Abstract
Despite the increasing incidence of vancomycin-intermediate Staphylococcus aureus (VISA) infections, few studies have examined the impact of delay in receipt of appropriate antimicrobial therapy on outcomes in VISA patients. We examined the effects of timing of appropriate antimicrobial therapy in a cohort of patients with sterile-site methicillin-resistant S. aureus (MRSA) and VISA infections. In this single-center, retrospective cohort study, we identified all patients with MRSA or VISA sterile-site infections from June 2009 to February 2015. Clinical outcomes were compared according to MRSA/VISA classification, demographics, comorbidities, and antimicrobial treatment. Thirty-day all-cause mortality was modeled with Kaplan-Meier curves. Multivariate logistic regression analysis (MVLRA) was used to determine odds ratios for mortality. We identified 354 patients with MRSA (n = 267) or VISA (n = 87) sterile-site infection. Fifty-five patients (15.5%) were nonsurvivors. Factors associated with mortality in MVLRA included pneumonia, unknown source of infection, acute physiology and chronic health evaluation (APACHE) II score, solid-organ malignancy, and admission from skilled care facilities. Time to appropriate antimicrobial therapy was not significantly associated with outcome. Presence of a VISA infection compared to that of a non-VISA S. aureus infection did not result in excess mortality. Linezolid use was a risk for mortality in patients with APACHE II scores of ≥14. Our results suggest that empirical vancomycin use in patients with VISA infections does not result in excess mortality. Future studies should (i) include larger numbers of patients with VISA infections to confirm the findings presented here and (ii) determine the optimal antibiotic therapy for critically ill patients with MRSA and VISA infections.
INTRODUCTION
Broad-spectrum antimicrobial coverage for patients with severe infections followed by de-escalation when culture data are available is now a standard of care (1). Vancomycin has historically been the empirical therapy of choice for coverage of possible methicillin-resistant Staphylococcus aureus (MRSA) infection. The emergence of vancomycin-intermediate S. aureus (VISA) infections (vancomycin MIC of 4 or 8 μg/ml) threatens the efficacy of vancomycin as empirical therapy in the management of critically ill patients (2 – 5). Delays in appropriate antimicrobial treatment of greater than 24 h in patients with MRSA sterile-site infections are known to increase mortality (6). Despite the increasing incidence of VISA infections (7) in a population that still predominantly receives vancomycin as the first-line empirical therapy for MRSA, no studies have specifically examined the impact of delay in receipt of appropriate antimicrobial therapy on outcomes in VISA patients. The primary objective of our study was to determine risk factors for mortality in patients with MRSA (non-VISA) and VISA sterile-site infections, particularly whether time to appropriate antimicrobial therapy was associated with mortality. Our secondary objectives were to (i) determine predictors of VISA infection and (ii) determine the impact of definitive MRSA or methicillin-resistant, vancomycin-intermediate S. aureus (MRVISA) therapy on outcome.
MATERIALS AND METHODS
Study location and patient population.
This study was conducted at Barnes-Jewish Hospital, a 1,250-bed academic medical center located in St. Louis, MO. The study period was June 2009 through February 2015, corresponding to the change of vancomycin MIC by Clinical and Laboratory Standards Institute (CLSI) in 2006 and the subsequent adoption of a screening agar in 2009 for detection of the vancomycin-intermediate phenotype in S. aureus isolates recovered from clinical specimens as previously described (8). Briefly, S. aureus isolates recovered from all blood cultures are inoculated onto brain heart infusion agar with 3 mg/liter vancomycin (BHI-V3) and the vancomycin MIC of any S. aureus isolates that grow on this agar is confirmed (the confirmatory methodology varied during the study period but included Microscan, Etest, and Vitek2) (8). All consecutive hospitalized patients with MRSA or VISA cultures from sterile sites were analyzed for eligibility. This study was approved by the Washington University School of Medicine Human Studies Committee.
Study design and data collection.
Utilizing a retrospective cohort study design, all patients ≥18 years of age with MRSA or VISA cultured from sterile sites were identified. Sterile sites were defined as blood, cerebrospinal fluid (CSF), pleural fluid (not taken from indwelling catheter), ascites fluid (not taken from indwelling catheter), pericardial fluid, bone marrow, and synovial fluid and surgical specimens from lymph nodes, brain, heart, liver, spleen, vitreous, kidney, pancreas, ovary, or vascular tissue. Patients with sterile sites which grew MRSA or VISA as part of a polymicrobial culture were excluded from the study. All vancomycin exposures within our health care system within 6 months of the first positive culture were considered. Receipt of vasopressors within 24 h of positive culture was used to define septic shock. The primary endpoint was 30-day all-cause mortality in the MRSA and VISA groups, with special attention to the timing of appropriate therapy. Secondary endpoints included hospital length of stay (LOS) and intensive care unit length of stay (ICU LOS) postinfection, effect of definitive antibiotic on mortality, predictors of VISA infection, and duration of prior vancomycin exposure. Baseline characteristics, including age, gender, race, place of origin, health care exposure, receipt of vancomycin within 6 months of positive culture, presence of immunosuppression, acute physiology and chronic health evaluation (APACHE) II (9) scores (calculated based on clinical data present during the 24 h after positive blood cultures were drawn), Charlson comorbidity index, and medical comorbidities, were obtained.
Definitions.
Patients were considered to have a VISA infection if S. aureus was isolated in culture and was determined to have a vancomycin MIC of 4 or 8 μg/ml, in accordance with CLSI standards (10).
Time to appropriate therapy was calculated from the time a positive blood culture was drawn to the time that the first appropriate antibiotic was administered. All isolates had to have test results showing that they were susceptible to the antibiotic in order for therapy to be considered appropriate. The definition of appropriate therapy was different for each subcategorization. For MRSA, appropriate therapy was defined as the receipt of linezolid, ceftaroline, daptomycin, telavancin, quinupristin-dalfopristin, or vancomycin. In patients with MRVISA, appropriate therapy was defined as the receipt of any of the antibiotics listed above for treatment of MRSA infection, with the exclusion of vancomycin. For patients with MSVISA, appropriate therapy was defined as the receipt of any of the antibiotics considered appropriate for MRVISA, with the addition of cefazolin, ceftriaxone, oxacillin, nafcillin, and dicloxacillin. For all subcategorizations to include MSVISA and MRVISA, in order for any antibiotic to be considered appropriate therapy, isolates had to test as susceptible to that antibiotic in accordance with CLSI standards (10). Additionally, daptomycin was not considered appropriate for patients with pulmonary infections. Definitive therapy was defined as the appropriate antibiotic therapy (see above) that patients received for the majority (>50%) of their treatment course.
Vancomycin trough levels were not available for all patients. It is institutional policy to administer vancomycin at a dose of 15 mg/kg of body weight, with a dosing interval appropriate for the patient's creatinine clearance. Target trough levels for all patients were 15 to 20 mg/dl. Institutional policy is to dose daptomycin for all S. aureus infections at 6 mg/kg of ideal body weight, 6 mg/kg of adjusted body weight if the BMI of the patient is >120% of the ideal BMI, and 6 mg/kg of total body weight for underweight patients. This dosing of daptomycin also applied to VISA infections.
Patients were identified by the first sterile site from which MRSA or VISA was isolated. Patients could be included in the study more than once only if a S. aureus infection occurred during a different hospitalization more than 30 days after completing a treatment course for a previous S. aureus infection.
We defined the septic shock group as receipt of blood pressure support with any of the following medications within 24 h of the first positive culture: norepinephrine, phenylephrine, epinephrine, dopamine, dobutamine, or vasopressin.
The following organisms were considered contaminants if not isolated in more than one blood culture within 72 h: coagulase-negative staphylococci, Corynebacterium spp., Propionibacterium acnes, or viridans group Streptococcus. Length of hospital stay postinfection was calculated from the time a positive sterile-site culture was drawn. Patients who never required intensive care unit (ICU) admission were considered to have an ICU stay length of 0 days. Health care exposure was defined as chemotherapy within the prior 30 days; residence in a nursing home (NH), skilled-nursing facility (SNF), or other long-term acute-care facility (LTACH); hospitalization in an acute care hospital for two or more days within the prior 90 days; or attendance at a hospital or hemodialysis clinic within the prior 30 days.
Immunosuppression was defined as receipt of any of the following drugs within 30 days: glucocorticoids (>20 mg prednisone equivalents/day for ≥3 months), cyclosporine, tacrolimus, sirolimus, temsirolimus, everolimus, mycophenolate mofetil, methotrexate, azathioprine, 6-mercaptopurine, cyclophosphamide, gold, d-penicillamine, sulfasalazine, antithymocyte globulin, rituximab, ofatumumab, muromonab, alemtuzumab, basiliximab, daclizumab, tocilizumab, belimumab, abatacept, belatacept, natalizumab, efalizumab, fingolimod, infliximab, adalimumab, certolizumab pegol, etanercept, golimumab, eculizumab, leflunomide, anakinra, adalimumab, auranofin, and any intravenous (i.v.) chemotherapeutic agents used in the treatment of malignancy.
Thirty-day mortality was assessed using the informatics database of BJC HealthCare, a large integrated health care system of both inpatient and outpatient care. Barnes-Jewish Hospital serves as the main teaching institution for BJC HealthCare. The system includes a total of 13 hospitals in a compact geographic region surrounding and including St. Louis, MO. Persons treated within this health care system are, in the majority of cases, readmitted to one of the system's participating hospitals or evaluated in a BJC HealthCare outpatient practice. If a patient who receives health care in the system presents to a nonsystem hospital, he/she is often transferred back into the integrated system because of issues of insurance coverage. Death certificate records and autopsy reports are included in the informatics database. All data were derived from the informatics database provided by the Center for Clinical Excellence, BJC HealthCare.
Statistical analysis.
Thirty-day all-cause mortality was modeled with Kaplan-Meier curves. Univariate analysis was performed by chi-square or Fisher's exact test where appropriate for categorical values. Student's t test or the Mann-Whitney U test was performed where appropriate for continuous variables. Continuous variables are reported as means with standard deviations. Categorical data are expressed as frequencies. A P value of <0.05 was considered significant. Factors associated with VISA infection in univariate analysis (P < 0.20) were entered into a multivariate logistic regression analysis (MVLRA) to determine odds ratios (ORs) for VISA infection. Factors associated with 30-day all-cause mortality in univariate analysis (P < 0.20) were subjected to non-stepwise MVLRA to determine odds ratios for death. All variables entered into the model were assessed for colinearity, and interaction terms were tested. Goodness of fit was assessed via the Hosmer-Lemeshow c-statistic method. All analyses were performed using SPSS v22. A 2-tailed P value of <0.05 was considered significant in all statistical tests.
We also planned to perform two sensitivity analyses of the cohort: one analysis limited to patients with APACHE II scores of ≥14 and one which excluded patients with infections due to central venous catheters.
RESULTS
Study cohort.
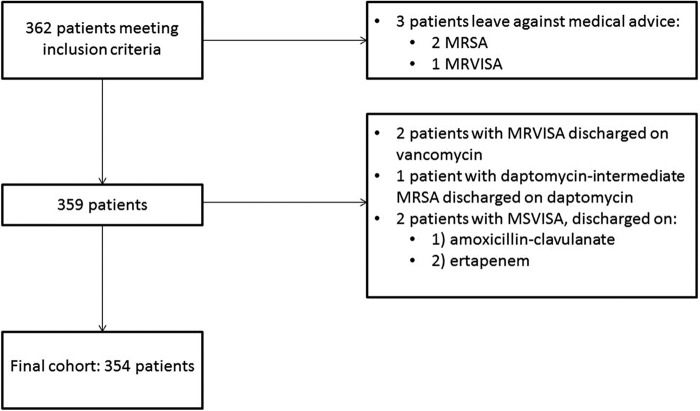
Following initial data collection, 362 patients met the inclusion criteria; among those patients, 8 were ultimately excluded (Fig. 1). Two patients on inappropriate antibiotics were discharged but were still included, as one patient was put on appropriate therapy as an outpatient and the other patient was readmitted for appropriate therapy. Two patients, both with MSVISA infections, died before receiving appropriate antibiotics; for these patients, the time to appropriate therapy was calculated as the time to death. Our final cohort consisted of 354 patients, 342 (96.6%) of whom had ≥1 positive blood culture(s). Six patients had negative blood cultures, and six patients had no blood cultures performed.
FIG 1.
Study cohort exclusion flowchart. MRSA, methicillin-resistant Staphylococcus aureus; MRVISA, methicillin-resistant vancomycin-intermediate Staphylococcus aureus; MSVISA, methicillin-susceptible vancomycin-intermediate Staphylococcus aureus; VISA, vancomycin-intermediate Staphylococcus aureus.
MRSA versus VISA.
A total of 267 MRSA (non-VISA) and a total of 87 VISA (68% MRSA, 32% MSSA) sterile-site infections met the inclusion criteria. Baseline characteristics of the patients are listed in Table 1. In univariate analysis, solid-organ malignancy, cirrhosis, APACHE II, origin NH/SNF/LTACH, hemodialysis (HD) or arteriovenous (AV) graft, pneumonia, and discitis/osteomyelitis data had P values of <0.20 in comparisons of MRSA patients to VISA patients. Time to appropriate antibiotics was significantly longer in the VISA group in univariate analysis (Table 1). Among patients who received any vancomycin, there was no significant difference between MRSA (n = 229) and VISA (n = 68) patients in the starting dose (Table 1). Surprisingly, there was no significant difference between the MRSA and VISA groups in total duration of antecedent vancomycin exposure within 6 months (Table 1). The percentage of MRSA patients who had had any vancomycin exposure within the prior 6 months was 35.2 (n = 94), and the percentage of VISA patients who had had any vancomycin exposure within the prior 6 months was 35.6 (n = 31). Analyzed at 1 day, 7 days, 2 weeks, 4 weeks, and 6 weeks of prior vancomycin exposure, there was still no significant difference between the MRSA and VISA groups in duration of exposure in analyses performed at the level of the whole cohort, nor when analyzed only amongst those that had any prior vancomycin exposure. The majority of MRSA and VISA patients (64.9% [n = 61] and 74.2% [n = 23], respectively) had less than 7 days of prior vancomycin exposure. Under half, 33.0% (n = 31) for MRSA and 48.4% (n = 15) for VISA, had less than 72 h of vancomycin exposure. In multivariate analysis, no factors were significantly predictive of VISA infection.
TABLE 1.
Comparison of patient characteristics according to S. aureus resistance pattern and survival statusa
| Characteristic | Value(s) |
|||||
|---|---|---|---|---|---|---|
| MRSA (n = 267) | VISA (n = 87) | P value | Survivors (30 days) (n = 299) | Nonsurvivors (30 days) (n = 55) | P value | |
| Age (yr) | 56.8 ± 16.7 | 55.3 ± 16.3 | 0.479 | 55.3 ± 16.2 | 62.4 ± 17.4 | 0.003 |
| Male sex | 59.9 (160) | 64.4 (56) | 0.461 | 61.9 (185) | 56.4 (31) | 0.441 |
| White ethnicity | 63.3 (169) | 60.9 (53) | 0.691 | 61.2 (183) | 70.9 (39) | 0.171 |
| Use of vasopressors | 31.5 (84) | 28.7 (25) | 0.633 | 26.1 (78) | 56.4 (31) | <0.001 |
| Bone marrow transplant | 3.7 (10) | 2.3 (2) | 0.738 | 3.0 (9) | 5.5 (3) | 0.409 |
| Solid-organ transplant | 4.9 (13) | 1.1 (1) | 0.202 | 3.7 (11) | 5.5 (3) | 0.464 |
| Cardiovascular disease | 34.8 (93) | 31.0 (27) | 0.516 | 31.1 (93) | 49.1 (27) | 0.010 |
| Congestive heart failure | 31.8 (85) | 27.6 (24) | 0.456 | 29.1 (87) | 40.0 (22) | 0.107 |
| Chronic respiratory failure | 24.3 (65) | 27.6 (24) | 0.545 | 25.1 (75) | 25.5 (14) | 0.954 |
| Diabetes mellitus, type 2 | 36.7 (98) | 33.3 (29) | 0.569 | 36.1 (108) | 34.5 (19) | 0.823 |
| CKD/RRT | 34.5 (92) | 36.8 (32) | 0.693 | 34.4 (103) | 38.2 (21) | 0.594 |
| Solid-organ malignancy | 13.1 (35) | 19.5 (17) | 0.141 | 12.4 (37) | 27.3 (15) | 0.004 |
| Leukemia | 8.2 (22) | 8.0 (7) | 0.954 | 8.4 (25) | 7.3 (4) | 1 |
| Lymphoma | 2.2 (6) | 2.3 (2) | 0.978 | 1.7 (5) | 5.5 (3) | 0.112 |
| Cirrhosis | 2.6 (7) | 6.9 (6) | 0.066 | 2.7 (8) | 9.1 (5) | 0.036 |
| Health care exposure | 83.9 (224) | 83.9 (73) | 0.998 | 82.3 (246) | 92.7 (51) | 0.070 |
| Time to appropriate antibiotics (days) | 0.23 [0.0, 0.70] | 2.55 [0.65, 3.77] | <0.001 | 0.40 [0.0, 1.34] | 0.40 [0.7, 1.04] | 0.918 |
| Immunosuppression | 18.7 (50) | 19.5 (17) | 0.866 | 19.1 (57) | 18.2 (10) | 0.878 |
| Charlson comorbidity score | 3.7 ± 3.0 | 3.5 ± 2.9 | 0.493 | 3.4 ± 2.8 | 5.0 ± 3.5 | <0.001 |
| APACHE II score | 14.1 ± 5.6 | 13.1 ± 4.8 | 0.172 | 13.1 ± 5.1 | 17.8 ± 5.8 | <0.001 |
| Patient origin, % (n) | ||||||
| NH, SNF, or LTACH | 13.5 (36) | 5.7 (5) | 0.054 | 10.0 (30) | 20.0 (11) | 0.034 |
| Community | 44.9 (120) | 40.2 (35) | 0.441 | 46.5 (139) | 29.1 (16) | 0.017 |
| OSH | 24.3 (65) | 31.0 (27) | 0.216 | 26.4 (79) | 23.6 (13) | 0.665 |
| In hospital | 16.1 (43) | 20.7 (18) | 0.325 | 15.7 (47) | 25.5 (14) | 0.079 |
| Infection source, % (n) | ||||||
| Endocarditis | 11.6 (31) | 11.5 (10) | 0.977 | 11.7 (35) | 10.9 (6) | 0.865 |
| Hemodialysis graft or AV fistula | 2.6 (7) | 5.7 (5) | 0.177 | 3.7 (11) | 1.8 (1) | 0.700 |
| Central venous catheter | 18.7 (50) | 19.5 (17) | 0.866 | 20.4 (61) | 10.9 (6) | 0.098 |
| Unknown | 27.3 (73) | 33.3 (29) | 0.284 | 25.1 (75) | 49.1 (27) | <0.001 |
| Pneumonia/empyema | 7.9 (21) | 2.3 (2) | 0.080 | 4.3 (13) | 18.2 (10) | <0.001 |
| Discitis/osteomyelitis | 14.2 (38) | 6.9 (6) | 0.072 | 13.7 (41) | 5.5 (3) | 0.117 |
| Septic arthritis | 3.7 (10) | 4.6 (4) | 0.753 | 4.7 (14) | 0 | 0.139 |
| Skin and soft tissue infection | 5.6 (15) | 5.7 (5) | 1 | 4.7 (14) | 0 | 0.139 |
| Otherb | Not significant | Not significant | ||||
| Initial vancomycin dose (μg)c | 1,143 ± 331 | 1,198 ± 316 | 0.222 | 1,163 ± 331 | 1,117 ± 316 | 0.380 |
| Total prior vancomycin exposure, days | 0 [0.0, 2.79] | 0 [0.0, 1.85] | 0.612 | 0 [0.0, 1.96] | 0 [0.0, 2.97] | 0.123 |
| LOS (days) | 12.0 [7, 13] | 10.0 [7, 21] | 0.598 | 11 [7, 23] | 12 [6, 19] | 0.200 |
| ICU LOS (h) | 0 [0.0, 146.3] | 0 [0.0, 149.7] | 0.487 | 0 [0.0, 116.65] | 97.6 [32.7, 288.5] | <0.001 |
| 30-day all-cause mortality, % | 16.9 | 11.5 | 0.231 | |||
| MRSA | 74.2 (222) | 81.8 (45) | 0.231 | |||
| MRVISA | 17.7 (53) | 10.9 (6) | 0.213 | |||
| MSVISA | 8.0 (24) | 7.3 (4) | 1 | |||
| Any VISA | 25.8 (77) | 18.2 (10) | 0.231 | |||
| Vancomycin treated | 41.8 (125) | 43.6 (24) | 0.801 | |||
| Linezolid treated | 10.0 (30) | 23.6 (13) | 0.005 | |||
Values are expressed as means ± standard deviations, percentages (numbers of patients), or medians [interquartile ranges]. For the MRSA and VISA groups, the origins of 3 and 2 patients, respectively, could not be definitively determined based on chart review. The origins of 4 survivors and 1 nonsurvivors could not be determined. Abbreviations: CKD, chronic kidney disease; RRT, renal replacement therapy; APACHE II, acute physiology and chronic health evaluation II; NH, nursing home; SNF, skilled-nursing facility; LTACH, long-term acute-care hospital; OSH, outside hospital; LVAD, left ventricular assist device; AICD, automatic implantable cardioverter defibrillators; PPM, permanent pacemaker; LOS, length of stay; ICU LOS, intensive care unit length of stay; MRSA, methicillin-resistant Staphylococcus aureus; MRVISA, methicillin-resistant vancomycin-intermediate Staphylococcus aureus; MSVISA, methicillin-susceptible vancomycin-intermediate Staphylococcus aureus; VISA, vancomycin-intermediate Staphylococcus aureus.
Data include the following types of infections: surgical site, LVAD, prosthetic joint, traumatic wound, thrombophlebitis, urine, AICD/PPM, endovascular graft. None of these categorizations were significantly different between groups. Each of these infection types had ≤8 infections in any column (MRSA, VISA, survivors, or nonsurvivors).
A total of 229 MRSA patients, 68 VISA patients, 250 survivors, and 47 nonsurvivors received vancomycin.
Survivors versus nonsurvivors.
At 30 days, there were 299 survivors and 55 nonsurvivors. Data corresponding to increased age, presence of shock, cardiovascular disease, solid-organ malignancy, APACHE II, Charlson comorbidity index, origin from a NH, SNF, or LTACH, origin from the community, unknown source of infection, pneumonia as infection source, and treatment with linezolid were significantly different between survivors and nonsurvivors in univariate analysis (Table 1). Time to appropriate antibiotic therapy had no effect on mortality, as assessed in MVLRA. There was no significant difference between survivors and nonsurvivors in time to appropriate therapy analyzed at the level of the whole cohort or by MRSA/VISA classification (Fig. 2). Among patients who received any vancomycin, there was no significant difference in the starting dose between survivors (n = 250) and nonsurvivors (n = 47) patients (Table 1). In multivariate analysis, patients who died were more likely to have had higher APACHE II scores, pneumonia or an unknown infection source, or a solid-organ malignancy or to have been admitted from a NH, SNF, or LTACH (Table 2). An attempt was made to incorporate the presence of VISA into the MVLRA data for mortality, but this did not significantly affect the model's performance.
FIG 2.
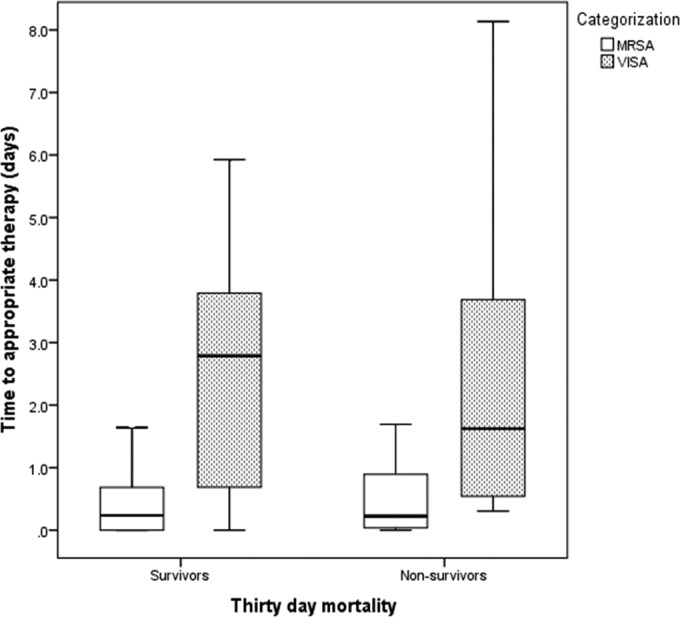
Time to appropriate therapy in survivors and nonsurvivors, classified by MRSA/VISA categorization. MRVISA, methicillin-resistant vancomycin-intermediate Staphylococcus aureus; VISA, vancomycin-intermediate Staphylococcus aureus.
TABLE 2.
Factors associated with 30-day all-cause mortality in multivariate logistic regression analysisa
| Factor | Odds ratio (95% confidence interval) |
|---|---|
| Pneumonia as infectious source | 5.08 (1.73–14.87) |
| Unknown source of infection | 5.03 (2.32–10.92) |
| APACHE II (1-point increments) | 1.18 (1.08–1.28) |
| Solid-organ malignancy | 3.16 (1.29–7.72) |
| Origin: SNF, LTACH, or NH | 3.11 (1.13–8.51) |
R-squared value, 0.364. Hosmer-Lemeshow c statistic, 0.958. APACHE II, acute physiology and chronic health evaluation II; NH, nursing home; SNF, skilled-nursing facility; LTACH, long-term acute-care hospital.
On Kaplan-Meier analysis, there was no difference in 30-day all-cause mortality between patients with MRSA (non-VISA) and those with VISA (Fig. 3). Data corresponding to the definitive therapy for MRVISA patients are shown in Table 3. All VISA isolates had a vancomycin MIC of 4 μg/ml. Table 4 shows the results of our sensitivity analyses in patients with an APACHE II score of ≥14 and in all patients with non-central venous catheter infections.
FIG 3.
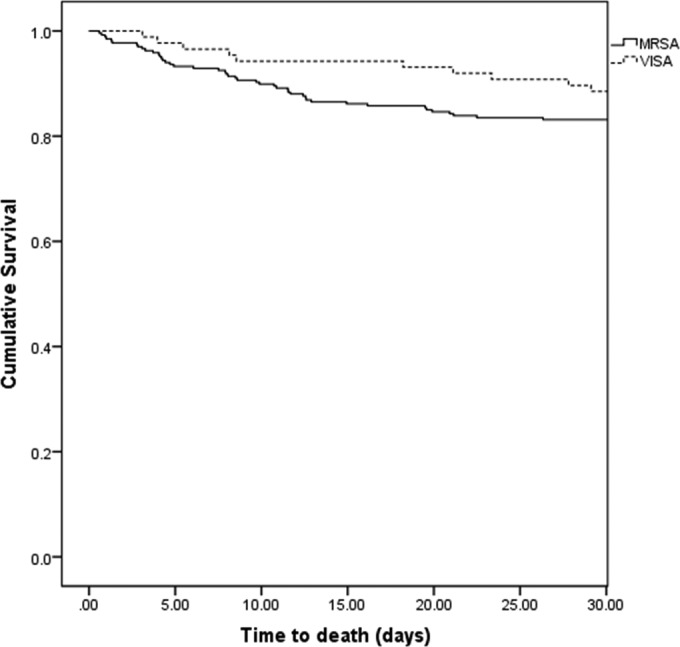
Kaplan-Meier curve comparing mortality rates of MRSA and VISA patients. There was no statistically significant difference in mortality (P = 0.231). MRVISA, methicillin-resistant vancomycin-intermediate Staphylococcus aureus; VISA, vancomycin-intermediate Staphylococcus aureus.
TABLE 3.
Definitive therapy
| Patient category (no. of patients) and antibiotic | No. (%) of patients |
|
|---|---|---|
| Total | 30-day mortality | |
| MRVISA (59)a | ||
| Daptomycin | 33.9 (20) | 15 (3) |
| Ceftaroline | 28.8 (17) | 5.9 (1) |
| Linezolid | 20.3 (12) | 8.3 (1) |
| Telavancin | 11.9 (7) | 14.3 (1) |
| Doxycycline | 1.7 (1) | 0 |
| Quinupristin-dalfopristin | 1.7 (1) | 0 |
| Trimethoprim-sulfamethoxazole | 1.7 (1) | 0 |
| MSVISA (28) | ||
| Ceftriaxone | 35.7 (10) | 0 |
| Cefazolin | 25.0 (7) | 0 |
| Oxacillin | 14.3 (4) | 25.0 (1) |
| Linezolid | 7.1 (2) | 50.0 (1) |
| Vancomycin | 7.1 (2) | 100 (2) |
| Ceftaroline | 3.6 (1) | 0 |
| Daptomycin | 3.6 (1) | 0 |
| Trimethoprim-sulfamethoxazole | 3.6 (1) | 0 |
| MRSA (267) | ||
| Vancomycin | 55.1 (147) | 15.0 (22) |
| Ceftaroline | 19.9 (53) | 15.1 (8) |
| Daptomycin | 12.4 (33) | 9.1 (3) |
| Linezolid | 10.9 (29) | 37.9 (11) |
| Doxycycline | 1.1 (3) | 33.3 (1) |
| Trimethoprim-sulfamethoxazole | 0.4 (1) | 0 |
| Telavancin | 0.4 (1) | 0 |
MRVISA, methicillin-resistant vancomycin-intermediate Staphylococcus aureus.
TABLE 4.
Factors associated with 30-day all-cause mortality in multivariate logistic regression analysis, subdivided by sensitivity analysis category
| Sensitivity analysis category and factor | Odds ratio (95% confidence interval) |
|---|---|
| Patients with APACHE II ≥ 14a | |
| Pneumonia as infectious source | 4.23 (1.26–14.25) |
| Linezolid as definitive therapy | 3.26 (1.07–9.9) |
| Exclusion of patients with central venous catheter infectionb | |
| Pneumonia as infectious source | 4.86 (1.35–17.42) |
| Unknown source of infection | 3.77 (1.41–10.07) |
| APACHE II (1-point increments) | 1.18 (1.07–1.30) |
APACHE II, acute physiology and chronic health evaluation II. R-squared value, 0.237. Hosmer-Lemeshow c statistic, 0.380.
R-squared value, 0.410. Hosmer-Lemeshow c statistic, 0.770.
DISCUSSION
The mortality rate in our cohort (15.5%) was similar to that seen in prior studies of S. aureus infections of sterile body sites. We found that the time to appropriate antibiotic therapy was not a predictor of 30-day all-cause mortality. This finding held true for analysis of that parameter as a dichotomous variable at cutoffs of 1, 6, 12, 24, and 48 h (data not shown). Time to appropriate therapy was almost 2 days longer in patients with VISA infections, without an increased risk of mortality. To our knowledge, no studies have been performed that specifically examined time to appropriate therapy as a risk factor for mortality in patients with VISA. Even among studies that have compared outcomes of infections by MRSA with reduced vancomycin susceptibility, cutoffs for reduced vancomycin MICs have been variable. Some studies in patients with MRSA infection have found an increased risk of mortality when appropriate therapy was delayed for more than 2 days, though with disparate conclusions regarding the role of vancomycin MIC in outcomes (11, 12).
One possible explanation for the lack of increased mortality in the VISA group in our cohort despite a 48-h delay in appropriate antibiotic therapy relative to the MRSA group is that vancomycin resistance comes with a fitness cost, as has been suggested previously (13, 14). Another possible explanation is that we are witnessing a “90–60 rule” (15) effect in patients with VISA who are treated with vancomycin; vancomycin usage may not cure the infection all the time but does at least decrease the risk of mortality while the patient is awaiting appropriate definitive therapy. Further studies need to be conducted to assess this possibility. If true, it would mean that vancomycin can remain an empirical first-line therapy for MRSA without risking adverse outcomes. It would also reduce the need to empirically prescribe nonvancomycin MRSA therapy in patients with a history of VISA infection when they are readmitted with concern for infection. It should be noted that the risk of readmission with VISA after an initial VISA infection is not known. Of the 87 VISA patients in our cohort, 41 (47%) were readmitted within 120 days of discharge from the date of VISA admission; only 3, all of whom had VISA infections, had a recurrent S. aureus infection.
The impact on mortality of an infection by a VISA or S. aureus strain with a phenotype of heterogeneous resistance to vancomycin (hVISA) is unclear, as there is significant heterogeneity of results and study designs in the literature. Evidence can be found to suggest that VISA infection results in reduced, similar, or increased mortality relative to MRSA or MSSA infection (2, 16 – 19). Even several recent meta-analyses came to differing conclusions; three studies associated elevated vancomycin MICs with an increased risk of mortality, though with different MIC cutoffs in each study (20 – 22), and a fourth meta-analysis found no evidence of increased mortality risk based on vancomycin MIC (23).
Pneumonia, unknown infection source, APACHE II score, origin from a NH, SNF, or LTACH, and solid-organ malignancy have all been shown previously to be risk factors for mortality in patients with S. aureus. It is not surprising that these factors, which are likely all surrogates for severity of illness, were associated with increased risk of mortality.
Few studies have specifically examined exposure rates and differences in duration of prior vancomycin administration in VISA and MRSA patients, though what data are available suggest that about 25% of patients with MRSA infections have had prior vancomycin exposure (2, 4, 5, 16, 24), a rate about 10% lower than that determined for our cohort (35.3%). The higher prevalence of antecedent vancomycin exposure in our cohort may be a reflection of variations in local antibiograms or better availability of data on prior vancomycin exposure. Studies that have attempted to address the role of prior vancomycin use in reduced vancomycin susceptibility have come to mixed conclusions (25, 26). Interestingly, hVISA may occur in populations never before exposed to vancomycin or as a result of exposure to beta-lactam antibiotics (27, 28). Larger studies are required to better assess the role that vancomycin plays in selecting for a VISA or hVISA phenotype.
MRSA prediction scores have been previously validated in patients with pneumonia (29). It was our goal to identify risk factors for VISA infection in order to help guide empirical therapy in these patients. Unfortunately, we were unable to find any factors independently associated with VISA infection.
Interestingly, definitive therapy with linezolid was associated with an increased OR for mortality in patients with an APACHE II score of ≥14. Of the 13 patients who died with linezolid therapy, 10 had an unknown source of infection, 1 had endocarditis, 1 had discitis/osteomyelitis, and 1 had pneumonia as the primary source of infection. None of the patients had catheter-related bloodstream infection. All 13 patients were bacteremic, 11 with MRSA, 1 with MRVISA, and 1 with MSVISA. None of the patients treated with linezolid as the definitive therapy had linezolid-resistant S. aureus isolates. Concerns about increases in mortality with use of linezolid have been raised before, though in the context of empirical usage in patients with Gram-negative infections (30, 31). The FDA issued a warning about linezolid in 2007 on the basis of the aforementioned data (32). In contrast, prior studies have found a trend not reaching statistical significance toward reduced mortality in patients treated with linezolid (33). Linezolid as a salvage therapy for patients with MRSA infections failing vancomycin therapy has shown success rates superior to and mortality lower than those seen with vancomycin combination therapy, albeit in a cohort of only 35 patients (34). Other studies looking at linezolid as a salvage therapy have shown trends toward improved mortality that were not statistically significant (35). Clinical response was shown to be superior for linezolid compared to vancomycin for nosocomial MRSA pneumonia, though there was no difference in mortality (36). Our results suggest that future studies looking at linezolid use in a large cohort of patients with high APACHE II scores are needed to better understand the efficacy of linezolid in critically ill patients.
One strength of our study is that the presence of VISA was determined prospectively, which is important as frozen isolates have been shown to have falsely low vancomycin MICs (37). In addition to having a high incidence of VISA infections (4% to 6% of all S. aureus isolates at our institution), we screen for VISA using a method that is likely more sensitive and specific than previously utilized methods (8, 38). Many previous studies have determined vancomycin MICs retrospectively from stored samples using VISA detection methods that are less sensitive and less specific than our own, which could affect conclusions about the relationship between vancomycin MIC and mortality.
The limitations of our study include its retrospective nature, in which unmeasured confounders could have biased the outcome measures. This was a single-center study, and results may not be generalizable to other centers. Due to the limitations of retrospective chart review, the ability to attain source control could not be assessed, a data point that could have influenced outcomes. However, it is standard practice at our institution to remove central venous catheters if they are felt to be an infectious nidus. Another limitation is the method of determining 30-day mortality. It is possible that some patients died outside the BJC HealthCare network and that we were unable to capture their mortality status. However, it is unlikely that this would have influenced our results given the almost identical mean times to appropriate therapy for survivors and nonsurvivors. We were also limited by a lack of antimicrobial MIC data for MRSA isolates to determine if the gradation of vancomycin MICs influenced mortality at a cutoff lower than 4 μg/ml. Confirmatory testing for vancomycin MIC changed over the course of the study, which was another potential limitation, as different methods are known to produce disparate MIC results. Vancomycin trough levels were not available for all patients, which was another limitation of the study, as differences in target trough attainment could influence outcomes.
In conclusion, VISA infection was not a predictor of mortality, and neither was time to appropriate antibiotic therapy in the VISA group. If this finding is reproduced in larger studies, important implications would include the conclusions that (i) vancomycin can remain an empirical first-line therapy for MRSA without risking adverse outcomes and (ii) empirical prescription of nonvancomycin MRSA therapy in patients with a history of VISA infection when they are readmitted with concern for infection would be unnecessary, thus preserving nonvancomycin MRSA agents for use only when necessary. Further studies are required to better understand the relationship between linezolid usage and outcome in patients with severe S. aureus infections. More data are needed to determine risk factors for VISA infection in order to minimize their occurrence, though VISA infections do not appear to be occurring with a risk of increased mortality at this time. The influence of a VISA phenotype on the microbiome and in the environment is unexplored and could have greater implications than we recognize at present. As the prevalence of antibiotic resistance increases, surveillance of drug-resistant pathogens will become increasingly important and will likely facilitate the collection of more robust data to understand the complex interaction between the host, the pathogen, and the antibiotics we use.
ACKNOWLEDGMENTS
The work was performed at Barnes-Jewish Hospital, St. Louis, MO.
J.P.B. has no conflicts of interest to report. C.-A.D.B. has no conflicts of interest to report. D.K.W. has no conflicts of interest to report. The effort of M.H.K. was supported by the Barnes-Jewish Hospital Foundation.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 3.Kullar R, Davis SL, Levine DP, Rybak MJ. 2011. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis 52:975–981. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 4.Khatib R, Jose J, Musta A, Sharma M, Fakih MG, Johnson LB, Riederer K, Shemes S. 2011. Relevance of vancomycin-intermediate susceptibility and heteroresistance in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 66:1594–1599. doi: 10.1093/jac/dkr169. [DOI] [PubMed] [Google Scholar]
- 5.Casapao AM, Leonard SN, Davis SL, Lodise TP, Patel N, Goff DA, Laplante KL, Potoski BA, Rybak MJ. 24 June 2013. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) bloodstream infection. Antimicrob Agents Chemother doi: 10.1128/aac.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schramm GE, Johnson JA, Doherty JA, Micek ST, Kollef MH. 2006. Methicillin-resistant Staphylococcus aureus sterile-site infection: the importance of appropriate initial antimicrobial treatment. Crit Care Med 34:2069–2074. doi: 10.1097/01.CCM.0000227655.41566.3E. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Sun X, Chang W, Dai Y, Ma X. 2015. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS One 10:e0136082. doi: 10.1371/journal.pone.0136082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnham CA, Weber CJ, Dunne WM Jr. 2010. Novel screening agar for detection of vancomycin-nonsusceptible Staphylococcus aureus. J Clin Microbiol 48:949–951. doi: 10.1128/JCM.02295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 10.CLSI. 2015. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement, vol 25 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Lee HY, Chen CL, Liu SY, Yan YS, Chang CJ, Chiu CH. 2015. Impact of molecular epidemiology and reduced susceptibility to glycopeptides and daptomycin on outcomes of patients with methicillin-resistant Staphylococcus aureus bacteremia. PLoS One 10:e0136171. doi: 10.1371/journal.pone.0136171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasch O, Camoez M, Dominguez MA, Padilla B, Pintado V, Almirante B, Molina J, Lopez-Medrano F, Ruiz E, Martinez JA, Bereciartua E, Rodriguez-Lopez F, Fernandez-Mazarrasa C, Goenaga MA, Benito N, Rodriguez-Bano J, Espejo E, Pujol M. 2013. Predictive factors for mortality in patients with methicillin-resistant Staphylococcus aureus bloodstream infection: impact on outcome of host, microorganism and therapy. Clin Microbiol Infect 19:1049–1057. doi: 10.1111/1469-0691.12108. [DOI] [PubMed] [Google Scholar]
- 13.Noto MJ, Fox PM, Archer GL. 2008. Spontaneous deletion of the methicillin resistance determinant, mecA, partially compensates for the fitness cost associated with high-level vancomycin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 52:1221–1229. doi: 10.1128/AAC.01164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foucault ML, Courvalin P, Grillot-Courvalin C. 2009. Fitness cost of VanA-type vancomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 53:2354–2359. doi: 10.1128/AAC.01702-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rex JH, Pfaller MA. 2002. Has antifungal susceptibility testing come of age? Clin Infect Dis 35:982–989. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 16.van Hal SJ, Jones M, Gosbell IB, Paterson DL. 2011. Vancomycin heteroresistance is associated with reduced mortality in ST239 methicillin-resistant Staphylococcus aureus blood stream infections. PLoS One 6:e21217. doi: 10.1371/journal.pone.0021217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SY, Oh IH, Lee HJ, Ihm CG, Son JS, Lee MS, Kim MN. 2013. Impact of reduced vancomycin MIC on clinical outcomes of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 57:5536–5542. doi: 10.1128/AAC.01137-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han JH, Mascitti KB, Edelstein PH, Bilker WB, Lautenbach E. 2012. Effect of reduced vancomycin susceptibility on clinical and economic outcomes in Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 56:5164–5170. doi: 10.1128/AAC.00757-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Cortés LE, Velasco C, Retamar P, del Toro MD, Gálvez-Acebal J, de Cueto M, García-Luque I, Caballero FJ, Pascual A, Rodríguez-Baño J. 2015. Is reduced vancomycin susceptibility a factor associated with poor prognosis in MSSA bacteraemia? J Antimicrob Chemother 70:2652–2660. doi: 10.1093/jac/dkv133. [DOI] [PubMed] [Google Scholar]
- 20.van Hal SJ, Lodise TP, Paterson DL. 2012. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 54:755–771. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 21.Mavros MN, Tansarli GS, Vardakas KZ, Rafailidis PI, Karageorgopoulos DE, Falagas ME. 2012. Impact of vancomycin minimum inhibitory concentration on clinical outcomes of patients with vancomycin-susceptible Staphylococcus aureus infections: a meta-analysis and meta-regression. Int J Antimicrob Agents 40:496–509. doi: 10.1016/j.ijantimicag.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Jacob JT, DiazGranados CA. 2013. High vancomycin minimum inhibitory concentration and clinical outcomes in adults with methicillin-resistant Staphylococcus aureus infections: a meta-analysis. Int J Infect Dis 17:e93–e100. doi: 10.1016/j.ijid.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalil AC, Van Schooneveld TC, Fey PD, Rupp ME. 2014. Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. JAMA 312:1552–1564. doi: 10.1001/jama.2014.6364. [DOI] [PubMed] [Google Scholar]
- 24.Moise PA, Smyth DS, El-Fawal N, Robinson DA, Holden PN, Forrest A, Sakoulas G. 2008. Microbiological effects of prior vancomycin use in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 61:85–90. [DOI] [PubMed] [Google Scholar]
- 25.Mascitti KB, Edelstein PH, Fishman NO, Morales KH, Baltus AJ, Lautenbach E. 2012. Prior vancomycin use is a risk factor for reduced vancomycin susceptibility in methicillin-susceptible but not methicillin-resistant Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 33:160–166. doi: 10.1086/663708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong RK, Low J, Koh TH, Kurup A. 2009. Clinical features and treatment outcomes of vancomycin-intermediate Staphylococcus aureus (VISA) and heteroresistant vancomycin-intermediate Staphylococcus aureus (hVISA) in a tertiary care institution in Singapore. Eur J Clin Microbiol Infect Dis 28:983–987. doi: 10.1007/s10096-009-0741-5. [DOI] [PubMed] [Google Scholar]
- 27.Yamakawa J, Aminaka M, Okuzumi K, Kobayashi H, Katayama Y, Kondo S, Nakamura A, Oguri T, Hori S, Cui L, Ito T, Jin J, Kurosawa H, Kaneko K, Hiramatsu K. 2012. Heterogeneously vancomycin-intermediate Staphylococcus aureus (hVISA) emerged before the clinical introduction of vancomycin in Japan: a retrospective study. J Infect Chemother 18:406–409. doi: 10.1007/s10156-011-0330-2. [DOI] [PubMed] [Google Scholar]
- 28.Katayama Y, Murakami-Kuroda H, Cui L, Hiramatsu K. 2009. Selection of heterogeneous vancomycin-intermediate Staphylococcus aureus by imipenem. Antimicrob Agents Chemother 53:3190–3196. doi: 10.1128/AAC.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shorr AF, Myers DE, Huang DB, Nathanson BH, Emons MF, Kollef MH. 2013. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect Dis 13:268. doi: 10.1186/1471-2334-13-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcox MH, Tack KJ, Bouza E, Herr DL, Ruf BR, Ijzerman MM, Croos-Dabrera RV, Kunkel MJ, Knirsch C. 2009. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 48:203–212. doi: 10.1086/595686. [DOI] [PubMed] [Google Scholar]
- 31.Lustberg ME, Schlesinger LS, Mangino JE. 2009. Concerns about “Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study”. Clin Infect Dis 49:313, author reply 314–315. doi: 10.1086/600056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FDA. 14 August 2013. Information for healthcare professionals: linezolid (marketed as Zyvox). Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm085249.htm Accessed 6 December 12/6/2015. [Google Scholar]
- 33.Chong YP, Park KH, Kim ES, Kim MN, Kim SH, Lee SO, Choi SH, Jeong JY, Woo JH, Kim YS. 2015. Clinical and microbiologic analysis of the risk factors for mortality in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 59:3541–3547. doi: 10.1128/AAC.04765-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang HC, Kim SH, Kim KH, Kim CJ, Lee S, Song KH, Jeon JH, Park WB, Kim HB, Park SW, Kim NJ, Kim EC, Oh MD, Choe KW. 2009. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin Infect Dis 49:395–401. doi: 10.1086/600295. [DOI] [PubMed] [Google Scholar]
- 35.Park HJ, Kim SH, Kim MJ, Lee YM, Park SY, Moon SM, Park KH, Chong YP, Lee SO, Choi SH, Woo JH, Kim YS. 2012. Efficacy of linezolid-based salvage therapy compared with glycopeptide-based therapy in patients with persistent methicillin-resistant Staphylococcus aureus bacteremia. J Infect 65:505–512. doi: 10.1016/j.jinf.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig F, Edwards B, Lawes T, Gould IM. 2012. Effects of storage on vancomycin and daptomycin MIC in susceptible blood isolates of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 50:3383–3387. doi: 10.1128/JCM.01158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riederer K, Shemes S, Chase P, Musta A, Mar A, Khatib R. 2011. Detection of intermediately vancomycin-susceptible and heterogeneous Staphylococcus aureus isolates: comparison of Etest and agar screening methods. J Clin Microbiol 49:2147–2150. doi: 10.1128/JCM.01435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]