Abstract
Alterations of intestinal microflora may significantly contribute to the pathogenesis of different inflammatory and autoimmune disorders. There is emerging interest on the role of selective modulation of microflora in inducing benefits in inflammatory intestinal disorders, by as probiotics, prebiotics, synbiotics, antibiotics, and fecal microbiota transplantation (FMT). To summarize recent evidences on microflora modulation in main intestinal inflammatory disorders, PubMed was searched using terms microbiota, intestinal flora, probiotics, prebiotics, fecal transplantation. More than three hundred articles published up to 2015 were selected and reviewed. Randomized placebo-controlled trials and meta-analysis were firstly included, mainly for probiotics. A meta-analysis was not performed because of the heterogeneity of these studies. Most of relevant data derived from studies on probiotics, reporting some efficacy in ulcerative colitis and in pouchitis, while disappointing results are available for Crohn’s disease. Probiotic supplementation may significantly reduce rates of rotavirus diarrhea. Efficacy of probiotics in NSAID enteropathy and irritable bowel syndrome is still controversial. Finally, FMT has been recently recognized as an efficacious treatment for recurrent Clostridium difficile infection. Modulation of intestinal flora represents a very interesting therapeutic target, although it still deserves some doubts and limitations. Future studies should be encouraged to provide new understanding about its therapeutical role.
Keywords: Microbiota, Inflammation, Gut, Probiotic, Prebiotic
Core tip: Alterations of intestinal microflora may significantly contribute to the pathogenesis of different inflammatory and autoimmune disorders. It is conceivable that selective modulation of intestinal microflora may induce benefits. In this article we tried to summarize recent evidences on microflora modulation in main intestinal inflammatory disorders, providing practical perspectives on its therapeutical role in these conditions.
INTRODUCTION
Gut microbiota
Definition: The human intestinal microflora, known as “microbiota”, includes 100 trillion (1014) bacteria, quadrillion viruses, fungi, parasites, and archaea for a total weight of about 1 kg[1]. The stomach and small intestine are colonized only by a few species of bacteria, mainly because of the acid environment, the presence of bile and pancreatic secretions, and the peristaltic activity limiting bacteria stable colonization[2]. On the contrary, the colon harbours about 1012 microorganisms[3]. Also several yeasts (“gut mycome”) are included in the gut microbiota, but their role is still not well established[1,4,5].
Composition: Colonization of human gut starts few days after birth. Pattern of intestinal microbiota may be firstly influenced by type of delivery and type of diet[2] and, later, by immune stimulaton and environmental factors, becoming more stable after the first two years[6], even though it can be modified under some circumstances, such as diarrhea, antibiotic treatment, or dietary interventions. Gut microflora may be characterized by conventional culturing techniques which fail to detect more than 30% of total bacteria in the gut, therefore molecular approaches, such as metagenomic analysis and 16S ribosomal RNA gene sequencing, are now commonly used[7].
Bacteria in stomach, duodenum, and jejunum are mostly represented by oropharyngeal origin aerobic gram-positive ones, whereas in the ileum coliforms are the predominant species. Post ileocecal valve, there is a growing of bacterial anaerobic species, mainly Bacteroides, Bifidobacteria, Clostridi and Lactobacilli[8].
Functions: The main metabolic function is represented by the fermentation of large polysaccharides and some oligosaccharides, unabsorbed sugars, and host-derived carbohydrates from mucus glycoproteins[9]. The major products of carbohydrate metabolism are gasses (hydrogen and carbon dioxide), ethanol and short chain fatty acids (SCFAs)[2,9]. These latter, represented by butyric, propionic, and acetic acid, are important energy sources for colonocytes, and may inflence glucose metabolism[10]. Colonic bacteria are also involved in vitamin synthesis, absorption of calcium, magnesium, and iron[11,12].
Differentiation and proliferation of epithelial cells is greatly influenced by interaction with resident microflora. This effect is mainly mediated by the SCFAs which promote the development of intestinal microvilli[13].
Intestinal microbiota plays also a pivotal role in development of the gut immune system, especially during early life, establishing an efficacious “cross-talk” with the host[14,15]. Germ-free animals are characterized by a different and less complex gut immune system than normal animals, showing defects in gut-associated lymphoid tissue and antibody production[15]. On the contrary, immediately after exposure to luminal microbes, development of a complete and helpful immune system is stimulated by increase in the number of intraepithelial lymphocytes and production of both mucosal immunoglobulin in germinal centers than in serum[2,15]. It is known that cells of innate immunity are able to discriminate between pathogenic and harmless microbial components by “pattern recognition receptors”. Among them, toll-like receptors, mainly present on macrophages, neutrophils, dendritic cells (DCs), intestinal epithelial cells, enable these cells to recognize typical molecules present on microorganism, like lipopolysaccharides, peptidoglycans, flagellins, described as pathogen-associated molecular patterns, or better, microbe-associated molecular patterns[16-19]. It is probably that different populations of DCs are responsible for induction of tolerance for commensal while stimulating immune response for pathogens, by differentiation of naïve T cells into either regulatory T cells or effectors cells indispensable for clearing infections[15,19-22]. Components of normal microflora thus induce in the gut a state of “physiological” inflammatory response, maintained by balanced and controlled responses[19].
Non-pathogenic bacteria can also avoid access of pathogen bacteria into intestinal lumen by attachment to the epithelial cells[2]. Microbiota can regulate the production of the mucins from intestinal goblet cells, thus limiting access to pathogens. Moreover, commensal bacteria compete for nutrient availability in ecological niches[15].
RATIONAL AND APPROACHES OF MODULATION OF INTESTINAL MICROBIOTA
It is now generally accepted that the intestinal flora plays a key role in maintaining the host’s health status, while its alteration or a dysregulation of the intestinal immune response to normal bacterial environment may significantly contribute to the pathogenesis of different inflammatory and autoimmune disorders[23,24]. As for example, broad-spectrum antibiotics may suppress components of normal microbiota, leading other pathogenic organisms to grow up and cause disease. Otherwise, normal cross-talk host-microbiota may be altered and the constituents of the normal intestinal flora may be recognized not as friends, thus triggering an inappropriate inflammatory response. Moreover, when gut mucosa is injured, the “leaky gut” lets bacteria to pass from the gastrointestinal tract through the epithelial layer to the submucosa and even to the circulation, potentially disseminating throughout the body and causing sepsis, shock and multisystem organ failure[2,23].
For all these reasons, in the last years, interest on the eventual role of selective modulation of microflora in inducing benefits in inflammatory intestinal disorders has been growing.
At this regards, different approaches have been investigated, mainly represented by probiotics, but also prebiotics, synbiotics, antibiotics, and, lastly, fecal transplantation.
Probiotics
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the consumer”[25]. Probiotics include both bacteria and yeast. Most of probiotics include Lactobacillus species, Bifidobacterium species, Escherichia coli (E. coli), Streptococcus species, Lactococcus lactis and some Enterococcus species[26]. The most commonly used yeast is Saccharomyces boulardii. Probiotics must survive gastric acid and bile, so they can exert their effect in the small and large intestine[27,28]. Probiotics colonize the gut temporarily and act modifying colonic environment according to the fecal persistence of the ingested strains[28]. Different mechanisms are involved in the protective role of probiotics and are represented both by direct interaction with the host and by indirect modulation of the intestinal microbiota[29] (Figure 1 and Table 1).
Figure 1.
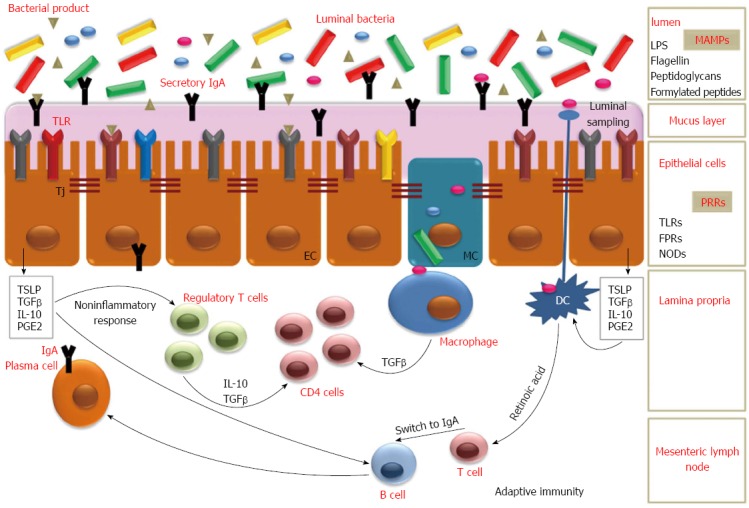
Interactions between luminal bacteria and intestine. The interaction between luminal bacteria and EC causes the production of cytokines, such as TGF-β, TSLP, IL-10 that may induce inflammatory cytokines synthesis from DC and mononuclear cells. These substances, together with retinoic acid produced by DC, can also promote the IgA switch in B cells. In Peyer’s patches DC and macrophages uptake bacterial antigens. The TSLP produced by TLR signaling, enhance APRIL and BAFF production from DC. Anti-inflammatory response is mainly mediated by TGF-β production by epithelial cells and IL-10 from mononuclear cells. Moreover, the interaction with commensal bacteria can result in T-cell expansion and regulation of Th-cells. Probiotics may modulate intestinal function, and in particular mucosal immunity, by secreted factors and by direct interactions with immune cells and the intestinal epithelium. EC: Enterocytes; MC: M cells; Tj: Tight junctions; TGF: Transforming growth factor; TNF: Tumor necrosis factor; TSLP: Thymic stromal lymphopoietin; DC: Dendritic cells; TLR: Toll-like receptors; APRIL: A proliferation-inducing factor; BAFF: B-cell activating factor; IL-10: Interleukin-10; MAMPs: Microbial Associated Molecular Patterns; LPS: Lipopolysaccharide; PRRs: Pattern Recognition Receptors; FPRs: Formylated peptide receptors; NODs: Nucleotide-binding oligomerization domain-like receptors.
Table 1.
Mechanisms of probiotics
| Increase in barrier function |
| Maintenance of epithelial tight junctions integrity |
| Increase mucin production (Globet cells) |
| Increase in trefoil factors and defensins production (Paneth Cells) |
| Modulation of immune response |
| Increase secretory IgA |
| Production of anti-inflammatory cytokines and inhibition of pro-inflammatory cytokines |
| Promotion tolerogenic dendritic cells and regulatory T cells |
| Enhance of natural killer activity |
| Antagonism of pathogens |
| Direct killing of bacteria |
| Reduction of pathogen adherence |
| Inhibition of pathogenic bacteria growth by antimicrobial and antitoxin compound production (i.e., SCFA, bacteriocins and microcins) |
| Production of substances |
| Production of enzymatic activities and/or beneficial metabolites to the host |
| Promotion pain relief in visceral hyperalgesia |
SCFA: Short chain fatty acids.
These mechanisms include: (1) Enhancement of the natural gastrointestinal barrier function providing a physical barrier, also know as “colonization resistance”; in particular at level of: (a) tight junctions: probiotics can induce structural changes in epithelial tight junctions, mainly by upregulating the expression of zona-occludens 1, a tight junction protein, or by preventing redistribution of the other proteins, so stabilizing the intercellular integrity[27,30,31]; (b) mucus barrier: probiotics can increase mucin expression and secretion by goblet cells, thereby creating a mucus barrier for bacterial passage toward intestinal surface[32] and by trefoil factors and defensins produced by intestinal Paneth cells; (2) Modulation of the local and systemic immune responses; in particular by production of: (a) secretory IgA: probiotics can stimulate production of IgA in the lamina propria and secretion of IgA into the luminal mucous layer, thus limiting bacterial colonization by binding with their antigens[28]; (b) anti-inflammatory cytokines: probiotics may have many other immunomodulatory effects in the gut, helping to keep intestinal homeostasis. They can modulate the immune response, including promoting tolerogenic DCs and regulatory T cell phenotypes, improving activities of macrophages and NK cells, regulating the nuclear factor-κB (NF-κB) pathway, inducing the apoptosis of T cells, and reduce the secretion of proinflammatory factors[32-34]; (3) Antagonism of pathogens: some probiotics can directly antagonize pathogenic bacteria or their growth via expression of antimicrobial factors such as SCFA and “bacteriocins” or “microcins”[35]. SCFA can disrupt the outer membranes of gram-negative pathogens such as Enterohemorrhagic E. coli, P. aeruginosa, and S. typhimurium, causing inhibition of pathogen growth[34]. Bacteriocins can either permeabilize the inner membrane of gram-negative bacteria, leading to disruption, or interfere with cell wall synthesis and cause the formation of pores[34]. Finally, probiotics may decrease luminal pH, thus creating an inhospitable environment for pathogens[26-29]; and (4) production of helpful substances: some probiotics may produce enzymatic activities and/or beneficial metabolites for the host, may activate receptors in the enteric nervous system as to alter pain responses and promote pain relief in the setting of visceral hyperalgesia[28,29].
There is great variation in the number and combination (single or multiple strains) of probiotic organisms provided in various supplements. The effects of various probiotics may reflect species-specific properties; however, whether a probiotic mixture may yield more beneficial effects because of a synergistic action among the individual organisms, in respect to a single strain, is still matter of debate[27].
Prebiotics
Prebiotic are currently defined as “selectively fermented ingredients that result in specific changes in the composition and/or activity of the GI microbiota, thus conferring benefits upon host health”[36].
Some examples of prebiotics are dietary fiber (as for example arabinoxylan, a non-starch polysaccharide found in many cereal grains[37]) and some types of oligosaccharides, although only inulin-type fructans and galacto-oligosaccharides fulfill all the criteria for prebiotic classification[23,38]. Some polysaccharides can also be found in seaweeds and microalgae[36]. These food ingredients may modify the gut microbiota, mainly at the level of individual strain, selectively stimulating growth of health-promoting species already residing in the colon[23,39-41].
Because of their chemical structure and consequent lack of the host’s capability to digest them, prebiotics are directly fermented in the colon by endogenous bacteria to SCFA[42], with consequent decrease of pH. By this process, they can exert antinflammatory effects, stimulating for example increase of T-regulatory cells (Treg) and reduction of IFN-γ[43,44]. Animal models also showed that some heteropolysaccharide (for example isolated from the fruit body of L. edodes) can restore the age-attenuated immune responses by increasing cytokine levels in peripheral blood[45]. Prebiotics can also inhibit the adherence of pathogens to gut epithelium, preventing them from translocating across the epithelial GI cells[36,37].
Finally, animals studies reported that prebiotics trigger an increase in the mucosa layer, with elongation of the microvilli, and an increase in the number of epithelial cells[36].
Synbiotics
About 20 years ago, the term “synbiotic” was firstly introduced to describe “a mixture of probiotics and prebiotics that beneficially affects the host by improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract, by selectively stimulating the growth and/or by activating the metabolism of one or a limited number of health-promoting bacteria, and thus improving host welfare”[28,46]. Commonly combinations include Bifidobacteria and fructooligosaccharides (FOS), Lactobacillus rhamnosus GG (LGG) and inulin, and Bifidobacteria and Lactobacilli with FOS or inulin[28,46,47].
Antibiotics
Manipulation of the enteric microbial flora by means of antibiotics represents a possible therapeutic option[48]. The most used systemic antibiotics in intestinal disorders are represented by metronidazole and ciprofloxacin, efficacious respectively against anaerobic bacteria and some parasites, and Gram-positive e Gram-negative bacteria such as E. coli and Enterobacteriacee[49]. Nevertheless, use of antibiotics may have some disadvantages, since the occurrence of side effects, antibiotic resistance and Clostridium difficile (C. difficile) superinfection. Metronidazole is known to be associated with various untoward effects (peripheral neuropathy, metallic taste, gastrointestinal disturbances etc.) with a reported incidence of 50% or more[48,50]. Ciprofloxacin is better tolerated, but more expensive, and can induce nausea, diarrhea, skin rashes, with an increasing evidence of tendinitis and tendon rupture[48,51]. In the last years, growing interest has been reserved to rifaximin, a minimally absorbed (< 0.4%) antibiotic, associated with a more favourable safety/tolerability profile than systemic antibiotics[52]. Rifaximin has also demonstrated a broad spectrum antibiotic efficacy, in particular against both Gram-positive and Gram-negative aerobic and anaerobic intestinal bacteria, with minimal potential for development of bacterial resistance[52].
Fecal transplantation
Fecal microbiota transplantation (FMT) consists of the infusion of a fecal suspension from a healthy individual into gut of another one[53].
FMT can be performed by various way, that are nasogastric or nasoduodenal tube, colonoscope, enema, or capsule[54]. The suggested mechanisms of action include the competition for nutrients, the direct inhibition of growth of the pathogen, the modulation the immune system of the host by interaction with normal flora[52-55].
It has been speculated that FMT may be more effective than probiotics in the restoration of altered gut microbiota, since fecal infusion overcomes the intrinsic quantitative gap of probiotics by a durable alteration of the recipient’s gut microbiota while probiotics are able to colonize the gut lumen only for a temporary period[56]. Molecular techniques demonstrated that intestinal flora of the host and the donor closely resembled about 2 wk after FMT and persisted for more than a month, with a predominance of Bacteroides spp.[57].
MODULATION OF MICROFLORA IN INTESTINAL INFLAMMATORY DISORDERS
Inflammatory bowel disease
The involvement of an aberrant immune response to intestinal flora in the pathogenesis of the inflammatory bowel disease (IBD), mainly in susceptible individuals, has been widely suggested[58]. Moreover, IBD is not seen in germ-free animals[26] and different studies reported a decline in bacterial diversity microbial in IBD subjects[58-60]. Nevertheless, disease-specific bacterial or fungal communities have not been identified, although Mycobacterium paratuberculosis and invasive E. coli, have been etiologically linked to Crohn’s disease (CD)[60]. Pathogens may trigger intestinal inflammation in genetically predisposed individuals by disruption of the mucosal barrier and consequent increase of translocation of luminal antigens, mimicry of self-antigens, and activation of the mucosal immune system by modulation of transcription factors such as NF-κB[61].
An extensive literature supports the impact of various probiotics in experimental models of IBD[26,61-64]. Up to now, most of studies in humans were focused on induction of remission and prevention of relapse[65-90]. The strongest evidences derive from clinical trials conducted with VSL#3, a probiotic mixture containing 900 billion viable lyophilized bacteria represented by four strains of Lactobacilli (L. casei, L. Plantarum, L. acidophilus, L. bulgaricus), three of Bifidobacteria (B. longuum, B. breve and B. infantis), and one of Streptococcus salivaris subspecie thermophilus[69-71].
As regarding ulcerative colitis (UC), previous meta-analysis reported insufficient evidence about the efficacy of probiotics both for induction[86] or maintenance of remission[87], while the more recent data support their role[88-90] (Table 2). In particular VSL#3 was effective in maintaining remission in mild to moderately active disease, conversely than for moderate-severe disease[88-90]. Probiotics also showed similarity of efficacy when compared with mesalazine in preventing the relapse of disease[88-90].
Table 2.
Role of probiotics in inflammatory bowel diseases
| Ref. | No. (intervention/control) | Probiotic | Control | Results |
| Remission induction in UC | ||||
| Kato et al[68] | 20 (10/10) | B. breve, B. bifidum, L. acidophilus + 5ASA/SASP | Placebo | slight ↑ |
| Tursi et al[69] | 90 (30/60) | VSL#3 + balsalazide | Balsalazide alone | ↑ |
| 5ASA | ||||
| Miele et al[70] | 29 (14/15) | VSL#3 | Placebo | ↑ |
| Sood et al[71] | 147 (77/70) | VSL#3 | Placebo | ↑ |
| Matthes et al[72] | 57 (46/11) | E. coli Nissle 1917 | Placebo | ↑ |
| Tursi et al[73] | 144 (71/73) | VSL#3 | Placebo | ↑ |
| Maintenance of remission | ||||
| Kruis et al[74] | 103 (50/53) | E. coli Nissle 1917 | 5ASA | Similar efficacy |
| Kruis et al[75] | 327 (162/165) | E. coli Nissle 1917 | 5ASA | Similar efficacy |
| Zocco et al[76] | 187 (127/60) | Lactobacillus GG | 5ASA | Similar efficacy (LGG more effective than 5ASA alone in prolonging relapse free time) |
| Lactobacillus GG + 5ASA | ||||
| Miele et al[70] | 29 (14/15) | VSL#3 | Placebo | ↑ |
| Wildt et al[77] | 32 (20/12) | L. acidophilus La5, B. animalis subs P. lactis BB-12 | Placebo | Similar effect |
| Maintenance of remission in Pouchitis | ||||
| Gionchetti et al[78] | 40 (crossover) | VSL#3 | Placebo | ↑ |
| Gionchetti et al[79] | 40 (20/20) | VSL#3 | Placebo | ↑ |
| Mimura et al[80] | 36 (20/16) | VSL#3 | Placebo | ↑ |
| Maintenance of remission in CD | ||||
| Prantera et al[81] | 45 (23/22) | Lactobacillus GG | Placebo | Similar effect |
| Bousvaros et al[82] | 75 (39/36) | Lactobacillus GG | placebo | Similar effect |
| Raju et al[83] | 90 (43/47) | L. johnsonii | placebo | Similar effect |
| Van Gossum et al[84] | 70 (34/36) | L. johnsonii | placebo | Similar effect |
| Induction of remission in CD | ||||
| Steed et al[85] | 24 (13/11) | B. longum, Synergy 1 + conventional therapy | Placebo + conventional therapy | Similar effect |
All article are randomized controlled trials. 5ASA: Mesalazine; UC: Ulcerative colitis; CD: Crohn’s disease.
Nevertheless, there are many limitations in the different meta-analysis linked to the heterogeneity of the cited studies, including variations in the selection of subjects, dosage, type and concentrations of the probiotics, duration of therapy, concomitant medications.
As for example, Kruis et al[75] found equivalent percentages of efficacy and safety in maintaining remission in 162 UC patients giving probiotic Escherichia coli Nissle 1917 (200 mg once daily) in respect to 165 patients giving mesalazine (500 mg three times daily) for 12 mo[75]. Twenty-nine newly diagnosed UC children were randomized by Miele et al[70] to receive either VSL#3 (weight-based dose, range: 450-1800 billion bacteria/day) or placebo together with steroid (induction period) or mesalamine (remission period). Results showed achievement of remission in 13 patients (92.8%) treated with VSL#3 vs 4 patients (36.4%) treated with placebo[70]. Relapse within 1 year of follow-up occurred in 3 of 14 (21.4%) patients on probiotic vs 11 of 15 (73.3%) patients on placebo[70]. Sood et al[71] enrolled adult patients with mild-to-moderate UC who were randomly given 3.6 × 1012 CFU VSL#3 (n = 77) or placebo (n = 70), twice daily for 12 wk, showing that both at week 6 than week 12, improvement in clinical score was significantly higher in the probiotic group (25; 32.5%) than in the placebo group (32.5% vs 10% and 42.9% vs 15.7%, respectively)[71]. In conclusion, these data are interesting and encouraging, but well-designed randomized controlled trials are still limited about this topic and further works are still warranted to support these promising results
Patients undergoing ileo pouch-anal anastomosis for UCs may develop pouchitis up to 50% of cases[91]. Only a few studies have been published about the use of probiotics in the treatment of active pouchitis. Instead many studies are available on the assessment of the potential of probiotics in the prevention of relapses. For this indication the most studied is the multispecies preparation VSL#3[26,75-79] (Table 2).
Gionchetti et al[79] studied 40 patients with ileal pouch-anal anastomosis receiving VSL#3 (n.20) or placebo (n.20), reporting occurrence of pouchitis in 10% of patients giving probiotic vs 14% patients receiving placebo. In the study by Mimura et al[80] on 36 patients with pouchitis, randomly assigned to receive VSL#3 6 g (20 patients) or placebo, maintenance of remission after one year was achieved in 17 patients (85%) on VSL#3 and in one patient (6%) on placebo[80]. Protective role of probiotics vs placebo in the management of pouchitis was also confirmed in a meta-analysis by Elahi et al[92] and Nikfar et al[93]. Based on these results, also confirmed by European guidelines[94], use of VSL#3 is suggested for the prevention and the maintenance of remission of pouchitis[94].
In contrast to the encouraging findings in UC, data on probiotics in CD patients are still disappointing[22,58,81-85,89,90] (Table 2). Butterworth et al[95] in the Cochrane Collaboration review concluded for not sufficient evidences supporting role of probiotics in inducing remission in CD[95]. These findings were confirmed in meta-analysis by Rahimi et al[96], as well as, later, by Fujiya et al[89] and Shen et al[90]. The most of the analyzed studies showed no significant effects on either the induction or maintenance of remission in CD. In their meta-analysis, Shen et al[90] proposed that the different efficacy of probiotics between UC and CD patients may be explained by the dissimilar inflammatory pattern in the two conditions. For example an impaired production of the regulatory cytokine interleukin (IL)-10 (not influcenced by probiotics) has been showed in CD4+ T cells of CD patients, as well as presence of circulating antibodies against bacterial antigens and deficiency in human defenses[90].
In conclusion, to date, probiotics have some efficacy in UC and in pouchitis, while disappointing results are available for CD.
Evidences regarding the use of prebiotics for IBD therapy are less stronger than for probiotics[58]. The efficacy of prebiotics in IBD, showed in in vitro and animal models[97,98], was investigated only in few human studies involving a small number of patients[99,100]. In 2002, Bamba et al[100] reported that germinated barley foodstuff (at doses of 20-30 g of Germinated Barley Foodstuff daily) may induce remission in 11 patients with mild to moderate active UC in respect to 7 patients receiving standard therapy[100]. However, sample study was too small to draw any final conclusion. In a more recent study[101], 103 CD patients were treated with 15 g/d FOS or placebo for 4 wk. Results showed no clinical improvement in patients on prebiotics, although differences in inflammatory pattern of lamina propria DCs, with reduced of levels of IL-6-in and increased IL-10 were described[101].
As regarding patients with pouchitis, Preidis et al[101] demonstrated that inulin ingestion may induce increased level of butyrate, lower concentration of Bacteroides fragilis and reduced pH and bile acids.
As for prebiotics, also for role of synbiotics in IBD, there are only few available and inconclusive studies[67,101,102]. It has been speculated that enteral nutrition may influence composition and activity of the gut microbial flora, but it deserves further exploration[103,104]. Leach et al[103] found in six CD children that enteral nutrition may significantly change intestinal bacterial composition, when compared with controls. Tjellström et al[104] found that 79% of the 13 children with small bowel/colonic CD responded clinically positively to enteral nutrition treatment, by a modulation of gut microflora activity with decreased levels of pro-inflammatory acetic acid as well as increased concentrations of anti-inflammatory butyric acids and also of valeric acids.
In patients with IBD, antimicrobial therapy, mainly by ciprofloxacin and metronidazole, is usually reserved for those with septic complications[105]. As regarding human studies, data on antibacterial agents, such as oral vancomycin and intravenous metronidazole in UC subjects, are limited and results are still controversial[64].
Also for pouchitis, available data are not conclusive[64,93,106]. As for example, a single study by Shen et al[107] showed that therapy with either metronidazole (1000 mg/d) or ciprofloxacin (20 mg/kg per day) for 2 wk induced a significant improvement in clinical and endoscopic scores of pouchitis, while a meta-analysis by Nikfar et al[93] showed that antibiotic were not significantly more effective than placebo in managment of pouchitis.
The role of antibiotics in uncomplicated CD has not been clearly demonstrated[49]. Different randomized trials have shown that metronidazole is efficacious in inducing remission in patients with active CD with colic and ileo-colic localization[49,108,109], while results on ciprofloxacin, alone or in association with metronidazole, in patients with active CD, remain uncertain[49,110-115]. A recent meta-analysis by Khan et al[116] suggested role of antibiotcs as primary therapy of CD, although the 2010 “Consensus on the diagnosis and management of Crohn’s disease”, did not recommend their use except for the treatment of septic complications, symptoms linked to bacterial overgrowth, or perianal disease[117].
Only few data are available for FMT in IBD[57]. Patients with refractory UC may benefit from FMT, although multiple infusions seem to be required. Nevertheless, only few cases have been reported yet and large clinical trials are lacking[57]. Moayyedi et al[118] in the first randomized trial demonstrate efficacy of FMT in UC. FMT induces remission in a significantly greater percentage of patients with active UC than placebo, with no difference in adverse events. Fecal donor and time of UC appear to affect outcomes. Wei et al[119] in an uncontrolled pilot clinical trial in fourteen IBD patients (11 UC and 3 CD) demonstrate that FT improves quality of life in patients with IBD. In conclusion, available data are too scanty and further and well-designed study are necessary.
Infectious diarrhea
Different probiotics were tested in regard to their efficacy for preventing nosocomial diarrhea and, in particular, rotavirus-associated diarrhea[101]. Most of data derived from pediatric studies. A systematic review of 23 RCTs, involving a total of 1917 participants[120], reported beneficial effects of probiotics in reducing the duration of acute gastroenteritis compared with placebo. Three RCTs (including 1043 children) tested LGG supplementation showing a significant lower incidence of nosocomial rotavirus diarrhea, with a concomitant shortening of the duration of the illness[121-123]. Moreover, a recent meta-analysis including 15 RCTs (2963 participants) showed that LGG significantly reduced the duration of diarrhoea when compared with placebo or no treatment, mainly when used at a daily dose ≥ 1010 CFU than at a daily dose < 1010 CFU[124]. Also a meta-analysis by Salari et al[125] showed that probiotics decrease the duration of diarrhea and fever significantly in children, while these results were not confirmed in adults’ diarrhea, amoebiasis, C. difficile-associated diarrhea, diarrhea in HIV positive patients, radiation-induced diarrhea, and chemotherapy-induced diarrhea[125]. On the other hand, few other trials[126,127] did not show any benefit. Taken together, these findings may suggest that that not all probiotics are equally effective for preventing nosocomial diarrhea.
Therefore, up to now, probiotic use may be advised in acute gastroenteritis, mainly rotavirus-associated diarrhea, while it is not recommended for critically ill hospitalized patients in prevention of nosocomial infections.
Clinical studies of both prebiotic and synbiotic preparations in the treatment of specific acute gastroenteritis are limited. One prospective RCT enrolling 496 children in day care centres tested a synbiotic blend of Bifidobacterium lactis oligofructose, and acacia gum, showing a 20% reduction in the number of days with diarrhea[128].
Further studies are necessary to confirm these preliminary results.
Antibiotic treatment strategies are limited, can worsen clinical course of disease, exacerbating toxin-mediated effects. For treatment of travelers’ diarrhea (TD), the Infectious Diseases Society of America (IDSA) guidelines recommended three antibiotics, that are the fluoroquinolones, rifaximin, and azythromicyn[129]. Nevertheless, there is a growing concern about using of systemic antibiotics since the occurrence of side effects; therefore, increasing evidences support the use of rifaximin, which is currently recommended for the empiric treatment of afebrile, non-dysenteric forms of TD[101,129].
Antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) and C. difficile-associated diarrhea (CDAD) are common complication linked to antibiotic use, mainly cephalosporins, clindamycin, broad-spectrum penicillins and fluoroquinolones[130].
A large systematic review and meta-analysis on role of probiotics in preventing AAD, including 63 randomized clinical trials and involving almost 12000 participants, showed a significant reduction in AAD in the probiotic groups compared with the control group[131]. However, heterogeneity in effectiveness among the patients, differences in the antibiotics and probiotic strains should be considered.
In more recent meta-analysis, Szajewska et al[132] evaluated the efficacy of LGG in preventing AAD in children and adults. They showed significant results only in children and in the subgroup of adult patients receiving antibiotics for Helicobacter pylori eradication. Also in this work, some questions remained answered, as the best treatment regimens, duration of LGG therapy and the optimal dose of LGG for preventing AAD[132].
Moreover, since AAD does not occur in the majority of patients and when it occurs, it is usually self-limiting, identifying populations most likely to benefit from adjunct probiotics should be desirable.
At this regards, only a small number of RCTs were performed on elderly participants. In a double-blind RCT of 135 hospital elderly patients, administration of L. casei, L. bulgaricus, and S. thermophilus, in combination with antibiotics and for one week after cessation, significantly reduced the risk of developing antibiotic-associated diarrhea[133].
Otherwise, Ehrhardt et al[134] in a multicenter, randomized, placebo-controlled trial, showed no beneficial effect of S. boulardii in preventing AAD or CDAD in a population of hospitalized patients giving systemic antibiotics. As the same, the large PLACIDE trial did not find a lower incidence of AAD or CDAD in elderly inpatients receiving supplementation with Lactobacilli and Bifidobacteria[135].
Finally, a recent meta-analysis by Xie et al[136], showed that probiotics may not reduce the risk of AAD and C. difficile-associated diarrhea in older patients, while Szajewska et al[137] showed that S. boulardii significantly reduces the risk of diarrhoea in patients treated with antibiotics in general, also in those of extreme ages. However, since S. boulardii can cause fungaemia, particular concern is required for more susceptible populations[137].
In conclusion, although available data on probiotics are encouraging, a more prudent and selected use of antibiotics represents the best strategy of preventing AAD.
Antibiotic use can lead to dysbiosis (microbial imbalance), and this allows C. difficile to flourish[138]. Up to now, studies documenting a reduction in C. difficile-associated diarrhea (CDAD) by probiotics are far fewer and remain the subject of controversy[65]. A series of systematic reviews showed promising but no definite results[125,139]. In particular, Johnston et al[139], in a meta-analysis including twenty trials and 3818 participants, concluded that moderate-quality evidence suggests probiotic prophylaxis as strategy in reducing CDAD without an increase in clinically important adverse events.
Waiting for more consistent data, actual clinical practice guidelines do not recommend the use of probiotics for the primary prevention of C. difficile infection (CDI)[27,65,101].
As regarding prebiotics and synbiotics in preventing CDAD, only few and RCT are available and data are not consistent to support this strategy[140,141].
In the last years, FMT has been recognized as an efficacious treatment for recurrent C. difficile infection (rCDI) by remodulation of intestinal microflora via donor feces. Patients with rCDI showed reduced levels fecal of Bacteroidetes and Firmicutes as compared to patients with just one episode of CDI or antibiotic-associated diarrhoea[57]. There has been an average 87%-90% cure rate (defined by resolution of diarrhea) for the over 500 cases that have been reported in the literature[142,143].
A systematic review on 36 studies evaluating a total of 536 patients receiving with FMT for CDI, showed that 467 (87%) experienced resolution of diarrhea following the first FMT procedure. The higher response rate (93%) was achieved by fecal infusion via colonoscopy[56]. The literature to date supports FMT for use in CDI as a safe, well-tolerated, effective treatment with few adverse events[144].
Irritable bowel syndrome
Evidences suggest alterations in gut microbiota in patients with irritable bowel syndrome (IBS)[132] with a dysbiosis of the luminal and mucosal colonic microbiota that is frequently characterised by a reduction in species of Bifidobacteria[145].
This is also supported by the hypothesis that IBS may be induced by bacterial gastroenteritis (postinfectious IBS)[23]. Therefore, targeting the intestinal microbiota for the treatment of this condition has been hypothesized as a valid approach.
Recent pre-clinical data on mouse reported beneficial effects of probiotics on visceral nociceptive reflexes[23,146]. As regarding human studies, different probiotic species have been shown to improve individual symptoms in IBS, such as bloating, flatulence, and constipation, but only a few products affect pain and global symptoms[147-170]. Results of more relevant studies were summarized in Table 3.
Table 3.
Role of probiotics in irritable bowel syndrome
| Ref. | Diagnostic criteria | No. participant | Probiotic | Time (wk) | Results (vs placebo) |
| O’Sullivan et al[147] | Rome | 25 | Lactobacillus GG | 20 | No difference |
| Nobaek et al[148] | Rome | 60 | L. plantarum | 4 | ↓ pain and flatulence |
| Kim et al[149] | Rome II | 25 | VSL#3 | 8 | ↓ bloating |
| O’Mahony et al[150] | Rome II | 80 | L. salivarius UCC4331, B. infantis 35624 | 8 | ↓ symptoms and pro-inflammatory cytokines (only for B. infantis) |
| Kajarder et al[151] | Rome/Rome II | 103 | Mixture of L. rhamnosus GG, L. rhamnosus LC705, B. breve Bb99, P. freudenreichii | 24 | ↓ symptoms |
| Kim et al[152] | Rome II | 48 | VSL#3 | 8 | ↓ flatulence |
| Niv et al[153] | Rome II | 54 | L. reuteri ATCC55730 | 24 | No difference |
| Whorwell et al[154] | Rome II | 362 | B. infantis 35624 | 4 | ↓ symptoms (only at dose of 1 × 108 CFU) |
| Guyonnet et al[155] | Rome II (C-IBS) | 274 | B. animalis DN173010 | 4 | ↑ QoL and bloating score |
| Kajander et al[156] | Rome II | 86 | L. rhamnosus GG, L. rhamnosus Lc705, P. freudenreichii sp P. Shermanii JS, B. animalis sp P. lactis Bb-12 | 20 | ↓ bloating and abdominal pain |
| Zeng et al[157] | Rome II (D-IBS) | 29 | S. thermophilus, L. bulgaricus, B. longum | 4 | ↓intestinal permeability and symptoms |
| Drouault-Holowazcz et al[158] | Rome II | 100 | B. longum LA101, L. acidophilus LA102, Lactocuccus lactis LA103, S. thermophilus LA104 | 4 | ↑ QoL |
| ↓ flatulence, pain and bloating | |||||
| Sinn et al[159] | Roma III | 40 | L. acidophilus SDC 2012, 2013 | 4 | ↓ symptoms |
| Simrén et al[160] | Rome II | 74 | L. paracasei F19, L. acidophilus La5, B. lactis Bb-12 | 8 | No difference |
| Søndergaard et al[161] | Rome II | 52 | L. paracasei F19, L. acidophilus La5, B. lactis Bb-12 | 8 | No difference |
| Guglielmetti et al[162] | Rome III | 122 | B. bifidum MIMBb75 | 4 | ↓ symptoms ↑ QoL |
| Ducrotté et al[163] | Rome III (D-IBS) | 214 | L. plantarum 299v | 4 | ↓ pain and bloating |
| Kruis et al[164] | Rome II (D-IBS) | 120 | E. coli Nissle 1917 | 12 | No difference |
| Ki Cha et al[165] | Roma III (D-IBS) | 50 | L. acidophilus, L. plantarum, L. rhamnosus, B. breve, B. lactis, B. longum, S.thermophilus | 8 | ↑ QoL |
| Dapoigny et al[166] | Rome III | 50 | L. caseirhamnosus | 4 | ↓ symptoms |
| Roberts et al[167] | Rome III | 179 | B. lactis CNCMI-2494 | 12 | No difference |
| Shavatehi et al[168] | Rome II | 160 | Lactobacillus, Bifidobacterium strains and Streptococcus thermophiles | 2 | No difference |
| Yoon et al[169] | Rome III | 80 | L. acidophilus, L. rhamnosus, B. breve, B. actis, B. longum, S.thermophilus | 4 | ↓ symptoms |
| Pineton de Chambrun et al[170] | Rome III | 179 | S. cerevisiae | 8 | ↓ pain and discomfort |
All the studies are randomized controlled trials vs placebo. QoL: Quality of life.
Results of meta-analyses were still controversial, mainly since the clinical and statistical heterogeneity. Demographic features of the study populations, length of follow up and dosage and type of probiotics used were very different among the reviewed studies. Moreover, clinical score was evaluated by different scales.
In a meta-analysis by Nikfar et al[171] including 8 randomized placebo controlled clinical trials, probiotics were reported to significantly increase clinical improvement compared to placebo[171]. One large systematic review including 19 studies on IBS patients concluded that probiotics significantly improved clinical outcomes[172]. This finding was confirmed by Moayyedi et al[173], who reviewed 19 RCT performed in 1650 patients with IBS, and Didari et al[174], who analyzed 1793 IBS patients.
On the other hand, two trials and a meta-analysis by Brenner et al[175] in adults did not show clinical benefit above placebo[160,176]. Nevertheless, it is possible that a subgroup of IBS subjects, such as those who developed the disease following gastroenteritis or an antibiotic course, show better beneficial effects by probiotics, as reported in a recent study involving 120 IBS patients[164], thus supporting the hypothesis of a greater efficacy of probiotics in patients with a dysbiosis as main plausible cause of their IBS[177].
In conclusion, probiotics seem to have a beneficial therapeutic role in IBS patients if administered accurately, although these data need to be confirmed by further well-designed trials. Recent United Kingdom guidelines formulated by the British Dietetic Association have made strain specific recommendations; in particular Bifidobacteria lactis DN 173010 showed limited weak evidence in improving overall symptoms, abdominal pain and urgency in constipation-predominant IBS, while VSL#3 showed limited weak evidence in reducing flatulence[178].
Few studies evaluated role of prebiotics for IBS. Few available data suggest that higher doses of prebiotics may have a negative impact on symptoms, maybe because increase of luminal gas production following fermentation and consequent worsening of intestinal symptoms[179]. Otherwise, some recent studies suggest that prebiotics may have some benefits in IBS[180,181]. Nevertheless, there are insufficient prospective and adequately powered studies to permit definitive conclusions to be drawn[23].
Also for synbiotics there are promising but too scanty results in IBS management[182].
Based on the hypothesis that alteration of intestinal microbiota could represent a cause of IBS, rifaximin, a nonabsorbable antibiotic, has been studied in IBS subjects and now represents the only antibiotic with a proven long-term benefit in these subjects[101,183]. Two double-blind, placebo-controlled trials (TARGET 1 and TARGET 2), involving more than 1200 patients with IBS (without constipation), showed that treatment with rifaximin for 2 wk provides significant relief of IBS symptoms[183]. It is conceivable that rifaximin exerts its effect by reducing bacterial products that negatively influence the host, or by reducing local immune responses of the host, or both of them[183].
FMT used in the treatment of IBS has been reported in some clinical studies and mainly in case series. Conclusions on the effectiveness cannot be made because the available data are limited and subject to bias[142].
Other conditions
Intestinal bacteria could play a role in initiation of colorectal cancer (CRC) through production of carcinogens, cocarcinogens, or procarcinogens[2,184]. Up to now, only a human study showed reduction of recurrence of adenoma atypia after 4 years of Lactobacillus casei administration[185]. Also for prebiotic and synbiotics, studies in CRC patients are not only insufficient but, in several aspects, inconclusive[186]. Therefore, available data are too scanty to draw any final conclusion.
Radiotherapy and chemotherapy kill replicating cells in small and large intestine. Probiotics can reduce side effects of these therapies, with some successful results in mice and animals[187-191] and also some trials on patients undergoing radiation and chemo therapy showed a reduction of radiation induced diarrhea and bowel toxicity at the end of the treatment[188-191]. Also in allergic disorders alterations of gut microbiota have been reported, with an imbalance between “beneficial” and potentially harmful bacteria, dysbiosis is not only a consequence but also a cause of allergy[192]. It has been reported that children with food allergies are found to exhibit, i.e., decreased Lactobacilli, Bifidobacteria and Enterococcus species and increased coliforms, Staphylococcus aureus and Clostridium species, suggesting that microbial inhabitants of the human body, may play either a pathogenic or protective role in allergies[192].
Probiotics have been studied as possible dietary interventions for allergic disorders, although the majority of studies have evaluated eczema as the main outcome rather than food or other allergies[192-194]. However, recently, the World Allergy Organization stated that “probiotics do not have an established role in the prevention or treatment of pediatric allergy. No single probiotic supplement or class of supplements has been demonstrated to efficiently influence the course of any allergic manifestation or long-term disease or to be sufficient to do so”[195].
For prebiotics and synbiotics, very few and not definitive results are available.
Intestinal bacteria are essential for the development of NSAID-induced small bowel lesions, since “germ-free” animals were found to be resistant to indomethacin injuries[196]. Moreover, NSAID ingestion may modify composition of intestinal flora, inducing the overgrowth of Gram-negative and anaerobic bacterial species, responsible for lower mucosal defense and an increase susceptibility to damage[197,198].
Therefore, modulation of intestinal flora could provide protection against NSAID-induced enteropathy, as already reported in vitro studies[199,200]. So far, only few studies have been performed in humans and results are not all concordant[201-204]. Gotteland et al[202] found in 16 healthy volunteers that the ingestion of live LGG may preserve the integrity of the gastric mucosal barrier against indomethacin, but has no effect at intestinal level. More recently, in a double-blind, cross-over trial on twenty healthy volunteers receiving indomethacin, the concomitant ingestion of a probiotic mixture (VSL#3) reduced small bowel inflammation, evaluated by fecal calprotectin concentrations[203]. Later, in a randomized, controlled trial on 35 chronic users of low-dose enteric-coated aspirin (100 mg daily) plus omeprazole (20 mg daily), the Authors reported a significant decrease in the number of mucosal breaks evaluated by videocapsule endoscopy in the L. casei group than in the control group[204]. Nevertheless, as stated in a recent review on this topic, current evidences supporting the role of probiotics in NSAID enteropathy are promising, but still weak e further and larger studies are necessary[14]. No significant data are available as regarding prebiotics, synbiotics and other therapeutic approaches targeting intestinal microflora in NSAID enteropathy.
CONCLUSION
Gut microbiota has a pivotal role in inducing a state of “physiological”, balanced and controlled, inflammatory response. Alterations of intestinal bacterial flora, or a dysregulation of the intestinal immune response to normal bacterial environment, may significantly contribute to the pathogenesis of different inflammatory and autoimmune disorders. For all these reasons interest is rising on the eventual role of selective modulation of microflora in inducing benefits in inflammatory intestinal disorders, mainly by probiotics, prebiotics, synbiotics, antibiotics, and, lastly, by fecal transplantation.
Most of larger and well-conducted studies, including review and meta-analysis, evaluated the role of probiotics in different gastrointestinal disease. However, several limitations are important to consider in interpreting the results, since the great heterogeneity in probiotic strains, dosage, age groups, treatment lengths, and outcomes.
Probiotics have been demonstrated of some efficacy in induction and maintaining of remission in UC and in particular in pouchitis, while disappointing results are available for CD. Moreover a definite role of antibiotics has been established for treatment of pouchitis. It is possible that probiotic supplementation may significantly reduce rates of rotavirus diarrhea. Efficacy of probiotic in IBS is still controversial, while a significant benefit has been reported for rifaximin in IBS patients without constipation. For food allergic disorders, modulation of intestinal microflora is not convincing and use of probiotics and prebiotics is not recommended. The role of probiotics in NSAID enteropathy is promising but still weak. Finally, FMT has been recently recognized as an efficacious treatment for recurrent C. difficile infection but these finding need to be confirmed by larger studies.
In conclusion, while representing a very interesting therapeutic target, modulation of intestinal flora still deserves some doubts and limitations. Firstly, the actual gut microbiota composition in healthy individuals is still unclear, since high cost for more specific methods of characterization and the lack of a standardization. Then, despite increasing evidences for probiotics, intestinal bioavailability of bacterial strains, the optimal dose, and treatment time are still matter of debate.
It is conceivable that future studies will provide a better gut microbiota characterization, leading to a more selective modulation by the above described means, that could improve its composition and, consequently, the host’s health status.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 26, 2016
First decision: May 12, 2016
Article in press: August 1, 2016
P- Reviewer: Abdollahi M, Cario E, Zhang XW S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Mondot S, de Wouters T, Doré J, Lepage P. The human gut microbiome and its dysfunctions. Dig Dis. 2013;31:278–285. doi: 10.1159/000354678. [DOI] [PubMed] [Google Scholar]
- 2.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 3.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Suau A, Bonnet R, Sutren M, Godon JJ, Gibson GR, Collins MD, Doré J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999;65:4799–4807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ianiro G, Bruno G, Lopetuso L, Beghella FB, Laterza L, D’Aversa F, Gigante G, Cammarota G, Gasbarrini A. Role of yeasts in healthy and impaired gut microbiota: the gut mycome. Curr Pharm Des. 2014;20:4565–4569. doi: 10.2174/13816128113196660723. [DOI] [PubMed] [Google Scholar]
- 6.Round JL, O’Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2010;34:J220–J225. doi: 10.1016/j.jaut.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi H, Sakamoto M, Benno Y. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol Immunol. 2002;46:535–548. doi: 10.1111/j.1348-0421.2002.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 8.D’Aversa F, Tortora A, Ianiro G, Ponziani FR, Annicchiarico BE, Gasbarrini A. Gut microbiota and metabolic syndrome. Intern Emerg Med. 2013;8 Suppl 1:S11–S15. doi: 10.1007/s11739-013-0916-z. [DOI] [PubMed] [Google Scholar]
- 9.Cummings JH, Beatty ER, Kingman SM, Bingham SA, Englyst HN. Digestion and physiological properties of resistant starch in the human large bowel. Br J Nutr. 1996;75:733–747. doi: 10.1079/bjn19960177. [DOI] [PubMed] [Google Scholar]
- 10.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conly JM, Stein K, Worobetz L, Rutledge-Harding S. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am J Gastroenterol. 1994;89:915–923. [PubMed] [Google Scholar]
- 12.Younes H, Coudray C, Bellanger J, Demigné C, Rayssiguier Y, Rémésy C. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br J Nutr. 2001;86:479–485. doi: 10.1079/bjn2001430. [DOI] [PubMed] [Google Scholar]
- 13.Gordon JI, Hooper LV, McNevin MS, Wong M, Bry L. Epithelial cell growth and differentiation. III. Promoting diversity in the intestine: conversations between the microflora, epithelium, and diffuse GALT. Am J Physiol. 1997;273:G565–G570. doi: 10.1152/ajpgi.1997.273.3.G565. [DOI] [PubMed] [Google Scholar]
- 14.Srikanth CV, McCormick BA. Interactions of the intestinal epithelium with the pathogen and the indigenous microbiota: a three-way crosstalk. Interdiscip Perspect Infect Dis. 2008;2008:626827. doi: 10.1155/2008/626827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purchiaroni F, Tortora A, Gabrielli M, Bertucci F, Gigante G, Ianiro G, Ojetti V, Scarpellini E, Gasbarrini A. The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci. 2013;17:323–333. [PubMed] [Google Scholar]
- 16.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 17.Medzhitov R, Janeway C. Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 18.Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R, Kozáková H, Rossmann P, Bártová J, Sokol D, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110–120. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 21.Kamada N, Núñez G. Role of the gut microbiota in the development and function of lymphoid cells. J Immunol. 2013;190:1389–1395. doi: 10.4049/jimmunol.1203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiffrin EJ, Blum S. Interactions between the microbiota and the intestinal mucosa. Eur J Clin Nutr. 2002;56 Suppl 3:S60–S64. doi: 10.1038/sj.ejcn.1601489. [DOI] [PubMed] [Google Scholar]
- 23.Quigley EM. Prebiotics and probiotics: their role in the management of gastrointestinal disorders in adults. Nutr Clin Pract. 2012;27:195–200. doi: 10.1177/0884533611423926. [DOI] [PubMed] [Google Scholar]
- 24.Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep. 2007;9:497–507. doi: 10.1007/s11894-007-0066-4. [DOI] [PubMed] [Google Scholar]
- 25.Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba: FAO/WHO; 2001. Report of the Joint Food and Agriculture Organization (FAO) of the United Nations/World Health Organization (WHO) Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. [Google Scholar]
- 26.Andrews JM, Tan M. Probiotics in luminal gastroenterology: the current state of play. Intern Med J. 2012;42:1287–1291. doi: 10.1111/imj.12015. [DOI] [PubMed] [Google Scholar]
- 27.Balakrishnan M, Floch MH. Prebiotics, probiotics and digestive health. Curr Opin Clin Nutr Metab Care. 2012;15:580–585. doi: 10.1097/MCO.0b013e328359684f. [DOI] [PubMed] [Google Scholar]
- 28.Ceapa C, Wopereis H, Rezaïki L, Kleerebezem M, Knol J, Oozeer R. Influence of fermented milk products, prebiotics and probiotics on microbiota composition and health. Best Pract Res Clin Gastroenterol. 2013;27:139–155. doi: 10.1016/j.bpg.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opin Gastroenterol. 2007;23:679–692. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- 30.Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol. 2004;286:G613–G626. doi: 10.1152/ajpgi.00341.2003. [DOI] [PubMed] [Google Scholar]
- 31.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 32.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 33.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7:503–514. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 35.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 36.de Jesus Raposo MF, de Morais AM, de Morais RM. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar Drugs. 2016;14:pii E27. doi: 10.3390/md14020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendis M, Leclerc E, Simsek S. Arabinoxylans, gut microbiota and immunity. Carbohydr Polym. 2016;139:159–166. doi: 10.1016/j.carbpol.2015.11.068. [DOI] [PubMed] [Google Scholar]
- 38.Vieira AT, Teixeira MM, Martins FS. The role of probiotics and prebiotics in inducing gut immunity. Front Immunol. 2013;4:445. doi: 10.3389/fimmu.2013.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X, Xu P, Ma C, Tang J, Zhang X. Gut microbiota, host health, and polysaccharides. Biotechnol Adv. 2013;31:318–337. doi: 10.1016/j.biotechadv.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Chung WS, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D, Duncan SH, Flint HJ. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 2016;14:3. doi: 10.1186/s12915-015-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conlon MA, Topping DL. Dietary polysaccharides and polyphenols can promote health by influencing gut microbiota populations. Food Funct. 2016;7:1730. doi: 10.1039/c6fo90009g. [DOI] [PubMed] [Google Scholar]
- 42.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Gibson PR, Rosella O, Wilson AJ, Mariadason JM, Rickard K, Byron K, Barkla DH. Colonic epithelial cell activation and the paradoxical effects of butyrate. Carcinogenesis. 1999;20:539–544. doi: 10.1093/carcin/20.4.539. [DOI] [PubMed] [Google Scholar]
- 44.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 45.Xu X, Yang J, Ning Z, Zhang X. Lentinula edodes-derived polysaccharide rejuvenates mice in terms of immune responses and gut microbiota. Food Funct. 2015;6:2653–2663. doi: 10.1039/c5fo00689a. [DOI] [PubMed] [Google Scholar]
- 46.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Zhu H, Lu C, Kang Z, Luo Y, Feng L, Lu X. Fermented milk supplemented with probiotics and prebiotics can effectively alter the intestinal microbiota and immunity of host animals. J Dairy Sci. 2012;95:4813–4822. doi: 10.3168/jds.2012-5426. [DOI] [PubMed] [Google Scholar]
- 48.Guslandi M. Antibiotics for inflammatory bowel disease: do they work? Eur J Gastroenterol Hepatol. 2005;17:145–147. doi: 10.1097/00042737-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Scribano ML, Prantera C. Antibiotics and inflammatory bowel diseases. Dig Dis. 2013;31:379–384. doi: 10.1159/000354704. [DOI] [PubMed] [Google Scholar]
- 50.Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R, Kerremans R. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108:1617–1621. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 51.Prantera C, Berto E, Scribano ML, Falasco G. Use of antibiotics in the treatment of active Crohn’s disease: experience with metronidazole and ciprofloxacin. Ital J Gastroenterol Hepatol. 1998;30:602–606. [PubMed] [Google Scholar]
- 52.Layer P, Andresen V. Review article: rifaximin, a minimally absorbed oral antibacterial, for the treatment of travellers’ diarrhoea. Aliment Pharmacol Ther. 2010;31:1155–1164. doi: 10.1111/j.1365-2036.2010.04296.x. [DOI] [PubMed] [Google Scholar]
- 53.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- 54.Choi HH, Cho YS. Fecal Microbiota Transplantation: Current Applications, Effectiveness, and Future Perspectives. Clin Endosc. 2016;49:257–265. doi: 10.5946/ce.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borgia G, Maraolo AE, Foggia M, Buonomo AR, Gentile I. Fecal microbiota transplantation for Clostridium difficile infection: back to the future. Expert Opin Biol Ther. 2015;15:1001–1014. doi: 10.1517/14712598.2015.1045872. [DOI] [PubMed] [Google Scholar]
- 56.Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693–702. doi: 10.1097/MCG.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 57.Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29:79–84. doi: 10.1097/MOG.0b013e32835a4b3e. [DOI] [PubMed] [Google Scholar]
- 58.Orel R, Kamhi Trop T. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J Gastroenterol. 2014;20:11505–11524. doi: 10.3748/wjg.v20.i33.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kotlowski R, Bernstein CN, Sepehri S, Krause DO. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut. 2007;56:669–675. doi: 10.1136/gut.2006.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 61.Reiff C, Kelly D. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int J Med Microbiol. 2010;300:25–33. doi: 10.1016/j.ijmm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Sheil B, Shanahan F, O’Mahony L. Probiotic effects on inflammatory bowel disease. J Nutr. 2007;137:819S–824S. doi: 10.1093/jn/137.3.819S. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh S, van Heel D, Playford RJ. Probiotics in inflammatory bowel disease: is it all gut flora modulation? Gut. 2004;53:620–622. doi: 10.1136/gut.2003.034249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andoh A, Fujiyama Y. Therapeutic approaches targeting intestinal microflora in inflammatory bowel disease. World J Gastroenterol. 2006;12:4452–4460. doi: 10.3748/wjg.v12.i28.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanders ME, Guarner F, Guerrant R, Holt PR, Quigley EM, Sartor RB, Sherman PM, Mayer EA. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–796. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jonkers D, Penders J, Masclee A, Pierik M. Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs. 2012;72:803–823. doi: 10.2165/11632710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 67.Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I, Bruno G, Petito V, Laterza L, Cammarota G, et al. Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int. 2013;2013:435268. doi: 10.1155/2013/435268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004;20:1133–1141. doi: 10.1111/j.1365-2036.2004.02268.x. [DOI] [PubMed] [Google Scholar]
- 69.Tursi A, Brandimarte G, Giorgetti GM, Forti G, Modeo ME, Gigliobianco A. Low-dose balsalazide plus a high-potency probiotic preparation is more effective than balsalazide alone or mesalazine in the treatment of acute mild-to-moderate ulcerative colitis. Med Sci Monit. 2004;10:PI126–PI131. [PubMed] [Google Scholar]
- 70.Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437–443. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 71.Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–1209, 1209.e1. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 72.Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN) BMC Complement Altern Med. 2010;10:13. doi: 10.1186/1472-6882-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218–2227. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11:853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 75.Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, Ojetti V, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–1574. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 77.Wildt S, Nordgaard I, Hansen U, Brockmann E, Rumessen JJ. A randomised double-blind placebo-controlled trial with Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 for maintenance of remission in ulcerative colitis. J Crohns Colitis. 2011;5:115–121. doi: 10.1016/j.crohns.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 79.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 80.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gut. 2002;51:405–409. doi: 10.1136/gut.51.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, Goldin B, Hartigan L, Kugathasan S, Levy J, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm Bowel Dis. 2005;11:833–839. doi: 10.1097/01.mib.0000175905.00212.2c. [DOI] [PubMed] [Google Scholar]
- 83.Raju KS, Wolfe CD. Is stage I epithelial ovarian cancer overtreated both surgically and systemically? Results of a five-year cancer registry review. Br J Obstet Gynaecol. 1992;99:530–531. doi: 10.1111/j.1471-0528.1992.tb13808.x. [DOI] [PubMed] [Google Scholar]
- 84.Van Gossum A, Dewit O, Louis E, de Hertogh G, Baert F, Fontaine F, DeVos M, Enslen M, Paintin M, Franchimont D. Multicenter randomized-controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn’s disease after lleo-caecal resection. Inflamm Bowel Dis. 2007;13:135–142. doi: 10.1002/ibd.20063. [DOI] [PubMed] [Google Scholar]
- 85.Steed H, Macfarlane GT, Blackett KL, Bahrami B, Reynolds N, Walsh SV, Cummings JH, Macfarlane S. Clinical trial: the microbiological and immunological effects of synbiotic consumption - a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol Ther. 2010;32:872–883. doi: 10.1111/j.1365-2036.2010.04417.x. [DOI] [PubMed] [Google Scholar]
- 86.Sang LX, Chang B, Zhang WL, Wu XM, Li XH, Jiang M. Remission induction and maintenance effect of probiotics on ulcerative colitis: a meta-analysis. World J Gastroenterol. 2010;16:1908–1915. doi: 10.3748/wjg.v16.i15.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naidoo K, Gordon M, Fagbemi AO, Thomas AG, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443. doi: 10.1002/14651858.CD007443.pub2. [DOI] [PubMed] [Google Scholar]
- 88.Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562–1567. doi: 10.1097/MIB.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 89.Fujiya M, Ueno N, Kohgo Y. Probiotic treatments for induction and maintenance of remission in inflammatory bowel diseases: a meta-analysis of randomized controlled trials. Clin J Gastroenterol. 2014;7:1–13. doi: 10.1007/s12328-013-0440-8. [DOI] [PubMed] [Google Scholar]
- 90.Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: meta-analysis of randomized controlled trials. Inflamm Bowel Dis. 2014;20:21–35. doi: 10.1097/01.MIB.0000437495.30052.be. [DOI] [PubMed] [Google Scholar]
- 91.Pardi DS, D’Haens G, Shen B, Campbell S, Gionchetti P. Clinical guidelines for the management of pouchitis. Inflamm Bowel Dis. 2009;15:1424–1431. doi: 10.1002/ibd.21039. [DOI] [PubMed] [Google Scholar]
- 92.Elahi B, Nikfar S, Derakhshani S, Vafaie M, Abdollahi M. On the benefit of probiotics in the management of pouchitis in patients underwent ileal pouch anal anastomosis: a meta-analysis of controlled clinical trials. Dig Dis Sci. 2008;53:1278–1284. doi: 10.1007/s10620-007-0006-z. [DOI] [PubMed] [Google Scholar]
- 93.Nikfar S, Darvish-Damavandi M, Abdollahi M. A review and meta-analysis of the efficacy of antibiotics and probiotics in management of pouchitis. Int J Pharmacol. 2010;6:826–835. [Google Scholar]
- 94.Floch MH. The power of poop: probiotics and fecal microbial transplant. J Clin Gastroenterol. 2012;46:625–626. doi: 10.1097/MCG.0b013e3182667a93. [DOI] [PubMed] [Google Scholar]
- 95.Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;(3):CD006634. doi: 10.1002/14651858.CD006634.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rahimi R, Nikfar S, Rahimi F, Elahi B, Derakhshani S, Vafaie M, Abdollahi M. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease. Dig Dis Sci. 2008;53:2524–2531. doi: 10.1007/s10620-007-0171-0. [DOI] [PubMed] [Google Scholar]
- 97.Casellas F, Borruel N, Torrejón A, Varela E, Antolin M, Guarner F, Malagelada JR. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25:1061–1067. doi: 10.1111/j.1365-2036.2007.03288.x. [DOI] [PubMed] [Google Scholar]
- 98.Langlands SJ, Hopkins MJ, Coleman N, Cummings JH. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53:1610–1616. doi: 10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benjamin JL, Hedin CR, Koutsoumpas A, Ng SC, McCarthy NE, Hart AL, Kamm MA, Sanderson JD, Knight SC, Forbes A, et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut. 2011;60:923–929. doi: 10.1136/gut.2010.232025. [DOI] [PubMed] [Google Scholar]
- 100.Bamba T, Kanauchi O, Andoh A, Fujiyama Y. A new prebiotic from germinated barley for nutraceutical treatment of ulcerative colitis. J Gastroenterol Hepatol. 2002;17:818–824. doi: 10.1046/j.1440-1746.2002.02709.x. [DOI] [PubMed] [Google Scholar]
- 101.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chermesh I, Tamir A, Reshef R, Chowers Y, Suissa A, Katz D, Gelber M, Halpern Z, Bengmark S, Eliakim R. Failure of Synbiotic 2000 to prevent postoperative recurrence of Crohn’s disease. Dig Dis Sci. 2007;52:385–389. doi: 10.1007/s10620-006-9549-7. [DOI] [PubMed] [Google Scholar]
- 103.Leach ST, Mitchell HM, Eng WR, Zhang L, Day AS. Sustained modulation of intestinal bacteria by exclusive enteral nutrition used to treat children with Crohn’s disease. Aliment Pharmacol Ther. 2008;28:724–733. doi: 10.1111/j.1365-2036.2008.03796.x. [DOI] [PubMed] [Google Scholar]
- 104.Tjellström B, Högberg L, Stenhammar L, Magnusson KE, Midtvedt T, Norin E, Sundqvist T. Effect of exclusive enteral nutrition on gut microflora function in children with Crohn’s disease. Scand J Gastroenterol. 2012;47:1454–1459. doi: 10.3109/00365521.2012.703234. [DOI] [PubMed] [Google Scholar]
- 105.Isaacs KL, Sartor RB. Treatment of inflammatory bowel disease with antibiotics. Gastroenterol Clin North Am. 2004;33:335–45, x. doi: 10.1016/j.gtc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 106.Dickinson RJ, O’Connor HJ, Pinder I, Hamilton I, Johnston D, Axon AT. Double blind controlled trial of oral vancomycin as adjunctive treatment in acute exacerbations of idiopathic colitis. Gut. 1985;26:1380–1384. doi: 10.1136/gut.26.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen B, Achkar JP, Lashner BA, Ormsby AH, Remzi FH, Brzezinski A, Bevins CL, Bambrick ML, Seidner DL, Fazio VW. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis. 2001;7:301–305. doi: 10.1097/00054725-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 108.Blichfeldt P, Blomhoff JP, Myhre E, Gjone E. Metronidazole in Crohn’s disease. A double blind cross-over clinical trial. Scand J Gastroenterol. 1978;13:123–127. doi: 10.3109/00365527809179816. [DOI] [PubMed] [Google Scholar]
- 109.Ambrose NS, Allan RN, Keighley MR, Burdon DW, Youngs D, Barnes P, Lennard-Jones JE. Antibiotic therapy for treatment in relapse of intestinal Crohn’s disease. A prospective randomized study. Dis Colon Rectum. 1985;28:81–85. doi: 10.1007/BF02552649. [DOI] [PubMed] [Google Scholar]
- 110.Sutherland L, Singleton J, Sessions J, Hanauer S, Krawitt E, Rankin G, Summers R, Mekhjian H, Greenberger N, Kelly M. Double blind, placebo controlled trial of metronidazole in Crohn’s disease. Gut. 1991;32:1071–1075. doi: 10.1136/gut.32.9.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Turunen U, Farkkila M, Hakala K, Seppälä K, Sivonen A, Ogren M, Vuoristo M, Valtonen VV, Miettinen TA. Ciprofloxacin treatment combined with conventional therapy in Crohn’s disease: a prospective, double blind, placebo controlled study. Gut. 1995;37:A193. doi: 10.1016/s0016-5085(98)70076-9. [DOI] [PubMed] [Google Scholar]
- 112.Prantera C, Zannoni F, Scribano ML, Berto E, Andreoli A, Kohn A, Luzi C. An antibiotic regimen for the treatment of active Crohn’s disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol. 1996;91:328–332. [PubMed] [Google Scholar]
- 113.Colombel JF, Lémann M, Cassagnou M, Bouhnik Y, Duclos B, Dupas JL, Notteghem B, Mary JY. A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn’s disease. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID) Am J Gastroenterol. 1999;94:674–678. doi: 10.1111/j.1572-0241.1999.935_q.x. [DOI] [PubMed] [Google Scholar]
- 114.Arnold GL, Beaves MR, Pryjdun VO, Mook WJ. Preliminary study of ciprofloxacin in active Crohn’s disease. Inflamm Bowel Dis. 2002;8:10–15. doi: 10.1097/00054725-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 115.Steinhart AH, Feagan BG, Wong CJ, Vandervoort M, Mikolainis S, Croitoru K, Seidman E, Leddin DJ, Bitton A, Drouin E, et al. Combined budesonide and antibiotic therapy for active Crohn’s disease: a randomized controlled trial. Gastroenterology. 2002;123:33–40. doi: 10.1053/gast.2002.34225. [DOI] [PubMed] [Google Scholar]
- 116.Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, Talley NJ, Moayyedi P. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:661–673. doi: 10.1038/ajg.2011.72. [DOI] [PubMed] [Google Scholar]
- 117.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 118.Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102–109.e6. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 119.Wei Y, Zhu W, Gong J, Guo D, Gu L, Li N, Li J. Fecal Microbiota Transplantation Improves the Quality of Life in Patients with Inflammatory Bowel Disease. Gastroenterol Res Pract. 2015;2015:517597. doi: 10.1155/2015/517597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allen SJ, Okoko B, Martinez E, Gregorio G, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database Syst Rev. 2004;(2):CD003048. doi: 10.1002/14651858.CD003048.pub2. [DOI] [PubMed] [Google Scholar]
- 121.Szajewska H, Kotowska M, Mrukowicz JZ, Armańska M, Mikołajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr. 2001;138:361–365. doi: 10.1067/mpd.2001.111321. [DOI] [PubMed] [Google Scholar]
- 122.Mastretta E, Longo P, Laccisaglia A, Balbo L, Russo R, Mazzaccara A, Gianino P. Effect of Lactobacillus GG and breast-feeding in the prevention of rotavirus nosocomial infection. J Pediatr Gastroenterol Nutr. 2002;35:527–531. doi: 10.1097/00005176-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 123.Szajewska H, Wanke M, Patro B. Meta-analysis: the effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare-associated diarrhoea in children. Aliment Pharmacol Ther. 2011;34:1079–1087. doi: 10.1111/j.1365-2036.2011.04837.x. [DOI] [PubMed] [Google Scholar]
- 124.Szajewska H, Skórka A, Ruszczyński M, Gieruszczak-Białek D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children--updated analysis of randomised controlled trials. Aliment Pharmacol Ther. 2013;38:467–476. doi: 10.1111/apt.12403. [DOI] [PubMed] [Google Scholar]
- 125.Salari P, Nikfar S, Abdollahi M. A meta-analysis and systematic review on the effect of probiotics in acute diarrhea. Inflamm Allergy Drug Targets. 2012;11:3–14. doi: 10.2174/187152812798889394. [DOI] [PubMed] [Google Scholar]
- 126.Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology. 2010;98:156–163. doi: 10.1159/000280291. [DOI] [PubMed] [Google Scholar]
- 127.Honeycutt TC, El Khashab M, Wardrop RM, McNeal-Trice K, Honeycutt AL, Christy CG, Mistry K, Harris BD, Meliones JN, Kocis KC. Probiotic administration and the incidence of nosocomial infection in pediatric intensive care: a randomized placebo-controlled trial. Pediatr Crit Care Med. 2007;8:452–458; quiz 464. doi: 10.1097/01.PCC.0000282176.41134.E6. [DOI] [PubMed] [Google Scholar]
- 128.Binns CW, Lee AH, Harding H, Gracey M, Barclay DV. The CUPDAY Study: prebiotic-probiotic milk product in 1-3-year-old children attending childcare centres. Acta Paediatr. 2007;96:1646–1650. doi: 10.1111/j.1651-2227.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 129.Paredes-Paredes M, Flores-Figueroa J, Dupont HL. Advances in the treatment of travelers’ diarrhea. Curr Gastroenterol Rep. 2011;13:402–407. doi: 10.1007/s11894-011-0208-6. [DOI] [PubMed] [Google Scholar]
- 130.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 131.Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, Johnsen B, Shekelle PG. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959–1969. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 132.Szajewska H, Kołodziej M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149–1157. doi: 10.1111/apt.13404. [DOI] [PubMed] [Google Scholar]
- 133.Hickson M, D’Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, Bulpitt CJ. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ehrhardt S, Guo N, Hinz R, Schoppen S, May J, Reiser M, Schroeder MP, Schmiedel S, Keuchel M, Reisinger EC, et al. Saccharomyces boulardii to Prevent Antibiotic-Associated Diarrhea: A Randomized, Double-Masked, Placebo-Controlled Trial. Open Forum Infect Dis. 2016;3:ofw011. doi: 10.1093/ofid/ofw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Allen SJ, Wareham K, Wang D, Bradley C, Hutchings H, Harris W, Dhar A, Brown H, Foden A, Gravenor MB, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249–1257. doi: 10.1016/S0140-6736(13)61218-0. [DOI] [PubMed] [Google Scholar]
- 136.Xie C, Li J, Wang K, Li Q, Chen D. Probiotics for the prevention of antibiotic-associated diarrhoea in older patients: a systematic review. Travel Med Infect Dis. 2015;13:128–134. doi: 10.1016/j.tmaid.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 137.Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793–801. doi: 10.1111/apt.13344. [DOI] [PubMed] [Google Scholar]
- 138.Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T, Wu G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology. 2015;149:223–237. doi: 10.1053/j.gastro.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile-associated diarrhea. In response. Ann Intern Med. 2013;158:706–707. doi: 10.7326/0003-4819-158-9-201305070-00019. [DOI] [PubMed] [Google Scholar]
- 140.Lewis S, Burmeister S, Brazier J. Effect of the prebiotic oligofructose on relapse of Clostridium difficile-associated diarrhea: a randomized, controlled study. Clin Gastroenterol Hepatol. 2005;3:442–448. doi: 10.1016/s1542-3565(04)00677-9. [DOI] [PubMed] [Google Scholar]
- 141.Orrhage K, Sjöstedt S, Nord CE. Effect of supplements with lactic acid bacteria and oligofructose on the intestinal microflora during administration of cefpodoxime proxetil. J Antimicrob Chemother. 2000;46:603–612. doi: 10.1093/jac/46.4.603. [DOI] [PubMed] [Google Scholar]
- 142.Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol. 2016;9:229–239. doi: 10.1177/1756283X15607414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 144.Jeffery IB, Quigley EM, Öhman L, Simrén M, O’Toole PW. The microbiota link to irritable bowel syndrome: an emerging story. Gut Microbes. 2012;3:572–576. doi: 10.4161/gmic.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tojo R, Suárez A, Clemente MG, de los Reyes-Gavilán CG, Margolles A, Gueimonde M, Ruas-Madiedo P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, Corthésy-Theulaz I, Fioramonti J, Bueno L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901–1907. doi: 10.1093/jn/137.8.1901. [DOI] [PubMed] [Google Scholar]
- 147.O’Sullivan MA, O’Morain CA. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Dig Liver Dis. 2000;32:294–301. doi: 10.1016/s1590-8658(00)80021-3. [DOI] [PubMed] [Google Scholar]
- 148.Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 149.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, Zinsmeister AR. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 150.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 151.Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–394. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 152.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 153.Niv E, Naftali T, Hallak R, Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome--a double blind, placebo-controlled, randomized study. Clin Nutr. 2005;24:925–931. doi: 10.1016/j.clnu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 154.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 155.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475–486. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 156.Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, Zoetendal EG, de Vos WM, Vapaatalo H, Korpela R. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 157.Zeng J, Li YQ, Zuo XL, Zhen YB, Yang J, Liu CH. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 158.Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147–152. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 159.Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, Kim YH, Kim JJ, Rhee JC, Rhee PL. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53:2714–2718. doi: 10.1007/s10620-007-0196-4. [DOI] [PubMed] [Google Scholar]
- 160.Simrén M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–227. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 161.Søndergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46:663–672. doi: 10.3109/00365521.2011.565066. [DOI] [PubMed] [Google Scholar]
- 162.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 163.Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012–4018. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kruis W, Chrubasik S, Boehm S, Stange C, Schulze J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int J Colorectal Dis. 2012;27:467–474. doi: 10.1007/s00384-011-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–227. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 166.Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot JM, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol. 2012;18:2067–2075. doi: 10.3748/wjg.v18.i17.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. doi: 10.1186/1471-230X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shavakhi A, Minakari M, Farzamnia S, Peykar MS, Taghipour G, Tayebi A, Hashemi H, Shavakhi S. The effects of multi-strain probiotic compound on symptoms and quality-of-life in patients with irritable bowel syndrome: A randomized placebo-controlled trial. Adv Biomed Res. 2014;3:140. doi: 10.4103/2277-9175.135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yoon H, Park YS, Lee DH, Seo JG, Shin CM, Kim N. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Biochem Nutr. 2015;57:129–134. doi: 10.3164/jcbn.15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pineton de Chambrun G, Neut C, Chau A, Cazaubiel M, Pelerin F, Justen P, Desreumaux P. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Dig Liver Dis. 2015;47:119–124. doi: 10.1016/j.dld.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 171.Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775–1780. doi: 10.1007/s10350-008-9335-z. [DOI] [PubMed] [Google Scholar]
- 172.Hungin AP, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Rubin G, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Aliment Pharmacol Ther. 2013;38:864–886. doi: 10.1111/apt.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 174.Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072–3084. doi: 10.3748/wjg.v21.i10.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033–1049; quiz 1050. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 176.Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010;10:16. doi: 10.1186/1471-230X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Whelan K, Quigley EM. Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:184–189. doi: 10.1097/MOG.0b013e32835d7bba. [DOI] [PubMed] [Google Scholar]
- 178.McKenzie YA, Alder A, Anderson W, Wills A, Goddard L, Gulia P, Jankovich E, Mutch P, Reeves LB, Singer A, et al. British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet. 2012;25:260–274. doi: 10.1111/j.1365-277X.2012.01242.x. [DOI] [PubMed] [Google Scholar]
- 179.Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care. 2011;14:581–587. doi: 10.1097/MCO.0b013e32834b8082. [DOI] [PubMed] [Google Scholar]
- 180.Depeint F, Tzortzis G, Vulevic J, I’anson K, Gibson GR. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study. Am J Clin Nutr. 2008;87:785–791. doi: 10.1093/ajcn/87.3.785. [DOI] [PubMed] [Google Scholar]
- 181.Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508–518. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- 182.Andriulli A, Neri M, Loguercio C, Terreni N, Merla A, Cardarella MP, Federico A, Chilovi F, Milandri GL, De Bona M, et al. Clinical trial on the efficacy of a new symbiotic formulation, Flortec, in patients with irritable bowel syndrome: a multicenter, randomized study. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 2:S218–S223. doi: 10.1097/MCG.0b013e31817fadd6. [DOI] [PubMed] [Google Scholar]
- 183.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP; TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 184.Sears CL, Pardoll DM. Perspective: alpha-bugs, their microbial partners, and the link to colon cancer. J Infect Dis. 2011;203:306–311. doi: 10.1093/jinfdis/jiq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ishikawa H, Akedo I, Otani T, Suzuki T, Nakamura T, Takeyama I, Ishiguro S, Miyaoka E, Sobue T, Kakizoe T. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int J Cancer. 2005;116:762–767. doi: 10.1002/ijc.21115. [DOI] [PubMed] [Google Scholar]
- 186.Peitsidou K, Karantanos T, Theodoropoulos GE. Probiotics, prebiotics, synbiotics: is there enough evidence to support their use in colorectal cancer surgery? Dig Surg. 2012;29:426–438. doi: 10.1159/000345580. [DOI] [PubMed] [Google Scholar]
- 187.Delia P, Sansotta G, Donato V, Frosina P, Salatino A, Messina G, De Renzis C, Famularo G. Use of probiotics for prevention of radiation-induced diarrhea. Tumori. 2007;93:suppl 1–6. [PubMed] [Google Scholar]
- 188.Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33:761–767. doi: 10.1016/j.clnu.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 189.Visich KL, Yeo TP. The prophylactic use of probiotics in the prevention of radiation therapy-induced diarrhea. Clin J Oncol Nurs. 2010;14:467–473. doi: 10.1188/10.CJON.467-473. [DOI] [PubMed] [Google Scholar]
- 190.Wedlake LJ, Shaw C, Whelan K, Andreyev HJ. Systematic review: the efficacy of nutritional interventions to counteract acute gastrointestinal toxicity during therapeutic pelvic radiotherapy. Aliment Pharmacol Ther. 2013;37:1046–1056. doi: 10.1111/apt.12316. [DOI] [PubMed] [Google Scholar]
- 191.Stein K, Borowicki A, Scharlau D, Schettler A, Scheu K, Obst U, Glei M. Effects of synbiotic fermentation products on primary chemoprevention in human colon cells. J Nutr Biochem. 2012;23:777–784. doi: 10.1016/j.jnutbio.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 192.Compare D, Nardone G. The role of gut microbiota in the pathogenesis and management of allergic diseases. Eur Rev Med Pharmacol Sci. 2013;17 Suppl 2:11–17. [PubMed] [Google Scholar]
- 193.Hardy H, Harris J, Lyon E, Beal J, Foey AD. Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients. 2013;5:1869–1912. doi: 10.3390/nu5061869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kuitunen M. Probiotics and prebiotics in preventing food allergy and eczema. Curr Opin Allergy Clin Immunol. 2013;13:280–286. doi: 10.1097/ACI.0b013e328360ed66. [DOI] [PubMed] [Google Scholar]
- 195.Fiocchi A, Burks W, Bahna SL, Bielory L, Boyle RJ, Cocco R, Dreborg S, Goodman R, Kuitunen M, Haahtela T, et al. Clinical Use of Probiotics in Pediatric Allergy (CUPPA): A World Allergy Organization Position Paper. World Allergy Organ J. 2012;5:148–167. doi: 10.1097/WOX.0b013e3182784ee0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Reuter BK, Davies NM, Wallace JL. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology. 1997;112:109–117. doi: 10.1016/s0016-5085(97)70225-7. [DOI] [PubMed] [Google Scholar]
- 197.Lanas A, Scarpignato C. Microbial flora in NSAID-induced intestinal damage: a role for antibiotics? Digestion. 2006;73 Suppl 1:136–150. doi: 10.1159/000089789. [DOI] [PubMed] [Google Scholar]
- 198.Scarpignato C. NSAID-induced intestinal damage: are luminal bacteria the therapeutic target? Gut. 2008;57:145–148. doi: 10.1136/gut.2007.134502. [DOI] [PubMed] [Google Scholar]
- 199.Wallace JL. Prevention of NSAID-Enteropathy: A Soluble Problem? Dig Dis Sci. 2016;61:1–3. doi: 10.1007/s10620-015-3963-7. [DOI] [PubMed] [Google Scholar]
- 200.Montalto M, Maggiano N, Ricci R, Curigliano V, Santoro L, Di Nicuolo F, Vecchio FM, Gasbarrini A, Gasbarrini G. Lactobacillus acidophilus protects tight junctions from aspirin damage in HT-29 cells. Digestion. 2004;69:225–228. doi: 10.1159/000079152. [DOI] [PubMed] [Google Scholar]
- 201.Montalto M, Gallo A, Gasbarrini A, Landolfi R. NSAID enteropathy: could probiotics prevent it? J Gastroenterol. 2013;48:689–697. doi: 10.1007/s00535-012-0648-2. [DOI] [PubMed] [Google Scholar]
- 202.Gotteland M, Cruchet S, Verbeke S. Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indometacin in humans. Aliment Pharmacol Ther. 2001;15:11–17. doi: 10.1046/j.1365-2036.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- 203.Montalto M, Gallo A, Curigliano V, D’Onofrio F, Santoro L, Covino M, Dalvai S, Gasbarrini A, Gasbarrini G. Clinical trial: the effects of a probiotic mixture on non-steroidal anti-inflammatory drug enteropathy - a randomized, double-blind, cross-over, placebo-controlled study. Aliment Pharmacol Ther. 2010;32:209–214. doi: 10.1111/j.1365-2036.2010.04324.x. [DOI] [PubMed] [Google Scholar]
- 204.Endo H, Higurashi T, Hosono K, Sakai E, Sekino Y, Iida H, Sakamoto Y, Koide T, Takahashi H, Yoneda M, et al. Efficacy of Lactobacillus casei treatment on small bowel injury in chronic low-dose aspirin users: a pilot randomized controlled study. J Gastroenterol. 2011;46:894–905. doi: 10.1007/s00535-011-0410-1. [DOI] [PubMed] [Google Scholar]


