Abstract
Aims
This study tested the hypothesis that changes in momentary affect, abstinence motivation, and confidence would predict lapse risk over the next 12–24 hours using Ecological Momentary Assessment (EMA) data from smokers attempting to quit smoking.
Method
103 adult, daily, treatment-seeking smokers recorded their momentary affect, motivation to quit, abstinence confidence, and smoking behaviors in near real time with multiple EMA reports per day using electronic diaries post-quit.
Results
Multilevel models indicated that initial levels of negative affect were associated with smoking, even after controlling for earlier smoking status, and that short-term increases in negative affect predicted lapses up to 12, but not 24, hours later. Positive affect had significant effects on subsequent abstinence confidence, but not motivation to quit. High levels of motivation appeared to reduce increases in lapse risk that occur over hours while momentary changes in confidence did not predict lapse risk over 12 hours.
Conclusion
Negative affect had short-lived effects on lapse risk, whereas higher levels of motivation protected against the risk of lapsing that accumulates over hours. An increase in positive affect was associated with greater confidence to quit, but such changes in confidence did not reduce short-term lapse risk, contrary to expectations. Relations observed among affect, cognitions, and lapse seem to depend critically on the timing of assessments.
Keywords: smoking cessation, affect, motivation, confidence, ecological momentary assessment
Despite the advent and dissemination of effective psychosocial and pharmacological treatments for tobacco dependence (Fiore et al., 2008), relapse (i.e., a return to regular smoking) remains the most common outcome of smoking cessation attempts (Brownell et al., 1986; Piasecki, 2003; Shiffman et al., 2008). While the dynamic process leading to relapse varies greatly across and within individuals (Tindle et al, 2006), approximately 85–95% of lapsers ultimately relapse even with an effort to reestablish abstinence (e.g., Brandon, et al., 1986; Kenford, et al, 1994). Moreover, the majority of single lapse episodes progress to relapse relatively quickly (Brandon et al., 1990; Kenford et al., 1994). This is a critical problem with deadly consequences. Studying lapse allows us to examine the initial transition in smoking status from abstinence to smoking that must precede a return to regular smoking (relapse). Identifying the proximal affective and cognitive processes that lead to lapses may help us predict and ultimately prevent the initial lapses that typically culminate in relapse (Kassel et al., 2003, Gwaltney, et al., 2005b, Piasecki et al., 2002, Shiffman, 2005). The current project focuses on predicting smoking behavior during a quit attempt from proximal reports of both negative and positive affect. More specifically, we test the hypothesis that affect influences smoking during a cessation attempt both directly and indirectly by undermining cessation motivation and confidence.
Negative Affect
The role of negative affect in smoking cessation has been extensively studied. Stress and negative affect often precede lapses during cessation attempts (Marlatt & Gordon, 1980; O’Connell & Martin, 1987; Shiffman, 1982; see Kassel et al., 2003 for a review). A modified negative reinforcement drug motivation model proposed by Baker et al. (2004) asserts that escape from or avoidance of negative affect plays a central role in the maintenance of addictive behavior. The model also posits that non-withdrawal aversive affect (e.g., anxiety or distress induced by external events) may trigger the same responses (i.e., craving and smoking) that negative affect from withdrawal does. As such, smokers may smoke to escape aversive affective states even when these states are not related to withdrawal symptoms (Baker et al., 2004). In fact, many individuals identify smoking as their way of dealing with stressful situations (Brandon et al., 1999; Copeland et al., 1995).
Whether part of withdrawal or prompted by stressful events, negative affect plays a significant role in smoking behavior. Yet, the cognitive pathways linking negative affect to smoking outcomes are little studied and poorly understood. It may be that negative affect increases smoking risk directly, as asserted by the reformulated negative affect model (Baker et al. 2004), and indirectly, by altering smoking-relevant cognitions, such as motivation to quit smoking and confidence in one’s ability to quit smoking. Affective distress, for example, may erode one’s willingness to work at quitting and confidence that one can cope with the stress of quitting to abstain successfully.
Positive Affect
Recent evidence also points to the importance of positive affect in smoking motivation and behavior change processes, independent of negative affect. For example, recent research (Doran et al., 2008) found that the effect of anhedonia (i.e., diminished capacity to experience pleasure) on heightened urges to smoke post-quit is mediated by decreased positive affect rather than increased negative affect. Looking at only negative affect may lead to an incomplete picture of the process of addictive behavior change.
The broaden-and-build model proposed by Fredrickson (2000, 2003) suggests that positive affect sparks changes primarily in cognitive activity, rather than directly influencing physical action (Fredrickson & Branigan, 2001). The positive affect model asserts that openness to novel experiences and an active search for resources promote desired changes (Wagner & Ingersoll, 2008). When a person experiences interest or surprise, his/her attention is broadened and, in turn, he/she is able to consider choices that previously had been disregarded or rejected. Resolution of ambivalence may be facilitated by this increased flexibility in perception which may guide one toward change (Fredrickson & Branigan, 2001).
The roles of positive affect in health behavior and goal-oriented behavior have been demonstrated in various studies. For example, recent prospective studies showed that positive affect responses to a brief exercise trial were associated with more stable motivation to exercise (Kwan, 2010) and subsequent exercise behavior (Standage, 2010). That is, those who experienced an increase in positive affect during a bout of exercise were more likely to have steady intentions to exercise and actually exercise in the future. Furthermore, increases in positive affect are associated with confidence and performance (e.g., exercise confidence, Ostir et al, 2003; test performance, Nelson, 2010).
Motivation to Quit
Motivation is a critical determinant of behavior change (Ajzen, 1991; Miller & Rollnick, 2002). In smoking cessation studies, motivation to quit is rarely treated as a dynamic construct, despite the fact that research has shown that motivation levels fluctuate even within a short-term period (e.g., Berman et al., 2010; Lavigne et al., 2009). Using EMA data, Piasecki et al. (2002) showed that smokers’ motivation to quit following their quit date was dynamic and that abstainers and relapsers showed different growth patterns of motivation over a 7-week post-quit period. Despite the conceptual and empirical bases for treating motivation as dynamic, research on real-time relations among affect, motivation to quit, and smoking behavior is lacking. Such research is needed to understand the effects of motivational drives on smoking behavior in naturalistic environments. Assessing changes in motivation following changes in affect during a quit attempt may help identify proximal precipitants of smoking lapses. Such information about predictors of motivational lapses that, in turn, predict behavioral lapses may facilitate intervention development. Just-in-time interventions that bolster motivation to quit may reduce lapse vulnerability during a quit attempt, for example.
Abstinence Confidence
The important roles of cognitions, particularly confidence, in intended behavioral change have been the focus of much research (e.g., Bandura, 1977; Shiffman et al., 2005) and the role of confidence in successful smoking cessation has been extensively studied (e.g., Condiotte & Lichtenstein, 1981; Shiffman, 2005). Smoking cessation research and treatments shaped by a social learning model of relapse focus on enhancing confidence and maintaining the perceived importance of quitting (Abrams et al., 2003; Marlatt & Donovan, 2005).
Some smoking cessation research has treated confidence as a dynamic construct. For example, Gwaltney et al. (2005a) assessed abstinence confidence and related constructs using ecological momentary assessment (EMA) and found that heightened cigarette craving and negative affect were related to decreases in confidence, especially in those with low baseline abstinence confidence. Furthermore, Shiffman (2005) demonstrated that day-to-day changes in abstinence confidence predicted relapse following a first lapse and concluded that negative affect predicted smoking behavior, at least partially, through undermining momentary confidence. Taken together, these findings indicate that changes in quitting confidence elicited by situational factors (e.g., affective distress) signal increased risk for smoking during a change attempt.
Roles of Motivation and Confidence in a Cessation Attempt
A recent study that examined the efficacy of sustained-release bupropion as a smoking cessation treatment revealed that motivation and confidence mediated the effect of bupropion SR on smoking outcomes (McCarthy et al., 2008). Moreover, a controlled laboratory study that aimed to assess how drug motivation influences health beliefs indicated that cigarette craving reduces confidence and intention to quit (Nordgren et al., 2008). Although this was not a smoking cessation study, the finding supports the notion that cognitions related to health behavior are dynamic. Moreover, McCarthy et al. (2008) demonstrated that confidence and motivation to quit smoking changed over the first week post-quit, although only the initial level of post-quit motivation, not the rate of change, was predictive of abstinence in one month. To date, the dynamic nature of motivation to change specific behaviors has been studied primarily by assessing day-to-day changes. The effects of acute motivation change on shorter-term behavioral outcomes are not well understood, however, and are in need of further study.
Study Hypotheses
We tested a complex set of hypotheses regarding the short-term effects of negative and positive affect on smoking behavior within 12 to 24 hours through changes in motivation to quit and quitting confidence in the context of an attempt to quit smoking, as depicted in Figures 1–3. We predicted that increases in negative affect would erode motivation and confidence, while positive affect would have the opposite effects on these cognitions. Second, we predicted that declines in momentary confidence and motivation would predict increased risk of a smoking lapse.
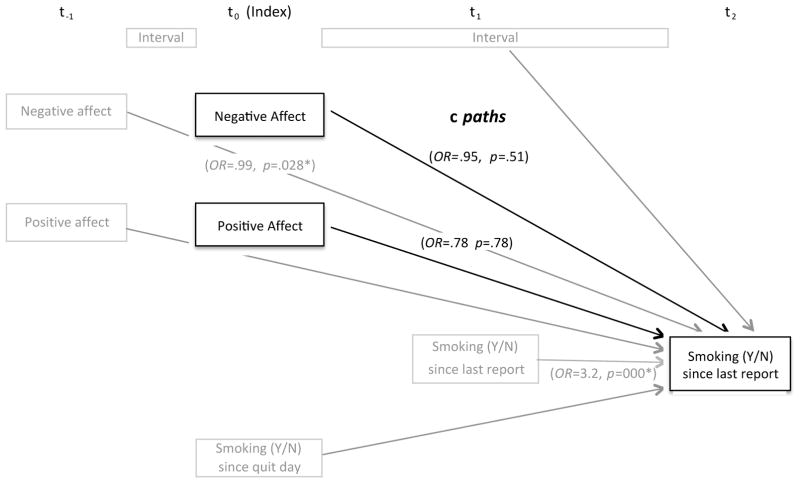
Figure 1. Model of hypothesized relations between negative and positive affect, and smoking lapse (c paths).
The model was fit to test the hypothesized direct effects of affect change on smoking behavior using HLM analysis. Odds ratios and p values were indicated only for variables of primary interest (shown in black) and control variables (shown in gray) that were significantly associated with smoking outcomes. * p < .05
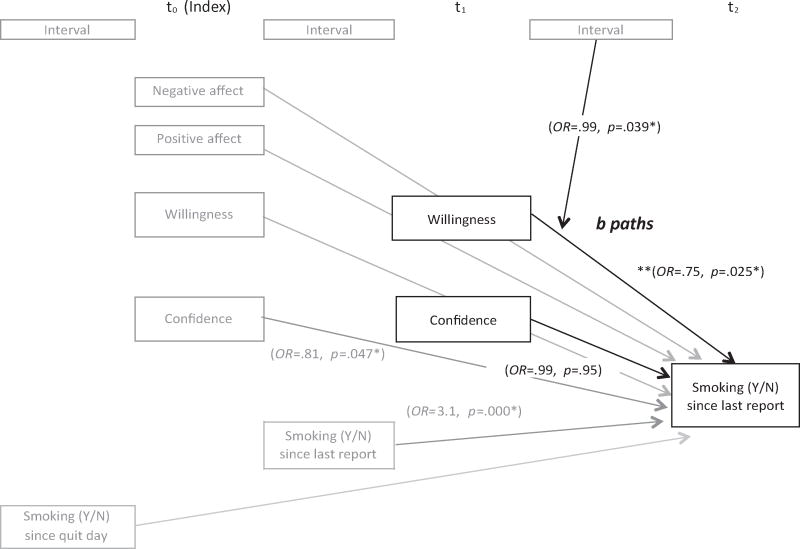
Figure 3. Model of hypothesized relations between cognitions and smoking lapse (b paths).
The model was fit to test the hypothesized the effects of willingness to work and confidence at t1 on smoking behavior using HLM analysis. Odds ratios and p values were indicated only for variables of primary interest (shown in black) and control variables (shown in gray) that were significantly associated with smoking outcomes. * p < .05. ** Odds ratio and p value were obtained from the model without a willingness X time interaction term.
Results from the proposed study may add to the literature regarding 1) the time course and mediators of affective influences on smoking lapses and 2) the role of explicit cognitions during a quit attempt, particularly in relation to affect and later smoking.
Method
Participants
For this study, 130 adult smokers were recruited in central New Jersey via mass media calls for smoking cessation research participants. Participants were screened for the following inclusion criteria: 18 years of age or older, English literacy, smoking a minimum of 10 cigarettes per day for at least 6 months, an expired carbon monoxide (CO) level of 8 parts per million or greater, motivation to quit smoking of at least 6 on a 10-point scale, and willingness to fulfill study requirements. Exclusion criteria included: living with someone enrolled in the study; contraindications to the use of nicotine lozenges (e.g., recent heart attack or heart surgery, heart disease, angina, irregular heartbeat, pregnancy, breastfeeding, past problems using the lozenge); serious psychiatric conditions (i.e., bipolar disorder or psychosis); and current use of other forms of tobacco, smoking cessation treatments, marijuana, or other illegal drugs.
Procedures
All study procedures were approved by an Institutional Review Board. Interested volunteers responding to mass media were first screened for eligibility over the telephone. Eligible individuals were invited to a group orientation session at which they received a detailed description of the study and written informed consent was obtained. Baseline data collection, CO testing, and electronic diary (ED) training were also performed at the orientation session. All participants in this study received standard smoking cessation treatment including four brief individual counseling sessions based on the Clinical Practice Guideline Treating Tobacco Use and Dependence (Fiore et al., 2008) and the Treating Tobacco Dependence Handbook (Abrams et al., 2003) and a 12-week course of nicotine lozenges for use beginning on a target quit day set by the researchers. Participants attended five study visits at weekly intervals beginning one week pre-quit and ending three weeks post-quit. Fifteen-minute counseling sessions were offered at the first four office visits and the nicotine lozenges were dispensed one week pre-quit, with instruction to begin lozenge use the morning of the quit day one week later. Individuals who smoked within 30 minutes of waking received 4-mg nicotine lozenges whereas those who waited more than 30 minutes before smoking received 2-mg lozenges (Shiffman, Dresler, & Rohay, 2004). Participants chose their preferred lozenge flavor (cherry or mint). The key constructs were assessed within a larger battery of assessments.
Participants carried EDs from day −10 to 21, relative to the quit date. CO testing was conducted at all visits and again 12-weeks post-quit for participants who reported seven-day point-prevalence abstinence at the 12-week follow-up call. Maximum remuneration for completing the 5 scheduled office visits, a follow-up call, and a follow-up visit, was $130, contingent upon return of the ED after the recording period (if the ED was not returned, $125 was deducted from participants’ compensation). Up to a maximum of $545 were possible in additional rewards depending on participants’ performance on behavioral measures of impulsiveness in the laboratory and on the ED. Actual bonuses averaged $317 and ranged from $27 to $513. The total amount of compensation works out to roughly $25 per hour for those who completed all study activities.
Measures
Baseline Assessment
At an initial group orientation session, participants provided breath samples for carbon monoxide testing and completed the self-report measures described below. These measures were used as baseline covariates in the current longitudinal models of data.
The Fagerström Test for Nicotine Dependence (FTND) consists of 6 items (e.g., “How soon after waking do you smoke your first cigarette?”) and has a maximum score of 10. A higher score indicates greater physical dependence on nicotine and a score of five indicates moderate dependence (Fagerström, Heatherton & Kozlowski, 1992). The FTND has fair internal consistency (Cronbach’s α = .61) (Heatherton et al, 1991) and high test–retest correlations (r =.85 to .88; Etter et al., 1999; Pomerleau et al., 1994).
The Positive and Negative Affect Scale (PANAS) is a 20-item self-report measure of affective state (10 items assessing positive affect and 10 assessing negative affect) rated on a 5-point scale (ranging from 1 = very slightly to 5 = extremely) during a specified period of time (e.g., past week). The PANAS has good internal consistency (α = .84 to .90) and validity as a measure of subjective affect (Watson et al., 1988, Crawford & Henry, 2004).
The Wisconsin Smoking Withdrawal Scale (WSWS) is a 28-item scale that taps the central elements of the nicotine withdrawal syndrome. It consists of seven subscales (i.e., anger, anxiety, sadness, concentration, hunger, sleep, and craving). Internal consistencies range from α = .75 to α = .93 for the subscales and α = .90 for the total score (Welsh et al., 1999). Validity analyses also show that the WSWS negative affect scales correlate with the negative affect items of the PANAS (r = .46–.59) and the WSWS scales significantly predict smoking outcomes (Welsh et al., 1999).
Ecological Momentary Assessment
Participants were asked to carry palmtop computers, or electronic diaries (EDs, Palm Z22 Palmtop Computers, Palm, Inc., Santa Clara, CA) for 31 days, including a 3-day practice period, one week pre-quit, and three-weeks post-quit. Each day during this assessment period, participants were prompted at four pseudo-random times throughout the day (the alarms were set at randomly selected times within four equal intervals between the participant’s wake-up and bed times and were at least 30 minutes apart). We excluded reports that were completed within 15 minutes of a previous report from the analyses to preserve the temporal separation of reports. The prompts signaled participants to complete a brief delay discounting task before completing reports of negative and positive affect, withdrawal symptoms, craving, restlessness, willingness to work hard at quitting (i.e., motivation to quit), confidence in ability to quit smoking for good, smoking since last report, and use of nicotine lozenges. The ED reports took approximately three to five minutes to complete and were time-stamped to indicate starting and completion times. Participants who completed a behavioral measure of impulsiveness after the report could earn up to $1.20 per report, based on their responses. This served as an incentive to complete ED reports. The behavioral measures of impulsiveness were not included in this study.
The ED assessed momentary affect and withdrawal symptoms (in the past 15 minutes) using items derived from the PANAS and the WSWS. Past factor analyses of items from both PANAS and WSWS assessed in EMA showed that negative affect and cognitive withdrawal symptoms loaded on one factor, whereas cravings to smoke loaded on another, and thoughts about food did not load on either factor (McCarthy et al., 2008). The items included in the current ED reports were selected because they were the top-performing items in a confirmatory factor analysis in a previous unpublished study. The negative affect items included were, from the WSWS: “I have been TENSE or ANXIOUS,” “I have felt SAD or DEPRESSED,” and “I have felt IMPATIENT” and from the PANAS: “I have felt DISTRESSED” and “I have felt UPSET.” Key words were presented in capital letters to facilitate easy comprehension of the question when subjects were completing the ED reports in the field. For momentary positive affect, the PANAS items, “I have felt ENTHUSIASTIC,” and “I have felt INTERESTED, “ were highly correlated (r = 0.83 in McCarthy et al., 2008). The timeframe of these questions was the 15 minutes preceding the prompt and participants rated their agreement on a 5-point scale ranging from 1 (very slightly or not at all) to 5 (extremely) for the PANAS items and 1 (disagree) to 5 (agree) for the WSWS items. The validity of such brief EMA measures of negative and positive affect is supported by research showing that stressful event reports predicted an increase in momentary negative affect and a decrease in momentary positive affect (Minami et al., 2011) and that affect ratings change at the outset of a quit attempt (McCarthy et al., 2006, 2008).
A confirmatory factor analysis of the affect and withdrawal items assessed in this study was conducted in MPlus 5.0 (Muthén & Muthén, 1998–2008, Los Angeles, CA) with weighting of subjects’ data based on the number of reports they contributed. A good fitting model (RMSEA = .023, 95% CI = .020–.025) specified that both the PANAS- and WSWS-derived negative affect items (tense or anxious, sad or depressed, impatient, distressed, and upset) loaded on single latent variable. This factor loaded onto a higher order factor that also comprised low positive affect and high urges to smoke and hunger.
Participants’ momentary confidence to quit smoking (“How CONFIDENT are you that you could quit smoking for good?” and motivation (“How WILLING are you TO WORK HARD at quitting smoking?”) were assessed using single 7-point scales where 1 was “not at all” and 7 was “extremely.” A past study showed that the latter motivation item was influenced by treatment and predictive of later abstinence (McCarthy et al., 2008).
The number of cigarettes smoked in the last two hours and since the last report was assessed (0–20 cigarettes). Lapse (yes/no) was considered to have occurred if participants reported smoking at least one cigarette since last report.
Final Sample
For the current study, 103 (79.2% out of 130 enrolled) participants who attended the quit day visit and provided at least 3 post-quit reports were included in the analyses. Demographic characteristics of the 103 individuals included in the analyses are summarized in Table 1. During the post-quit period, these participants provided 6,351 random report records (an average of 61.7 reports per person, or 3 out of 4 reports per day in the 21-day post-quit assessment period). There were no differences between the excluded and included participants in terms of age, gender, minority status, cigarettes smoked per day, years smoking, baseline CO level, or number of past quit attempts (all ps > .05). A total of 94 participants (91.3%) reported at least one smoking lapse during the post-quit assessment period. These subjects reported a total of 1,467 reports (an average of 15.61 lapse reports per person, range = 1–80, SD = 18.97). A total of 103 subjects had sufficient data to be included in the analysis.
Table 1.
Demographic characteristics of final sample (N=103).
| Variable | Value | n (%) |
|---|---|---|
| Sex (N=103) | Female | 50 (48.5%) |
|
| ||
| Race/Ethnicity (N=103) | Hispanic | 6 (5.9%) |
| White | 68 (66.0%) | |
| African-American | 23 (22.3 %) | |
| Asian, Pacific Islander | 6 (5.8%) | |
| American Indian | 1 (1.0%) | |
| Other | 4 (3.9%) | |
|
| ||
| Marital Status (N=103) | Married | 40 (38.8%) |
| Divorced | 11 (10.7%) | |
| Never married | 29 (28.2%) | |
| Cohabitating | 10 (9.7%) | |
| Separated | 7 (6.8%) | |
| Widowed | 6 (5.8%) | |
|
| ||
| Education (N=103) | < High school graduate | 1 (1.0%) |
| High school graduate | 25 (24.3%) | |
| Some college | 45 (43.7%) | |
| College degree | 32 (31.1%) | |
|
| ||
| Employment Status (N=103) | Employed for wages | 58 (56.3%) |
| Self-employed | 12 (11.7%) | |
| Unemployed <1 year | 12 (11.7%) | |
| Unemployed >1 year | 8 (7.8 %) | |
| Homemaker | 3 (2.9%) | |
| Student | 10 (9.7%) | |
| Retired | 5 (4.9%) | |
| Disabled | 9 (8.7%) | |
|
| ||
| Household Income (N=100) | < $25,000 | 33 (33.0%) |
| $25,00–$34,999 | 6 (6.0%) | |
| $35,000–$49,999 | 13 (13.0%) | |
| $50,000–$74.999 | 20 (20.0%) | |
| >$75.000 | 28 (28.0%) | |
|
| ||
| M (SD) | ||
|
| ||
| Age (N=103) | 45.1 (12.0) | |
| Age at first cigarette (N=103) | 15.1 (3.77) | |
| Cigarettes smoked per day (N=103) | 18.6 (6.81) | |
| Previous quit attempts (N=103) | 4.33 (9.77) | |
| Baseline CO level (N=103) | 21.7 (11.3) | |
| Baseline FTND Score (N=103) | 5.32 (1.92) | |
Analytic Plan
A series of multilevel random coefficient models was tested using Hierarchical Linear Modeling (HLM) Version 6.08 software (Raudenbush, Bryk, & Congdon, 2007). In this study, random reports (i.e., reports of positive and negative affect, confidence to quit for good, willingness to work hard at quitting, and smoking) made up the first level of data nested within individuals at the second level. Continuous predictors (i.e., positive and negative affect, confidence level, and willingness to work hard at quitting) were centered around the grand means prior to entry in the models. As such, when all other predictors are zero, estimated coefficients reflect the probability of lapsing at the overall average level of cognitive variables and positive and negative affect.
Three sets of models were fit to test the hypothesized relations shown in Figures 1–3. Variables and paths of primary interest are shown in black whereas control variables are shown in gray. First, the direct effects of affect change on smoking behavior 12 (t1) to 24 (t2) hours later were assessed (separately for negative and positive affect), controlling for previous affect (at t−1) and smoking (both at the last report and to that point in the quit attempt). These are the c paths shown in Figure 1. A Bernoulli distribution was specified, as smoking was coded as a dichotomous outcome (smoke free = 0, any smoking = 1) in non-linear models. Second, models of negative and positive affective influences on willingness to work at quitting and confidence related to quitting up to 12 hours later (between t0 and t1) were fit to the data. These are the a paths shown in Figure 2. These models controlled for previous levels of affect (t−1) and cognition (t0) and smoking status at the last report and up to that point in the quit attempt. Third, willingness to work and confidence at t1 were added to the c path models in order to test the b paths hypothesized, controlling for initial affect, earlier cognitions, and smoking status (Figure 3). Random effects were specified to allow regression coefficients to vary across individuals, as long as doing so improved model fit. Deviance statistics for the models were compared in order to determine which models better fit the data (Raudenbush & Bryk, 2002). A significant reduction in deviance in a model, relative to another, indicates an improvement in model fit.
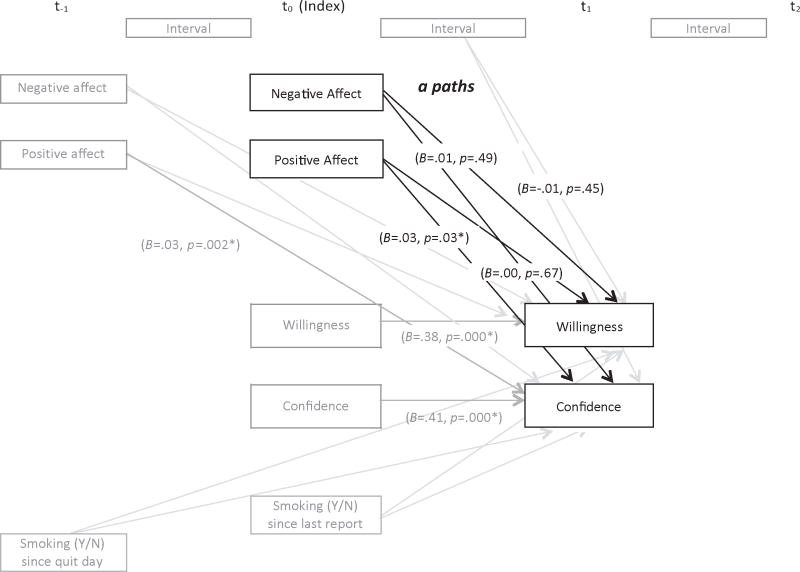
Figure 2. Model of hypothesized relations between negative and positive affect, and cognitions (a paths).
The model was fit to test the hypothesized effects of negative and positive affective on willingness to work at quitting and confidence related to quitting using HLM analysis. Unstandardized coefficients and p values were indicated only for variables of primary interest (shown in black) and control variables (shown in gray) that were significantly associated with smoking outcomes. * p < .05
Power to detect the hypothesized relations among affect, cognitions, and smoking is difficult to estimate, particularly for the binary smoking outcomes of greatest interest. Power estimation for multilevel models is complex and guidelines are unclear. Power to detect relations between affect and cognition (treated as continuously scaled in this analysis) was moderate. An analysis with Power IN Two-level designs (PINT) software (Bosker et al., 2003) indicated that 103 subjects with an average of 60 reports per subject should be sufficient to detect small size effects with power = .80 at alpha = .05 (two-tailed). Power to detect relations between affect or cognition and later smoking is likely to be lower, given the binary nature of these data.
Results
Affect and Lapse Risk (c paths)
First, we examined whether affect at the index report, controlling for affect at the previous report, prospectively predicted lapse risk between 0.5 and 24 hours later. Time (in minutes) between the index report (t0) and report at t2 (M = 758, SD = 333) was included to control for the effect of time on lapse risk (Figure 1). Only the model intercept was allowed to vary across individuals since setting additional parameters (i.e., negative and positive affect) to random did not significantly reduce deviance (10.74 df = 5, p = .06), indicating no improvement to model fit. Results showed that neither changes in negative nor positive affect predicted changes in lapse risk within 24 hours, contrary to our hypothesis (Table 2, top panel). However, higher background negative affect assessed at t−1, but not positive affect, was significantly associated with greater risk of lapse risk, controlling for recent smoking. Moreover, no significant time by affect (positive and negative) interaction effect was observed, indicating that the impact of changes in negative or positive affect on later lapse risk did not differ as a linear function of time.1 Significant level-2 variables for the intercept indicated that those with higher baseline nicotine withdrawal (assessed by WSWS) and baseline CO level had a significantly higher likelihood of smoking. Baseline nicotine dependence (assessed by FTND) was not related with smoking odds, after controlling for baseline CO.
Table 2.
Trimmed HLM analysis of the effects of changes in negative and positive affect (t0) on lapse risk over 24 hours (t2) and over 12 hours (t2).
| Predictor | Ratio Odds | 95% CI | Approx. d.f, | P-value |
|---|---|---|---|---|
| Direct effect c path (24 hours) | ||||
|
| ||||
| Mean P (Smoking ) 30 min–24hrs from index**a | 0.034 | (0.014, 0.082) | 100 | 0.000* |
| Baseline CO | 1.053 | (1.017, 1.089) | 100 | 0.005* |
| Baseline WSWS | 1.027 | (1.009, 1.152) | 100 | 0.043* |
| Recent Smoking (between index to t1 :Y/N) | 3.191 | (2.549, 3.994) | 4,582 | 0.000* |
| Negative Affect preceded Index (t−1) | 1.283 | (1.103, 1.491) | 4,582 | 0.002* |
| Index Negative Affect (t0) | 0.950 | (0.815, 1.109) | 4,582 | 0.518 |
| Positive Affect preceded Index (t−1) | 1.081 | (0.926, 1.261) | 4,582 | 0.326 |
| Index Positive Affect (t0) | 0.978 | (0.837, 1.144) | 4,582 | 0.783 |
|
| ||||
| Direct effect c path (12 hours) | ||||
|
| ||||
| Mean P (Smoking ) 30 min–24hrs from index**b | 0.037 | (0.016, 0.086) | 98 | 0.000* |
| Baseline CO | 1.053 | (1.018, 1.090) | 98 | 0.004* |
| Baseline WSWS | 1.029 | (1.002, 1.056) | 98 | 0.036* |
| Recent Smoking (between index to t1: Y/N) | 3.280 | (2.537, 4.239) | 3,646 | 0.000* |
| Index Negative Affect (t0) | 0.916 | (0.770, 1.090) | 3,646 | 0.325 |
| Negative Affect (t1) w/in 12 hours of index | 1.297 | (1.087, 1.548) | 3,646 | 0.004* |
| Index Positive Affect (t0) | 0.942 | (0.788,1.126) | 3,646 | 0.511 |
| Positive Affect (t1) w/in 12 hours of index | 1.151 | (0.961,1.379) | 3,646 | 0.125 |
| Time Interval (from t1 to t2) | 1.001 | (1.001, 1.002) | 3,646 | 0.000* |
Random coefficient, df = 100, reliability = .805.
Random coefficient, df = 98, reliability = .775.
All other predictors were treated as fixed to facilitate model convergence. CO: expired carbon monoxide; WSWS: Wisconsin Smoking Withdrawal Scale.
p<.05
t−1 = Report preceding index report
t0 = Index report
t1 = Next report within 12 hours of index report
t2 = Next report within 12 hours of t1 report (and within 24 hours of index report)
Although changes in negative and positive affect did not predict a lapse within 24 hours, analyses indicated that negative affect changes had shorter-lived effects on lapse risk for up to 12 hours (i.e., the interval between reports t1 and t2). A Bernoulli distribution was specified and the same time-varying covariates were entered along with previous negative and positive affect levels. Results revealed that an increase in negative affect at t1 predicted greater lapse risk in the next report t2 whereas change in positive affect was not related to change in lapse risk (Table 2, bottom panel).
Affect and Cognitive Mediators (a paths)
Multilevel models were built in which confidence and willingness to work hard (t1) were regressed on positive and negative affect recorded in the previous reports (t0) completed within the past 12 hours (at least 15 minutes apart). The mean interval between these reports was 273 minutes (SD = 172). Previous (t0) levels of cognitive variables were included as control variables in the model in order to assess changes in cognitive variables following changes in affect (Figure 2). Other time-varying covariates included: smoking status since the quit date (t0), recent smoking (t1), and the time interval between the index (t0) and second (t1) reports. No significant interaction effects between time interval and positive or negative affect were found in either the confidence or motivation models.
Motivation
In the a path model for willingness to work hard at quitting, the intercept (within-individual average level of willingness to work hard at t1) and the coefficient for earlier willingness at t0 were allowed to vary across individuals. This model yielded a statistically significant reduction in deviance (552, df = 2, p = .000) relative to the model in which only the intercept was specified as random, indicating an improvement of fit. Neither negative nor positive affect predicted willingness at the next report (Table 3, top panel). Male gender, greater baseline willingness to work hard, and higher baseline positive affect (level 2-individual variables) predicted higher average willingness post-quit.
Table 3.
Trimmed HLM analysis of the effects of changes in negative and positive affect (t0) on willingness to work hard and confidence to quit within 12 hours (t1).
| Fixed Effect | Coefficient | Standard Error | T-ratio | Approx. d.f. | P-value |
|---|---|---|---|---|---|
| a paths (Willingness) | |||||
|
| |||||
| Mean willingness (t1) 15 min–12 hrs from index**a | 0.109 | 0.049 | 2.223 | 100 | 0.028* |
| Baseline Willingness | 0.144 | 0.045 | 3.181 | 100 | 0.002* |
| Baseline Positive Affect | 0.018 | 0.007 | 2.603 | 100 | 0.011* |
| Recent Smoking (between index to t1: Y/N) | − 0.080 | 0.018 | − 4.499 | 4,816 | 0.000* |
| Index Willingness to Work Hard ** | 0.380 | 0.040 | 9.542 | 102 | 0.000* |
| Positive Affect preceded Index (t−1) | 0.010 | 0.009 | 1.150 | 4,816 | 0.250 |
| Index Positive Affect (t0) | 0.002 | 0.001 | 0.252 | 4,816 | 0.801 |
| Negative Affect preceded Index (t−1) | 0.004 | 0.009 | 0.427 | 4,816 | 0.669 |
| Index Negative Affect (t0) | − 0.008 | 0.009 | − 0.761 | 4,816 | 0.447 |
|
| |||||
| a paths (Confidence) | |||||
|
| |||||
| Mean confidence (t1) 15 min–12 hrs from index**b | 0.042 | 0.088 | 0.482 | 101 | 0.630 |
| Baseline Confidence | 0.239 | 0.063 | 3.800 | 101 | 0.000* |
| Recent Smoking (between index to t1: Y/N) | − 0.063 | 0.022 | − 2.793 | 4,817 | 0.006* |
| Index Confidence (t0) ** | 0.414 | 0.033 | 12.514 | 102 | 0.000* |
| Positive Affect preceded Index (t−1) | 0.026 | 0.011 | 2.386 | 4,817 | 0.017* |
| Index Positive Affect (t0) | 0.025 | 0.011 | 2.192 | 4,817 | 0.028* |
| Negative Affect preceded Index (t−1) | − 0.001 | 0.011 | − 0.121 | 4,817 | 0.904 |
| Index Negative Affect (t0) | 0.008 | 0.012 | 0.698 | 4,817 | 0.485 |
Random coefficient, df = 100/102. All other predictors were treated as fixed, with df=4,816, to facilitate model convergence. Reliability: Intercept = .895, Willingness = .715, N=63.
Random coefficient, df = 101/102.
All other predictors were treated as fixed, with df=4,817, to facilitate model convergence.
Reliability: Intercept = .924, Confidence = .634
p<.05.
Confidence
The intercept (within-individual average confidence level at t1) and the coefficient for the confidence level at t0 were allowed to vary across individuals in this model. Deviance for this model was 6622.04, which represents a statistically significant reduction in deviance (change in deviance = 305.51 df = 2, p = .000) from the model in which only the intercept was specified as random, indicating a superior fit to the data. Results (Table 3, bottom panel) indicated that an increase in positive affect at the index report was positively associated with an increase in confidence level at the next report within 12 hours while a change in negative affect was not associated with changes in confidence at the next report. Moreover, higher baseline confidence level (level 2) predicted greater average ED confidence level.
Cognitive variables and lapse risk (b paths)
Changes in confidence and willingness to work hard at t1 were simultaneously entered in the model as predictors of lapse likelihood within 12 hours along with the following covariates: recent smoking (t1), smoking status since quit date (t0), and time (minutes) between the t1 and t2 reports (Figure 3). A Bernoulli distribution was specified for this model and the intercept was allowed to vary across individuals given that allowing additional parameters (i.e., confidence and willingness at t1) did not significantly reduce deviance (6.3 df = 5, p = .28), indicating no improvement in model fit. As in the direct model (c paths), higher baseline CO (level 2- individual variables) predicted greater lapse risk, but nicotine withdrawal scores (WSWS) no longer predicted smoking lapse (Table 4). Greater time elapsed since the previous reports (t1) and recent smoking (between index and t1) were associated with greater lapse risk between t1 and t2. Inclusion of cognitive mediators did not change the non-significant relations between affect at t0 and lapse risk at t2.
Table 4.
HLM analysis of the effects of changes in confidence and willingness (t1) on lapse risk within 12 hours (t2).
| Predictor | Odds Ratio | 95% CI | Approx. d.f. | P-value |
|---|---|---|---|---|
| b paths | ||||
|
| ||||
| Mean P (Smoking ) 30 min–24hrs from index** | 0.025 | (0.010, 0.062) | 98 | 0.000* |
| Baseline CO | 1.055 | (1.018, 1.093) | 98 | 0.004* |
| Baseline WSWS | 1.024 | (0.997, 1.052) | 98 | 0.086 |
| Smoke Free (quit date till index: Y/N) | 0.857 | (0.473, 1.552) | 3,643 | 0.610 |
| Recent Smoking (between index to t1: Y/N) | 3.121 | (2.409, 4.042) | 3,643 | 0.000* |
| Index Positive Affect (t0) | 0.998 | (0.838, 1.188) | 3,643 | 0.983 |
| Index Negative Affect (t0) | 0.978 | (0.830, 1151) | 3,643 | 0.786 |
| Time Interval (from t1 to t2) | 1.001 | (1.001, 1.002) | 3,643 | 0.000* |
| Index Confidence (t0) | 0.805 | (0.649, 0.998) | 3,643 | 0.047* |
| Confidence (t1) w/in 12 hours of index | 0.993 | (0.800, 1.233) | 3,643 | 0.950 |
| Index Willingness to work hard | 1.063. | (0.820, 1.382) | 3,643 | 0.638 |
| Willingness to work ( t1 ) w/in 12 hours of index | 0.880 | (0.652, 1.187) | 3,643 | 0.402 |
| Willingness (t1) X Interval (from t1 to t2) | 0.999 | (0.999, 1.000) | 3,643 | 0.039* |
Random coefficient, df = 98, reliability = .777 All other predictors were treated as fixed to facilitate model convergence.
p<.05.
Motivation
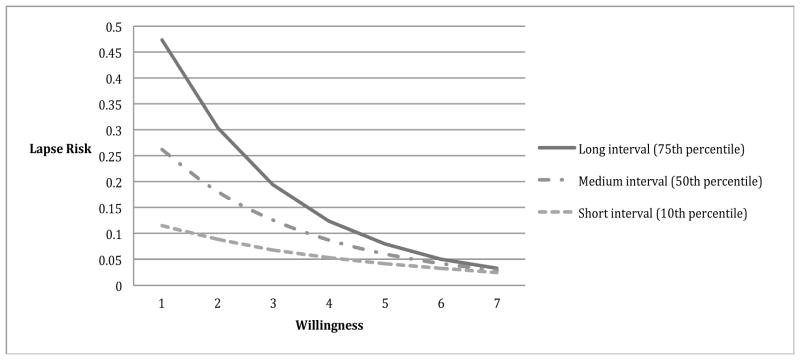
Results revealed a significant main effect of changes in willingness on later lapse risk (p = .025). A significant interaction between willingness to work hard at quitting at t1 and the time interval between t1 to t2 (Table 4) was also found. The protective effects of willingness on lapse risk emerged only as more time elapsed (Figure 4). That is, motivation mattered less when there was less opportunity to lapse (because less time had elapsed), but became more protective with more time (and presumably greater opportunity to smoke).
Figure 4. Willingness to Work at Quitting X Time Elapsed Interaction Effects in Model of Lapse Risk.
Moderating effects of time on relations between abstinence motivation and lapse risk (up to 12 hours). Each line represents different time intervals. The protective effect of increases in motivation on lapse risk is observed only with the longer interval. The coefficients used to create this graph were from the HLM analysis of the b-path model, controlling for confidence level at the previous report.
Confidence
Results showed that while lower background confidence (t0) significantly predicted greater lapse risk, a momentary change in confidence (t1) was not associated with a change in lapse risk within 12 hours, contrary to our hypothesis (Table 4). We further examined whether the relation between confidence and later lapse was moderated by baseline level of confidence, affect, or demographic characteristics, and found no such effects. Moreover, a follow-up analysis showed that removing previous confidence level (t0) from the model resulted in a significant association between confidence (t1) and lapse risk within 12 hours, confirming that the background levels of confidence (either over the past 12 or 24 hours), rather than hourly fluctuations in confidence, were predictive of smoking.
Discussion
The aim of this study was to prospectively examine the role of abstinence motivation and confidence in relation to earlier changes in negative and positive affect and later smoking behavior during a quit attempt. Results provided mixed support for the model. The direct effect of momentary changes in negative affect on later lapse risk was observed up to 12 hours, but not 24 hours later. An increase in positive affect had a significant effect on confidence within 12 hours, but not on willingness to work hard at quitting. While time-dependent significant relations between motivation and later lapse risk were found, increases in confidence were not protective against later lapse.
Affect and smoking behavior
Models of lagged relations between real-time affect and smoking behavior indicated that spikes in negative affect were associated with short-term elevated risk of smoking in models that controlled for earlier affect and smoking status. The relation between increased negative affect and later risk of lapse seemed to last only 12 hours, as this was not evident in reports as many as 24 hours later. This is consistent with earlier research showing that increases in negative affect over hours, but not days, were predictive of lapsing (Shiffman, 2005). The decay in the strength of the relation between negative affect and later smoking does not appear to be linear, as we did not find an interaction between negative affect and the interval between reports in models predicting lapses. Thus, there seems to be a qualitative difference in the strength of the relation between spikes in affective distress and smoking risk that merits further exploration.
Affect and cognitive variables
Increases in positive affect were significantly associated with increases in confidence to quit, but not willingness to work hard at quitting up to 12 hours later. Neither baseline nor changes in negative affect had detectable impact on confidence or willingness. This may suggest that momentary confidence is more likely to be influenced by positive affect, rather than negative affect. Furthermore, those with higher baseline positive affect reported greater average levels of post-quit willingness to work hard at quitting. It seems that stable positive affectivity, rather than acute changes, may influence levels of willingness to work hard toward a goal. Overall, the results indicated that positive affect may have a greater influence on cognitions than negative affect. This is consistent with the models of positive affect which assert that positive emotions expand one’s openness to new experience and prompt changes in perspectives and cognitions such as motivation to change (Fredrickson & Branigan, 2001, Wagner & Ingersoll, 2008). Increasing positive affect, rather than merely focusing on reduction or avoidance of negative affect may help enhance confidence and motivation to work toward a specific goal.
Willingness to work hard at quitting
An increase in motivation to quit was protective against lapse, especially when adequate time had elapsed. This suggests that the positive effects of enhanced willingness to work hard during a quit attempt last at least 12 hours. Daily motivational boosters such as reminders of reasons to quit (i.e., negative consequences of smoking, benefits of quitting, values inconsistent with smoking) may counter a decline in motivation over time (i.e., motivation fatigue) that may contribute to an increased risk of relapse (Piasecki et al., 2002).
Confidence to quit
Contrary to expectations, an increase in confidence did not predict lower lapse risk within 12 hours. This result seems inconsistent with the prevalent notion that abstinence confidence is protective against lapse risk and earlier findings (Gwaltney et al., 2005b, Shiffman, 2005) indicating that lower self-efficacy predicted smoking lapse or relapse over days. However, while these studies prospectively examined relations between dynamic changes in self-efficacy and subsequent lapse/relapse risk, such changes were assessed daily (using average confidence within a day), unlike this study that assessed changes in confidence over hours within a day. In fact, our results showed that levels of abstinence confidence, rather than acute fluctuations in confidence, were predictive of subsequent lapse risk, indicating that higher background confidence is more important in preventing lapse than acute increases in confidence.
Furthermore, a recent review of relations between self-efficacy/confidence and smoking outcomes underscored the importance of considering smoking status at the time of the self-efficacy assessment (Gwaltney et al., 2009). The review found that controlling for smoking status diminished the predictive value of self-efficacy on later smoking risk. Moreover, the bidirectional relations between self-efficacy and smoking status (i.e., abstinence predicts greater confidence while greater confidence predicts abstinence) have been empirically demonstrated (e.g., Perkins et al., 2012). Given that recent smoking status was controlled in all the models examined in this study, there may have been little unique, residual variance in later smoking to be explained by the part of self-efficacy that was not affected by earlier smoking.
Taken together, our findings highlight the differential roles that affective states may play in smoking cessation and suggest several clinical implications. Positive affect, but not negative affect, appears to influence cognitive determinants of smoking lapse. The important role of positive affect in the process of behavioral change has garnered much attention (e.g., Kwan, 2010; Standage, 2010; Ostir et al, 2003) and this study provided support for a potential pathway though which positive affect may impact subsequent behavior. At the same time, negative affect, confidence and willingness to work hard at quitting seem to have independent relations with subsequent smoking, suggesting that the associations between negative affect and smoking may be independent of smoking-related cognitions. These results suggest the importance of sustained positive affectivity as well as reducing acute increases in negative affect in successful quitting. In addition, given that short-lived spikes in confidence associated with acute increases in positive affect did not have protective effects against lapses, interventions that are designed to both enhance affective states and sustain these over time may improve cessation rates.
Limitations
There are several limitations that warrant caution when interpreting the results. First, the psychometric properties of the data may be limited; the extent to which brief EMA assessments are sensitive to momentary fluctuations in affect and cognition is unclear, although Shiffman and colleagues have shown that changes in affect and confidence assessed by EMA predicted later smoking behaviors (e.g., Gwaltney et al., 2005b, Shiffman, 2005). Second, possible reporting biases should be considered since there is no way to identify systematic missing reports. That is, some individuals may have missed reports only when they were in distress, had just smoked, or were particularly demoralized about quitting smoking. The non-experimental nature of this study is another limitation. Since none of the variables of interest (e.g., affect, confidence) was manipulated, the interpretation of causal relations should be tempered. Finally, the best time-frames (seconds, minutes, days, etc.) to study the effects of affect on cognitive variables as well as the impact of cognitive variables on smoking behavior is not clear. However, the results from this study suggest that a shorter timeframe (less than 12 hours) is more suitable for studying relations among affect, cognitions, and smoking. Unfortunately, analyses using shorter timeframes were not possible in this study because participants, on average, completed 3–4 reports per day and sufficient reports for the analysis were not available (three consecutive reports were completed within 12 hours less than 47% of the time). Therefore, the time-frame used in this study may not be optimal for the study of cognitive variables during a quit attempt. Further studies investigating the acute impact of affect and cognitions on smoking cessation may be needed to better elucidate these relations. In addition, although extremely low rates of lozenge use were observed in this study (an average of 3.26 lozenges (SD = 3.13) per day during the first 6 weeks of the quit attempt), the lack of a no treatment control group is another limitation. Finally, we believe that willingness to participate in the study is a marker of readiness, as participants in this study were treatment seekers who signed up for a study knowing that a quit day would be set for them within two weeks. However, it is possible that changes in willingness to work hard at quitting may not fully capture changes in readiness to quit. As such, including additional constructs associated with smoking cessation success such as readiness to quit may improve our understanding of the interrelations among cognitive constructs and their impact on smoking behavior.
Conclusions
This study examined the dynamic relations among affect, confidence, motivation, and smoking behavior of adult smokers during an attempt to quit smoking. Multilevel models supported the general idea that negative and positive affect have detectable short-term effects on cognitions and behavior. The results also indicated, however, that the magnitude of relations among key constructs varies across assessment timeframes. Additional research is needed to identify the optimal timeframes for studying the proximal determinants of lapse risk. Studies of dynamic relations among affect, cognition, and behavior have the potential to provide a better understanding of crucial determinants and time courses of behavioral change that may facilitate the development of effective smoking cessation interventions.
Acknowledgments
Funding: The project described was supported by Award Number RC1DA028129 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
The time lag between the index and t2 reports clustered around 6 and 12 hours (a bimodal distribution). This may reflect the differences in the time of day that the reports were completed. The last reports of a day will inevitably have a longer delay till the next report due to suspension of recording during the overnight hours. In order to take this difference into account, an interaction term between a binary variable for time of day (capturing whether a report occurred within 8 hours of waking or more than 8 hours after waking) and the time lag between reports was entered in this model. However, no significant main effect of time of day or interaction effect was observed and these terms were pruned from the final model. Thus, it did not appear as though relations between affect and lapsing depended on the time of day reports were completed or the interaction between this and the interval between reports.
References
- Abrams DB, Niaura R, Brown RB, Emmons KM, Goldstein MG, Monti PM, Linnan LA. The tobacco dependence treatment handbook: a guide to best practices. New York: The Guilford Press; 2003. [Google Scholar]
- Ajzen I. Perceived behavioral control, self-efficacy, locus of control, and the theory of planned behavior. Journal of Applied Social Psychology. 2002;32:665–683. [Google Scholar]
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- Bandura A. Self-efficacy: Towards a unifying theory of behavior change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Berman AH, Forsberg L, Durbeej N, Kallmen H, Hermansson U. Single-session motivational interviewing for drug detoxification inpatients: Effects on self efficacy, stages of change and substance use. Substance Use & Misuse. 2010;45(3):384–402. doi: 10.3109/10826080903452488. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Juliano LM, Copeland AL. Expectancies for tobacco smoking. In: Kirsch I, editor. How expectancies shape experience. Washington, DC: American Psychological Association; 1999. pp. 263–299. [Google Scholar]
- Bosker RJ, Snijders TAB, Guldemond H. PINT (Power IN Two-level designs): Estimating standard errors of regression coefficients in hierarchical linear models for power calculations. The Netherlands: University of Groningen; 2003. User’s manual (Version 2.1) [Google Scholar]
- Cohen S, Pressman SD. Positive Affect and Health. Current Directions in Psychological Science. 2006;15(3):122–125. [Google Scholar]
- Condiotte M, Lichtenstein E. Self-efficacy and relapse in smoking cessation programs. Journal of Consulting and Clinical Psychology. 1981;49:648–658. doi: 10.1037//0022-006x.49.5.648. [DOI] [PubMed] [Google Scholar]
- Conners CK, Staff MHS, editors. Conners’ Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. North Tonwanda, NY: Mutli-Health Systems; 2000. [Google Scholar]
- Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire – Adult: measurement of smoking outcome expectancies of experienced smokers. Psychological Assessment. 1995;7:484–94. [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Doran N, Cook J, McChargue D, Myers M, Spring B. Cue-elicited negative affect in impulsive smokers. Psychology of Addictive Behaviors. 2008;22(2):249–256. doi: 10.1037/0893-164X.22.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Duc TV, Perneger TV. Validity of the Fagerstrom test for nicotine dependence and of the heaviness of smoking index among relatively light smokers. Addiction. 1990;94:269–281. doi: 10.1046/j.1360-0443.1999.94226910.x. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear, Nose, and Throat Journal. 1992;69:763–767. [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- Fredrickson BL. Cultivating positive emotions to optimize health and well-being. Prevention & Treatment. 2000;3:1–25. [Google Scholar]
- Fredrickson BL. The value of positive emotions: The emerging science of positive psychology is coming to understand why it’s good to feel good. American Scientist. 2003;91:330–336. [Google Scholar]
- Fredrickson BL, Branigan C. Positive emotion. In: Mayne TJ, Bonnano GA, editors. Emotion: Current issues and future directions. New York: Gilford Press; 2001. pp. 123–151. [Google Scholar]
- Gwaltney CJ, Shiffman S, Sayette MA. Situational correlates of abstinence self-efficacy. Journal of Abnormal Psychology. 2005a;114:649–660. doi: 10.1037/0021-843X.114.4.649. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman J, Balabanis MH, Paty JA. Dynamic Self-Efficacy and Outcome Expectancies: Prediction of Smoking Lapse and Relapse. Journal of Abnormal Psychology. 2005b;114(4):661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Metrik J, Kahler CW, Shiffman S. Self-efficacy and smoking cessation: a meta-analysis. Psychology of Addictive Behaviors. 2009;23(1):56–66. doi: 10.1037/a0013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Marlatt A, George WH. Relapse prevention and the maintenance of optimal health. In: Shumaker S, Ockene JK, Riekert K, editors. The Handbook of Behavior Change. 3. New York: Springer Publishing Co; 2009. [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kwan BM, Bryan AD. Affective response to exercise as a component of exercise motivation: Attitudes, norms, self-efficacy, and temporal stability of intentions. Psychology of Sport and Exercise. 2010;11(1):71–79. doi: 10.1016/j.psychsport.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne GL, Hauw N, Vallerand RJ, Brunel P, Blanchard C. International Journal of Sport and Exercise Psychology. 2009;7(2):147–168. [Google Scholar]
- Marlatt GA, Donovan D. Relapse prevention. 2. New York, NY: Guilford Press; 2005. [Google Scholar]
- Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavioral medicine: Changing health lifestyles. New York: Bunner/Mazel; 1980. [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: An electronic diary study. Journal of Abnormal Psychology. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained-release treatment for smoking cessation. Addiction. 2008;103:1521–1533. doi: 10.1111/j.1360-0443.2008.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, Baker TB. A multi-level analysis of non-significant counseling effects in a randomized smoking cessation trial. Addiction. 2010;105(12):2195–2208. doi: 10.1111/j.1360-0443.2010.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. New York: Guilford Press; 2002. [Google Scholar]
- Minami H, McCarthy DE, Jorenby DE, Baker TB. An ecological momentary assessment of relations among affect, coping, and smoking during a quit attempt. Addition. 2011;106(2):641–650. doi: 10.1111/j.1360-0443.2010.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. 5. Los Angeles, CA: Muthen & Muthen; 1998–2008. [Google Scholar]
- Nelson DW, Knight AE. The power of positive recollections: Reducing test anxiety and enhancing college student efficacy and performance. Journal of Applied Social Psychology. 2010;40(3):732–745. [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedrazza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Nordgren LF, van der Pligt J, van Harreveld F. The Instability of Health Cognitions: Visceral States Influence Self-efficacy and Related Health Beliefs. Health Psychology. 2008;27(6):22–727. doi: 10.1037/0278-6133.27.6.722. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Leveille S, Volpato S, Cohen-Mansfield J, Guralnik JM. The association of positive and negative affect and exercise self-efficacy in older adults. Journal of Aging and Physical Activity. 2003;11:265–274. [Google Scholar]
- O’Connell KA, Hosein VL, Schwartz JE, Leibowitz RQ. How does coping help people resist lapses during smoking cessation? Health Psychology. 2007;26(1):77–84. doi: 10.1037/0278-6133.26.1.77. [DOI] [PubMed] [Google Scholar]
- Patrick C, Curtin J, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14:150–63. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Parzynski C, Mercincavage M, Conklin CA, Fonte CA. Is self-efficacy for smoking abstinence a cause of, or a reflection on, smoking behavior change? Experimental and Clinical Psychopharmacology. 2012;20(1):56–62. doi: 10.1037/a0025482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? the need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97(9):1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerstrom tolerance questionnaire and the Fagerstrom test for nicotine dependence. Addictive Behavior. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Newbury Park: Sage Publications; 2002. [Google Scholar]
- Raudenbush S, Bryk A, Congdon R. HLM for Windows (Version 6.04) Lincolnwood IL: Scientific Software International; 2007. [Google Scholar]
- Shiffman S. Dynamic Influences on Smoking Relapse Process. Journal of Personality. 2005;73(6):1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50(1):71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking cessation treatments in the United States. American Journal of Preventive Medicine. 2008;34:102–11. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dresler CM, Rohay JM. Successful treatment with a nicotine lozenge of smokers with prior failure in pharmacological therapy. Addiction. 2004;99(1):83–92. doi: 10.1111/j.1360-0443.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Standage M. Make it happen! Journal of Sport & Exercise Psychology. 2010;32(2):263–264. [Google Scholar]
- Wagner CC, Ingersoll KS. Beyond cognition: Broadening the emotional base of motivational interviewing. Journal of Psychotherapy Integration. 2008;18(2):248–258. doi: 10.1037/1053-0479.18.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress, and distress: Exploring the central role of negative affectivity. Psychological Review. 1989;96:234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the wisconsin smoking withdrawal scale. Experimental and Clinical Psychopharmacology. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. Journal of Abnormal Psychology. 1992;101(4):617–629. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]