Abstract
The T cell Ig and mucin domain–containing molecule (TIM) family of receptors have emerged as potential therapeutic targets to correct abnormal immune function in chronic inflammatory conditions. TIM-3 serves as a functional receptor in structural cells of the airways and via the ligand galectin-9 (Gal-9) can modulate the inflammatory response. The aim of this study was to investigate TIM-3 expression and function in neutrophils, focusing on its potential role in cystic fibrosis (CF) lung disease. Results revealed that TIM-3 mRNA and protein expression values of circulating neutrophils were equal between healthy controls (n = 20) and people with CF (n = 26). TIM-3 was detected on resting neutrophil membranes by FACS analysis, and expression levels significantly increased post IL-8 or TNF-α exposure (p < 0.05). Our data suggest a novel role for TIM-3/Gal-9 signaling involving modulation of cytosolic calcium levels. Via TIM-3 interaction, Gal-9 induced neutrophil degranulation and primed the cell for enhanced NADPH oxidase activity. Killing of Pseudomonas aeruginosa was significantly increased upon bacterial opsonization with Gal-9 (p < 0.05), an effect abrogated by blockade of TIM-3 receptors. This mechanism appeared to be Gram-negative bacteria specific and mediated via Gal-9/ LPS binding. Additionally, we have demonstrated that neutrophil TIM-3/Gal-9 signaling is perturbed in the CF airways due to proteolytic degradation of the receptor. In conclusion, results suggest a novel neutrophil defect potentially contributing to the defective bacterial clearance observed in the CF airways and suggest that manipulation of the TIM-3 signaling pathway may be of therapeutic value in CF, preferably in conjunction with antiprotease treatment.
Cystic fibrosis (CF) is the most common lethal genetic disease in whites, affecting at least 60,000 individuals worldwide (1). CF is caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR) chloride channel (2, 3), and <1500 CFTR mutations leading to defective chloride transport have been identified to date (4, 5). CFTR absence or malfunction causes defective ion transport across the epithelium, a reduction in periciliary liquid volume, and persistent mucus hypersecretion. As a consequence, mucus accumulation on airway surfaces leads to chronic bacterial infection, exacerbated airway inflammation, lung injury, and ultimately death (6).
The hallmark of CF lung disease is exacerbated inflammation accompanied by high neutrophil influx, which paradoxically cannot eradicate bacterial infections. As a result, people with CF (PWCF) suffer from chronic bacterial infection and inflammation in the lower airways. Indeed, bronchoalveolar lavage fluid (BALF) of PWCF contains elevated levels of neutrophils and proinflammatory cytokines such as IL-8, IL-6, and TNF-α. The mechanisms linking CFTR dysfunction to chronic bacterial infection and enhanced inflammation in the lung are still not fully understood. The median age at the onset of Pseudomonas colonization is 10 y, and once Pseudomonas aeruginosa colonization occurs, complete bacterial eradication is infrequent (7). Whether neutrophils in CF are intrinsically abnormal or if dysregulated cell activity is a result of chronic inflammation is still a matter of debate (8). However, potential defects in CF neutrophils have been proposed for several of the main neutrophil functions including cell chemotaxis (9), phagocytosis (10), oxidant production, and protease degranulation (11, 12).
T cell Ig and mucin domain–containing molecules (TIMs) are key regulators of immune responses (13–16). TIM proteins are associated with several human inflammatory conditions (15, 17), including rheumatoid arthritis (18), asthma (19), systemic lupus erythematosus (13), multiple sclerosis (20), diabetes (21), and antimicrobial immunity (22). However, the role of TIMs in regulation of the immune response in CF remains underexplored. TIM-3 has been shown to play a role in T cell exhaustion during chronic viral infections and in tumor immunity (23). In line with its lectin nature, Gal-9 binds to TIM-3 in a carbohydrate-dependent manner, interacting with the N-glycosylated site in the IgV domain. By engagement with Gal-9, TIM-3 has been shown to play a role in apoptosis of Th1 cells (24), and inhibition of Gal-TIM-3 interaction induced an exacerbation of Th1-driven immune response in a mouse model of autoimmune disease (25). Most recently, a repressor of TIM-3 function has been reported, namely HLA-B–associated transcript 3, which protects Th1 cells from Gal-9–mediated cell death (26). Moreover, by use of TIM-3–blocking mAbs, human TIM-3 has been shown to regulate cytokine expression at the transcriptional level (27). In line with these results, we have found that physiological relevant levels of Gal-9 induced IL-8 production by CF bronchial epithelial cells, indicating that TIM-3 may initiate the early neutrophil-dominated inflammation in the CF lung. Furthermore, constitutive upregulation of this receptor and its ligand may exacerbate the proinflammatory response of CF bronchial epithelial cells (28, 29). There is mounting evidence suggesting that TIM-3 can modulate the function of several immune cell types, and although a population of TIM-3+CD11b+Ly-6G+ cells (neutrophils) in peritoneal lavage fluid from septic mice has been described (30), the role of TIM-3–mediated signaling in neutrophils has not been determined. Moreover, apart from one study reporting the inability of Gal-9 to bind neutrophil extracellular traps (31), there are virtually no studies on the role of Gal-9 signaling in neutrophils. Therefore, within this study, we investigated whether TIM-3 was expressed in neutrophils and whether disruption of TIM-3/Gal-9 signaling played a role in impaired neutrophil-mediated bacterial killing in the CF airways.
Materials and Methods
Reagents
Unless stated otherwise, chemical reagents were purchased from Sigma-Aldrich (Dublin, Ireland) and were of the highest purity available.
BALF sample collection
BALF from adult PWCF (n = 4) was collected from individuals undergoing bronchoscopy for clinical reasons. Full informed consent was obtained preprocedure according to a protocol approved by Beaumont Hospital Ethics Committee. Bronchoscopy was performed with the bronchoscope directed to the lingula and right middle lobe. BALF was performed by instilling 1 ml/kg sterile normal saline per lobe. All BALF samples were centrifuged at 1000 × g for 10 min at 4°C, and cell-free supernatants were aliquoted and stored at −80°C for subsequent analysis.
Peripheral blood neutrophil isolation
Human neutrophils were isolated from heparinized (10 U/ml; Sarstedt, Numbrecht, Germany) venous blood as previously described (32). Cells were resuspended in PBS containing 5 mM glucose (PBSG) unless spec-ified otherwise. The purity of the neutrophil population was confirmed by flow cytometry (as described below) measuring the neutrophil membrane marker CD16b and was found to be <99% (33). Cell viability was systematically monitored before and after each treatment by trypan blue exclusion or by MTT assay and found to be <98%.
Neutrophils were isolated from control volunteers (n = 20; mean age 33.26 ± 1.73 y) who had no underlying medical illnesses and were not receiving any medication. Prior to recruitment, PWCF (n = 26; mean age 22.50 ± 2.7 y, either homozygous or heterozygous for the ΔF508 mutation) were exacerbation free over the preceding 6-wk period. In a subset of experiments, neutrophils were isolated from CF BALF as previously described (34). Informed patient consent was obtained for all procedures, and ethical approval for this study was obtained from the Beaumont Hospital Ethics Review Board.
Isolation of mouse peritoneal exudate neutrophils
Female BALB/c mice (Harlan Laboratories) between 8 and 10 wk of age were used in all experiments, which were performed according to regulations under the Home Office (U.K.) Animals (Scientific Procedures) Act 1986 for care and use of animals in biomedical research. Peritoneal elicited neutrophils were obtained as previously described (32) with minor modifications. Briefly, mice were injected i.p. with 2 ml sterile thioglycollate broth 3% (w/v). After 6 h, mice were euthanized by CO2 asphyxiation. Peritoneal exudate cells were harvested in 10 ml ice-cold enzyme-free PBS-based Cell Dissociation Buffer (Life Technologies). Cells were washed by centrifugation at 470 × g for 5 min at 4°C (two times) and resuspended in PBSG.
Quantitative real-time PCR
Total RNA was extracted from neutrophils (1 × 106) using TRI reagent following the manufacturer's instructions. cDNA (2 μl) was amplified with SYBR green I Master mix (Roche, Basel, Switzerland) using the Light-Cycler 480 PCR system (Roche). PCR was performed as previously described (35) using TIM-3 primer sets (amplicon size 96 bp) as follows: forward primer 5′-TCC AAG GAT GCT TAC CAC CAG-3′ and reverse primer 5′-GCC AAT GTG GAT ATT TGT GTT AGA TT-3′. All samples were carried out in duplicate 20-μl reactions in 96-well plates, and a negative control with no cDNA template was included in every run. Specificity of the amplicon products was confirmed by visual inspection of melting curves. The relative expression of the gene was determined using the 2−ΔΔ threshold cycle method (36) with GAPDH as an internal control. The primer set for GAPDH (amplicon size 113 bp) was as follows: forward primer 5′-CAT GAG AAG TAT GAC AAC AGC CT-3′ and reverse primer 5′-AGT CCT TCC ACG ATA CCA AAG T-3′.
SDS-PAGE and Western blotting
For preparation of neutrophil whole cell lysates for SDS-PAGE analysis, 1 × 107 cells was precipitated with 10% TCA on ice. Electrophoresis of samples was conducted according to Laemmli's method (37). Denatured protein samples (20 μl) were resolved on 10 or 12.5% (w/v) resolving gel and 4% (w/v) stacking gel. SeeBlue Plus2 Prestained molecular mass markers (4 μl; Invitrogen) were loaded on each gel for determination of m. w. Gels were run in an ATTO AE6450 electrophoresis tank (ATTO Corporation, Tokyo, Japan), and electrophoresis was carried out for 60–90 min at 150 V.
Following electrophoresis, proteins were transferred onto nitrocellulose membrane at 150 mA for 60 min using a semidry blotting apparatus. Following transfer, membranes were blocked with 5% (w/v) nonfat powdered milk in PBS containing 0.1% (v/v) Tween-20 for 1 h at room temperature. For immunological detection of the desired proteins, blots were incubated overnight at 4°C in blocking buffer containing Ab against TIM-3 (goat polyclonal, 1 μg/ml; R&D Systems, Abingdon, U.K.), hCAP-18 (rabbit polyclonal, 1 μg/ml; Invitrogen, Lund, Sweden), matrix metalloprotease-9 (MMP-9; goat polyclonal, 1 μg/ml; R&D Systems), and myeloperoxidase (MPO; goat polyclonal, 1 μg/ml; R&D Systems). In a subset of experiments, rabbit polyclonal anti-human TIM-3 Ab (1 μg/ml) was used that was purchased from MBL International (Woburn, MA). Mouse monoclonal anti–p-tyrosine (clone 4G10 Ab; 1 μg/ml) was purchased from Millipore. The secondary Abs were HRP-linked anti-rabbit, anti-mouse, or anti-goat IgG (Cell Signaling Technology, Danvers, MA). Blots were developed with Immobilon Western chemiluminescent HRP substrate (Millipore) and visualized on the Syngene G:Box chemi XL gel documentation system (Syn-optics, Cambridge, U.K.) or by exposing the membrane to Kodak X-Omat LS film (Kodak).
Flow cytometry experiments
Flow cytometry was carried out to evaluate the membrane expression of CD16b as a measure of cell purity (33). Neutrophils were then fixed (4% (w/v) paraformaldehyde) and blocked (2% (w/v) BSA) for 30 min at room temperature. After washing (PBS ×2), neutrophils (1 × 106) were incubated with 1 μg/100 μl mouse monoclonal anti-CD16b (Santa Cruz Biotechnology). Control samples were exposed to relevant nonspecific isotype control IgG or secondary-labeled Ab alone (FITC-labeled bovine anti-mouse). For TIM-3 expression studies, human or mouse purified neutrophils (1 × 106/ml) were left untreated or exposed to IL-8 (10 ng/ml) for 5 min or TNF-α (10 ng/ml) for 10 min at 37°C in PBSG. Healthy control neutrophils were also treated with CF BALF (200 μg) or 100 nM neutrophil elastase (NE) for 1 or 2 h, respectively, at 37°C in PBS. In a subset of experiments, neutrophils from CF BALF were also screened for TIM-3 expression. Cells were fixed, blocked, and incubated with PE-labeled anti-human TIM-3 Ab or PE–anti-mouse TIM-3 Ab (rat monoclonal, 1 μg/106 cells; eBioscience, Hatfield, U.K.) or PE isotype control Ab overnight at 4°C. Samples were analyzed on an FACScalibur flow cytometer (BD Biosciences, San Jose, CA). BALF neutrophils were gated on by using CD66b, a marker of neutrophil activation, and a forward light scatter and side scatter plot (38). At least 10,000 events were acquired, and the mean fluorescence intensity (MFI) for each experiment was determined using BD CellQuest Pro software (BD Biosciences).
TIM-3 immunoprecipitation
TIM-3 immunoprecipitation was performed exactly as previously described (35). Neutrophils (1 × 107) were resuspended in 1 μl Lamberts Break Buffer (10 mM KCl, 3 mM NaCl, 4 mM MgCl2, and 10 mM PIPES [pH 7.2]) supplemented with protease inhibitors Complete Mini tablets and phosphatase inhibitors PhosStop Mini tablets (Roche, Basel, Switzerland) at 4°C. Cell membranes were purified by ultracentrifugation as previously described (35) and the membrane pellet resuspended in 1 ml radio-immunoprecipitation assay buffer (150 mM NaCl, 50 mM Tris-HCl [pH 8], 0.5% [w/v] sodium deoxycholate, 1% Triton X-100 [v/v], and 0.1% [w/v] SDS) containing protease and phosphatase inhibitors. Samples were precleared with 6 μg normal goat IgG (Santa Cruz Biotechnology) and 50 μl protein-G Dynabeads (Invitrogen) for 1 h at 4°C with rotation. The precleared samples were incubated with 6 μg goat anti–TIM-3 Ab for 2 h at 4°C with rotation, and the immunocomplex was captured by incubation with 50 μl protein-G Dynabeads as previously described (35). The activation of TIM-3 by Gal-9 was analyzed by Western blot of immunoprecipitated cell membranes probed for p-tyrosine. Blots were stripped with Restore Western blot stripping buffer (Pierce Biotechnology) according to the manufacturer's instructions and reprobed with rabbit anti–TIM-3 Ab to confirm equal amounts of TIM-3 being immunoprecipitated.
Intracellular Ca2+ mobilization measurements
Intracellular Ca2+ mobilization was monitored using a Fluo-4 NW kit (Invitrogen) as previously described (33). Neutrophils were resuspended in sterile polypropylene tubes (Sarstedt) at 2.5 × 106 cells/ml in assay buffer and left untreated or incubated with 30 mM lactose or 30 mM sucrose for 30 min at 37°C and 5% CO2. A total of 100 μl neutrophil suspension (1.25 × 105 cells) was pipetted into a black 96-well plate in duplicate, and fluorescence was recorded at room temperature every 5 s in a Victor X3 2030 Multilabel reader (PerkinElmer) using excitation and emission wavelengths of 485 and 535 nm, respectively. After 60–90 s, Gal-9 was added (final assay concentrations of 0, 50, 100, and 500 nM), and intracellular Ca2+ fluorescence was monitored for up to 10min. Proteolytically stable human Gal-9 was a kind gift from GalPharma (Kagawa, Japan). Expression and purification of stable human Gal-9 was as previously described (18).
Neutrophil superoxide production
Neutrophil superoxide (O2−) generation was measured by the cytochrome c reduction assay as previously described (39). Neutrophils were resuspended at 2.5 × 106 cells/ml in cytochrome c assay buffer (0.5 mM MgCl2, 0.5 mM CaCl2, 5 mM glucose, and 100 μM cytochrome c from bovine heart in PBS) prewarmed at 37°C. Cells were exposed to Gal-9 at increasing concentrations (0, 50, 100, 250, and 500 nM), and reduction of cytochrome c was measured at 550 nm every minute. In a subset of experiments after 10 min, fMLF (final concentration 1 μM) was added to cells to assess whether Gal-9 was capable of neutrophil priming. O2− production was monitored every minute for an additional 30 min and the concentration of O2− produced calculated using the Beer-Lambert law with an extinction coefficient (ε) of 21.1 nM−1 cm−1. In this set of experiments, PMA (500 ng/ml) was employed as a positive control.
Neutrophil degranulation assays
Neutrophils (5 − 106/ml) suspended in PBSG remained untreated or were incubated with Gal-9 (0, 50, and 500 nM) or fMLF (1 μM) and cytochalasin B (Cyto-B; 5 μM), and cell aliquots were taken at 0, 15, or 30 min. Cell-free supernatants were harvested following centrifugation at 500 × g for 5 min at 4°C and analyzed for degranulated proteins: MPO as a marker of primary granule release, hCAP-18 as a marker of secondary granule release, and MMP-9 as a marker of tertiary granule release by Western blotting. Degranulation of neutrophil elastase to the outside of the cell was quantified by performing an NE activity assay using the NE-specific substrate N-methoxysuccinyl-ala-ala-proval-p-nitroanilide, as previously described (40). Samples (10 μl) were mixed with 90 μl 3 mM substrate in assay buffer (0.5 M NaCl, 0.1% [v/v] Brij-35, and 0.1 M HEPES [pH 7.5]). OD was recorded at 405 nm for 5 min at 1-min intervals at 37°C using a Wallac 1420 Victor2 multilabel counter (Wallac).
Determination of bactericidal activity
P. aeruginosa strain (PAO1) and Staphylococcus aureus strain (8325–4) were used in this study. Bacteria were stored at −80°C as glycerol stocks (50% [v/v] glycerol in tryptic soy broth) and streaked onto tryptic soy agar plates to obtain single colonies after an overnight incubation at 37°C. A single colony was then suspended in BBL trypticase soy broth (BD Biosciences) and cultured at 37°C and 200 rpm in a shaker incubator (New Brunswick Scientific, Eppendorf, Cambridge, U.K.). Bacterial quantification was achieved by measuring absorbance at 600 nm using a Biophotometer (Eppendorf) according to the following conversion values: P. aeruginosa PAO1, OD600 of 0.185 = 1 × 108 bacteria/ml and S. aureus strain (8325–4), OD600 of 0.170 = 1 × 108 bacteria/ml.
The role of TIM-3 and Gal-9 in bacterial killing was assessed in vitro following a previously described method (32) using a neutrophil/bacteria ratio of 1:1. In brief, bacteria (5 × 107) were pelleted by centrifugation at 20,000 × g for 10 min at room temperature, resuspended in 500 μl 50% (v/v) autologous plasma in PBS, and opsonized for 30 min at 37°C. In a subset of experiments, Gal-9 in PBS (0, 50, 100, or 500 nM) was used as the opsonin. Freshly isolated healthy control neutrophils (1 × 107) were resuspended in a total volume of 500 μl PBSG, in the presence or absence of TIM-3–Fc fusion protein (rhTIM-3–Fc; 5 μg/ml) or control fusion protein (rhIgG1Fc; 5 μg/ml). rhTIM-3–Fc and human IgG1–Fc (110-HG) were both purchased from R&D Systems. Opsonized bacteria (1 × 107/100 μl) were added to neutrophils in a rapidly stirring oxygenated chamber, and 100-μl aliquots were removed at 2, 4, and 8 min. Serial dilutions of the bacteria/neutrophil suspensions were plated in triplicate on tryptic soy agar plates and incubated at 37°C. Viable bacterial CFUs were counted the following day. Bacterial viability was expressed as a percentage of bacterial counts at time 0 min, the latter representing 100% viability. In a subset of experiments to determine if TIM-3 was involved in Gal-9–mediated bacterial killing, neutrophils were preincubated with TIM-3–blocking Ab (mouse monoclonal derived from a hybridoma [clone 1G5]; 10 μg/ml) or mouse IgG control (Santa Cruz Biotechnology; 10 μg/ml) for 10 min at room temperature prior to the addition of bacteria.
Neutrophil phagocytosis assays
Phagocytosis assays were carried out as previously described with some modifications (41). In brief, P. aeruginosa (2 × 108 bacteria) were resuspended in 1 μl labeling buffer (50 mM Na2CO3 and 100 mM NaCl [pH 9]) containing 0.5 μg/ml FITC and incubated for 20 min at room temperature, pelleted by centrifugation (20,000 × g for 10 min) and then washed three times in 1 ml PBS. FITC-labeled bacteria were either untreated or opsonized with Gal-9 (50 nM) for 30 min and then washed with PBS. For analyzing neutrophil phagocytosis, FITC-labeled bacteria (5 × 107 of either Gal-9 opsonized or unopsonized) and neutrophils were mixed at 37°C in a rapidly stirring oxygenated chamber at a 10:1 ratio. Aliquots were removed at 0 and 5 min and placed in 0.4% (v/v) trypan blue in PBS to quench extracellular and membrane-adhered FITC-labeled bacteria. Cells were then analyzed by flow cytometry for phagocytosed fluorescent bacteria as previously described (41).
Gal-9/LPS binding assays
Interaction between P. aeruginosa LPS and Gal-9 was determined by an in-house developed solid-phase binding assay based on previously published Gal-3/LPS binding studies (42–44). High-binding, Immulon 2HB flat-bottom polystyrene microtiter plates (Thermo Scientific, Dublin, Ireland) were coated overnight at 4°C with 100 μl/well commercial LPS purified from P. aeruginosa, serotype 10 (50 μg/ml in Vollers buffer). Following three washes with PBS containing 1% (v/v) Tween-20, 100 μl PBS containing Gal-9 (0–500 nM) was added to each well and incubated for 2 h at 37°C. The plates were then washed (five times), incubated for 2 h with 100 μl 10 μg/ml Gal-9 Ab (clone FG9-M; Galpharma) in PBS, washed, and then incubated for 1 h with anti-mouse–HRP Ab (1:1000 in PBS). After a final wash, plates were incubated for 1 h with ABTS, and Gal-9/LPS binding was confirmed by measuring absorbance at 405 nm. Controls included uncoated LPS wells, wells without Gal-9, and wells treated with Abs only.
Gal-9 binding to bacteria was also measured by flow cytometry. P. aeruginosa and S. aureus (5 × 107) were pelleted by centrifugation, fixed in 3.7% (w/v) paraformaldehyde in PBS for 15 min at room temperature, washed in 1 μl PBS (three times), and resuspended in 1 ml PBS. Bacteria (1 × 106) were incubated for 1 h at 4°C with end-to-end rotation in 500 μl PBS containing 500 nM Gal-9 or PBS alone. Following centrifugation, bacteria were washed in PBS (three times) and incubated with 20 μl PE-labeled Gal-9 Ab or PE-labeled mouse IgG control for 30 min at room temperature in the dark. After a final wash, the bacteria were resuspended in PBS and Gal-9 binding assessed by flow cytometry.
Statistical analysis
Data were analyzed with GraphPad Prism 4.0 software package (GraphPad Software, San Diego, CA). Results of cytochrome c assays, intracellular Ca2+ measurements, and killing assays were analyzed by two-way ANOVA. For data sets less than n = 6, the Student t test was employed (45). Data are expressed as mean ± SE of at least three independent experiments performed in triplicate unless otherwise stated. Results were considered significant (*) when p was <0.05.
Results
TIM-3 is equally expressed by circulating healthy control and CF neutrophils
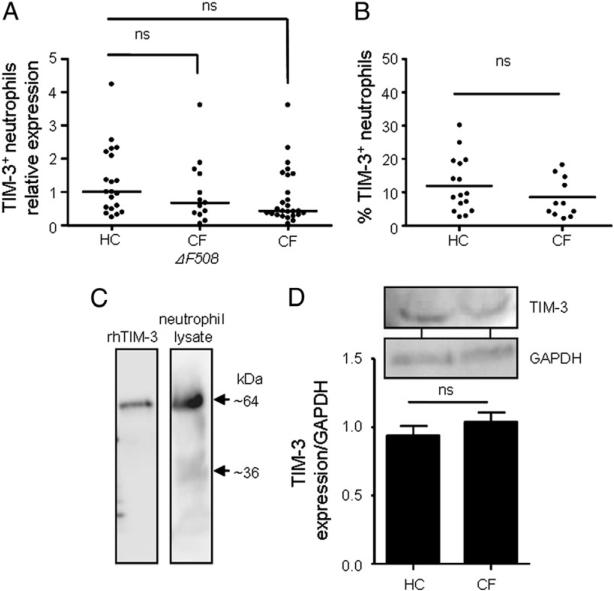
Altered TIM-3 expression in peripheral monocytic cells has been reported associated with a number of respiratory diseases (46); however, whether TIM-3 is expressed by human neutrophils and how TIM-3 expression is affected by CF are not known. To address this, we employed purified peripheral blood neutrophils from healthy controls and PWCF, whom were either homozygous or heterozygous for the ΔF508 mutation, and examined TIM-3 expression by quantitative real-time PCR. TIM-3 was found to be present in all cell types, with a slight decrease in TIM-3 mRNA expression in neutrophils from PWCF homozygous for the ΔF508 mutation, although this did not reach statistical significance (Fig. 1A).
FIGURE 1.
TIM-3 gene and protein expression is not altered in CF circulating neutrophils. (A) Differential TIM-3 mRNA expression in peripheral blood neutrophils from healthy controls (HC; n = 20), stable PWCF homozygous for the ΔF508 (CF ΔF508; n = 13), or stable PWCF carrying one ΔF508 allele (CF; n = 26) was analyzed by quantitative RT-PCR. TIM-3 mRNA levels were not significantly different among groups. Horizontal bars indicate median values. Data analyzed by nonparametric one-way ANOVA. (B) Neutrophils from HC (n = 16) or stable CF patients (n = 11) were probed with PE-labeled anti–TIM-3 Ab and analyzed by flow cytometry for TIM-3 protein expression. Graph shows the percentage of TIM-3–positive cells. Horizontal bars indicate the median. Data analyzed by Student t test. (C) rhTIM-3 (100 ng) or total neutrophil protein from 4 × 106 cells obtained by TCA precipitation was analyzed for TIM-3 expression (64 kDa) by Western blotting using polyclonal goat TIM-3 Ab. Image shown is representative of results obtained from five different neutrophil donors. (D) Neutrophil (4 × 105 cells) protein was obtained by TCA precipitation from HC or stable ΔF508 homozygous PWCF. TIM-3 and GAPDH (loading control) expression was analyzed by Western blotting, and representative immunoblots are shown. Bar graph depicts densitometric analysis of TIM-3 expression in HC (n = 4) and PWCF (n = 4). Data shown as mean ± SE and analyzed by Student t test. ns, p > 0.05.
Next, we investigated TIM-3 expression at the protein level by flow cytometry analysis. All neutrophils were fixed in paraformaldehyde prior to incubation with TIM-3 Ab. TIM-3 expression was detected on the membranes of peripheral blood neutrophils from PWCF and healthy controls, and there was no significant difference in the number of TIM-3–positive cells (Fig. 1B). This result is in contrast to our previous observation of increased TIM-3 on CF bronchial epithelial cells (35), and therefore, to consolidate this result, the expression of TIM-3 was examined in whole-cell lysates obtained by TCA precipitation. Western blot analysis of whole neutrophil lysates identified two characteristic bands for TIM-3 of 64 and 36 kDa (Fig. 1C). In line with previous reports on the molecular mass of TIM-3 (35, 47), the fully glycosylated form of the protein was found present in neutrophils (64 kDa) and also a second band indicative of nonglycosylated TIM-3 (36 kDa) (Fig. 1C). Moreover, to evaluate potential differential expression of TIM-3 protein by circulating neutrophils from PWCF compared with healthy controls, cell lysates were obtained by TCA precipitation of neutrophils from healthy and ΔF508 homozygous PWCF. Expression of mature TIM-3 (64 kDa) was analyzed by densitometry of immunoblots, and in line with results demonstrating equal mRNA expression, no significant difference in TIM-3 protein expression was detected (Fig. 1D).
TIM-3 membrane expression is increased post–proinflammatory challenge
As expression of TIM-3 on circulating human neutrophil membranes was established (Fig. 1B), additional studies were conducted to determine whether inflammatory stimuli could further influence TIM-3 expression of purified cells. Neutrophils were treated with IL-8 (10 ng/ml) or TNF-α (10 ng/ml), and TIM-3 expression was measured by flow cytometry after 5 and 10 min treatment, respectively. The MFI of TIM-3 on peripheral unstimulated neutrophils was 4.8 ± 0.16 (Fig. 2A, 2B), and the percentage of TIM-3–positive cells following stimulation with IL-8 or TNF-α was 28.47 ± 2.22 and 29.98 ± 1.20, respectively (Fig. 2C). These data revealed that inflammatory stimuli can modulate TIM-3 membrane levels and that IL-8 (p < 0.05) and TNF-α (p < 0.05) induced a significant increase in the percentage of TIM-3–positive neutrophils (Fig. 2C).
FIGURE 2.
TIM-3 protein membrane expression is upregulated postexposure to proinflammatory stimuli. Isolated neutrophils (1 × 106/ml) remained untreated (Con) or exposed to IL-8 (10 ng/ml) for 5 min (A) or TNF-α (10 ng/ml) for 10 min (B). Cells were then fixed with 4% (w/v) paraformaldehyde, blocked, and incubated with PE-labeled anti–TIM-3 Ab or PE isotype control Ab (dark gray, filled). TIM-3 expression was analyzed by flow cytometry, and results are representative images of three independent experiments. (C) Analysis of TIM-3 expression illustrating percentage of TIM-3–positive cell values for untreated (Con) and IL-8– or TNF-α–treated neutrophils (n = 3; statistical analysis by Student t test, *p < 0.05). (D) Neutrophils were elicited in BALB/c mice (n = 6) by i.p. injection of 3% (w/v) thioglycollate. After 6 h, neutrophils were recovered from the peritoneal cavity by lavage, fixed in 4% (w/v) paraformaldehyde, blocked, and stained with 1 μg/1 × 106 cells with PE-anti–TIM-3 Ab or isotype control (black, filled). TIM-3 expression was analyzed by flow cytometry. Image shown is representative of three independent experiments. (E) Neutrophils were either left untreated or treated with 50 nM Gal-9 for 15 min. TIM-3 was immunoprecipitated (IP) from cell membranes with goat anti–TIM-3 Ab. Normal goat IgG was used as a control for nonspecific binding. Activation of TIM-3 was analyzed by Western blotting (WB) employing a mouse monoclonal anti–p-tyrosine Ab (top panel). Blots were stripped and reprobed with rabbit anti–TIM-3 Ab to confirm equal levels of immunoprecipitated TIM-3 (bottom panel). Representative blot of three experiments is shown, and the level of TIM-3 phosphorylation was normalized to total immunoprecipitated TIM-3 (F). *p < 0.05 compared with untreated (Con) by Student t test.
Subsequent experiments considered whether TIM-3 was expressed post–neutrophil migration by employing the thioglycollate-induced sterile peritonitis model. Female BALB/c mice were injected i.p. with sterile thioglycollate for 6 h, following which neutrophils were recovered from the peritoneal cavity. As illustrated in Fig. 2D, elicited neutrophils retained TIM-3 surface expression. This result suggests that TIM-3 would be expressed on neutrophils post-migration to the site of inflammation/infection and be capable of exerting a putative function following translocation from the systemic circulation.
We next investigated if TIM-3 expressed on neutrophils was a functional receptor for Gal-9. For this purpose, neutrophils were treated with Gal-9, which has previously been shown to activate TIM-3 by phosphorylation of the cytosolic tyrosine motif Y265 (47). After 15 min stimulation with Gal-9 (50 nM), TIM-3 was immunoprecipitated from neutrophil cell membrane extracts with goat anti–TIM-3 Ab, and normal goat IgG was used as a control for nonspecific binding. Western blot analysis of immunoprecipitates revealed that TIM-3 exhibited a substantial increase in the level of tyrosine phosphorylation in response to Gal-9 treatment compared with untreated controls (Fig. 2E, 2F). Background levels of phosphorylation were detected, suggesting constitutive activation of TIM-3, but levels of phosphorylation were significantly higher post–Gal-9 exposure (p < 0.05) (Fig. 2F). A blot probed with anti–TIM-3 served as a control for equal amounts of immunoprecipitated TIM-3 (Fig. 2E, bottom panel), and levels of TIM-3 phosphorylation were normalized to total immunoprecipitated TIM-3 protein (Fig. 2F). This result shows that TIM-3 in neutrophils can become activated by Gal-9 challenge, subsequently leading to tyrosine phosphorylation of the receptor.
Gal-9 induces neutrophil degranulation via TIM-3 interaction
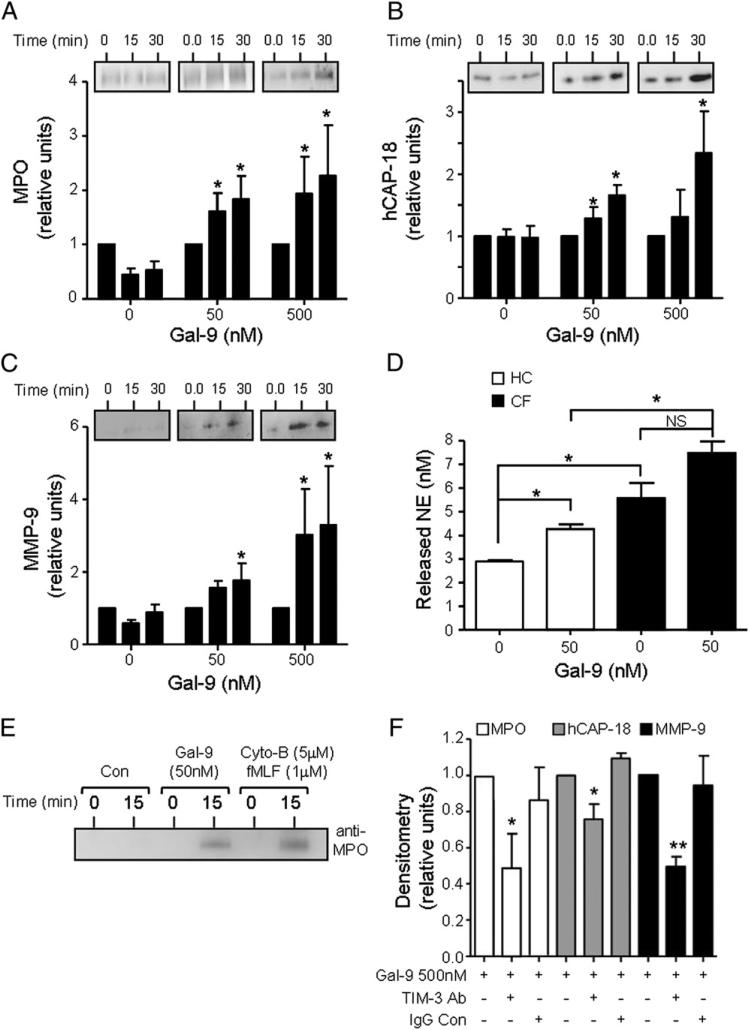
As TIM-3 was found on membranes of resting and IL-8– or TNF-α–challenged cells and was also present on the surface of trans-migrating neutrophils, we next set out to investigate whether TIM-3 played a role in neutrophil antimicrobial function. The degranulation of proteolytic enzymes and peptides is required for the bactericidal activity of neutrophils. For this reason, we evaluated the kinetics of degranulation upon cell challenge with the TIM-3 ligand Gal-9. Upon exposure, Gal-9–induced (50 or 500 nM) release of granule proteins was quantified in the extracellular supernatant by immunoblotting. Levels of cell released MPO from primary granules (Fig. 3A), hCAP-18 from secondary granules (Fig. 3B), and MMP-9 from tertiary granules (Fig. 3C) were significantly increased in the presence of Gal-9 (50 or 500 nM) post–15- or 30-min exposure (p < 0.05). Ensuing experiments were designed to investigate Gal-9–induced degranulation of NE and compare the difference between CF and healthy control cells. Post–15-min exposure to 50 nM Gal-9, there was a significant increase in the release of NE by healthy control cells compared with untreated cells (2.9 ± 0.05 to 4.2 ± 0.19 nM; p < 0.05) and an increased release by CF neutrophils, although this did not reach significance (5.57 ± 0.64 to 7.49 ± 0.48 nM) (Fig. 3D). Moreover, the level of NE released by CF cells in response to Gal-9 was significantly higher than healthy controls (p < 0.05), which is in agreement with previous reports on the increased degranulation of primary granules by CF neutrophils (12, 48) (Fig. 3D). Furthermore, as a positive control for degranulation assays, a comparison was made between the responses induced by Gal-9 (50 nM) and that of fMLF (1 μM) in combination with Cyto-B (5 μM). Post-activation for 15 min, a strong MPO immunoband indicative of primary granule release was observed in both Gal-9 and fMLF/Cyto-B–challenged cells (Fig. 3E).
FIGURE 3.
Gal-9 induces neutrophil degranulation via TIM-3 interaction. (A–C) Neutrophils were treated with Gal-9 (0, 50, and 500 nM), and aliquots were taken at 0, 15, or 30 min. Cells were centrifuged and super-natants were resolved on 12% (w/v) SDS-PAGE gradient gels. Immunoblots were probed for MPO (A), hCAP-18 (B), or MMP-9 (C) as markers of primary, secondary, and tertiary granule release, respectively. Blots shown are representative of three independent experiments carried out with different blood donors. Bar graphs depict immunoband densitometry values of degranulated proteins. *p < 0.05 compared with respective 0 min control by Student t test. (D) Neutrophils from PWCF (n = 3) and healthy control volunteers (HC; n = 3) were untreated or treated with Gal-9 (50 nM) and aliquots removed after 15-min incubation at 37°C. Levels of released NE were quantified by an NE activity assay. *p < 0.05 by Student t test. NS, p > 0.05. (E) Neutrophils were treated with Gal-9 (50 nM) or a combination of fMLF (1 μM) and Cyto-B (5 μM), and aliquots were taken at 0 and 15 min. Immunoblots were probed for MPO. Blot shown is a representative of three independent experiments carried out with different blood donors. (F) Neutrophils (1 × 106/ml) were incubated for 10 min in the presence or absence of 10 μg/ml TIM-3–blocking Ab or mouse IgG1Fc control Ab (IgG Con) followed by Gal-9 (500 nM), and aliquots were taken after 30 min. Cell supernatants were analyzed for MPO, hCAP-18, or MMP-9. Results shown are mean ± SE of n = 3 independent experiments. *p < 0.05, **p < 0.01 compared with Gal-9 treatment by Student t test.
Subsequent experiments were performed to confirm that Gal-9–evoked neutrophil degranulation was TIM-3 receptor mediated. Neutrophils were incubated with a human TIM-3–blocking Ab or relevant IgG control for 10 min subsequent to Gal-9 (500 nM) induced degranulation for 15 min. TIM-3 Ab blockade significantly reduced Gal-9–evoked MPO (p < 0.05), hCAP-18 (p < 0.05), and MMP-9 (p < 0.01) release, whereas the IgG control did not abrogate this effect (Fig. 3F). Taken together, these results indicate that Gal-9 acts as a potent inducer of neutrophil degranulation via interaction with TIM-3.
The TIM-3/Gal-9 signaling axis confers a novel neutrophil-priming mechanism
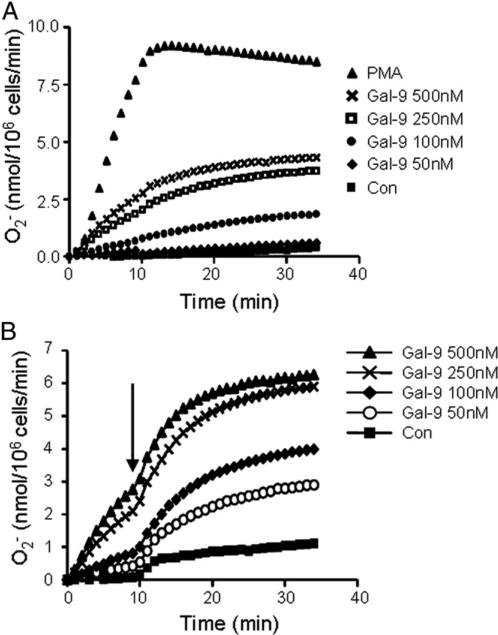
Intravacuolar killing in neutrophils occurs not only due to release of proteolytic enzymes, but also requires NADPH oxidase activity, which results in O2− generation. Prompted by the data indicating a role for Gal-9 and TIM-3 as a novel signaling mechanism leading to degranulation, ensuing experiments focused on the impact of Gal-9/TIM-3 signaling on NADPH oxidase activation. Neutrophils were challenged with increasing concentrations of Gal-9 (50, 100, 250, or 500 nM), and O2− generation was monitored itored over 30 min by cytochrome c reduction (Fig. 4A). Within these experiments, PMA (500 ng/ml) was chosen as a positive control with maximal production of O2− observed after ~8 min. In contrast, O2− generation was not produced by unstimulated cells or those exposed to low Gal-9 treatment (50 nM). Of interest, however, 100–500 nM Gal-9 induced significant O2− production in a dose-dependent manner (p < 0.05) (Fig. 4A).
FIGURE 4.
The priming effect of Gal-9 on O2− production. (A) O2− production was analyzed in neutrophils stimulated in the presence or absence of Gal-9 (0 [Con], 50, 100, 250, or 500 nM). PMA (500 ng/ml) was used as a positive control. Kinetics of cytochrome c reduction were measured at 550 nm over 40 min. Results shown are average of n = 3 independent experiments. O2− production following 50 nM Gal-9 treatment was not significantly different from control. All other treatments were significantly different. Differences among treatments were analyzed by two-way ANOVA compared with untreated control cells, p < 0.05. (B) O2− production was analyzed in the presence or absence of Gal-9 (0 [Con], 50, 100, 250, or 500 nM). Reduction of cytochrome c was measured at 550 nm and after 10 min, fMLF was added to cells (final concentration 1 μM, indicated by arrow) to assess whether Gal-9 primes the respiratory burst. O2− production was monitored every min for an additional 30 min. O2− production in all Gal-9 treatments was significantly different compared with control (Con) neutrophils not pretreated with Gal-9. Differences among treatments were analyzed by two-way ANOVA compared with Gal-9 untreated cells, p < 0.05.
As Gal-3 has previously been shown to prime neutrophils for O2− production (49), we next tested if Gal-9 could also act as a priming agent. Neutrophils were treated for 10 min with increasing levels of Gal-9 (0, 50, 100, 250, and 500 nM), fMLF (1 μM final concentration) was then added, and cytochrome c reduction as a measure of O2− production was monitored for 25 min (Fig. 4B). In line with previous reports, fMLF alone is a poor inducer of O2− production (33). However, treatment of cells with Gal-9 enhanced NADPH oxidase activity in a dose-dependent manner. Of note, the 50 nM dose of Gal-9, which did not trigger O2− production alone (Fig. 4A), induced a marked release of O2− following fMLF treatment, indicating that Gal-9 can act as a neutrophil-priming agent.
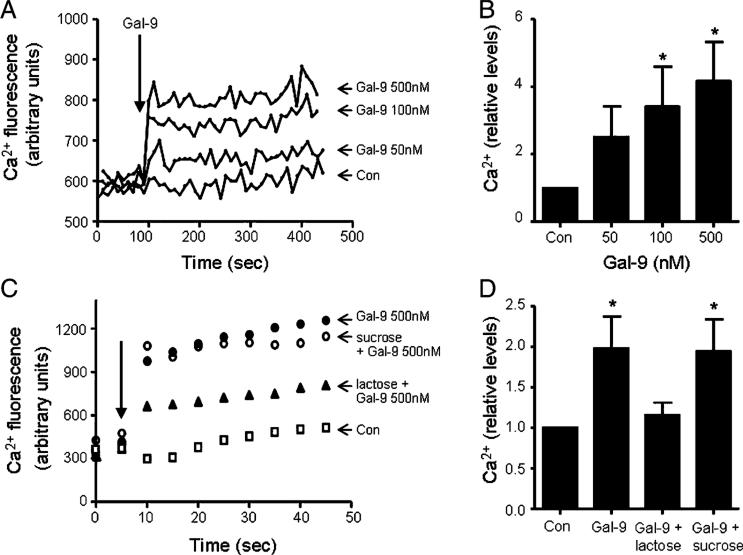
An increase in intracellular cytosolic Ca2+ is a feature of neutrophil priming and activation (50). Moreover, Gal-9 has been shown to promote intracellular Ca2+ mobilization via TIM-3 in mouse Th1 cells (24) and a number of human cell lines including HL-60 cells (51). To determine whether Gal-9 can trigger a Ca2+ flux in neutrophils, intracellular Ca2+ mobilization was monitored following stimulation with 50, 100, or 500 nM Gal-9 (Fig. 5A). A rapid increase in cytoplasmatic Ca2+ was observed immediately after addition of Gal-9, reaching a maximum after ~20 s without returning to basal levels. All Gal-9 treatments triggered intracellular Ca2+ mobilization, but most significantly by use of 100 or 500 nM Gal-9 (p < 0.05) (Fig. 5B). To confirm that this effect was caused by Gal-9 binding to β-galactoside in the TIM-3 receptor, neutrophils were preincubated with lactose (30 mM), which has been shown to prevent Gal-9 binding to TIM-3 (52), or sucrose (30 mM) as a negative control. Cotreatment with lactose significantly decreased Gal-9–evoked intracellular Ca2+ flux, whereas control sucrose treatment did not reverse the effects of Gal-9 (Fig. 5C, 5D).
FIGURE 5.
Galectin-9–evoked intracellular Ca2+ mobilization is dose dependent and inhibited by lactose. (A) Neutrophils (1.25 × 105/100 μl) were incubated with Fluo-4 (5 mM) according to the manufacturer's instructions for 30 min at 37°C. Calcium mobilization was monitored after addition of Gal-9 (indicated by arrow) at 0 (Con), 50, 100, or 500 nM. Data shown are representative of three independent experiments. The kinetics of Ca2+ flux are illustrated in (A), and data of the 30-s time point post–Gal-9 challenge of triplicate experiments are plotted in (B). (C) Neutrophils (1.25 × 105/100 μl) were incubated with Fluo-4 (5 mM) in PBS (Con) or in the presence of lactose (30 mM) or sucrose (30 mM) before addition of Gal-9 (500 nM) (indicated by arrow). Blockade of Gal-9 with lactose abrogated the Gal-9–evoked intracellular Ca2+ flux in neutrophils. The kinetics of Ca2+ flux are illustrated in (C), and data of triplicate experiments of the 20-s time point are plotted in (D). For data presented in (B) and (D), each bar is the mean ± SE. *p < 0.05 calculated by Student t test between Gal-9–treated and untreated control (Con) samples.
Collectively, results demonstrate TIM-3 expression on resting and IL-8– or TNF-α–primed neutrophil cell surfaces. Gal-9 was shown to stimulate signal transduction in the neutrophil, leading to intracellular Ca2+ mobilization, neutrophil degranulation, and NADPH oxidase activity via TIM-3 interactions. Taken together, this study has identified a novel role for TIM-3/Gal-9 in neutrophil function with potentially important consequences in neutrophil antimicrobial activity.
TIM-3/Gal-9 signaling plays an essential role in opsonization and phagocytosis of P. aeruginosa
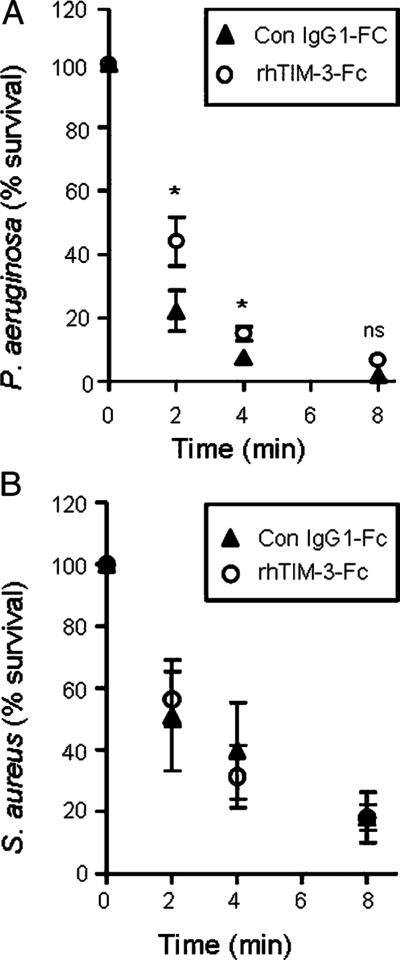
A direct role in bacterial killing has recently been revealed for TIM-3/Gal-9 host resistance to Mycobacterium tuberculosis (22). Moreover, a recent study has shown that killing of Escherichia coli by monocytic cells was TIM-3 dependent, as phagocytosis and killing mechanisms were inhibited in the presence of soluble TIM-3 (53). Within this study, we investigated whether Gal-9 plays a role in neutrophil-mediated bacterial killing via TIM-3 interaction. As Gal-9 is found in human serum of healthy individuals (54, 55), bacterial opsonization with serum was performed, and killing assays were carried out in the presence or absence of rhTIM-3–Fc fusion protein (5 μg/ml). As shown in Fig. 6A, the presence of rhTIM-3–Fc protein significantly (p < 0.05) increased survival of serum-opsonized P. aeruginosa compared with controls at the earlier time points of 2 min (44 versus 22% survival) and 4 min (22 versus 7% survival). This result is in agreement with the previous study in human immune cells that demonstrated that blocking TIM-3 interaction with serum-opsonized bacteria resulted in decreased bacterial killing (53). In contrast, rhTIM-3–Fc treatment did not significantly alter S. aureus neutrophil-mediated killing (Fig. 6B). These results suggest that TIM-3 expression on neutrophils is directly involved in a killing mechanism specific for P. aeruginosa, but not present in S. aureus.
FIGURE 6.
Blockade of neutrophil TIM-3 reduces P. aeruginosa killing. Neutrophils (1 × 107/ml) were preincubated in 5 μg/ml rhTIM-3–Fc or control IgG1Fc (Con IgG1-FC) and then exposed to 1 × 107 serum-opsonized P. aeruginosa (A) or S. aureus (B). Aliquots were removed at 0, 2, 4, and 8 min. Serial dilutions of the bacterial/neutrophil suspensions were plated in triplicate on tryptic soy agar plates and viable bacterial CFUs counted after overnight incubation at 37°C. Data shown as mean ± SE of n = 3 independent experiments with neutrophils from different donors. Statistical significance analyzed by Student t test at each time point, *p < 0.05.
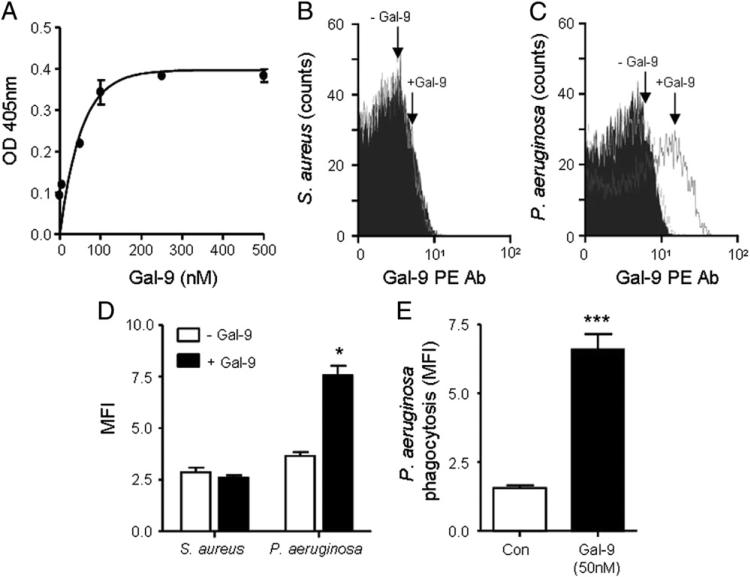
The previous experiment has demonstrated that TIM-3 on neutrophils is involved in bacterial killing. However, there are no known bacterial components that can directly bind to TIM-3. Thus, it is likely that the interaction blocked by soluble rhTIM-3 is TIM-3/Gal-9, because Gal-9 is the only known ligand for TIM-3. Indeed, Gal-9 has been reported to bind to Leishmania major through specific interaction with poly-β-galactosyl epitopes, and Gal-3 has also been found to bind to glycans expressed on the surface of parasites such as L. major (56) and fungi such as Candida albicans (57). Thus, galectins present in serum can potentially act as pathogen recognition molecules against a varied range of microorganisms. Within this study we assessed whether Gal-9 was capable of binding to LPS from P. aeruginosa by a modified solid-binding ELISA. Commercially available LPS purified from P. aeruginosa (50 μg/well) was employed to coat a 96-well plate, and wells were incubated overnight with increasing levels of Gal-9 (0–500 nM). Binding was determined by using a Gal-9–specific Ab, and a positive response could only be obtained if direct interaction between LPS and Gal-9 occurred. Fig. 7A shows that Gal-9 binds to P. aeruginosa LPS and that maximal binding was obtained between 50 and 100 nM Gal-9. Once Gal-9 was shown capable of binding P. aeruginosa–derived LPS, we next investigated whether Gal-9 could directly bind to bacteria. To this end, both S. aureus and P. aeruginosa cells were fixed with paraformaldehyde and incubated overnight with Gal-9 (50 nM). Importantly, Gal-9 did not bind to S. aureus (Fig. 7B, 7D), but in contrast, Gal-9 was detected on the surface of P. aeruginosa bacteria (Fig. 7C, 7D). Subsequent experiments were designed to investigate whether Gal-9 opsonization of P. aeruginosa–enhanced phagocytosis of bacteria. For analyzing neutrophil phagocytosis, FITC-labeled bacteria (5 × 107 of either Gal-9 opsonized or unopsonized) and neutrophils were mixed at 37°C at a 10:1 ratio. The fluorescence of bacteria that were either unphagocytosed or adhered to the outer membrane of the cell was quenched by trypan blue prior to analyses by flow cytometry. Results revealed a significant 4-fold increase in the level of phagocytosed Gal-9–opsonized bacteria compared with unopsonized (p < 0.0001) (Fig. 7E). Thus, Gal-9 was revealed as a novel pathogen recognition molecule, specific for Gram-negative bacteria, and in particular P. aeruginosa.
FIGURE 7.
Gal-9 binds to P. aeruginosa but not to S. aureus. (A) High-binding 96-well plates were coated with commercial P. aeruginosa LPS (50 μg/ ml). Gal-9 (0–500 nM) was added, and binding was assessed after incubation with Gal-9 Ab (10 μg/ml) followed by an HRP-conjugated Ab. After a final wash, ABTS was added and Gal-9/LPS binding confirmed by measuring absorbance at 405 nm. Results are shown as mean ± SE and representative of two independent experiments performed in triplicate. (B and C) Paraformaldehyde-fixed S. aureus or P. aeruginosa (1 × 106) was incubated overnight at 4°C in PBS in the presence or absence of 50 nM Gal-9. After washing, bacteria were incubated with 20 μl PE-labeled Gal-9 Ab or PE-labeled mouse IgG control (black filled) for 30 min. Gal-9 binding to S. aureus (B) or P. aeruginosa (C) was assessed by flow cytometry, and results are representative of three independent experiments. (D) Quantification of Gal-9 binding to bacteria expressed as MFI. Data shown as mean MFI ± SE of three independent experiments and analyzed by Student t test compared with Gal-9–untreated samples, *p < 0.05. (E) Phagocytosis of either unopsonized or Gal-9 (50 nM) opsonized FITC-labeled P. aeruginosa was analyzed by flow cytometry. Data shown as mean MFI ± SE of n = 3 independent experiments with neutrophils from different donors. Statistical significance analyzed by Student t test, ***p < 0.0001.
Gal-9 opsonization of P. aeruginosa induces neutrophil TIM-3–mediated bacterial killing, an antimicrobial mechanism potentially perturbed in the CF airways
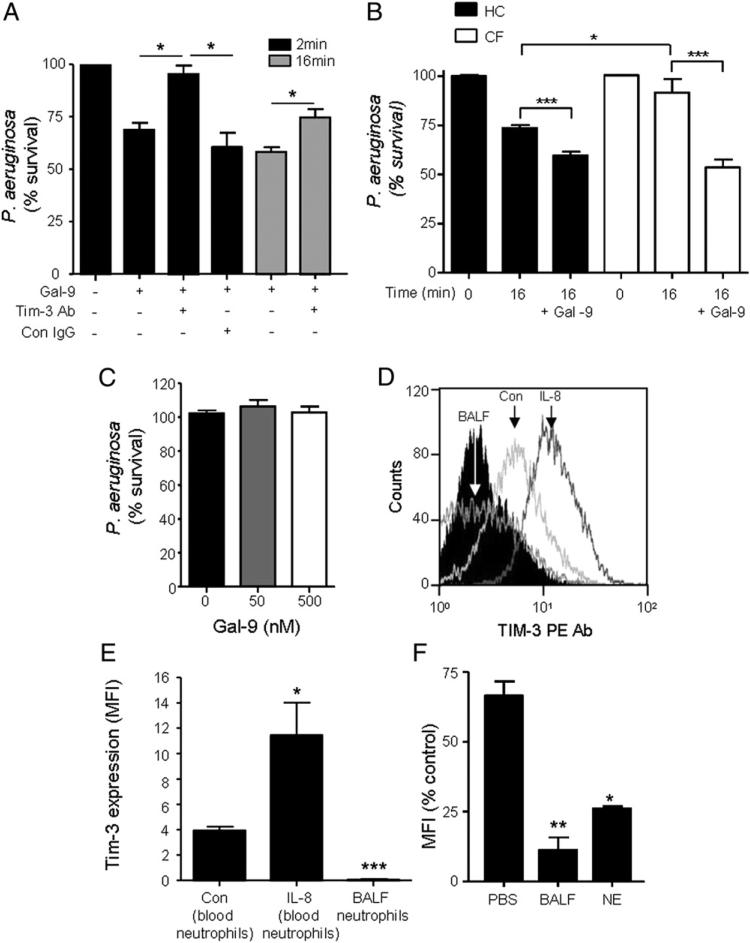
Having established that Gal-9 can bind to Gram-negative bacteria via interaction with LPS on P. aeruginosa, we next investigated whether Gal-9 opsonization would be sufficient to induce bacterial killing by neutrophils. To this end, P. aeruginosa bacteria were opsonized with Gal-9 instead of serum and then employed in a neutrophil-killing assay. As shown in Fig. 8A, opsonization with 50 nM Gal-9 was sufficient to induce on average 28% more killing after 2 min compared with nonopsonized bacteria (p < 0.05). This enhanced killing promoted by opsonization with Gal-9 was sustained after 16-min incubation with an average increase in P. aeruginosa killing of 46% observed (p < 0.05). Conversely, blockade of TIM-3 by use of a TIM-3–blocking Ab abrogated the promotion of Gal-9–opsonized (50 nM) P. aeruginosa killing. This effect was not observed when neutrophils were pretreated with a relevant mouse IgG control. Moreover, increasing the dose of Gal-9 during the opsonization process to 100 or 500 nM did not increase bacterial killing (result not shown).
FIGURE 8.
Gal-9–evoked enhanced P. aeruginosa killing is TIM-3 mediated. (A) Neutrophils (1 × 107) were incubated for 10 min in the presence or absence of 10 μg/ml TIM-3–blocking Ab (TIM-3 Ab) or mouse IgG control Ab (Con IgG). P. aeruginosa remained unopsonized or opsonized with 50 nM Gal-9 and were then incubated with neutrophils at a ratio of 1:1 for 2 or 16 min. Serial dilutions were plated on tryptic soy agar plates in triplicate. Blockade of TIM-3 reversed the enhanced killing induced by P. aeruginosa opsonization with Gal-9. Results shown are mean ± SE of n = 3 independent experiments. Percentage P. aeruginosa survival in samples treated with TIM-3–blocking Ab was not significantly different from nonopsonized bacteria. All other treatments were statistically significant. Data analyzed compared with Gal-9 unopsonized bacteria by Student t test. (B) Neutrophils from healthy individuals (HC) or PWCF (n = 3) were incubated with either unopsonized or Gal-9 (50 nM) opsonized bacteria. Aliquots were removed at 0 and 16 min and P. aeruginosa percentage survival determined. Data shown as mean MFI ± SE of n = 3 independent experiments with neutrophils from different donors. Statistical significance calculated by Student t test, *p < 0.05, ***p < 0.0001. (C) P. aeruginosa was incubated with and without Gal-9 (50 and 500 nM) for 30 min and bacterial survival determined. (D and E) Flow cytometry analysis of TIM-3 membrane expression on unstimulated (Con) and IL-8–stimulated purified blood neutrophils and BALF neutrophils from PWCF (n = 3). Relative isotype control Ab, rat IgG2A, is illustrated (black filled histogram). (F) Neutrophils (1 × 106/ml) were incubated in PBS, 200 μg of CF BALF, or 100 nM NE at 37°C. Levels of TIM-3 on the cell surface were measured by flow cytometry. Bar graph shows MFI peak values for each treatment. Data shown in (E) and (F) are mean ± SE; n = 3. Statistical significance calculated by Student t test. *p < 0.05, **p < 0.01 compared with control or PBS cells, respectively.
Of major relevance to bacterial killing in CF, a previous study reported defective neutrophil intraphagosomal killing of P. aeruginosa. Thus, it was important to extend our experiments to compare the effect of Gal-9 on the level of bacterial killing by healthy control and CF neutrophils (Fig. 8B). By using unopsonized bacteria, the pattern of in vitro killing was in agreement with previous reports describing increased killing of P. aeruginosa by control cells compared pared with CF neutrophils (bacterial survival 73.66 ± 1.39 and 91.64 ± 6.82 for healthy control and CF cells after 16 min incubation, respectively; p < 0.05). Opsonization of bacteria with Gal-9 (50 nM) increased killing by ~14 and 38% by control and CF cells, respectively, with no significant difference in the level of killing observed between the two cell types after 16-min incubation (bacterial survival 59.52 ± 1.9 and 53.5 ± 4.1 for healthy control and CF cells, respectively). In addition, the observed increased bactericidal activity prompted us to evaluate whether Gal-9 itself exhibited bactericidal or bacteriostatic properties. Our results showed that Gal-9 at physiological (50 nM) or a high dose (500 nM) did not display bactericidal properties against P. aeruginosa, indicating that Gal-9 was not directly involved in bacterial killing, and the bactericidal properties required the involvement of phagocytes (Fig. 8C).
We have previously shown that TIM-3 and Gal-9 are completely absent in the adult CF lung due to proteolytic degradation by neutrophil-derived proteases (NE and proteinase 3) (35). The lack of bacterial killing via the TIM-3/Gal-9 mechanism may have a negative impact on the eradication of Gram-negative bacterial infections, which may in part explain chronic colonization by P. aeruginosa of the CF lower airways. Therefore, we next evaluated the fate of neutrophil-associated TIM-3 under conditions prevailing in the CF airways. For this experiment, circulating and BALF neutrophils were isolated from PWCF (n = 3). TIM-3 expression was detected on membranes of peripheral blood neutrophils from PWCF, and the expression was significantly increased postexposure to IL-8 (p < 0.05) (Fig. 8D). The MFI of TIM-3 on peripheral neutrophils from PWCF was 4.8 ± 0.16 and following stimulation with IL-8 was 11.45 ± 2.56 (Fig. 8E). In contrast, negligible levels of TIM-3 were detected on neutrophils isolated from CF BALF (Fig. 8D, 8E). To understand whether the lack of TIM-3 on CF BALF neutrophils was due to proteolytic degradation by neutrophil-derived proteases, healthy control neutrophils were treated with CF BALF (200 μg) or 100 nM NE for 1 or 2 h, respectively, at 37°C in PBS. The remaining levels of TIM-3 on the cell outer surface were measured by flow cytometry. Both CF BALF and NE significantly decreased the level of TIM-3 cell outer surface expression (p < 0.01 and p < 0.05, respectively) (Fig. 8F). These results confirm the ability of neutrophil-derived serine proteases to degrade native neutrophil TIM-3 in vivo.
Collectively, the results of this study demonstrate that TIM-3 expressed on neutrophils plays a direct role in bacterial killing. Opsonization of P. aeruginosa with a physiologically relevant dose of Gal-9 promoted significant bacterial killing that could be reversed by blockade of neutrophil TIM-3 receptors. This previously unidentified mechanism for neutrophil killing of P. aeruginosa is likely to be disrupted in the CF lung due to protease action with important consequences for bacterial clearance.
Discussion
TIM-3 was initially described as a marker of Th1 cells (25), and since then, it has been shown to be expressed in a variety of immune cells including Th17 (27), dendritic cells (58), NK cells (59), NKT cells (20), monocytes (20), macrophages (53, 60), and mast cells (61). A study also examined whether TIM-3 was expressed on neutrophils and found a population of TIM-3+ CD11b+Ly-6G+ cells (neutrophils) in peritoneal lavage fluid from septic mice; however, the role of TIM-3–mediated signaling in neutrophils was not determined (30). Moreover, specific studies on the role of Gal-9 signaling via TIM-3 are rare within the literature. Indeed, to the best of our knowledge, the only report on the effect of Gal-9 in human neutrophils is a study on the lack of neutrophil chemotactic activity as opposed to eosinophils (62), although TIM-3 involvement was not investigated. TIM-3 has been shown to play a role in activation and release of inflammatory mediators including IL-1β (22), NO (53), TNF-α (58), and IL-12 (63). Of major interest to the present project, TIM-3 has also been implicated in bacteria killing (53), and TIM-3/Gal-9 involvement in control of intracellular pathogenic M. tuberculosis in human macrophages has been demonstrated (64). Moreover, data have shown that E. coli killing by human peripheral monocytic cells was TIM-3 dependent, as phagocytosis and killing mechanisms were inhibited in the presence of soluble TIM-3 (53). Within the current study, we investigated TIM-3/Gal-9–mediated neutrophil activity with focus on the prototypical neutrophil role as an antimicrobial mediator.
Our data demonstrate TIM-3 expression in resting neutrophils by Western blot analysis of whole neutrophil protein obtained by TCA precipitation. This technique preserves neutrophil proteins and protects them from rapid proteolytic degradation (65). These results were further confirmed by flow cytometric analysis of resting neutrophils or cells exposed to proinflammatory stimuli, which indicated that TIM-3 is present on the neutrophil membrane. brane. In line with studies indicating intracellular storage of receptors, with prompt upregulation on the neutrophil surface upon exposure to inflammatory stimuli (66), results revealed up-regulation of TIM-3 in response to both IL-8 and TNF-α. However, in contrast with the observed upregulation of TIM-3 in CF bronchial epithelial cells (35), CF circulating neutrophils did not show significantly altered expression compared with healthy control cells, supporting the notion of differential TIM-3 expression depending on cell type and activation stage. Moreover, surface expression of neutrophil receptors change following recruitment to sites of inflammation. For example, C5a receptor CD11b and FcγRII have been shown to be upregulated in human extravasated neutrophils, whereas l-selectin was down-regulated on the surface of recruited cells (67). Therefore, to assess whether TIM-3 would still be present on neutrophils recruited to a site of infection/inflammation, TIM-3 expression was examined in extravasated peritoneal neutrophils obtained after thioglycollate injection. TIM-3 surface expression was confirmed in this context, suggesting that TIM-3 is likely present on the surface of trans-migrating cells, thus supporting previous studies (30). Moreover, Gal-9 has been shown to induce tyrosine phosphorylation in several cell types (47, 58) and in this study, TIM-3 phosphorylation upon Gal-9 stimulation was demonstrated in neutrophils, indicating the presence of a functional TIM-3 receptor. This observation is of importance, as it suggests that epithelial TIM-3 plays an active role and does not merely function as a scavenger receptor as reported for TIM-1 in kidney cells (68).
Although a role for Gal-9 in the neutrophil respiratory burst has not been reported to date, galectins have been shown to be capable of directly inducing O2− production in primed neutrophils (69, 70). Indeed, Gal-3 has been shown to induce O2− release in unstimulated neutrophils, albeit at micromolar concentrations (49). In this study, Gal-9 could only promote O2− generation at supraphysiological concentrations .50 nM. However, a low dose of Gal-9 (50 nM) was shown to be sufficient to enhance fMLF-induced O2− production, suggesting that Gal-9 can act as a priming agent for the NADPH oxidase at physiological concentrations. Additionally, our data show that Gal-9 is capable of inducing neutrophil degranulation via signaling through TIM-3. These results are consolidated by experiments providing evidence that Gal-9 can induce intracellular Ca2+ mobilization, a perquisite for both oxidase and degranulation functionality. Taken together, we have identified a novel role for TIM-3/Gal-9 in neutrophil function with potentially important consequences in neutrophil antimicrobial activity.
Having established a role for TIM-3/Gal-9 interactions in neutrophil activity, ensuing experiments focused on the bactericidal role of TIM-3 and Gal-9. TIM-3 has previously been implicated in bacterial killing although the proposed mechanisms of action are diverse. In a mouse model of tuberculosis infection, M. tuberculosis–infected macrophages increased intracellular killing following stimulation by TIM-3–expressing T cells (22). In this paradigm, Gal-9 served as a cell-surface receptor in macrophages, and TIM-3 was the ligand. Gal-9 activation by TIM-3 promoted caspase-1 processing and release of IL-1β, which stimulated intracellular killing in an autocrine manner. In contrast, soluble TIM-3 markedly reduced E. coli killing by human peripheral blood monocytic cells (53). In this model, TIM-3 expressed in phagocytes appeared to be directly involved in phagocytosis and intracellular production of reactive oxygen and nitrogen species. In the current study, to clarify if soluble TIM-3 enhanced or blocked bacterial killing by neutrophils, neutrophils isolated from whole blood were incubated with 5 μg/ml recombinant TIM-3 fusion protein, a dose within the range employed in previous studies (22, 53). In the current study, TIM-3 treatment inhibited P. aeruginosa neutrophil-mediated killing, whereas it exerted no effect on the level of S. aureus killing. Our data suggest that TIM-3 expressed in neutrophils is directly involved in bacterial killing, in agreement with previous results linking TIM-3 function on phagocytes with E. coli killing (53). Moreover, the inclusion of recombinant TIM-3 significantly reduced killing of serum opsonized bacteria, in line with reports on plasma levels of Gal-9. Indeed, galectins can act as pathogen recognition molecules against a wide range of microorganisms, although different affinities for specific glycan epitopes may determine the immune response in the host. For instance, both Gal-3 and Gal-9 bind to L. major poly-β-galactosyl epitopes, but only Gal-9 induced uptake of the parasite by macrophages (71). Moreover, LPS from different bacterial species has been shown to bind to Gal-3, including Klebsiella pneumonia (44), E. coli, Salmonella typhimurium (43), and, notably, P. aeruginosa (42). LPS interaction with Gal-3 appeared to occur at specific sites, including recognition of the lipid A/inner core region or the o-polysaccharide chain (44). The N-terminal part of Gal-3 has also been shown to bind specifically to the rough form of LPS (LPS devoid of side chains), and in contrast, the C-terminal part of Gal-3 specifically interacted with β-galactoside present on side chains of smooth LPS (LPS containing the o-polysaccharide chain). To the best of our knowledge, Gal-9 has not previously been reported to bind to LPS, and data demonstrating that Gal-9 bound to P. aeruginosa, but not S. aureus, corroborated the selective role of Gal-9 in P. aeruginosa uptake and killing and potentially other Gram-negative bacteria expressing LPS.
The data from this study indicated that Gal-9 had no direct effect on viability of bacteria, but Gal-9 opsonization could promote bacterial killing by neutrophils from healthy control and PWCF. This effect was achieved with 50 nM opsonization, and surprisingly, incubation with higher levels of Gal-9 did not increase bacterial killing. Concentrations of Gal-9 previously recorded in the airways are between 1 and 18 nM (35, 72), which is within the range employed in the current study. Blockade of TIM-3 abrogated the neutrophil-mediated killing of P. aeruginosa promoted by Gal-9 opsonization, confirming the implication of TIM-3 in the observed bactericidal effects. Our data suggest that TIM-3 is involved in killing of Gram-negative bacteria, in particular P. aeruginosa via interaction with Gal-9 that binds to bacterial LPS. Of note, the best intracellular killing effect and calcium flux was observed at ~2 min, yet two activation processes involving O2− and degranulation, which were measured extracellular, were recorded up to 15 min. The discrepancy in the timing of the resultant cellular processes is likely due to the difference in intracellular and extracellular fluid volumes, and for example, the amount of protein that must be degranulated to the outside of the cell before detection by Western blotting.
We have previously shown that both TIM-3 and Gal-9 are completely absent in the adult CF lung due to proteolytic degradation (35), and in the current study, we observed reduced levels of TIM-3 on CF BALF neutrophils and healthy control cells exposed to CF BALF or NE. The lack of bacterial killing via this novel TIM-3/Gal-9 mechanism may have a negative impact on the eradication of Gram-negative bacterial infections, which may in part explain why P. aeruginosa is a successful colonizer of the CF lower airways. CF infants are initially infected with S. aureus and H. influenzae, but soon become infected with P. aeruginosa (73). Initially, these infections may be sporadic and alternate different strains, usually presenting a nonmucoid phenotype and responsive to antibiotic therapy (74). Eventually, chronic colonization ensues, generally by a single infecting strain with a mucoid phenotype (75, 76). The median age for the onset of P. aeruginosa colonization has been traditionally established at ~10 y of age (75); however, P. aeruginosa presence in the lungs of preschool children has been reported in several studies (74, 77, 78). Interestingly, the onset of P. aeruginosa infection coincides in time with the appearance of elevated levels of NE and a concomitant decline in Gal-9 presence in BALF (35). The absence of Gal-9 and/or TIM-3 may result in impaired P. aeruginosa clearance by neutrophils and promote colonization. Therefore, early intervention with aerosolized proteolytic-resistant Gal-9 could be beneficial in preventing bacterial colonization in the CF airways, preferably in conjunction with NE inhibitors such as α-1 antitrypsin (AAT). Aerosolization of AAT has previously been shown to increase AAT levels and restore anti-NE capacity in lung epithelium lining fluid of PWCF (79). Aerosolized AAT was found to positively impact upon neutrophil-mediated killing of Pseudomonas (80), possibly by preventing cleavage of complement receptors by serine proteases (81) or by preventing cleavage of CXCR1 (82). The results of the current study further support aerosolization of AAT for maintaining neutrophil membrane levels of TIM-3.
In conclusion, our data suggest a novel role for TIM-3/Gal-9 in neutrophil function and demonstrate that TIM-3 expressed on neutrophils plays a direct role in bacterial killing. Enhanced neutrophil-mediated killing of Gal-9–opsonized Pseudomonas was observed, an antimicrobial effect perturbed in the CF airways. The rapid degradation of membrane TIM-3 by serine proteases could potentially contribute to the defective bacterial clearance observed within the CF lung despite the high neutrophilic presence.
Acknowledgments
We thank Prof. A. W. Segal and Dr. P. Behe, The Rayne Institute, Centre for Molecular Medicine, University College London, for tremendous help on TIM-3 murine studies. We also thank all of the healthy volunteers and PWCF who participated in this study.
This work was supported by the Health Research Board, Science Foundation Ireland (Grant 11/RFP/BMT/3094) and the Program for Research in Third Level Institutes administered by the Higher Education Authority Ireland.
Abbreviations used in this article
- AAT
a-1 antitrypsin
- BALF
bronchoalveolar lavage fluid
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- Cyto-B
cytochalasin B
- Gal-9
galectin-9
- MFI
mean fluorescence intensity
- MMP-9
matrix metalloprotease-9
- MPO
myeloperoxidase
- NE
neutrophil elastase
- O2−
superoxide
- PBSG
PBS containing 5 mM glucose
- PWCF
people with cystic fibrosis
- rhTIM-3
recombinant human T cell Ig and mucin domain– containing molecule-3
- TIM-3
T cell Ig and mucin domain–containing molecule-3
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 4.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann. Hum. Genet. 2003;67:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 5.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 6.Davis PB. Cystic fibrosis since 1938. Am. J. Respir. Crit. Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 7.Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes E, Pohl K, McElvaney NG, Reeves EP. The cystic fibrosis neutrophil: a specialized yet potentially defective cell. Arch. Immunol. Ther. Exp. (Warsz.) 2011;59:97–112. doi: 10.1007/s00005-011-0113-6. [DOI] [PubMed] [Google Scholar]
- 9.Brennan S, Cooper D, Sly PD. Directed neutrophil migration to IL-8 is increased in cystic fibrosis: a study of the effect of erythromycin. Thorax. 2001;56:62–64. doi: 10.1136/thorax.56.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexis NE, Muhlebach MS, Peden DB, Noah TL. Attenuation of host defense function of lung phagocytes in young cystic fibrosis patients. J. Cyst. Fibros. 2006;5:17–25. doi: 10.1016/j.jcf.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koller DY, Urbanek R, Götz M. Increased degranulation of eosinophil and neutrophil granulocytes in cystic fibrosis. Am. J. Respir. Crit. Care Med. 1995;152:629–633. doi: 10.1164/ajrccm.152.2.7633718. [DOI] [PubMed] [Google Scholar]
- 12.Taggart C, Coakley RJ, Greally P, Canny G, O'Neill SJ, McElvaney NG. Increased elastase release by CF neutrophils is mediated by tumor necrosis factor-alpha and interleukin-8. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L33–L41. doi: 10.1152/ajplung.2000.278.1.L33. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Meng J, Wang X, Liu S, Shu Q, Gao L, Ju Y, Zhang L, Sun W, Ma C. Expression of human TIM-1 and TIM-3 on lymphocytes from systemic lupus erythematosus patients. Scand. J. Immunol. 2008;67:63–70. doi: 10.1111/j.1365-3083.2007.02038.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat. Rev. Immunol. 2008;8:577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 15.Su EW, Lin JY, Kane LP. TIM-1 and TIM-3 proteins in immune regulation. Cytokine. 2008;44:9–13. doi: 10.1016/j.cyto.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol. Rev. 2009;229:259–270. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recalcati S, Invernizzi P, Arosio P, Cairo G. New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J. Autoimmun. 2008;30:84–89. doi: 10.1016/j.jaut.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, Ito K, Takeshita K, Niki T, Saita N, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin. Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Rennert PD, Ichimura T, Sizing ID, Bailly V, Li Z, Rennard R, McCoon P, Pablo L, Miklasz S, Tarilonte L, Bonventre JV. T cell, Ig domain, mucin domain-2 gene-deficient mice reveal a novel mechanism for the regulation of Th2 immune responses and airway inflammation. J. Immunol. 2006;177:4311–4321. doi: 10.4049/jimmunol.177.7.4311. [DOI] [PubMed] [Google Scholar]
- 20.Khademi M, Illés Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris RA, et al. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J. Immunol. 2004;172:7169–7176. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, Kuchroo VK, Behar SM. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 2010;207:2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:345–349. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 25.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 26.Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3–mediated cell death and exhaustion. Nat. Med. 2012;18:1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vij N, Mazur S, Zeitlin PL. CFTR is a negative regulator of NFkappaB mediated innate immune response. PLoS ONE. 2009;4:e4664. doi: 10.1371/journal.pone.0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter MJ, Treharne KJ, Winter AK, Cassidy DM, Land S, Mehta A. Expression of wild-type CFTR suppresses NF-kappaB-driven inflammatory signalling. PLoS ONE. 2010;5:e11598. doi: 10.1371/journal.pone.0011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, Zhou T, Li Y, Guo X, Xiao H, et al. T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. J. Immunol. 2013;190:2068–2079. doi: 10.4049/jimmunol.1202661. [DOI] [PubMed] [Google Scholar]
- 31.Schorn C, Janko C, Krenn V, Zhao Y, Munoz LE, Schett G, Herrmann M. Bonding the foe - NETting neutrophils immobilize the proinflammatory monosodium urate crystals. Front Immunol. 2012;3:376. doi: 10.3389/fimmu.2012.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 33.Bergin DA, Reeves EP, Meleady P, Henry M, McElvaney OJ, Carroll TP, Condron C, Chotirmall SH, Clynes M, O'Neill SJ, McElvaney NG. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J. Clin. Invest. 2010;120:4236–4250. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirouvanziam R, Gernez Y, Conrad CK, Moss RB, Schrijver I, Dunn CE, Davies ZA, Herzenberg LA, Herzenberg LA. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc. Natl. Acad. Sci. USA. 2008;105:4335–4339. doi: 10.1073/pnas.0712386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vega-Carrascal I, Reeves EP, Niki T, Arikawa T, McNally P, O'Neill SJ, Hirashima M, McElvaney NG. Dysregulation of TIM-3-galectin-9 pathway in the cystic fibrosis airways. J. Immunol. 2011;186:2897–2909. doi: 10.4049/jimmunol.1003187. [DOI] [PubMed] [Google Scholar]
- 36.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 37.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 38.Watt AP, Courtney J, Moore J, Ennis M, Elborn JS. Neutrophil cell death, activation and bacterial infection in cystic fibrosis. Thorax. 2005;60:659–664. doi: 10.1136/thx.2004.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergin DA, Greene CM, Sterchi EE, Kenna C, Geraghty P, Belaaouaj A, Taggart CC, O'Neill SJ, McElvaney NG. Activation of the epidermal growth factor receptor (EGFR) by a novel metalloprotease pathway. J. Biol. Chem. 2008;283:31736–31744. doi: 10.1074/jbc.M803732200. [Published erratum appears in 2009. J. Biol. Chem. 284: 9624.] [DOI] [PubMed] [Google Scholar]
- 41.Weingart CL, Broitman-Maduro G, Dean G, Newman S, Peppler M, Weiss AA. Fluorescent labels influence phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 1999;67:4264–4267. doi: 10.1128/iai.67.8.4264-4267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta SK, Masinick S, Garrett M, Hazlett LD. Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect. Immun. 1997;65:2747–2753. doi: 10.1128/iai.65.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Komai-Koma M, Gilchrist DS, Hsu DK, Liu F-T, Springall T, Xu D. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J. Immunol. 2008;181:2781–2789. doi: 10.4049/jimmunol.181.4.2781. [DOI] [PubMed] [Google Scholar]
- 44.Mey A, Leffler H, Hmama Z, Normier G, Revillard JP. The animal lectin galectin-3 interacts with bacterial lipopolysaccharides via two independent sites. J. Immunol. 1996;156:1572–1577. [PubMed] [Google Scholar]
- 45.Janusonis S. Comparing two small samples with an unstable, treatment-independent baseline. J. Neurosci. Methods. 2009;179:173–178. doi: 10.1016/j.jneumeth.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Idali F, Wahlström J, Dahlberg B, Khademi M, Olsson T, Eklund A, Grunewald J. Altered expression of T cell immunoglobulin-mucin (TIM) molecules in bronchoalveolar lavage CD4+ T cells in sarcoidosis. Respir. Res. 2009;10:42. doi: 10.1186/1465-9921-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem. Biophys. Res. Commun. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 48.Witko-Sarsat V, Allen RC, Paulais M, Nguyen AT, Bessou G, Lenoir G, Descamps-Latscha B. Disturbed myeloperoxidase-dependent activity of neutrophils in cystic fibrosis homozygotes and heterozygotes, and its correction by amiloride. J. Immunol. 1996;157:2728–2735. [PubMed] [Google Scholar]
- 49.Yamaoka A, Kuwabara I, Frigeri LG, Liu FT. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J. Immunol. 1995;154:3479–3487. [PubMed] [Google Scholar]
- 50.Kelher MR, Ambruso DR, Elzi DJ, Anderson SM, Paterson AJ, Thurman GW, Silliman CC. Formyl-Met-Leu-Phe induces calcium-dependent tyrosine phosphorylation of Rel-1 in neutrophils. Cell Calcium. 2003;34:445–455. doi: 10.1016/s0143-4160(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 51.Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, Nakamura T, Hirashima M. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J. Immunol. 2003;170:3631–3636. doi: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- 52.Nagahara K, Arikawa T, Oomizu S, Kontani K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M, et al. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J. Immunol. 2008;181:7660–7669. doi: 10.4049/jimmunol.181.11.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao J, Lei Z, Liu Y, Li B, Zhang L, Fang H, Song C, Wang X, Zhang GM, Feng ZH, Huang B. Human pregnancy up-regulates Tim-3 in innate immune cells for systemic immunity. J. Immunol. 2009;182:6618–6624. doi: 10.4049/jimmunol.0803876. [DOI] [PubMed] [Google Scholar]
- 54.Chagan-Yasutan H, Saitoh H, Ashino Y, Arikawa T, Hirashima M, Li S, Usuzawa M, Oguma S, O Telan EF, Obi CL, Hattori T. Persistent elevation of plasma osteopontin levels in HIV patients despite highly active antiretroviral therapy. Tohoku J. Exp. Med. 2009;218:285–292. doi: 10.1620/tjem.218.285. [DOI] [PubMed] [Google Scholar]
- 55.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M, et al. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS ONE. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pelletier I, Sato S. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J. Biol. Chem. 2002;277:17663–17670. doi: 10.1074/jbc.M201562200. [DOI] [PubMed] [Google Scholar]
- 57.Fradin C, Poulain D, Jouault T. beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect. Immun. 2000;68:4391–4398. doi: 10.1128/iai.68.8.4391-4398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 59.Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao D, Liu Y, Zhu F, Zhang L, Sun W, et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J. Hepatol. 2010;52:322–329. doi: 10.1016/j.jhep.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113:3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- 61.Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, Galli SJ. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumoto R, Matsumoto H, Seki M, Hata M, Asano Y, Kanegasaki S, Stevens RL, Hirashima M. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J. Biol. Chem. 1998;273:16976–16984. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Ma CJ, Wang JM, Ji XJ, Wu XY, Moorman JP, Yao ZQ. Tim-3 regulates pro- and anti-inflammatory cytokine expression in human CD14+ monocytes. J. Leukoc. Biol. 2012;91:189–196. doi: 10.1189/jlb.1010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sada-Ovalle I, Chávez-Galán L, Torre-Bouscoulet L, Nava-Gamiño L, Barrera L, Jayaraman P, Torres-Rojas M, Salazar-Lezama MA, Behar SM. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. J. Immunol. 2012;189:5896–5902. doi: 10.4049/jimmunol.1200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Painter RG, Valentine VG, Lanson NA, Jr., Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef WM, Wang G, Wang G. CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45:10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sengeløv H, Boulay F, Kjeldsen L, Borregaard N. Subcellular localization and translocation of the receptor for N-formylmethionyl-leucylphenylalanine in human neutrophils. Biochem. J. 1994;299:473–479. doi: 10.1042/bj2990473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch J. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin. Exp. Immunol. 2009;155:559–566. doi: 10.1111/j.1365-2249.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Almkvist J, Dahlgren C, Leffler H, Karlsson A. Activation of the neutrophil nicotinamide adenine dinucleotide phosphate oxidase by galectin-1. J. Immunol. 2002;168:4034–4041. doi: 10.4049/jimmunol.168.8.4034. [DOI] [PubMed] [Google Scholar]
- 70.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- 71.Farnworth SL, Henderson NC, Mackinnon AC, Atkinson KM, Wilkinson T, Dhaliwal K, Hayashi K, Simpson AJ, Rossi AG, Haslett C, Sethi T. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am. J. Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katoh S, Nobumoto A, Matsumoto N, Matsumoto K, Ehara N, Niki T, Inada H, Nishi N, Yamauchi A, Fukushima K, Hirashima M. Involvement of galectin-9 in lung eosinophilia in patients with eosinophilic pneumonia. Int. Arch. Allergy Immunol. 2010;153:294–302. doi: 10.1159/000314371. [DOI] [PubMed] [Google Scholar]
- 73.Cystic Fibrosis Foundation Patient Registry . 2009 Annual Data Report to the Center of Directors. Cystic Fibrosis Foundation; Bethesda, MD.: 2010. [Google Scholar]
- 74.Burns JL, Gibson RL, McNamara S, Yim D, Emerson J, Rosenfeld M, Hiatt P, McCoy K, Castile R, Smith AL, Ramsey BW. Longi tudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 2001;183:444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 75.Cramer N, Wiehlmann L, Tümmler B. Clonal epidemiology of Pseudomonas aeruginosa in cystic fibrosis. Int. J. Med. Microbiol. 2010;300:526–533. doi: 10.1016/j.ijmm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Govan JR, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dakin CJ, Numa AH, Wang HE, Morton JR, Vertzyas CC, Henry RL. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2002;165:904–910. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- 78.Pittman JE, Calloway EH, Kiser M, Yeatts J, Davis SD, Drumm ML, Schechter MS, Leigh MW, Emond M, Van Rie A, Knowles MR. Age of Pseudomonas aeruginosa acquisition and subsequent severity of cystic fibrosis lung disease. Pediatr. Pulmonol. 2011;46:497–504. doi: 10.1002/ppul.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hubbard RC, McElvaney NG, Sellers SE, Healy JT, Czerski DB, Crystal RG. Recombinant DNA-produced alpha 1-antitrypsin administered by aerosol augments lower respiratory tract antineutrophil elastase defenses in individuals with alpha 1-antitrypsin deficiency. J. Clin. Invest. 1989;84:1349–1354. doi: 10.1172/JCI114305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McElvaney NG, Hubbard RC, Birrer P, Chernick MS, Caplan DB, Frank MM, Crystal RG. Aerosol alpha 1-antitrypsin treatment for cystic fibrosis. Lancet. 1991;337:392–394. doi: 10.1016/0140-6736(91)91167-s. [DOI] [PubMed] [Google Scholar]
- 81.Berger M, Sorensen RU, Tosi MF, Dearborn DG, Döring G. Complement receptor expression on neutrophils at an inflammatory site, the Pseudomonas-infected lung in cystic fibrosis. J. Clin. Invest. 1989;84:1302–1313. doi: 10.1172/JCI114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hartl D, Latzin P, Hordijk P, Marcos V, Rudolph C, Woischnik M, Krauss-Etschmann S, Koller B, Reinhardt D, Roscher AA, et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat. Med. 2007;13:1423–1430. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]