Abstract
Exposure to ionizing radiation induces not only apoptosis but also senescence. While the role of endothelial cell apoptosis in mediating radiation-induced acute tissue injury has been extensively studied, little is known about the role of endothelial cell senescence in the pathogenesis of radiation-induced late effects. Senescent endothelial cells exhibit decreased production of nitric oxide and expression of thrombomodulin, increased expression of adhesion molecules, elevated production of reactive oxygen species and inflammatory cytokines and an inability to proliferate and form capillary-like structures in vitro. These findings suggest that endothelial cell senescence can lead to endothelial dysfunction by dysregulation of vasodilation and hemostasis, induction of oxidative stress and inflammation and inhibition of angiogenesis, which can potentially contribute to radiation-induced late effects such as cardiovascular diseases (CVDs). In this article, we discuss the mechanisms by which radiation induces endothelial cell senescence, the roles of endothelial cell senescence in radiation-induced CVDs and potential strategies to prevent, mitigate and treat radiation-induced CVDs by targeting senescent endothelial cells.
INTRODUCTION
An increasing amount of clinical evidence has demonstrated that exposing the heart to ionizing radiation increases the risks of cardiovascular diseases (CVDs). Radiation-induced CVDs first became apparent in patients who received high doses of radiation to the heart during treatment of Hodgkin’s disease (1) and breast cancer (2, 3). The manifestations of radiation-induced CVDs are usually observed more than a decade after radiotherapy, and include accelerated atherosclerosis, myocardial fibrosis, conduction abnormalities and injuries to cardiac valves (4, 5). In addition, long-term epidemiological studies conducted in Japanese atomic bomb survivors revealed that individuals who received an acute single dose of 1–2 Gy total-body irradiation (TBI) showed a significant increase in mortality from myocardial infarction >40 years postirradiation. The risk of CVD-related death in these survivors was increased by 17% per Gy after 0–4 Gy TBI (6). It has also been shown that the risk of CVD-related mortality increases with low-dose occupational exposures (e.g., radiologists, nuclear power plant workers and emergency workers from the Chernobyl accident) (7–9). Collectively, these findings suggest that the heart and vasculature might be more radiosensitive than was previously thought (10).
Currently, the only available clinical approach to reduce late cardiac complications of radiotherapy is to reduce cardiac exposure during the therapy, but this is not always possible. Although radiotherapy has been improved with treatment planning and radiation delivery, a significant subset of patients with thoracic cancers, including those of the lung, esophagus and proximal stomach, still receives considerable radiation doses to the heart (11–13). There are similar risks from the increasing radiological and nuclear threats that exist today. Therefore, a better understanding of the pathophysiology of radiation-induced CVDs is needed to develop more effective therapeutic strategies to prevent, mitigate and treat radiation-induced CVDs.
The heart is a multicellular organ. The adult human ventricles consist of about 33% cardiomyocytes, 24% endothelial cells and 43% mesenchymal cells (14). The cell turnover rate for cardiomyocytes is highest in early childhood and gradually declines to <1% per year in adulthood. In contrast, endothelial cell turnover in the heart is high throughout life (>15% per year), whereas mesenchymal cell exchange is relatively low in adulthood (<4% per year). Cardiomyocytes are terminally differentiated, quiescent and highly radioresistant. Endothelial cells are relatively resistant to radiation-induced apoptosis, but they readily undergo senescence or permanent cell-cycle arrest after exposure to a moderate or high radiation dose (0.5–10 Gy) (Fig. 1) (15–30). While the role of endothelial cell apoptosis in mediating radiation-induced acute tissue injury has been extensively studied (29–37), little is known about the role of endothelial cell senescence in the pathogenesis of late effects caused by radiation (Fig. 2). Because endothelial cells are one of the major constituents of heart microvasculature and macrovasculature, induction of endothelial cell senescence may play an important role in radiation-induced CVDs. Therefore, in this article we focus our discussion on the mechanisms by which radiation induces endothelial cell senescence, effects of endothelial cell senescence on cardiovascular function and potential strategies for targeting senescent endothelial cells to prevent, mitigate and treat radiation-induced CVDs.
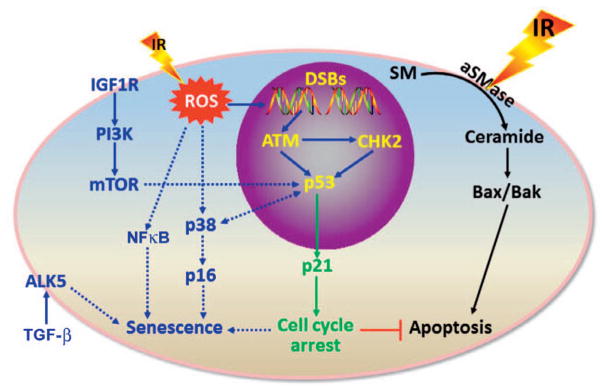
FIG. 1.
Ionizing radiation induces endothelial cell apoptosis and senescence in a dose-dependent manner. Exposure of endothelial cells to a very high dose (>10 Gy) of ionizing radiation (IR) induces apoptosis via activation of the acidic sphingomyelinase (aSMase) that hydrolyzes sphingomyelin (SM) on the plasma membrane to generate ceramide and to induce Bax and Bak. However, exposure of endothelial cells to a moderate (>0.5 Gy) or high dose (<10 Gy) of radiation primarily induces senescence via multiple pathways, as shown. ALK5, TGF-β type 1 receptor kinase; ATM, ataxia-telangiectasia mutated protein kinase; CHK2, checkpoint kinase 2; DSBs, DNA double-strand breaks; IGF1R, insulin-like factor-1 receptor; mTOR, mechanistic target of rapamycin; NFκB, nuclear factor κB; p38, p38 mitogen-activated protein kinases; PI3K, phosphtidylinositol-3-kinase; ROS, reactive oxygen species; and TGF-β, tumor growth factor β.
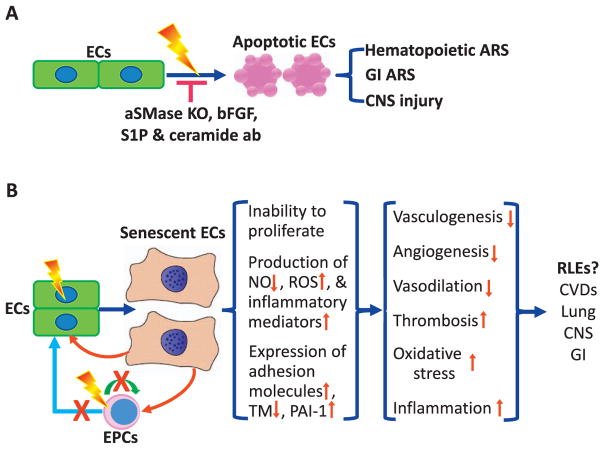
FIG. 2.
Role of endothelial cell in radiation-induced acute tissue injuries and late effects. Panel A: Role of endothelial cell apoptosis in acute radiation syndrome (ARS) in the hematopoietic system, gastrointestinal (GI) system, and central nerve system (CNS). Acidic sphingomyelinase knockout (aSMase KO), basic fibroblast growth factor (bFGF), sphingosine-1-phosphate (S1P), and antibodies against ceramide (ceramide ab) can inhibit radiation-induced apoptosis in endothelial cells (ECs) and reduce radiation-induced acute injuries to various tissues. Panel B: Hypothetical roles of endothelial cell senescence in radiation-induced late effects (RLEs). EPCs, endothelial progenitor cells; NO, nitric oxide; ROS, reactive oxygen species; TM, thrombomodulin; PAI-1, plasminogen activator inhibitor-1; and CVDs, cardiovascular diseases.
CELLULAR SENESCENCE: A DOUBLE-EDGED SWORD IN THE FIGHT AGAINST CANCER
Cells undergo senescence after extensive cell division or exposure to oncogenic or genotoxic stress such as radiation (38–41). Induction of cellular senescence is considered an important tumor-suppressive mechanism because it permanently arrests growth of genetically deranged cells with critically shortened telomeres or persistent DNA damage that may lead to genetic instability and cell transformation. More importantly, an increasing body of evidence indicates that induction of cellular senescence can stimulate the immune system to rapidly eliminate these genetically distorted cells (42–44). In addition, induction of senescence is an important function of radiation in cancer treatment, since it can potently induce senescence not only in cancer cells but also in endothelial cells (45–47). Induction of endothelial cell senescence inhibits angiogenesis, which may also contribute to the therapeutic effects of radiation on cancer.
However, if the rate of senescent cell production exceeds the immune system’s capacity to clear them, or if the immune system is compromised and cannot efficiently remove senescent cells, senescent cells can accumulate in tissues (39, 48), which has been observed in animals and humans during aging and after radiotherapy. Under such circumstances, senescent cells can promote various age- and therapy-related pathologies, including CVDs, in a cell-autonomous and a non-cell-autonomous manner. For example, senescent cells produce increased levels of reactive oxygen species (ROS), which can induce oxidative damage to neighboring cells. In addition, senescent cells secrete a plethora of inflammatory mediators (e.g., cytokines and chemokines) and extracellular proteases, termed the senescence-associated secretory phenotype (SASP), which causes chronic inflammation and disruption of tissue structure and function (38, 39, 49, 50). Moreover, persistent accumulation of senescent cells in tissues can cause a decline in tissue stem and progenitor cells (51–55). This can occur intrinsically, if tissue stem and progenitor cells themselves become senescent, or extrinsically, if the cells making up the stem-cell niche undergo senescence and express SASP. Senescent tissue stem and progenitor cells exhibit significant defects in self-renewal, proliferation and differentiation, rendering them incapable of tissue maintenance, regeneration and repair. Therefore, induction of cellular senescence is a double-edged sword in the fight against cancer. Inhibiting the induction of senescence can be detrimental by increasing tumorigenesis and decreasing tumor response to radiotherapy. In contrast, promoting clearance of senescent cells during aging and after irradiation can prevent their accumulation within tissues, and it may delay the onset and progression of age-related diseases and reduce late effects of radiation, such as CVDs. This hypothesis is supported by a recently reported finding that genetically selective elimination of p16Ink4a (p16)-positive senescent cells in normal INK-ATTAC transgenic mice via administration of the drug AP20187 significantly extended the animals’ lifespans by delaying the onset and progression of age-related pathologies, including cardio-dysfunction (56). However, whether selective clearance of senescent cells can prevent and mitigate radiation-induced CVDs has not yet been investigated.
ENDOTHELIAL CELL SENESCENCE INDUCED BY IONIZING RADIATION
Endothelial cells from a variety of tissue origins and species, including human coronary artery, can undergo senescence after exposure to a moderate-to-high dose of radiation in vitro (15–28). In addition, emerging evidence indicates that cardiac endothelial cells senesce in vivo after local exposure to radiation (57). However, the underlying mechanisms by which radiation induces endothelial senescence have not been fully established. It has been suggested that diverse stimuli can induce cellular senescence in different cells via various upstream signal transduction cascades (including the p53-p21 pathway) that eventually converge on the p16-Rb pathway, whose activation inescapably prevents senescent cells from re-entering the cell cycle. The importance of the p53-p21 pathway is supported by the finding that activation of p53 and induction of p21 are transient events during the onset of senescence that subside when expression of p16 starts rising (58–60). Induction of senescence can be prevented by inactivation of p53 prior to upregulation of p16; however, once p16 is highly expressed, downregulation of p53 cannot reverse cell cycle arrest (60, 61). This indicates that activation of the p53-p21 pathway is an important role in initiation of senescence and that upregulation of p16 is required for maintenance of senescence.
However, endothelial cells are unique for the induction of senescence. Unlike other cells, it appears that the p53-p21 pathway is more important than the p16-Rb pathway for the induction of endothelial cell senescence, because knockdown of p53 expression, but not knockdown of p16 expression, inhibits endothelial cell senescence induced by a variety of stimuli (Fig. 1) (16, 62–64). The p53-p21 pathway may be activated in endothelial cells via induction of unrepairable DNA damage, persistent oxidative stress and expression of X-linked inhibitor of apoptosis-associated factor 1 and growth differentiation factor 15 (16, 23, 62, 65–67). Recently, it was reported that activation of the insulin/ insulin-like growth factor 1 (IGF1)-phosphtidylinositol-3-kinase (PI3K)-Akt/mechanistic target of rapamycin (mTOR) pathway acts upstream of the p53-p21 pathway in mediating endothelial cell senescence induced by radiation and high glucose (Fig. 1) (21, 27, 28, 57, 68). Radiation-induced senescence of endothelial cells was suppressed by specific inhibition of IGF1 receptor (IGF1R), PI3K or mTOR. The activation of the IGF1-PI3K-Akt/mTOR pathway may be attributable to downregulation of sirtuin 1 (SIRT1) (22, 68, 69). In addition, radiation-induced endothelial cell senescence also may involve: activation of p38, NFκB and TGF-β type 1 receptor ALK5; induction of endoplasmic reticulum stress; and downregulation of telomerase reverse transcriptase (15, 22, 70–74).
Radiation-induced senescent endothelial cells exhibit a variety of senescence-like phenotypes. These include changes in cell morphology, permanent cell-cycle arrest, increased staining for senescence-associated β-galactosidase (SA-β-gal) and elevated expression of p16 and p21. The cells are also defective in angiogenesis, having reduced ability to sprout, migrate and invade to form capillary-like structures in Matrigel® (15, 70). In addition, senescent endothelial cells produce increased levels of ROS, probably due in part to upregulation of NADPH oxidases, downregulation and/or upcoupling of endothelial nitric oxide synthase (eNOS) and induction of mitochondrial dysfunction (75–78). They acquire SASP by expressing increased levels of inflammatory cytokines and adhesion molecules (15, 18, 25, 26, 57, 77, 79). Radiation-induced senescent endothelial cells expressed decreased levels of thrombomodulin (80, 81) and increased levels of plasminogen activator inhibitor-1 (PAI-1) (82, 83). All these changes in senescent endothelial cells lead to endothelial dysfunction, which results in inhibition of angiogenesis, induction of oxidative stress and inflammation and dysregulation of vasodilation and hemostasis.
ROLE OF ENDOTHELIAL CELL SENESCENCE IN RADIATION-INDUCED CVDS
Although it has been extensively implicated in the pathogenesis of age-related CVDs (82, 84–88), the role of endothelial cell senescence in radiation-induced CVDs has yet to be determined (89–91). Radiation-induced CVDs may be in part attributable to a combination of effects on microvasculature and macrovasculature (89–91). Senescent endothelial cells are incapable of regenerating new cells to maintain the homeostasis of vasculatures and repair damaged blood vessels, which may contribute to the decreased density of cardiac capillaries and small coronary arterioles and to the accelerated atherosclerosis of large blood vessels, including rodent and human coronary arteries (89, 90, 92–94). Moreover, senescent endothelial cells can potentially impede the angiogenic activity of endothelial progenitor cells via SASP and increased production of ROS. Increased production of ROS, along with reduced production of NO due to downregulation of eNOS expression in senescent endothelial cells, can lead to a further decrease in NO bioavailability, which can impair endothelial-mediated vasodilation and cause hypertension, a major contributing factor of fatal age-related CVDs in humans (75–78, 86, 95). With increased levels of various inflammatory cytokines, adhesion molecules and PAI-1, and decreased levels of thrombomodulin, senescent endothelial cells are proinflammatory, prothrombotic and proatherogenic (15, 18, 25, 26, 57, 77, 79–83). Therefore, they are likely to play a very important role in radiation-induced atherosclerosis. Collectively, the combined deleterious effects of senescent endothelial cells on cardiac microvasculature and macrovasculature may lead to radiation-induced CVDs.
APPROACHES FOR TARGETING ENDOTHELIAL CELL SENESCENCE TO PREVENT, MITIGATE AND TREAT RADIATION-INDUCED CVDS
Because senescent endothelial cells likely play an important role in radiation-induced CVDs, targeting them with a therapeutic agent may be a novel strategy to prevent, mitigate and treat radiation-induced CVDs. While no such therapies are currently available, several strategies and candidate approaches are under investigation, as shown in Fig. 3. One strategy for targeting senescent endothelial cells is to prevent radiation from inducing senescence of endothelial cells. This could be achieved by inhibiting the IGF1-PI3K-Akt/mTOR pathway because its activation is important in mediating radiation-induced endothelial senescence. The pathway can be inhibited with a specific inhibitor of IGF1R, PI3k and mTOR, or with an activator of SIRT1 (21, 22, 68, 69). In addition, radiation-induced endothelial cell senescence could be inhibited by using antioxidants to scavenge ROS or by using a specific inhibitor to block activation of p38, NFκB and ALK5 (15, 70). However, these preventive strategies can be effective only if applied before or shortly after irradiation, when endothelial cells have not yet undergone senescence.
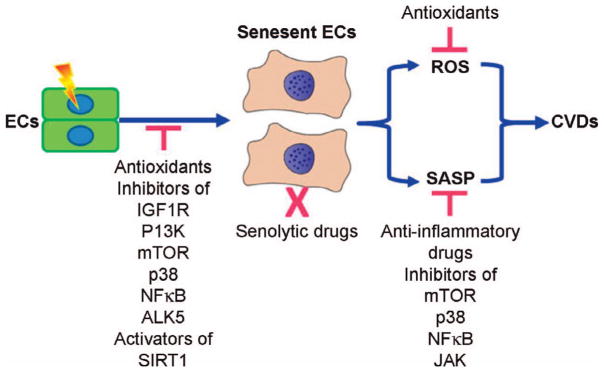
FIG. 3.
Potential strategies to prevent, mitigate, and treat radiation-induced CVDs by targeting senescent endothelial cells. Antioxidants, inhibitors of insulin/insulin-like growth factor I receptor (IGF1R), phosphtidylinositol-3-kinase (PI3K), mechanistic target of rapamycin (mTOR), p38, NFκB and TGF-β type 1 receptor (ALK5), and the activators of sirtuin 1 (SIRT1) may be used to prevent radiation-induced CVDs by inhibiting the induction of endothelial cell (EC) senescence. Clearance of senescent cells with a senolytic drug that can selectively kill senescent cells including senescent endothelial cells has the potential to be developed as novel therapeutic strategy to mitigate and treat radiation-induced CVDs. Antioxidants and inhibitors of mTOR, p38, NFκB, and Janus kinase (JAK) may prevent, mitigate, and treat radiation-induced CVDs by scavenging senescent cell-produced reactive oxygen species (ROS) and inhibiting senescence-associated secretory phenotype (SASP), respectively.
Alternatively, inhibition of SASP could be used to mitigate or treat radiation-induced CVDs because SASP likely mediates most of the deleterious effects of senescent endothelial cells on the cardiovascular system (96). The pathways involved in induction of cellular senescence substantially overlap those of SASP (e.g., p38, NFκB and mTOR). Specific inhibition of these pathways would not only block induction of senescence, as discussed above, but also suppress SASP (97–101). Targeting the Janus kinase (JAK) pathway, an upstream regulatory pathway of SASP, is another potential strategy for mitigating the effects of radiation-induced CVDs. It was recently reported that using RNAi or JAK inhibitors to target the JAK pathway suppressed development of SASP in human umbilical vein endothelial cells and human preadipocytes after exposure to radiation (102). Further, treating aged mice with a specific inhibitor of JAK-1 and JAK-2 (ruxolitinib) reduced systemic inflammation and frailty (102). It will be of great interest to determine whether inhibiting pathways such as JAK, which mediate radiation induction of SASP, can also prevent and mitigate radiation-induced CVDs. However, these approaches may require continuous treatment because the senescent endothelial cells remain in the heart and can express SASP again as soon as the pathway is no longer being inhibited.
The limitations associated with inhibition of senescence induction and SASP to prevent and mitigate radiation-induced CVDs could be overcome by clearing senescent endothelial cells with a small molecule that selectively kills senescent cells. This approach to mitigate and treat radiation-induced CVDs can be used after irradiation, when endothelial cells have already become senescent, and can be effective with an intermittent treatment. The seminal finding, discussed above, that AP20187 selectively eliminated senescent cells in transgenic mice, which prolonged their lifespans by delaying the onset of age-related pathologies, prompted efforts to identify senolytic drugs, small molecules that can selectively kill senescent cells without depending on a transgene (103, 104). Senolytic drugs have the potential for use not only as novel antiaging drugs but also as new medical countermeasures against radiation to mitigate and treat radiation-induced CVDs. However, finding a senolytic drug has been challenging because senescent cells are highly resistant to induction of apoptosis (105). We and others have used high-throughput screening of libraries, each containing thousands of compounds, to identify small molecules that selectively kill senescent cells, but only two nonspecific, cell-type-selective senolytic drugs have been discovered (104). We, therefore, took a targeted approach to identify senolytic drugs by individually titrating the cytotoxicity of hundreds of small molecules that participate in pathways predicted to be important for viability of senescent cells or maintenance of their phenotype. With this approach, ABT263, a Bcl-2/xL-specific inhibitor (106), was identified as the most potent broad-spectrum senolytic drug that selectively, potently and rapidly kills senescent cells in culture, regardless of cell type or species (48), including radiation-induced senescent human umbilical vein endothelial cells (preliminary data not shown). More importantly, oral administration of ABT263 to sublethally irradiated and normally aged mice effectively cleared senescent cells in several tissues, including senescent bone marrow hematopoietic stem cells and muscle stem cells, and it suppressed SASP in the lungs (48). We plan next to investigate in thoracic-irradiated mice whether ABT263 can effectively clear senescent endothelial cells and suppress SASP in the heart and whether ABT263 can mitigate and treat radiation-induced CVDs.
Using senolytic drugs such as ABT263 for mitigation and treatment of radiation-induced CVDs and other radiation-induced late effects (RLEs) has several advantages over conventional anti-inflammatory treatments. Senolytic drugs target the cells that may be fundamentally responsible for initiating and driving radiation-induced CVDs and other RLEs (Fig. 4) (96, 107, 108). Therefore, senolytic drugs should be more effective than anti-inflammatory therapeutics that target individual harmful inflammatory molecules (e.g., cytokine and chemokine) and less harmful, because, unlike anti-inflammatory agents, they do not interfere with the normal immune functions of the molecules. In addition, senolytic drugs may invigorate tissue stem and progenitor cells to improve tissue repair and regeneration because space occupied by senescent cells will be freed, allowing normal tissue stem and progenitor cells to repopulate and because SASP will be suppressed, improving the microenvironment for these cells. Therefore, senolytic drug treatment could be expected to have a much broader therapeutic effect than conventional anti-inflammatory treatments. Finally, senolytic drug treatment has the potential not only to mitigate radiation-induced CVDs and other RLEs, but also to prevent radiation-induced secondary malignancies and reduce cancer relapse and metastasis, because senescent cells are known to promote malignant transformation in neighboring cells and to stimulate proliferation and metastasis of resistant tumor cells, possibly in part through SASP (79, 96, 107, 108).
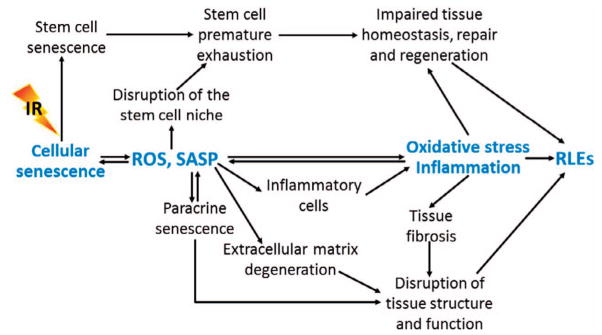
FIG. 4.
Role of cellular senescence in radiation-induced late effects (RLEs). Senescent cells may mediate RLEs in part via increased production of reactive oxygen species (ROS) and expression of senescence-associated secretory phenotype (SASP).
Acknowledgments
The authors apologize for the omission of work by colleagues due to space constraints. The studies on senescent cells conducted in Zhou’s laboratory were supported in part by the National Institutes of Health (grant nos. R01CA122023 and P20GM109005), a scholarship from the Arkansas Research Alliance and the Rockefeller Leukemia and Lymphoma Research Endowment; and those on radiation-induced cardiovascular tissue injury in Boerma’s laboratory were supported by the National Space Biomedical Research Institute through NCC 9-58. The manuscript was edited by the Office of Grants and Scientific Publications at the University of Arkansas for Medical Sciences. Drs. Zhou and Wang are inventors of a pending patent application for use of Bcl-2 and/or Bcl-xL inhibitors as anti-aging agents. Dr. Zhou is a cofounder and an advisor of Unity Biotechnology that sponsors some of his research to develop senolytic drugs for age-related diseases.
References
- 1.Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 2.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–65. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–70. [PubMed] [Google Scholar]
- 4.Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95:252–8. doi: 10.1136/hrt.2008.149088. [DOI] [PubMed] [Google Scholar]
- 5.Martinou M, Gaya A. Cardiac complications after radical radiotherapy. Semin Oncol. 2013;40:178–85. doi: 10.1053/j.seminoncol.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 7.McGale P, Darby SC. Low doses of ionizing radiation and circulatory diseases: a systematic review of the published epidemiological evidence. Radiat Res. 2005;163:247–57. doi: 10.1667/rr3314. [DOI] [PubMed] [Google Scholar]
- 8.Hauptmann M, Mohan AK, Doody MM, Linet MS, Mabuchi K. Mortality from diseases of the circulatory system in radiologic technologists in the United States. Am J Epidemiol. 2003;157:239–48. doi: 10.1093/aje/kwf189. [DOI] [PubMed] [Google Scholar]
- 9.Ivanov VK, Maksioutov MA, Chekin SY, Petrov AV, Biryukov AP, Kruglova ZG, et al. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- 10.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 11.Kole TP, Aghayere O, Kwah J, Yorke ED, Goodman KA. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;83:1580–6. doi: 10.1016/j.ijrobp.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 12.Tillman GF, Pawlicki T, Koong AC, Goodman KA. Preoperative versus postoperative radiotherapy for locally advanced gastro-esophageal junction and proximal gastric cancers: a comparison of normal tissue radiation doses. Dis Esophagus. 2008;21:437–44. doi: 10.1111/j.1442-2050.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 13.Wu WC, Chan CL, Wong YW, Cuijpers JP. A study on the influence of breathing phases in intensity-modulated radiotherapy of lung tumours using four-dimensional CT. Br J Radiol. 2009;83:252–6. doi: 10.1259/bjr/33094251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161:1566–75. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Dong X, Tong F, Qian C, Zhang R, Dong J, Wu G, et al. NEMO modulates radiation-induced endothelial senescence of human umbilical veins through NF-kappaB signal pathway. Radiat Res. 2015;183:82–93. doi: 10.1667/RR13682.1. [DOI] [PubMed] [Google Scholar]
- 16.Heo JI, Kim W, Choi KJ, Bae S, Jeong JH, Kim KS. XIAP-associating factor 1, a transcriptional target of BRD7, contributes to endothelial cell senescence. Oncotarget. 2016;7:5118–30. doi: 10.18632/oncotarget.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KS, Kim JE, Choi KJ, Bae S, Kim DH. Characterization of DNA damage-induced cellular senescence by ionizing radiation in endothelial cells. Int J Radiat Biol. 2014;90:71–80. doi: 10.3109/09553002.2014.859763. [DOI] [PubMed] [Google Scholar]
- 18.Lowe D, Raj K. Premature aging induced by radiation exhibits pro-atherosclerotic effects mediated by epigenetic activation of CD44 expression. Aging Cell. 2014;13:900–10. doi: 10.1111/acel.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendonca MS, Chin-Sinex H, Dhaemers R, Mead LE, Yoder MC, Ingram DA. Differential mechanisms of x-ray-induced cell death in human endothelial progenitor cells isolated from cord blood and adults. Radiat Res. 2011;176:208–16. doi: 10.1667/rr2427.1. [DOI] [PubMed] [Google Scholar]
- 20.Oh CW, Bump EA, Kim JS, Janigro D, Mayberg MR. Induction of a senescence-like phenotype in bovine aortic endothelial cells by ionizing radiation. Radiat Res. 2001;156:232–40. doi: 10.1667/0033-7587(2001)156[0232:ioaslp]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Panganiban RA, Day RM. Inhibition of IGF-1R prevents ionizing radiation-induced primary endothelial cell senescence. PLoS One. 2013;8:e78589. doi: 10.1371/journal.pone.0078589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panganiban RA, Mungunsukh O, Day RM. X-irradiation induces ER stress, apoptosis, and senescence in pulmonary artery endothelial cells. Int J Radiat Biol. 2013;89:656–67. doi: 10.3109/09553002.2012.711502. [DOI] [PubMed] [Google Scholar]
- 23.Park H, Kim CH, Jeong JH, Park M, Kim KS. GDF15 contributes to radiation-induced senescence through the ros-mediated p16 pathway in human endothelial cells. Oncotarget. 2016;7:9634–44. doi: 10.18632/oncotarget.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrache I, Petrusca DN, Bowler RP, Kamocki K. Involvement of ceramide in cell death responses in the pulmonary circulation. Proc Am Thorac Soc. 2011;8:492–6. doi: 10.1513/pats.201104-034MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sermsathanasawadi N, Ishii H, Igarashi K, Miura M, Yoshida M, Inoue Y, et al. Enhanced adhesion of early endothelial progenitor cells to radiation-induced senescence-like vascular endothelial cells in vitro. J Radiat Res. 2009;50:469–75. doi: 10.1269/jrr.09036. [DOI] [PubMed] [Google Scholar]
- 26.Ungvari Z, Podlutsky A, Sosnowska D, Tucsek Z, Toth P, Deak F, et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci. 2013;68:1443–57. doi: 10.1093/gerona/glt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yentrapalli R, Azimzadeh O, Sriharshan A, Malinowsky K, Merl J, Wojcik A, et al. The PI3K/Akt/mTOR pathway is implicated in the premature senescence of primary human endothelial cells exposed to chronic radiation. PLoS One. 2013;8:e70024. doi: 10.1371/journal.pone.0070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yentrapalli R, Azimzadeh O, Barjaktarovic Z, Sarioglu H, Wojcik A, Harms-Ringdahl M, et al. Quantitative proteomic analysis reveals induction of premature senescence in human umbilical vein endothelial cells exposed to chronic low-dose rate gamma radiation. Proteomics. 2013;13:1096–107. doi: 10.1002/pmic.201200463. [DOI] [PubMed] [Google Scholar]
- 29.Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–35. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CL, Moding EJ, Cuneo KC, Li Y, Sullivan JM, Mao L, et al. p53 functions in endothelial cells to prevent radiation-induced myocardial injury in mice. Sci Signal. 2012;5:ra52. doi: 10.1126/scisignal.2002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 32.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–7. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 33.Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–7. [PubMed] [Google Scholar]
- 34.Kirsch DG, Santiago PM, di TE, Sullivan JM, Hou WS, Dayton T, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–6. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–74. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doan PL, Russell JL, Himburg HA, Helms K, Harris JR, Lucas J, et al. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells. 2013;31:327–37. doi: 10.1002/stem.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muramoto GG, Chen B, Cui X, Chao NJ, Chute JP. Vascular endothelial cells produce soluble factors that mediate the recovery of human hematopoietic stem cells after radiation injury. Biol Blood Marrow Transplant. 2006;12:530–40. doi: 10.1016/j.bbmt.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 38.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–35. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim G, Meriin AB, Gabai VL, Christians E, Benjamin I, Wilson A, et al. The heat shock transcription factor Hsf1 is downregulated in DNA damage-associated senescence, contributing to the maintenance of senescence phenotype. Aging Cell. 2012;11:617–27. doi: 10.1111/j.1474-9726.2012.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaglom JA, McFarland C, Mirny L, Sherman MY. Oncogene-triggered suppression of DNA repair leads to DNA instability in cancer. Oncotarget. 2014;5:8367–78. doi: 10.18632/oncotarget.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoenicke L, Zender L. Immune surveillance of senescent cells–biological significance in cancer- and non-cancer pathologies. Carcinogenesis. 2012;33:1123–6. doi: 10.1093/carcin/bgs124. [DOI] [PubMed] [Google Scholar]
- 43.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–51. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 44.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acosta JC, Gil J. Senescence: a new weapon for cancer therapy. Trends Cell Biol. 2012;22:211–9. doi: 10.1016/j.tcb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Citrin DE, Shankavaram U, Horton JA, Shield W, III, Zhao S, Asano H, et al. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst. 2013;105:1474–84. doi: 10.1093/jnci/djt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki M, Boothman DA. Stress-induced premature senescence (SIPS)–influence of SIPS on radiotherapy. J Radiat Res. 2008;49:105–12. doi: 10.1269/jrr.07081. [DOI] [PubMed] [Google Scholar]
- 48.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohtani N, Takahashi A, Mann DJ, Hara E. Cellular senescence: a double-edged sword in the fight against cancer. Exp Dermatol. 2012;21(Suppl 1):1–4. doi: 10.1111/j.1600-0625.2012.01493.x. [DOI] [PubMed] [Google Scholar]
- 50.Luo X, Suzuki M, Ghandhi SA, Amundson SA, Boothman DA. ATM regulates insulin-like growth factor 1-secretory clusterin (IGF-1-sCLU) expression that protects cells against senescence. PLoS One. 2014;9:e99983. doi: 10.1371/journal.pone.0099983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–22. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marusyk A, Casas-Selves M, Henry CJ, Zaberezhnyy V, Klawitter J, Christians U, et al. Irradiation alters selection for oncogenic mutations in hematopoietic progenitors. Cancer Res. 2009;69:7262–9. doi: 10.1158/0008-5472.CAN-09-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010;8:e1000324. doi: 10.1371/journal.pbio.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao L, Feng W, Li H, Gardner D, Luo Y, Wang Y, et al. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood. 2014;123:3105–15. doi: 10.1182/blood-2013-07-515619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–66. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16Ink4a-positive cells shorten heathy lifespan. Nature. 2016;530:184–9. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azimzadeh O, Sievert W, Sarioglu H, Merl-Pham J, Yentrapalli R, Bakshi MV, et al. Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction. J Proteome Res. 2015;14:1203–19. doi: 10.1021/pr501141b. [DOI] [PubMed] [Google Scholar]
- 58.Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–23. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- 59.te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–83. [PubMed] [Google Scholar]
- 60.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–22. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J, Huang X, Halicka D, Brodsky S, Avram A, Eskander J, et al. Contribution of p16INK4a and p21CIP1 pathways to induction of premature senescence of human endothelial cells: permissive role of p53. Am J Physiol Heart Circ Physiol. 2006;290:H1575–86. doi: 10.1152/ajpheart.00364.2005. [DOI] [PubMed] [Google Scholar]
- 63.Cho JH, Kim MJ, Kim KJ, Kim JR. POZ/BTB and AT-hook-containing zinc finger protein 1 (PATZ1) inhibits endothelial cell senescence through a p53 dependent pathway. Cell Death Differ. 2012;19:703–12. doi: 10.1038/cdd.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HJ, Cho JH, Kim JR. Downregulation of Polo-like kinase 1 induces cellular senescence in human primary cells through a p53-dependent pathway. J Gerontol A Biol Sci Med Sci. 2013;68:1145–56. doi: 10.1093/gerona/glt017. [DOI] [PubMed] [Google Scholar]
- 65.Igarashi K, Miura M. Inhibition of a radiation-induced senescence-like phenotype: a possible mechanism for potentially lethal damage repair in vascular endothelial cells. Radiat Res. 2008;170:534–9. doi: 10.1667/rr1423.1. [DOI] [PubMed] [Google Scholar]
- 66.Paneni F, Osto E, Costantino S, Mateescu B, Briand S, Coppolino G, et al. Deletion of the activated protein-1 transcription factor JunD induces oxidative stress and accelerates age-related endothelial dysfunction. Circulation. 2013;127:1229–21. doi: 10.1161/CIRCULATIONAHA.112.000826. [DOI] [PubMed] [Google Scholar]
- 67.Zhan H, Suzuki T, Aizawa K, Miyagawa K, Nagai R. Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence. J Biol Chem. 2010;285:29662–70. doi: 10.1074/jbc.M110.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang E, Guo Q, Gao H, Xu R, Teng S, Wu Y. Metformin and resveratrol inhibited high glucose-induced metabolic memory of endothelial senescence through SIRT1/p300/p53/p21 Pathway. PLoS One. 2015;10:e0143814. doi: 10.1371/journal.pone.0143814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wan YZ, Gao P, Zhou S, Zhang ZQ, Hao DL, Lian LS, et al. SIRT1-mediated epigenetic downregulation of plasminogen activator inhibitor-1 prevents vascular endothelial replicative senescence. Aging Cell. 2014;13:890–9. doi: 10.1111/acel.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imaizumi N, Monnier Y, Hegi M, Mirimanoff RO, Ruegg C. Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting. PLoS One. 2010;5:e11084. doi: 10.1371/journal.pone.0011084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang H, Rha SY, Jeung HC, Park KH, Kim TS, Kim YB, et al. Telomerase- and angiogenesis-related gene responses to irradiation in human umbilical vein endothelial cells. Int J Mol Med. 2013;31:1202–8. doi: 10.3892/ijmm.2013.1300. [DOI] [PubMed] [Google Scholar]
- 72.Spallarossa P, Altieri P, Barisione C, Passalacqua M, Aloi C, Fugazza G, et al. p38 MAPK and JNK antagonistically control senescence and cytoplasmic p16INK4A expression in doxorubicin-treated endothelial progenitor cells. PLoS One. 2010;5:e15583. doi: 10.1371/journal.pone.0015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–44. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Kellner J, Liu L, Zhou D. Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev. 2011;20:1143–52. doi: 10.1089/scd.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koziel R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, et al. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J. 2013;452:231–9. doi: 10.1042/BJ20121778. [DOI] [PubMed] [Google Scholar]
- 76.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015;89:122–35. doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muller M. Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal. 2009;11:59–98. doi: 10.1089/ars.2008.2104. [DOI] [PubMed] [Google Scholar]
- 79.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18:1123–9. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pathak R, Shao L, Ghosh SP, Zhou D, Boerma M, Weiler H, et al. Thrombomodulin contributes to gamma tocotrienol-mediated lethality protection and hematopoietic cell recovery in irradiated mice. PLoS One. 2015;10:e0122511. doi: 10.1371/journal.pone.0122511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol (1985) 2009;106:326–32. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abderrahmani R, Francois A, Buard V, Tarlet G, Blirando K, Hneino M, et al. PAI-1-dependent endothelial cell death determines severity of radiation-induced intestinal injury. PLoS One. 2012;7:e35740. doi: 10.1371/journal.pone.0035740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian XL, Li Y. Endothelial cell senescence and age-related vascular diseases. J Genet Genomics. 2014;41:485–95. doi: 10.1016/j.jgg.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Minamino T, Miyauchi H, Yoshida T, Tateno K, Kunieda T, Komuro I. Vascular cell senescence and vascular aging. J Mol Cell Cardiol. 2004;36:175–83. doi: 10.1016/j.yjmcc.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 86.Minamino T, Komuro I. Vascular cell senescence: contribution to atherosclerosis. Circ Res. 2007;100:15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- 87.Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123:1900–10. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 88.Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular senescence, vascular disease, and aging: Part 1 of a 2-part review. Circulation. 2011;123:1650–60. doi: 10.1161/CIRCULATIONAHA.110.007021. [DOI] [PubMed] [Google Scholar]
- 89.Korpela E, Liu SK. Endothelial perturbations and therapeutic strategies in normal tissue radiation damage. Radiat Oncol. 2014;9:266–73. doi: 10.1186/s13014-014-0266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39–46. doi: 10.3389/fonc.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zagar TM, Cardinale DM, Marks LB. Breast cancer therapy-associated cardiovascular disease. Nat Rev Clin Oncol. 2016;13:172–84. doi: 10.1038/nrclinonc.2015.171. [DOI] [PubMed] [Google Scholar]
- 92.Stewart FA, Heeneman S, Te PJ, Kruse J, Russell NS, Gijbels M, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE–/– mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol. 2006;168:649–58. doi: 10.2353/ajpath.2006.050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yeung TK, Lauk S, Simmonds RH, Hopewell JW, Trott KR. Morphological and functional changes in the rat heart after X irradiation: strain differences. Radiat Res. 1989;119:489–99. [PubMed] [Google Scholar]
- 94.Baker JE, Fish BL, Su J, Haworth ST, Strande JL, Komorowski RA, et al. 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int J Radiat Biol. 2009;85:1089–100. doi: 10.3109/09553000903264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higashi Y, Kihara Y, Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res. 2012;35:1039–47. doi: 10.1038/hr.2012.138. [DOI] [PubMed] [Google Scholar]
- 96.Tchkonia T, Zhu Y, van DJ, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–72. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17:1205–17. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17:1049–61. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu Z, Yu Y, Liu C, Xiong Y, Montani JP, Yang Z, et al. Role of p38 mitogen-activated protein kinase in vascular endothelial aging: interaction with Arginase-II and S6K1 signaling pathway. Aging (Albany NY) 2015;7:70–81. doi: 10.18632/aging.100722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–36. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–48. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112:E6301–10. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–6. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–58. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15:1139–53. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 107.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–96. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 108.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–46. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]