083.2007.02492.x. [DOI] [PubMed] [Google Scholar]
- 83.Hamann I., Hofmann P., Schmutzler C., Mentrup B., Huhne K., Jarry H., Seidlová-Wuttke D., Wuttke W., KöHrle J. 4MBC and OMC, components of UV-sunscreens, exert organ specific alterations on type I 5′-Deiodinase activity and expression in female rats. Exp. Clin. Endocrinol. Diabetes. 2005:113–138. doi: 10.1055/s-2005-862997. [DOI] [Google Scholar]
- 84.Zhuang S., Wang H., Ding K., Wang J., Pan L., Lu Y., Liu Q., Zhang C. Interactions of benzotriazole UV stabilizers with human serum albumin: Atomic insights revealed by biosensors, spectroscopies and molecular dynamics simulations. Chemosphere. 2016;144:1050–1059. doi: 10.1016/j.chemosphere.2015.09.085. [DOI] [PubMed] [Google Scholar]
- 85.Ding K., Zhang H., Wang H., Lv X., Pan L., Zhang W., Zhuang S. Atomic-scale investigation of the interactions between tetrabromobisphenol A, tetrabromobisphenol S and bovine trypsin by spectroscopies and molecular dynamics simulations. J. Hazard. Mater. 2015;299:486–494. doi: 10.1016/j.jhazmat.2015.07.050. [DOI] [PubMed] [Google Scholar]


