Abstract
Fluconazole is an effective agent for prophylaxis of invasive candidiasis in premature infants. The objective of this study was to characterize the population pharmacokinetics (PK) and dosing requirements of fluconazole in infants with birth weights of <750 g. As part of a randomized clinical trial, infants born at <750 g birth weight received intravenous (i.v.) or oral fluconazole at 6 mg/kg of body weight twice weekly. Fluconazole plasma concentrations from samples obtained by either scheduled or scavenged sampling were measured using a liquid chromatography-tandem mass spectrometry assay. Population PK analysis was conducted using NONMEM 7.2. Population PK parameters were allometrically scaled by body weight. Covariates were evaluated by univariable screening followed by multivariable assessment. Fluconazole exposures were simulated in premature infants using the final PK model. A population PK model was developed from 141 infants using 604 plasma samples. Plasma fluconazole PK were best described by a one-compartment model with first-order elimination. Only serum creatinine was an independent predictor for clearance in the final model. The typical population parameter estimate for oral bioavailability in the final model was 99.5%. Scavenged samples did not bias the parameter estimates and were as informative as scheduled samples. Simulations indicated that the study dose maintained fluconazole troughs of >2,000 ng/ml in 80% of simulated infants at week 1 and 59% at week 4 of treatment. Developmental changes in fluconazole clearance are best predicted by serum creatinine in this population. A twice-weekly dose of 6 mg/kg achieves appropriate levels for prevention of invasive candidiasis in extremely premature infants.
INTRODUCTION
Invasive candidiasis is a common cause of death and neurodevelopmental impairment in extremely premature infants (1). Fluconazole, a triazole antifungal drug that exhibits fungistatic activity against a variety of Candida species, is an effective agent as a prophylaxis for treatment of invasive candidiasis in this population (2 – 4). Fluconazole exhibits pharmacokinetic (PK) characteristics that make it an attractive candidate for prevention of Candida infections. It has a long half-life allowing infrequent administration, is minimally (12%) bound to plasma proteins, penetrates the cerebrospinal fluid, and achieves saliva and lung concentrations that are 1.3 and 1.2 times the plasma concentrations, respectively, thereby providing higher concentrations at key areas of colonization (5 – 7). Additionally, in adults, fluconazole has very high (>90%) oral bioavailability (8, 9).
Previous PK studies in infants suggested increasing fluconazole clearance (CL) over the first postnatal weeks (10 – 13). However, the PK of fluconazole in infants of <750 g birth weight have not been extensively evaluated, and it is this population that has the highest risk for invasive candidiasis where prophylaxis has the most potential for benefit (14). Therefore, the objective of this study was to characterize the population PK and dosing requirements of fluconazole in infants of <750 g birth weight.
MATERIALS AND METHODS
Study design.
This PK study was associated with a multicenter, randomized, placebo-controlled trial that evaluated the efficacy and safety of fluconazole in preventing death or invasive candidiasis in premature infants weighing <750 g at birth (4). Inclusion criteria required that infants were <120 h old at the time of randomization and of <750 g birth weight and that informed consent was received from a legally authorized representative. Participants were excluded from the trial if they had significant liver dysfunction (aspartate aminotransferase [AST] and alanine aminotransferase [ALT], >250 U/liter), renal dysfunction (serum creatinine [SCR], >2 mg/dl), a diagnosis of invasive candidiasis or congenital Candida infection, or a history of hypersensitivity to any azole antifungal. Participants randomized to fluconazole therapy received 6 mg/kg of body weight twice weekly (Tuesdays and Fridays) for up to 42 days of treatment. The fluconazole dose was administered either by an intravenous (i.v.) infusion given over approximately 60 min or orally (in infants that were receiving enteral medications). Clinical data were collected and included demographic information (gestational age [GA], postnatal age [PNA], birth weight, current weight, race, sex, and ethnicity), laboratory values collected within the 72 h prior to the first fluconazole dose and during treatment (serum creatinine [SCR], total bilirubin, alanine transaminase [ALT], and albumin [ALB]), details of concomitant medications of interest (all antimicrobials and vasopressors), intubation status, mode of delivery (Cesarean section or vaginal), and microbiological cultures from sterile sites. The study was approved by the Institutional Review Boards at each center, and informed consent was obtained from a legally authorized representative.
PK sample collection.
Participants were randomized to 1 of 8 sampling schemes with a maximum of 3 timed blood samples. Each infant had two PK samples drawn after a single dose taken around the time of administration of dose 3, 5, 7, or 9 and one sample taken around the time of administration of the final dose. Samples obtained from infants receiving placebo were used for other purposes. One additional plasma PK sample was requested from infants who developed invasive candidiasis. Up to 10 scavenged samples left over from laboratory blood samples obtained per routine care were also collected for PK evaluation. Sample collection consisted of whole blood (200 μl) collected in EDTA tubes. Timed blood samples were processed within 6 h of collection, and plasma was stored at −80°C until analysis for fluconazole concentration determination.
Bioanalytical assay.
Plasma samples were analyzed for fluconazole concentrations using a validated liquid chromatography method with tandem mass spectrometric detection (LC-MS/MS). The lower limit of quantitation for fluconazole was 10 ng/ml. The precision determined at each concentration level did not exceed 8.5% of the coefficient of variation, including the lower limit of quantification.
Population PK analysis.
Concentration-time data were analyzed with nonlinear mixed-effect modeling using NONMEM version 7.2 (Icon; Ellicott City, MD, USA). Plasma samples were excluded from the analysis if they (i) were below the limit of quantitation (<10 ng/ml), (ii) were collected >120 h after the last recorded fluconazole dose, (iii) demonstrated increased fluconazole concentrations (compared to prior samples) without a recorded dose, or (iv) were extreme outliers (>10-fold difference between observed and predicted concentrations). A 1-compartment model (ADVAN2, TRANS2 subroutine) and a first-order conditional estimation method (FOCE with interaction) were used to describe the fluconazole concentration data. Plasma concentrations following intravenous and oral administration were simultaneously analyzed, allowing estimation of absolute oral bioavailability. Parameters of the model were the absorption rate constant (ka), volume of distribution (V), clearance (CL), and bioavailability (F1). Diagnostic plots were executed in PLT Tools 5.1.0 (PLTSoft; San Francisco, CA), SAS 9.3 (Cary NC), and R Project 3.0.1 (downloaded from a website of the University of California, Los Angeles, Los Angeles, CA). The bootstrap procedure was performed using WINGS for NONMEM version 7.2 (Auckland, NZ), and 1,000 bootstrap sample data sets were generated. Consistent with prior PK analyses of fluconazole, a 1-compartment model with first-order absorption was selected for development. Population PK parameters were scaled by body size prior to evaluation of potential covariates. Clearance was scaled by allometric weight (WT0.75), and volume of distribution was scaled by weight (WT1.0). The initial model used a combined proportional error and additive residual error value. Diagnostic plots were used to assess the appropriateness of this structure for the base model.
Once the base model was identified, covariates were investigated for their potential influence on PK parameters, CL, and volume (V). Continuous covariates were evaluated by normalization to median values and included PNA, GA, postmenstrual age (PMA), SCR, and ALB. Categorical covariates included race and ethnicity, intubation status, and mode of delivery (Cesarean section or vaginal). Missing covariate values were imputed using the closest value available for that participant and either a carry-forward approach or a backfill approach, depending on which date was closest. The investigation of the relationship between potential covariates and PK parameters proceeded by developing the base population PK model and post hoc generation of the Bayesian estimates of individual PK parameters. Individual subject etas (η), representing deviation from the typical population parameter values, were generated. Graphical assessment of the relationships between PK parameters and potential covariates was performed by plotting etas versus potential clinically relevant covariates. Covariates with an evident graphical relationship to ηCL and ηV were evaluated for inclusion in the final model. A forward-addition, backward-elimination approach to covariate selection was used when two or more covariates were found to be significant for CL or V. The threshold for the significance of a single covariate was reduction of the objective function value (OFV) by >7.88.
Model evaluation.
Model evaluation included successful minimization, goodness-of-fit plots, precision-of-parameter estimates, bootstrap procedures, and visual predictive checks. The precision of the final population PK model parameter estimates was evaluated using nonparametric bootstrapping (1,000 replicates) to generate the 95% confidence intervals for parameter estimates. The final model was used to perform Monte Carlo simulations in 14,100 virtual subjects with demographic and laboratory characteristics simulated from the same distribution as the study population. The simulated trough fluconazole concentrations were determined during an 8-week course of fluconazole and predose concentrations compared to a minimum target of 2,000 ng/ml. This target was selected based upon typical drug MICs for Candida species in infants.
RESULTS
Study population and PK samples.
A total of 141 premature infants weighing <750 g at birth who received i.v. or oral fluconazole were included (Table 1), and fluconazole concentrations were determined from 619 plasma samples obtained from the infants. Of these, 15 samples (2.4%) were excluded, resulting in 604 plasma samples available for population PK modeling. Samples were excluded for the following reasons: concentration below the limit of quantitation (n = 7); collection >120 h after the last dose, indicating an incomplete dosing history (n = 3); outlier concentrations based on individual predictions (IPRED) and population predictions (PRED) (n = 3); and increased concentrations compared to prior samples without a documented dose (n = 2). The majority of PK samples (n = 368, 61%) were from scavenged samples. The PK collection time after dose was most frequently the first 6 h postdose, with an overall median (range) of 30 h (0 to 115 h). The medians (ranges) of the fluconazole concentrations were 4,144 ng/ml (491 to 14,050 ng/ml) from scavenged samples and 6,154 ng/ml (320 to 13,167 ng/ml) from timed samples.
TABLE 1.
Patient demographic and clinical data at first PK evaluationa
| Parameter | Value |
|---|---|
| PNA (days) | 23 (3–47) |
| GA (wks) | 24.7 (22.6–28.7) |
| PMA (wks) | 28.3 (23.7–35.1) |
| Wt (g) | 710 (345–2,680) |
| Serum creatinine (mg/dl) | 0.7 (0.1–3.6) |
| Albumin (g/dl) | 2.5 (1.0–4.7) |
| Male | 40 |
| Intubation status | 81 |
| Delivery by Cesarean section | 67 |
| Race | |
| American Indian or Alaska Native | 5 |
| Asian | 1 |
| Black or African American | 53 |
| White | 40 |
n = 141. Values presented as median (range), except for sex, intubation status, delivery by Cesarean section, and race, which are presented as percentages.
Population PK model building.
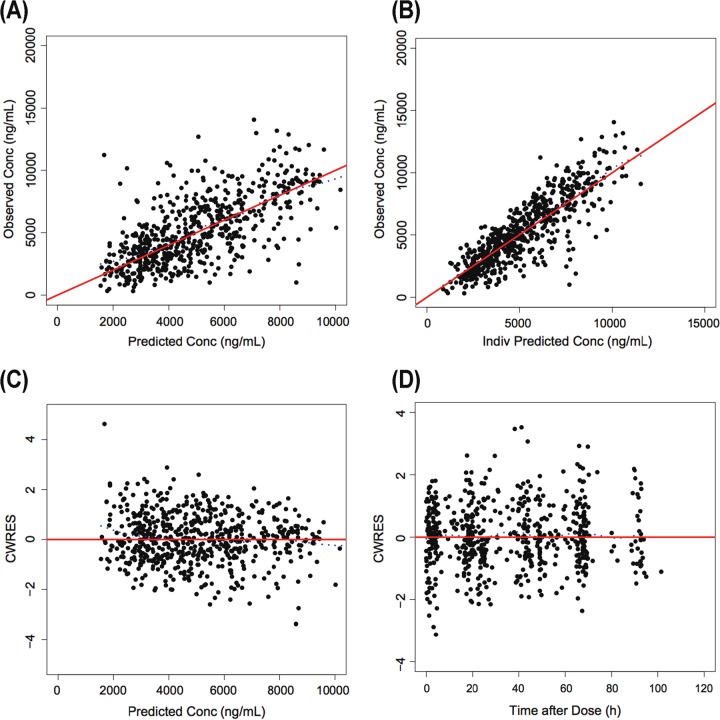
A 1-compartment model with first-order absorption and with combined proportional error plus additive residual error adequately described the concentration versus time data (Fig. 1). Due to limited numbers of early samples after oral administration in the data set, between-subject variability (BSV) was not estimated for the absorption rate constant (ka). Data from the base fluconazole population PK model of observed and conditionally weighted residuals versus population and individual-predicted concentrations were evenly scattered at the line of unity with no obvious biases.
FIG 1.
Goodness-of-fit plots for the base model. (A) Predicted versus observed concentrations (Conc). (B) Individual (Indiv) predicted versus observed concentrations. (C) Conditional weighted residuals (CWRES) versus population predictions. (D) Conditional weighted residuals versus time after dose. For panels A and B, the line of identity is included as a reference. For panels C and D, a solid line at y = 0 is included as a reference.
Graphical plots of GA, PNA, PMA, SCR, and mode of delivery were suggestive of a relationship with ηCL, which was consistent with the significant changes in OFV that resulted during the univariable screen assessment of these covariates for CL (Table 2). Plots of PMA and SCR were suggestive of a relationship with ηV, and both of these covariates met the OFV reduction criteria. All of the potential covariates identified in the univariate screen were incorporated into a combined model for backward elimination assessment. Although PMA had the second greatest impact on OFV during the univariable screen, it is a function of PNA and GA, both of which were also identified as potential covariates. The multivariable process started with the elemental components of PMA (PNA and GA) rather than PMA itself. Sequential removal was performed in the reverse order of the magnitude of OFV change seen with the covariate in the univariable screening process. Attempts to remove GA (CL), PNA (CL), and SCR (CL) each resulted in increases of >10 in the OFV, and the parameters were thus deemed significant independent covariates. Finally, a model using SCR (CL) and PMA (CL) as a function of GA and PNA was assessed and performed better than the model with SCR (CL), GA (CL), and PNA (CL) with an OFV reduction of 37.3 despite having one fewer covariate. The model-estimated absolute oral bioavailability of fluconazole was 100% in the final model, which is in agreement with prior adult data.
TABLE 2.
Summary of key univariable population PK model building processa
| Model description | Population model | OFV | Change in OFV from base model |
|---|---|---|---|
| CL (base model) | CL = θCL * (WT)0.75 | 9,624 | |
| PNA | CL = θCL * (WT)0.75 * (PNA/25)θCL-PNA | 9,492 | −132 |
| GA | CL = θCL * (WT)0.75 * (GA/25)θCL-GA | 9,599 | −25 |
| PMA | CL = θCL * (WT)0.75 * (PMA/28)θCL-PMA | 9,450 | −174 |
| SCR | CL = θCL * (WT)0.75 * (SCR/0.8)θCL-SCR | 9,405 | −219 |
| CSCT | CL = θCL * (WT)0.75 * θCL-CSCTCSCT | 9,617 | −7 |
| V (base model) | V = θV * (WT)1.0 | 9,624 | |
| PMA | V = θV * (WT)1.0 * (PMA/28)θV-PMA | 9,620 | −4 |
| SCR | V = θV * (WT)1.0 * (SCR/0.8)θV-SCR | 9,600 | −24 |
OFV, objective function value; CSCT, delivery by Cesarean section.
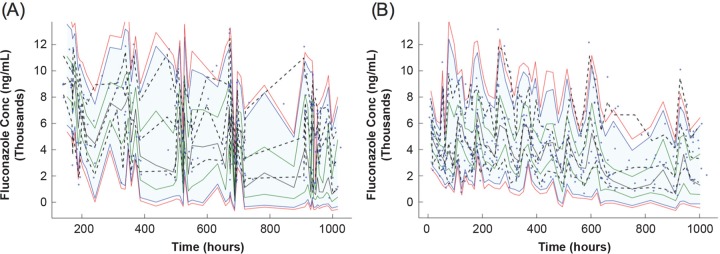
No significant relationships were observed between fluconazole CL or V and sex, race, ethnicity, intubation, or mode of infant delivery. The typical population PK parameter estimates in the final model were as follows: CL (liters/h/kg0.75) = 0.0127 * (SCR/0.8)−0.41 * (PMA/28)2.05; V (liters/kg) = 1.00; ka (1/h) = 0.96; F1 = 100% (where SCR is in milligrams per deciliter and PMA is in weeks). BSV was estimated as 23% for CL, 13% for V, and 25% for F1. The final PK parameters are displayed in Table 3. The model was evaluated using a 1,000-set bootstrap analysis with the program WINGS for NONMEM; 98.7% of bootstrap data sets converged to ≥3 significant digits. The medians of bootstrap fixed-effect parameter estimates were within 1% of population estimates from the original data set for all parameters (Table 3). The visual predictive check indicated that the model adequately described the data, with 3.1% of observed values >95th percentile and 4.1% <5th percentile (Fig. 2).
TABLE 3.
Final PK model parametersa
| Parameter | Symbol | Point estimate | SEE | Bootstrap CI |
||
|---|---|---|---|---|---|---|
| 2.5% | Median | 97.5% | ||||
| V (liters/kg) | θV | 1.00 | 0.0378 | 0.93 | 1.00 | 1.08 |
| CL (liters/h/kg0.75) | θCL | 0.0127 | 0.00033 | 0.0120 | 0.0127 | 0.0133 |
| F1 (%) | θF1 | 1.00 | 0.065 | 0.86 | 1.00 | 1.13 |
| ka (1/h) | θKA | 0.96 | 0.25 | 0.52 | 0.96 | 1.81 |
| SCR-CL | θSCR | −0.410 | 0.0498 | −0.53 | −0.41 | −0.32 |
| PMA-CL | θPMA | 2.05 | 0.35 | 1.23 | 2.05 | 2.62 |
| Interindividual variance (CV%) | ||||||
| V | ω2V | 13 | 61 | 1 | 13 | 18 |
| CL | ω2CL | 23 | 29 | 15 | 22 | 27 |
| F1 | ω2F1 | 31 | 73 | 1 | 22 | 50 |
| Residual variance (CV%) | σ2 | 46 | 27 | 37 | 46 | 51 |
| Additive value (ng/ml) | σ2 | 505 | 329 | 5 | 495 | 858 |
CI, confidence interval; CV%, percent coefficient of variation; SEE, standard error of estimate.
FIG 2.
Visual predictive check of the final model for scheduled samples (A) and scavenged samples (B), displaying time after first dose.
The potential influence of the collection type on the final model was assessed by fitting a reduced data set that contained only timed samples to the final model structure. The parameter estimates using this reduced data set were within 10% of those estimated with the full data set, indicating that the scavenged samples did not bias the PK parameter estimates and were as informative as the scheduled samples. Further, the visual predictive check demonstrated that the model adequately captured fluconazole concentrations irrespective of the sample type (Fig. 2). While the residual error was somewhat smaller when limiting the analysis to scheduled samples, of particular interest, the CL estimated from timed samples was altered by only 6% compared to that estimated from the full data set (0.0119 versus 0.0127 liters/h/kg0.75).
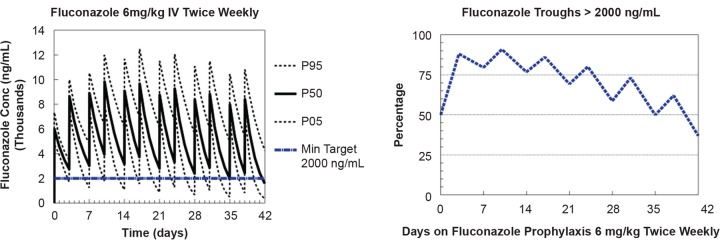
Using Monte Carlo simulations, the fluconazole exposure from doses of 6 mg/kg twice weekly was assessed. The trough fluconazole concentrations were determined during an 8-week course of fluconazole, and predose concentrations were compared to a minimum target of 2,000 ng/ml. This threshold was exceeded in 80% of simulated infants at week 1 and in 59% of simulated infants at week 4 of fluconazole prophylaxis. This is consistent with 95.7% of the first measured fluconazole concentrations being >2,000 ng/ml and with 89.9% of the overall fluconazole concentrations being >2,000 ng/ml (Fig. 3).
FIG 3.
Monte Carlo simulations of fluconazole given at 6 mg/kg intravenously (IV) twice weekly using the final population PK model displaying simulated fluconazole concentrations (left) and proportion of fluconazole troughs of >2,000 ng/ml (right). Min, minimum.
DISCUSSION
The present study evaluated the population PK of fluconazole in 141 premature infants of <750 g birth weight receiving twice-weekly fluconazole for 42 days for candidiasis prophylaxis. This represents a particularly difficult study population for collecting PK samples, and the use of scavenged samples more than doubled the data set size, leading to improved parameter estimates. In this population, fluconazole CL is low and is associated with a long half-life, which allows infrequent administration. Oral bioavailability appears to be high, as has been demonstrated in older populations, and fluconazole may therefore be given at the same dose in young infants and administered either as an oral suspension or intravenously. The population PK model identified SCR as the best predictor of developmental changes in fluconazole CL. Given fluconazole's renal CL in adults, this is not surprising. Although SCR levels decrease with age and are significantly correlated with measures of maturation, PMA was also independently associated with fluconazole CL. The individual impacts of GA and PNA on CL were modest and not of a magnitude to justify dose stratification in this population during the first 30 days of life. Overall, CL and V were in the range expected based on prior neonatal fluconazole PK studies. In the study by Wade et al., the typical CL was 0.015 liters/h/kg0.75 for a typical infant (GA, 26 weeks; PNA, 2 days) (10). This is similar to our typical CL value of 0.0127 liters/h/kg0.75 at 28 weeks PMA. Wade et al. also found that GA, PNA, and SCR were significant covariates for fluconazole CL in infants (10).
This study employed scavenged PK sampling, which is a minimal-risk approach that uses leftover blood collected in the course of routine clinical care that would otherwise be discarded (15). The scavenged samples were as informative as the scheduled samples and did not bias parameter estimates. Scavenged PK sampling is particularly effective in populations that are difficult to study, such as infants, and has been successfully used in population PK studies of anti-infection drugs (16, 17). In addition, scavenged sampling is useful for drugs with long half-lives where traditional sampling schemes may not capture the full PK profile.
Effective prophylaxis dosing in adults suggests that fluconazole concentrations of >2,000 ng/ml would be beneficial. Further, fluconazole MICs for Candida species in infants typically range from 250 to 4,000 ng/ml (18 – 20). In this study, 90% of measured fluconazole concentrations were >2,000 ng/ml, and the Monte Carlo simulations predict that trough concentrations would be maintained at >2,000 ng/ml in a high proportion of participants receiving 6 mg/kg twice weekly for the first few weeks of life. Maturation of renal function and other developmental processes would result in lower fluconazole concentrations at later PNA. Another commonly used fluconazole regimen for the prophylaxis of invasive candidiasis is 3 mg/kg given twice weekly (2, 3). Although the lower fluconazole regimen of 3 mg/kg twice weekly may be effective for Candida species with an MIC of ≤2,000 ng/ml, our results suggest that the higher 6 mg/kg twice-weekly regimen would be needed to optimize fluconazole exposure for the first few weeks of life, when the fluconazole MIC range for Candida species is >2,000 ng/ml.
This study had several limitations. Only 2 infants with PK data had invasive candidiasis, which precluded any meaningful exploration regarding fluconazole exposure and outcomes. Additionally, on the basis of typical MICs of Candida species in infants, we chose a pharmacodynamic target of 2,000 ng/ml by which to evaluate fluconazole dosing regimens. Alternative fluconazole dosing strategies may be appropriate in instances where the MIC differs significantly from 2,000 ng/ml. Additionally, MIC-based approaches to guide antifungal drug selection and dosing do not take into consideration the complexity of host-drug-microbe interactions, which may limit the power of correlation with patient outcomes. Finally, since only about 20% of the PK samples were collected following oral administration and since there was limited sampling available during the absorption phase, no BSV was estimated for ka.
In summary, the population PK of fluconazole were successfully characterized in premature infants of <750 g birth weight using sparse sampling that included scavenged samples. Much of the variability in fluconazole CL was explained by SCR and, to a lesser extent, by PMA. SCR was confounded with PNA and GA, and SCR explained most of the BSV in CL, limiting the impact of age on CL. The 6 mg/kg twice-weekly dosage given by either i.v. or oral administration maintained trough fluconazole concentrations of >2,000 ng/ml in the vast majority of infants for the first few weeks of life. While the clinical outcomes of this trial did not show a survival benefit (4), the fluconazole prophylaxis dosage used appears to be appropriate for maintaining fluconazole trough concentrations above 2,000 ng/ml in extremely premature infants.
ACKNOWLEDGMENTS
This work was funded by 5R01HD057956-05, 5R01FD003519-04, the Thrasher Research Fund, and the Best Pharmaceuticals for Children Act under the guidance of the National Institute of Child Health and Human Development via contract HHSN2752010000031 for the Pediatric Trials Network. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1TR001117.
M.L. is supported by the U.S. government for his work in pediatric and neonatal clinical pharmacology (HHSN267200700051C [Principal Investigator: D.K.B.J.]), the NICHD (K23 HD068497), and the National Heart, Lung, and Blood Institute (R34 HL124038). P.B.S. receives salary support for research from the National Institutes of Health (NIH) and the National Center for Advancing Translational Sciences of the NIH (1R21HD080606-01A1 and UL1TR001117), the National Institute of Child Health and Human Development (HHSN275201000003I and 1R01-HD081044-01), and the Food and Drug Administration (1R18-FD005292-01); he also receives research support from Cempra Pharmaceuticals (subaward to HHS0100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). D.K.B.J. receives support from the National Institutes of Health (NIH) (award 2K24HD058735-06, National Center for Advancing Translational Sciences award UL1TR001117, National Institute of Child Health and Human Development contract HHSN275201000003I, and National Institute of Allergy and Infectious Diseases contract HHSN272201500006I); he also receives research support from Cempra Pharmaceuticals (subaward to HHSO100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
All other authors report no disclosures or conflicts of interest.
Fluconazole Prophylaxis Study Team Investigators and Study Coordinators included the following: at the University of Miami Miller School of Medicine, Miami, FL (28 patients enrolled), Shahnaz Duara (Principal Investigator [PI]) and Karina Lifschitz (Site Coordinator [SC]); at UF Health, Jacksonville, FL (25 patients enrolled), Michele Burke (SC) and Renee Prince (SC); at the University of Alabama—Birmingham (25 patients enrolled), Robert L. Schelonka, David A. Randolph (PI), and Claire Roane (SC); at the Duke University Medical Center, Durham, NC (24 patients enrolled), Margarita Bidegain (PI) and Elizabeth Bradsher (SC); at Kings County Hospital, Brooklyn, NY (23 patients enrolled), Sukhvinder Ranu (Coinvestigator) and Subhatra Limbu (SC); at Pennsylvania Hospital, Philadelphia, PA (20 patients enrolled), Toni Mancini (SC); at Wayne State University, Detroit, MI (18 patients enrolled), Seetha Shankaran (PI) and Melanie Lulic (SC); at Memorial Hospital of South Bend, South Bend, IN (16 patients enrolled), Robert D. White (PI) and Mashelle Monhaut (SC); at the University of Florida Health Shands Hospital, Gainesville, FL (16 patients enrolled), Matthew Saxonhouse and David J. Burchfield (PI) and Cindy Miller (SC); athe University of Texas Medical Branch, Galveston, TX (16 patients enrolled), Karen E. Smith and Karen E. Shattuck (PI) and Kristin Pollock (SC); at Columbia University, New York, NY (14 patients enrolled), Natalie Neu (PI), Erin Humel-Amadori (SC), and Glen Bona (SC); at Brookdale University Hospital, Brooklyn, NY (12 patients enrolled), Roger Kim (PI) and Chika Iwuchukwu (SC); at the University of Minnesota Masonic Children's Hospital, Minneapolis, MN (12 patients enrolled), Catherine Bendel (PI), Marla Mills (SC), and Nichole Birge (SC); at the University of Louisville, Louisville, KY (11 patients enrolled), Dan L. Stewart (PI) and Karen Kernen (SC); at the University of California-San Diego Medical Center, San Diego, CA (11 patients enrolled), Neil N. Finer (PI) and Wade Rich (SC); at Wolfson Children's Hospital, Jacksonville, FL (11 patients enrolled), Michele Burke (SC) and Renee Prince (SC); at Children's Hospital of Orange County, Orange, CA (9 patients enrolled), Antonio C. Arrieta (PI), Ofelia Vargas-Shiraishi (SC), Kathy Shea (SC); at Children's Hospital Medical Center of Akron, Akron, OH (8 patients enrolled), Judy Ohlinger (SC); at the University of Tennessee Health Science Center, Memphis, TN (7 patients enrolled), Sheila Dempsey (SC); at the University of Texas Health Science Center at Houston, Houston, TX (7 patients enrolled), Kathleen Kennedy (PI), Georgia McDavid (SC), and Peggy Robichaux (SC); at the University of Texas Southwestern Medical Center, Dallas, TX (7 patients enrolled), Pablo Sanchez (PI), Theresa Barton (PI), Deborah McElroy (SC), and Luz Muniz (SC); at Cook Children's Health Care System, Fort Worth, TX (6 patients enrolled), Barbara Austin (SC) and Sara Scott (SC); at East Carolina University, Greenville, NC (6 patients enrolled), Scott MacGilvray (PI) and Sherry Moseley (SC); at the University of Arkansas for Medical Sciences, Little Rock, AK (5 patients enrolled), Ashley Ross (PI), Michelle Hart (SC), and Howard Lee (SC); at Virtua West Jersey Hospital, Voorhees, NJ (5 patients enrolled), Paresh Pandit (PI) and Christine Catts (SC); at Texas Children's Hospital, Houston, TX (4 patients enrolled), Mohan Pammi (PI), Eric Eichenwald (PI), and Teresa Falk (SC); at Riley Hospital for Children at Indiana University, Indianapolis, IN (4 patients enrolled), Brenda Poindexter (PI) and Leslie Wilson (SC); at SUNY Downstate Medical Center, Brooklyn, NY (4 patients enrolled), Agnes Perenyi (PI), Susan Sullivan (SC), and Sara Higgerson (SC); at the University of Nevada School of Medicine, Reno, NV (3 patients enrolled): Echezona Ezeanolue (PI) and Aaron Hunt (SC); at the Tulane University School of Medicine, New Orleans, LA (2 patients enrolled), Phillip Gordon (PI) and Jane Reynolds (SC); at Wesley Medical Center, Wichita, KS (2 patients enrolled), Barry Bloom (PI) and Paula Delmore (SC); at Arkansas Children's Hospital, Little Rock, AK (1 patient enrolled), Ashley Ross (PI), Michelle Hart (SC), and Howard Lee (SC); and at Duke Clinical Research Institute, Durham, NC, Katherine Berezny (Project Leader), Kristi Prather (Biostatistician), Debbe Blackwell (Clinical Research Associate), Kristy Hwang (Data Manager), Elizabeth Vandyne (Safety Associate), Debbie Hendrick (Clinical Trials Assistant), and Tedryl Gentry-Bumpass (Lead Clinical Research Associate).
Oversight of the work of the study team was provided by the following members of the Best Pharmaceuticals for Children Act—Pediatric Trials Network Administrative Core Committee: Jeffrey Barrett, Children's Hospital of Philadelphia, Philadelphia, PA; Edmund Capparelli, University of California, San Diego, San Diego, CA; Michael Cohen-Wolkowiez, Duke Clinical Research Institute, Durham, NC; Gregory L. Kearns, Children's Mercy Hospital, Kansas City, MO; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Andre Muelenaer, Virginia Tech Carilion School of Medicine, Roanoke, VA; T. Michael O'Shea, Wake Forest Baptist Medical Center, Winston Salem, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; John van den Anker, George Washington University School of Medicine and Health, Washington, DC; and Thomas J. Walsh, Weill Cornell Medical College of Cornell University, New York, NY. Oversight of the work of the study team was provided by the following members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development: David Siegel, Perdita Taylor-Zapata, Anne Zajicek, and Alice Pagan. Oversight of the work of the study team was provided by the following employees of the EMMES Corporation (Data Coordinating Center): Ravinder Anand, Traci Clemons, and Gina Simone.
Funding Statement
This work was funded by 5R01HD057956-05, 5R01FD003519-04, the Thrasher Research Fund, and the Best Pharmaceuticals for Children Act under the guidance of the National Institute of Child Health and Human Development via contract HHSN2752010000031 for the Pediatric Trials Network. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study sponsor was consulted concerning study design, data collection, analysis and interpretation, the writing of the report, and the decision to submit the manuscript for publication.
Footnotes
See Acknowledgments for a list of the members of the Fluconazole Prophylaxis Study Team.
REFERENCES
- 1.Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, Duara S, Poole K, Laptook A, Goldberg R; National Institute of Child Health and Human Development Neonatal Research Network. 2006. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. 2001. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med 345:1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 3.Manzoni P, Stolfi I, Pugni L, Decembrino L, Magnani C, Vetrano G, Tridapalli E, Corona G, Giovannozzi C, Farina D, Arisio R, Merletti F, Maule M, Mosca F, Pedicino R, Stronati M, Mostert M, Gomirato G; Italian Task Force for the Study and Prevention of Neonatal Fungal Infections; Italian Society of Neonatology. 2007. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med 356:2483–2495. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin DK Jr, Hudak ML, Duara S, Randolph DA, Bidegain M, Mundakel GT, Natarajan G, Burchfield DJ, White RD, Shattuck KE, Neu N, Bendel CM, Kim MR, Finer NN, Stewart DL, Arrieta AC, Wade KC, Kaufman DA, Manzoni P, Prather KO, Testoni D, Berezny KY, Smith PB; Fluconazole Prophylaxis Study Team. 2014. Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial. JAMA 311:1742–1749. doi: 10.1001/jama.2014.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphrey MJ, Jevons S, Tarbit MH. 1985. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother 28:648–653. doi: 10.1128/AAC.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koks CH, Crommentuyn KM, Hoetelmans RM, Mathot RA, Beijnen JH. 2001. Can fluconazole concentrations in saliva be used for therapeutic drug monitoring? Ther Drug Monit 23:449–453. doi: 10.1097/00007691-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Vaden SL, Heit MC, Hawkins EC, Manaugh C, Riviere JE. 1997. Fluconazole in cats: pharmacokinetics following intravenous and oral administration and penetration into cerebrospinal fluid, aqueous humour and pulmonary epithelial lining fluid. J Vet Pharmacol Ther 20:181–186. doi: 10.1111/j.1365-2885.1997.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 8.Tett S, Moore S, Ray J. 1995. Pharmacokinetics and bioavailability of fluconazole in two groups of males with human immunodeficiency virus (HIV) infection compared with those in a group of males without HIV infection. Antimicrob Agents Chemother 39:1835–1841. doi: 10.1128/AAC.39.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debruyne D, Ryckelynck JP. 1993. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet 24:10–27. doi: 10.2165/00003088-199324010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Wade KC, Wu D, Kaufman DA, Ward RM, Benjamin DK Jr, Sullivan JE, Ramey N, Jayaraman B, Hoppu K, Adamson PC, Gastonguay MR, Barrett JS; National Institute of Child Health and Development Pediatric Pharmacology Research Unit Network. 2008. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother 52:4043–4049. doi: 10.1128/AAC.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenzl TG, Schefels J, Hornchen H, Skopnik H. 1998. Pharmacokinetics of oral fluconazole in premature infants. Eur J Pediatr 157:661–662. doi: 10.1007/s004310050906. [DOI] [PubMed] [Google Scholar]
- 12.Nahata MC, Tallian KB, Force RW. 1999. Pharmacokinetics of fluconazole in young infants. Eur J Drug Metab Pharmacokinet 24:155–157. doi: 10.1007/BF03190361. [DOI] [PubMed] [Google Scholar]
- 13.Saxén H, Hoppu K, Pohjavuori M. 1993. Pharmacokinetics of fluconazole in very low birth weight infants during the first two weeks of life. Clin Pharmacol Ther 54:269–277. doi: 10.1038/clpt.1993.147. [DOI] [PubMed] [Google Scholar]
- 14.Smith PB, Steinbach WJ, Benjamin DK Jr. 2005. Neonatal candidiasis. Infect Dis Clin North Am 19:603–615. doi: 10.1016/j.idc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Ku LC, Smith PB. 2015. Dosing in neonates: special considerations in physiology and trial design. Pediatr Res 77:2–9. doi: 10.1038/pr.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen-Wolkowiez M, Benjamin DK Jr, Ross A, James LP, Sullivan JE, Walsh MC, Zadell A, Newman N, White NR, Kashuba AD, Ouellet D. 2012. Population pharmacokinetics of piperacillin using scavenged samples from preterm infants. Ther Drug Monit 34:312–319. doi: 10.1097/FTD.0b013e3182587665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen-Wolkowiez M, Ouellet D, Smith PB, James LP, Ross A, Sullivan JE, Walsh MC, Zadell A, Newman N, White NR, Kashuba AD, Benjamin DK Jr. 2012. Population pharmacokinetics of metronidazole evaluated using scavenged samples from preterm infants. Antimicrob Agents Chemother 56:1828–1837. doi: 10.1128/AAC.06071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wade KC, Benjamin DK Jr, Kaufman DA, Ward RM, Smith PB, Jayaraman B, Adamson PC, Gastonguay MR, Barrett JS. 2009. Fluconazole dosing for the prevention or treatment of invasive candidiasis in young infants. Pediatr Infect Dis J 28:717–723. doi: 10.1097/INF.0b013e31819f1f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Grossman LB. 2005. Twice weekly fluconazole prophylaxis for prevention of invasive Candida infection in high-risk infants of <1000 grams birth weight. J Pediatr 147:172–179. doi: 10.1016/j.jpeds.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Kicklighter SD, Springer SC, Cox T, Hulsey TC, Turner RB. 2001. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics 107:293–298. doi: 10.1542/peds.107.2.293. [DOI] [PubMed] [Google Scholar]