Abstract
Purpose/Objectives
Re-irradiation (re-RT) is the only potentially curative treatment option for patients with locally recurrent head and neck cancer (HNC). Given the significant morbidity with head and neck re-irradiation, interest in proton beam radiotherapy (PBRT) has increased. Herein, we report the first multi-institutional clinical experience using curative intent PBRT for re-RT in recurrent HNC.
Materials/Methods
A retrospective analysis of ongoing prospective data registries from 2-hybrid community practice and academic proton centers was conducted. Patients with recurrent HNC who had at least one prior course of definitive intent external beam RT were included. Acute and late toxicities were assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 and by the Radiation Therapy Oncology Group late radiation morbidity scoring system, respectively. The cumulative incidence of locoregional failure was calculated with death as a competing risk. The actuarial twelve-month freedom from distant metastasis (FFDM) and overall survival (OS) rates were calculated with the Kaplan-Meier method.
Results
Ninety-two consecutive patients were treated with curative intent re-RT with PBRT between 2011 and 2014. Median follow-up among surviving patients was 13.3 months and among all patients was 10.4 months (interquartile range, 5.3-17.5 months). The median time between last RT and PBRT was 34.4 months. There were 76 patients with one prior RT course and 16 with two or more courses. Median PBRT dose was 60.6 Gy (RBE). Eighty-five percent of patients had prior HNC RT for an oropharynx primary and 39% had salvage surgery prior to re-RT. The cumulative incidence of locoregional failure at 12-months, with death as a competing risk, was 25.1%. Actuarial 12-month FFDM and OS were 84.0% and 65.2%, respectively.
Acute grade ≥3 toxicities included mucositis (9.9%), dysphagia (9.1%), esophagitis (9.1%), and dermatitis (3.3%). There was one death during PBRT secondary to disease progression. Grade 3 or greater late skin and dysphagia toxicity were noted in 6 (8.7%) and 4 (7.1%) of patients, respectively. Two patients had grade 5 toxicity secondary to treatment-related bleeding.
Conclusions
Proton beam re-irradiation of the head and neck can provide effective tumor control with acceptable acute and late toxicity profiles likely secondary to the decreased dose to the surrounding normal, albeit previously irradiated tissue, though longer follow up is needed to confirm these findings.
Keywords: proton, head and neck cancer, re-irradiation, recurrent
INTRODUCTION
The treatment of head and neck cancer (HNC) requires a multimodality approach [1]. The majority of HNC patients will receive radiation therapy (RT) as part of their initial therapy, including the roughly 70% of patients with locally advanced disease [2,3]. Despite noteworthy advances in the treatment of HNC, a significant portion of patients will develop locoregional recurrences, and some will survive long enough to develop a metachronous malignancy [4,5]. The management of recurrent disease in patients with prior RT to the head and neck remains challenging [5,6].
Despite significant advances in the initial treatment of HNC, outcomes in recurrent HNC have remained largely unchanged [7]. Surgical resection or re-irradiation (re-RT) are two potentially curative options. When feasible, surgical resection is preferred and postoperative re-irradiation is often indicated if high-risk features are present. With recurrent disease, the best reported outcomes in non-surgical patients have been reported with chemoradiation therapy, but many patients are not offered re-RT due to concerns of serious toxicity with a second course of radiation and instead are treated with palliative chemotherapy alone.
In randomized trials such as RTOG 9911, the high rates of Grade 4 or greater acute and late toxicities that resulted when using conventional re-RT techniques were of significant concern [8]. Compared to 3D conformal RT (3D-CRT), IMRT demonstrated improved rates of two-year locoregional control (LRC) and freedom from locoregional progression and lower rates of treatment associated toxicity suggesting that the therapeutic index can be improved with more conformal radiation techniques [9-11].
Given the unique physical properties of proton beam radiation therapy (PBRT), higher doses can be delivered to the tumor, while minimizing the dose to the previously irradiated normal tissues [5]. The unique properties of PBRT can be explained by a comprehensive understanding of energy deposition upon entrance in the body. Protons enter with low energy deposition until a rapid increase in energy deposition is achieved within the ‘Bragg Peak’. This allows for both sparing of normal tissue, and intensified tumor doses. The capability of PBRT to maximize a focused dose of radiation to the tumor is complemented by the minimal exit dose delivered to surrounding non-target tissues [12]. Due to the dosimetric advantages of PBRT, there are an increasing number of centers being built both within the United States and worldwide. While a commonly cited indication for PBRT is recurrent HNC, to-date there has been minimal evidence supporting the use of PBRT in the setting of re-RT in the head and neck [13]. Here we present the first multi-institutional and largest clinical report on the use of PBRT in curative intent re-irradiation for recurrent HNC.
MATERIALS/METHODS
Patients and Pre-Treatment Workup
This retrospective analysis of a prospectively managed multi-institutional proton database (NCT01255748) was approved by either the institutional review board with a waiver of informed consent and/or by direct patient consent at time of PBRT registration. Ninety-eight consecutive patients with recurrent HNC treated with curative intent PBRT between February 2011 and September 2014 with a history of at least one prior course of definitive intent head and neck external beam RT (EBRT) were identified. Six patients were excluded from the study cohort including 2 patients treated in the re-RT setting with a combination of PBRT and brachytherapy and 3 patients who did not consent to inclusion on a prospective registry database. Patients were treated at two referral based hybrid community and academic practice proton centers by 19 independent radiation oncologists. While patients were referred from multiple institutions, 31 patients (33.7%) were referred from a single institution. Further review of all patients treated with definitive re-irradiation at this single institution, the largest referral base, identified only 3 patients not treated with proton re-irradiation but rather IMRT photon based re-irradiation due to logistical issues and not physician preference.
Before PBRT, patients were evaluated by a multidisciplinary team, which usually included a medical oncologist, head and neck surgeon, dentist, speech and swallow pathologist, and radiation oncologist. All patients underwent a history and physical with a focused head and neck examination including a fiberoptic nasopharyngoscopy when appropriate, complete blood count and basic metabolic panel, and imaging (computed tomography (CT), magnetic resonance imaging (MRI), and/or positron emission tomography (PET)). Chemotherapy was delivered at the discretion of the medical oncologist, which prompted additional pre-treatment tests, which could include liver function tests, audiogram, and/or an electrocardiogram.
Radiation Therapy
Patients were simulated in the supine position with individualized head and neck immobilization masks in a base of skull frame. A CT scan with ≤3mm slice thickness was acquired through the target region. The gross tumor volume (GTV) consisted of the gross tumor based on clinical examination, CT, PET and/or MRI findings. Primary fusion to the simulation CT was preferred when feasible. For surgical cases, the entire postoperative bed was delineated as the clinical target volume (CTV). Surgical margins were considered close when they were within 5 mm, or within 1 mm for the larynx or tonsil. A 3mm margin was typically added for the planning target volume (PTV) to account for setup error and motion. As many of the patients’ previous RT plans were unavailable digitally, it was often assumed that the brainstem, spinal cord, and optic structures were treated to tolerance. While no specific dose constraints were used an additional 10-20Gy (RBE) were allotted for the brainstem, spinal cord, and optic structures, depending in part on the interval since prior RT.
Proton therapy was delivered with uniform scanning beams and planned in CMS XIO (Elekta, Stockholm Sweden) at both regional proton centers. The beam arrangement varied depending on target volume geometry, and dose limits to neighboring organs at risk, such as those with prior radiation exposure. Three-field plans were typically used (2–4 beams). For some cases, matched and/or patched fields were used. Physical and biological uncertainties were evaluated and taken into account, and worst- and best-case scenarios with the range uncertainties (2.5% * range + 2mm) were evaluated. Special care was taken to avoid ranging out into critical structures through selection of beam angles and range feathering if necessary. Dental artifacts were overridden, and as much as possible, attempts were made to avoid beams traversing through dental hardware. A RBE of 1.1 and a smear of 3–5mm was used for most cases. Setup accuracy was confirmed with daily x-ray orthogonal verification of the isocenter based on bony anatomy. A verification CT scan was typically performed at least once in the course of treatment to assess for changes in anatomy, most often in the first week of treatment.
Acute and Late Toxicity Management
During treatment, patients were evaluated weekly by the radiation oncologist of record. After completing treatment, patients were evaluated every 2-3 months for the first two years, and every 4-6 months thereafter by the multidisciplinary head and neck team, which includes a medical, surgical, and radiation oncologist. Between 2-4 months following treatment, patients would receive routine surveillance imaging with PET, MRI, or CT, and as clinically indicated afterward.
Acute and late toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0) and the Radiation Therapy Oncology Group (RTOG) late radiation morbidity scoring system, respectively. Late toxicity was assessed beginning at 90 days after completion of PBRT.
Statistical Methods
The cumulative incidence of locoregional control (LRC) was calculated with death as a competing risk. Freedom from distant metastasis (FDM) and overall survival (OS) actuarial rates were calculated by the Kaplan Meier method [14]. Time to event was determined from start of PBRT. All analyses were performed in SPSS statistics version 21 (IBM, Armonk, NY; USA).
RESULTS
Patient and tumor characteristics
Ninety-two evaluable patients were included in the final proton beam re-RT cohort. The median follow-up was 13.3 months (interquartile range, 8.2-19.2 months) among surviving patients and 10.4 months (interquartile range, 5.3-17.5 months) for all patients. There were 65 males (70.7%) and 27 females (29.3%), with a median patient age of 63.0 years (interquartile range, 51.5– 70.0 years). The majority of patients (70.7%, n = 70) had a KPS of ≥80 or greater at the time of treatment. Complete patient and tumor characteristics are summarized in Table 1.
Table 1.
Patient and Tumor Characteristics
| All | SCC | non-SCC | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Gender | |||
| Male | 65 (70.7%) | 38 (73.1%) | 27 (67.5%) |
| Female | 27 (29.3%) | 14 (26.9%) | 13 (32.5%) |
| KPS | |||
| ≥80 | 65 (70.7%) | 41 (78.8%) | 24 (60.0%) |
| <80 | 18 (19.6%) | 7 (13.5%) | 11 (27.5%) |
| Not recorded | 9 (9.8%) | 4 (7.7%) | 5 (12.5%) |
| Initial disease site | |||
| Oropharynx | 17 (18.5%) | 17 (32.7%) | 0 (0.0%) |
| Nasal cavity/paranasal sinuses | 12 (13.0%) | 4 (7.7%) | 8 (20.0%) |
| Oral cavity | 12 (13.0%) | 9 (17.3%) | 3 (7.5%) |
| Salivary glands | 11 (12.0%) | 0 (0.0%) | 11 (27.5%) |
| Larynx/ hypopharynx | 10 (10.9%) | 9 (17.3%) | 1 (2.5%) |
| Nasopharynx | 9 (9.8%) | 5 (9.6%) | 4 (10.0%) |
| Other | 21 (22.8%) | 8 (15.4%) | 13 (32.5%) |
| New primary | |||
| Yes | 12 (13.0%) | 7 (13.5%) | 5 (9.6%) |
| No (Recurrence) | 80 (87.0%) | 45 (86.5%) | 35 (67.3%) |
| Histology | |||
| SCC | 52 (56.5%) | 52 (100.0%) | 0 (0.0%) |
| Adenocarcinoma | 9 (9.8%) | 0 (0.0%) | 9 (22.5%) |
| Nasopharyngeal carcinoma | 4 (4.3%) | 0 (0.0%) | 4 (10.0%) |
| Sarcoma | 5 (5.4%) | 0 (0.0%) | 5 (12.5%) |
| Adenoid cystic carcinoma | 2 (2.2%) | 0 (0.0%) | 2 (5.0%) |
| Sinonasal undifferentiated carcinoma | 3 (3.3%) | 0 (0.0%) | 3 (7.5%) |
| Acinic cell carcinoma | 2 (2.2%) | 0 (0.0%) | 2 (5.0%) |
| Olfactory neuroblastoma | 2 (2.2%) | 0 (0.0%) | 2 (5.0%) |
| Myoepithelial carcinoma | 2 (2.2%) | 0 (0.0%) | 2 (5.0%) |
| Merkel cell carcinoma | 1 (1.1%) | 0 (0.0%) | 1 (2.5%) |
| Basal cell carcinoma | 1 (1.1%) | 0 (0.0%) | 1 (2.5%) |
| Esthesioneuroblastoma | 2 (2.2%) | 0 (0.0%) | 2 (5.0%) |
| Other | 7 (7.6%) | 0 (0.0%) | 7 (17.5%) |
| Number of prior EBRT | |||
| 1 | 76 (82.6%) | 43 (82.7%) | 33 (82.5%) |
| 2 | 13 (14.1%) | 8 (15.4%) | 5 (12.5%) |
| ≥3 | 3 (3.2%) | 1 (1.9%) | 2 (5.0%) |
| Prior Chemotherapy | |||
| Yes | 45 (48.9%) | 32 (61.5%) | 13 (32.5%) |
| No | 40 (43.5%) | 17 (32.7%) | 23 (57.5%) |
| Unknown | 7 (7.6%) | 3 (5.8%) | 4 (10.0%) |
| Smoking | |||
| ≥10 pack years | 35 (38.0%) | 24 (46.2%) | 11 (27.5%) |
| <10 pack years | 8 (8.7%) | 6 (11.5%) | 2 (5.0%) |
| None | 43 (46.7%) | 17 (32.7%) | 26 (65.0%) |
| Not recorded | 6 (6.5%) | 5 (9.6%) | 1 (2.5%) |
| G-tube placement | |||
| Prior to PBRT | 26 (28.3%) | 20 (38.5%) | 6 (15.0%) |
| Reactive | 6 (6.5%) | 6 (11.5%) | 0 (0.0%) |
| After PBRT | 3 (3.3%) | 2 (3.8%) | 1 (2.5%) |
| None | 57 (62.0%) | 24 (46.2%) | 33 (82.5%) |
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Age (years) | 63.0 (51.5-70.0) | 63.5 (54.8-71.0) | 59.0 (46.8-68.0) |
| Prior radiation dose (Gy RBE) | 61.4 (54.0-69.9) | 66.0 (58.2-70.0) | 58.9 (49.8-65.1) |
| Interval between first RT and re-RT (months) | 54.4 (22.2-126.4) | 50.8 (20.5-97.5) | 57.1 (30.8-159.0) |
| Interval between last RT and re-RT (months) | 34.4 (14.2-92.5) | 24.6 (13.1-83.4) | 54.4 (21.1-121.9) |
The most common initial tumor site was the oropharynx (n = 17, 85.5%), followed by the nasal cavity and paranasal sinuses (n = 12, 13.0%), oral cavity (n = 12, 13.0%), larynx/hypopharynx (n = 10, 10.9%), salivary glands (n = 11, 12.0%), and nasopharynx (n = 9, 9.8%). The remaining disease sites were less common; this heterogeneous group included various skin malignancies (n = 5), skull base tumors (n = 8), and others (n = 8) including the unilateral or bilateral neck, jugular fossa, and external auditory canal.
The most common recurrent histologic subtype treated with PBRT was squamous cell carcinoma (SCC) (n=52; 56.5%). Other less common histologies included adenocarcinoma (n=9; 9.8%), nasopharyngeal carcinoma (n=4, 4.3%), sarcoma (n=5; 5.4%), and others (n=22; 23.9%), Table 1. Most patients (n = 80, 87.0%) received PBRT for recurrent disease, but 12 (13.0%) were treated for biopsy-proven metachronous malignancies.
Previous Treatment Characteristics
There were 76 patients with one prior RT course (82.6%) and 16 with two or more prior courses (17.4%). The median prior RT dose was 61.4 Gy (RBE) (interquartile range, 54.0-69.9 Gy (RBE)). Forty-five patients (48.9%) received chemotherapy as part of their prior treatment. Previous treatment characteristics are summarized in Table 1.
PBRT Re-irradiation Treatment Characteristics
The median time between PBRT and the most recent prior RT treatment was 34.4 months (interquartile range, 14.2–92.5 months). Prior to re-RT, 36 patients (39.1%) had a salvage surgery, with positive margins found in 50.0% (n = 18), close margins (<5 mm) in 156.7% (n=6), negative margins in 13.9% (n = 5), and unavailable margin status in 19.4% (n=7). Median time between salvage surgery and PBRT was 2.5 months (interquartile range, 1.9– 3.3 months). Patients were treated with PBRT to a median dose of 60.6 Gy (RBE) (interquartile range, 50.0– 66.1 Gy (RBE)). Forty-four (47.8%) patients were treated with concurrent chemotherapy at the discretion of the medical oncologist during PBRT, with cetuximab being the most common regimen, followed by cisplatin and carboplatin. RE-irradiation PBRT treatment characteristics are summarized in Table 2.
Table 2.
Proton re-RT Tumor and Treatment Characteristics
| All | SCC | non-SCC | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Re-RT site | |||
| Oropharynx | 14 (15.2%) | 14 (26.9%) | 0 (0.0%) |
| Nasal cavity/paranasal sinuses | 13 (14.1%) | 5 (9.6%) | 8 (20.0%) |
| Oral cavity | 16 (17.4%) | 12 (23.1%) | 4 (10.0%) |
| Salivary glands | 12 (13.0%) | 0 (0.0%) | 12 (30.0%) |
| Larynx/ hypopharynx | 9 (9.8%) | 9 (17.3%) | 0 (0.0%) |
| Nasopharynx | 12 (13.0%) | 8 (15.4%) | 4 (10.0%) |
| Other | 16 (17.4%) | 4 (7.7%) | 12 (30.0%) |
| Chemotherapy | |||
| Neoadjuvant | 4 (4.3%) | 3 (5.8%) | 1 (2.5%) |
| Neoadjuvant and Concurrent | 8 (8.7%) | 6 (11.5%) | 2 (5.0%) |
| Concurrent | 36 (39.1%) | 26 (50.0%) | 10 (25.0%) |
| None | 44 (47.8%) | 17 (32.7%) | 27 (67.5%) |
| Salvage surgery | |||
| Yes | 36 (39.1%) | 22 (42.3%) | 14 (35.0%) |
| No | 56 (60.9%) | 30 (57.7%) | 26 (65.0%) |
| Median (IQR) | Median (IQR) | Median (IQR) | |
| CTV volume (cc) | 78.2 (31.7-162.3) | 90.8 (33.4-188.3) | 69.1 (26.8-138.1) |
| PTV volume (cc) | 135.0 (67.8-280.8) | 158.8 (72.3-294.9) | 106.5 (54.2-193.8) |
| Prescribed total dose (Gy RBE) | 60.6 (50.0-66.1) | 66.0 (53.1-66.2) | 60.1 (50.0-66.0) |
| Prescribed dose/ fraction (Gy RBE) | 2.0 (2.0-2.0) | 2.0 (2.0-2.0) | 2.0 (2.0-2.0) |
| CTV D95 (Gy RBE) | 60.3 (49.3-66.2) | 62.2 (53.3-67.2) | 54.5 (43.1-65.8) |
| PTV D95 (Gy RBE) | 58.9 (44.9-65.3) | 60.1 (50.2-65.7) | 54.3 (36.6-63.0) |
| Number of fields | 3 (3-4) | 4 (3-4) | 3 (3-4) |
| Fields treated per day | 3 (2-3) | 3 (2-3) | 3 (2-3) |
Locoregional control
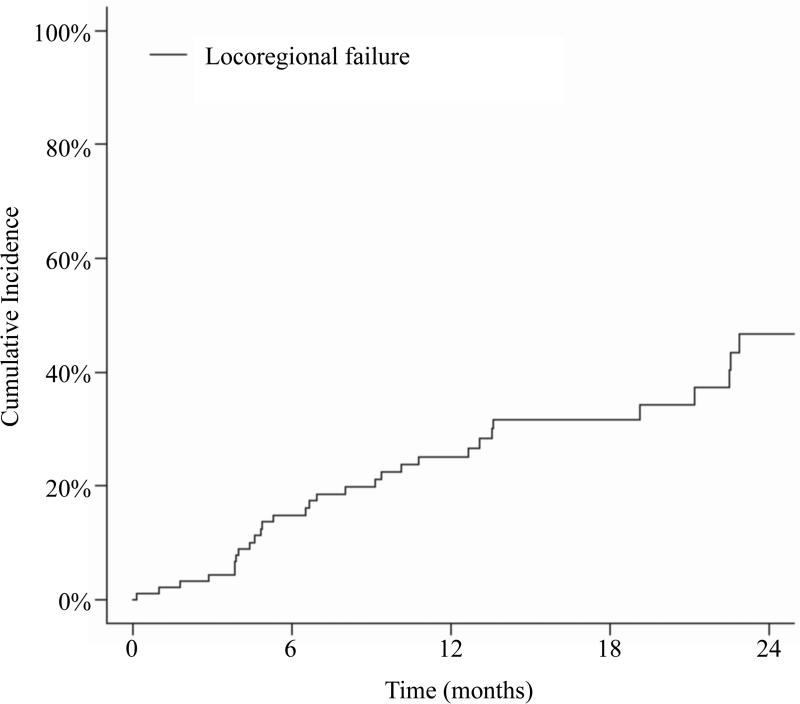
The 12- month cumulative incidence of locoregional failure with death as a competing risk was 25.1%, Figure 1a, with no difference between patients with SCC histology and non-SCC histology (p=0.570). Thirty-one patients developed a locoregional failure (LRF) including 24 in-field, 6 out-of-field, and 1 marginal miss. The median time to LRF was 7.0 months (interquartile range, 4.2–13.3 months). The most common site of local failure was the base of skull (n=8).
Figure 1.
Cumulative incidence of locoregional failure with death as a competing risk.
Freedom from distant metastasis
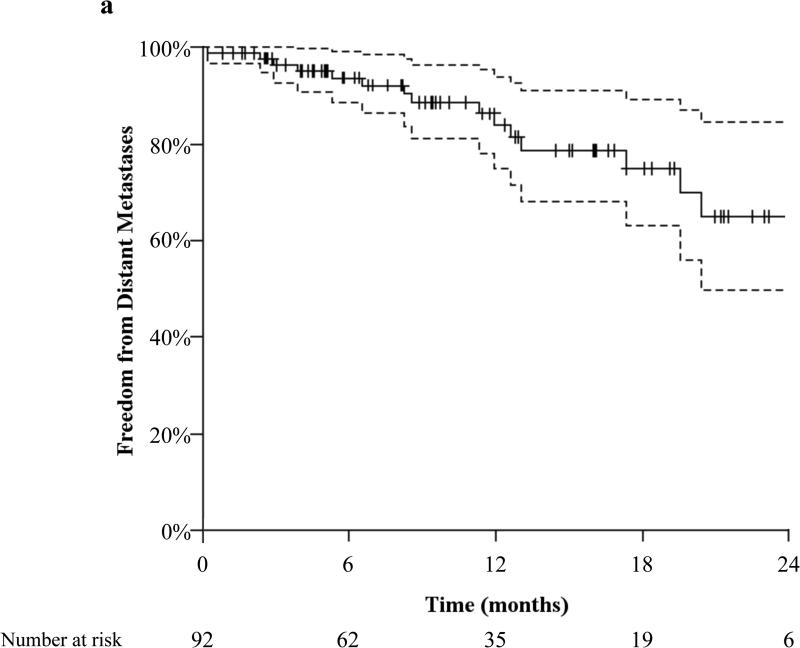
The actuarial 12-month freedom from distant metastasis (FFDM) was 84.0%, Figure 2a, with no difference between patients with SCC histology and non-SCC histology (p=0.217). There were 15 patients who developed distant metastases (DM) at a median time of 8.6 months (interquartile range, 4.6–12.8 months). The lung was the most common site of DM (n=6).
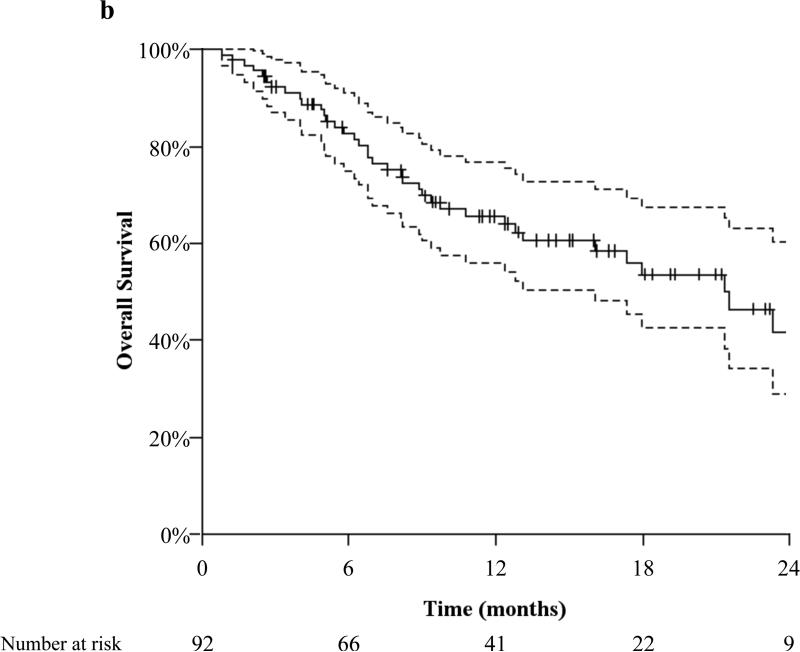
Figure 2.
(2a) actuarial distant metastasis free survival and (2b) overall survival both with 95% confidence interval and number needed to treat.
Overall survival
The actuarial 12-month OS was 65.2%, Figure 2b, with no difference between patients with SCC histology and non-SCC histology (p=0.129). Forty patients died at a median of 7.3 months (interquartile range, 4.7–12.9 months) from the start of PBRT. There was one death during PBRT secondary to disease progression. Of the 41 patients alive at 12 months, 20 were without evidence of disease, while 14 were alive with locoregionally recurrent only, 3 with metastatic only disease, and 4 with both locoregionally recurrent and metastatic disease.
Acute toxicity
Eighty-seven patients (94.6%) completed their PBRT course. Five patients (5.4%) did not complete re-RT due to tumor progression in 4 patients and severe nausea in 1 patient undergoing hypofractionated re-RT regimen of 14 Gy (RBE) in four fractions given twice daily for two consecutive days (‘Quad Shot’) followed by a 2-3 week break for 4 planned cycles with concurrent ifosfamide. An additional patient progressed during treatment and after 47.3 Gy (RBE), was switched to ‘Quad Shot’ and subsequently completed treatment [15]. Grade 3 or greater acute toxicities included mucositis (9.9%), dysphagia (9.1%), esophagitis (9.1%), and dermatitis (3.3%), Table 3.
Table 3.
Acute toxicity1
| Total | Grade 0 | (%) | Grade 1 | (%) | Grade 2 | (%) | Grade 3 | (%) | Grade 4 | (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dysphagia2 | 66 | 25 | 37.9% | 19 | 28.8% | 16 | 24.2% | 6 | 9.1% | 0 | 0.0% |
| Mucositis | 91 | 37 | 40.7% | 29 | 31.9% | 16 | 17.6% | 9 | 9.9% | 0 | 0.0% |
| Nausea | 91 | 63 | 69.2% | 21 | 23.1% | 7 | 7.7% | 0 | 0.0% | 0 | 0.0% |
| Dysgeusia | 91 | 50 | 54.9% | 23 | 25.3% | 18 | 19.8% | 0 | 0.0% | 0 | 0.0% |
| Esophagitis2 | 66 | 41 | 62.1% | 12 | 18.2% | 7 | 10.6% | 6 | 9.1% | 0 | 0.0% |
| Dermatitis | 91 | 10 | 11.0% | 38 | 41.8% | 40 | 44.0% | 3 | 3.3% | 0 | 0.0% |
Data unavailable for one patient, admitted during treatment
Limited to patients without G-tube in place or symptoms prior to treatment
Twenty-four patients had a gastrostomy tube (g-tube) in place prior to consultation for re-RT with PBRT and 2 patients were referred for a prophylactic g-tube. Of the remaining 66 patients, 6 patients (9.1%) were referred for a reactive g-tube (either during or within 90 days of PBRT completion). The first patient had developed severe trismus in the setting of progressive disease; a second patient was admitted for pneumonia on the last day of treatment, with subsequent g-tube placement due to aspiration risk; and the remaining four patients required a g-tube due to Grade 3 mucositis (n=2), dysphagia (n=1), or both (n=1).
Six patients had other severe acute events, either treatment- or disease-related. One patient developed a right maxillary sinocutaneous fistula secondary to tumor response after 21 of 35 treatments, which was managed conservatively with wound care, antibiotics, and pain management. A 2nd patient experienced bleeding from the tumor site that required embolization, and developed a pharyngocutaneous fistula 1-month following treatment, which in the setting of progressive disease was managed conservatively. A 3rd patient developed severe ear pain likely secondary to PBRT-induced nerve swelling, which resolved with morphine and steroid administration. A 4th patient, who had a salvage right maxillectomy 89 days prior to PBRT, developed dehiscence of the anterolateral thigh flap at the nasal border and required a revision surgery with flap placement one month after PBRT treatment. A 5th patient developed a nonhealing temporal skin defect seven weeks after PBRT, and was referred to plastic surgery for flap placement. Lastly, a 6th patient developed painful thyroid cartilage necrosis after the first 2 Gy (RBE) PBRT treatment; the patient had a laryngectomy with consequent removal of the cartilage.
Late toxicity
Of the 75 patients with follow-up of greater than three months after the end of PBRT, 69 had toxicity data available, Table 4. One patient developed a small open ulcer on the right neck 3.5 months after treatment, with plans for a future closure. Two patients developed mucocutaneous fistulas (cheek and floor of mouth) from tumor resolution. Neither were considered for surgical intervention as they had poor performance status. One patient developed a chronic neck wound eight months after treatment, and was successfully managed with hyperbaric oxygen treatments and wound care. Importantly, in this patient, there was no fresh skin flap placed on the re-RT fields during a salvage modified radical neck surgery performed at an outside hospital prior to PBRT, as would be routinely done at our institution(s) to prevent this complication. Another 2 patients developed soft tissue necrosis from treatment in the pharyngeal regions. Both had surgical repair with flap reconstruction with one of the patients having attempted hyperbaric oxygen to assist with the healing prior to surgery. One patient developed pneumocephalus from a skull base defect in the setting of recurrent/progressive disease 4.5 months after treatment, who subsequently deceased. Two patients had aspiration pneumonia that required hospitalization with IV antibiotics leading to resolution of their infection. One patient developed osteoradionecrosis of the mandible 7.5 months after treatment that required a mandibulectomy with free flap reconstruction. Another patient developed asymptomatic necrosis of the temporal lobe 8.5 months after PBRT, which did not necessitate intervention. Two patients developed mild unilateral hearing loss and 1 patient developed mild bilateral hearing loss. One patient had late mild tearing. No retina, vision, or larynx late toxicity was appreciated. There were 2 patients with grade 5 toxicity secondary to treatment-related nasopharyngeal or parapharyngeal bleeding/ carotid rupture, both patients were without evidence of disease on PET imaging.
Table 4.
Late toxicity1
| Total | Grade 0 | (%) | Grade 1 | (%) | Grade 2 | (%) | Grade 3 | (%) | Grade 4 | (%) | Grade 5 | (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | 69 | 44 | 63.8% | 16 | 23.2% | 3 | 4.3% | 1 | 1.4% | 5 | 7.2% | 0 | 0.0% |
| Induration/fibrosis2 | 67 | 45 | 67.2% | 22 | 32.8% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Xerostomia | 69 | 40 | 58.0% | 26 | 37.7% | 3 | 4.3% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Trismus2 | 65 | 45 | 69.2% | 16 | 24.6% | 4 | 6.2% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Dysphagia3 | 56 | 41 | 73.2% | 10 | 17.9% | 1 | 1.8% | 4 | 7.1% | 0 | 0.0% | 0 | 0.0% |
| Bleeding | 69 | 67 | 97.1% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 2.9% |
Toxicity data available for 69 out of 75 patients with follow-up >90 days
Limited to patients without symptoms prior to treatment
Limited to patients without G-tube in place
DISCUSSION
The present multi-institutional study examines the preliminary toxicity data and clinical outcomes of the largest proton re-RT cohort for HNC to date. We demonstrate the feasibility of using PBRT, with a minimal acute toxicity profile, even in patients who have had multiple prior courses of RT.
Our overall rate of acute Grade 3 dermatitis (3%) and mucositis (10%) favorably compares to photon re-RT rates with IMRT, which have been reported to be between 13–32% and 13–43%, respectively [11,16,17]. Only 9% of patients in the present study required a reactive g-tube. This compares favorably to patients undergoing definitive chemoradiation for HNC [18]. Furthermore, a recent case-control study of patients undergoing RT for newly diagnosed oropharyngeal carcinoma reported fewer reactive g-tubes in patients treated with PBRT (19%) compared to those treated with IMRT (46%) [19]. The rates of reactive g-tubes in the re-RT setting in the present study are similar, which reflects the correspondingly low rates of severe acute mucositis and dysphagia seen in this cohort.
We had two Grade 5 events in patients’ with no evidence of disease, but who developed bleeding in the setting of significant ulceration and necrosis. This phenomenon was likely the result of RT-induced blood vessel injury and fragility, which has been associated with a greater risk of bleeding [20].
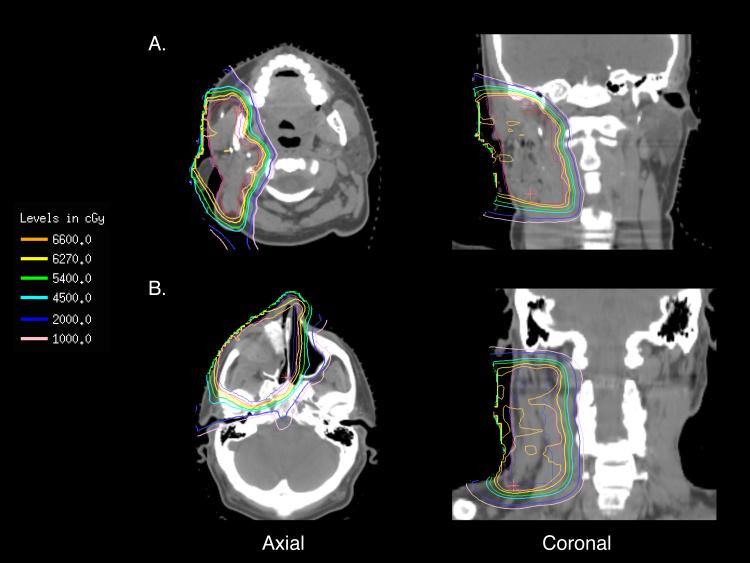
The inherent physical properties of PBRT, which allow for the sparing of previously irradiated normal tissue, are particularly important for minimizing toxicity for patients treated for brain tumors and other head and neck malignancies [21,22]. Representative plans of 2 patients with isodose lines are depicted in Figure 3. Especially in these regions, critical normal structures such as the brainstem, optic nerves, optic chiasm, and spinal cord can often receive doses greater than their respective tissue tolerances when exposed to multiple courses of RT [13]. In 53 patients with recurrent NPC treated with conventional photon re-RT, 15% of patients developed severe toxicities, which included significant bone, tissue, and brain necrosis, and cervical myelopathy, with a cumulative RT dose of greater than 100 Gy [21]. The available late toxicity reports in the present study were encouraging; while 80% of patients received cumulative RT doses of over 100 Gy, there were only three cases of radionecrosis: one was asymptomatic, while the other two were successfully managed surgically. Importantly, there was no brainstem necrosis or visual complications in our report. Even while sparing normal tissue, it is critical that an adequate re-RT dose be delivered, as these cells are presumably becoming increasingly radioresistant [6].
Figure 3.
Representative isodose plans for patients under going re-RT for (3a) recurrent poorly differentiated carcinoma with squamous features previously treated to 48Gy with concurrent cisplatin and etoposide after a superficial parotidectomy who developed a local recurrence ~3 years later who underwent re-irradiation with PBRT to 66Gy (RBE) in 33 fractions with concurrent cisplatin in the post-operative setting for a positive margin after a right parotidectomy with right masseter muscle resection, rectus abdominus free flap reconstruction, and selective right neck dissection and (3b) recurrent olfactory neuroblastoma previously treated to 55.8 Gy after endoscopic resection who developed a local recurrence ~2.5 years later who underwent re-irradiation with PBRT to 66Gy (RBE) in 33 fractions in the post-operative setting for a positive margin after a right maxillectomy, extranasal ethmoidectomy, dacryocystorhinostomy, and right modified neck dissection for a positive margin, cortical bone invasion, perineural invasion, and four positive nodes with extracapsular extension. The PTV is in magenta and CTV is in red.
Some have questioned the use of an expensive technology such as PBRT for patients with recurrent HNC because of their very limited prognosis. This series and others demonstrate that the majority of patients will not develop metastatic disease within the first couple of years after re-RT and that these patients truly have localized disease that can potentially be cured. Locoregional disease control remains the biggest challenge for this group of patients and that many can survive if treated with aggressive locoregional therapy. Certainly, palliative chemotherapy has been shown to result in limited temporary response rates, but surgery and/or radiation therapy are needed for durable control. Because the majority of these patients have potentially curable disease and are at risk for serious complications due to the high cumulative doses to critical structures, we believe these types of patients are ideally suited for PBRT, if PBRT can indeed make re-RT delivery safer. Currently we offer curative intent proton re-RT to patients with non-metastatic recurrent disease with a KPS >60. Cost effectiveness studies would be interesting and are planed to determine if the reduction in complications with PBRT would prove cost effective as compared to IMRT.
While, the focus of this multi-institutional report was to assess early treatment outcomes and toxicity rates in the setting of re-RT with PBRT, certain limitations merit discussion. While histologies were heterogeneous, the location and consequently the local radiation complications and toxicities can still be expected to be comparable throughout this group of patients [6]. This multi-institutional study was retrospective in nature and as such is subject to the standard limitations of retrospective reviews. Despite its retrospective nature, however, patient outcomes and toxicities were tracked in a prospective database, thereby improving the integrity of the data as compared with traditional retrospective analyses. The disadvantages of the variety of practice patterns and heterogeneity of the patients in our cohort, we believe, are offset by their greater generalizability as compared with a single institution report. As patients were treated at 2 independent referral-based hybrid academic/ community practice centers we were unable to quantify the number of patients treated with palliative chemotherapy or surgery alone. The combined academic/ community based experience likely improves the generalizability of our outcomes, demonstrating pragmatic real world outcomes rather than a report from a single quaternary care center as is often reported. Although multiple studies have shown the dosimetric advantages of PBRT when treating HNC, in both the upfront and recurrent settings, clinic outcomes with HNC for PBRT are scant [23]. Comparison of our treatment outcomes and acute and late toxicity rates with historical reports, however, is predominantly hypothesis generating. Accordingly, randomized clinical data to demonstrate improved outcomes and decreased toxicity in the setting of re-RT with PBRT is needed; however, there are many challenges to conducting large randomized clinical trials for a rare disease and a technology with limited availability [24,25].
As the clinical data remains limited, many expert panels and treatment guidelines advocate/ support the use of proton therapy in patients with prior radiation for recurrent HNC based almost exclusively on the dosimetric advantages of PBRT [23]. It is remarkable that proton beam therapy has been used for several decades in the US and around the world, and that re-RT for HNC is an often-cited indication, yet there are few clinical outcomes reports in the literature. This is the largest report, to our knowledge, of curative intent PBRT in HNC reirradiation. We have demonstrated a favorable toxicity profile as compared with historical controls with IMRT and 3D-CRT. Since a randomized trial is very unlikely, reports of clinical outcomes from large multi-institutional experiences are needed to further define the benefits of PBRT in this setting.
CONCLUSION
We have shown that PBRT is a safe and effective treatment for patients with recurrent HNC who have previously undergone head and neck irradiation. As the physical properties of PBRT allow the delivery of higher radiation doses to the tumor, while minimizing the dose to the previously irradiated normal tissues, we have been able to achieve adequate disease control and survival with reasonably low rates of acute, and to-date late toxicity. Further evaluation is warranted and we plan to prospectively validate these findings.
SUMMARY.
This multi-institutional retrospective study assessed the early treatment outcomes and toxicity in the setting of proton beam re-irradiation to the head and neck. Proton beam re-irradiation is a safe and effective treatment for patients with recurrent head and neck cancer allowing the delivery of higher radiation doses to the tumor, while minimizing the dose to the previously irradiated normal tissues.
Acknowledgments
Funding: there is no specific funding to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentations: This work was presented in part at the inaugural meeting for the Particle Therapy Co-Operative Group of North American (PT-COG NA) in Houston, TX, October 27-29th, 2014. This work was submitted for consideration to the 57th Annual meeting for the American Society of Radiation Oncology (ASTRO) in San Antonio, TX, October 18-21st, 2015.
Disclaimers: Oren Cahlon has a minority investment in ProCure
REFERENCES
- 1.Glenny AM, Furness S, Worthington HV, Conway DI, Oliver R, Clarkson JE, Macluskey M, Pavitt S, Chan KK, Brocklehurst P. Interventions for the treatment of oral cavity and oropharyngeal cancer: Radiotherapy. The Cochrane Library. 2010 doi: 10.1002/14651858.CD006387.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brizel DM, Albers ME, Fisher SR, Scher RL, Richtsmeier WJ, Hars V, George SL, Huang AT, Prosnitz LR. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. New England Journal of Medicine. 1998;338:1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 3.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. New England Journal of Medicine. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 4.Riaz N, Hong JC, Sherman EJ, Morris L, Fury M, Ganly I, Wang TJ, Shi W, Wolden SL, Jackson A, Wong RJ, Zhang Z, Rao SD, Lee NY. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2014;111:382–387. doi: 10.1016/j.radonc.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salama JK, Vokes EE. Concurrent chemotherapy and re-irradiation for locoregionally recurrent head and neck cancer. Seminars in oncology. 2008;35:251–261. doi: 10.1053/j.seminoncol.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Jensen AD, Nikoghosyan A, Ellerbrock M, Ecker S, Debus J, Munter MW. Re-irradiation with scanned charged particle beams in recurrent tumours of the head and neck: Acute toxicity and feasibility. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2011;101:383–387. doi: 10.1016/j.radonc.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Lowe VJ, Boyd JH, Dunphy FR, Kim H, Dunleavy T, Collins BT, Martin D, Stack BC, Hollenbeak C, Fletcher J. Surveillance for recurrent head and neck cancer using positron emission tomography. Journal of clinical oncology. 2000;18:651–651. doi: 10.1200/JCO.2000.18.3.651. [DOI] [PubMed] [Google Scholar]
- 8.Langer CJ, Harris J, Horwitz EM, Nicolaou N, Kies M, Curran W, Wong S, Ang K. Phase ii study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: Results of radiation therapy oncology group protocol 9911. Journal of Clinical Oncology. 2007;25:4800–4805. doi: 10.1200/JCO.2006.07.9194. [DOI] [PubMed] [Google Scholar]
- 9.Lee N, Chan K, Bekelman JE, Zhung J, Mechalakos J, Narayana A, Wolden S, Venkatraman ES, Pfister D, Kraus D. Salvage re-irradiation for recurrent head and neck cancer. International Journal of Radiation Oncology* Biology* Physics. 2007;68:731–740. doi: 10.1016/j.ijrobp.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 10.Sulman EP, Schwartz DL, Le TT, Ang KK, Morrison WH, Rosenthal DI, Ahamad A, Kies M, Glisson B, Weber R, Garden AS. Imrt reirradiation of head and neck cancer—disease control and morbidity outcomes. International Journal of Radiation Oncology*Biology*Physics. 2009;73:399–409. doi: 10.1016/j.ijrobp.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Sher DJ, Haddad RI, Norris CM, Posner MR, Wirth LJ, Goguen LA, Annino D, Balboni T, Allen A, Tishler RB. Efficacy and toxicity of reirradiation using intensity-modulated radiotherapy for recurrent or second primary head and neck cancer. Cancer. 2010;116:4761–4768. doi: 10.1002/cncr.25305. [DOI] [PubMed] [Google Scholar]
- 12.Mendenhall NP, Malyapa RS, Su Z, Yeung D, Mendenhall WM, Li Z. Proton therapy for head and neck cancer: Rationale, potential indications, practical considerations, and current clinical evidence. Acta oncologica. 2011;50:763–771. doi: 10.3109/0284186X.2011.590147. [DOI] [PubMed] [Google Scholar]
- 13.Lin R, Slater JD, Yonemoto LT, Grove RI, Teichman SL, Watt DK, Slater JM. Nasopharyngeal carcinoma: Repeat treatment with conformal proton therapy—dose-volume histogram analysis 1. Radiology. 1999;213:489–494. doi: 10.1148/radiology.213.2.r99nv29489. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:25. [Google Scholar]
- 15.Corry J, Peters LJ, Costa ID, Milner AD, Fawns H, Rischin D, Porceddu S. The ‘quad shot’— a phase ii study of palliative radiotherapy for incurable head and neck cancer. Radiotherapy and Oncology. 2005;77:137–142. doi: 10.1016/j.radonc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Wang TJC, Riaz N, Tam M, Zhang C, Youssef B, Hong JC, Born H, Wolden S, Rao S, Lee NY. Patterns of failure after salvage re-irradiation for recurrent head and neck cancer: Implications for field design and consolidation therapy. Journal of Radiation Oncology. 2014;3:139–145. [Google Scholar]
- 17.Lee N, Chan K, Bekelman JE, Zhung J, Mechalakos J, Narayana A, Wolden S, Venkatraman ES, Pfister D, Kraus D, Shah J, Zelefsky MJ. Salvage re-irradiation for recurrent head and neck cancer. International Journal of Radiation Oncology*Biology*Physics. 2007;68:731–740. doi: 10.1016/j.ijrobp.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 18.Romesser PB, Romanyshyn JC, Schupak KD, Setton J, Riaz N, Wolden SL, Gelblum DY, Sherman EJ, Kraus D, Lee NY. Percutaneous endoscopic gastrostomy in oropharyngeal cancer patients treated with intensity-modulated radiotherapy with concurrent chemotherapy. Cancer. 2012;118:6072–6078. doi: 10.1002/cncr.27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank SJ, Rosenthal DI, Ang K, Sturgis EM, Chambers MS, Gunn GB, Hutcheson KA, Zhu XR, Palmer MB, Garden AS. Gastrostomy tubes decrease by over 50% with intensity modulated proton therapy (impt) during the treatment of oropharyngeal cancer patients: A case–control study. International Journal of Radiation Oncology • Biology • Physics. 87:S144. [Google Scholar]
- 20.Chen H-y, Ma X-m, Ye M, Hou Y-l, Xie H-Y, Bai Y-r. Effectiveness and toxicities of intensity-modulated radiotherapy for patients with locally recurrent nasopharyngeal carcinoma. PloS one. 2013;8:e73918. doi: 10.1371/journal.pone.0073918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pryzant RM, Wendt CD, Delclos L, Peters LJ. Re-treatment of nasopharyngeal carcinoma in 53 patients. International Journal of Radiation Oncology*Biology*Physics. 1992;22:941–947. doi: 10.1016/0360-3016(92)90792-g. [DOI] [PubMed] [Google Scholar]
- 22.Mizumoto M, Okumura T, Ishikawa E, Yamamoto T, Takano S, Matsumura A, Oshiro Y, Ishikawa H, Sakurai H, Tsuboi K. Reirradiation for recurrent malignant brain tumor with radiotherapy or proton beam therapy. Strahlentherapie und Onkologie. 2013;189:656–663. doi: 10.1007/s00066-013-0390-6. [DOI] [PubMed] [Google Scholar]
- 23.Chan AW, Liebsch NJ. Proton radiation therapy for head and neck cancer. Journal of surgical oncology. 2008;97:697–700. doi: 10.1002/jso.21013. [DOI] [PubMed] [Google Scholar]
- 24.Glimelius B, Ask A, Bjelkengren G, Björk-Eriksson T, Blomquist E, Johansson B, Karlsson M, Zackrisson B. Number of patients potentially eligible for proton therapy. Acta oncologica. 2005;44:836–849. doi: 10.1080/02841860500361049. [DOI] [PubMed] [Google Scholar]
- 25.Cozzi L, Fogliata A, Lomax A, Bolsi A. A treatment planning comparison of 3d conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiotherapy and Oncology. 2001;61:287–297. doi: 10.1016/s0167-8140(01)00403-0. [DOI] [PubMed] [Google Scholar]