Abstract
Colorectal anastomotic leakage (CAL) remains a major complication after colorectal surgery. Despite all efforts during the last decades, the incidence of CAL has not decreased. In this review, we summarize the available strategies regarding prevention, prediction and intervention of CAL and categorize them into three categories: communication, infection and healing disturbances. These three major factors actively interact during the onset of CAL. We aim to provide an integrated approach to CAL based on its etiology. The intraoperative air leak test, intraoperative endoscopy, radiological examinations and stoma construction mainly aim to detect and to prevent communication between the intra- and extra-luminal content. Other strategies including postoperative drainage, antibiotics, and infectious-parameter evaluation are intended to detect and prevent anastomotic or peritoneal infection. Most currently available interventions for CAL focus on the control of communication and infection, while strategies targeting the healing disturbances such as lifestyle changes, oxygen therapy and evaluation of metabolic biomarkers still lack wide clinical application. This simplified categorization may contribute to an integrated understanding of CAL. We strongly believe that this integrated approach should be taken into consideration during clinical practice. An integrated approach to CAL could contribute to a better understanding of the etiology of CAL and eventually better patient outcome.
Keywords: Colorectal anastomotic leakage, Integrated approach, Prevention, Prediction, Intervention
Core tip: Colorectal anastomotic leakage (CAL) remains the most dangerous complication after colorectal surgery. In this review, we propose an integrated approach for CAL, consisting of three major parts, communication, infection, and healing disturbances. This simplified categorization is based on the etiology of leakage and may contribute to our integrated understanding of CAL, and eventually facilitate an integrated approach to CAL and eventually better patient outcome.
INTRODUCTION
Colorectal anastomotic leakage (CAL) still remains a frequent and dangerous complication after gastrointestinal surgery, occurring in 4%-33% of patients and contributing to one third of postoperative mortality[1]. An anastomotic defect causes leakage of colonic content into the abdominal or pelvic cavity leading to peritonitis, abscess formation or sepsis[2]. CAL substantially prolongs hospital stay - by one to two weeks - and increases medical costs by as much as $24000 within the first period of hospitalization, thereby approximately tripling the expenditure relative to that of normal recovery[3,4]. Moreover, CAL is identified as a risk factor for local recurrence of colorectal cancer and is reported to reduce long-term cancer specific survival[5]. The need for more effective strategies to prevent and detect CAL is undoubtedly urgent. Many previous studies have explored techniques targeting the prevention, detection and intervention of CAL, but little attention has been paid to the systematic categorization of these strategies. To this end, we aim to provide surgeons with an integrated understanding of these strategies by a categorization based on CAL etiology.
INTEGRATED ETIOLOGY
In research, many efforts have been devoted to identifying risk factors of CAL such as being male[6], smoking[7], alcohol abuse[7], obesity[8], a high American Society of Anesthesiologists (ASA) score[9], low level (e.g., rectal) anastomosis[10], late tumor stage[6], urgent operation[9], increased blood loss[11], after-hours surgery[12], corticosteroids administration[13], and prolonged duration of surgery[14]. However, these risk factors seem to cover most patients, and thus do not contribute to the understanding of the etiology of CAL.
Doctors and researchers still do not understand the detailed etiology of CAL. In many previous studies, CAL was attributed to technical failure or ischemia[15,16], but neither of these seem to explain the whole mechanism[17]. This emphasizes the need for an integrated approach regarding the etiology of CAL.
Based on previous literature and our investigations, we categorized the etiology of CAL into three major components: communication, infection, and healing disturbances (Figure 1).
Figure 1.
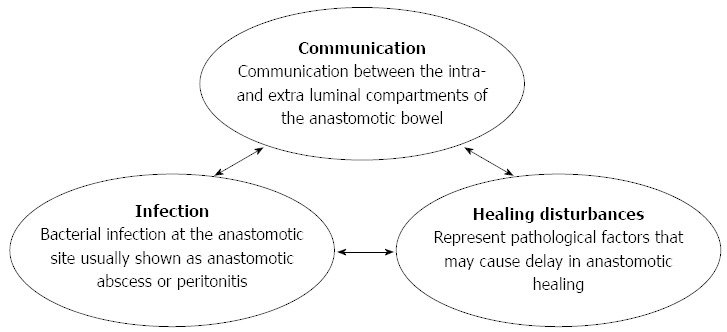
Integrated approach with proposed categorization based on the etiology of colorectal anastomotic leakage (communication, infection and healing disturbances).
Communication represents the classic definition of CAL: “communication between the intra- and extra-luminal compartments of the anastomotic bowel”[2]. Infection indicates bacterial infection at the anastomotic site, which is usually shown as anastomotic abscess or peritonitis. Healing disturbances represent pathological factors that may cause delay in wound healing.
We propose these three major components mainly due to two reasons. First, based on our observations and previous studies, evidence regarding these three aspects was always observed in patients with leakage such as lower anastomotic bursting pressure, anastomotic abscess, peritonitis, ischemia or anastomotic hypoxia[18-21]. Second, we also found that at least one of these factors can be identified as the main cause in CAL cases, which may also cause the other two as these factors actively interact with each other. For instance, it is known that severe infection significantly reduces organ perfusion[22], which may further worsen the healing process of the anastomosis, resulting in CAL. Furthermore, bacterial endotoxins activate the inflammatory response and cause infiltration of inflammatory cells, including subtype-I macrophages, which produce nitric oxide by inducible nitric oxide synthase (iNOS)[20,23]. This overexpression of iNOS is associated with a decrease in collagen deposition[24,25], which eventually causes a delay in wound healing and subsequent communication between intra- and extra-luminal bowel compartments.
PREVENTION
Nowadays, several techniques are available which could contribute to the prevention of CAL. In previous studies, surgeons and researchers have often categorized these strategies based on the time of application (e.g., preoperative, intraoperative and postoperative)[26]. In addition to that, we divided these strategies into the three proposed categories, which further reveals their underlying mechanism (Figure 2).
Figure 2.
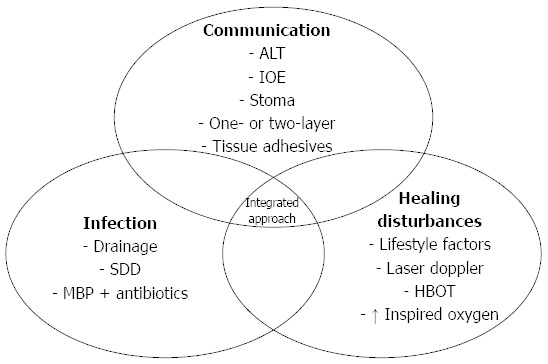
Preventive strategies for colorectal anastomotic leakage with regard to the proposed categorization based on the etiology of colorectal anastomotic leakage (communication, infection and healing disturbances). ALT: Air leaking test; IOE: Intraoperative endoscopy; SDD: Selective decontamination of the digestive tract; MBP: Mechanical bowel preparation; HBOT: Hyperbaric oxygen therapy.
Prevent communication
Many preventive strategies aim to prevent communication between intra- and extra-luminal compartments of the anastomosis.
The air leak test (ALT) is most frequently used as an intraoperative test in colorectal surgery to identify a technically failed anastomosis, which may cause direct communication between intra- and extra-luminal compartments. The rate of this intraoperative test varies greatly in studies evaluating the ALT[27]. Surprisingly, our on-going study shows that meta-analysis of previous studies did not find a significant decrease in the rate of CAL in patients who underwent the ALT[28]. This may partly be due to marked variation in ALT methodology. However, we also found a much higher CAL rate in patients who had a leak during the test[28], thus the ALT is still necessary in our daily practice.
Similar to the ALT, intraoperative endoscopy (IOE) is another intraoperative test which, ideally, could allow immediate diagnostic and therapeutic interventions. However, relevant studies on this topic are very limited and show a low level of evidence. Several authors suggest the selective use of IOE in patients during surgery based on their retrospective data. However, there are at least two studies which show that routine IOE does not reduce the CAL rate compared to selective use[29,30]. Since performing IOE requires certain facilities and equipment, it still seems too early to draw conclusions regarding this technique, especially for routine use[31]. Further research on this topic is required.
Another way to prevent communication is to reinforce the anastomosis. One conventional strategy is to perform a second layer anastomosis. This technique has been used for decades, if not centuries, and was once considered the standard technique for colorectal anastomosis. However, studies have shown that the one-layer anastomosis does not result in a higher CAL rate, hence it is as safe as the double-layer technique[32,33]. Due to these non-inferior results, both the one-layer and the double-layer techniques have their own followers and are being used by different surgeons.
Reinforcing an anastomosis with tissue adhesives is used as another strategy and may serve as a sealant and prevent possible microscopic leakage. The most frequently used tissue adhesive in clinical practice is fibrin glue, which is considered to both reinforce the strength of the anastomosis and facilitate wound healing due to its ingredients[34]. However, analysis of the clinical data shows no actual beneficial influence of the intraoperative application of fibrin glue[35].
Our ex vivo research demonstrated that fibrin glue, together with many other sealants, were very weak in mechanical tests[36]. Many animal studies have also shown that fibrin glue does not accelerate wound healing[37,38]. Nevertheless, one type of tissue adhesive, cyanoacrylates, has emerged from our series of experiments[39]. This glue is preferred over the other glues in mechanical tests, as it increases the mechanical strength of colorectal anastomosis in both normal and technically insufficient situations[40]. Although animal studies have suggested many promising applications of various tissue adhesives[20,23], clinical data are limited and inconclusive. Further clinical research on this topic is planned by our group.
A temporary stoma is also a technique which prevents communication by diverting the intra-luminal content. Although the effect of preventing CAL with diversion seems unquestionable[41], previous studies on this topic have resulted in different conclusions[42-45]. We should be careful with the unselective use of stomas to prevent CAL as stomas are associated with high complication and comorbidity rates[46]. Therefore, routine diversion with a “temporary” stoma should not be recommended in regions with sufficient follow-up of the patients.
Prevent infection
Preventing infection is another major area in CAL prevention. One important technique is drainage placement. The purpose of drainage placement seems evident: it helps to eliminate localized toxins and thus prevents infection and its further advancement. Nowadays, drainage placement is omitted in more and more colonic surgeries especially in centers applying the ERAS (Early Recovery After Surgery) program, while in most centers it remains routine practice after anterior rectal resection. However, several contradictory meta-analyses are available regarding the effect of drainage[47-49]. The most recent meta-analysis indicates that a pelvic drain reduces the incidence of extra peritoneal CAL and the rate of re-intervention after anterior rectal resection. These findings are based on the analysis of observational studies. In contrast, the analysis of RCTs did not indicate any benefit of drainage[48].
Another strategy to prevent infection is the application of preoperative selective decontamination of the digestive tract (SDD), which aims to eradicate pathogenic microorganisms with oral antibiotics before elective resection. There is currently one on-going randomized controlled trial, the SELECT trial[50], which is investigating the use of SDD. The results of this trial are expected to further modify the current clinical regimen.
Bowel preparation also follows the concept of preventing infection by eliminating intraluminal pathogens. However, the conventional “mechanical bowel preparation” has been greatly challenged by accumulating evidence which suggests that it may not reduce the risk of CAL, but only substantially delays the return of bowel function[51]. However, evidence for or against the use of oral mechanical bowel preparation is still too weak to change this worldwide clinical practice. Whether bowel preparation should be included into routine preparation for colorectal surgery still requires data from future investigations.
Prevent healing disturbances
Many healing disturbances have been identified as pre-operative risk factors of CAL such as diabetes mellitus and smoking. Therefore, a preoperative assessment of the patient’s condition is important in the prevention of CAL. Many life-style changes and medical interventions should be arranged before admission. However, the clinical influence of many of these strategies remains unclear and is yet to be determined.
Of course, not all healing disturbances are reversible before surgery. Bowel ischemia contributes to the occurrence of CAL[16,52,53], and therefore intraoperative measurement of the cutting edges may help to detect ischemic edges and may theoretically assist surgeons in the alternative management of the anastomosis (reconstruction or diversion)[54]. However, it is important to note that there is no solid (i.e., high level) evidence supporting such an application. Although observational studies have demonstrated the safety of such a device, it remains unclear whether those detected “ischemic” edges would eventually cause any clinical side effects. Further studies on this topic are necessary before further wide application.
Perioperative tissue oxygen tension measurement could also provide information on anastomotic perfusion[55]. In 1985, it was demonstrated in rabbits that lower tissue oxygen tension was associated with CAL[56]. Therefore, several animal experiments were performed to establish whether Hyperbaric Oxygen Therapy (HBOT) could prevent CAL[57-59]. All studies demonstrated that HBOT increases tissue oxygen tension and improves anastomotic healing. In addition, it is known that high intraoperative inspired oxygen fraction reduces surgical site infections[60,61]. A double-blinded RCT indicated that perioperative supplemental oxygen administration reduced postoperative anastomotic dehiscence after total gastrectomy[62]. The same study group performed a RCT on major rectal cancer surgery and found similar results[63]. With these data, the perioperative application of oxygen therapy seems promising; however, its application is still limited in current clinical practice.
PREDICTION AND EARLY DETECTION
CAL is usually detected between day 5 and day 8 postoperatively, or even later after surgery[64], with more than 50% of cases requiring a reoperation[2,65]. This suggests that with the current strategy many early stages of leakage are not detected until they progress to a severe status. Early diagnosis is necessary as delayed diagnosis of CAL increases postoperative mortality[66]. Figure 3 provides an overview of the methods of prediction and early detection, which have been assessed during the last decades.
Figure 3.
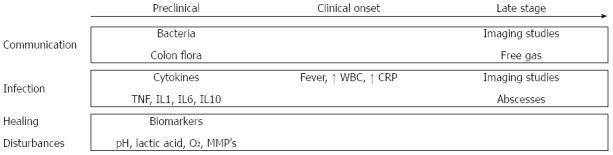
Overview of the methods of prediction and early detection of colorectal anastomotic leakage with regard to the proposed categorization based on the etiology of colorectal anastomotic leakage (communication, infection and healing disturbances). TNF: Tumor necrosis factor; IL: Interleukin; WBC: White blood cell; CRP: C-reactive protein; MMP: Matrix metalloproteinase.
In most cases, conventional radiological examinations are still required to confirm the occurrence of CAL. However, decision-making on radiological examinations depends on the surgeon’s awareness, which is based on clinical manifestations and laboratory tests. Fever, abdominal pain and prolonged ileus are considered clinical manifestations of CAL but are common after colorectal surgery[67,68]. Based on risk factor analysis and expert opinions, several scoring systems have been developed to predict the individual risk of developing CAL after surgery[69-71]. Dekker et al[69] proposed the Colorectal Leakage Score based on the literature and expert opinions. In 2013 den Dulk et al[70] suggested the modified DULK score, which evaluated postoperative factors to estimate the risk of CAL. These scores may help the surgeon make an individualized decision, but prospective evaluation of these scores is still limited to date.
Early detection of communication
Imaging studies aim to show whether communication exists between the intra- and extra-luminal compartments of the anastomosis. Routine imaging studies may decrease the interval between diagnosis and treatment of CAL, but are not ideal due to radiation exposure, costs, patient discomfort and false positives because of subclinical CAL[66,72]. In addition, the diagnostic accuracy of imaging tests is still under debate. The sensitivity of CT-scanning for the early detection of CAL varies from 15% to 52%. The main problem for routine use of CT-scanning is the high reported rate of false negatives[73-75]. The other option for radiological evaluation of colorectal anastomoses is contrast radiography. The sensitivity and specificity of this alternative imaging test range from 20% to 52% and from 85% to 87%, respectively, when performed routinely at postoperative day 7 or 8[76,77]. When contrast radiography is performed in patients with clinical symptoms, the diagnostic accuracy is reported to be higher, with a sensitivity of 68% and a specificity of 94%[73]. Nevertheless, we should be aware that the interval between operation and the examination is often more than a week, indicating that this technique may not be adequate in detecting CAL at an early stage, but only when leakage has already progressed to a severe state in which abscesses or free gas are already present and indicated by imaging studies.
Recent studies focus on innovative strategies to detect CAL as routine radiological examinations are not preferred because leakage is detected at a relatively late stage. An early screening tool for CAL could be the detection of colon flora in drain fluid. The presence of colon flora in drain fluid is suggestive of communication between intra- and extra-luminal compartments and causes infection at the anastomotic site in patients with leakage[2]. Although promising, there are few studies which have considered the predictive value of bacterial measurement in drain fluid. Fouda et al[78] evaluated intraperitoneal bacterial colonization using cultures during the early postoperative period after rectal surgery. Escherichia coli, Bacteroides and Pseudomonas showed significant differences between leaking and non-leaking patients at postoperative day 1, 3 and 5. These results indicate that this method may decrease the period to diagnosis of CAL. However, it takes at least 48 hours before bacteria can be identified on quantitate cultures, resulting in an inevitable delay in diagnosis. Therefore, Komen et al[79] proposed the use of RT-PCR techniques for the detection of bacteria in drain fluid. This technique is much faster, more sensitive and less susceptible to contamination than culture. It achieved a negative predictive value of 98.7%, although its positive predictive value was unsatisfactory (31.6%).
Early detection of infection
Leukocyte count and serum C-reactive protein (CRP) levels are often abnormal after surgery both in CAL patients and in a substantial number of patients with uncomplicated recovery. Therefore, these parameters have a limited predictive value for CAL[67,80]. In 2014, a meta-analysis by Singh et al[81] was published which assessed the predictive value of serum CRP levels for CAL. Rather than determining the positive predictive value, this article reported a negative predictive value of 97% for CRP on postoperative day 3-5, while the corresponding positive predictive value for leakage ranged between 21% and 23%.
In addition to white blood cell count and CRP levels, other innovative inflammatory biomarkers have also been tested in several studies for the early detection of CAL. Inflammatory cytokines such as TNF-a, IL-1b, and IL-6 have been evaluated in both peritoneal drain fluid and blood samples. Cini et al[82] performed a meta-analysis and found that cytokine levels in drain fluid were significantly higher in CAL cases. However, Ellebæk et al[83] reported that serum levels of inflammatory cytokines were the same in patients with CAL and in those with normal recovery. This is because the onset of CAL is a progressive process. A localized response at the site of the anastomosis occurs before systemic changes such as fever, leukocytosis and septic symptoms become manifest[84]. Therefore, monitoring changes in cytokine levels in drain fluid could contribute to the early detection of CAL[85], while systematic changes remain latent until CAL is at an advanced stage[86]. The data from these studies seem promising; however, the main problem with the available literature is that these reports provide a low level of evidence due to low sample sizes, poor patient selection and lack of standardization[78,87-91]. Further examination of these parameters may be an interesting topic for future studies.
Early detection of healing disturbances
As many metabolic biomarkers represent healing disturbances, the detection of metabolic parameters may be another strategy for the early detection of CAL. However, clinical data on this topic are very limited, mainly due to a lack of proper sensors[18]. Daams et al[92] showed promising results using the minimally invasive method of intraperitoneal microdialysis. This technique enabled measurement of real-time local ischemia and changes in metabolism by establishing dialysate levels of lactate, pyruvate, glucose and glycerol[93-95]. Due to a lack of clinical data, how to correctly interpret these metabolic data and associate them with anastomotic healing remains difficult and requires further investigation[96,97].
INTERVENTION
Once leakage has occurred, an effective intervention should be undertaken to control morbidity and mortality. The ultimate goal of prediction or early detection of CAL is to initiate timely treatment to improve patient outcome. The type of intervention strongly depends on the severity of CAL, which as discussed above, is hard to determine and therefore the choice of intervention for a suspicious leakage is quite complex with very limited evidence available at present[2].
Despite individual experience from surgeons, the best knowledge regarding intervention in CAL came from a Delphi analysis, which used an expert panel and aimed to emphasize consensus[98] and to construct evidence-based guidelines[99]. Phitayakorn et al[100] used this technique to develop a treatment algorithm for CAL.
Interventions for CAL can be divided into two main groups: treatment of infection and treatment of communication. Interventions which prevent communication also contribute to infection control, therefore most interventions for CAL require an integrated approach.
Administration of antibiotics is often the first intervention when CAL is suspected. Antibiotics are usually modified after the results of the susceptibility test are obtained when drainage or blood samples are cultured. A retrospective study showed that both surgical and non-surgical interventions based on the presentation of CAL are both effective and safe[101]. There are several surgical intervention options: drainage, repair of the anastomosis, deviating ileostomy or permanent colostomy. It is known that a stoma after colorectal surgery moderates quality of life. Moreover, half of patients who undergo the formation of a stoma due to CAL are left with a permanent stoma[102]. Given that routine construction of a stoma for CAL repair should not be recommended, alternative surgical strategies should be discussed and considered before reoperation[103].
If surgical re-intervention is indicated, and the surgeon decides to construct a stoma, the choice between diversion of the anastomosis with a loop ileostomy and resection of the anastomosis with end colostomy should be made. A questionnaire completed by members of the Dutch Society for Gastrointestinal Surgery showed that Dutch colorectal surgeons prefer to preserve the anastomosis in young non-septic patients, whereas the anastomosis is broken down and a colostomy is constructed in older patients or in those with abdominal sepsis[104]. Despite the surgeon’s experience, this choice strongly depends on the severity of leakage and comorbidities in the patient[105]. Some data suggest that diversion with loop ileostomy is safe and is associated with less mortality and morbidity if no sepsis or fecal contamination is present[106,107], but no solid evidence or consensus is available in this regard.
Most re-interventions were initiated with an open approach until recently when two retrospective cohort studies showed that laparoscopic re-intervention for CAL was safe and feasible[108,109]. With more and more surgeons experienced in the laparoscopic approach, we may expect laparoscopy as the first choice for re-interventions in the future.
CONCLUSION
CAL remains the most dangerous complication after colorectal surgery. Surgeons still have to deal with this critical issue mainly based on their experience and limited knowledge from the literature. In this review, we proposed an integrated etiology of CAL, consisting of three major parts including communication, infection, and healing disturbances. Based on the etiology, we categorized the currently available strategies into at least one of these major factors. This simplified categorization may contribute to our integrated understanding of CAL. All three aspects should be taken into consideration during clinical practice regarding prevention, prediction, early detection and intervention of CAL, which we believe will eventually facilitate an integrated approach for CAL and result in better patient outcome.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Authors declare no conflict of interest for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 28, 2016
First decision: May 12, 2016
Article in press: June 15, 2016
P- Reviewer: Lakatos PL, Uggeri F S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Ma S
References
- 1.Snijders HS, Wouters MW, van Leersum NJ, Kolfschoten NE, Henneman D, de Vries AC, Tollenaar RA, Bonsing BA. Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol. 2012;38:1013–1019. doi: 10.1016/j.ejso.2012.07.111. [DOI] [PubMed] [Google Scholar]
- 2.Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Hammond J, Lim S, Wan Y, Gao X, Patkar A. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg. 2014;18:1176–1185. doi: 10.1007/s11605-014-2506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemi L, Mukherjee N, Morseon M, Sirkar R. SAGES 2012 Meeting, San Diego, CA, United States; 2012. Economic impact of anastomotic leaks in colectomy procedures in the USA: 2005-2009. [Google Scholar]
- 5.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 6.Eriksen MT, Wibe A, Norstein J, Haffner J, Wiig JN. Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Colorectal Dis. 2005;7:51–57. doi: 10.1111/j.1463-1318.2004.00700.x. [DOI] [PubMed] [Google Scholar]
- 7.Sørensen LT, Jørgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille-Jørgensen P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg. 1999;86:927–931. doi: 10.1046/j.1365-2168.1999.01165.x. [DOI] [PubMed] [Google Scholar]
- 8.Nickelsen TN, Jørgensen T, Kronborg O. Lifestyle and 30-day complications to surgery for colorectal cancer. Acta Oncol. 2005;44:218–223. doi: 10.1080/02841860510029707. [DOI] [PubMed] [Google Scholar]
- 9.Choi HK, Law WL, Ho JW. Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon Rectum. 2006;49:1719–1725. doi: 10.1007/s10350-006-0703-2. [DOI] [PubMed] [Google Scholar]
- 10.Vignali A, Fazio VW, Lavery IC, Milsom JW, Church JM, Hull TL, Strong SA, Oakley JR. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg. 1997;185:105–113. doi: 10.1016/s1072-7515(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 11.Daams F, Monkhorst K, van den Broek J, Slieker JC, Jeekel J, Lange JF. Local ischaemia does not influence anastomotic healing: an experimental study. Eur Surg Res. 2013;50:24–31. doi: 10.1159/000348411. [DOI] [PubMed] [Google Scholar]
- 12.Komen N, Dijk JW, Lalmahomed Z, Klop K, Hop W, Kleinrensink GJ, Jeekel H, Ruud Schouten W, Lange JF. After-hours colorectal surgery: a risk factor for anastomotic leakage. Int J Colorectal Dis. 2009;24:789–795. doi: 10.1007/s00384-009-0692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slieker JC, Komen N, Mannaerts GH, Karsten TM, Willemsen P, Murawska M, Jeekel J, Lange JF. Long-term and perioperative corticosteroids in anastomotic leakage: a prospective study of 259 left-sided colorectal anastomoses. Arch Surg. 2012;147:447–452. doi: 10.1001/archsurg.2011.1690. [DOI] [PubMed] [Google Scholar]
- 14.Ho YH, Ashour MA. Techniques for colorectal anastomosis. World J Gastroenterol. 2010;16:1610–1621. doi: 10.3748/wjg.v16.i13.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricciardi R, Roberts PL, Marcello PW, Hall JF, Read TE, Schoetz DJ. Anastomotic leak testing after colorectal resection: what are the data? Arch Surg. 2009;144:407–411; discussion 411-2. doi: 10.1001/archsurg.2009.43. [DOI] [PubMed] [Google Scholar]
- 16.Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum. 2000;43:76–82. doi: 10.1007/BF02237248. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z. An Integrated Approach to Colorectal Anastomotic Leakage. 2015. Available from: http://repub.eur.nl/pub/77525/ [DOI] [PMC free article] [PubMed]
- 18.Wu Z, Boersema GS, Taha D, Fine I, Menon A, Kleinrensink GJ, Jeekel J, Lange JF. Postoperative Hemodynamic Index Measurement With Miniaturized Dynamic Light Scattering Predicts Colorectal Anastomotic Healing. Surg Innov. 2016;23:115–123. doi: 10.1177/1553350615618286. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z, Daams F, Boersema GSA, Jeekel J, Lange JF. Hoboken: Wiley-Blackwell; 2013. A rat model of anastomotic leakage created by insufficient sutures after partial colectomy. Proceedings of the British journal of surgery; p. 211. [Google Scholar]
- 20.Wu Z, Vakalopoulos KA, Boersema GS, Kroese LF, Lam KH, van der Horst PH, Mulder IM, Bastiaansen-Jenniskens YM, Kleinrensink GJ, Jeekel J, et al. The prevention of colorectal anastomotic leakage with tissue adhesives in a contaminated environment is associated with the presence of anti-inflammatory macrophages. Int J Colorectal Dis. 2014;29:1507–1516. doi: 10.1007/s00384-014-2012-x. [DOI] [PubMed] [Google Scholar]
- 21.Wu Z, Vakalopoulos KA, Boersema GSA, Jeekel J, Lange JF. Prevention of Anastomotic Leakage with Tissue Adhesives in Contaminated Environment. Proceedings of the British journal of surgery. Hoboken: Wiley-Blackwell; 2013. p. 5. [Google Scholar]
- 22.Bone RC. Sepsis, the sepsis syndrome, multi-organ failure: a plea for comparable definitions. Ann Intern Med. 1991;114:332–333. doi: 10.7326/0003-4819-114-4-332. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Boersema GS, Kroese LF, Taha D, Vennix S, Bastiaansen-Jenniskens YM, Lam KH, Kleinrensink GJ, Jeekel J, Peppelenbosch M, et al. Reducing colorectal anastomotic leakage with tissue adhesive in experimental inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1038–1046. doi: 10.1097/MIB.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 24.Ahrendt GM, Tantry US, Barbul A. Intra-abdominal sepsis impairs colonic reparative collagen synthesis. Am J Surg. 1996;171:102–107; discussion 107-108. doi: 10.1016/S0002-9610(99)80082-8. [DOI] [PubMed] [Google Scholar]
- 25.Thornton FJ, Ahrendt GM, Schäffer MR, Tantry US, Barbul A. Sepsis impairs anastomotic collagen gene expression and synthesis: a possible role for nitric oxide. J Surg Res. 1997;69:81–86. doi: 10.1006/jsre.1997.5034. [DOI] [PubMed] [Google Scholar]
- 26.Daams F, Wu Z, Lahaye MJ, Jeekel J, Lange JF. Prediction and diagnosis of colorectal anastomotic leakage: A systematic review of literature. World J Gastrointest Surg. 2014;6:14–26. doi: 10.4240/wjgs.v6.i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachiappan S, Askari A, Currie A, Kennedy RH, Faiz O. Intraoperative assessment of colorectal anastomotic integrity: a systematic review. Surg Endosc. 2014;28:2513–2530. doi: 10.1007/s00464-014-3520-z. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, van de Haar RC, Sparreboom CL, Boersema GS, Li Z, Ji J, Jeekel J, Lange JF. Is the intraoperative air leak test effective in the prevention of colorectal anastomotic leakage? A systematic review and meta-analysis. Int J Colorectal Dis. 2016;31:1409–1417. doi: 10.1007/s00384-016-2616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li VK, Wexner SD, Pulido N, Wang H, Jin HY, Weiss EG, Nogeuras JJ, Sands DR. Use of routine intraoperative endoscopy in elective laparoscopic colorectal surgery: can it further avoid anastomotic failure? Surg Endosc. 2009;23:2459–2465. doi: 10.1007/s00464-009-0416-4. [DOI] [PubMed] [Google Scholar]
- 30.Shamiyeh A, Szabo K, Ulf Wayand W, Zehetner J. Intraoperative endoscopy for the assessment of circular-stapled anastomosis in laparoscopic colon surgery. Surg Laparosc Endosc Percutan Tech. 2012;22:65–67. doi: 10.1097/SLE.0b013e3182401e20. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Menon A, Jeekel J, Lange J. With routine air leak testing of low colorectal anastomosis is routine intra-operative flexible sigmoidoscopy necessary? Colorectal Dis. 2015;17:265. doi: 10.1111/codi.12877. [DOI] [PubMed] [Google Scholar]
- 32.Everett WG. A comparison of one layer and two layer techniques for colorectal anastomosis. Br J Surg. 1975;62:135–140. doi: 10.1002/bjs.1800620214. [DOI] [PubMed] [Google Scholar]
- 33.Wayand W, Rieger R, Umlauft M. [Single or double layer? A controlled prospective study on the comparison of 2 suture technics in gastrointestinal anastomoses] Chirurg. 1984;55:650–652. [PubMed] [Google Scholar]
- 34.Vakalopoulos KA, Daams F, Wu Z, Timmermans L, Jeekel JJ, Kleinrensink GJ, van der Ham A, Lange JF. Tissue adhesives in gastrointestinal anastomosis: a systematic review. J Surg Res. 2013;180:290–300. doi: 10.1016/j.jss.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 35.Vakalopoulos KA, Wu Z, Kroese LF, van der Horst PH, Lam KH, Dodou D, Jeekel JJ, Lange JF. Clinical, mechanical, and immunohistopathological effects of tissue adhesives on the colon: An in-vivo study. J Biomed Mater Res B Appl Biomater. 2016 doi: 10.1002/jbm.b.33621. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Vakalopoulos KA, Wu Z, Kroese L, Kleinrensink GJ, Jeekel J, Vendamme R, Dodou D, Lange JF. Mechanical strength and rheological properties of tissue adhesives with regard to colorectal anastomosis: an ex vivo study. Ann Surg. 2015;261:323–331. doi: 10.1097/SLA.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 37.van der Ham AC, Kort WJ, Weijma IM, Jeekel H. Transient protection of incomplete colonic anastomoses with fibrin sealant: an experimental study in the rat. J Surg Res. 1993;55:256–260. doi: 10.1006/jsre.1993.1137. [DOI] [PubMed] [Google Scholar]
- 38.van der Ham AC, Kort WJ, Weijma IM, van den Ingh HF, Jeekel H. Healing of ischemic colonic anastomosis: fibrin sealant does not improve wound healing. Dis Colon Rectum. 1992;35:884–891. doi: 10.1007/BF02047878. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z, Boersema GS, Vakalopoulos KA, Daams F, Sparreboom CL, Kleinrensink GJ, Jeekel J, Lange JF. Critical analysis of cyanoacrylate in intestinal and colorectal anastomosis. J Biomed Mater Res B Appl Biomater. 2014;102:635–642. doi: 10.1002/jbm.b.33039. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Vakalopoulos KA, Kroese LF, Boersema GS, Kleinrensink GJ, Jeekel J, Lange JF. Reducing anastomotic leakage by reinforcement of colorectal anastomosis with cyanoacrylate glue. Eur Surg Res. 2013;50:255–261. doi: 10.1159/000350383. [DOI] [PubMed] [Google Scholar]
- 41.Hüser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J, Friess H. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg. 2008;248:52–60. doi: 10.1097/SLA.0b013e318176bf65. [DOI] [PubMed] [Google Scholar]
- 42.Graffner H, Fredlund P, Olsson SA, Oscarson J, Petersson BG. Protective colostomy in low anterior resection of the rectum using the EEA stapling instrument. A randomized study. Dis Colon Rectum. 1983;26:87–90. doi: 10.1007/BF02562579. [DOI] [PubMed] [Google Scholar]
- 43.Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246:207–214. doi: 10.1097/SLA.0b013e3180603024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pakkastie TE, Ovaska JT, Pekkala ES, Luukkonen PE, Järvinen HJ. A randomised study of colostomies in low colorectal anastomoses. Eur J Surg. 1997;163:929–933. [PubMed] [Google Scholar]
- 45.Pimentel JM, Duarte A, Patricio J. The role of a protecting stoma in low anterior resection with TME and colonic J-pouch for rectal cancer; results of a prospective randomized trial. Colorectal Dis. 2003;5 Suppl 2:P83. [Google Scholar]
- 46.Gooszen AW, Geelkerken RH, Hermans J, Lagaay MB, Gooszen HG. Temporary decompression after colorectal surgery: randomized comparison of loop ileostomy and loop colostomy. Br J Surg. 1998;85:76–79. doi: 10.1046/j.1365-2168.1998.00526.x. [DOI] [PubMed] [Google Scholar]
- 47.Karliczek A, Jesus EC, Matos D, Castro AA, Atallah AN, Wiggers T. Drainage or nondrainage in elective colorectal anastomosis: a systematic review and meta-analysis. Colorectal Dis. 2006;8:259–265. doi: 10.1111/j.1463-1318.2006.00999.x. [DOI] [PubMed] [Google Scholar]
- 48.Rondelli F, Bugiantella W, Vedovati MC, Balzarotti R, Avenia N, Mariani E, Agnelli G, Becattini C. To drain or not to drain extraperitoneal colorectal anastomosis? A systematic review and meta-analysis. Colorectal Dis. 2014;16:O35–O42. doi: 10.1111/codi.12491. [DOI] [PubMed] [Google Scholar]
- 49.Urbach DR, Kennedy ED, Cohen MM. Colon and rectal anastomoses do not require routine drainage: a systematic review and meta-analysis. Ann Surg. 1999;229:174–180. doi: 10.1097/00000658-199902000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abis GS, Oosterling SJ, Stockmann HB, van der Bij GJ, van Egmond M, Vandenbroucke-Grauls CM, Bonjer HJ. Perioperative selective decontamination of the digestive tract and standard antibiotic prophylaxis versus standard antibiotic prophylaxis alone in elective colorectal cancer patients. Dan Med J. 2014;61:A4695. [PubMed] [Google Scholar]
- 51.Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, Ljungqvist O, Soop M, Ramirez J. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg. 2013;37:285–305. doi: 10.1007/s00268-012-1787-6. [DOI] [PubMed] [Google Scholar]
- 52.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Law WI, Chu KW, Ho JW, Chan CW. Risk factors for anastomotic leakage after low anterior resection with total mesorectal excision. Am J Surg. 2000;179:92–96. doi: 10.1016/s0002-9610(00)00252-x. [DOI] [PubMed] [Google Scholar]
- 54.Ambrosetti P, Robert J, Mathey P, Rohner A. Left-sided colon and colorectal anastomoses: Doppler ultrasound as an aid to assess bowel vascularization. A prospective evaluation of 200 consecutive elective cases. Int J Colorectal Dis. 1994;9:211–214. doi: 10.1007/BF00292253. [DOI] [PubMed] [Google Scholar]
- 55.Sheridan WG, Lowndes RH, Young HL. Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum. 1987;30:867–871. doi: 10.1007/BF02555426. [DOI] [PubMed] [Google Scholar]
- 56.Shandall A, Lowndes R, Young HL. Colonic anastomotic healing and oxygen tension. Br J Surg. 1985;72:606–609. doi: 10.1002/bjs.1800720808. [DOI] [PubMed] [Google Scholar]
- 57.Hamzaoğlu I, Karahasanoğlu T, Aydin S, Sahin DA, Carkman S, Sariyar M, Alemdaroğlu K. The effects of hyperbaric oxygen on normal and ischemic colon anastomoses. Am J Surg. 1998;176:458–461. doi: 10.1016/s0002-9610(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 58.Kimberger O, Fleischmann E, Brandt S, Kugener A, Kabon B, Hiltebrand L, Krejci V, Kurz A. Supplemental oxygen, but not supplemental crystalloid fluid, increases tissue oxygen tension in healthy and anastomotic colon in pigs. Anesth Analg. 2007;105:773–779. doi: 10.1213/01.ane.0000277490.90387.96. [DOI] [PubMed] [Google Scholar]
- 59.Yildiz R, Can MF, Yagci G, Ozgurtas T, Guden M, Gamsizkan M, Ozturk E, Cetiner S. The effects of hyperbaric oxygen therapy on experimental colon anastomosis after preoperative chemoradiotherapy. Int Surg. 2013;98:33–42. doi: 10.9738/CC130.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hovaguimian F, Lysakowski C, Elia N, Tramèr MR. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2013;119:303–316. doi: 10.1097/ALN.0b013e31829aaff4. [DOI] [PubMed] [Google Scholar]
- 61.Qadan M, Akça O, Mahid SS, Hornung CA, Polk HC. Perioperative supplemental oxygen therapy and surgical site infection: a meta-analysis of randomized controlled trials. Arch Surg. 2009;144:359–366; discussion 366-367. doi: 10.1001/archsurg.2009.1. [DOI] [PubMed] [Google Scholar]
- 62.Schietroma M, Cecilia EM, Carlei F, Sista F, De Santis G, Piccione F, Amicucci G. Prevention of anastomotic leakage after total gastrectomy with perioperative supplemental oxygen administration: a prospective randomized, double-blind, controlled, single-center trial. Ann Surg Oncol. 2013;20:1584–1590. doi: 10.1245/s10434-012-2714-7. [DOI] [PubMed] [Google Scholar]
- 63.Schietroma M, Carlei F, Cecilia EM, Piccione F, Bianchi Z, Amicucci G. Colorectal Infraperitoneal anastomosis: the effects of perioperative supplemental oxygen administration on the anastomotic dehiscence. J Gastrointest Surg. 2012;16:427–434. doi: 10.1007/s11605-011-1717-1. [DOI] [PubMed] [Google Scholar]
- 64.Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245:254–258. doi: 10.1097/01.sla.0000225083.27182.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cong ZJ, Hu LH, Bian ZQ, Ye GY, Yu MH, Gao YH, Li ZS, Yu ED, Zhong M. Systematic review of anastomotic leakage rate according to an international grading system following anterior resection for rectal cancer. PLoS One. 2013;8:e75519. doi: 10.1371/journal.pone.0075519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Macarthur DC, Nixon SJ, Aitken RJ. Avoidable deaths still occur after large bowel surgery. Scottish Audit of Surgical Mortality, Royal College of Surgeons of Edinburgh. Br J Surg. 1998;85:80–83. doi: 10.1046/j.1365-2168.1998.00554.x. [DOI] [PubMed] [Google Scholar]
- 67.Erb L, Hyman NH, Osler T. Abnormal vital signs are common after bowel resection and do not predict anastomotic leak. J Am Coll Surg. 2014;218:1195–1199. doi: 10.1016/j.jamcollsurg.2013.12.059. [DOI] [PubMed] [Google Scholar]
- 68.Wu Z, Boersema GS, Dereci A, Menon AG, Jeekel J, Lange JF. Clinical endpoint, early detection, and differential diagnosis of postoperative ileus: a systematic review of the literature. Eur Surg Res. 2015;54:127–138. doi: 10.1159/000369529. [DOI] [PubMed] [Google Scholar]
- 69.Dekker JW, Liefers GJ, de Mol van Otterloo JC, Putter H, Tollenaar RA. Predicting the risk of anastomotic leakage in left-sided colorectal surgery using a colon leakage score. J Surg Res. 2011;166:e27–e34. doi: 10.1016/j.jss.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 70.den Dulk M, Witvliet MJ, Kortram K, Neijenhuis PA, de Hingh IH, Engel AF, van de Velde CJ, de Brauw LM, Putter H, Brouwers MA, et al. The DULK (Dutch leakage) and modified DULK score compared: actively seek the leak. Colorectal Dis. 2013;15:e528–e533. doi: 10.1111/codi.12379. [DOI] [PubMed] [Google Scholar]
- 71.Rojas-Machado SA, Romero-Simó M, Arroyo A, Rojas-Machado A, López J, Calpena R. Prediction of anastomotic leak in colorectal cancer surgery based on a new prognostic index PROCOLE (prognostic colorectal leakage) developed from the meta-analysis of observational studies of risk factors. Int J Colorectal Dis. 2016;31:197–210. doi: 10.1007/s00384-015-2422-4. [DOI] [PubMed] [Google Scholar]
- 72.Goligher JC, Lee PW, Simpkins KC, Lintott DJ. A controlled comparison one- and two-layer techniques of suture for high and low colorectal anastomoses. Br J Surg. 1977;64:609–614. doi: 10.1002/bjs.1800640902. [DOI] [PubMed] [Google Scholar]
- 73.Doeksen A, Tanis PJ, Wüst AF, Vrouenraets BC, van Lanschot JJ, van Tets WF. Radiological evaluation of colorectal anastomoses. Int J Colorectal Dis. 2008;23:863–868. doi: 10.1007/s00384-008-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khoury W, Ben-Yehuda A, Ben-Haim M, Klausner JM, Szold O. Abdominal computed tomography for diagnosing postoperative lower gastrointestinal tract leaks. J Gastrointest Surg. 2009;13:1454–1458. doi: 10.1007/s11605-009-0925-4. [DOI] [PubMed] [Google Scholar]
- 75.Nicksa GA, Dring RV, Johnson KH, Sardella WV, Vignati PV, Cohen JL. Anastomotic leaks: what is the best diagnostic imaging study? Dis Colon Rectum. 2007;50:197–203. doi: 10.1007/s10350-006-0708-x. [DOI] [PubMed] [Google Scholar]
- 76.Akyol AM, McGregor JR, Galloway DJ, George WD. Early postoperative contrast radiology in the assessment of colorectal anastomotic integrity. Int J Colorectal Dis. 1992;7:141–143. doi: 10.1007/BF00360354. [DOI] [PubMed] [Google Scholar]
- 77.Hoffmann J, Jensen RH, Shokouh-Amiri MH, Damm P. Clinical value of water-soluble contrast enema in assessing the integrity of left colonic anastomoses. J R Coll Surg Edinb. 1988;33:23–24. [PubMed] [Google Scholar]
- 78.Fouda E, El Nakeeb A, Magdy A, Hammad EA, Othman G, Farid M. Early detection of anastomotic leakage after elective low anterior resection. J Gastrointest Surg. 2011;15:137–144. doi: 10.1007/s11605-010-1364-y. [DOI] [PubMed] [Google Scholar]
- 79.Komen N, Slieker J, Willemsen P, Mannaerts G, Pattyn P, Karsten T, de Wilt H, van der Harst E, van Leeuwen W, Decaestecker C, et al. Polymerase chain reaction for Enterococcus faecalis in drain fluid: the first screening test for symptomatic colorectal anastomotic leakage. The Appeal-study: analysis of parameters predictive for evident anastomotic leakage. Int J Colorectal Dis. 2014;29:15–21. doi: 10.1007/s00384-013-1776-8. [DOI] [PubMed] [Google Scholar]
- 80.Alves A, Panis Y, Pocard M, Regimbeau JM, Valleur P. Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg. 1999;189:554–559. doi: 10.1016/s1072-7515(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 81.Singh PP, Zeng IS, Srinivasa S, Lemanu DP, Connolly AB, Hill AG. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg. 2014;101:339–346. doi: 10.1002/bjs.9354. [DOI] [PubMed] [Google Scholar]
- 82.Cini C, Wolthuis A, D’Hoore A. Peritoneal fluid cytokines and matrix metalloproteinases as early markers of anastomotic leakage in colorectal anastomosis: a literature review and meta-analysis. Colorectal Dis. 2013;15:1070–1077. doi: 10.1111/codi.12192. [DOI] [PubMed] [Google Scholar]
- 83.Ellebæk MB, Baatrup G, Gjedsted J, Fristrup C, Qvist N. Cytokine response in peripheral blood indicates different pathophysiological mechanisms behind anastomotic leakage after low anterior resection: a pilot study. Tech Coloproctol. 2014;18:1067–1074. doi: 10.1007/s10151-014-1204-2. [DOI] [PubMed] [Google Scholar]
- 84.Wiik H, Karttunen R, Haukipuro K, Syrjälä H. Maximal local and minimal systemic cytokine response to colorectal surgery: the influence of perioperative filgrastim. Cytokine. 2001;14:188–192. doi: 10.1006/cyto.2001.0870. [DOI] [PubMed] [Google Scholar]
- 85.Slotwiński R, Olszewski WL, Chaber A, Slodkowski M, Zaleska M, Krasnodebski IW. The soluble tumor necrosis factor receptor I is an early predictor of local infective complications after colorectal surgery. J Clin Immunol. 2002;22:289–296. doi: 10.1023/a:1020022006043. [DOI] [PubMed] [Google Scholar]
- 86.Sparreboom CL, Wu Z, Dereci A, Boersema GS, Menon AG, Ji J, Kleinrensink GJ, Lange JF. Cytokines as Early Markers of Colorectal Anastomotic Leakage: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2016;2016:3786418. doi: 10.1155/2016/3786418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alonso S, Pascual M, Salvans S, Mayol X, Mojal S, Gil MJ, Grande L, Pera M. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol. 2015;41:208–214. doi: 10.1016/j.ejso.2014.10.052. [DOI] [PubMed] [Google Scholar]
- 88.Bertram P, Junge K, Schachtrupp A, Götze C, Kunz D, Schumpelick V. Peritoneal release of TNFalpha and IL-6 after elective colorectal surgery and anastomotic leakage. J Invest Surg. 2003;16:65–69. [PubMed] [Google Scholar]
- 89.Matthiessen P, Strand I, Jansson K, Törnquist C, Andersson M, Rutegård J, Norgren L. Is early detection of anastomotic leakage possible by intraperitoneal microdialysis and intraperitoneal cytokines after anterior resection of the rectum for cancer? Dis Colon Rectum. 2007;50:1918–1927. doi: 10.1007/s10350-007-9023-4. [DOI] [PubMed] [Google Scholar]
- 90.Uğraş B, Giriş M, Erbil Y, Gökpinar M, Citlak G, Işsever H, Bozbora A, Oztezcan S. Early prediction of anastomotic leakage after colorectal surgery by measuring peritoneal cytokines: prospective study. Int J Surg. 2008;6:28–35. doi: 10.1016/j.ijsu.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto T, Umegae S, Matsumoto K, Saniabadi AR. Peritoneal cytokines as early markers of peritonitis following surgery for colorectal carcinoma: a prospective study. Cytokine. 2011;53:239–242. doi: 10.1016/j.cyto.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 92.Daams F, Wu Z, Cakir H, Karsten TM, Lange JF. Identification of anastomotic leakage after colorectal surgery using microdialysis of the peritoneal cavity. Tech Coloproctol. 2014;18:65–71. doi: 10.1007/s10151-013-1020-0. [DOI] [PubMed] [Google Scholar]
- 93.Jansson K, Ungerstedt J, Jonsson T, Redler B, Andersson M, Ungerstedt U, Norgren L. Human intraperitoneal microdialysis: increased lactate/pyruvate ratio suggests early visceral ischaemia. A pilot study. Scand J Gastroenterol. 2003;38:1007–1011. doi: 10.1080/00365520310004065. [DOI] [PubMed] [Google Scholar]
- 94.Sommer T, Larsen JF. Intraperitoneal and intraluminal microdialysis in the detection of experimental regional intestinal ischaemia. Br J Surg. 2004;91:855–861. doi: 10.1002/bjs.4586. [DOI] [PubMed] [Google Scholar]
- 95.Ungerstedt J, Nowak G, Ericzon BG, Ungerstedt U. Intraperitoneal microdialysis (IPM): a new technique for monitoring intestinal ischemia studied in a porcine model. Shock. 2003;20:91–96. doi: 10.1097/01.shk.0000070904.21762.36. [DOI] [PubMed] [Google Scholar]
- 96.Baker EA, Leaper DJ. Profiles of matrix metalloproteinases and their tissue inhibitors in intraperitoneal drainage fluid: relationship to wound healing. Wound Repair Regen. 2003;11:268–274. doi: 10.1046/j.1524-475x.2003.11406.x. [DOI] [PubMed] [Google Scholar]
- 97.Pasternak B, Matthiessen P, Jansson K, Andersson M, Aspenberg P. Elevated intraperitoneal matrix metalloproteinases-8 and -9 in patients who develop anastomotic leakage after rectal cancer surgery: a pilot study. Colorectal Dis. 2010;12:e93–e98. doi: 10.1111/j.1463-1318.2009.01908.x. [DOI] [PubMed] [Google Scholar]
- 98.de Villiers MR, de Villiers PJ, Kent AP. The Delphi technique in health sciences education research. Med Teach. 2005;27:639–643. doi: 10.1080/13611260500069947. [DOI] [PubMed] [Google Scholar]
- 99.Roddy E, Zhang W, Doherty M, Arden NK, Barlow J, Birrell F, Carr A, Chakravarty K, Dickson J, Hay E, et al. Evidence-based clinical guidelines: a new system to better determine true strength of recommendation. J Eval Clin Pract. 2006;12:347–352. doi: 10.1111/j.1365-2753.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 100.Phitayakorn R, Delaney CP, Reynolds HL, Champagne BJ, Heriot AG, Neary P, Senagore AJ; International Anastomotic Leak Study Group. Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J Surg. 2008;32:1147–1156. doi: 10.1007/s00268-008-9468-1. [DOI] [PubMed] [Google Scholar]
- 101.Blumetti J, Chaudhry V, Cintron JR, Park JJ, Marecik S, Harrison JL, Prasad LM, Abcarian H. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg. 2014;38:985–991. doi: 10.1007/s00268-013-2340-y. [DOI] [PubMed] [Google Scholar]
- 102.Khan AA, Wheeler JM, Cunningham C, George B, Kettlewell M, Mortensen NJ. The management and outcome of anastomotic leaks in colorectal surgery. Colorectal Dis. 2008;10:587–592. doi: 10.1111/j.1463-1318.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 103.Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg. 2014;259:916–923. doi: 10.1097/SLA.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 104.Daams F, Slieker JC, Tedja A, Karsten TM, Lange JF. Treatment of colorectal anastomotic leakage: results of a questionnaire amongst members of the Dutch Society of Gastrointestinal Surgery. Dig Surg. 2012;29:516–521. doi: 10.1159/000346348. [DOI] [PubMed] [Google Scholar]
- 105.Moghadamyeghaneh Z, Hanna MH, Alizadeh RF, Carmichael JC, Mills S, Pigazzi A, Stamos MJ. Contemporary management of anastomotic leak after colon surgery: assessing the need for reoperation. Am J Surg. 2016;211:1005–1013. doi: 10.1016/j.amjsurg.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 106.Fraccalvieri D, Biondo S, Saez J, Millan M, Kreisler E, Golda T, Frago R, Miguel B. Management of colorectal anastomotic leakage: differences between salvage and anastomotic takedown. Am J Surg. 2012;204:671–676. doi: 10.1016/j.amjsurg.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 107.Hedrick TL, Sawyer RG, Foley EF, Friel CM. Anastomotic leak and the loop ileostomy: friend or foe? Dis Colon Rectum. 2006;49:1167–1176. doi: 10.1007/s10350-006-0602-6. [DOI] [PubMed] [Google Scholar]
- 108.Vennix S, Abegg R, Bakker OJ, van den Boezem PB, Brokelman WJ, Sietses C, Bosscha K, Lips DJ, Prins HA. Surgical re-interventions following colorectal surgery: open versus laparoscopic management of anastomotic leakage. J Laparoendosc Adv Surg Tech A. 2013;23:739–744. doi: 10.1089/lap.2012.0440. [DOI] [PubMed] [Google Scholar]
- 109.Wind J, Koopman AG, van Berge Henegouwen MI, Slors JF, Gouma DJ, Bemelman WA. Laparoscopic reintervention for anastomotic leakage after primary laparoscopic colorectal surgery. Br J Surg. 2007;94:1562–1566. doi: 10.1002/bjs.5892. [DOI] [PubMed] [Google Scholar]


