Abstract
Background
Prader-Willi syndrome (PWS) is a rare obesity-related genetic disorder often caused by a deletion of the chromosome 15q11–q13 region inherited from the father or by maternal disomy 15. Growth hormone deficiency with short stature, hypogonadism, cognitive and behavioral problems, analgesia, decreased gastric motility and decreased ability to vomit with hyperphagia are common in PWS leading to severe obesity in early childhood, if not controlled. Substance P (SP) and beta-endorphin (BE) are neuropeptides involved with centrally and peripherally mediated pain perception, emotional regulation, and gastric motility impacting nausea, emesis and feeding patterns.
Objective
The goal of this study was to investigate potential mechanisms for PWS symptom development for pain, emotion and gastric motility and plasma levels of substance P and beta-endorphin between PWS and unrelated unaffected children.
Methodology
Plasma samples were collected from 23 Caucasian children with PWS and 18 unrelated, unaffected siblings with an average age of 8.2 ±2.0 years and age range of 5 to 11 years following an overnight fast and neuropeptide substance p and beta-endorphin levels were assessed using Multiplex sandwich immunoassays using the Luminex magnetic-bead based platform. Linear regression analysis was carried out on log-transformed values adjusted for age, sex, and body mass index (BMI).
Results
The mean plasma SP (57 ± 23 pg/ml) and BE (592 ± 200 pg/ml) levels in PWS were significantly higher than SP (35 ± 20 pg/ml, F=10.5, P<0.01) and BE (402 ± 162 pg/ml, F=10.8, P<0.01) levels found in unrelated, unaffected siblings suggesting a previously uncharacterized neuroendocrine pathophysiology in PWS.
Conclusions
The increased BE and SP plasma levels relative to unrelated, unaffected siblings may contribute to hyperphagia, abnormal pain sensation and adrenal insufficiency seen in PWS. Increases in SP levels may be modulated by central and/or peripheral actions of BE on opioid, GABA or POMC precursors and may reflect loss of feedback inhibitory control. Further studies are needed to confirm and elucidate the biochemical basis for observed disturbances in neuropeptide levels seen in our study and may impact on the development and persistence of symptoms commonly seen in PWS.
INTRODUCTION
Prader-Willi syndrome (PWS) is a rare disorder due to errors in genomic imprinting most often from a de novo paternal chromosome 15q11–q13 deletion seen in about 70% of cases, maternal disomy 15 (both 15s from the mother) in about 25% of cases or a small percentage of cases caused by imprinting defects. An initial finding in PWS is decreased fetal activity followed by severe infantile hypotonia1,2 with most babies exhibiting feeding difficulties with diminished swallowing and sucking reflexes. These symptoms resolve slowly in early childhood with the development of overeating and hyperphagia leading to severe obesity and corresponding comorbidities, if not controlled. Other symptoms of PWS include hypogenitalism/hypogonadism; cognitive impairment (average IQ of 65); temper tantrums, obsessive compulsive disorder, and skin picking; abnormal temperature regulation; analgesia; decreased gastric motility and growth hormone deficiency leading to short stature and small hands and feet.1,3 Central adrenal insufficiency is also reported in about 10% of cases.4 Enamel hypoplasia and dry, sticky saliva are common. PWS occurs in about 1 in 10,000 live births.1
While PWS was first described by Prader et al. in 19565, the mechanism(s) of symptom development and persistence have not yet been elucidated fully. Although dozens of genes have been localized to the 15q11–q13 region, the definitive genetic disturbances and causative pathophysiology in this rare obesity-related disorder have escaped characterization. In addition, there is a paucity of laboratory evaluations of neuro-related peptides that may contribute to PWS.
Beta-endorphin (BE) is a 31 amino acid peptide which is primarily synthesized and stored in the anterior pituitary gland. It is one of ten peptides produced from processing of proopiomelanocortin (POMC) by prohormone convertases.6 Additional products of POMC are adrenocorticotropic hormone (ACTH); N-terminal peptide of proopiomelanocortin (pro-γ-MSH); α-, β-, and γ-melanotropin; corticotropin-like intermediate peptide; β- and γ-lipotropin; and [Met]-enkephalin. Mutations of the POMC gene which is located on chromosome 2 are associated with onset of early childhood obesity, adrenal insufficiency, and changes in pigmentation (e.g., red hair); all of which can be seen in PWS.7 BE is stored in the hypothalamus and anterior pituitary gland and released in response to corticotropin releasing hormone secretion after stress.6 Gene expression studies from human lymphoblast cells from PWS subjects and whole brain specimens from a PWS mouse model show POMC gene disturbances (e.g., 34 fold increase in the PWS mouse model compared to normal littermates).8,9 BE functions as an opioid receptor agonist producing strong analgesic effects, also a common finding in PWS and act on the central nervous system (CNS) to produce overeating in rats.10
Substance P (SP), an 11 amino acid peptide derived from the preprotachykinin-A gene and secreted by enterochromaffin cells, is a member of the tachykinin neuropeptide family. It functions as a neurotransmitter and neuromodulator.11 This neuropeptide is associated with a variety of effects including neurogenic inflammation, stimulation of cell growth, vasodilation, pain perception, gastric motility and regulation of mood disorders, stress, anxiety, reinforcement, respiratory rhythm and nausea/emesis. It also plays a role in neurotoxicity and neurogenesis12–16 many of these features are present in PWS. In addition, exposure of mouse adipocytes to SP can produce altered regulation in adiposity and adipocytokine profiles. SP is also thought to function in transmission of pain signals with elevated levels associated with hyperalgesia. Therefore, a goal for this study was to explore whether substance P and beta-endorphin, both neuropeptides that modulate adiposity and function with complementary effects on pain perception, may contribute to the constellation of symptoms seen in PWS by comparing plasma levels in a cohort of PWS and control children.
METHODS
Subjects
Twenty-three American Caucasian children (13 males, 10 females) were diagnosed with PWS and confirmed by genetic testing with 15 having a paternal chromosome 15q11–q13 deletion and 8 showing maternal uniparental disomy 15 having an average age of 8.2 ± 2.0 years with an age range of 5 to 11 years. Eighteen American Caucasian children and 18 (10 males, 8 females) were unaffected, unrelated siblings having an average age of 8.2 ± 2.3 years within an age range of 5 to 11 years. These children were recruited from a large, ongoing, multi-site rare disease consortium on PWS. Consent forms were approved by the local Human Subjects Committee and signed. All children with PWS were receiving growth hormone, but were otherwise healthy. On an average less than ten percent of individuals with PWS present with adrenal gland insufficiency4 or thyroid problems.17 Peripheral blood was collected in anti-coagulant EDTA tubes in the morning following a supervised overnight fast and plasma separated immediately then stored at −80°C until used. Height (cm) and weight (kg) were also routinely obtained on each subject and body mass index (BMI) calculated using a wall mounted stadiometer and calibrated electronic weight balance, respectively, in the clinical setting. The average BMI ± SD for the 23 children diagnosed with PWS was 20.7 ± 5.0 while the average BMI ± SD for the 18 unaffected, unrelated siblings was 18.2 ± 2.3. Additional information including PWS genetic subtype, age, gender, weight, height, BMI and BMI-z score and total body fat percentage by dual-energy x-ray absorptiometry in relationship to selected plasma chemokine levels on each participant within this study cohort have previously been reported.18 Demographic, subject description and neuropeptide data are shown in Table 1.
Table 1.
Summary of Substance P and Beta-endorphin Levels
| Prader-Willi syndrome (n = 23) | Unrelated Siblings (n = 18) | F-value | P-value | |
|---|---|---|---|---|
| Age (yrs) | 8.2 ± 2.0 | 8.2 ± 2.3 | 0.01 | 0.92 |
| BMI (kg/m2) | 20.7 ± 5.0 | 18.2 ± 3.3 | 3.1 | 0.08 |
| Substance P (pg/ml) | 57.0 ± 23.1 | 34.8 ± 20.0 | 10.5 | < 0.01 |
| Beta-endorphin (pg/ml) | 592 ± 200 | 402 ± 162 | 10.8 | < 0.01 |
| Male (N=23) | Female (N=18) | |||
| Substance P (pg/ml) | 47.8 ± 24.7 | 46.5 ± 24.4 | 0.03 | 0.86 |
| Beta-endorphin (pg/ml) | 495 ± 197 | 526 ± 221 | 0.2 | 0.64 |
| Deletion (N=15) | UPD (N=8) | |||
| Substance P (pg/ml) | 52.5 ± 24.1 | 65.6 ± 19.9 | 1.7 | 0.20 |
| Beta-endorphin (pg/ml) | 555 ± 199 | 662 ± 197 | 1.5 | 0.23 |
Analysis carried out using uncontrolled analysis of variance by diagnostic subgroup, gender or PWS genetic subtype. UPD=uniparental maternal disomy 15
BETA-ENDORPHIN AND SUBSTANCE P ASSAYS
BE and SP levels were determined from plasma samples using the Milliplex Human Neuropeptide Kit (Millipore; Billerica, MA) and multiplex sandwich immunoassays following established protocols.19 Blood plasma (25ul) combined with Milliplex quality control standards were mixed with assay buffer and pre-mixed antibody-coupled magnetic beads followed by overnight incubation at 4°C. Incubation was carried out using a micro-titer plate shaker at 300 RPM. The biological samples were then washed on the following day and incubated for 1 hour at room temperature in the presence of secondary detection antibodies. Another series of washes were carried out then followed by the addition of a fluorescent Streptavidin-Phycoerythrin detection solution. The entire mixture was incubated at room temperature for 30 minutes. Each sample was run in duplicate. The plate was then read on the Luminex 200™ instrument (Luminex Molecular Diagnostic; Toronto, ON) based on magnetic-bead technology after sheath fluid was added to each sample then incubated. The level of magnetic field separates the beads. BE and SP levels were analyzed in both PWS and control plasma specimens using the Luminex 200™ v2.3 software with indicated minimal detectable concentration levels in pg/ml for each analyte. Plasma BE and SP concentrations were calculated using a standard curve derived from the reference BE and SP concentration standards supplied by the manufacturer. The inter-assay coefficient of variation for both BE and SP was < 20% and the intra-assay coefficient of variation was < 15%. Plasma samples were analyzed using numbers and blinded as to gender and control versus PWS status during each assay run.
STATISTICAL ANALYSIS
Data were presented as mean ± standard deviation for raw and/or log-transformed BE and SP levels by diagnosis (PWS or unrelated siblings), gender and PWS subtype. Pearson correlation and one-way analysis of variance (ANOVA) was used to test for significant differences in age, gender, BMI and diagnosis using log-transformed BE and SP levels (Table 1). Final linear regression analysis with Bonferroni correction was controlled for the effects of age, gender and BMI. Laboratory data falling below the detection limits were replaced with values at one half of the minimum detection level for the respective neuropeptide as reported in previous studies.19–21 Log-transformed data met the necessary statistical criteria for assumption of normality by showing equal variance and near linear residual plots. Findings with p-values of <0.05 were considered significant. Statistical analyses including descriptive statistics were generated using SAS statistical analysis software version 9.4 (SAS Inc., Cary, NC).
RESULTS
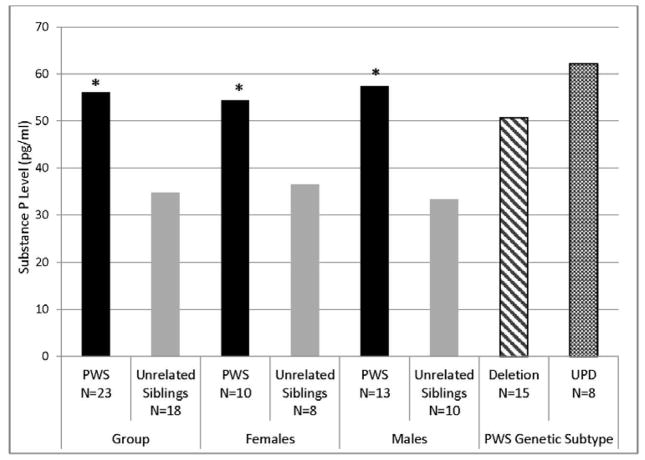
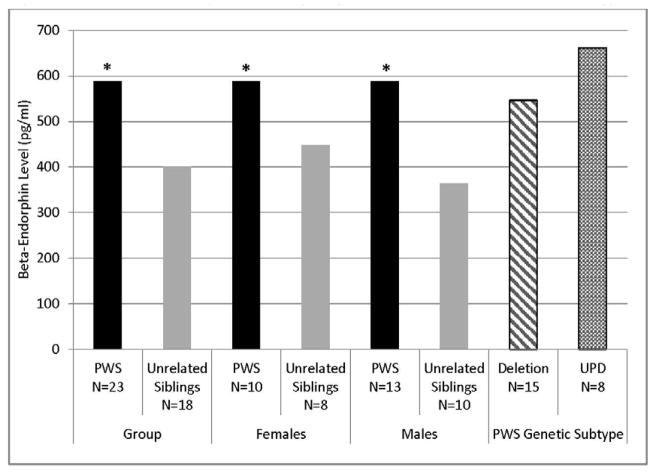
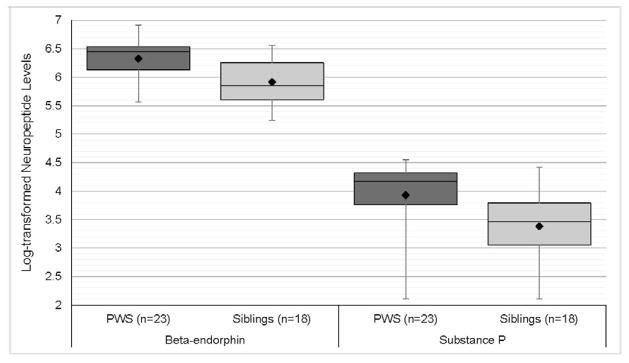
There was no significant difference in age, or BMI between PWS and unaffected, unrelated sibling groups (Table 1). There was also no significant difference in SP or BE levels between male and female subjects or between PWS deletion or uniparental maternal disomy 15 genetic subtypes (Table 1) or relevant correlations between plasma SP or BE levels with age, sex or body fat content. Plasma SP levels were significantly higher in PWS children (mean = 57.0 pg/ml) compared with unaffected, unrelated siblings (mean = 34.8 pg/ml) for log-transformed SP levels controlled for age, sex and BMI (F=4.3, P<0.01, Figure 1). The range of SP plasma levels in children with PWS was 8–95 pg/ml and 8–83 pg/ml for unaffected, unrelated siblings. Plasma BE levels were also significantly higher in PWS children (mean = 592 pg/ml) compared with unaffected, unrelated siblings (mean = 402 pg/ml) for log-transformed BE levels controlled for age, sex and BMI (F=6.5, P<0.001, Figure 2). The range of plasma BE levels in PWS children was 261–998 pg/ml and 188–705 pg/ml in the unaffected, unrelated siblings. Mean plasma BE levels were also determined for PWS genetic subtypes and subject subgroups with data shown in Figure 2. Box and whisker plot of log-transformed plasma BE and SP levels are shown in Figure 3.
Figure 1.
Plasma substance P levels by diagnosis, gender and PWS genetic subtype
Figure 2.
Plasma beta-endorphin levels by diagnosis, gender and PWS genetic subtype
Figure 3.
Plasma neuropeptide levels in Prader-Willi Syndrome and unrelated sibling controls
DISCUSSION
Children with PWS presented with statistically significant elevations in morning fasting plasma levels in both BE and SP compared to age and gender matched unaffected, unrelated siblings. Notable overlap exists between the actions of BE and SP and common features seen in PWS including increased pain threshold, hyperphagia (over-eating) and decreased gastric motility which may reflect a mechanism for pathogenicity observed in PWS. We focus our discussion on the complex regulatory interplay between BE and SP associated neuroendocrine pathways and their possible link to selective features seen in PWS.
Disturbances to POMC Influencing BE
Prohormone convertase enzymes function in the processing of POMC to yield BE and nine other proteins and precursor molecules. Disturbances in prohormone convertase enzyme function and subsequent disruptions in POMC gene expression may produce abnormal levels of proteins, which can result in phenotypically detectable symptoms. In 1994, Gabreël et al.22 described a subset of patients with PWS possessing altered immunoreactivity of pituitary polypeptide 7B2 [also called secretory granule neuroendocrine protein 1 (SGNE1)], a neuroendocrine chaperone that interacts with prohormone convertase 2 and is encoded on the human chromosome region 15q13–q14, distal to the PWS 15q11–q13 chromosome region.23 In those patients, the function of polypeptide 7B2 in the supraoptic nucleus and paraventricular nucleus was severely depressed or completely eliminated.22 A follow-up study confirmed that patients with PWS lacking polypeptide 7B2 immunoreactivity possessed slightly diminished prohormone convertase 1 immunoreactivity and demonstrated no prohormone convertase 2 immunoreactivity.24 Thus, at least a portion of patients with PWS are susceptible to changes in POMC levels and function, which could disturb BE levels. While polypeptide 7B2 mutations are unlikely to explain entirely the development and persistence of the symptoms seen in PWS, prior studies have shown associations between POMC disturbances and phenotypic manifestations common to PWS, such as early-onset obesity, adrenal insufficiency, and light pigmentation.25–28 In addition, Bittel et al.8 reported elevations in POMC gene expression in whole brain specimens of the PWS imprinting center deletion neonatal mice indicating an important genetic factor for survival of these mice. This may affect eating behavior regulating energy homeostasis. Also, POMC knockout mice have also been shown to develop obesity.
Analgesia
BE is present in CNS neurons and in the peripheral nervous system, where it acts as an opioid receptor agonist.10 In the peripheral nervous system, BE binds to pre- and post-synaptic nerve terminals of the primary afferent neurons, peripheral sensory nerve fibers, and dorsal root ganglia; attachment to pre-synaptic terminals is more common than post-synaptic.29 Binding of BE to opioid receptors in the peripheral nervous system leads to a reduction of SP levels.29,30
SP coexists with glutamate in primary afferents that respond to painful stimuli and is thought to participate in the transmission of pain signals to the CNS.13,31 Therefore, decreased SP levels following BE binding to opioid receptors may produce an analgesic state as SP, in contrast to BE, functions to enhance pain sensation. Indeed, mice lacking the neurokinin 1 receptor [NK1R or tachykinin receptor 1 (TACR1)] for SP demonstrated statistically significant decreases in hot-plate latency (45% reduction) and tail-flick latency (70% reduction) following cold water swims, which produces a non-opiate, NMDA-dependent analgesia.13 Conversely, elevated levels of SP should be associated with increased pain perception, i.e. hyperalgesia, in patients with PWS. In our PWS cohort, there was significant elevation of SP coupled with a history of decreased pain sensation or analgesia common in PWS indicating a potential loss of SP function in the CNS.
In the CNS, BE binds to opioid receptors at the pre- and post-synaptic nerve terminals of the amygdala, rostral ventral medulla, periaqueductal gray matter, and mesencephalic reticular formation; like in the peripheral nervous system, pre-synaptic binding predominates.32 Instead of reducing SP levels, the binding of BE to CNS receptors leads to inhibition of GABA release, which causes excess production of dopamine and downstream analgesia.32
The antinociceptive effect of BE in the CNS was first tested by Loh et al.33, whose team injected 1 μl of BE into rats and 5 μl of BE into mice intracerebrally. The results of BE injection were a two-fold increase in latency to the hot-plate and tail-flick response tests in mice; elimination of writing response following intraperitoneal acetic acid injection for as little as 25 minutes in mice; and significant decrease in shaking response following cold water immersion in rats.33 Quantification of BE potency has shown that its analgesic effect is 18–33 times stronger than morphine33 suggesting that persistently elevated BE levels in PWS might contribute to lack of pain perception. Simultaneous elevation of SP and BE levels may indicate a complex interaction and potential disruption of neuropeptide function in PWS, specifically the role of these neuropeptides in determining the levels of analgesia. However, our study did not contain measures on pain threshold in the study participants to permit correlation analysis with plasma neuropeptide levels but decreased pain sensation is a common finding in PWS.34
Obesity
Studies on both neuropeptides (BE and SP) have been associated with abnormal regulation of adiposity or overeating in animal models. The effects of SP on preadipocytes and mature adipocytes in mouse cells tested by Miegueu et al.35 and when treated with SP demonstrated decreased fatty acid uptake and storage; increased lipolysis; significantly reduced expression of differentiation-related transcription factors peroxisome proliferator-activated receptor gamma – 2 (PPARγ2) and CCAAT/enhancer binding protein, alpha (CEBPα) as well as fatty acid binding protein 4 (FABP4) and diacylglycerol O-acyltransferase-1. Significantly elevated CD36 (thrombospondin receptor) expression, increased secretion of complement component C3, monocyte chemoattractant protein-1 (MCP-1), and keratinocyte-derived chemokine, and downregulation of insulin receptor substrate-1, insulin-responsive glucose transporter (SLC2A4), adiponectin mRNA expression, and insulin-mediated action were also noted.34 Observed alterations to transcription factors led to exploration into the effects of SP on preadipocyte differentiation, which was decreased by SP exposure.35 In our children with PWS, abnormal elevations of SP were detected that may have contributed to dysregulation of adiposity, although further experiments would be required to determine if their adipocytokine profiles match those detected by Miegueu et al.35
BE levels may also be involved in the development and persistence of obese states. For example, genetically obese (ob/ob) mice showed a 14-fold elevation in corticotropin levels has been reported by Edwardson and Hough.36 Beloff-Chain et al.37 also detected an increase in corticotropin-like intermediate peptide levels. Elevations in both corticotropin and corticotropin-like intermediate peptide are significant as both neuropeptides share a common precursor with BE. Corticotropin and BE are both released concomitantly in vivo and in vitro from rat pituitary glands, but the synthesis and/or degeneration of these neuropeptides may be uncoupled.6,38 Functionally, injection of BE into the rat brain is associated with overeating39 and conversely, administration of naloxone, an opiate antagonist, in ob/ob mice and fa/fa rats yielded a significant reduction of food intake without affecting lean littermates. The pituitaries of the obese mice contained twice as much BE as well.38 Therefore, in PWS with elevated BE levels and exaggerated neuropeptide effects contributing to overeating in PWS. Although hyperphagia is a cardinal feature in PWS, our study did not include hyperphagia measurement data such as diet records or PWS nutritional phases40 to judge the level of overeating or hyperphagia in relationship to the neuropeptide levels in the PWS children.
Adrenal Insufficiency
Regulation of the hypothalamic corticotropin releasing hormone neurons in rats was tested in 1988 by Calogero et al.41 particularly the effects of corticotropin releasing hormone, glucocorticoids, and products of POMC processing – corticotropin, BE, α-melanocyte-stimulating hormone, corticotropin-like intermediate peptide, β-lipotropin, and dexamethasone. POMC products generate inhibitory effects on corticotropin, dexamethasone and BE, α-melanocyte-stimulating hormone, and corticotropin-like intermediate peptide with BE as the strongest inhibitor of the three.41 The results of the Calogero et al. study implied the existence of several complicated negative feedback loops indicating the potential effect that elevated BE levels may have on adrenal function in individuals with PWS. A limitation of our study included the lack of information about the adrenal insufficiency status in each individual PWS child in our cohort and comparison with individual neuropeptide levels. However, an average of only 10 percent of children with PWS are reported to have adrenal insufficiency4.
CONCLUSIONS
BE and SP act in complicated facilitatory and inhibitory pathways, sometimes antagonistically, and may contribute to common features seen in PWS, particularly obesity/overeating, abnormal pain sensation, and adrenal insufficiency. However, a limitation of our study included the lack of markers for hyperphagia, pain sensation and adrenal insufficiency with the prior two features considered common in individuals with PWS. Experiments on mouse adipocytes demonstrated that persistent SP exposure can produce altered regulation of adiposity and adipocytokine profiles, whereas BE can act in the CNS to produce overeating in rats; blocking opiate receptors with naloxone significantly reduced food intake in obese mice and rats. SP is also thought to function in transmission of pain signals, and elevated levels may be associated with hyperalgesia. Conversely, BE binding to opioid receptors in the peripheral nervous system leads to reduced SP levels, decreasing pain perception; centrally, BE binding causes a reduction of GABA release that produces analgesia. Lastly, disturbances to POMC, the precursor molecule to BE, are associated with early-onset obesity, light pigmentation, and adrenal insufficiency; high levels of BE found in PWS children in our study compared with unaffected, unrelated siblings may produce feedback inhibition of serotonin-stimulated release of corticotropin releasing hormone.
Hence, children with PWS in our study presented with significantly elevated plasma levels of BE and SP compared to age and gender matched unaffected, unrelated siblings. Elevations of BE levels may be partly explained by the work of Gabreël et al.22,24, who found that altered 7B2 immunoreactivity in a group of patients with PWS was associated with reduced or eliminated prohormone convertase 2 function, but this is unlikely to encompass all patients with PWS and supported by POMC gene expression in human PWS subjects and PWS mouse models.8,9 A paradoxical elevation of SP also complicates interpretation of the role of these neuropeptides in PWS pathophysiology. While more work must be done to determine if BE and SP are functioning properly in the central and peripheral nervous system, the functions of BE coalesce with the constellation of symptoms often seen in PWS, particularly in decreasing pain perception, producing overeating, and inhibiting release of corticotropin releasing hormone. If SP is involved in PWS symptom development, it may be in modifying adipocytokine profiles, but SP otherwise seems unable to function properly in the central nervous system of PWS. Our study describes a preliminary approach to examine differences in plasma neuropeptide levels in children with and without PWS, future studies should include objective measures of hyperphagia, pain sensation and adrenal insufficiency in relationship to the plasma concentration levels for these neuropeptides.
Acknowledgments
FUNDING
Partial funding support was received from the Angelman, Rett and Prader-Willi Syndrome Consortium (U54 HD06122) which is a part of the National Institute of health (NIH) Rare Disease Clinical Research Network (RDCRN) supported through collaboration between the NIH Office of Rare Disease Research (ORDR) at the National Center of Advancing Translational Science (NCATS) and the National Institute of Child Health and Human Development (NICHD). NICHD grant number HD02528 is also acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the office views of the National Institutes of Health.
We thank Carla Meister for preparation of the manuscript and Carlos Sulsona for technical assistance.
Footnotes
CONFLICT OF INTEREST
None of the authors have any commercial or other associations that might pose a conflict in connection with the submitted manuscript.
References
- 1.Butler MG. Prader–Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990;35:319–332. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bittel DC, Butler MG. Prader–Willi syndrome: clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med. 2005;7:1–20. doi: 10.1017/S1462399405009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MG. Genomics of childhood obesity. Curr Genomics. 2011;12(3):153. doi: 10.2174/138920211795677949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler MG, Brandau DT, Theodoro M, Garg U. Cortisol levels in Prader-Willi syndrome support changes in routine care. Am J Med Genet A. 2009;149A:138–9. doi: 10.1002/ajmg.a.32633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prader A, Labhart A, Willi H. Ein syndrome von adipositas, kleinwuchs, kryptorchismus und oligophrenie nach myatonieartigem zustand im neugeborenenalter. Schweizerishe Med Wochen. 1956;86:1260–1261. [Google Scholar]
- 6.Guillemin R, Vargo T, Rossier J, et al. Beta-endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science. 1977;4311:1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- 7.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency, and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;2:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 8.Bittel DC, Kibiryeva N, McNulty SG, Driscoll DJ, Butler MG, White RA. Whole genome microarray analysis of gene expression in an imprinting center deletion mouse model of Prader-Willi syndrome. Am J Med Genet A. 2007a;143A(5):422–429. doi: 10.1002/ajmg.a.31504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bittel DC, Kibiryeva N, Sell SM, Strong TV, Butler MG. Whole genome microarray analysis of gene expression in Prader-Willi syndrome. Am J Med Genet A. 2007b;143A(5):430–42. doi: 10.1002/ajmg.a.31606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CH, Lemaire S, Yamashiro D, Doneen B. The synthesis and opiate activity of β-endorphin. Biochem Bioph Res Co. 1976;1:19–25. doi: 10.1016/0006-291x(76)90243-6. [DOI] [PubMed] [Google Scholar]
- 11.Krause JE, Chirgwin JM, Carter MS, Xu ZS, Hershey AD. Three rate preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci USA. 1987;3:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossaller C, Reither K, Hehlert-Friedrich C, et al. In vivo measurement of endothelium-dependent vasodilation with substance P in man. Herz. 1992;5:284–290. [PubMed] [Google Scholar]
- 13.De Felipe C, Herrero JF, O’Brien JA, et al. Altered nociception, analgesia, and aggression in mice lacking the receptor for substance P. Nature. 1998;6674:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 14.Donkin JJ, Nimmo AJ, Cernak I, Blumbergs PC, Vink R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J Cerebr Blood F Met. 2009;8:1388–1398. doi: 10.1038/jcbfm.2009.63. [DOI] [PubMed] [Google Scholar]
- 15.Ebner K, Muigg P, Singewald G, Singewald N. Substance P in stress and anxiety. Ann NY Acad Sci. 2008;1:61–73. doi: 10.1196/annals.1418.018. [DOI] [PubMed] [Google Scholar]
- 16.Krowicki ZK, Hornby PJ. Substance P in the dorsal motor nucleus of the vagus evokes gastric motor inhibition via neurokinin 1 receptor in rat. J Pharmacol Exp Ther. 2000;1:214–221. [PubMed] [Google Scholar]
- 17.Butler MG, Theodoro M, Skouse JD. Thyroid function studies in Prader-Willi syndrome. Am J Med Genet A. 2007;143A:488–92. doi: 10.1002/ajmg.a.31683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler MG, Hossain W, Sulsona C, Driscoll DJ, Manzardo AM. Increased plasma chemokine levels in children with Prader-Willi syndrome. Am J Med Genet A. 2015;167:563–571. doi: 10.1002/ajmg.a.36908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manzardo AM, Henkhaus R, Dhillon S, Butler MG. Plasma cytokine levels in children with autistic disorder and unrelated siblings. Int J Dev Neurosci. 2012;30(2):121–127. doi: 10.1016/j.ijdevneu.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashwood P, Nguyen DV, Hessl D, Hagerman RJ, Tassone F. Plasma cytokine profiles in Fragile X subjects: is there a role for cytokines in the pathogenesis? Brain Behav Immun. 2010;24:898–902. doi: 10.1016/j.bbi.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zerbo O, Yoshida C, Grether JK, et al. Neonatal cytokines and chemokines and risk of Autism Spectrum Disorder: the Early Markers for Autism (EMA) study: a case-control study. J Neuroinflam. 2014;111:113. doi: 10.1186/1742-2094-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabreël BA, Swaab DF, Seidah NG, Van Duynhoven HLP, Martens GJM, Van Leeuwen FW. Differential expression of the neuroendocrine polypeptide 7B2 in hypothalami of Prader-(Labhart)-Willi syndrome patients. Brain Res. 1994;1:281–293. doi: 10.1016/0006-8993(94)90978-4. [DOI] [PubMed] [Google Scholar]
- 23.Roebroek AJ, Dehaen MR, van Bokhoven A, et al. Regional mapping of the human gene encoding the novel pituitary polypeptide 7B2 to chromosome 15q13–q14 by in situ hybridization. Cytogenet Cell Genet. 1989;50(2–3):158–160. doi: 10.1159/000132749. [DOI] [PubMed] [Google Scholar]
- 24.Gabreël BA, Swaab DF, de Kleijn DPV, et al. Attenuation of the polypeptide 7B2, prohormone convertase PC2, and vasopressin in the hypothalamus of some Prader-Willi patients: indications for a processing defect. J Clin Endocr Metab. 1998;2:591–599. doi: 10.1210/jcem.83.2.4542. [DOI] [PubMed] [Google Scholar]
- 25.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency, and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;2:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 26.Kuehnen P, Mischke M, Wiegand S, et al. An Alu element-associated hypermethylation variant of the POMC gene is associated with childhood obesity. Plos Genet. 2012;3:e1002543. doi: 10.1371/journal.pgen.1002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millington GWM. The role of proopiomelanocortin (POMC) neurons in feeding behavior. Nutr Metab. 2007;4(1):18. doi: 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler MG. Hypopigmentation: a common feature of Prader-Labhart-Willi syndrome. Am J Hum Genet. 1989;45:140–146. [PMC free article] [PubMed] [Google Scholar]
- 29.Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman S. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;5769:155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- 30.Yaksh TL. Substance P release from knee joint afferent terminals: modulation by opioids. Brain Res. 1988;458:319–324. doi: 10.1016/0006-8993(88)90474-x. [DOI] [PubMed] [Google Scholar]
- 31.Yaksh TL, Wallace MS. Chapter 18: Opioids, Analgesia, and Pain Management. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill; 2011. p. 12e. [Google Scholar]
- 32.Stein C, Kopf A. Section V. Anesthesia and Treatment of Chronic Pain. In: Miller RD, editor. Miller’s Anesthesia. Vol. 5. 7e Philadelphia, PA: Elsevier/Churchill Livingstone; 2010. pp. 1798–1800. [Google Scholar]
- 33.Loh HH, Tseng LF, Wei E, Li CH. Beta-endorphin is a potent analgesic agent. Proc Natl Acad Sci USA. 1976;8:2895–2898. doi: 10.1073/pnas.73.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler MG, Lee PDK, Whitman BY, editors. Management of Prader-Willi Syndrome. 3. New York: Springer; 2006. [Google Scholar]
- 35.Miegueu P, St-Pierre DH, Lapointe M, et al. Substance P decreases fat storage and increases adipocytokine production in 3T3-L1 adipocytes. Am J Physiol Gastrointest Liver Physiol. 2013;304:G420–427. doi: 10.1152/ajpgi.00162.2012. [DOI] [PubMed] [Google Scholar]
- 36.Edwardson JA, Hough CAM. The pituitary-adrenal system of the genetically obese (ob/ob) mouse. J Endocrinol. 1975;1:99–107. doi: 10.1677/joe.0.0650099. [DOI] [PubMed] [Google Scholar]
- 37.Beloff-Chain A, Edwardson JA, Hawthorn J. Influence of the pituitary gland on insulin secretion in the genetically obese (ob/ob) mouse. J Endocrinol. 1975;1:109–116. doi: 10.1677/joe.0.0650109. [DOI] [PubMed] [Google Scholar]
- 38.Margules DL, Moisset B, Lewis MJ, Shibuya H, Pert CB. β-endorphin is associated with overeating in genetically obese mice (ob/ob) and rats (fa/fa) Science. 1978;4371:988–991. doi: 10.1126/science.715455. [DOI] [PubMed] [Google Scholar]
- 39.Grandison Ll, Guidotti A. Stimulation of food intake by muscimol and beta endorphin. Neuropharmacol. 1977;7:533–536. doi: 10.1016/0028-3908(77)90019-3. [DOI] [PubMed] [Google Scholar]
- 40.Miller JL, Lynn CH, Driscoll DC, Goldstone AP, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A. 2011;155A:1040–1049. doi: 10.1002/ajmg.a.33951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calogero AE, Gallucci WT, Gold PW, Chrousos GP. Multiple feedback regulatory loops upon rat hypothalamic corticotropin-releasing hormone secretion. Potential clinical implications. J Clin Invest. 1988;3:767–774. doi: 10.1172/JCI113677. [DOI] [PMC free article] [PubMed] [Google Scholar]