Abstract
Radiotherapy techniques have substantially improved in the last two decades. After the introduction of 3-dimensional conformal radiotherapy, radiotherapy has been increasingly used for the treatment of hepatocellular carcinoma (HCC). Currently, more advanced techniques, including intensity-modulated radiotherapy (IMRT), stereotactic ablative body radiotherapy (SABR), and charged particle therapy, are used for the treatment of HCC. IMRT can escalate the tumor dose while sparing the normal tissue even though the tumor is large or located near critical organs. SABR can deliver a very high radiation dose to small HCCs in a few fractions, leading to high local control rates of 84%-100%. Various advanced imaging modalities are used for radiotherapy planning and delivery to improve the precision of radiotherapy. These advanced techniques enable the delivery of high dose radiotherapy for early to advanced HCCs without increasing the radiation-induced toxicities. However, as there have been no effective tools for the prediction of the response to radiotherapy or recurrences within or outside the radiation field, future studies should focus on selecting the patients who will benefit from radiotherapy.
Keywords: Hepatocellular carcinoma, Radiotherapy, 3D-conformal radiotherapy, Intensity-modulated radiotherapy, stereotactic ablative body radiotherapy, Charged particle therapy, Image-guided radiotherapy
Core tip: Radiotherapy techniques have greatly improved in the last two decades. After the introduction of 3-dimensional conformal radiotherapy, the use of radiotherapy for hepatocellular carcinoma (HCC) has increased substantially. Currently, more advanced techniques including intensity-modulated radiotherapy, stereotactic ablative body radiotherapy, charged particle therapy, and image-guided radiotherapy are increasingly used for the treatment of HCCs. These techniques facilitate the delivery of higher dose radiotherapy for early to advanced HCCs, while minimizing radiation-induced toxicities. This review will cover the technical aspects of modern radiotherapy techniques along with their clinical applications.
INTRODUCTION
In the past, radiotherapy (RT) had a limited role in the treatment of hepatocellular carcinoma (HCC) due to the poor tolerance of the normal liver and the poor RT technique. As a result, the well-known Barcelona Clinic Liver Cancer guidelines for the treatment of HCC did not recommend RT as a treatment option for all stages of HCC[1]. This guideline recommends surgical treatments or local ablative therapies such as percutaneous ethanol injection or radiofrequency ablation (RFA) for the treatment of early small tumor(s) of stage 0 or A. Transarterial chemoembolization (TACE) is recommended for stage B large or multifocal HCCs and new agents like sorafenib are recommended for advanced stage C HCCs, which includes portal vein invasion or lymph node metastases. However, as many patients are not candidates for curative treatment or are not effectively treated with TACE or sorafenib, the use of other effective local modalities are warranted.
With the advancement of RT technologies, including 3-dimensional conformal radiotherapy (3D-CRT), intensity-modulated radiotherapy (IMRT), stereotactic ablative body radiotherapy (SABR), charged particle therapy, and image-guided radiotherapy (IGRT), delivering a higher radiation dose to the tumor in a safer way than before has become possible. To date, many institutions have reported good clinical outcomes for HCC patients receiving high dose radiation[2]. Moreover, increased understanding of the dose-response relationship and radiation-induced liver disease (RILD) facilitates the use of RT for patients with early to advanced HCCs[3-5]. In this topic highlight, we focused on the technical aspects of modern RT techniques for HCC along with their clinical applications.
3D-CRT
In contrast to the conventional 2D-RT technique, which usually uses opposing anterior and posterior radiation fields, 3D-CRT uses multiple coplanar or non-coplanar fields in order to reduce the high-dose exposure of normal tissues including the liver and bowels and to increase the tumor dose coverage (Figure 1). With the use of computed tomography (CT) images for RT planning and a computerized treatment planning system, the tumor and surrounding normal liver can be delineated accurately; the delivered dose and irradiated volume of the tumor and normal liver can be precisely evaluated. As a result, experience in the response of the tumor and normal liver to certain dose levels shapes the current decision making process for the RT regimen.
Figure 1.
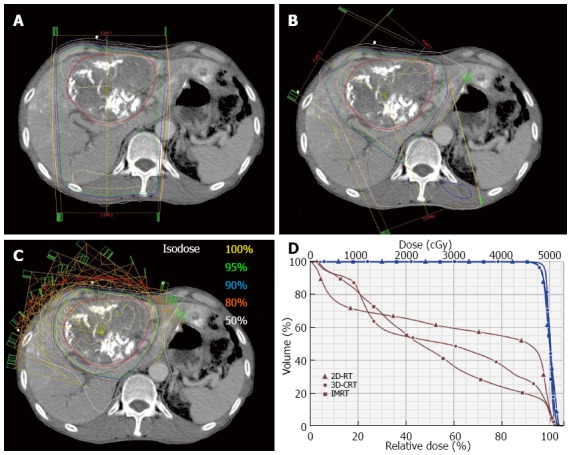
The radiotherapy plans of different radiotherapy techniques for hepatocellular carcinoma. A: 2-dimensional radiotherapy (2D-RT); B: 3-dimensional conformal radiotherapy (3D-CRT); C: Intensity-modulated radiotherapy (IMRT); D: dose volume histogram of the tumor (blue) and normal liver (brown). IMRT plan shows the best liver sparing while 2D-RT shows the worst liver sparing.
A high 92% response rate (80% complete response and 12% partial response) was achieved in a French phase 2 trial conducted in 27 patients having Child -Pugh class A or B liver function with a single tumor sized ≤ 5 cm or 2 tumors sized ≤ 3 cm after 66 Gy of 3D-CRT delivered in 33 fractions[6]. A Korean multicenter retrospective patterns of care study conducted in 398 HCC patients showed that a biologic effective dose of ≥ 53.1 Gy10 was associated with an improved 2-year overall survival[3]. Seong et al[7] treated 158 HCC patients with a dose of 25.2-60 Gy (1.8 Gy per fraction). In their study, the RT dose was identified by multivariate analysis as the only significant factor for survival. The median survival times in patients who received < 40 Gy, 40-50 Gy, and > 50 Gy were 6, 8, and 13 mo, respectively. Other studies also showed that a total RT dose of > 40-50 Gy achieved higher response or survival rates[8-10].
The Korean Practice Guidelines for the Management of Hepatocellular Carcinoma recommend RT for HCC patients as follows: (1) RT can be performed in HCC patients if liver functions indicate Child-Pugh class A or superb B and the irradiated total liver volume receiving ≥ 30 Gy is ≤ 60% (evidence level B1); (2) RT can be considered for HCC patients ineligible for surgical resection, liver transplantation, RFA, percutaneous ethanol injection, or TACE (C1); (3) RT can be considered for HCC patients who show incomplete response to TACE when the dose-volume criteria in Recommendation 1 are met (B2); (4) RT can be considered for HCC patients with portal vein invasion when the dose-volume criteria in Recommendation 1 are met (C1); and (5) RT is performed to alleviate symptoms caused by primary HCC or its metastases (B1)[11]. In the meta-analysis of 5 randomized and 12 non-randomized trials, TACE combined with RT achieved a better tumor response and survival than TACE alone[12]. Patients with portal vein thrombosis (PVT) responded to RT in about 45% of the cases[13,14].
However, the tolerance dose of the normal liver often limits the use of higher dose RT for HCC despite the availability of the modern 3D-CRT technique. Many factors including poor liver function with a Child-Pugh B or C score, prior TACE, PVT, and hepatitis B carrier status are known to be associated with a higher risk of RILD[15,16]. Nonetheless, these factors are unavoidable when RT is indicated. Radiation dose modification is recommended according to the liver function, the relative size of the tumor to the whole liver, and the normal liver dose[17,18]. Therefore, more advanced RT techniques are warranted to overcome these unavoidable obstacles and improve the clinical outcomes in terms of tumor control and normal tissue toxicity.
IMRT
IMRT is an advanced form of conformal RT that facilitates the delivery of a higher radiation dose compared to 3D-CRT. A computer-aided automated optimization process, known as inverse treatment planning, modulates the intensity of each beam to gain the desired target coverage while minimizing the dose to the normal organs (Figure 1). At present, various forms of IMRT, including volumetric-modulated arc therapy (VMAT) and helical tomotherapy (HT), are available. VMAT delivers intensity-modulated beams during gantry rotation, and HT delivers the radiation dose in slices with the help of a rotating gantry similar to a helical CT scanner. Furthermore, IMRT can deliver different doses to different targets at the same time, which is called simultaneous integrated boost-IMRT. Using this technique, a higher dose can be delivered to the gross tumor volume concurrently with a lower dose to areas of subclinical disease. Even though radiation oncologists have been reluctant in using IMRT for moving tumors due to the dosimetric and radiobiological uncertainties related to respiratory movement, recent experimental and clinical studies have rationalized its use for the treatment of HCC.
The distortion of calculated dose distribution of the IMRT plan on the static CT images is inevitable if the target moves during the IMRT beam delivery. The difference between the calculated and measured doses of a single IMRT field or a single fraction with doses of multiple IMRT fields was unacceptably high; however, repeated irradiation negated the effect of motion[19-21]. After the delivery of 30 fractions, the mean dose to a moving tumor differed slightly (< 2%-3%) from that of a static tumor[19]. Volumetric dose measurements by Duan et al[21] revealed that the 5-fraction isodose line for the moving phantom was fairly well matched with that of the stationary phantom, and the difference between the tumor control probabilities of the stationary and moving tumors for ≥ 2 fractions was small (< 2.3%). Kuo et al[20] reported that this difference was larger at higher amplitudes of tumor motion and higher dose rates of irradiation (500 MU/min vs 300 MU/min); however, it did not differ between the IMRT delivery modes (sliding window vs step-and-shoot).
The dosimetric advantages of IMRT over 3D-CRT and the importance of the IMRT techniques were previously reported. Early dosimetric studies comparing IMRT to 3D-CRT suggested that IMRT enabled dose escalation without the risk of increased liver toxicity and potentially reduced the normal tissue complication probability in HCC patients previously diagnosed with RILD after 3D-CRT[16,22]. Chen et al[23] compared the techniques of 3D-CRT, fixed-angle IMRT, and VMAT in small to large HCCs and suggested that VMAT might carry the lowest risk of RILD with the lowest V20 and V30 compared to 3D-CRT or IMRT for right lobe tumors. However, the results of comparisons between different RT techniques (3D-CRT vs fixed-angle IMRT vs VMAT vs HT) have been variable. Although some studies reported that the mean liver dose was higher for fixed-angle IMRT or VMAT plan compared to 3D-CRT[16,23], these results could be caused by suboptimal IMRT beam configuration or the routine application of constraints to IMRT planning as a planning study. HT has been reported to provide a better uniformity for the target coverage than fixed-angle IMRT; however, the low dose volume of the normal liver that is related to the risk of RILD was higher for HT compared to that of fixed-angle IMRT[24,25]. Park et al[26] reported that the dose-volumetric parameters of VMAT vs fixed-angle IMRT differed according to the target location within the liver; central tumors showed higher mean liver dose and lower liver volume receiving 30 Gy for VMAT than for IMRT; however, peripheral tumors showed no difference. When using fixed-angle coplanar IMRT, using fields entering the body near the tumor might be better at reducing the normal liver dose by decreasing the length of beam path through the normal liver compared to the equidistant beam array[27].
The clinical outcomes of IMRT have been reported recently. Yoon et al[28] reported that IMRT could deliver higher doses (median, 50 Gy in 20 fractions) and achieved higher 3-year overall survival and progression-free survival than 3D-CRT without the increased risk of RILD in stage III or IVA HCC patients. Hou et al[29] reported similar results in advanced HCC patients with portal vein and/or inferior vena cava tumor thrombi with IMRT of a median total dose of 60 Gy with a fraction size of 2.5-4.0 Gy. Several authors reported that delivering a high dose IMRT was feasible in patients with small to large HCCs without a high incidence of RILD. Wang et al[30] delivered 45, 60, or 66 Gy in 1.8 or 2.0 Gy per fraction depending on tumor stage, target location, and the sizes of small to large HCCs ineligible for surgery or ablative treatments. The mean normal liver dose was 19.4 ± 6.3 Gy, and nonconventional RILD was observed in 13% of patients. Kang et al[31] delivered a median dose of 50.4 Gy to advanced HCCs with an equivalent sphere size of 11.4 ± 2.6 cm. There was no grade ≥ 3 RILD in patients treated with IMRT without combination with TACE or intra-arterial chemotherapy. McIntosh et al[4] conducted an accelerated IMRT with concurrent capecitabine in 20 patients with unresectable HCC with a mean tumor size of 9 cm (range, 1.3-17.4 cm). The prescribed dose was 50 Gy in 20 fractions and there were no grade > 2 acute or late toxicities. Kim et al[32] reported that an accelerated RT with simultaneous integrated boost-IMRT was feasible and safe for patients with inoperable HCC. The tumor and the surrounding area with subclinical disease received 66 Gy and 55 Gy in 22 fractions, respectively. When the tumor was located within < 1 cm of the gastrointestinal structures, 55 Gy and 44 Gy in 22 fractions to the tumor and the surrounding area with subclinical disease, respectively, was delivered.
The results discussed thus far indicated that IMRT has the potential of dose escalation for HCC without an increased risk of RILD, which signals the potential for improved survival and quality of life in patients with HCC. However, because there is no standard technique for IMRT delivery and because the IMRT plan is not always better than the 3D-CRT plan, it is important to individualize the treatment plan for every patient.
SABR
SABR is generally defined as a treatment method for delivering a high dose of radiation to the target in a few fractions (typically 1-5 fractions) with a high degree of precision. SABR with a common linear accelerator usually utilizes multiple coplanar or noncoplanar static beams or multiple arc beams (Figure 2). The CyberKnife system (Accuray, Inc., Sunnyvale, CA, United States) and the VERO system (BrainlLab AG, Feldkirchen, Germany) are specialized machines for SABR, and HT is also used for SABR. To irradiate the tumor more accurately and to increase the sparing of the normal organs, SABR is performed in combination with at least one kind of IGRT technique integrated into the treatment machine; the different IGRT techniques are described in the subsequent section. During the last decade, the use of SABR for HCC has increased substantially and the practice guidelines from the National Cancer Center and Korean Liver Cancer Study Group recommend SABR as an alternative to the ablation/embolization techniques, or when these therapies have failed or are contraindicated[11,33].
Figure 2.
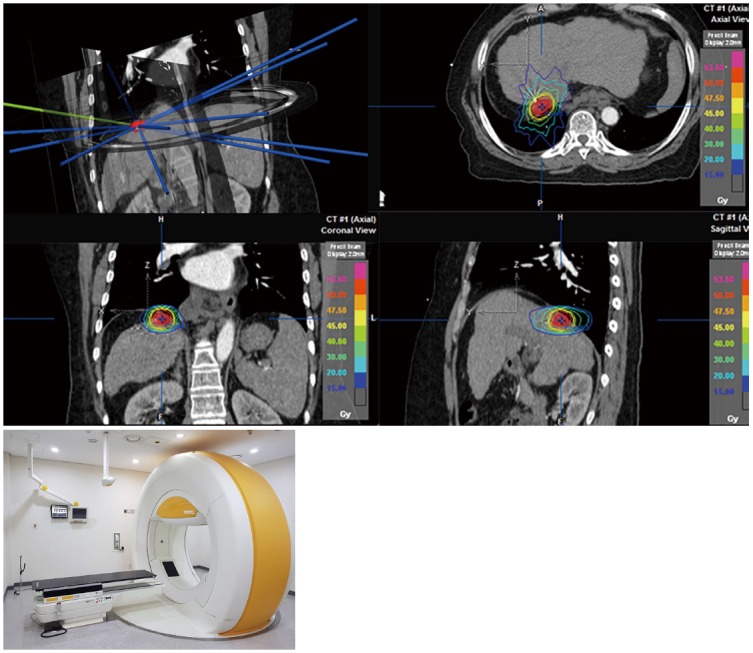
The stereotactic ablative body radiotherapy plan using the VERO system.
Since the reporting of the first clinical experience with SABR by Blomgren et al[34] in 1995, many prospective and retrospective studies have been conducted (Table 1). Generally, SABR was used for the treatment of a few, small HCCs (< 5-6 cm) in patients with Child-Pugh class A or B disease. Local control rates at 2-3 years were 84%-100%, excluding two studies in which a relatively low dose was used[35] or large tumors were treated[36]. However, the overall survival and the incidence of severe hepatic toxicities varied due to the heterogeneity of patient and tumor characteristics such as liver function, tumor location, and tumor size. The most recent study by Wahl et al[37] showed comparable results between SABR (249 tumors in 161 patients) and RFA (83 tumors in 63 patients) in 224 patients with inoperable, nonmetastatic HCC. The rates of freedom from local progression at 1 and 2 years were 83.6% and 80.2% for RFA vs 97.4% and 83.8% for SABR. Notably, increase in tumor size was a predictor of local progression in patients who underwent RFA, but not in patients who underwent SABR.
Table 1.
The summary of the trials conducted using stereotactic ablative body radiotherapy for hepatocellular carcinoma
| Study | Year | n | Dose/fraction (Gy/Fr) | Local control | Overall survival | Severe hepatic toxicity |
| Cárdenes et al[65] | 2010 | 17 | 36–48 Gy/3 or 5 Fr | 2-yr, 100% | 2-yr, 60% | 27% in CPC-B |
| Kwon et al[35] | 2010 | 42 | 30–39 Gy/3 Fr | 3-yr, 68% | 3-yr, 59% | Gr ≥ 3, 2% |
| Andolino et al[5] | 2011 | 60 | 40–48 Gy/3 or 5 Fr | 2-yr, 90% | 2-yr, 67% | Gr ≥ 3, 0% |
| Bujold et al[36] | 2013 | 102 | 24–54 Gy/6 Fr | 2-yr, 74% | 2-yr, 34% | Gr ≥ 3, 17% |
| Kang et al[66] | 2012 | 47 | 42–60 Gy/3 Fr | 2-yr, 95% | 2-yr, 69% | Gr ≥ 3, 19% |
| Yoon et al[67] | 2013 | 93 | 30–60 Gy/3-4 Fr | 3-yr, 92% | 3-yr, 54% | Gr ≥ 3, 7% |
| Sanuki et al[68] | 2014 | 185 | 35–40 Gy/5 Fr | 3-yr, 91% | 3-yr, 70% | Gr 5, 7% in CPC-B |
| Kimura et al[69] | 2015 | 65 | 48 Gy/4 Fr | 2-yr, 100% | 2-yr, 76% | Gr ≥ 3, 23% |
| Wahl et al[37] | 2016 | 63 | 27–60 Gy/3 or 5 Fr | 2-yr, 84% | 2-yr, 46% | Gr ≥ 3, 2% |
CPC-B: Child-Pugh class B; Gr: Grade.
Although there have been no prospective trials comparing SABR to other ablative modalities, recent data supports the use of SABR as an alternative ablative treatment option for the treatment of inoperable HCC. However, because SABR cannot be repeated unlike the other treatment modalities and because RILD occurs more frequently in patients with poor liver function, the decision on the best ablative modality should be made using a multidisciplinary approach.
CHARGED PARTICLE THERAPY
Charged particle therapy such as proton and carbon ion therapy offers distinct physical properties. The absorbed dose rapidly increases and suddenly rises to a peak before the proton is ultimately stopped, called the “Bragg peak effect”. This facilitates increased sparing of normal tissues surrounding the tumor compared to conventional photon beam therapy, and thus, dose escalation for HCC can be achieved.
Some retrospective[38-40] and prospective[41-43] studies have reported encouraging outcomes with proton or carbon beam therapy in patients with HCC (Table 2). Local control rates were 88%-98% at 2-5 years with a very low incidence of severe toxicity. Hata et al[39] reported that patients with Child-Pugh C cirrhosis also showed no therapy-related toxicity of grade ≥ 3. Bush et al[41] reported that 6 patients showed pathologic complete response and 7 patients showed microscopic residual disease in 18 patients who underwent liver transplantation after proton beam therapy. Recently, the interim analysis of a randomized trial comparing proton beam therapy to TACE for HCC was reported[43]. At the time of analysis, 36 patients in the TACE group and 33 patients in the proton group were available for analysis. Pathologic complete response was achieved in 10% of the 10 patients from the TACE group and 25% of the 12 patients from the proton group, who underwent liver transplantation after treatment. There was a trend toward improved 2-year local control (88% vs 45%, P = 0.06) and progression-free survival (48% vs 31%, P = 0.06) favoring proton beam therapy.
Table 2.
The summary of trials conducted using charged particle therapy for hepatocellular carcinoma
| Study | Year | Particle | n | Dose/fraction (Gy/fr) | Local control | Overall survival | Grade ≥ 3 Liver toxicity |
| Nakayama et al[38], retrospective | 2009 | Proton | 318 | 55–79.2 CGE/10–22 Fr | NA | 5-yr, 45% | None |
| Komatsu et al[40], retrospective | 2011 | Proton | 242 | 52.8–84 CGE/4–38 Fr | 5-yr, 90% | 5-yr, 38% | 1% |
| Carbon | 101 | 52.8–76 CGE/4–20 Fr | 5-yr, 93% | 5-yr, 36% | 3% | ||
| Hata et al[39], retrospective | 2006 | Proton | 19 | 50–84 Gy/10–24 Fr | 1 failure | 2-yr, 42% | None |
| Bush et al[70], phase 2 | 2011 | Proton | 76 | 63 Gy/15 Fr | NA | 3-yr, 60%1 | None |
| Hong et al[42], phase 2 | 2016 | Proton | 44 | 58.05–67.5 CGE/15 Fr | 2-yr, 95% | 2-yr, 63% | 2% |
| Bush et al[43], randomized | 2016 | Proton | 33 | 70.2 Gy/15 Fr | 2-yr, 88% | 2-yr, 48%1 | None |
Progression-free survival. CGE: Cobalt gray equivalent; NA: Not available.
Charged particle therapy generally showed better local control and survival rates than the photon-based RT series, although a direct comparison is impossible due to the differences in patient characteristics. Moreover, a recent interim analysis of a randomized trial comparing TACE and proton beam therapy favored the proton beam therapy. Although the facilities for charged particle therapy have been limited thus far, it is anticipated that the use of charged particle therapy will increase in the near future.
IGRT
IGRT is defined as RT that employs imaging to maximize accuracy and precision throughout the whole process, which includes target and normal tissue delineation, radiation delivery, and adaptation of therapy to anatomic and biological changes over time in individual patients[44]. Of these, accurate target delineation, target relocalization to allow proper patient repositioning, and respiratory motion management have been the most challenging in patients with HCC.
Target delineation
The initial step of IGRT is precise tumor delineation. The specific enhancement pattern of HCC (enhancement in arterial phase and washout in portal venous or late delayed phase) can help radiation oncologists delineate gross tumor volume. A radiologic-pathologic correlation study showed that microscopic invasion from HCC was observed up to 4 mm from the gross tumor, and the distance was correlated with the alpha-feto protein level, tumor size, PVT, and TNM stage[45]. This study suggested that a margin of < 5 mm from the gross tumor volume is required for the clinical target volume. The planning target volume (PTV) is defined as the volume that is used for the RT planning to ensure the tumor dose in the presence of breathing motion and set-up uncertainties. The PTV margin from the clinical target volume ranges 5-10 mm or more, depending on the methods of simulation and in-room IGRT.
For the tumor delineation of HCC, 4D-CT images, which are synchronized with the patient’s respiratory cycle, are usually acquired to capture the whole trajectory of the moving tumor. Brock[46] recommended the acquisition of contrast breath-hold CT scans followed by 4D-CT in the HCC patients to capture both the early enhancement and washout phases. However, 4D-CT cannot acquire the same quality achieved with the diagnostic scans[47] and have many artifacts preventing accurate tumor delineation[48]. Therefore, diagnostic CT or magnetic resonance imaging (MRI), which shows the extent of HCC better, should be used for tumor delineation. Rigid or deformable registration between the diagnostic and RT planning images can be used. Based on our experience of rigid registration, it is important to match the fiducial markers (e.g., lipiodol) or anatomical landmarks (e.g., liver contour and vessels) near the tumor, instead of the whole liver. Although a difference in target size by a few millimeters was observed after the deformable registration between MRI and CT images[49], deformable registration between diagnostic MRI and RT planning images could be helpful for target delineation; however, it is still at the investigational phase.
Every effort should be taken to delineate the target precisely using the currently available imaging modalities, and further research is required for the combination of these modalities in order to make the whole trajectory of the tumor more clearly visible on the RT planning system.
Target relocalization and tumor surrogates
Before the radiation beam is turned on, bony landmarks are usually used to position the tumor to its original location at the time of simulation. However, as HCC moves during the respiratory cycle and is often invisible on in-room images, surrogates for the tumor are required for the application of IGRT. High-density materials (e.g., inserted fiducial markers, packed lipiodol, surgical clips), the diaphragm, large vessels, and the entire liver can be used as surrogates. With the help of these surrogates, PTV margins can be reduced and normal tissue doses can be further spared.
Various techniques involving 2D or 3D volumetric image guidance are now available to verify and reposition the location of surrogates[46]. Kilovoltage (kV) or megavoltage (MV) radiography can help visualize the location of diaphragm or fiducial markers, which is subsequently compared to their location on the planning CT image at the specific phase of respiration (e.g., breath-hold or gated). The kV fluoroscopic imaging can show the tumor motion during respiration or breath-hold. Using volumetric imaging by a CT scanner in the treatment room, soft tissues, including the liver, adjacent structures, or fiducial markers, can be used for image guidance. Because the long acquisition time for CT images can lead to image blurring, breath-hold or respiration sorting techniques can be used as well. The specific technique for the target relocalization can be chosen according to the RT delivery technique (free-breathing, breath-hold, gated, or tracking). Recently, non-invasive MRI has been used for IGRT[50,51].
Gold fiducial markers are preferred over other surrogates because they provide better visibility on a standard MV imaging device as well as on kV X-ray images. They can be used for real-time tumor tracking (for gated or tracking treatment) as well as for confirming PTV margins in 2D or 3D images. Interestingly, Wahl et al[37] reported that the local failure rate was higher in SABR-treated patients without fiducial markers compared to those with fiducial markers (10% vs 0%, respectively, P = 0.15), which highlights the importance of using an accurate IGRT technique.
The management of respiratory motion
Another issue in RT for HCC is the control of the respiratory motion, because the liver moves in a significant range during respiration[52]. The ways to treat a moving tumor can be classified into motion-encompassing, forced shallow breathing with abdominal compression, respiratory gating, and real-time tumor tracking[53]. The motion-encompassing method refers to the covering of all possible positions of the moving tumor through the whole breathing cycle and subsequently a large volume of normal tissue may be irradiated. Although breath-hold and forced shallow breathing can reduce the respiratory motion for liver tumors, this might result in significant patient discomfort or inconvenience during treatment. Presently, respiratory gating and real-time tumor tracking are the most advanced techniques. Figure 3 shows the dose distribution for the three techniques of motion management.
Figure 3.
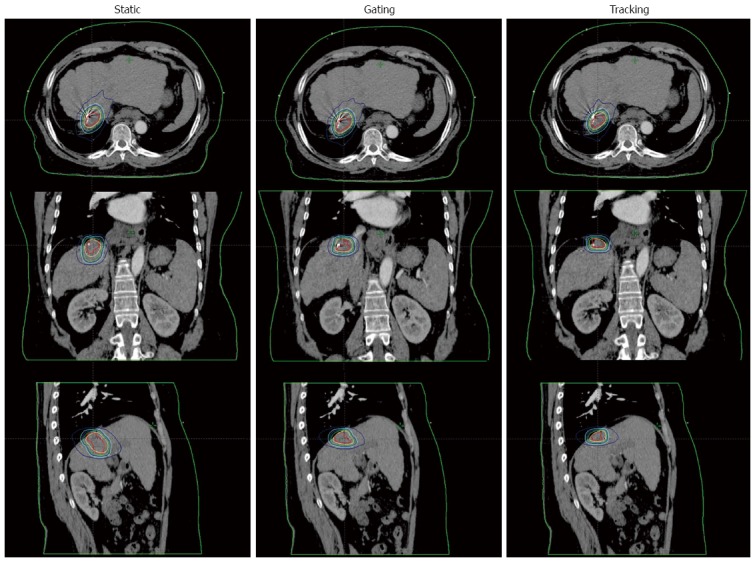
The comparison of dose distributions in 3 stereotactic ablative body radiotherapy plans for a representative case. The static plan using the motion encompassing technique is shown in the left column. The gating and tracking plans are shown in the middle and right column, respectively.
The respiratory gating method involves turning on the radiation beam when the tumor is at a given location, which leads to a smaller PTV volume. The current commercially available Real-time Position Management system (Varian Medical system, Palo Alto, CA) detects the respiratory signal via the movement of the surrogate on the abdominal surface, which can be correlated with the respiratory movement of the tumor inside the body. The position and width of the gate within a patient’s respiratory cycle are determined by monitoring the tumor’s respiratory motion that was captured on 4D-CT images. This gating method using an external breathing signal is easy, noninvasive, and radiation-free; however, a potential error might be that the signal does not accurately correlate with the internal target position[54-56]. For this reason, the Hokkaido group developed the real-time tumor tracking radiation therapy system that combines both the external breathing signals and the internal tumor motion signals via implanted fiducial markers[57]. Kubo et al[58] reported the feasibility of gated IMRT as well. A disadvantage of the gating techniques is the reduced efficiency of radiation delivery, resulting in a prolonged treatment time (or reduced duty cycle). For SABR, where a larger dose is delivered at each treatment, this prolonged treatment time could decrease the patient compliance.
An alternative strategy is to reposition the radiation beam while tracking the tumor’s changing position dynamically. Ideally, this method can eliminate the need to compensate for the movement of the tumor and achieve a 100% duty cycle for dose delivery. Iizuka et al[59] showed that the tracking technique could reduce the PTV volume by 35% in 11 liver cases, compared to the motion-encompassing method. Currently, there have been two treatment machines capable of tumor tracking: the CyberKnife system and the VERO system. The clinical feasibility of the CyberKnife system has been shown in several studies[60-63]. The CyberKnife system consists of a pair of fluoroscopes in the ceiling coupled to a small X-band linear accelerator mounted on a robotic arm, which can move according to the movement of the inserted fiducial markers. The VERO system uses a pair of fluoroscopes mounted in the machine to monitor the movement of inserted fiducial markers and allows the treatment head, gimbal, to pivot in two dimensions according to the movement of the fiducial markers[64].
CONCLUSION
Recent advances in the RT techniques facilitate dose escalation for small to large tumors with the hope of improved local tumor control without increasing normal tissue toxicity. However, local failure is still problematic, especially in advanced HCCs, and intrahepatic or distant metastases often develop, which could offset the impact of increased local control and render the given treatments meaningless. Unfortunately, reliable methods that can predict the tumor response to RT or recurrences within or outside the RT field have not been developed. Therefore, future research should focus on the prediction of the outcomes after treatment to determine the patients who will benefit from RT as well as the novel biologic agents that can prevent recurrences outside the RT field.
ACKNOWLEDGMENTS
We thank Jonathan Lawler for the English editing and audio recording.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 2, 2016
First decision: May 30, 2016
Article in press: July 21, 2016
P- Reviewer: Lau WY, Wang YG S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 2.Feng M, Ben-Josef E. Radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2011;21:271–277. doi: 10.1016/j.semradonc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Seong J, Lee IJ, Shim SJ, Lim DH, Kim TH, Kim JH, Jang HS, Kim MS, Chie EK, Kim JH, et al. A multicenter retrospective cohort study of practice patterns and clinical outcome on radiotherapy for hepatocellular carcinoma in Korea. Liver Int. 2009;29:147–152. doi: 10.1111/j.1478-3231.2008.01873.x. [DOI] [PubMed] [Google Scholar]
- 4.McIntosh A, Hagspiel KD, Al-Osaimi AM, Northup P, Caldwell S, Berg C, Angle JF, Argo C, Weiss G, Rich TA. Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer. 2009;115:5117–5125. doi: 10.1002/cncr.24552. [DOI] [PubMed] [Google Scholar]
- 5.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Mornex F, Girard N, Beziat C, Kubas A, Khodri M, Trepo C, Merle P. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies--mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152–1158. doi: 10.1016/j.ijrobp.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55:329–336. doi: 10.1016/s0360-3016(02)03929-9. [DOI] [PubMed] [Google Scholar]
- 8.Liu MT, Li SH, Chu TC, Hsieh CY, Wang AY, Chang TH, Pi CP, Huang CC, Lin JP. Three-dimensional conformal radiation therapy for unresectable hepatocellular carcinoma patients who had failed with or were unsuited for transcatheter arterial chemoembolization. Jpn J Clin Oncol. 2004;34:532–539. doi: 10.1093/jjco/hyh089. [DOI] [PubMed] [Google Scholar]
- 9.Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150–155. doi: 10.1016/s0360-3016(02)02864-x. [DOI] [PubMed] [Google Scholar]
- 10.Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, Yoo BC, Lee JE, Kang MK, Park YJ, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Korean Liver Cancer Study Group (KLCSG); National Cancer Center, Korea (NCC) 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015;9:267–317. doi: 10.5009/gnl14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng MB, Cui YL, Lu Y, She B, Chen Y, Guan YS, Zhang RM. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2009;92:184–194. doi: 10.1016/j.radonc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Zeng ZC, Fan J, Tang ZY, Zhou J, Qin LX, Wang JH, Sun HC, Wang BL, Zhang JY, Jiang GL, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–443. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 15.Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, Ten Haken RK. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94–100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng JC, Wu JK, Huang CM, Liu HS, Huang DY, Tsai SY, Cheng SH, Jian JJ, Huang AT. Dosimetric analysis and comparison of three-dimensional conformal radiotherapy and intensity-modulated radiation therapy for patients with hepatocellular carcinoma and radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2003;56:229–234. doi: 10.1016/s0360-3016(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 17.Seong J. Challenge and hope in radiotherapy of hepatocellular carcinoma. Yonsei Med J. 2009;50:601–612. doi: 10.3349/ymj.2009.50.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng JC, Chuang VP, Cheng SH, Huang AT, Lin YM, Cheng TI, Yang PS, You DL, Jian JJ, Tsai SY, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000;47:435–442. doi: 10.1016/s0360-3016(00)00462-4. [DOI] [PubMed] [Google Scholar]
- 19.Jiang SB, Pope C, Al Jarrah KM, Kung JH, Bortfeld T, Chen GT. An experimental investigation on intra-fractional organ motion effects in lung IMRT treatments. Phys Med Biol. 2003;48:1773–1784. doi: 10.1088/0031-9155/48/12/307. [DOI] [PubMed] [Google Scholar]
- 20.Kuo HC, Chuang KS, Liu WS, Wu A, Lalonde R. Analysis of organ motion effects on the effective fluences for liver IMRT. Phys Med Biol. 2007;52:4227–4244. doi: 10.1088/0031-9155/52/14/014. [DOI] [PubMed] [Google Scholar]
- 21.Duan J, Shen S, Fiveash JB, Popple RA, Brezovich IA. Dosimetric and radiobiological impact of dose fractionation on respiratory motion induced IMRT delivery errors: a volumetric dose measurement study. Med Phys. 2006;33:1380–1387. doi: 10.1118/1.2192908. [DOI] [PubMed] [Google Scholar]
- 22.Eccles CL, Bissonnette JP, Craig T, Taremi M, Wu X, Dawson LA. Treatment planning study to determine potential benefit of intensity-modulated radiotherapy versus conformal radiotherapy for unresectable hepatic malignancies. Int J Radiat Oncol Biol Phys. 2008;72:582–588. doi: 10.1016/j.ijrobp.2008.06.1496. [DOI] [PubMed] [Google Scholar]
- 23.Chen D, Wang R, Meng X, Liu T, Yan H, Feng R, Liu S, Jiang S, Xu X, Zhu K, et al. A comparison of liver protection among 3-D conformal radiotherapy, intensity-modulated radiotherapy and RapidArc for hepatocellular carcinoma. Radiat Oncol. 2014;9:48. doi: 10.1186/1748-717X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh CH, Liu CY, Shueng PW, Chong NS, Chen CJ, Chen MJ, Lin CC, Wang TE, Lin SC, Tai HC, et al. Comparison of coplanar and noncoplanar intensity-modulated radiation therapy and helical tomotherapy for hepatocellular carcinoma. Radiat Oncol. 2010;5:40. doi: 10.1186/1748-717X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song JH, Son SH, Kay CS, Jang HS. Identification of Biologically Effective Dose-Volumetric Parameters That Predict Radiation-Induced Hepatic Toxicity in Patients Treated With Helical Tomotherapy for Unresectable Locally Advanced Hepatocellular Carcinoma. Medicine (Baltimore) 2015;94:e1904. doi: 10.1097/MD.0000000000001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JM, Kim K, Chie EK, Choi CH, Ye SJ, Ha SW. RapidArc vs intensity-modulated radiation therapy for hepatocellular carcinoma: a comparative planning study. Br J Radiol. 2012;85:e323–e329. doi: 10.1259/bjr/19088580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SH, Kang MK, Yea JW, Kim SK, Choi JH, Oh SA. The impact of beam angle configuration of intensity-modulated radiotherapy in the hepatocellular carcinoma. Radiat Oncol J. 2012;30:146–151. doi: 10.3857/roj.2012.30.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon HI, Lee IJ, Han KH, Seong J. Improved oncologic outcomes with image-guided intensity-modulated radiation therapy using helical tomotherapy in locally advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014;140:1595–1605. doi: 10.1007/s00432-014-1697-0. [DOI] [PubMed] [Google Scholar]
- 29.Hou JZ, Zeng ZC, Wang BL, Yang P, Zhang JY, Mo HF. High dose radiotherapy with image-guided hypo-IMRT for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombi is more feasible and efficacious than conventional 3D-CRT. Jpn J Clin Oncol. 2016;46:357–362. doi: 10.1093/jjco/hyv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang PM, Hsu WC, Chung NN, Chang FL, Fogliata A, Cozzi L. Radiotherapy with volumetric modulated arc therapy for hepatocellular carcinoma patients ineligible for surgery or ablative treatments. Strahlenther Onkol. 2013;189:301–307. doi: 10.1007/s00066-012-0298-6. [DOI] [PubMed] [Google Scholar]
- 31.Kang MK, Kim MS, Kim SK, Ye GW, Lee HJ, Kim TN, Eun JR. High-dose radiotherapy with intensity-modulated radiation therapy for advanced hepatocellular carcinoma. Tumori. 2011;97:724–731. doi: 10.1177/030089161109700608. [DOI] [PubMed] [Google Scholar]
- 32.Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, Kim SS, Lee WJ, Kim DY, Kim CM. Simultaneous integrated boost-intensity modulated radiation therapy for inoperable hepatocellular carcinoma. Strahlenther Onkol. 2014;190:882–890. doi: 10.1007/s00066-014-0643-z. [DOI] [PubMed] [Google Scholar]
- 33.NCCN Clinical Practice Guidelines in Oncology, Hepatobiliary Cancers. 2016, version 1. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. [DOI] [PMC free article] [PubMed]
- 34.Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 35.Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 37.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakayama H, Sugahara S, Tokita M, Fukuda K, Mizumoto M, Abei M, Shoda J, Sakurai H, Tsuboi K, Tokuuye K. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115:5499–5506. doi: 10.1002/cncr.24619. [DOI] [PubMed] [Google Scholar]
- 39.Hata M, Tokuuye K, Sugahara S, Fukumitsu N, Hashimoto T, Ohnishi K, Nemoto K, Ohara K, Matsuzaki Y, Akine Y. Proton beam therapy for hepatocellular carcinoma patients with severe cirrhosis. Strahlenther Onkol. 2006;182:713–720. doi: 10.1007/s00066-006-1564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, Hori Y, Hishikawa Y, Ku Y, Murakami M. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011;117:4890–4904. doi: 10.1002/cncr.26134. [DOI] [PubMed] [Google Scholar]
- 41.Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127:S189–S193. doi: 10.1053/j.gastro.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 42.Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Goyal L, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol. 2016;34:460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bush DA, Smith JC, Slater JD, Volk ML, Reeves ME, Cheng J, Grove R, de Vera ME. Randomized Clinical Trial Comparing Proton Beam Radiation Therapy with Transarterial Chemoembolization for Hepatocellular Carcinoma: Results of an Interim Analysis. Int J Radiat Oncol Biol Phys. 2016;95:477–482. doi: 10.1016/j.ijrobp.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 44.American College of Radiology. ACR-ASTRO Practice Parameter for Image-Guided Radiation Therapy (IGRT) 2014. Available from: http://www.acr.org/~/media/69e10ccca4784452b9ffa174529d0843.pdf. [DOI] [PubMed]
- 45.Wang MH, Ji Y, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, Bi AH, Tan YS. Impact factors for microinvasion in patients with hepatocellular carcinoma: possible application to the definition of clinical tumor volume. Int J Radiat Oncol Biol Phys. 2010;76:467–476. doi: 10.1016/j.ijrobp.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 46.Brock KK. Imaging and image-guided radiation therapy in liver cancer. Semin Radiat Oncol. 2011;21:247–255. doi: 10.1016/j.semradonc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beddar AS, Briere TM, Balter P, Pan T, Tolani N, Ng C, Szklaruk J, Krishnan S. 4D-CT imaging with synchronized intravenous contrast injection to improve delineation of liver tumors for treatment planning. Radiother Oncol. 2008;87:445–448. doi: 10.1016/j.radonc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto T, Langner U, Loo BW, Shen J, Keall PJ. Retrospective analysis of artifacts in four-dimensional CT images of 50 abdominal and thoracic radiotherapy patients. Int J Radiat Oncol Biol Phys. 2008;72:1250–1258. doi: 10.1016/j.ijrobp.2008.06.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voroney JP, Brock KK, Eccles C, Haider M, Dawson LA. Prospective comparison of computed tomography and magnetic resonance imaging for liver cancer delineation using deformable image registration. Int J Radiat Oncol Biol Phys. 2006;66:780–791. doi: 10.1016/j.ijrobp.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 50.Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Fallone BG. The rotating biplanar linac-magnetic resonance imaging system. Semin Radiat Oncol. 2014;24:200–202. doi: 10.1016/j.semradonc.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Nishioka T, Nishioka S, Kawahara M, Tanaka S, Shirato H, Nishi K, Hiromura T. Synchronous monitoring of external/internal respiratory motion: validity of respiration-gated radiotherapy for liver tumors. Jpn J Radiol. 2009;27:285–289. doi: 10.1007/s11604-009-0332-5. [DOI] [PubMed] [Google Scholar]
- 53.Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, Kapatoes JM, Low DA, Murphy MJ, Murray BR, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33:3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 54.Berbeco RI, Nishioka S, Shirato H, Chen GT, Jiang SB. Residual motion of lung tumours in gated radiotherapy with external respiratory surrogates. Phys Med Biol. 2005;50:3655–3667. doi: 10.1088/0031-9155/50/16/001. [DOI] [PubMed] [Google Scholar]
- 55.Seppenwoolde Y, Berbeco RI, Nishioka S, Shirato H, Heijmen B. Accuracy of tumor motion compensation algorithm from a robotic respiratory tracking system: a simulation study. Med Phys. 2007;34:2774–2784. doi: 10.1118/1.2739811. [DOI] [PubMed] [Google Scholar]
- 56.Wu H, Zhao Q, Berbeco RI, Nishioka S, Shirato H, Jiang SB. Gating based on internal/external signals with dynamic correlation updates. Phys Med Biol. 2008;53:7137–7150. doi: 10.1088/0031-9155/53/24/009. [DOI] [PubMed] [Google Scholar]
- 57.Shirato H, Shimizu S, Kitamura K, Nishioka T, Kagei K, Hashimoto S, Aoyama H, Kunieda T, Shinohara N, Dosaka-Akita H, et al. Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor. Int J Radiat Oncol Biol Phys. 2000;48:435–442. doi: 10.1016/s0360-3016(00)00625-8. [DOI] [PubMed] [Google Scholar]
- 58.Kubo HD, Wang L. Compatibility of Varian 2100C gated operations with enhanced dynamic wedge and IMRT dose delivery. Med Phys. 2000;27:1732–1738. doi: 10.1118/1.1287110. [DOI] [PubMed] [Google Scholar]
- 59.Iizuka Y, Matsuo Y, Ishihara Y, Akimoto M, Tanabe H, Takayama K, Ueki N, Yokota K, Mizowaki T, Kokubo M, et al. Dynamic tumor-tracking radiotherapy with real-time monitoring for liver tumors using a gimbal mounted linac. Radiother Oncol. 2015;117:496–500. doi: 10.1016/j.radonc.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 60.van der Voort van Zyp NC, Prévost JB, Hoogeman MS, Praag J, van der Holt B, Levendag PC, van Klaveren RJ, Pattynama P, Nuyttens JJ. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol. 2009;91:296–300. doi: 10.1016/j.radonc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Brown WT, Wu X, Fayad F, Fowler JF, García S, Monterroso MI, de la Zerda A, Schwade JG. Application of robotic stereotactic radiotherapy to peripheral stage I non-small cell lung cancer with curative intent. Clin Oncol (R Coll Radiol) 2009;21:623–631. doi: 10.1016/j.clon.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Collins BT, Erickson K, Reichner CA, Collins SP, Gagnon GJ, Dieterich S, McRae DA, Zhang Y, Yousefi S, Levy E, et al. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol. 2007;2:39. doi: 10.1186/1748-717X-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nuyttens JJ, Prévost JB, Praag J, Hoogeman M, Van Klaveren RJ, Levendag PC, Pattynama PM. Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: Marker placement and early results. Acta Oncol. 2006;45:961–965. doi: 10.1080/02841860600902205. [DOI] [PubMed] [Google Scholar]
- 64.Depuydt T, Poels K, Verellen D, Engels B, Collen C, Haverbeke C, Gevaert T, Buls N, Van Gompel G, Reynders T, et al. Initial assessment of tumor tracking with a gimbaled linac system in clinical circumstances: a patient simulation study. Radiother Oncol. 2013;106:236–240. doi: 10.1016/j.radonc.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 65.Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 66.Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung DH, Kim KB, Lee DH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 67.Yoon SM, Lim YS, Park MJ, Kim SY, Cho B, Shim JH, Kim KM, Lee HC, Chung YH, Lee YS, et al. Stereotactic body radiation therapy as an alternative treatment for small hepatocellular carcinoma. PLoS One. 2013;8:e79854. doi: 10.1371/journal.pone.0079854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanuki N, Takeda A, Oku Y, Mizuno T, Aoki Y, Eriguchi T, Iwabuchi S, Kunieda E. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53:399–404. doi: 10.3109/0284186X.2013.820342. [DOI] [PubMed] [Google Scholar]
- 69.Kimura T, Aikata H, Takahashi S, Takahashi I, Nishibuchi I, Doi Y, Kenjo M, Murakami Y, Honda Y, Kakizawa H, et al. Stereotactic body radiotherapy for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Hepatol Res. 2015;45:378–386. doi: 10.1111/hepr.12359. [DOI] [PubMed] [Google Scholar]
- 70.Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053–3059. doi: 10.1002/cncr.25809. [DOI] [PubMed] [Google Scholar]


