Abstract
Understanding the relationship between antibiotic exposure and amplification of bacterial subpopulations with reduced drug susceptibility over time is important for evaluating the adequacy of dosing regimens. We utilized a hollow-fiber infection model to identify the fosfomycin intravenous dosing regimens that prevented the amplification of Escherichia coli bacterial subpopulations with reduced fosfomycin susceptibility. The challenge isolate was E. coli ATCC 25922 (agar MIC with glucose-6-phosphate, 1 mg/liter; agar MIC without glucose-6-phosphate, 32 mg/liter). The fosfomycin dosing regimens studied were 1 to 12 g every 8 h for 10 days to approximate that planned for clinical use. The studies included a no-treatment control regimen. Two bacterial subpopulations were identified, one with reduced susceptibility with agar MIC values ranging from 32 to 128 mg/liter and the other resistant with agar MIC values of 256 to >1,024 mg/liter on plates containing 5× and 256× the baseline MIC value, respectively. An inverted-U-shaped function best described the relationship between the amplification of the two bacterial subpopulations and drug exposure. The lowest fosfomycin dosing regimen that did not amplify a bacterial subpopulation with reduced susceptibility was 4 g administered every 8 h. Nearly immediate amplification of bacterial subpopulations with reduced susceptibility was observed with fosfomycin dosing regimens consisting of 1 to 2 g every 8 h. These data will be useful to support the selection of fosfomycin dosing regimens that minimize the potential for on-therapy amplification of bacterial subpopulations with reduced susceptibility.
INTRODUCTION
Resistance of Enterobacteriaceae to antibiotics remains a global clinical concern. One risk factor for the emergence of antibiotic resistance is suboptimal drug exposure, which may be further exacerbated in at-risk patient populations. Those patient populations at risk for the development of antibiotic-resistant Enterobacteriaceae infections include but are not limited to those with prolonged hospitalization, immunocompromised status, and a neurological diagnosis (1). One way to reduce the likelihood of the emergence of on-therapy antibiotic resistance is to utilize dosing regimens that result in exposures sufficient to prevent the amplification of bacterial subpopulations with reduced susceptibility.
The hollow-fiber infection model has been utilized to characterize the relationship between drug exposure and resistance amplification over time (2, 3, 4). For ceftolozane-tazobactam, the relationship between drug exposure and resistance amplification of CTX-M-15-producing Escherichia coli resembled an inverted-U-shape function (3). Using this relationship, dosing regimens less likely to amplify resistant bacterial subpopulations were identified.
There has been considerable interest in the use of fosfomycin oral and intravenous (ZTI-01) formulations for infections associated with multidrug-resistant bacteria (5, 6). Fosfomycin has a broad spectrum of in vitro activity, including Enterobacteriaceae producing extended-spectrum β-lactamase (ESBL) enzymes (7). Given the renewed clinical interest and spectrum of fosfomycin in vitro activity, the goal of the studies described herein was to use an in vitro hollow-fiber infection model to identify the fosfomycin dosing regimen that prevented the amplification of Escherichia coli subpopulations with reduced fosfomycin susceptibility.
MATERIALS AND METHODS
Bacteria, antimicrobials, and β-lactamase inhibitor.
The fosfomycin intravenous solution (ZTI-01) was provided by Zavante Therapeutics, Inc. (San Diego, CA). The challenge isolate was Escherichia coli ATCC 25922 (Manassas, VA).
Media and in vitro susceptibility studies.
All studies described here were completed using Mueller-Hinton (MH) broth and MH agar media (BD Laboratories, Franklin Lakes, NJ) with or without supplementation with 25 mg/liter of glucose-6-phosphate (Sigma-Aldrich, St. Louis, MO). Susceptibility studies were performed in triplicate over a 2-day period using agar dilution methodology as described in Clinical and Laboratory Standards Institute guidelines (8). In order to understand the conditions represented within the dynamic in vitro model, broth dilution susceptibilities were also determined. The MIC results presented represent the modal values for these studies.
Mutation frequency studies.
The mutation frequency of drug resistance (density) was estimated using a previously described methodology (3, 9). In brief, the ratio of the total bacterial population to the bacterial subpopulations with reduced fosfomycin susceptibility found within a bacterial suspension was determined by plating 4 ml of log-phase growth suspension onto fosfomycin-containing agar plates. The growth medium was MH agar supplemented with 25 mg/liter of glucose-6-phosphate, and fosfomycin concentrations were either 5× or 256× the baseline fosfomycin agar MIC value. The concentration of fosfomycin on the drug-containing plates was based upon the results of the previously described susceptibility studies (10), which represented drug concentrations greater than baseline MIC values with and without the presence of glucose-6-phosphate. The bacterial concentration within the suspension was determined by quantitative culture, and the ratio of growth found on the drug-containing plates to that of the starting inoculum was used to provide an estimate of the frequency of drug resistance within the total population. This assay was performed in duplicate, and for each study, a subset of isolates was taken from the drug-containing plates and evaluated for change in the MIC from baseline to confirm decreased susceptibility using agar dilution methodology (8).
Hollow-fiber infection model.
The hollow-fiber infection model has been described previously (3, 11). In brief, this pharmacodynamic system allows for a challenge isolate to be exposed to simulated human pharmacokinetic profiles of a study compound. The system consists of a peripheral chamber, which is separated from the central compartment by semipermeable membranes. These membranes contain pores with diameters that are large enough to allow nutrients, drugs, and bacterial metabolites to transverse freely into and out of the peripheral compartment but too small for bacteria to leave the peripheral compartment. Fresh drug-free medium is circulated through the hollow-fiber cartridge from the central compartment using peristaltic pumps. The challenge compound is pumped into the central compartment under computer control and is continually diluted in the central compartment, simulating a targeted half-life, without diluting the pathogen in the peripheral compartment. Due to the high surface area-to-volume ratio, drug concentrations equilibrate rapidly in the periphery. The peripheral compartment contains sampling ports which allow for the sampling of the target organism over multiple time points throughout the study duration.
Fosfomycin dose-ranging studies.
In the dose-ranging studies, the initial inoculum of the challenge isolate was prepared using a previously described methodology (3, 4). In brief, the challenge inoculum was grown from an overnight culture in Trypticase soy agar supplemented with 5% sheep blood (BD Laboratories) at 35°C. Colonies were removed from the overnight growth plate and suspended in MH broth medium supplemented with glucose-6-phosphate. The bacterial concentration within the flask of MH broth was determined by optical density and a growth curve for the challenge isolate. A bacterial suspension, representing a concentration of 1.0 × 108 CFU/ml, was inoculated into the extracapillary space of the hollow-fiber cartridges (FiberCell Systems, Frederick, MD). Within the hollow-fiber cartridge, bacteria were exposed to fluctuating free-drug fosfomycin concentrations that simulated a human half-life of 2 h and exposures representing doses of 1, 2, 4, 8, and 12 g administered every 8 h (q8h) (12). A no-treatment control regimen was evaluated for comparison. All studies were performed in duplicate.
Over the course of the 10-day experiment, 1.5-ml samples were taken from the extracapillary space, washed twice with sterile normal saline to prevent drug carryover, serially diluted, and quantitatively cultured on drug-free Trypticase soy agar supplemented with 5% sheep blood to determine the effect of treatment on the total bacterial population. A portion of each sample was plated on MH agar plates containing 5× and 256× the baseline agar MIC of fosfomycin for confirmation of the bacterial subpopulations with reduced susceptibility. MIC values were determined for a subset of isolates found growing on the drug-containing plates on days 1, 3, 6, and 10 of each study using agar dilution methodology supplemented with glucose-6-phosphate.
Pharmacokinetic validation.
Over the first 48 h of each of the above-described studies, 1-ml samples were collected from the peripheral compartment at 1, 3, 5, 7, 9, 23, 25, 27, 29, and 48 h. All samples were immediately frozen at −80°C until assayed for concentration of fosfomycin.
Bioanalytical method.
Fosfomycin concentrations were determined via biological assay. Two hundred microliters of E. coli ATCC 25922 was grown to log phase in MH broth supplemented with 25 mg/liter of glucose-6-phosphate. The suspension was diluted to a concentration of 1.0 × 106 CFU/ml and inoculated onto the surface of an MH agar plate supplemented with 25 mg/liter of glucose-6-phosphate. A series of 4.8-mm-diameter wells were aseptically bored into the agar of each plate. A volume of 15 μl was taken from each pharmacokinetic sample, deposited into the individual wells, and incubated for 18 h at 35°C. The fosfomycin standard curve was logarithmic over concentrations ranging from 10 to 500 mg/liter, with a lower quantification limit of 10 mg/liter.
RESULTS
In vitro susceptibility studies.
The fosfomycin susceptibility test results against E. coli ATCC 25922 are shown in Table 1. When tested using the agar dilution methodology with glucose-6-phospate supplementation, per Clinical and Laboratory Standards Institute guidelines (8), the fosfomycin MIC value (1 mg/liter) was within the quality control range (0.5 to 2 mg/liter) (13). When tested without glucose-6-phospate, the fosfomycin MIC value for E. coli ATCC 25922 increased to 32 mg/liter.
TABLE 1.
Fosfomycin susceptibility test results in broth and agar media, with and without supplementation with 25 mg/liter glucose-6-phosphate
| Isolate | MIC (mg/liter)a |
|||
|---|---|---|---|---|
| Microbroth medium |
Agar medium |
|||
| With 25 mg/liter glucose-6-phosphate | Without glucose-6-phosphate | With 25 mg/liter glucose-6-phosphate | Without glucose-6-phosphate | |
| Escherichia coli ATCC 25922b | 2 | 64 | 1 | 32 |
The MIC represents the modal value based on the results of studies performed in triplicate.
The Clinical and Laboratory Standards Institute quality control range for fosfomycin is 0.5 to 2 mg/liter, as determined via agar dilution methodology using growth medium supplemented with 25 mg/liter glucose-6-phosphate (12).
Mutation frequency studies.
The average densities of the bacterial subpopulations with reduced susceptibility, which were determined at 5× and 256× the baseline MIC value, were 1 fosfomycin-resistant CFU in every 2.5 × 108 and 1 fosfomycin-resistant in every >9.5 × 108 CFU/ml, respectively. The MIC value of the subpopulation at 5× the baseline MIC was 16 to 64 mg/liter. The MIC value of the subpopulation at 256× the baseline MIC could not be determined due to lack of growth on the drug-containing plates.
Pharmacokinetic validation of targeted fosfomycin regimens.
The targeted fosfomycin pharmacokinetic profiles were well simulated within the hollow-fiber infection model (Fig. 1), as evidenced by the good agreement between the observed and targeted concentration-time profiles (r2 = 0.98, slope = 0.927, intercept = 9.46).
FIG 1.
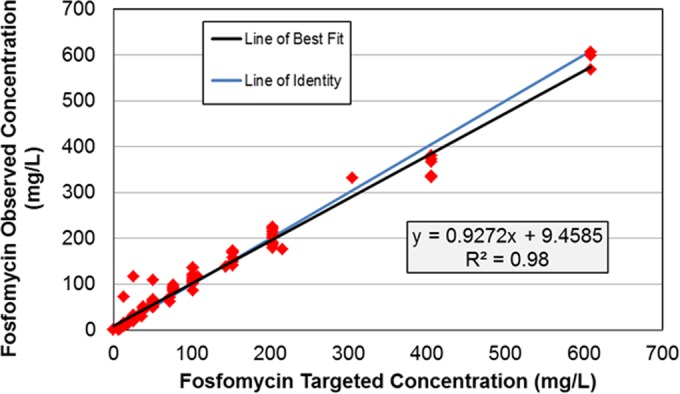
Relationship between the observed and targeted fosfomycin concentrations simulated in the in vitro hollow-fiber infection model.
Fosfomycin dose-ranging studies.
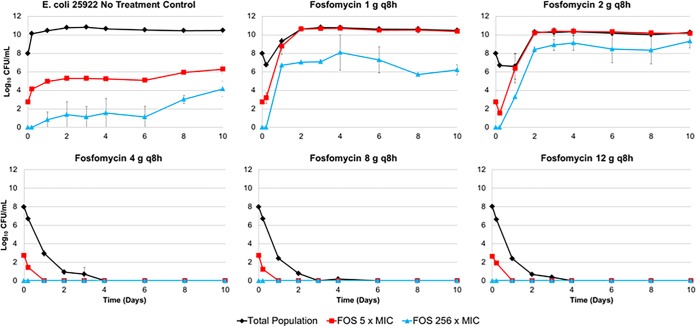
Figure 2 shows the average bacterial density of the total population and colonies observed on agar plates containing 5× and 256× the MIC over 10 days for each dosing regimen examined. The bacteria grew well in the no-treatment control regimens, as evidenced by the increase in average bacterial density from 1.0 × 107.9 to 1.0 × 1010.7 CFU/ml over 2 days. Two bacterial subpopulations emerged over time. The initial average density of the bacterial subpopulation with decreased fosfomycin susceptibility that grew on plates containing 5× the baseline MIC of fosfomycin was 1.0 × 102.7 CFU/ml; the average density of this bacterial subpopulation increased to 1.0 × 106.3 CFU/ml on day 10. The initial density of the resistant subpopulation that grew on plates containing 256× the baseline fosfomycin MIC was not detected but increased to 1.0 × 104.2 CFU/ml by day 10.
FIG 2.
Average bacterial density of the total bacterial population and the bacterial subpopulations with reduced fosfomycin susceptibility (5× and 256× the baseline MIC) over 10 days for each fosfomycin dosing regimen.
As shown in Fig. 2, there was a wide range of drug effect across the fosfomycin dosing regimens evaluated. The lowest fosfomycin dosing regimen (1 g q8h) produced an initial decrease in bacterial burden of 1 log10 CFU/ml after 5 h of therapy. This initial reduction in bacterial burden was observed only in the total population, which was replaced by the bacterial subpopulation with reduced susceptibility on day 1. The bacterial subpopulation observed on the agar plates containing 256× the baseline MIC increased in density but never achieved densities matching the total population. The majority of the total population (approximately 99.99%) represented the bacterial subpopulation observed on the agar plates containing 5× the baseline MIC. A similar pattern of results was observed for the fosfomycin 2 g q8h dosing regimen. The larger fosfomycin dosing regimens (4, 8, and 12 g q8h) resulted in a rapid reduction of the total bacterial population and prevented the emergence of both bacterial subpopulations. On day 10, the three most intensive fosfomycin dosing regimens sterilized the hollow-fiber model system.
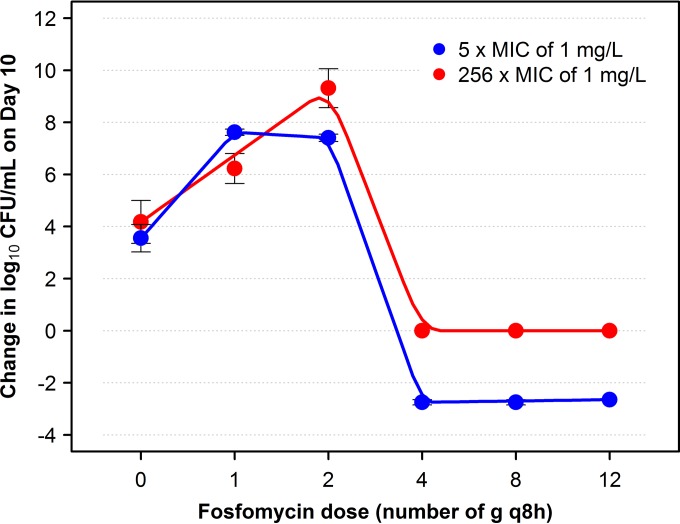
Figure 3 shows the relationship between the change in log10 CFU/ml from baseline on day 10 of both bacterial subpopulations with reduced susceptibility and the fosfomycin dosing regimen. The relationship between fosfomycin dosing regimen and change in log10 CFU/ml from baseline on day 10 of each subpopulation took the form of an inverted U. The fosfomycin MIC values of the isolates grown on plates supplemented with 5× and 256× the baseline MIC of fosfomycin increased in parallel with duration of therapy and were determined to be 32 to 128 and 256 to >1,024 mg/liter, respectively.
FIG 3.
Relationship between fosfomycin dosing regimen and change in log10 CFU/ml from baseline on day 10 of bacterial subpopulations with reduced fosfomycin susceptibility identified on drug-containing plates with 5× and 256× the baseline MIC. For each plotted point, which represents the average of two studies, the bars extend across the range of observed data.
DISCUSSION
The objective of these studies was to use an in vitro hollow-fiber infection model to identify the fosfomycin dosing regimen that prevented the amplification of E. coli subpopulations with reduced fosfomycin susceptibility. The q8h dosing interval was selected to match the dosing frequency to be used in an upcoming clinical trial involving patients with complicated urinary tract infections. E. coli was selected as the challenge organism due to the prevalence of this pathogen in patients with complicated urinary tract infections (14).
We successfully discriminated among fosfomycin dosing regimens and identified those that prevented the emergence of bacterial subpopulations with reduced susceptibility from those that amplified such subpopulations. The least intensive fosfomycin dosing regimens (1 and 2 g q8h) failed, with replacement of the susceptible population with the bacterial subpopulation with reduced susceptibility for the 1 g q8h dosing regimen and with the bacterial subpopulation with reduced susceptibility and the resistant bacterial subpopulation for the 2 g q8h dosing regimen. The bacterial subpopulation found on the agar plates with 5× the baseline fosfomycin MIC replaced the susceptible population by day 1 of the study. The bacterial subpopulation observed on the plates containing 256× the baseline MIC never fully replaced the total bacterial population for the 1 g q8h dosing regimen; for the 2 g q8h dosing regimen, the bacterial subpopulation reached that of the total population. The MIC values of the isolates collected from the supplemented agar plates containing 5× and 256× the baseline MIC ranged from 32 to 128 mg/liter and from 256 to >1,024 mg/liter, respectively. Of note, fosfomycin MIC values of ≥256 mg/liter for Enterobacteriaceae are resistant per the Clinical Laboratory and Standards Institute (13). These results are concordant with those observed by Nilsson et al., in which the number of resistance determinants increased and the biological fitness decreased as an isolate's fosfomycin MIC increased (15).
The most intensive fosfomycin dosing regimens (4, 8, and 12 g q8h) prevented the emergence and amplification of the bacterial subpopulations with reduced susceptibility. Moreover, these dosing regimens all resulted in a rapid reduction of the total bacterial population and ultimately sterilized the hollow-fiber infection model. A marked difference in the time to model sterilization for the 4, 8, and 12 g q8h dosing regimens was not detected. The number of CFU recovered in each of the more intensive dosing regimens approached zero by day 4 of the study.
The relationship between fosfomycin dose and the amplification of bacterial subpopulations with reduced susceptibility was hormetic (inverted U) in nature. Such relationships were first described in 1888 by Hugo Paul Friedrich Schulz (16) in studies involving yeast exposed to formic acid and more recently by others (2, 3, 4). The explanation for this phenomenon with the data described here is the presence of two bacterial subpopulations with different drug susceptibilities, which over certain exposure ranges drives the response in opposite directions (cell growth versus cell kill). The in vitro hollow-fiber infection model has been proven to be a useful system for the examination of exposure-response relationships of this nature (2, 3, 4, 17, 18). Indeed, this phenomenon has also been observed using data from daptomycin-treated patients with Staphylococcus aureus bacteremia with or without infective endocarditis. At 30 days after the start of therapy, the relationship between the probability of decreased susceptibility and AUC/MIC ratio resembled an inverted-U shape (19). The strength of the in vitro hollow-fiber infection model is that drug exposures beyond those that can be attained clinically can be examined. Given this capability, drug exposures can be identified that not only amplify resistance but also inhibit the growth of resistant subpopulations over a clinically relevant duration of therapy. Conducting such study earlier in drug development allows for discrimination among dosing regimens and the identification of those dosing regimens with a reduced probability of amplifying bacterial subpopulations with reduced susceptibility before clinical trials are conducted. The use of this approach is predicted to increase the duration of time that a given dosing regimen will be clinically useful.
The major limitation of the studies described herein was that they involved the evaluation of a single isolate. Thus, additional work with multiple E. coli isolates that manifest a broad range of resistance determinants is warranted. Moreover, studies involving other Enterobacteriaceae and Pseudomonas aeruginosa will enrich our understanding of the fosfomycin exposures necessary to decrease the probability of on-therapy resistance emergence. A second limitation of these studies is that the effect of the immune system on the eradication of the bacterial population and subpopulation was not considered. However, despite this limitation, studies have shown concordant findings with respect to the development of resistance under in vitro conditions and clinical settings after administration of the same dosing regimen (20, 21).
In conclusion, we successfully discriminated among fosfomycin dosing regimens that prevented the emergence of E. coli subpopulations with reduced drug susceptibility and identified those dosing regimens that amplified the emergence of such subpopulations. Fosfomycin dosing regimens of 4, 8, and 12 g q8h prevented the emergence of bacterial subpopulations with reduced drug susceptibility and, by day 4, drove the entire bacterial population toward extinction. These data will be useful to support the selection of fosfomycin dosing regimens that minimize the potential for on-therapy amplification of bacterial subpopulations with reduced fosfomycin susceptibility.
ACKNOWLEDGMENTS
We thank Kim A. Charpentier from the Institute for Clinical Pharmacodynamics (Schenectady, NY, USA) for manuscript assistance and technical support.
This study was sponsored by Zavante Therapeutics.
The Institute for Clinical Pharmacodynamics (B.V., J.M., S.M.B., and P.G.A.) has received research support from Achaogen, Basilea Pharmaceutica, Cempra Pharmaceuticals, Cellceutix Corporation, Cubist Pharmaceuticals, Fedora Pharmaceuticals, GlaxoSmithKline, Meiji Seika Pharma, Melinta Therapeutics, Merck & Co., Nabriva Therapeutics, Nimbus, Pfizer, Roche Bioscience, Tetraphase Pharmaceuticals, and the Medicines Company.
REFERENCES
- 1.Bhavnani SM, Hammel JP, Forrest A, Jones RN, Ambrose PG. 2003. Relationships between patient- and institution-specific variables and decreased antimicrobial susceptibility of Gram-negative pathogens. Clin Infect Dis 37:344–350. doi: 10.1086/375817. [DOI] [PubMed] [Google Scholar]
- 2.Tam VH, Louie A, Deziel MR, Liu W, Leary R, Drusano GL. 2005. Bacterial-population responses to drug-selective pressure: examination of garenoxacin's effect on Pseudomonas aeruginosa. J Infect Dis 192:420–428. doi: 10.1086/430611. [DOI] [PubMed] [Google Scholar]
- 3.VanScoy B, Mendes RE, Castanheira M, McCauley J, Bhavnani SM, Forrest A, Jones RN, Ambrose PG. 2013. Relationship between ceftolozane-tazobactam exposure and drug resistance amplification in a hollow-fiber infection model. Antimicrob Agents Chemother 57:4134–4138. doi: 10.1128/AAC.00461-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanScoy BD, Mendes RE, Castanheira M, McCauley J, Bhavnani SM, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2014. Relationship between ceftolozane-tazobactam exposure and selection for Pseudomonas aeruginosa resistance in a hollow-fiber infection model. Antimicrob Agents Chemother 58:6024–6031. doi: 10.1128/AAC.02310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Bano J, Alcala JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, Tortola T, Mirelis B, Navarro G, Cuenca M, Esteve M, Pena C, Llanos AC, Canton R, Pascual A. 2008. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med 168:1897–1902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 6.Pullukcu H, Tasbakan M, Sipahi OR, Yamazhan T, Aydemir S, Ulusoy S. 2007. Fosfomycin in the treatment of extended spectrum beta-lactamase-producing Escherichia coli-related lower urinary tract infections. Int J Antimicrob Agents 29:62–65. doi: 10.1016/j.ijantimicag.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Kastoris AC, Lapaskelis AM, Karageogopoulos DE. 2010. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum β-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis 10:43–50. doi: 10.1016/S1473-3099(09)70325-1. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Drusano GL, Bonomo RA, Bahniuk N, Bulitta JB, VanScoy B, Defiglio H, Fikes S, Brown D, Drawz SM, Kulawy R, Louie A. 2012. Resistance emergence mechanism and mechanism of resistance suppression by tobramycin for cefepime for Pseudomonas aeruginosa. Antimicrob Agents Chemother 56:231–242. doi: 10.1128/AAC.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanScoy BD, McCauley J, Ellis-Grosse EJ, Okusana OO, Bhavnani SM, Forrest A, Ambrose PG. 2015. Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother 59:7170–7177. doi: 10.1128/AAC.04955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie A, Heine HS, VanScoy B, Eichas A, Files K, Fikes S, Brown DL, Liu W, Kinzig-Schippers M, Sogel F, Drusano GL. 2011. Use of an in vitro pharmacodynamics model to derive a moxifloxacin regimen that optimizes kill of Yersinia pestis and prevents emergence of resistance. Antimicrob Agents Chemother 55:822–830. doi: 10.1128/AAC.00818-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laboratorios ERN.1997. Fosfocina intravenosa (package insert). Laboratorios ERN, Madrid, Spain. [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Foxman B. 2010. The epidemiology of urinary tract infection. Nat Rev Urol 7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson AI, Berg OG, Aspevall O, Kahmeter G, Andersson DI. 2003. Biological costs and mechanisms of fosfomycin resistance in Escherichia coli. Antimicrob Agents Chemother 47:2850–2858. doi: 10.1128/AAC.47.9.2850-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz H. 2003. Contemporary medicine as presented by its practitioners themselves, Leipzig, 1923:217–250. Nonlinearity Biol Toxicol Med 1:295–318. doi: 10.1080/15401420390249880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam VH, Louie A, Deziel MR, Liu W, Drusano GL. 2007. The relationship between quinolone exposures and resistance amplification is characterized by an inverted U: a new paradigm for optimizing pharmacodynamics to counterselect resistance. Antimicrob Agents Chemother 51:744–747. doi: 10.1128/AAC.00334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drusano GL, Neely M, VanGuilder M, Schumitzky A, Brown D, Fikes S, Peloquin C, Louie A. 2014. Analysis of combination drug therapy to develop regimens with shortened duration of treatment for tuberculosis. PLoS One 9(7):e101311. doi: 10.1371/journal.pone.0101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhavnani SM, Ambrose PG, Hammel JP, Rubino CM, Drusano GL. 2016. Evaluation of daptomycin exposure and efficacy and safety endpoints to support risk versus benefit considerations. Antimicrob Agents Chemother 60:1600–1607. doi: 10.1128/AAC.02967-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanScoy BD, Bulik CC, Moseley C, Scangarella-Oman NE, Miller LA, Bhavnani SM, Ambrose PG. 2013. Hollow fiber infection model (HFIM) mimics both the time-to-resistance emergence and magnitude of E. coli resistance to GSK052 occurring in a phase 2b clinical study, poster A-016. 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO. [Google Scholar]
- 21.O'Dwyer K, Spivak AT, Ingraham K, Min S, Holmes DJ, Jakielaszek C, Rittenhouse S, Kwan AL, Livi GP, Sathe G, Thomas E, Van Horn S, Miller LA, Twynholm M, Tomayko J, Dalessandro M, Caltabiano M, Scangarella-Oman NE, Brown JR. 2015. Bacterial resistance to leucyl-tRNA synthetase inhibitor GSK2251052 develops during treatment of complicated urinary tract infections. Antimicrob Agents Chemother 59:289–298. doi: 10.1128/AAC.03774-14. [DOI] [PMC free article] [PubMed] [Google Scholar]