Abstract
Colorectal cancer (CRC) development represents a multistep process starting with specific mutations that affect proto-oncogenes and tumour suppressor genes. These mutations confer a selective growth advantage to colonic epithelial cells that form first dysplastic crypts, and then malignant tumours and metastases. All these steps are accompanied by deep mechanical changes at the cellular and the tissue level. A growing consensus is emerging that such modifications are not merely a by-product of the malignant progression, but they could play a relevant role in the cancer onset and accelerate its progression. In this review, we focus on recent studies investigating the role of the biomechanical signals in the initiation and the development of CRC. We show that mechanical cues might contribute to early phases of the tumour initiation by controlling the Wnt pathway, one of most important regulators of cell proliferation in various systems. We highlight how physical stimuli may be involved in the differentiation of non-invasive cells into metastatic variants and how metastatic cells modify their mechanical properties, both stiffness and adhesion, to survive the mechanical stress associated with intravasation, circulation and extravasation. A deep comprehension of these mechanical modifications may help scientist to define novel molecular targets for the cure of CRC.
Keywords: Colorectal cancer, Biomechanics, Pressure, Mechanical signalling, Atomic force microscopy, Wnt
Core tip: Physical forces, either within tissues or externally applied, affect all tissues of the body. Cell mechanotransduction converts such forces into cellular responses that affect gene expression, protein synthesis, proliferation and morphogenesis. Here, we focused on recent studies covering the impact of physical stimuli such as compression, shear stress, adhesion and stiffness, in the development of colorectal cancer. We highlight that such stimuli play a major role in the tumor progression, affecting the Wnt pathway, being involved in the differentiation of non-invasive cells into metastatic variants and helping metastatic cells to survive the mechanical stress associated with intravasation, circulation and extravasation.
INTRODUCTION
Colorectal cancer (CRC) is the 3th most commonly diagnosed malignancy and the 4th cause of cancer death in the world, with approximately 1.4 million new cases and almost 700000 deaths in 2012. Its burden is expected to increase by 60% by 2030[1].
CRC development is a multistep process that results from genetic alterations that underlie the transformation of normal cells into malignant cells, conferring them growth advantages such as anomalous multiplication, self-sufficiency with respect to growth signals, insensitivity to growth-inhibitor signals and evasion of apoptosis[2].
The earliest mutations that occur in CRC are usually in components of the Wnt pathway that regulates colon cell homeostasis, being involved in the control of cell proliferation, differentiation and adhesion (Figure 1). A recently published genetic study performed on 224 colorectal tumours indeed confirmed that in 94% of cases a mutation in one or more members of the Wnt signalling pathway is detected[3]. Subsequent mutations occur at the level of the RAS-MAPK, P13K, TGF-β, p53 and DNA mismatch-repairs pathways[4].
Figure 1.
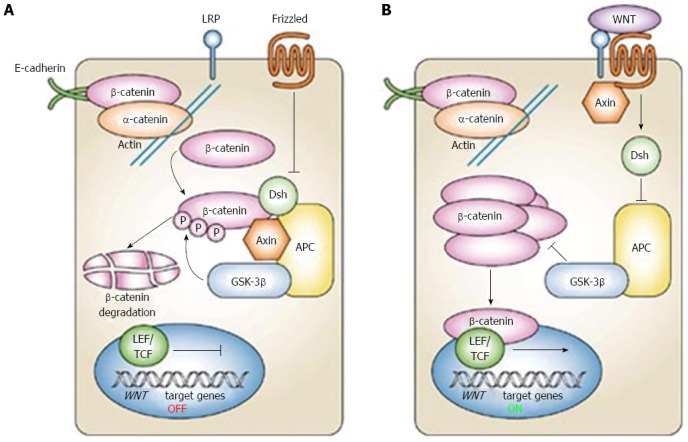
Canonical Wnt signaling pathway (reproduced with permission from[95]). the WNT pathway consist of two states, one referred to as “off” state where the small lipid modified Wnt protein does not bind to frizzled receptors (A), the other refereed as to “on state” when Wnt binds to frizzled (B). In the off state a large destruction complex is formed by the APC, Axin and GSk3β proteins. This complex binds to free β-catenin, phosphorylates it, thus triggering its degradation and preventing it from entering the nucleus. In the on-state dishevelled is activated, inhibits the formation of the destruction complex and leads to an abundance of cytoplasmic β-catenin, some of which enters the nucleus, binds with TCF, leading to cell proliferation.
These genetic mutations are accompanied by changes in the behaviour of cells which result in deep structural and biomechanical alterations that may occur at the tissue level, such as crypt buckling[5-19] or to more subtle modifications occurring at the cellular level[5,6,20-27] and in the extracellular matrix (ECM)[2,28]. Such modifications are not only a mere consequence of genetic alterations. In fact, there is a growing consensus that an evolving balance between mechanical and genetic cues exists and plays a key role in the genesis and the development of malignancies[2,29-41]. Indeed while the malignant potential is mainly dictated by the intrinsic genetic state of the cells, the tumour phenotype is regulated by a complex interplay between the biomechanical and biochemical properties of the cellular constituents and the ECM, which synergistically alters cellular behaviour stimulating migration, invasion, proliferation and survival[42].
During colorectal cancer development, cells within tissues are exposed to a highly heterogeneous and continuously evolving mechanical landscape. To provide a more in-depth understanding of this complex mechanical behaviour, a large number of studies have focused on isolated cell lines cultured in well-defined in vitro systems where each biomechanical cue, such as compression[6,20,21,24,43,44], ECM stiffness[24,25,45-48], flow conditions could be precisely controlled[26,27,49-51]. These in vitro studies opened the way to more advanced in vivo studies showing how biomechanical cues contribute to the malignant behaviour of colon epithelium by activating detrimental biochemical and genetic signalling pathways[5,42].
In this review, we focus on the most recent studies investigating the role of the biomechanical signals in the development of colorectal cancer. A particular attention is paid to highlight how the modifications of the tumour microenvironment and the extracellular matrix actively contribute to this process. A deep comprehension of the mechanism by which the mechanical cues modulate the onset and the development of the pathology may help to define novel molecular targets for the cure of colorectal cancer.
MECHANICAL SIGNALS CONTRIBUTE TO SHAPE HEALTHY COLON CRYPTS THROUGH A STRESS-RELAXATION MECHANISM
The epithelial layer of the human colon consists of a single sheet of columnar epithelial cells, which are arranged into finger-like invaginations in the underlying connective tissue of the lamina propria forming crypts, the basic functional unit of the intestine[52]. Three different types of cells are found in the epithelium, the goblet cells (secreting mucin into the crypt and intestinal lumen), the enterocytes and the neuroendocrine cells. The base of the crypts contains stem cells, which proliferate continuously producing transit cells, which divided several times before differentiating into the different type of cells that constitute the epithelium[53,54].
Crypt development occurs approximately seven days after birth in mice; before to this, the intestinal wall is smooth[53]. However, the mechanism through which these structures are formed is still not fully understood. It has been hypothesized that crypt growth could be regarded as a stress-relaxation phenomenon. Similarly to what happens with solid inorganic materials, where a tensile layer is coupled with a compressive one[55,56], the epithelial layer coating the intestinal wall might induce compressive residual stress in a tissue that can in turn be relaxed via a buckling instability, which can triggers the formation of crypts[18,57].
The above-described phenomenon has been investigated by using continuous mechanics. Edwards and Chapman[18] modelled a cross-section of an unfolded (smooth) colorectal crypt as a beam connected to the underlying tissue by a series of viscoelastic springs. This model was able to predict that an increase in the cellular proliferation rate can initiate buckling.
A similar method was used by Nelson et al[58] that modelled the unfolded crypt as a bilayer in which a growing cell layer adheres to a thin compressible elastic beam. Authors confirmed that the buckling instability could be induced as a consequence of the stress relaxation driven by the epithelial cells proliferation. Moreover, it was pointed out that non-uniformities in cell growth and variations in cell-substrate adhesion are predicted to have minimal effect on the shape of resulting buckled states. Interestingly the authors provided also an experimental verification of their theoretical model, by culturing a monolayer of epithelial cells on a flexible PDMS-based surface and showing by optical microscopy that cell growth could cause out-of-plane substrate deflection. These results provide another piece of understanding on how mechanical signals has a key role, both, in physiological and pathological processes.
For the sake of completeness, we deem appropriate to mention other mathematical models, such as cell-based methods or lattice-based models[13-17], that characterize the position and behaviour of individual cells within the crypt, lattice-free models[7-12], that allow for a more realistic approach considering interaction between adjacent cells, and kinetic continuum models that take into account stem cells proliferation[19]. These models are deeply described in the comprehensive review from van Leeuwen et al[59].
MECHANICAL CUES COULD HAVE A ROLE IN THE ONSET OF COLORECTAL CANCER THROUGH THE CONTROL OF THE WNT SIGNALING PATHWAY
An altered tissue mechanics is one of the key hallmark of cancer. A large body of evidence is emerging that a modified mechanical landscape might be not merely a by-product of the malignant progression, but it could contribute to cancer onset and/or accelerate its progression[29-41].
This is particularly interesting for colon cancer, because gastrointestinal (GI) tract is naturally submitted to significant endogenous mechanical stress as a consequence of intestinal transit[60]. The high-amplitude propagating contractions that periodically move luminal contents from the ascending colon toward the sigmoid, for instance, generate luminal pressures in excess of 80 mmHg (approximately 10 kPa). In pathological conditions, the increase of cell mass due to the deregulated cell proliferation, apoptosis resistance and neoangiogenesis, exerts a considerable stress on adjacent healthy tissues. Moreover, cancer cells of the primary neoplasm are embedded in the tumour “reactive stroma” that is associated with an increased number of fibroblasts, enhanced capillary density and anomalous ECM-molecules deposition, rich in collagen-I and fibrin[61]. This “reactive stroma”, together with the uncontrolled cells proliferation, modifies tissue topography, density and stiffness, exerting a mechanical stress of a few kPa on the tumour itself and the adjacent normal tissues[5]. High abdominal pressure are also common during insufflations for laparoscopy and after surgery, as a result of tissue edemas, whereas pressure during surgical manipulations can be as high as 1500 mmHg or more[62].
Many experimental findings suggested that repetitively applied physical forces, such as those related to GI transit, or constantly applied forces might contribute to initiate intracellular signals capable of altering intestinal epithelial proliferation[5,6,27,43,44,60,63-65]. Some of these studies are summarized in Table 1.
Table 1.
List of experimental studies investigating the effect of external pressure on colon cancer cells proliferation
| Ref. | Applied load | Pressure loading system and experimental conditions | Results |
| Hirokawa et al[44], 1997 | 40-120 mmHg (5-16 kPa) | The pressure-loading apparatus consists of a flask of which cap was pierced and connected to a tubing by which compressed He gas was introduced to raise internal pressure. | Pressurization from 40 to 120 mmHg for 48 h significantly increased cell (IEC18) number with peak proliferation at 80 mmHg. Pressure-induced DNA synthesis was further enhanced by the addition of interleukin-2, suggesting the regulation of intestinal epithelial growth by pressure could be dependent on cytokines. |
| Hirokawa et al[43], 2001 | Applied pressure for 48 h induced proliferation of IEC18 cell, with a significantly peak at 80 mmHg. The pattern of F-actin distribution was not significantly altered. The pressure-induced increase in phosphorylation of Elk-1 fusion protein corresponding to the activation of MAPK. | ||
| Basson et al[63], 2000 | 15 mmHg (2 kPa) | Cell plates was positioned in an airtight acrylic box, in which pressurized gas was introduced by a tubing to increase pressure. | Increasing ambient pressure stimulated the adhesion of human Caco-2, SW1116, SW620, and HT-29 cells to Matrigel, type I collagen, laminin, and fibronectin. |
| Whitehead et al[6], 2008 | 0.8 kPa | A controlled mechanical strain was applied on short segments of colon explants obtained from normal and APC1638N/+ mice. Tissues were placed into a mechanical deformation box and compressed in the z-direction of approximately half of their relaxed thickness for 20 min. | APC1638N/+ mice showed the expression of the two oncogenes Myc and Twist1, not observed in wild-type colon explants. Myc and Twist1 activation was found to be correlated with an increased presence of nuclear β-catenin . Almost no nuclear β-catenin was detected in the wild-type colon epithelium. |
| The mechanical stimulation of APC1638N/+ tissue leads to the phosphorylation of β-catenin at tyrosine 654, the site of interaction with E-cadherin, affecting cell adhesions properties. | |||
| Fernández-Sánchez et al[5], 2015 | 1.2 kPa | A controlled pressure was applied in vivo in APC1638N/+ and control mice by subcutaneously inserting a magnet close to the mouse colon. The magnet generates a magnetic force on ultra-magnetic liposomes, stabilized in the mesenchymal cells of the connective tissue surrounding colonic crypts. | The magnetically induced load led to a rapid Ret activation and the phosphorylation of β-catenin on Tyr654, impairing its interaction with E-cadherin. |
| β-catenin nuclear translocation was observed after 15 days with a consequent increased expression of β-catenin-target genes at 1 month, together with crypt enlargement accompanying the formation of early tumorous aberrant crypt foci. | |||
| Such malignant behavior was induced in, both, APC1638N/+ and control mice, irrespective of the presence of prior genetic abnormalities. | |||
| Avvisato et al[27], 2007 | 1.5 kPa | Cells were plated on 38 mm × 76 mm slides and subjected to a laminar shear stress in a rectangular flow channel for 12 h. | β-catenin signalling of SW480 cells decreased to 22% of control values. The β-catenin signalling were measured for 0-24 h during shear stress exposure, it decreased significantly following 12 h of flow, reaching a minimum after 24 h. |
Hirokawa et al[43,44] investigated the effect of intraluminal pressure on cultured intestinal epithelial cells (IEC18 cell line). Pressure was applied to cells by helium gas in a culture flask, up to reach a load of 80 mmHg (approximately 10 kPa). Authors showed that such an external pressure induces cell proliferation, probably via the activation of Myc expression, a β-catenin related oncogene[43,44]. Similarly, a pressure of 15 mmHg applied to colon 26 cells implanted in rat model increases liver metastasis suggesting that even a low pressure increase might influence malignant cell proliferation[66].
Other than an altered cellular proliferation, extracellular pressure can influence cancer growth by promoting cell adhesion[60,63-65]. In this regard, Basson and co-workers showed that the exposure of non-adherent primary human colon cancer and SW620 cells to 15 mmHg of extracellular pressure increases cell adhesion via src-mediated or cytoskeleton-mediated FAK activation. Both mechanisms promote FAK association with integrin, altering its binding affinity and facilitating colon cancer cell adhesion[64,65].
As stated above, loss of APC function triggers the chain of molecular and histological changes leading to colorectal tumours. In this context, Whitehead et al[6] applied a controlled mechanical strain on short segments of colon explants from normal and APC deficient mice (APC1638N/+). Differently from humans, where GI tumours are found primarily in colon, mice develop cancer predominantly in the small intestine. Therefore APC1638N/+ mice colon tissues are both, morphologically normal and APC deficient, thus providing an ideal model system to study the earliest event in colorectal tumorigenesis[6]. Both control and APC deficient tissues were placed into a mechanical deformation box and compressed in the z-direction of approximately half of their relaxed thickness for 20 min with an applied load of approximately 800 Pa. Compressed tissues showed elongated crypt openings hinting at some shape changes at the cellular level. Such modifications were accompanied by the expression of the two oncogenes Myc and Twist1 in APC deficient colon tissue explants, but not in wild-type colon explants. Authors showed that Myc and Twist1 activation is strongly dependent on the presence of nuclear β-catenin, in agreement with[43,44]. In response to mechanical strain, the APC deficient colon tissues showed an increased number of β-catenin positive nuclei per crypt, whereas almost no nuclear β-catenin was detected in the wild-type colon epithelium. The mechanical stimulation of APC1638N/+ tissues was found to induce a phosphorylation of β-catenin at tyrosine 654, the site of interaction with E-cadherin, thereby dramatically affecting cell adhesions properties. These data demonstrate that, when APC is down expressed, mechanical strain, such as that associated with intestinal transit, presence of polyps or tumour growth, can be interpreted by cells of pre-neoplastic colon tissue as a signal to initiate a β-catenin dependent transcriptional program characteristics of cancer[6].
Even though the above mentioned in vitro experiments provided convincing data that establish a clear correlation between endogenous mechanical pressure and tumorigenesis, they cannot take properly into account all factors contributing to the mechanical environment in vivo. To overcome this limitation, Fernández-Sánchez et al[5] developed a novel and effective method that allows the delivery of a defined mechanical pressure in vivo by subcutaneously inserting a magnet close to the mouse colon. The implanted magnet generates a magnetic force on ultra-magnetic liposomes, stabilized in the mesenchymal cells of the connective tissue surrounding colonic crypts after intravenous injection (Figure 2).
Figure 2.
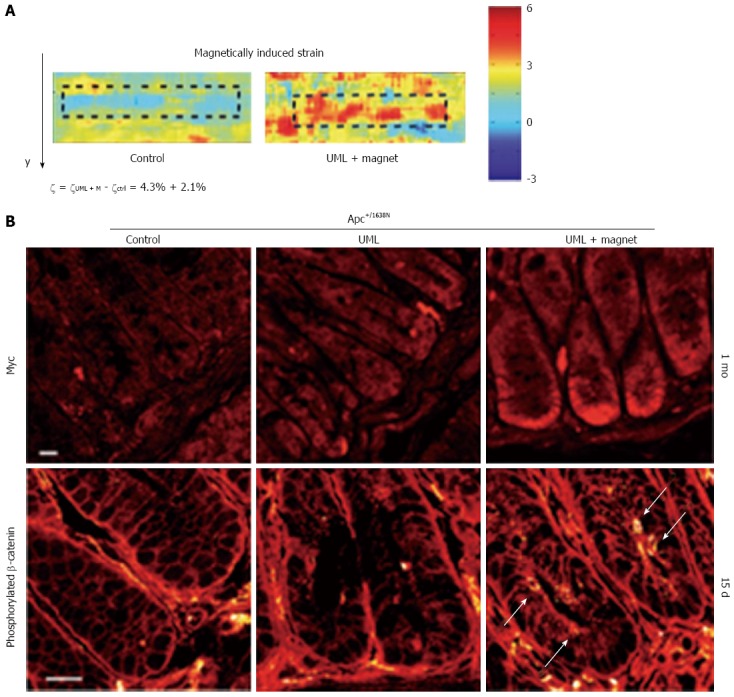
Novel method by Fernández-Sánchez et al[5] to deliver a defined mechanical pressure in vivo (reproduced with permission from[5]). A: Strain maps of non-magnetized (left) and magnetized (right) colon crypt injected with ultra-magnetic liposomes; B: Top: immunefluorescence of Myc expression in APC deficient crypts 1 mo after the ULM injection, in control (left), non-magnetized (middle) and magnetized (left) tissues; Bottom: β-catenin Y654 phosphorylation after 15 d under UML in control (left), non-magnetized (middle) and magnetized (left) tissues.
Such method appears to be a significant breakthrough in the field of cancer biomechanics as it permits to control mechanical stimuli in vivo with same precision that can be achieved in vitro[42]. As pointed out in the recent review by Ou and Weaver, this novel technique has the potential to boost a new era in tissue biomechanics, providing a direct link between mechanical perturbation occurring in vivo and tumorigenic cell modifications[42].
The authors used this revolutionary method to induce a controlled pressure of approximately 1.2 kPa, mimicking the endogenous stress produced by the early tumour growth on healthy tissues. The applied strain led to a rapid Ret activation and downstream phosphorylation of β-catenin on Tyr654, impairing its interaction with the E-cadherin in adherents junction and promoting β-catenin nuclear translocation. Consequently, authors observed an increased expression of β-catenin-target genes, together with the formation of aberrant crypt foci. Interestingly the authors showed that the mechanical induction of a malignant behaviour in normal tissues adjacent to the tumour does not depend on the presence of prior genetic abnormalities, adding another piece of understanding to the growing consensus that the mechanical environment intrinsic to cancerous tissues has the potential to directly modify cells behaviour even in absence of genetic mutations.
Taken together, these results show how mechanical signals can contribute to the onset of a malignancy by affecting the Wnt pathway and triggering the consequent disruption of the physiological crypt dynamics. Interestingly, this behaviour may be propagated by a positive feedback loop in which mechanical pressure from the primary tumour and the stroma induce a breakdown of the normal Wnt signalling pathway in non-transformed adjacent cells. This event, in turn, can trigger an abnormal cell growth that generates further mechanical stress.
For the sake of completeness we want to stress that the Wnt/β-catenin pathway - being one of most important regulators of proliferation in various systems - can be modulated by a wide range of factors other than mechanical stimuli. To give an example, recent experimental findings showed that α6A(β4) integrin regulates cell proliferation and the Wnt/β-catenin pathway through the control of DVL2/GSK3β[67].
MECHANICAL CUES COULD PLAY AN IMPORTANT ROLE IN THE EARLY PHASES OF METASTASES BY TUNING THE TUMOUR MICROENVIRONMENT STIFFNESS
An effective identification of metastasis triggering-signals appears to be a crucial step in the fight against cancer since metastasis accounts for the most of cancer deaths. The process leading to the formation of metastasis is strongly mediated and supported by the tumour microenvironment, which consists of different structures with different mechanical responses, such as tumour-infiltrating cells, blood vessels, extracellular matrix (ECM) and other matrix-associated proteins[2,28].
A large body of evidence suggests that the tumour microenvironment with its mechanical stimuli, including stiffness, might play a major role in initiation of metastasis. For instance, a highly aggressive metastatic variant of murine B16-F1 melanoma cells is produced by culturing cells in a soft fibrin scaffold[22]. Weaver and collaborators showed indeed that ECM stiffening obtained through collagen crosslinking promotes malignant behaviour in mammary epithelial cells by modulating integrin’s expression[68,69].
These results suggest that a fine tuning of the microenvironment stiffness might be involved in the differentiation of non-invasive cells into metastatic variants[70,71]. A confirmation of this hypothesis is provided by the experimental findings of Tang and collaborators[23-25], carried out mainly on HCT-8 cells, a low metastatic colon cancer cell line (Grade I), epithelial in phenotype (E-cells). Previous experimental works demonstrated that when cultured on conventional stiff plastic substrates, low grade HCT-8 E-cells adhere and proliferate, resulting in monolayers covering the entire dish with occasional mounds consisting of 2-3 layers of cells. On top of these mounds, a small number of rounded-shaped metastatic variants of these cells can be detected (1 variant per 2 × 105 epithelial-shaped cells). Due to their shape, such metastatic-like variants are called rounded cells (R-cells)[46-48,72].
By culturing E-cells on polyacrylamide (PA) substrates of well-defined stiffness, Tang et al[24] demonstrated that the proportion of the metastatic-like R-cells can be increased by several orders of magnitude up to reach 70%-90% of the original cell population. To this purpose, authors cultured HCT-8 cells on several fibronectin-coated substrates with a different Young’s modulus, namely a stiff 3.6 GPa polystyrene surface (PS) and a set of polyacrylamide (PA) substrates with a Young’s modulus lying in the range 21-47 kPa. Such stiffness range mimics the rigidity of the tumour microenvironment, thus being suitable to reproduce the mechanical stimuli sensed by cells in pathological conditions[24]. Cells cultured on 21 kPa PA substrates form colonies in 2-4 culture days; after 7 culture days cells begin to dissociate and after 11 d the entire colony dissociates in single round shaped cells. Similarly, HCT-116 cells cultured on 10 kPa sPA fibronectin-coated gel substrates form cell colonies in 2-5 culture days and begin to dissociate from colonies on the 10th day. The metastatic variant is not observed on the 3.6 GPa stiff PS substrate. Similar results were obtained on laminin coated substrates and by using other human colon cancer (SW480) and prostate cancer cell lines[23]. Interestingly, the stiffness-mediated E-to-R transition cannot be reversed by plating the dissociated cells on stiff substrates[24]. The irreversible nature of the transition is likely due to the fact that dissociated cells loose mechano-sensitivity to the substrate[25].
The shape modifications occurring in the E-to-R transition hint at a complex cellular remodeling at the cytoskeleton level. Not-dissociated cells cultured on hard PS substrates indeed show a well-organized cytoskeleton network made of aligned actin stress bundles. Such network is also associated to the presence of large intracellular tension forces that induces significant nuclear stretching[24]. Conversely, R-cells have an almost spherical-shaped nucleus and do not display intracellular stress bundles. The loss of stress bundle, in turn, is associated to a down-expression of E-cadherin along cell-cell contact borders in the metastatic variant[6].
Tang et al[23] studied also the invasive behavior of both cell variants, showing that HCT-8 R cells are remarkably more invasive and tumorigenic than E cells. R cells were found to express many of the molecular signatures associated with resistance to hypoxia, apoptosis, as well as genes linked to metastasis. Of particular interest is the reported down regulation of CKB gene that is linked to the epithelial-to-mesenchymal transition (EMT) in colon cancer[73]. One of the major elements that characterize EMT of carcinoma cells is the loss of E-cadherin-mediated cell-cell adhesion[74], a second characteristic that is in common with the E-to-R transition, providing further evidence that a metastasis-enhancing gene pattern is activated in R cells and this activation might be associated with the characteristics of in vivo EMT[23]. Some of these studies are summarized in Table 2.
Table 2.
List of cell properties that depends on substrate stiffness
| Substrate-related mechanical properties | Substrate stiffness | Substrate type and composition | Outcomes | Related-biochemical and genetic pathway | Ref. |
| E-to-R transition | 1 kPa | Laminin | The E to R transition is not observed. | Not applicable. | Tang et al[24], 2015 |
| or fibronectin coated PA gel | |||||
| 21 kPa | Laminin coated PA gel | Approximately 70%-90% of E cells start transiting to R cells after culturing for 7 d. Transition takes approximately 5-10 h. | E-Cadherin decreases in dissociated | ||
| R cell by a factor 4.73 ± 1.4. | |||||
| Fibronectin coated PA gel | Approximately 70%-90% of E cells start transiting to R cells after culturing for 15 d. Transition takes approximately 5-10 h. | Replanted cells retain their dissociated phenotype irrespective of the substrate stiffness. | |||
| 3.6 GPa | Fibronectin coated PA gel | Not observed. | Not applicable. | ||
| 20 kPa | E-cadherin coated PA gel | E cells transit to R cells in 6 h. | Vinculin in mainly located at the cell-cell junction. | ||
| Fibronectin coated PA gel | E cells transit to R cells in 6 h. | Vinculin in mainly located at the cell- substrate junction. | Ali et al[45], 2014 | ||
| ~70 GPa | E-cadherin coated stiff glass substrates | Transition is not observed. | Not applicable. | ||
| Extremely stiff1 | Plastic/glass stiff substrate | Occasionally E cells transit to R cell (1 cell over 2 × 105). | R cells are deficient in αE-catenin (protein linking the cell-cell adhesion molecule E-cadherin to the action cytoskeleton). | Vermeulen et al[46-48], 1995, 1998, 1999 | |
| Cell colony sizes | 1-20 kPa | Gradient stiffness fibronectin coated PA gel | E type: colony size positively correlated with substrate stiffness | Not applicable. | Tang et al[25], 2012 |
| R type: colony size (smaller than E-colony size) positively correlated with substrate stiffness. | |||||
| Soft1 | Agar gel | Equal numbers of E and R cells were plated and examined after 10 d, 75% of the E cells plated formed colonies while R cells formed no colonies. | Rosenthal et al[72], 1977 | ||
| Adhesion | 1-20 kPa | Gradient stiffness fibronectin coated PA gel | E type: cells show a strong cell-cell adhesion and cell-substrate adhesion evaluated through the measurement of the cell-substrate contact area (188.1 ± 80.7 μm2) by confocal microscopy. Moreover, a strong aspecific adhesion of ~250 nN is detected trough a novel MEMS system. | Reduced E-cadherin expression on R cells. | Tang et al[25], 2012 |
| R type: cells show a weak cell-cell adhesion on very soft substrate (1 kPa). No cell-cell contact is observed on stiffer substrate (5-10-15-20 kPa). A weak cell/substrate adhesion is demonstrated through the measurement of the cell/surface contact area (49.5 ± 20.9 μm2). | |||||
| A week aspecific adhesion of ~2.5 nN is measured through a MEMS system. |
Young’s modulus not provide.
COLON CANCER CELLS MODIFY THEIR MECHANICAL PROPERTIES TO RESIST INTRAVASATION, SHEAR STRESS ASSOCIATED WITH CIRCULATION AND EXTRAVASATION
Following the detachment from the primary tumour, cancer cells intravasate into blood vessels to disseminate. The process of intravasation is still not fully understood in the case of colon cancer. Several molecular steps involving matrix metalloproteinases and interaction between cancer cells and endothelial adhesion molecules have been described. Such processes are discussed in detail in the comprehensive review from Gout and Hout[2] and involve also the tumor-infiltrated macrophages (TAM) that are stimulated by cancer cells to secrete matrix metalloproteinase MMP-7, MMP-12 and vascular endothelial growth factor (VEGF)[28,75-77].
After ECM degradation, cancer cells can gain access to the blood vessels. At this step, the vessel diameter - often smaller then cell sizes - play a crucial role being a key parameter underling colon cancer intravasation via passive entry[78,79].
Moreover, once entered in the blood stream circulating cancer cells are usually not able to generate metastasis. In the most of the cases, indeed, they undergo disruption because of the mechanical stress imposed by circulation, which appears one of the major defence mechanism in the host microenvironment[80,81].
Metastatic cancer cells have developed several strategies to survive the mechanical stress related to both, the migration within the degraded ECM and the shear stress in the blood stream. Such strategies include the occurrence of major modifications at the cytoskeleton level deeply altering the cells viscoelastic properties.
In this context, recent in vitro studies compared the viscoelastic properties of different colon cancer cell lines[20,21,26,27]. To this purpose, two main techniques are used: micropipette aspiration (MA) and atomic force microscopy (AFM). The former permit to investigate the mechanical properties of the whole cell[82,83], whereas the latter provides information on the morphological and mechanical properties at the cellular and sub-cellular level[20,21,30-33,84-90]. Both methods can be coupled to advanced finite element simulation methods[26,91,92].
Pachenari et al[26] recently studied the viscoelastic properties of grade I (HT29) and grade IV (SW480) cancer cells trough micropipette aspiration (MA) method, showing that SW480 are significantly more deformable than HT29. The former are indeed characterized by instantaneous and an equilibrium Young’s modulus of E0 = 331.67 Pa and E∞ = 123.47 Pa, respectively, the latter by E0 = 574.72 Pa and E∞ = 84.76 Pa. The higher compliance of the metastatic cells is accompanied to deep modifications occurring in the cytoskeleton organization, mainly at the level of actin filaments. Authors indeed unveiled a significant decrease in the ratio of actin filaments to microtubules by western blot analysis and fluorescence measurements. Taken together these results confirm that cancer invasiveness is related with an increased cell deformability that, in turn, is instrumental to squeeze through slim capillaries with diameters less than cell sizes as well as to tolerate frictional forces arising between their outer surface and vessel walls.
Avvisato et al[27] recently investigated the behaviour of metastatic SW480 cell lines under shear stress. In particular, cells were cultured on glass and on fibronectin and laminin coated substrates, placed in a rectangular flow channel and exposed to a laminar shear stress lying in the range 0.4 Pa to 3.5 Pa, comparable to human blood shear stress[93]. After 12 h exposure, authors observed a decrease in β-catenin, showing that Wnt signalling pathway is also shear stress dependent (Table 1). Interestingly, such a decrease is greater on laminin-coated substrates, suggesting that the effect of shear stress could be mediated by integrin cell adhesion receptors that in turn have a key role in the intra- and extravasation processes. One way to escape the shear stress associated with circulation is the overexpression of integrin and E-cadherin that allow cells to adhere on the blood vessel wall and epithelial tissues, respectively, favouring extravasation[40,68].
Palmieri et al[20] recently compared the biomechanical properties of SW480 and SW620 colon carcinoma cell lines, derived from primary tumour and lymph-node metastasis of the same patient, respectively. The limited genetic variability of these cells makes them an ideal system to analyse phenotypic variations associated with the metastatic process. Authors studied by confocal microscopy the actin organization of both cell lines, demonstrating that SW620 cells show a decreased cytoskeleton organization with respect to SW480, as quantitatively evaluated by measuring the actin filament-junction density and coherency[20]. Such loss of structure affects also the overall mechanical properties of SW620 cells that appear to be significantly more compliant (480 Pa) than SW480 (1.06 kPa) as demonstrated by atomic force spectroscopy measurements. These results point out that cells extracted from metastases undergo a further destructuration process with the respect to those extracted from the primary tumour that might be related to the cell’s ability to escape from primary tumour mass, to resist to blood shear stress and to extravasate. Moreover, authors unveiled that cells from lymph-node metastasis (SW620) exhibit a higher non-specific adhesion force (95 pN) than SW480 (50 pN), suggesting that the non-specific adhesion forces could participate, together with the high specific one (receptor-ligand binding), in the attachment to the blood vessel walls, in the consequent extravasation and in the metastasis formation. Interestingly, two morphologically different sub-populations of SW480 cells having an elongated (E-type) and a rounded (R-type) shape were reported[20,21]. Similarly to HT29, E-type SW480 cells are significantly stiffer (E ~ 1 kPa) that R-type cells (E ~ 0.5 kPa), indicating a less-organized cytoskeleton in the latter case. At variance with the R-type HT29 cells, SW480 E-type cells do not show impaired adhesion properties with the respect to E-type cells[21] and consistently do not metastasize when injected in nude mice[94].
CONCLUSION
Physical forces either within tissues or externally applied, affect all tissues of the body. Cell mechanotransduction indeed converts biophysical forces into cellular responses that may influence gene expression, protein synthesis, proliferation and morphogenesis. In this review, we focused on recent studies covering the impact of physical stimuli such as compression, shear stress, adhesion and stiffness, in the development of colorectal cancer, showing that such stimuli may have a role in each step of the tumour progression.
An anomalous tissue compression due to a modified microenvironment or an altered abdominal pressure can indeed affect cell proliferation and adhesion properties. A large body of experimental evidence show that mechanical strain can activate a β-catenin dependent pathway, characteristic of cancer, that is able to disrupt the physiological crypt dynamics, leading to the formation of aberrant crypt foci. The mechanism behind this process was recently unveiled by a pioneering in vivo study. The application of a controlled strain in vivo was demonstrated to foster the phosphorylation of β-catenin on Tyr654, leading to an impaired interaction with E-cadherin and promoting β-catenin nuclear translocation with the consequent overexpression of β-catenin targeted oncogenes.
Mechanical cues have also the potential to affect the early phases of metastasis. Tumour progression is accompanied by deep modifications in the tumour microenvironment, which is characterized by a rapidly evolving mechanical landscape. In this context, microenvironment stiffness modifications was indicated as one of the signalling-pathways involved in the initiation of metastasis. This hypothesis was confirmed by recent in vitro studies carried out on a wide range of primary colon cancer cell lines cultured on artificial substrates of a given stiffness. Such studies showed that, in these experimental conditions, substrate stiffness is the main responsible of the differentiation of non-invasive cells into metastatic variants, irrespective of the surface chemistry.
We highlighted also how physical stimuli can support metastatic cells dissemination. Metastatic cells undergo deep structural and mechanical modifications occurring mainly at the cytoskeleton level, that allow them to resist the stress related to migration within the degraded ECM, to intravasate and to survive at the shear stress associated with circulation. To this purpose, actin molecules and microtubules are rearranged within the cell cytoskeleton to make the metastatic cell more compliant than the primary tumour.
Taken together, the experimental findings here reviewed show that mechanical forces are an important player in the development of colon cancer. Therefore, a deep comprehension of the role of physical forces may help scientist to develop both novel diagnostic tools and innovative pharmacological approaches.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 16, 2016
First decision: June 20, 2016
Article in press: August 1, 2016
P- Reviewer: Beaulieu JF, Chae SC S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2016 doi: 10.1136/gutjnl-2015-310912. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Gout S, Huot J. Role of cancer microenvironment in metastasis: focus on colon cancer. Cancer Microenviron. 2008;1:69–83. doi: 10.1007/s12307-008-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Sánchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon A, Brullé L, et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature. 2015;523:92–95. doi: 10.1038/nature14329. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead J, Vignjevic D, Fütterer C, Beaurepaire E, Robine S, Farge E. Mechanical factors activate beta-catenin-dependent oncogene expression in APC mouse colon. HFSP J. 2008;2:286–294. doi: 10.2976/1.2955566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smallwood RH, Holcombe WM, Walker DC. Development and validation of computational models of cellular interaction. J Mol Histol. 2004;35:659–665. doi: 10.1007/s10735-004-2660-1. [DOI] [PubMed] [Google Scholar]
- 8.Walker DC, Southgate J, Hill G, Holcombe M, Hose DR, Wood SM, Mac Neil S, Smallwood RH. The epitheliome: agent-based modelling of the social behaviour of cells. Biosystems. 2004;76:89–100. doi: 10.1016/j.biosystems.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Galle J, Loeffler M, Drasdo D. Modeling the effect of deregulated proliferation and apoptosis on the growth dynamics of epithelial cell populations in vitro. Biophys J. 2005;88:62–75. doi: 10.1529/biophysj.104.041459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meineke FA, Potten CS, Loeffler M. Cell migration and organization in the intestinal crypt using a lattice-free model. Cell Prolif. 2001;34:253–266. doi: 10.1046/j.0960-7722.2001.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel D, Marcelpoil R, Brugal G. A proliferation control network model: the simulation of two-dimensional epithelial homeostasis. Acta Biotheor. 2001;49:219–234. doi: 10.1023/a:1014201805222. [DOI] [PubMed] [Google Scholar]
- 12.Drasdo D, Loeffler M. Individual-based models to growth and folding in one-layered tissues: intestinal crypts and early development. Nonlinear Analysis: Theory, Methods & Applications. 2001;47:245–256. [Google Scholar]
- 13.van Leeuwen IM, Byrne HM, Jensen OE, King JR. Crypt dynamics and colorectal cancer: advances in mathematical modelling. Cell Prolif. 2006;39:157–181. doi: 10.1111/j.1365-2184.2006.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeffler M, Stein R, Wichmann HE, Potten CS, Kaur P, Chwalinski S. Intestinal cell proliferation. I. A comprehensive model of steady-state proliferation in the crypt. Cell Tissue Kinet. 1986;19:627–645. doi: 10.1111/j.1365-2184.1986.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 15.Loeffler M, Potten CS, Paulus U, Glatzer J, Chwalinski S. Intestinal crypt proliferation. II. Computer modelling of mitotic index data provides further evidence for lateral and vertical cell migration in the absence of mitotic activity. Cell Tissue Kinet. 1988;21:247–258. doi: 10.1111/j.1365-2184.1988.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 16.Paulus U, Loeffler M, Zeidler J, Owen G, Potten CS. The differentiation and lineage development of goblet cells in the murine small intestinal crypt: experimental and modelling studies. J Cell Sci. 1993;106(Pt 2):473–483. doi: 10.1242/jcs.106.2.473. [DOI] [PubMed] [Google Scholar]
- 17.Gerike TG, Paulus U, Potten CS, Loeffler M. A dynamic model of proliferation and differentiation in the intestinal crypt based on a hypothetical intraepithelial growth factor. Cell Prolif. 1998;31:93–110. doi: 10.1046/j.1365-2184.1998.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards CM, Chapman SJ. Biomechanical modelling of colorectal crypt budding and fission. Bull Math Biol. 2007;69:1927–1942. doi: 10.1007/s11538-007-9199-8. [DOI] [PubMed] [Google Scholar]
- 19.Boman BM, Fields JZ, Bonham-Carter O, Runquist OA. Computer modeling implicates stem cell overproduction in colon cancer initiation. Cancer Res. 2001;61:8408–8411. [PubMed] [Google Scholar]
- 20.Palmieri V, Lucchetti D, Maiorana A, Papi M, Maulucci G, Calapà F, Ciasca G, Giordano R, Sgambato A, De Spirito M. Mechanical and structural comparison between primary tumor and lymph node metastasis cells in colorectal cancer. Soft Matter. 2015;11:5719–5726. doi: 10.1039/c5sm01089f. [DOI] [PubMed] [Google Scholar]
- 21.Palmieri V, Lucchetti D, Maiorana A, Papi M, Maulucci G, Ciasca G, Svelto M, De Spirito M, Sgambato A. Biomechanical investigation of colorectal cancer cells. Appl Phys Lett. 2014;105:123701. [Google Scholar]
- 22.Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J, Poh YC, Tang K, Wang N, Huang B. Soft fibrin gels promote selection and growth of tumorigenic cells. Nat Mater. 2012;11:734–741. doi: 10.1038/nmat3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang X, Kuhlenschmidt TB, Li Q, Ali S, Lezmi S, Chen H, Pires-Alves M, Laegreid WW, Saif TA, Kuhlenschmidt MS. A mechanically-induced colon cancer cell population shows increased metastatic potential. Mol Cancer. 2014;13:131. doi: 10.1186/1476-4598-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang X, Kuhlenschmidt TB, Zhou J, Bell P, Wang F, Kuhlenschmidt MS, Saif TA. Mechanical force affects expression of an in vitro metastasis-like phenotype in HCT-8 cells. Biophys J. 2010;99:2460–2469. doi: 10.1016/j.bpj.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X, Wen Q, Kuhlenschmidt TB, Kuhlenschmidt MS, Janmey PA, Saif TA. Attenuation of cell mechanosensitivity in colon cancer cells during in vitro metastasis. PLoS One. 2012;7:e50443. doi: 10.1371/journal.pone.0050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pachenari M, Seyedpour SM, Janmaleki M, Babazadeh Shayan S, Taranejoo S, Hosseinkhani H. Mechanical properties of cancer cytoskeleton depend on actin filaments to microtubules content: investigating different grades of colon cancer cell lines. J Biomech. 2014;47:373–379. doi: 10.1016/j.jbiomech.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Avvisato CL, Yang X, Shah S, Hoxter B, Li W, Gaynor R, Pestell R, Tozeren A, Byers SW. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J Cell Sci. 2007;120:2672–2682. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]
- 28.Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010;3:149–166. doi: 10.1007/s12307-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lekka M. Atomic force microscopy: A tip for diagnosing cancer. Nat Nanotechnol. 2012;7:691–692. doi: 10.1038/nnano.2012.196. [DOI] [PubMed] [Google Scholar]
- 31.Lekka M, Gil D, Pogoda K, Dulińska-Litewka J, Jach R, Gostek J, Klymenko O, Prauzner-Bechcicki S, Stachura Z, Wiltowska-Zuber J, et al. Cancer cell detection in tissue sections using AFM. Arch Biochem Biophys. 2012;518:151–156. doi: 10.1016/j.abb.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Lekka M, Laidler P. Applicability of AFM in cancer detection. Nat Nanotechnol. 2009;4:72; author reply 72–73. doi: 10.1038/nnano.2009.004. [DOI] [PubMed] [Google Scholar]
- 33.Lekka M, Pogoda K, Gostek J, Klymenko O, Prauzner-Bechcicki S, Wiltowska-Zuber J, Jaczewska J, Lekki J, Stachura Z. Cancer cell recognition--mechanical phenotype. Micron. 2012;43:1259–1266. doi: 10.1016/j.micron.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibáñez T, Pellinen T, Echarri A, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 37.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi K, Iwata M. Stiffness of cancer cells measured with an AFM indentation method. J Mech Behav Biomed Mater. 2015;49:105–111. doi: 10.1016/j.jmbbm.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 39.Katira P, Bonnecaze RT, Zaman MH. Modeling the mechanics of cancer: effect of changes in cellular and extra-cellular mechanical properties. Front Oncol. 2013;3:145. doi: 10.3389/fonc.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. 2011;11:512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Tian M, Li Y, Liu W, Jin L, Jiang X, Wang X, Ding Z, Peng Y, Zhou J, Fan J, et al. The nanomechanical signature of liver cancer tissues and its molecular origin. Nanoscale. 2015;7:12998–13010. doi: 10.1039/c5nr02192h. [DOI] [PubMed] [Google Scholar]
- 43.Hirokawa M, Miura S, Kishikawa H, Yoshida H, Nakamizo H, Higuchi H, Nakatsumi RC, Suzuki H, Saito H, Ishii H. Loading of mechanical pressure activates mitogen-activated protein kinase and early immediate gene in intestinal epithelial cells. Dig Dis Sci. 2001;46:1993–2003. doi: 10.1023/a:1010607819842. [DOI] [PubMed] [Google Scholar]
- 44.Hirokawa M, Miura S, Shigematsu T, Yoshida H, Hokari R, Higuchi H, Kurose I, Kimura H, Saito H, Nakaki T, et al. Pressure stimulates proliferation and DNA synthesis in rat intestinal epithelial cells. Life Sci. 1997;61:667–672. doi: 10.1016/s0024-3205(97)00531-6. [DOI] [PubMed] [Google Scholar]
- 45.Ali MY, Saif MT. Substrate Stiffness Mediated Metastasis Like Phenotype of Colon Cancer Cells is Independent of Cell to Gel Adhesion. Cell Mol Bioeng. 2014;7:532–543. [Google Scholar]
- 46.Vermeulen SJ, Bruyneel EA, Bracke ME, De Bruyne GK, Vennekens KM, Vleminckx KL, Berx GJ, van Roy FM, Mareel MM. Transition from the noninvasive to the invasive phenotype and loss of alpha-catenin in human colon cancer cells. Cancer Res. 1995;55:4722–4728. [PubMed] [Google Scholar]
- 47.Vermeulen SJ, Chen TR, Speleman F, Nollet F, Van Roy FM, Mareel MM. Did the four human cancer cell lines DLD-1, HCT-15, HCT-8, and HRT-18 originate from one and the same patient? Cancer Genet Cytogenet. 1998;107:76–79. doi: 10.1016/s0165-4608(98)00081-8. [DOI] [PubMed] [Google Scholar]
- 48.Vermeulen SJ, Nollet F, Teugels E, Vennekens KM, Malfait F, Philippé J, Speleman F, Bracke ME, van Roy FM, Mareel MM. The alphaE-catenin gene (CTNNA1) acts as an invasion-suppressor gene in human colon cancer cells. Oncogene. 1999;18:905–915. doi: 10.1038/sj.onc.1202348. [DOI] [PubMed] [Google Scholar]
- 49.Hartono D, Liu Y, Tan PL, Then XY, Yung LY, Lim KM. On-chip measurements of cell compressibility via acoustic radiation. Lab Chip. 2011;11:4072–4080. doi: 10.1039/c1lc20687g. [DOI] [PubMed] [Google Scholar]
- 50.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giavazzi R, Foppolo M, Dossi R, Remuzzi A. Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J Clin Invest. 1993;92:3038–3044. doi: 10.1172/JCI116928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- 53.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 54.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ekins-Daukes N, Kawaguchi K, Zhang J. Strain-balanced criteria for multiple quantum well structures and its signature in X-ray rocking curves. Cryst Growth Des. 2002;2:287–292. [Google Scholar]
- 56.Ciasca G, De Seta M, Capellini G, Evangelisti F, Ortolani M, Virgilio M, Grosso G, Nucara A, Calvani P. Terahertz intersubband absorption and conduction band alignment in n-type Si/SiGe multiple quantum wells. Physic Rev B. 2009;79:085302. [Google Scholar]
- 57.Drasdo D. Buckling instabilities of one-layered growing tissues. Phys Rev Lett. 2000;84:4244–4247. doi: 10.1103/PhysRevLett.84.4244. [DOI] [PubMed] [Google Scholar]
- 58.Nelson MR, Howard D, Jensen OE, King JR, Rose FR, Waters SL. Growth-induced buckling of an epithelial layer. Biomech Model Mechanobiol. 2011;10:883–900. doi: 10.1007/s10237-010-0280-0. [DOI] [PubMed] [Google Scholar]
- 59.van Leeuwen IM, Edwards CM, Ilyas M, Byrne HM. Towards a multiscale model of colorectal cancer. World J Gastroenterol. 2007;13:1399–1407. doi: 10.3748/wjg.v13.i9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basson MD. Paradigms for mechanical signal transduction in the intestinal epithelium. Category: molecular, cell, and developmental biology. Digestion. 2003;68:217–225. doi: 10.1159/000076385. [DOI] [PubMed] [Google Scholar]
- 61.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 62.Dregelid E, Svendsen E. Endothelial cell injury in human saphenous veins after manipulation and tweezer grasping. J Cardiovasc Surg (Torino) 1988;29:464–469. [PubMed] [Google Scholar]
- 63.Basson MD, Yu CF, Herden-Kirchoff O, Ellermeier M, Sanders MA, Merrell RC, Sumpio BE. Effects of increased ambient pressure on colon cancer cell adhesion. J Cell Biochem. 2000;78:47–61. doi: 10.1002/(sici)1097-4644(20000701)78:1<47::aid-jcb5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 64.Basson MD, Modlin IM, Madri JA. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest. 1992;90:15–23. doi: 10.1172/JCI115828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thamilselvan V, Basson MD. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signaling. Gastroenterology. 2004;126:8–18. doi: 10.1053/j.gastro.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 66.Gutt CN, Kim ZG, Gessmann T, Lorenz M, Paolucci V. Hepatic tumor spread of colorectal cancer in a laparoscopic animal model. Surg Endosc. 2000;14:448–451. doi: 10.1007/s004640000159. [DOI] [PubMed] [Google Scholar]
- 67.Groulx JF, Giroux V, Beauséjour M, Boudjadi S, Basora N, Carrier JC, Beaulieu JF. Integrin α6A splice variant regulates proliferation and the Wnt/β-catenin pathway in human colorectal cancer cells. Carcinogenesis. 2014;35:1217–1227. doi: 10.1093/carcin/bgu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Brábek J, Mierke CT, Rösel D, Veselý P, Fabry B. The role of the tissue microenvironment in the regulation of cancer cell motility and invasion. Cell Commun Signal. 2010;8:22. doi: 10.1186/1478-811X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 72.Rosenthal KL, Tompkins WA, Frank GL, McCulloch P, Rawls WE. Variants of a human colon adenocarcinoma cell line which differ in morphology and carcinoembryonic antigen production. Cancer Res. 1977;37:4024–4030. [PubMed] [Google Scholar]
- 73.Mooney SM, Rajagopalan K, Williams BH, Zeng Y, Christudass CS, Li Y, Yin B, Kulkarni P, Getzenberg RH. Creatine kinase brain overexpression protects colorectal cells from various metabolic and non-metabolic stresses. J Cell Biochem. 2011;112:1066–1075. doi: 10.1002/jcb.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Black W, Chen Y, Matsumoto A, Thompson DC, Lassen N, Pappa A, Vasiliou V. Molecular mechanisms of ALDH3A1-mediated cellular protection against 4-hydroxy-2-nonenal. Free Radic Biol Med. 2012;52:1937–1944. doi: 10.1016/j.freeradbiomed.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233–1243. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mizukami Y, Kohgo Y, Chung DC. Hypoxia inducible factor-1 independent pathways in tumor angiogenesis. Clin Cancer Res. 2007;13:5670–5674. doi: 10.1158/1078-0432.CCR-07-0111. [DOI] [PubMed] [Google Scholar]
- 77.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsuji T, Sasaki Y, Tanaka M, Hanabata N, Hada R, Munakata A. Microvessel morphology and vascular endothelial growth factor expression in human colonic carcinoma with or without metastasis. Lab Invest. 2002;82:555–562. doi: 10.1038/labinvest.3780450. [DOI] [PubMed] [Google Scholar]
- 79.Tien YW, Jeng YM, Hu RH, Chang KJ, Hsu SM, Lee PH. Intravasation-related metastatic factors in colorectal cancer. Tumour Biol. 2004;25:48–55. doi: 10.1159/000077723. [DOI] [PubMed] [Google Scholar]
- 80.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 81.Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243–55, vii. [PubMed] [Google Scholar]
- 82.Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 83.Tomaiuolo G. Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics. 2014;8:051501. doi: 10.1063/1.4895755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma S, Gimzewski JK. Application of AFM to the Nanomechanics of Cancer. MRS Advances. 2016:1–11. [Google Scholar]
- 85.Ciasca G, Papi M, Di Claudio S, Chiarpotto M, Palmieri V, Maulucci G, Nocca G, Rossi C, De Spirito M. Mapping viscoelastic properties of healthy and pathological red blood cells at the nanoscale level. Nanoscale. 2015;7:17030–17037. doi: 10.1039/c5nr03145a. [DOI] [PubMed] [Google Scholar]
- 86.De Spirito M, Brunelli R, Mei G, Bertani FR, Ciasca G, Greco G, Papi M, Arcovito G, Ursini F, Parasassi T. Low density lipoprotein aged in plasma forms clusters resembling subendothelial droplets: aggregation via surface sites. Biophys J. 2006;90:4239–4247. doi: 10.1529/biophysj.105.075788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Gaspare L, Ciasca G, Pea M, Notargiacomo A. Ion and plasma based treatments for enhanced chemical speciation of metals in ferritin. Microelectron Eng. 2014;124:86–89. [Google Scholar]
- 88.Maiorana A, Bugli F, Papi M, Torelli R, Ciasca G, Maulucci G, Palmieri V, Cacaci M, Paroni Sterbini F, Posteraro B. Effect of alginate lyase on biofilm-grown Helicobacter pylori probed by atomic force microscopy. Int J Poly Sci. 2015:2015. [Google Scholar]
- 89.Papi M, Lauriola M, Palmieri V, Ciasca G, Maulucci G, De Spirito M. Plasma protein corona reduces the haemolytic activity of graphene oxide nano and micro flakes. RSC Advances. 2015;5:81638–81641. [Google Scholar]
- 90.Lekka M. Discrimination Between Normal and Cancerous Cells Using AFM. Bionanoscience. 2016;6:65–80. doi: 10.1007/s12668-016-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boccaccio A, Papi M, De Spirito M, Lamberti L, Pappalettere C. Effect of the residual stress on soft sample nanoindentation. Appl Phys Lett. 2013;102:133704. [Google Scholar]
- 92.Frassanito M, Papi M, De Spirito M, Lamberti L, Boccaccio A, Arcovito G, Pappalettere C. Investigation of Zona Pellucida hardening with Atomic Force Microscopy and nonlinear optimization. 2011. Available from: http://hdl.handle.net/10807/17321.
- 93.Doriot PA, Dorsaz PA, Dorsaz L, De Benedetti E, Chatelain P, Delafontaine P. In-vivo measurements of wall shear stress in human coronary arteries. Coron Artery Dis. 2000;11:495–502. doi: 10.1097/00019501-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 94.Yoon WH, Lee SK, Song KS, Kim JS, Kim TD, Li G, Yun EJ, Heo JY, Jung YJ, Park JI, et al. The tumorigenic, invasive and metastatic potential of epithelial and round subpopulations of the SW480 human colon cancer cell line. Mol Med Rep. 2008;1:763–768. doi: 10.3892/mmr_00000026. [DOI] [PubMed] [Google Scholar]
- 95.McDonald SA, Preston SL, Lovell MJ, Wright NA, Jankowski JA. Mechanisms of disease: from stem cells to colorectal cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3:267–274. doi: 10.1038/ncpgasthep0473. [DOI] [PubMed] [Google Scholar]


