Abstract
Stenotrophomonas maltophilia is an emerging multidrug-resistant (MDR) opportunistic pathogen for which new antibiotic options are urgently needed. We report our clinical experience treating a 19-year-old renal transplant recipient who developed prolonged bacteremia due to metallo-β-lactamase-producing S. maltophilia refractory to conventional treatment. The infection recurred despite a prolonged course of colistimethate sodium (colistin) but resolved with the use of a novel drug combination with clinical efficacy against the patient's S. maltophilia isolate.
CASE REPORT
Stenotrophomonas maltophilia is a Gram-negative, nonfermentative, environmental bacillus that has emerged as an important cause of nosocomial infections in immunocompromised hosts (1, 2). S. maltophilia characteristically manifests a multidrug-resistant (MDR) phenotype. Intrinsic antibiotic resistance is mediated by the expression of aminoglycoside-modifying enzymes; qnrB-like quinolone-resistant determinant, multidrug efflux pumps; and two β-lactamases (L1 and L2). The L1 inducible metallo-β-lactamase (MBL) hydrolyzes carbapenems and other β-lactams, with the exception of the monobactam aztreonam (ATM), and is resistant to all clinically available β-lactamase inhibitors (1, 3, 4). The L2 β-lactamase is a chromosomally encoded, inducible cephalosporinase that confers resistance to extended-spectrum cephalosporins and ATM but can be inhibited by commercially available serine-β-lactamase inhibitors such as clavulanic acid (1, 2, 5). Here, we report our experience treating an immunocompromised patient with unrelenting MDR S. maltophilia bacteremia.
CASE PRESENTATION
A 19-year-old male with end-stage renal disease secondary to autosomal recessive polycystic kidney disease, two renal transplants, asplenia, adrenal insufficiency, and a history of S. maltophilia bacteremia (21 months before) developed persistent, MDR S. maltophilia bacteremia. The patient's immunosuppressive regimen consisted of tacrolimus, mycophenolate, and physiologic dosing of hydrocortisone undergoing a slow taper because of adrenal insufficiency. His prophylactic antimicrobials included trimethoprim-sulfamethoxazole (TMP-SMX) at 160/800 mg thrice weekly (against Pneumocystis jirovecii), penicillin VK at 250 mg twice daily (asplenia), and ciprofloxacin at 750 mg daily (secondary prophylaxis after sepsis from ascending cholangitis) for the first 2 weeks of every month.
On day 1 of bacteremia, the patient presented with fever without focal symptoms of infection. Linezolid and cefepime were administered after blood cultures were obtained. A peripherally inserted central catheter (PICC) was placed on day 2. After 62 h of incubation, blood cultures grew Gram-negative rods. Final identification of the organism and susceptibilities was delayed because of slow growth of the isolate. The preliminary antibiotic susceptibility testing suggested an MDR phenotype with apparent susceptibility to aminoglycosides, and so, the empirical antibiotic regimen was changed to gentamicin on day 6. On day 8, the organism was identified as S. maltophilia that showed resistance to TMP-SMX, ceftazidime (CAZ), minocycline, meropenem, and levofloxacin (Table 1). The resistance profile of this isolate may have been related to the long-term antimicrobial prophylaxis posttransplantation (TMP-SMX, penicillin, ciprofloxacin). The patient was transitioned to intravenous (i.v.) colistimethate sodium (2.5 mg/kg once daily, dose adjusted on day 15 to 1.5 mg/kg every 36 h because of reduced creatinine clearance). Repeat blood cultures on day 10 showed growth of a Gram-negative bacillus, prompting removal of the PICC. After removal of the PICC, all blood cultures remained without growth. The patient was treated for 14 days with i.v. colistimethate, during which a new PICC was placed to complete treatment at home. The PICC was removed upon completion of therapy.
TABLE 1.
Culture results
| Day(s) | Culture result | Time (h) to positivity | Drug(s) (MIC[s] [μg/ml])a |
|||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | No breakpoint | |||
| PI | S. maltophiliab | 19 | TMP-SMX (<0.25/4.75) | Minocycline (6) | CAZ (>256), levofloxacin (>32), ticarcillin-clavulanate (256), meropenem (>32) | Amikacin (8), tobramycin (1.5) |
| 1 | S. maltophiliab | 62 | Minocycline (6) | ATM (>256), cefepime (>256), ciprofloxacin (>256), TMP-SMX (>32/608) | Colistin (0.09), tigecycline (12) | |
| 4 | S. maltophilia | 85 | Minocycline (8) | TMP-SMX (>32/608), meropenem (>32) | Colistin (0.09), tigecycline (16) | |
| 6–9 | NGd | |||||
| 10 | S. maltophilia | 118 | Minocycline (6) | TMP-SMX (>32/608), meropenem (>32) | Colistin (0.047) | |
| 11 | S. maltophilia | 53 | Minocycline (4) | TMP-SMX (>32/608), meropenem (>32) | Rifampin (>32) | |
| 13–15 | NG | |||||
| 39 | S. maltophilia | 75 | TMP-SMX (>32/608), minocycline (16), CAZ (>256), meropenem (>32) | Colistin (0.38), tigecycline (24) | ||
| 43 | S. maltophilia | 160 | Same as day 39 | Same as day 39 | Same as day 39 | |
| S. maltophiliab,c | 80 | Minocycline (8) | TMP-SMX (>32/608), CAZ (>256), meropenem (>32) | |||
| 44 | S. maltophilia | 58 | Same as day 43 | Same as day 43 | Same as day 43 | |
| S. maltophilia | 56 | Minocycline (8) | TMP-SMX (>32/608), CAZ (>256), meropenem (>32) | |||
| S. maltophiliac | 56 | Minocycline (3) | TMP-SMX (>32/608), CAZ (>256), meropenem (>32) | |||
| 45 | S. maltophiliab | 57 | Same as day 44 | Same as day 44 | Same as day 44 | |
| S. maltophilia | 61 | Same as day 44 | Same as day 44 | Same as day 44 | ||
| 47, 49 | NG | |||||
| 50 | S. maltophiliab,c | 64 | Minocycline (6) | TMP-SMX (>32/608), CAZ (>256), meropenem (>32) | ||
| 53 | NG | |||||
| 57 | S. maltophilia | 71 | Minocycline (8) | TMP-SMX (>32/608), CAZ (>256), meropenem (>32) | ||
| 58 | S. maltophilia | 84 | Same as day 57 | Same as day 57 | Same as day 57 | |
| 66 | NG | |||||
Isolates with similar identifications obtained from the same site within 72 h had susceptibility testing referred to the most recent prior isolate.
Isolate submitted for PFGE.
Isolate submitted for CZA-ATM in vitro susceptibility testing.
NG, no growth.
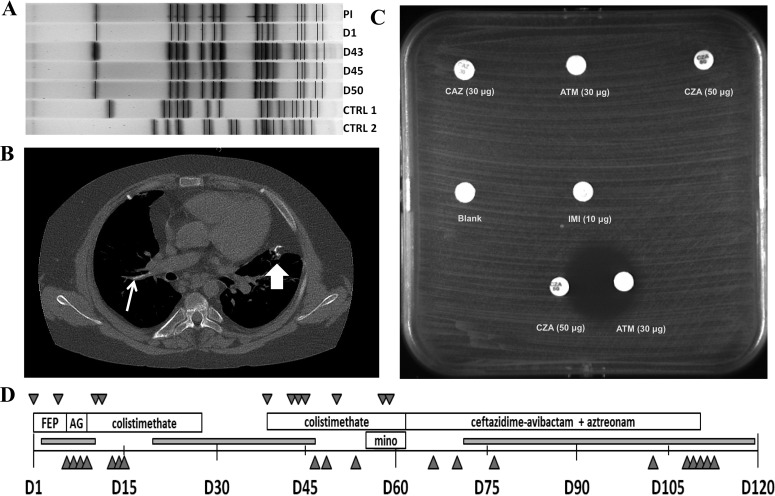
On day 39 after the initial bacteremia, he developed fever, hypotension, and tachycardia. A new PICC was placed, and intravenous colistimethate and linezolid were reinitiated empirically; blood cultures on admission demonstrated growth of S. maltophilia. Repeat cultures of blood taken on days 43, 44, and 45 also demonstrated growth of S. maltophilia. The MIC of colistimethate increased nearly 4-fold for isolates obtained from the first episode of bacteremia to those from the second bacteremic episode (Table 1). Pulsed-field gel electrophoresis (PFGE) was performed to assess the genetic similarity of the isolates from the separate bacteremic events (6). Results noted indistinguishable PFGE band patterns on samples obtained on days 1, 45, and 50 (Fig. 1A). A similar PFGE restriction band pattern was noted for an S. maltophilia isolate (past isolate [PI]) obtained 21 months prior to day 1 of the current episode, suggesting earlier infection with the same strain of S. maltophilia in this patient.
FIG 1.
Synopsis of the treatment of bacteremia due to MDR S. maltophilia. (A) PFGE of S. maltophilia clinical isolates using Xba1. CTRL 1 and 2, contemporaneous control isolates of S. maltophilia from different patients. Isolates obtained on day (D1), day 45, and day 50 (primary cluster) are indistinguishable on PFGE. The PI is different by one band, suggesting that it is probably the same strain as the primary cluster. The isolate obtained on day 43 has up to four bands different from the primary cluster, suggesting that it may be related to the primary cluster. The two control clusters are significantly different from the strains isolated from the patient. (B) Noncontrast chest CT scan demonstrating an intravascular calcific lesion. Line arrow, area of calcification in the right pulmonary artery, the suspected site of an endovascular focus. Block arrow, calcification at a prior surgical site of a lung biopsy. (C) Disk diffusion susceptibility testing of the MDR S. maltophilia isolate in Mueller-Hinton agar. Note the synergy between CZA and ATM. IMI, imipenem. (D) Time line of bacteremia, antimicrobial therapy, and indwelling vascular devices. FEP, cefepime; AG, aminoglycoside; mino, minocycline. Downward triangles represent positive blood cultures for S. maltophilia. Upright triangles represent blood cultures without growth. Gray boxes indicate times when indwelling vascular devices were present.
Given persistent bacteremia, the PICC was removed on day 47. Culture of the catheter tip was without growth. However, cultures from day 50 still grew S. maltophilia. A transthoracic echocardiogram on day 48 did not reveal evidence of vegetations on the cardiac valves. A computed tomography (CT) scan of the chest performed on day 54 revealed a calcified focus in the right main pulmonary artery (Fig. 1B) that was suspicious for a thrombus possibly serving as a nidus of infection. Minocycline (loading dose of 200 mg twice daily for 2 doses, followed by 100 mg twice daily) was added to the therapy with i.v. colistimethate on day 55. Repeat blood cultures on days 57 and 58 again demonstrated growth of S. maltophilia despite the absence of indwelling lines or other devices. As the bacteremia persisted during therapy with i.v. colistimethate and minocycline, alternative antibiotic options were considered and additional in vitro susceptibility testing, including determination of synergistic activity, was performed with several of the patient's recent isolates to identify drug combinations with potential efficacy against this MDR organism.
CHALLENGE QUESTION
Which antimicrobial(s) would be appropriate to treat the patient whose case is described?
A. Colistimethate (i.v.) and gentamicin
B. Ticarcillin-clavulanate (i.v.) and minocycline
C. Oral fosfomycin and extended-infusion meropenem
D. Oral TMP-SMX and ceftolozane-tazobactam (i.v.)
E. CAZ-avibactam (AVI) (CZA) (i.v.) and aztreonam (ATM)
F. Meropenem and colistimethate (i.v.)
TREATMENT AND OUTCOME
The presence of L1 and L2 β-lactamases was confirmed by PCR in all isolates. Disc diffusion susceptibility testing revealed in vitro resistance to CAZ, CZA, ATM, and imipenem (zone diameter, >6 mm). However, when discs of CZA and ATM were placed 20 mm apart, a zone of inhibition was observed on the side of the ATM disk facing CZA. This was interpreted as evidence of a synergy between CZA and ATM (Fig. 1C).
On day 63, the patient's antibiotic regimen was changed to CZA (2.5 g i.v. every 8 h) in combination with ATM (2 g i.v. every 8 h). Repeat blood cultures up to 113 days after completion of CZA-ATM therapy were without growth. He received a total of 48 days of therapy with CZA-ATM for the treatment of a presumptive endovascular infection in the pulmonary outflow tract caused by S. maltophilia (Fig. 1D). Since discontinuation of antibiotic therapy with CZA-ATM, further episodes of S. maltophilia bacteremia have not occurred (>90 days of observation). The answer to the challenge question is E.
Infections caused by S. maltophilia pose a therapeutic challenge because of intrinsic and acquired resistance to many agents (7). Exposure to β-lactams induces S. maltophilia to express two chromosomal β-lactamases, L1 and L2, which together confer resistance to all β-lactams and cannot be inhibited by commercially available inhibitors. CZA is a novel combination of the extended-spectrum cephalosporin CAZ with the diazabicyclooctane AVI, a non-β-lactam β-lactamase inhibitor that was recently approved for complicated intraabdominal and urinary infection in adults (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm435629.htm).
CZA demonstrates in vitro activity against bacteria possessing class A and C β-lactamases, such as extended-spectrum β-lactamases (ESBLs), Klebsiella pneumoniae carbapenemase, and AmpC cephalosporinases, as well as activity against some class D β-lactamases (e.g., OXA-48). Of important note, CZA does not demonstrate in vitro activity against isolates containing MBLs.
We hypothesized that a “triple combination” of antibiotics may be effective against MDR S. maltophilia if each one of its two β-lactamases were “occupied” with the right counterpart, leaving a third agent “free” to reach its target. The combination of CZA and ATM could target L2 with AVI, thus protecting ATM and CAZ from hydrolysis. The L1 MBL hydrolyzes CAZ and is not inhibited by AVI but cannot hydrolyze ATM. With coadministration of CZA and ATM, CAZ would serve as the primary substrate for L1, while AVI would inhibit L2 and allow ATM to bypass inactivation and successfully reach the penicillin-binding proteins (PBPs) of S. maltophilia, likely PBP3.
The results of molecular analysis and in vitro susceptibility testing supported our reasoning. Genes encoding L1 and L2 were detected by PCR, consistent with the observed in vitro resistance to CZA and ATM; synergy between CZA and ATM was demonstrated by disc diffusion testing, ultimately predicting therapeutic success. Unfortunately, methods to identify potentially synergistic drug combinations are not readily available (8, 9).
It is conceivable that ATM partnered with AVI (without CAZ) might have also been effective in treating this infection, although this combination has not been extensively tested in vitro against S. maltophilia. The coformulation of ATM and AVI has demonstrated in vitro and in vivo activity against Enterobacteriaceae producing MBLs; however, the activity of the ATM-AVI combination against Pseudomonas aeruginosa harboring MBLs is not as predictable (10, 11). Regardless, ATM-AVI is only in the early stages of clinical development (ClinicalTrials.gov Identifier: NCT01689207). Moreover, CAZ may contribute antimicrobial activity against certain MBLs, and the potential benefit of “dual β-lactam therapy” provided by ATM and CAZ cannot be discounted (12, 13). Therefore, in the absence of alternatives, the coadministration of CZA and ATM may offer an option for the treatment of serious infections caused by some carbapenem-resistant Gram-negative bacteria with a complex background of resistance determinants that includes the simultaneous production of MBLs and class A and C cephalosporinases.
COMMENTARY
S. maltophilia is an increasingly important pathogen in immunocompromised patients or those with cystic fibrosis. Characteristically displaying an MDR phenotype, including being inherently resistant to carbapenems, infections with S. maltophilia are difficult to treat, and there are sparse data providing guidance on the optimal regimen when commonly used antibiotics fail (1). Because of a combination of host factors and limited treatment options, the mortality rate associated with infections caused by S. maltophilia exceeds 30% (14). Additionally, as highlighted by this case, S. maltophilia can persist for years given the correct host (in this case, bacteremia recurred nearly 2 years after treatment), adding an additional layer of complexity to the treatment of S. maltophilia infections. The case presented here highlights these difficulties routinely encountered by clinicians in the treatment of patients with S. maltophilia infections and provides promising information on a novel “repurposing” of two marketed antibiotics, ATM and CZA.
In this case, neither the gold-standard therapy, TMP-SMX, nor any alternative agents (including levofloxacin, minocycline, and colistimethate sodium) were options because of in vitro resistance or clinical failure. The intrinsic resistance of S. maltophilia to most commercially available β-lactams is mediated by two chromosomally encoded inducible β-lactamases, L1 and L2. L1 is an MBL that, characteristically of all MBL enzymes, does not hydrolyze ATM. L2 is a clavulanate-sensitive Ambler class A cephalosporinase that complements the activity of L1 by hydrolyzing ATM in addition to extended-spectrum cephalosporins (1, 15). The authors hypothesized that the novel broad-spectrum β-lactamase inhibitor AVI (which inhibits Ambler class A and C β-lactamases) would inhibit the L2 β-lactamase, leaving ATM free to interact with the PBPs of S. maltophilia (10). As demonstrated by evidence of in vitro synergy of CZA and ATM by the double-disk diffusion test and the clinical response, this hypothesis was well founded. Despite recalcitrant bacteremia of several weeks duration, the combination of ATM and CZA led to rapid and sustained clearance of blood cultures through several months following the completion of therapy.
The methods used by the authors to identify synergy between ATM and CZA is worth noting. A double-disk synergy test requires no special equipment or training and can be routinely performed in many clinical microbiology labs to provide timely, clinically useful information. Overlaid E tests may provide similarly useful information with the added benefit of quantifying the degree of synergy observed (16). It is important to note that CZA is likely the only currently available β-lactam–β-lactamase inhibitor combination with this potential for synergy with ATM. While L2 is inhibited by clavulanate and the combination of ATM and ticarcillin-clavulanate is synergistic in vitro, ticarcillin-clavulanate is no longer commercially available (17). In contrast to other class A β-lactamases, tazobactam and sulbactam are less potent inhibitors of L2 with little to no benefit when combined with ticarcillin against S. maltophilia (18). As oral amoxicillin-clavulanate is the sole means of obtaining clavulanate in the United States, CZA appears to be the only viable option for synergism with ATM for serious S. maltophilia infections.
As discussed by the authors, the combination of ATM and CZA has utility extending beyond S. maltophilia and has significant potential in the management of infections caused by other MBL-producing Gram-negative organisms. MBL enzymes, including NDM, VIM, and IMP, have now been identified worldwide in diverse members of the family Enterobacteriaceae and P. aeruginosa (among others) (19). Although ATM is active against organisms harboring only MBL enzymes, these organisms frequently possess one or more ESBLs, carbapenemases, or AmpC-type enzymes, which readily hydrolyze ATM and limit its utility as a single agent to rare cases. AVI potently inhibits these class A and C enzymes, and the ATM-AVI combination is nearly universally active in vitro against MBL-producing Enterobacteriaceae (10, 19). In contrast, the addition of AVI to ATM provides only minimal benefit against MBL-producing P. aeruginosa and Acinetobacter baumannii, perhaps because of the prevalence of OXA-type enzymes or non-β-lactamase-mediated β-lactam resistance in these organisms. Since ATM-AVI is in early-phase clinical trials, CZA plus ATM is presently the only means to achieve this potentially effective combination against the increasingly prevalent MBL-producing Enterobacteriaceae. Additionally, the possibility of in vitro synergy or activity resulting from the CAZ component may result in additional salutary benefit beyond the inhibition of β-lactamases by AVI (12, 13). However, given the limited clinical data available on the utility and safety of ATM-CZA, the combination should be further studied prior to widespread use (13, 20).
ACKNOWLEDGMENTS
Research described in this case report was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under awards R01AI100560, R01AI072219, and R01AI063517 to R.A.B. Additionally, F.P. is supported through UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH Roadmap for Medical Research. The content of this case report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This case report was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs to R.A.B. and Veterans Affairs Merit Review Program award 1I01BX001974 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Service to R.A.B. This work was supported in part by funds provided by the Geriatric Research Education and Clinical Center VISN 10 to R.A.B. and F.P. The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
This Journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. An expert clinician then provides a commentary on the case.
REFERENCES
- 1.Brooke JS. 2012. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang YT, Lin CY, Chen YH, Hsueh PR. 2015. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol 6:893. doi: 10.3389/fmicb.2015.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crossman LC, Gould VC, Dow JM, Vernikos GS, Okazaki A, Sebaihia M, Saunders D, Arrowsmith C, Carver T, Peters N. 2008. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol 9:R74. doi: 10.1186/gb-2008-9-4-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin C-W, Huang Y-W, Hu R-M, Chiang K-H, and Yang T-C. 2009. The role of AmpR in regulation of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. Res Microbiol 160:152–158. doi: 10.1016/j.resmic.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Perez F, Adachi J, Bonomo RA. 2014. Antibiotic-resistant Gram-negative bacterial infections in patients with cancer. Clin Infect Dis 59:S335–S339. doi: 10.1093/cid/ciu612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denton M, Todd NJ, Kerr KG, Hawkey PM, Littlewood JM. 1998. Molecular epidemiology of Stenotrophomonas maltophilia isolated from clinical specimens from patients with cystic fibrosis and associated environmental samples. J Clin Microbiol 36:1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fihman V, Le Monnier A, Corvec S, Jaureguy F, Tankovic J, Jacquier H, Carbonnelle E, Bille E, Illiaquer M, Cattoir V. 2012. Worrisome threat among unusual non-fermentative Gram-negative bacilli from hospitalized patients: a prospective multicenter study. J Infect 64:391–398. doi: 10.1016/j.jinf.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Tamma PD, Cosgrove SE, Maragakis LL. 2012. Combination therapy for treatment of infections with Gram-negative bacteria. Clin Microbiol Rev 25:450–470. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. 2013. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biedenbach DJ, Kazmierczak K, Bouchillon SK, Sahm DF, Bradford PA. 2015. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob Agents Chemother 59:4239–4248. doi: 10.1128/AAC.00206-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-β-lactamase producers. Antimicrob Agents Chemother 57:3299–3306. doi: 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. Unexpected in vivo activity of ceftazidime alone and in combination with avibactam against New Delhi metallo-β-lactamase-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 58:7007–7009. doi: 10.1128/AAC.02662-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahme C, Butterfield JM, Nicasio AM, Lodise TP. 2014. Dual beta-lactam therapy for serious Gram-negative infections: is it time to revisit? Diagn Microbiol Infect Dis 80:239–259. doi: 10.1016/j.diagmicrobio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Falagas ME, Kastoris AC, Vouloumanou EK, Rafailidis PI, Kapaskelis AM, Dimopoulos G. 2009. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol 4:1103–1109. doi: 10.2217/fmb.09.84. [DOI] [PubMed] [Google Scholar]
- 15.Walsh TR, MacGowan AP, Bennett PM. 1997. Sequence analysis and enzyme kinetics of the L2 serine beta-lactamase from Stenotrophomonas maltophilia. Antimicrob Agents Chemother 41:1460–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White RL, Burgess DS, Manduru M, Bosso JA. 1996. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob Agents Chemother 40:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milne KE, Gould IM. 2012. Combination antimicrobial susceptibility testing of multidrug-resistant Stenotrophomonas maltophilia from cystic fibrosis patients. Antimicrob Agents Chemother 56:4071–4077. doi: 10.1128/AAC.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lecso-Bornet M, Bergogne-Berezin E. 1997. Susceptibility of 100 strains of Stenotrophomonas maltophilia to three beta-lactams and five beta-lactam-beta-lactamase inhibitor combinations. J Antimicrob Chemother 40:717–720. doi: 10.1093/jac/40.5.717. [DOI] [PubMed] [Google Scholar]
- 19.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, Sahm DF, Bradford PA. 2016. Multiyear, multinational survey of the incidence and global distribution of metallo-beta-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:1067–1078. doi: 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aitken SL, Tarrand JJ, Deshpande LM, Tverdek FP, Jones AL, Shelburne SA, Prince RA, Bhatti MM, Rolston KV, Jones RN, Castanheira M, Chemaly RF. 16 June 2016. High rates of non-susceptibility to ceftazidime-avibactam and identification of New Delhi metallo-beta-lactamase production in Enterobacteriaceae bloodstream infections at a major cancer center. Clin Infect Dis doi: 10.1093/cid/ciw398. [DOI] [PubMed] [Google Scholar]