Abstract
Portal vein tumor thrombosis (PVTT) is a common phenomenon in hepatocellular carcinoma (HCC). Compared to HCC without PVTT, HCC with PVTT is characterized by an aggressive disease course, worse hepatic function, a higher chance of complications related to portal hypertension and poorer tolerance to treatment. Conventionally, HCC with PVTT is grouped together with metastatic HCC during the planning of its management, and most patients are offered palliative treatment with sorafenib or other systemic agents. As a result, most data on the management of HCC with PVTT comes from subgroup analyses or retrospective series. In the past few years, there have been several updates on management of HCC with PVTT. First, it is evident that HCC with PVTT consists of heterogeneous subgroups with different prognoses. Different classifications have been proposed to stage the degree of portal vein invasion/thrombosis, suggesting that different treatment modalities may be individualized to patients with different risks. Second, more studies indicate that more aggressive treatment, including surgical resection or locoregional treatment, may benefit select HCC patients with PVTT. In this review, we aim to discuss the recent conceptual changes and summarize the data on the management of HCC with PVTT.
Keywords: Liver cancer, Vascular invasion, Targeted agent, Surgery, Radiotherapy
Core tip: Conventionally, the presence of portal vein tumor thrombosis (PVTT) indicated an extremely poor prognosis for hepatocellular carcinoma (HCC) patients and was considered a contraindication to both surgery and trans-arterial procedures. Recent studies indicate that HCC with PVTT represents a heterogeneous group with variable prognoses. Several classifications have been proposed to gauge the prognoses of PVTT. For selected patients with less severe PVTT, surgery with curative intent is feasible with favorable outcomes. Further, expanding treatment options, such as radiotherapy, radioembolization and systemic treatment, could improve the outcomes of patients with more severe forms of PVTT in patients with HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is characterized by its propensity to invade the vasculature within the liver. The invasion of the hepatic vasculature is of macroscopic or microscopic type. Macrovascular invasion refers to gross invasion into the main portal veins or their branches, hepatic veins or the inferior vena cava in the liver, while microscopic vascular invasion is defined as tumors within a vascular space lined by endothelium that is identifiable only by microscopy[1].
Portal vein tumor thrombosis (PVTT) is the most common form of macrovascular invasion of HCC. Multiple case series have suggested that the PVTT is a common phenomenon with a prevalence rate ranging from 10% to over 60%[2-5]. The presence of PVTT in patients with HCC has been consistently demonstrated by different series to be associated with poor prognoses, with a hazard ratio of death close to 2[5,6]. The poor prognosis of PVTT in HCC patients is the result of combined factors including impaired hepatic reserves, intrinsic aggressiveness of tumor, reduced intolerance to anti-neoplastic treatment and a high rate of developing complications related to portal hypertension. Clinically, PVTT is associated with large tumor size, increased tumor number, higher tumor grade, worse Child-Pugh class and higher serum alpha-fetoprotein (AFP)[7]. At the genetic level, next generation sequencing identified that mutations of the KDM6A, CUL9, FDG6, AKAP3, and RNF139 genes were associated with the development of PVTT in advanced Hepatitis B virus-related HCC[8].
It has to be noted that not all portal vein thrombosis in HCC patients is due to neoplastic thrombus. It is evident that a portion (ranging from 0.6% to 11%) of cirrhotic patients, particularly those with portal hypertension, are complicated by non-neoplastic portal vein thrombosis (NPVT)[9]. Up to 72.7% of portal thrombi in HCC patients are indeed NPVT[10]. Patients with NPVT have better prognoses than those with PVTT; therefore, the differentiation between NPVT and PVTT is of clinical relevance. Theoretically, image-guided percutaneous fine needle aspiration or a biopsy of portal vein thrombosis could provide a definite pathological diagnosis[9,11]. However, biopsy procedures are not frequently conducted in clinical practice to confirm PVTT because of concerns about life-threatening complications such as injuries to the bile ducts or hepatic arteries[11]. Instead, non-invasive imaging studies are the most frequently used diagnostic tool for PVTT. Contrast-enhanced ultrasonography, dynamic contrast enhanced computed tomography and gadoxetic acid-enhanced magnetic resonance could achieve a diagnosis of PVTT with sensitivities of 82.5%-98%, 68%-86% and 92%-95%, respectively[11-13].
Conventionally, the treatment algorithm proposed by the Barcelona Clinic Liver Cancer (BCLC) system considers the presence of PVTT, regardless of the degree of invasion, as a contra-indication to surgery or transarterial chemoembolization (TACE)[14]. Patients with PVTT are classified in the BCLC stage C category and are considered candidates for systemic agents. However, a recent concept has evolved, which considers HCC with PVTT to consist of heterogeneous populations with different disease behaviors and prognoses, and selected patients with PVTT may benefit from more aggressive treatment modalities. In the current review, we aim to discuss these changing concepts in the management of HCC with PVTT with a focus on the latest data on the adoption of a more aggressive treatment approach for this disease entity.
CLASSIFICATIONS OF PVTT
The management of patients with PVTT can be challenging because the clinical course is typically characterized by poor underlying liver reserve and high portal vein pressure. A complicated operation is usually required if an aggressive treatment approach is contemplated. To devise the best treatment strategy for patients with PVTT, a universally accepted classification of PVTT is necessary for the guidance of treatment and a comparison of outcomes between different groups. At present, various classification systems have been developed in different centers. The more conventional and better-known classification is the one proposed by the Liver Cancer Study Group of Japan (LCSGJ). In their General Rules for the Clinical and Pathological Study of Primary Liver Cancer[15], there is a macroscopic classification of HCC with PVTT: Vp0, no PVTT; Vp1, the presence of a PVTT distal to, but not in, the second-order branches of the portal vein; Vp2, the presence of a PVTT in the second-order branches of the portal vein; Vp3, the presence of a PVTT in the first-order branches of the portal vein; and Vp4, the presence of a PVTT in the main trunk of the portal vein or a contralateral portal vein branch or both. According to the guideline, resection is one of the feasible options for treatment in case of minor portal vein involvement (i.e., Vp1 and Vp2)[16]. For selected Vp3 or Vp4 patients, surgical resection would still be considered, and a 5-year survival of 18.3% has been reported[16]. In view of the guarded prognosis, few centers would adopt this aggressive approach. Moreover, the required expertise is not always available (Table 1).
Table 1.
Common classifications of portal vein tumor thrombosis in hepatocellular carcinoma
| Group | Types of PVTT | Survival |
| Ikai et al[80] | Vp0: Absent | 59% at 5 yr |
| Vp1: Distal to but not in second-order branches | 39.1% at 5 yr | |
| Vp2: In second-order branches | 23.3% at 5 yr | |
| Vp3: In first-order branches | 18.3% at 5 yr (Vp3 and Vp4) | |
| Vp4: In the main trunk or contralateral or both | ||
| Shi et al[17] | 10: Microscopic | |
| 1: In segmental branches or above | 26.7% at 3 yr (Type 10 included) | |
| 2: In the left or right branch | 16.9% at 3 yr | |
| 3: In the main trunk | 3.7% at 3 yr | |
| 4: In the superior mesenteric vein | 0% at 3 yr | |
| Xu et al[18] | A: In the main trunk or both the left and right branches | 0% at 5 yr |
| B: In only the left or right branch | 5.2% at 5 yr |
HCC: Hepatocellular carcinoma; PVTT: Portal vein tumor thrombosis.
In 2007, Shi et al[17] devised a classification of HCC with PVTT that incorporated microscopic PVTT as Type 1. In this classification, Type 1 is a PVTT involving segmental branches or above; Type 2 is a PVTT involving the right or left portal vein; Type 3 is a PVTT involving the main portal vein; and Type 4 is a PVTT involving the superior mesenteric vein. In general, surgical resection can be applied to Type 3 and to selected patients with Type 4. In their report, the numbers of patients with Types 1, 2, 3 and 4 were 144 (32.7%), 189 (42.9%), 86 (19.5%) and 22 (5.0%), respectively. The 1-, 2-, and 3-year overall survival rates were 54.8%, 33.9% and 26.7% for Type 1 patients, respectively, 36.4%, 24.9% and 16.9% for Type 2 patients, respectively, 25.9%, 12.9% and 3.7% for Type 3 patients, respectively, and 11.1%, 0% and 0% for Type 4 patients, respectively (P < 0.0001).
A simplified classification by Xu et al[18] divided patients with PVTT into two groups: Group A, with involvement of the main portal vein trunk or both the left and right portal veins, and Group B, with involvement of only the left or right portal vein. In their report, the Group A 1-year overall survival rate was 31.5% after resection, and the 1-, 3- and 5-year overall survival rates of Group B patients were 62.3%, 16.1% and 5.2%, respectively. This simple classification could be used as a quick reference when counseling patients (especially Group A patients) about surgical treatment. No matter which of the above classifications is used, the prognosis would be determined by the extent of the PVTT and its proximity to the main, or even the contralateral, portal vein.
SURGERY (EN BLOC PORTAL VEIN RESECTION VS TUMOR THROMBECTOMY)
The prognosis is notoriously poor for HCC patients with macrovascular PVTT, especially those whose PVTT has extended to the main or contralateral portal vein[19]. The PVTT could propagate further and obstruct the whole vein lumen, resulting in liver failure or life-threatening variceal bleeding. One of the treatment modalities is surgical resection, the two common modes of which are tumor thrombectomy and en bloc resection of the thrombus and the portal vein followed by portal vein reconstruction. Tumor thrombectomy is technically less demanding but might result in a residual tumor. As for the latter mode, it is associated with high morbidity and mortality rates despite a “perceived” better oncological outcome[20]. However, there has been no randomized controlled trial to determine the superiority of one over the other, and the choice rests with individual centers and individual surgeons (Figure 1).
Figure 1.
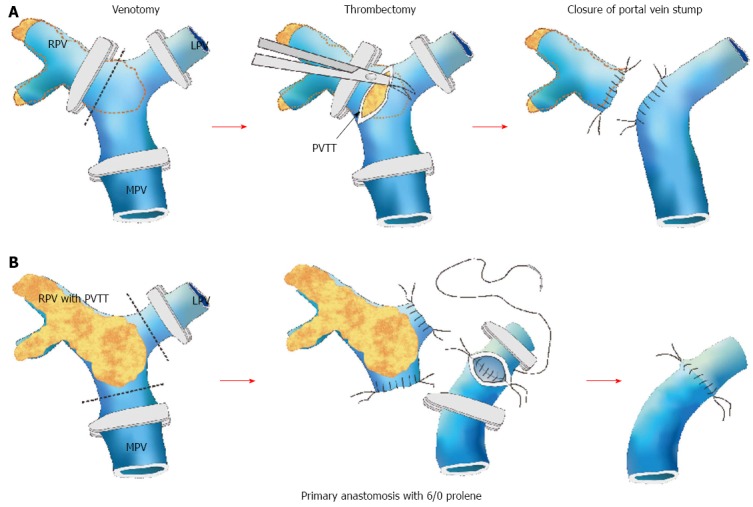
Schematic diagrams demonstrating different types of portal vein tumor thrombosis and the relevant surgical approaches. A: Thrombectomy; B: En bloc resection with portal vein reconstruction. RPV: Right portal vein; LPV: Left portal vein; MPV: Main portal vein. Courtesy of Chok et al[21].
A PVTT confined to the hepatic lobe harboring the HCC (ipsilateral PVTT) is usually resected when a hepatectomy is conducted to remove the HCC[21]. For the management of PVTT extending to the portal vein bifurcation or the main or contralateral portal vein, different approaches have been recommended. En bloc resection, including bifurcation with or without the main portal vein and/or the contralateral portal vein, is believed to produce good oncological outcomes[22]. However, it has been documented that thrombectomy can produce similar survival outcomes with lower operative mortality and morbidity[20-22]. In a study from Japan, 979 consecutive patients were evaluated, and 45 of them had Vp3 or Vp4 PVTT[23]. They received hepatectomy with tumor thrombectomy. The 3- and 5-year survival rates in the Vp3 and Vp4 groups were 35.3% and 41.8%, and 21.2% and 20.9%, respectively.
In a study by The University of Hong Kong trying to address the controversy about en bloc resection vs thrombectomy[21], patients were divided into three groups: Group 1 (n = 71), with ipsilateral PVTT resected in a hepatectomy; Group 2 (n = 10), with PVTT extending to or beyond the bifurcation, treated by en bloc resection followed by portal vein reconstruction; and Group 3 (n = 7), with PVTT extending to or beyond the bifurcation, treated by thrombectomy. The median overall survival duration was 10.91 mo in Group 1, 9.4 mo in Group 2, and 8.58 mo in Group 3, and it was shown that en bloc resection and thrombectomy were not significantly different in terms of hospital mortality and morbidity. The frequent practice of living donor liver transplantation at this center certainly contributed to the low morbidity rate after portal vein resection[24]. The 1-, 3- and 5-year overall survival rates were 50%, 12.5% and 12.5%, respectively, in Group 2 and 28.6%, 14.3% and 14.3%, respectively, in Group 3. The 1-year disease-free survival rates were 24.3, 0, and 14.3%, respectively. The 3-year DFS rates were 14.3, 0, and 14.3%, respectively. The 5-year DFS rates were 10.7, 0, and 14.3%, respectively. Again, the two approaches had no significant differences in terms of overall survival or disease-free survival. Patients with ipsilateral PVTT also had similar survival outcomes compared with patients with PVTT extending to or beyond the bifurcation. These survival outcomes were satisfactory when compared with those (a median survival duration of only 2.7 mo) of patients with PVTT who were untreated[1]. Peng et al[25] compared hepatic resection and transarterial chemoembolization (TACE) for patients with PVTT and found that the resection group had significantly longer overall survival. The 1-, 3-, and 5-year overall survival rates were 42.0%, 14.1% and 11.1%, respectively, in the resection group and 37.8%, 7.3% and 0.5%, respectively, in the TACE group (P < 0.001). In the subgroup analysis, the resection group had better overall survival in regard to Type 1 PVTT, Type 2 PVTT, single tumor, and tumor >5 cm (P < 0.001, P = 0.002, P < 0.001, and P < 0.001, respectively). No difference in overall survival was shown in regard to Type 3 PVTT, Type 4 PVTT, multiple tumors, and tumor < 5 cm (P = 0.541, P = 0.371, P = 0.264, P = 0.338, and P = 0.125, respectively).
In a number of reports concerning all degrees of PVTT, the median survival durations varied from 8.9 mo to 33 mo, and the operative mortality rates varied from 0% to 5.9%[22,26-30]. For patients with PVTT confined to the ipsilateral first-generation portal vein, resection of the PVTT in a hepatectomy is recommended. With a good resection margin, intrahepatic recurrence could be prevented. For PVTT extending to or beyond the bifurcation, an en bloc resection or thrombectomy should be considered. Nonetheless, if the liver is cirrhotic or if the resection would leave an inadequate liver remnant, major resection will not be possible.
TRANS-ARTERIAL CHEMOEMBOLIZATION
Trans-arterial chemoembolization takes advantage of the relatively selective arterial vascularization of hepatic tumors. Chemotherapeutic agents are delivered with simultaneous embolization to increase the local chemotherapeutic dwell time and induce tumor ischemia[31]. The technique of TACE varies; typically, super-selective cannulation of the artery supplying the tumor is performed whenever possible. An emulsion of cisplatin (1 mg/mL) and Lipiodol (Lipiodol Ultrafluide®; Laboratoire Guerbet, Aulnay-Sous-Bois, France) in a volume ratio of 1:1 is injected up to a maximum of 60 mL, depending on the size and number of the tumor[32,33], with or without embolization. TACE is repeated every 8 to 12 wk, and the treatment is to be stopped when there is progressive disease, extrahepatic disease, a severe life-threatening complication, or evidence of liver failure or decompensation (serum total bilirubin > 50 μmol/L, gross ascites with uncontrollable with diuretics, or hepatic encephalopathy)[34]. TACE is considered the primary treatment for patients who have inoperable HCC that is confined to the liver and in the absence of contraindications to TACE. Studies have reported that 35% of patients had a complete or progressive response to TACE, with < 2% of patients having a complete response[35,36]. Lo et al[37] compared TACE with symptomatic treatment and found that the patient survival rate at 2 years after treatment was higher with TACE (41%) than that with symptomatic treatment (27%).
The thought behind using TACE for the treatment of HCC with PVTT is evolving. Traditionally, TACE is generally not recommended for patients with PVTT because of the increased risk of liver failure[38,39], but there have been no large trials to validate this recommendation. For the past 5 years, a growing number of studies showed that TACE could be safely conducted in patients with PVTT, provided that there is an adequate hepatic reserve and the establishment of collateral blood circulation around the obstructed PVTT (Table 2)[40,41]. Of note, there are two randomized prospective studies comparing TACE to conservative management in patients with PVTT. Both studies consistently demonstrated improved overall survival in the TACE arm when compared to patients undergoing conservative management. Both studies have also conducted subgroup analyses in a population with different degrees of PVTT. Niu et al has shown that the survival benefits in PVTT type 1 (TACE: 19 mo vs conservative: 4 mo, P = 0.001); type II (TACE: 11 mo vs conservative: 1.43 mo, P < 0.001); type III (TACE: 7.1 mo vs conservative: 1.3 mo, P < 0.001) and type IV (TACE: 4 mo vs conservative: 1 mo, P = 0.005). Luo et al showed that the 6-mo survival rates of the TACE arm for branch and main PVTT were 75% and 38.7%, respectively, compared to 45.5% and 20% in the conservative arm. There are also prospective studies comparing TACE vs the hepatic arterial infusion of chemotherapy, of which superior survival outcomes were observed in the TACE arm.
Table 2.
Prospective trials on transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis
| Group | No. of patients | Treatments | Child-Pugh A | Survival |
| Luo et al[81] | 164 (84 vs 80) | TACE vs Control | Not known | 1-yr survival 30.9% vs 9.2% |
| Niu et al[82] | 150 (115 vs 35) | TACE vs Control | 88 vs 21 | Median survival 8.67 mo vs 14 mo |
| Kim et al[83] | 110 (49 vs 61) | TACE vs TACI | 30 vs 22 | Median survival 14.9 mo vs 4.4 mo |
| Peng et al[25] | 603 (402 vs 201) | TACE vs Resection | 389 vs 197 | Median survival 42 mo vs 14.1 mo |
TACE: Transarterial chemoembolization; PVTT: Portal vein tumor thrombosis; TACI: Transcatheter arterial chemoinfusion.
Currently, there are no prospective data from a head-to-head comparison between TACE and systemic therapy in patients with PVTT. However, taking the above studies into consideration, it is generally accepted that TACE is feasible and effective in select patients with branch PVTT because the overall survival appeared to be longer than that achieved in most clinical trials of systemic therapy. As shown in Table 2, many more Child-Pugh A patients underwent TACE, and the benefits of TACE were significantly less in Child-Pugh B patients with PVTT. Liver function remains the most important criteria for selecting patients to receive TACE treatment. The role of TACE in the treatment of main PVTT (Vp4 or type III/IV) is more controversial. Although randomized studies indicated the survival benefits of TACE in these groups of patients, overall survival (4-7 mo) was similar, if not inferior, to survival observed in clinical trials on systemic agents such as sorafenib (see below: systemic therapy).
RADIOTHERAPY
External radiotherapy
Historically, external radiotherapy (RT) is not considered a feasible treatment in the management of HCC. This is because the liver is highly radiosensitive, and the delivery of a sufficient high dose RT without excessive hepatotoxicity is challenging. However, as a result of the advances in RT technology, including the conformal RT planning and breathing motion management and image-guided radiation therapy, RT has emerged as a valid treatment option in the treatment of HCC[42,43]. A number of retrospective studies have studied the efficacy of RT as single treatment or in combination with other treatment modalities, particularly TACE, in treating HCC with PVTT (Table 3). These reports have consistently reported a favorable toxicity profile and modest efficacy of RT[44-57]. In one of the largest series, 412 patients with PVTT were treated with 21-60 Gy in 2- to 5-Gy fractions in combination with sequential TACE. The median survival was 10.6 mo with a 2-year survival rate of 23%, while grade 3 or above toxicity was observed in 10% of patients[44]. The radiologic response of PVTT to RT is the most significant prognostic factor, with a median overall survival of 19.4 mo in the responder group vs 7.0 mo in the non-responder group.
Table 3.
Studies on radiation therapy for hepatocellular carcinoma with portal vein tumor thrombosis
| n | Treatment | Total RT dose/fractional dose (in Gy) | Response rate (CR + PR, %) | Median survival (mo) | Toxicity grade ≥ 3 (%) | Ref. |
| 45 | 3D-CRT (+TACE/PEI/RFA; 7% RT only) | 38-65/1.8-2.5 | 62.3 (CR 6.7) | 11.2 | 2 | Rim et al[51] |
| 412 | 3D-CRT + TACE | 21-60/2-5 | 27.9 (CR 3.6) | 10.6 | 10 | Yoon et al[44] |
| 40 | IGRT + IA 5FU/IFN vs IA 5FU/IFN | 30-48/7-16 | 60 (CR 5) | 12 (RT) | 15 | Chuma et al[52] |
| 9.1 (non-RT) | ||||||
| 32 | IA 5FU/IFN + 3D-CRT vs IA 5FU/IFN | 30-45/3 | 75 (CR 19) | 7.5 (RT) | G4: 2 | Katamura et al[46] |
| 7.9 (non-RT) | G3: 7% (leucopenia)/6% (thrombocytopenia)/1 (anorexia) | |||||
| 45 | PV stenting + TACE + 3D-CRT vs PV stenting + TACE | 30-60/2 | 35.6 (CR 0) | 16.5 (RT) | 0 | Zhang et al[45] |
| 4.8 (non-RT) | ||||||
| 326 | 3D-CRT (IMRT 14.1%) | 60/2-3 | 18.1 (CR 5.8) | 4 | 0 | Huang et al[53] |
| 38 | 3D-CRT | 17.5-50.4/1.8-4 | 44.7 (CR 15.8) | 9.6 | 0 | Toya et al[47] |
| 59 | 3D-CRT | 30-54/2-3 | 45.8 (CR 6.8) | 7.8 | 0 | Kim et al[48] |
| 44 | RT + TACE | 36-60/2 | 45.5 (CR 34.1) | 8 | 0 | Kim et al[49] |
| 19 | 3D-CRT (+ TACE for liver tumor) | 46-60/2 | 57.9 (CR 0) | 7 | G3: 5% (thrombocytopenia)/2% (leucopenia)/2 (GI ulcers) | Yamada et al[54] |
| 20 | RT + TACE | 50/2 | 50 (CR 0) | 5.3 | 5 | Ishikura et al[55] |
| 24 | RT + TACE | 50/2 | 50 (CR 16.7) | CR/PR (9.7) | 13% | Tazawa et al[56] |
| NR/PD (3/8) | ||||||
| 281 | 3D-CRT + TACE | 30-54/1.8 -4.5 | 53.8 (CR 3.6) | 11.6 | 20% | Yu et al[57] |
3D-CRT: 3 dimensional conformal radiotherapy; GI: Gastrointestinal; PR: Partial response; RT: Radiotherapy; TACE: Transarterial chemoembolization; NR: Non-responder; PD: Progressive disease; PVTT: Portal vein tumor thrombosis.
There is also a growing body of evidence suggesting that the concurrent administration of RT and locoregional treatment could improve the response rate or treatment outcomes of PVTT. In a retrospective study reported by Zhang et al[45], patients with PVTT were treated with percutaneous transhepatic PV stenting and TACE, with or without RT. The median overall survival was 16.5 mo in the RT cohort compared with 4.8 mo in the non-RT cohort. In a matched cohort study by Katamura et al[46], a significant improvement in the objective response rate in PVTT was observed in the RT group compared with the non-RT group (75% vs 25%) in patients treated with intra-arterial 5-fluorouracil and interferon-alpha. Several studies had evaluated the dose-response of RT in the treatment of HCC with PVTT. The response rate was better when the biologically effective dose (BED) exceeded 58 Gy[47,48,50]. Therefore, an attempt to deliver a BED as high as possible is preferred during the planning of RT to HCC with PVTT. However, this can only be achieved in cases of small primary HCC, where both HCC and PVTT could be covered within a high dose target volume without compromising the normal liver. In clinical practice, because bulky HCC is frequently encountered, a combined approach with RT to focus on PVTT and TACE for the treatment of the intra-hepatic tumor is generally preferred to keep the normal liver radiation tolerance within a safe limit. Well-designed prospective studies are required to evaluate this combination for patients with PVTT.
Selective internal radiation therapy
Selective internal radiation therapy (SIRT), or transarterial radioembolization with Yttrium-90, involves the transarterial administration of therapeutic doses of radiation to the hepatic tumor via resin or glass particles. Although resin and glass are both considered permanent embolic agents, their embolic effects are less than those of a TACE procedure due to their small size, as is their effect on hepatic vascular dynamics[58]. During radioembolization, a radioactive microsphere is selectively injected into the hepatic artery or its branch, delivering intense local tumor radiation. SIRT provides adequate radiation to the tumor with little radiation to the rest of the liver and to the patient’s body. Because it does not cause ischemia, SIRT can be performed in patients with portal vein thrombosis, making SIRT a feasible choice for HCC with PVTT. Common complications of SIRT include fatigue and deranged liver function[59]. Serious complications such as radiation pneumonitis, radiation cholecystitis, liver abscess and radiation-induced liver disease occur in less than 1% of patients. In general, technetium-labeled macroaggregated albumin scanning is performed prior to SIRT to quantify the fraction of lung shunting and/or the tumor/normal uptake ratio. The accepted safe radiation dose to the lung is less than 30 Gy in a single procedure and less than 50 Gy in total over multiple procedures[59,60].
Studies on SIRT focusing on patients with HCC and PVTT are limited. However, numerous retrospective series have reported the safety and efficacy of SIRT in patients with HCC[61-64]. Subgroup analyses from the three largest series of HCC patients who underwent SIRT involving more than 200 patients with PVTT demonstrated the overall survival to be approximately 10 mo. Ozkan et al[65] reported a better median overall survival of 17 mo among 29 HCC patients treated with SIRT, with and without PVTT. There was no significant difference in survival between patients with and without PVT. A report from the largest group of PVT patients showed concordant results[63], in which it was also found that patients with Child-Pugh A cirrhosis, with or without PVT, benefited the most from SIRT. In a recent prospective phase II trial of 35 patients with PVT treated with SIRT, the overall survival of Child-Pugh A and B patients were 16 mo and 6 mo, respectively.
The efficacy of SIRT in unresectable HCC was compared with sorafenib in a recent study. Edeline et al[66] retrospectively reviewed the records of 151 HCC patients with PVTT. The overall survival of 34 patients treated with SIRT was compared with 117 patients treated with sorafenib only. SIRT was associated with a higher median overall survival compared to sorafenib (18.8 mo vs 6.5 mo, P < 0.001). A prospective randomized study comparing SIRT and sorafenib has already completed accrual with preliminary results expected to be available in late 2016 (NCT01135056). At present, there have been no randomized controlled trials directly comparing SIRT to TACE in patients with HCC and PVTT. Several small-scale studies have suggested that the efficacy of SIRT is comparable to TACE in unresectable HCC. Of note, She et al[67] performed survival analysis of 16 patients who underwent SIRT and compared it with another 16 patients in a matched cohort treated with TACE. Half of the patients in each group had major vascular invasion, and those treated with SIRT had an overall survival of 12.0 mo compared to 8.0 mo in the TACE cohort. These data provide preliminary clues that SIRT might be more effective than TACE in the setting of HCC with PVT, but this hypothesis requires further validation in prospective randomized clinical trials.
SYSTEMIC THERAPY
Compared to other cancer types, the progress in the development of systemic therapy is slower for HCC. Conventional cytotoxic chemotherapy, such as doxorubicin or doxorubicin-based combinations, could lead to significant toxicity, which limits the potential benefits in cirrhotic populations[68]. Other more “novel” regimens of systemic chemotherapy have also been evaluated. In particular, the combination of oxaliplatin and 5-fluorouracil and leucovorin, known as the FOLFOX4 regimen, has been compared to doxorubicin in patients with advanced HCC in an Asian population with HCC[69]. The overall study fails to demonstrate a statistically significant difference between the two regimens, but FOLFOX4 was found to have small survival benefits in the subgroup population of Chinese patients[70,71]. There have been a handful of case reports or series showing that cytotoxic chemotherapy could improve the severity of PVTT, but there are no dedicated prospective studies to validate such efficacy of systemic chemotherapy in the treatment of PVTT. Further, the toxicity of systemic chemotherapy has been shown to be higher in the presence of impaired liver function and portal hypertension, which frequently occur in patients with PVTT.
Sorafenib is a multi-targeted small molecule with specific activity against vascular endothelial growth factor receptor. Its use in the setting of BCLC stage C HCC, including patients with PVTT, extra-hepatic metastases, and ECOG performance status of 1 or higher, is known to modestly prolong median overall survival by approximately 2 mo, compared to a placebo[72,73]. In both Phase III clinical trials, namely the SHARP[72] and the Asian pacific study[73], there are no detailed analyses on the efficacy of sorafenib according to the different severities of PVTT. Nevertheless, the efficacy of sorafenib in PVTT may still be indirectly reflected by the subgroup analyses in patients with macrovascular invasion. In both studies, the proportion of macrovascular invasion in the sorafenib arm was 36%, and subgroup analyses unanimously indicated similar survival benefits between patients with and without macrovascular invasion[72,73]. Regarding the specific treatment for patients with Vp3 and Vp4 PVTT, a retrospective review was published by a Korean center, which analyzed the outcome of sorafenib in 6 patients with Vp3 and 24 patients with Vp4 PVTT[74]. It was shown that 10% (3 patients) had a partial response in the PVTT arm with revascularization, and the median overall survival was 3.1 mo. The above dataset suggested that sorafenib demonstrated a modest efficacy in the treatment of PVTT, but given the low response rate of likely lower than 10%, the treatment is mainly reserved as a palliative treatment for patients with Vp3 or Vp4 who are not amenable to more aggressive treatment.
Recently, the direction of the development of systemic treatment for HCC has shifted from targets along signaling pathways to immunotherapy and, in particular, to the check-point inhibitors. There have been one phase I clinical trial of a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, tremelimumab, and another ongoing trial on a programmed cell death protein-1 (PD-1) inhibitor, nivolumab, in patients with advanced HCC[75,76]. Both studies have suggested that check-point inhibitors are tolerable and potentially efficacious in HCC. In particular, some remarkable and durable radiological responses have been observed. Given the more mature and robust data of the immune check-point inhibitor in the treatment of HCC, its use in the treatment of PVTT can be better elucidated. Another direction is biomarker-driven drug development, which aims to improve the response via patient selection with predictive biomarkers in the tissue or plasma[77].
PORTAL VEIN STENTING
Theoretically, the placement of an endovascular stent into the portal vein could increase the portal blood flow into the liver parenchyma in HCC complicated with PVTT. This may, in turn, relieve the portal hypertension-related complications, especially variceal bleeding, and expand treatment options for the tumor. Most of the studies on portal vein stenting in the treatment of PVTT of HCC are case reports or small-scale series. Two large-scale case series suggest that portal vein stenting is feasible in select patients. One series come from a Japanese group that reported the percutaneous placement of stents in the portal vein following the administration of TACE or trans-arterial infusion of chemotherapy in 21 patients[78]. With a 100% success rate of placement, it was demonstrated that the portal pressure decreased by approximately 3 mmHg. A short course of an anticoagulant was administered in 14 patients, but it was not suitable for the others due to coagulopathy. The patency rate was 53.6% at 1 year with a mean patency period of 12.4 mo. Procedure-related complications appeared to be uncommon, with only a case of pseudoaneurysm reported. Another large case series from a French group reviewed the records of 54 patients with PVTT who underwent portal stenting under general anesthesia by a percutaneous approach or an open approach via catheterization of the superior mesenteric vein after exteriorization of the terminal ileum[79]. Twenty percent of patients developed complications after portal stenting, mostly in the form of hepatic failure, and 7% died within 30 d of stenting. Bilirubin levels > 30 μmol/L and an open surgical approach were predictors of short-term mortality. TACE was administered in 48% of patients after stenting, and the 12-mo survival rate was 44%. Both of these series indicate that the placement of portal stents is a feasible procedure with potential efficacy. However, because of the concern of bleeding complications during the anticoagulation period and the potential risk of tumor dissemination during the stenting procedures, in addition to the complicated procedures involved, portal vein stenting has not gained wide acceptance among HCC experts.
FUTURE PERSPECTIVES
The need for high-level evidence
Conventionally, HCC with PVTT is considered to be an advanced disease with clinical management similar to HCC with extra-hepatic metastases. There are no dedicated clinical trials to study the treatment of this population. As a result, the efficacy and safety of different treatment modalities on PVTT are mainly generated from retrospective studies or extrapolated from subgroup analyses of prospective clinical trials. Currently, it is evident that the prognoses and disease behavior of patients with PVTT differ from those of patients without PVTT. In addition, patients with PVTT are more prone to develop complications due to portal hypertension or hepatic failure. Therefore, to define a better treatment strategy for patients with HCC with PVTT, dedicated clinical trials in this population are warranted. Because the prognoses differ between patients with different severities and degrees of PVTT, it is important to accurately gauge the outcome of patients during the design of clinical trials for HCC with PVTT. To achieve this goal, a uniform classification of PVTT is required for the stratification of risk groups during randomization and to facilitate the comparison of results between different studies. At present, at least three classifications have been developed for PVTT in HCC. It is crucial to reach consensus on the classification of PVTT amongst the HCC experts.
Multi-disciplinary approach
Management of HCC with PVTT is a clinical dilemma with challenges. On one hand, despite the recommendation of sorafenib as the standard treatment for HCC with PVTT by the BCLC guidelines, emerging evidence clearly shows that select patients could benefit from more aggressive treatment approaches. On the other hand, not all patients may uniformly benefit from aggressive treatment. PVTT represents an adverse prognostic factor with an underlying more rapid disease course. Aggressive treatment may not lead to better outcomes in some patients; for example, in the setting of poor performance or with impaired hepatic function. Therefore, when facing patients with HCC with PVTT, clinicians have to balance the potential benefits and toxicity of different treatments in the individual patients. A multi-disciplinary tumor board is required to determine the most appropriate management of patients with PVTT. Furthermore, the cumulative evidence indicates that a single treatment modality is unlikely to achieve a remarkable effect in PVTT, and the combination of different treatment modalities, such as RT to PVTT and TACE or the addition of SIRT to systemic agents, may be more efficacious in select patients. Future research should focus on combinations of different established treatment modalities for PVTT in HCC.
CONCLUSION
The management of HCC with PVTT is evolving. The treatment modality for HCC with PVTT includes surgical resection, TACE, radiation therapy including external RT or SIRT of the liver lesions, and systemic agents, while portal vein stent treatment remains investigational. Dedicated clinical studies on HCC complicated with PVTT are inadequate. The decision of the optimal treatment for individual patients requires multi-disciplinary input. Future research should be geared towards the generation of high-level evidence of novel treatments and combinations of established treatments for this population.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Stephen L Chan has acted as an advisor to Novartis and MSD.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 1, 2016
First decision: May 30, 2016
Article in press: August 1, 2016
P- Reviewer: Cardinale V, Tchilikidi KY, Wei W, Zhang Q S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quirk M, Kim YH, Saab S, Lee EW. Management of hepatocellular carcinoma with portal vein thrombosis. World J Gastroenterol. 2015;21:3462–3471. doi: 10.3748/wjg.v21.i12.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirisi M, Avellini C, Fabris C, Scott C, Bardus P, Soardo G, Beltrami CA, Bartoli E. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol. 1998;124:397–400. doi: 10.1007/s004320050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan SL, Mo FK, Wong CS, Chan CM, Leung LK, Hui EP, Ma BB, Chan AT, Mok TS, Yeo W. A study of circulating interleukin 10 in prognostication of unresectable hepatocellular carcinoma. Cancer. 2012;118:3984–3992. doi: 10.1002/cncr.26726. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 6.Chan SL, Mo FK, Johnson PJ, Liem GS, Chan TC, Poon MC, Ma BB, Leung TW, Lai PB, Chan AT, et al. Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol. 2011;26:340–347. doi: 10.1111/j.1440-1746.2010.06329.x. [DOI] [PubMed] [Google Scholar]
- 7.Connolly GC, Chen R, Hyrien O, Mantry P, Bozorgzadeh A, Abt P, Khorana AA. Incidence, risk factors and consequences of portal vein and systemic thromboses in hepatocellular carcinoma. Thromb Res. 2008;122:299–306. doi: 10.1016/j.thromres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Deng Q, Wang Q, Li KY, Dai JH, Li N, Zhu ZD, Zhou B, Liu XY, Liu RF, et al. Exome sequencing of hepatitis B virus-associated hepatocellular carcinoma. Nat Genet. 2012;44:1117–1121. doi: 10.1038/ng.2391. [DOI] [PubMed] [Google Scholar]
- 9.Tarantino L, Francica G, Sordelli I, Esposito F, Giorgio A, Sorrentino P, de Stefano G, Di Sarno A, Ferraioli G, Sperlongano P. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging. 2006;31:537–544. doi: 10.1007/s00261-005-0150-x. [DOI] [PubMed] [Google Scholar]
- 10.Piscaglia F, Gianstefani A, Ravaioli M, Golfieri R, Cappelli A, Giampalma E, Sagrini E, Imbriaco G, Pinna AD, Bolondi L. Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation. Liver Transpl. 2010;16:658–667. doi: 10.1002/lt.22044. [DOI] [PubMed] [Google Scholar]
- 11.Rammohan A, Jeswanth S, Sukumar R, Anand L, Kumar PS, Srinivasan UP, Ravi R, Ravichandran P. Percutaneous ultrasound-guided fine-needle aspiration of portal vein thrombi as a diagnostic and staging technique for hepatocellular carcinoma. Abdom Imaging. 2013;38:1057–1060. doi: 10.1007/s00261-013-9997-4. [DOI] [PubMed] [Google Scholar]
- 12.Rossi S, Ghittoni G, Ravetta V, Torello Viera F, Rosa L, Serassi M, Scabini M, Vercelli A, Tinelli C, Dal Bello B, et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepatocellular carcinoma. Eur Radiol. 2008;18:1749–1756. doi: 10.1007/s00330-008-0931-z. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Lee JM, Yoon JH, Lee DH, Lee KB, Han JK, Choi BI. Portal Vein Thrombosis in Patients with Hepatocellular Carcinoma: Diagnostic Accuracy of Gadoxetic Acid-enhanced MR Imaging. Radiology. 2016;279:773–783. doi: 10.1148/radiol.2015150124. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 15.Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33:765–770. doi: 10.1159/000439101. [DOI] [PubMed] [Google Scholar]
- 16.Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines) Hepatol Res. 2015:45. doi: 10.1111/hepr.12464. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18:74–80. doi: 10.1007/s00534-010-0314-0. [DOI] [PubMed] [Google Scholar]
- 18.Xu JF, Liu XY, Wang S, Wen HX. Surgical treatment for hepatocellular carcinoma with portal vein tumor thrombus: a novel classification. World J Surg Oncol. 2015;13:86. doi: 10.1186/s12957-015-0493-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumada K, Ozawa K, Okamoto R, Takayasu T, Yamaguchi M, Yamamoto Y, Higashiyama H, Morikawa S, Sasaki H, Shimahara Y. Hepatic resection for advanced hepatocellular carcinoma with removal of portal vein tumor thrombi. Surgery. 1990;108:821–827. [PubMed] [Google Scholar]
- 20.Tanaka A, Morimoto T, Yamaoka Y. Implications of surgical treatment for advanced hepatocellular carcinoma with tumor thrombi in the portal vein. Hepatogastroenterology. 1996;43:637–643. [PubMed] [Google Scholar]
- 21.Chok KS, Cheung TT, Chan SC, Poon RT, Fan ST, Lo CM. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg. 2014;38:490–496. doi: 10.1007/s00268-013-2290-4. [DOI] [PubMed] [Google Scholar]
- 22.Wu CC, Hsieh SR, Chen JT, Ho WL, Lin MC, Yeh DC, Liu TJ, P’eng FK. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg. 2000;135:1273–1279. doi: 10.1001/archsurg.135.11.1273. [DOI] [PubMed] [Google Scholar]
- 23.Ban D, Shimada K, Yamamoto Y, Nara S, Esaki M, Sakamoto Y, Kosuge T. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg. 2009;13:1921–1928. doi: 10.1007/s11605-009-0998-0. [DOI] [PubMed] [Google Scholar]
- 24.Chan SC, Liu CL, Lo CM, Lam CM, Poon RT, Yuen WK, Fan ST, Wong J. Value of live donor liver transplantation experience in major hepatectomy for hepatocellular carcinoma. Arch Surg. 2003;138:265–271. doi: 10.1001/archsurg.138.3.265. [DOI] [PubMed] [Google Scholar]
- 25.Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118:4725–4736. doi: 10.1002/cncr.26561. [DOI] [PubMed] [Google Scholar]
- 26.Ohkubo T, Yamamoto J, Sugawara Y, Shimada K, Yamasaki S, Makuuchi M, Kosuge T. Surgical results for hepatocellular carcinoma with macroscopic portal vein tumor thrombosis. J Am Coll Surg. 2000;191:657–660. doi: 10.1016/s1072-7515(00)00740-7. [DOI] [PubMed] [Google Scholar]
- 27.Konishi M, Ryu M, Kinoshita T, Inoue K. Surgical treatment of hepatocellular carcinoma with direct removal of the tumor thrombus in the main portal vein. Hepatogastroenterology. 2001;48:1421–1424. [PubMed] [Google Scholar]
- 28.Pawlik TM, Poon RT, Abdalla EK, Ikai I, Nagorney DM, Belghiti J, Kianmanesh R, Ng IO, Curley SA, Yamaoka Y, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005;137:403–410. doi: 10.1016/j.surg.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Ikai I, Hatano E, Hasegawa S, Fujii H, Taura K, Uyama N, Shimahara Y. Prognostic index for patients with hepatocellular carcinoma combined with tumor thrombosis in the major portal vein. J Am Coll Surg. 2006;202:431–438. doi: 10.1016/j.jamcollsurg.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Le Treut YP, Hardwigsen J, Ananian P, Saïsse J, Grégoire E, Richa H, Campan P. Resection of hepatocellular carcinoma with tumor thrombus in the major vasculature. A European case-control series. J Gastrointest Surg. 2006;10:855–862. doi: 10.1016/j.gassur.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Liu CL, Fan ST. Nonresectional therapies for hepatocellular carcinoma. Am J Surg. 1997;173:358–365. doi: 10.1016/S0002-9610(96)00384-4. [DOI] [PubMed] [Google Scholar]
- 32.Ngan H, Lai CL, Fan ST, Lai EC, Yuen WK, Tso WK. Treatment of inoperable hepatocellular carcinoma by transcatheter arterial chemoembolization using an emulsion of cisplatin in iodized oil and gelfoam. Clin Radiol. 1993;47:315–320. doi: 10.1016/s0009-9260(05)81446-1. [DOI] [PubMed] [Google Scholar]
- 33.O’Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325–331. doi: 10.1002/bjs.4045. [DOI] [PubMed] [Google Scholar]
- 34.Yu SC, Hui JW, Hui EP, Chan SL, Lee KF, Mo F, Wong J, Ma B, Lai P, Mok T, et al. Unresectable hepatocellular carcinoma: randomized controlled trial of transarterial ethanol ablation versus transcatheter arterial chemoembolization. Radiology. 2014;270:607–620. doi: 10.1148/radiol.13130498. [DOI] [PubMed] [Google Scholar]
- 35.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 36.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 38.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397–401. doi: 10.1148/radiology.148.2.6306721. [DOI] [PubMed] [Google Scholar]
- 40.Leng JJ, Xu YZ, Dong JH. Efficacy of transarterial chemoembolization for hepatocellular carcinoma with portal vein thrombosis: a meta-analysis. ANZ J Surg. 2014 doi: 10.1111/ans.12803. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. doi: 10.1186/1471-230X-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87:22–32. doi: 10.1016/j.ijrobp.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 43.Dawson LA. Overview: Where does radiation therapy fit in the spectrum of liver cancer local-regional therapies? Semin Radiat Oncol. 2011;21:241–246. doi: 10.1016/j.semradonc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, Chung YH, Lee YS, Lee SG, Park JH, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XB, Wang JH, Yan ZP, Qian S, Du SS, Zeng ZC. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115:1245–1252. doi: 10.1002/cncr.24139. [DOI] [PubMed] [Google Scholar]
- 46.Katamura Y, Aikata H, Takaki S, Azakami T, Kawaoka T, Waki K, Hiramatsu A, Kawakami Y, Takahashi S, Kenjo M, et al. Intra-arterial 5-fluorouracil/interferon combination therapy for advanced hepatocellular carcinoma with or without three-dimensional conformal radiotherapy for portal vein tumor thrombosis. J Gastroenterol. 2009;44:492–502. doi: 10.1007/s00535-009-0033-y. [DOI] [PubMed] [Google Scholar]
- 47.Toya R, Murakami R, Baba Y, Nishimura R, Morishita S, Ikeda O, Kawanaka K, Beppu T, Sugiyama S, Sakamoto T, et al. Conformal radiation therapy for portal vein tumor thrombosis of hepatocellular carcinoma. Radiother Oncol. 2007;84:266–271. doi: 10.1016/j.radonc.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 49.Kim TH, Kim DY, Park JW, Kim YI, Kim SH, Park HS, Lee WJ, Park SJ, Hong EK, Kim CM. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Am J Clin Oncol. 2006;29:568–575. doi: 10.1097/01.coc.0000239147.60196.11. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Kim DH, Ki YK, Nam JH, Heo J, Woo HY, Kim DW, Kim WT. Three-dimensional conformal radiotherapy for portal vein tumor thrombosis alone in advanced hepatocellular carcinoma. Radiat Oncol J. 2014;32:170–178. doi: 10.3857/roj.2014.32.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA, Kim CY. Effectiveness of high-dose three-dimensional conformal radiotherapy in hepatocellular carcinoma with portal vein thrombosis. Jpn J Clin Oncol. 2012;42:721–729. doi: 10.1093/jjco/hys082. [DOI] [PubMed] [Google Scholar]
- 52.Chuma M, Taguchi H, Yamamoto Y, Shimizu S, Nakanishi M, Ogawa K, Sho T, Horimoto H, Kobayashi T, Nakai M, et al. Efficacy of therapy for advanced hepatocellular carcinoma: intra-arterial 5-fluorouracil and subcutaneous interferon with image-guided radiation. J Gastroenterol Hepatol. 2011;26:1123–1132. doi: 10.1111/j.1440-1746.2011.06745.x. [DOI] [PubMed] [Google Scholar]
- 53.Huang YJ, Hsu HC, Wang CY, Wang CJ, Chen HC, Huang EY, Fang FM, Lu SN. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–1163. doi: 10.1016/j.ijrobp.2008.06.1486. [DOI] [PubMed] [Google Scholar]
- 54.Yamada K, Izaki K, Sugimoto K, Mayahara H, Morita Y, Yoden E, Matsumoto S, Soejima T, Sugimura K. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 55.Ishikura S, Ogino T, Furuse J, Satake M, Baba S, Kawashima M, Nihei K, Ito Y, Maru Y, Ikeda H. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189–193. doi: 10.1097/00000421-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 56.Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, Miyasaka Y, Nagayama K, Enomoto N, Sato C. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660–665. doi: 10.1046/j.1440-1746.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- 57.Yu JI, Park HC, Lim DH, Park W, Yoo BC, Paik SW, Koh KC, Lee JH. Prognostic index for portal vein tumor thrombosis in patients with hepatocellular carcinoma treated with radiation therapy. J Korean Med Sci. 2011;26:1014–1022. doi: 10.3346/jkms.2011.26.8.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato K, Lewandowski RJ, Bui JT, Omary R, Hunter RD, Kulik L, Mulcahy M, Liu D, Chrisman H, Resnick S, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006;29:522–529. doi: 10.1007/s00270-005-0171-4. [DOI] [PubMed] [Google Scholar]
- 59.Coldwell D, Sangro B, Wasan H, Salem R, Kennedy A. General selection criteria of patients for radioembolization of liver tumors: an international working group report. Am J Clin Oncol. 2011;34:337–341. doi: 10.1097/COC.0b013e3181ec61bb. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, Benson A, Espat J, Bilbao JI, Sharma RA, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 61.D’Avola D, Lñarrairaegui M, Bilbao JI, Martinez-Cuesta A, Alegre F, Herrero JI, Quiroga J, Prieto J, Sangro B. A retrospective comparative analysis of the effect of Y90-radioembolization on the survival of patients with unresectable hepatocellular carcinoma. Hepatogastroenterology. 2009;56:1683–1688. [PubMed] [Google Scholar]
- 62.Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 63.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 65.Ozkan ZG, Poyanli A, Ucar A, Kuyumcu S, Akyuz F, Keskin S, Saglam S, Yilmaz E, Karaca C, Turkmen C. Favorable survival time provided with radioembolization in hepatocellular carcinoma patients with and without portal vein thrombosis. Cancer Biother Radiopharm. 2015;30:132–138. doi: 10.1089/cbr.2014.1748. [DOI] [PubMed] [Google Scholar]
- 66.Edeline J, Crouzet L, Campillo-Gimenez B, Rolland Y, Pracht M, Guillygomarc’h A, Boudjema K, Lenoir L, Adhoute X, Rohou T, et al. Selective internal radiation therapy compared with sorafenib for hepatocellular carcinoma with portal vein thrombosis. Eur J Nucl Med Mol Imaging. 2016;43:635–643. doi: 10.1007/s00259-015-3210-7. [DOI] [PubMed] [Google Scholar]
- 67.She WH, Cheung TT, Yau TC, Chan AC, Chok KS, Chu FS, Liu RK, Poon RT, Chan SC, Fan ST, et al. Survival analysis of transarterial radioembolization with yttrium-90 for hepatocellular carcinoma patients with HBV infection. Hepatobiliary Surg Nutr. 2014;3:185–193. doi: 10.3978/j.issn.2304-3881.2014.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan SL, Yeo W. Targeted therapy of hepatocellular carcinoma: present and future. J Gastroenterol Hepatol. 2012;27:862–872. doi: 10.1111/j.1440-1746.2012.07096.x. [DOI] [PubMed] [Google Scholar]
- 69.Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501–3508. doi: 10.1200/JCO.2012.44.5643. [DOI] [PubMed] [Google Scholar]
- 70.Qin S, Cheng Y, Liang J, Shen L, Bai Y, Li J, Fan J, Liang L, Zhang Y, Wu G, et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma: a subgroup analysis of the EACH study. Oncologist. 2014;19:1169–1178. doi: 10.1634/theoncologist.2014-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan SL. Drug development for hepatocellular carcinoma: knowing the past helps to understand the future. Oncologist. 2014;19:1115–1117. doi: 10.1634/theoncologist.2014-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 73.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 74.Jeong SW, Jang JY, Shim KY, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, et al. Practical effect of sorafenib monotherapy on advanced hepatocellular carcinoma and portal vein tumor thrombosis. Gut Liver. 2013;7:696–703. doi: 10.5009/gnl.2013.7.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 76.El-Khoueiry AB, Melero I, Crocenzi TS, Welling TH, Yau TC, Yeo W, Chopra A, Grosso J, Lang L, Anderson J, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol. 2015;33 Suppl:abstr LBA101. [Google Scholar]
- 77.Chan SL, Wong AM, Lee K, Wong N, Chan AK. Personalized therapy for hepatocellular carcinoma: Where are we now? Cancer Treat Rev. 2016;45:77–86. doi: 10.1016/j.ctrv.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 78.Yamakado K, Tanaka N, Nakatsuka A, Matsumura K, Takase K, Takeda K. Clinical efficacy of portal vein stent placement in patients with hepatocellular carcinoma invading the main portal vein. J Hepatol. 1999;30:660–668. doi: 10.1016/s0168-8278(99)80197-4. [DOI] [PubMed] [Google Scholar]
- 79.Vibert E, Azoulay D, Cunha AS, Adam R, Samuel D, Castaing D. Portal stenting for hepatocellular carcinoma extending into the portal vein in cirrhotic patients. J Surg Oncol. 2013;107:696–701. doi: 10.1002/jso.23306. [DOI] [PubMed] [Google Scholar]
- 80.Ikai I, Kudo M, Arii S, Omata M, Kojiro M, Sakamoto M, Takayasu K, Hayashi N, Makuuchi M, Matsuyama Y, et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res. 2010;40:1043–1059. doi: 10.1111/j.1872-034X.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 81.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 82.Niu ZJ, Ma YL, Kang P, Ou SQ, Meng ZB, Li ZK, Qi F, Zhao C. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012;29:2992–2997. doi: 10.1007/s12032-011-0145-0. [DOI] [PubMed] [Google Scholar]
- 83.Kim JH, Yoon HK, Kim SY, Kim KM, Ko GY, Gwon DI, Sung KB. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29:1291–1298. doi: 10.1111/j.1365-2036.2009.04016.x. [DOI] [PubMed] [Google Scholar]


