Abstract
Community antimicrobial resistance rates are high in communities with frequent use of nonprescription antibiotics. Studies addressing nonprescription antibiotic use in the United States have been restricted to Latin American immigrants. We estimated the prevalence of nonprescription antibiotic use in the previous 12 months as well as intended use (intention to use antibiotics without a prescription) and storage of antibiotics and examined patient characteristics associated with nonprescription use in a random sample of adults. We selected private and public primary care clinics that serve ethnically and socioeconomically diverse patients. Within the clinics, we used race/ethnicity-stratified systematic random sampling to choose a random sample of primary care patients. We used a self-administered standardized questionnaire on antibiotic use. Multivariate regression analysis was used to identify independent predictors of nonprescription use. The response rate was 94%. Of 400 respondents, 20 (5%) reported nonprescription use of systemic antibiotics in the last 12 months, 102 (25.4%) reported intended use, and 57 (14.2%) stored antibiotics at home. These rates were similar across race/ethnicity groups. Sources of antibiotics used without prescriptions or stored for future use were stores or pharmacies in the United States, “leftover” antibiotics from previous prescriptions, antibiotics obtained abroad, or antibiotics obtained from a relative or friend. Respiratory symptoms were common reasons for the use of nonprescription antibiotics. In multivariate analyses, public clinic patients, those with less education, and younger patients were more likely to endorse intended use. The problem of nonprescription use is not confined to Latino communities. Community antimicrobial stewardship must include a focus on nonprescription antibiotics.
INTRODUCTION
The potential harms of antibiotic use, especially the emergence of resistant pathogens, are garnering increasing global attention (1, 2). A major driver of antimicrobial resistance is the misuse of antibiotics (3). Community antimicrobial resistance rates are high in communities with frequent use of nonprescription antibiotics (4, 5). The World Health Organization encourages prescription-only use of antibiotics in an effort to improve the rational use of antimicrobials (6). Nonprescription use may be of concern for the development of resistant organisms because it may involve very short courses, inappropriate drug and dose choices, and unnecessary therapy (4). Other potential problems associated with nonprescription use include adverse drug reactions, drug interactions, masking of underlying infectious processes, superinfection, and other harms, including the effect of antibiotics on microbiota (4).
Surveys addressing nonprescription antibiotic use in the United States have been restricted to Latin American immigrants (7, 8). Within these ethnic groups, 19% of individuals acquired antibiotics in the United States without a prescription, and 16% transported nonprescribed antibiotics from another country (7). In another study, antibiotics were available without a prescription in all private, independent pharmacies or groceries in the Hispanic neighborhood studied in New York City (9). Other sources of nonprescription antibiotic use may include antibiotics “left over” from previous treatment courses or obtained from relatives or friends. Intended use (intention to use antibiotics without a prescription or medical guidance) and storage of unused antibiotics at home are facilitating factors for actual nonprescription use. In a study including 19 European countries, intended self-medication and storage of antibiotics were strong independent predictors of actual nonprescription use in the past 12 months (10).
We estimated the prevalences of nonprescription antibiotic use, intended use, and storage of antibiotics in a socioeconomically and ethnically diverse sample of adult patients from private and public primary care clinics. We also examined patient characteristics associated with nonprescription use, the types of antibiotics used, the sources of nonprescription use, and the symptoms for which the antibiotics were reportedly used.
MATERIALS AND METHODS
Sampling, setting, and participants.
The survey was conducted between April and August 2015 in the waiting rooms of three primary care clinics representing a public health care delivery system and a private practice network in a large, urban area. The two public clinics serve a diverse, predominantly uninsured and underinsured patient population. The one private clinic serves primarily managed care and privately insured patients.
Our multistage sampling design took into account the fact that many probability-based sampling designs (e.g., random-digit-dialing telephone survey or mail survey from a published address list) would not adequately access minority residents. Therefore, we selected private and public primary care clinics that serve ethnically and socioeconomically diverse patients and used race/ethnicity-stratified sampling to reflect the racial/ethnic makeup of the population of Harris County, TX. With more than 4.5 million residents, Harris County is the most populous county in the state and one of the largest in the country, with 42% of residents being Hispanic or Latino, 20% being black or African American, and 38% being non-Hispanic (including white, Asian, and other racial/ethnic groups) (11). Within the clinics, we used race/ethnicity-stratified systematic random sampling to choose a sample of adults. The clinic staff gave a flyer to every third patient who checked in for a primary care visit. The flyer introduced the study and asked the patient to approach the study research coordinator if the patient was interested in participating. The research coordinator invited study volunteers to complete a short questionnaire about their use of antibiotics. The questionnaires were completed anonymously in the respondent's preferred language (English or Spanish). For their reference, respondents were provided a list of brand and generic names of commonly used antibiotics in the United States and Latin American countries. Exclusion criteria were an age of <18 years and an inability or unwillingness to complete a short questionnaire. The study was approved by the Baylor College of Medicine Institutional Review Board (IRB).
Sample size.
The prevalence of nonprescription antibiotic use among Latin American immigrants in previous studies conducted in the United States was 19 to 26%. If the maximum expected prevalence is 26%, to obtain a precision of 0.05, the sample size needed is 296. To adjust for possible nonresponse, we selected 400 participants.
Instrument.
The self-administered questionnaire was modified from a previously pretested and standardized questionnaire used in a 19-country pan-European survey on the prevalence of self-medication with antibiotics (10). We created a Spanish version of the questionnaire using a combination of committee translation and standard back-translation strategies to achieve semantic equivalence. During this process, a panel of five bilingual translators from a community health research background provided feedback on any phrasing that was not comparable in meaning, formatting that could be confusing, and other concerns that could impact the ability of the patient to understand or respond to the questions. After this procedure was completed, the translators and two of the investigators reviewed the translation and reached a consensus on the final version of the questionnaire, which had a sixth-grade reading level, as assessed by the Flesch-Kincaid method (25). The questionnaire was pretested with a convenience sample of English- and Spanish-speaking individuals with various levels of education, and some minor changes were made prior to administration (see the supplemental material).
Questions asked about the respondent's use of antibiotics during the past 12 months, how the antibiotics were obtained, whether they were stored at home, and whether the respondent would consider using antibiotics without consulting a physician. Details of the antibiotics used (name of the medicine, symptom or disease, and duration of use) and demographic characteristics of the respondents were included. Only antibacterial drugs for systemic use were included in the analyses.
Nonprescription antibiotic use, storage of antibiotics, and intended use.
Nonprescription use is defined as the actual consumption of antibiotics that were not prescribed to that individual at that time, and intended use is defined as an intention or willingness to take antibiotics that had not been prescribed. Respondents were classified as nonprescription antibiotic users if they reported having taken any antibiotics in the previous 12 months without a prescription and as prescribed antibiotic users if antibiotics had been prescribed. Two estimates were used to assess storage of drugs: a maximum estimate, including all respondents who stored antibiotics, and a conservative estimate, which excluded those respondents who reported having taken an antibiotic for a prescribed course in the previous 12 months and reported having the same drug at home. We used this estimate to exclude any current users of prescribed antibiotics. Intended use was defined as an answer of “yes” or “maybe” to the question, “In general, would you use antibiotics for yourself without contacting a doctor/nurse/hospital?”
Statistical analyses.
Descriptive statistics were used to estimate the prevalence rates and 95% confidence intervals (CIs) for nonprescription use, prescribed use in the previous 12 months, storage, and intended use. Data were checked for normality. The effects of individual characteristics were analyzed by multivariate logistic regression analysis with intended use as a dependent variable and including factors that had a P value of <0.20 in univariate analyses. Factors were regarded as being significant in multivariate analyses when they had a P value of <0.05. Nonsignificant factors from the multivariate analyses were deleted from the model stepwise. Possible interactions between factors found to be significant in the multivariate analyses were tested.
Multivariate logistic regression was also used to study the relationship between intended use, storage, and actual nonprescription use in the previous 12 months. Data were analyzed by using SPSS (version 23) for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
A total of 400 respondents (response rate, 94%) completed the questionnaires. Table 1 shows the demographic characteristics of the sample. Our sample was comparable to Harris County census data relating to insurance coverage, employment status, race/ethnicity, and education. However, our sample included a higher percentage of women (68% versus 51%) and a higher percentage of respondents with a total annual household income of ≤$20,000 (38% versus 23%). Twenty-three percent of the respondents were uninsured but received county benefits allowing access to public clinics at either very low or no cost, and 19% had Medicaid insurance.
TABLE 1.
General characteristics of respondents
| Characteristic | Value |
|---|---|
| Median age (yr) (range) | 49 (18–89) |
| No. (%) of female respondents | 274 (68) |
| No. (%) of respondents of race/ethnicity | |
| Hispanic or Latino | 168 (42) |
| African American or black | 80 (20) |
| Non-Hispanica | 152 (38) |
| No. (%) of respondents with education level | |
| Less than high school | 43 (11) |
| High school or GEDg | 112 (28) |
| Some college and above | 245 (61) |
| No. (%) of respondents with insurance statusb | |
| Private | 177 (45) |
| Medicare | 50 (12) |
| Medicaid | 75 (19) |
| Uninsuredc | 90 (23) |
| Self-pay | 4 (1) |
| No. (%) of patients attending clinic type | |
| Private | 200 (50) |
| Public | 200 (50) |
| No. (%) of patients with chronic diseased | 138 (34) |
| No. of respondents with income/total no. of respondents (%)e | |
| ≤$20,000 | 141/372 (38) |
| >$20,000 but ≤$40,000 | 73/372 (20) |
| >$40,000 but ≤$60,000 | 57/372 (15) |
| >$60,000 but ≤$100,000 | 46 (12) |
| >$100,000 | 55 (15) |
| No. of respondents with employment status/total no. of respondents (%)f | |
| Employed | 232/363 (64) |
| Not working | 111/363 (31) |
| Retired | 20/363 (5) |
| No. (%) of questionnaires completed in Spanish | 80 (20) |
A total of 139 (91%) of whom are white and 8% of whom belong to other ethnic groups (Indian, Vietnamese, or Chinese, etc).
Data missing for 4 participants.
Includes those who have benefits from the county allowing access to public clinic providers at either very low cost or no cost.
Including any of the following: asthma, chronic bronchitis, emphysema, HIV, hepatitis C, cystic fibrosis, diabetes, endocarditis, tuberculosis, prostatitis, chronic urinary tract infection, chronic osteomyelitis, peptic ulcer disease, chronic pyelonephritis, or cancer.
Data missing for 28 participants.
Data missing for 37 participants.
GED, general educational development (high school equivalency test).
Prevalence of prescription and nonprescription use of systemic antibiotics.
Of the 400 respondents, 20 (5%) reported nonprescription antibiotic use in the last 12 months (Table 2). In total, there were 22 nonprescription courses. The prevalence rates of nonprescription use were not significantly different across race/ethnicity groups. The rates also were not significantly different between the respondents who completed the questionnaire in English and those who completed it in Spanish.
TABLE 2.
Prescription and nonprescription antibiotic use and storage of antibiotics stratified by race/ethnicity
| Respondent group (no. of respondents) | % of respondents (95% confidence interval) who reported: |
||||
|---|---|---|---|---|---|
| Nonprescription antibiotic use in the last 12 moa | Prescribed antibiotic use in the last 12 mob | Intended usec | Storaged (conservative estimate) | Storagee (maximum estimate) | |
| Total (400) | 5.0 (2.9–7.1) | 38.8 (34.0–43.6) | 25.4 (21.1–29.7) | 10.2 (7.2–13.2) | 14.2 (10.8–17.6) |
| Hispanic or Latino (168) | 7.7 (3.7–11.7) | 36.9 (29.6–44.2) | 29.2 (22.3–36.1) | 12.1 (7.2–17.0) | 14.5 (9.2–19.8) |
| African American or black (80) | 3.8 (0–8.0) | 38.8 (28.1–49.5) | 17.5 (9.2–25.8) | 8.9 (2.7–15.1) | 11.4 (4.4–18.4) |
| NonHispanic (152) | 2.6 (0–5.1) | 40.8 (33.0–48.6) | 25.7 (18.8–32.7) | 8.7 (4.2–13.2) | 15.3 (9.6–21.0) |
Respondents who used at least one course of nonprescription oral antibiotics.
Respondents who used at least one course of prescribed systemic antibiotics.
Respondents who would use antibiotics without contacting a doctor/nurse/hospital.
Including only those respondents who stored antibiotics and had not taken the same antibiotics for a prescribed course in the previous 12 months.
All respondents who stored antibiotics.
The rates of prescribed use were also similar across different race/ethnicity groups, with overlapping 95% CIs. In total, 25.4% (102/400) of the respondents were willing to use antibiotics without contacting a doctor/nurse/hospital (intended use), and 14.2% reported that they have antibiotics at home (Table 2). No difference was observed for intended use and storage of antibiotics across race/ethnicity groups.
Types and sources of antibiotics for nonprescription use and reported symptoms.
Amoxicillin was used in 10 of 22 nonprescription courses, followed by azithromycin (3/22), ciprofloxacin (3/22), ampicillin (2/22), and other antibiotics (including trimethoprim-sulfamethoxazole, tetracycline, ofloxacin, and amoxicillin clavulanate [1 for each course]).
The major source of antibiotics used without a prescription was a store or pharmacy in the United States (40%), followed by antibiotics obtained from another country (24%), antibiotics obtained from a relative or friend (20%), antibiotics left over from previous prescriptions (12%), and veterinary antibiotics (4%). Respiratory symptoms (including cough, sore throat, and sinus infection) were the most common reasons for using nonprescription antibiotics, followed by urinary tract infections (UTI), tooth pain, stomach pain, and infection in general. The median duration of nonprescription antibiotic use was 7 days (range, 2 to 45 days).
Intended use and storage of antibiotics.
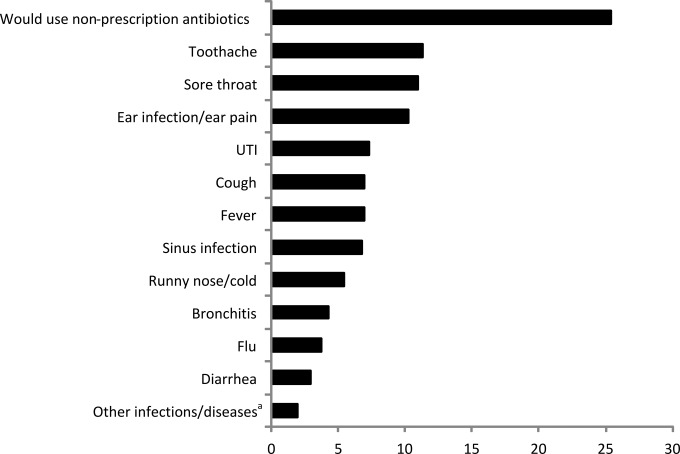
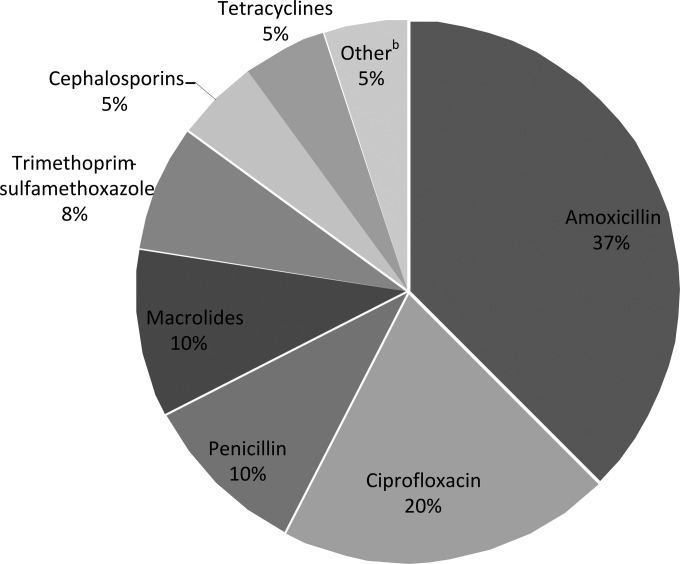
For intended use, toothache and sore throat were the most common symptoms, followed by ear infection/ear pain, UTI, and respiratory symptoms (Fig. 1). For storage of antibiotics (using the conservative estimate of storage to exclude users currently prescribed antibiotics), amoxicillin was the most commonly stored antibiotic, followed by ciprofloxacin, penicillin, and macrolides (Fig. 2). The sources of the drugs were leftover prescription antibiotics (74%), antibiotics stored after being obtained abroad (21%), and antibiotics obtained without a prescription in a store or pharmacy in the United States (5%).
FIG 1.
Prevalence of intended use per predefined symptom/disease. a, other infections/diseases included skin infection and “any infection.”
FIG 2.
Types of antibiotics that respondents reported storing at home. a, excluding those respondents who stored antimicrobial drugs and also reported having taken the same drugs for a prescribed course in the previous 12 months (we used this estimate to exclude any current users of prescribed antibiotics [conservative estimate of storage]); b, other antibiotics included cephalexin, cefaclor, metronidazole, and nitrofurantoin.
Relationship between intended use, storage of antibiotics, and actual nonprescription use.
Intended use was a significant predictor of actual nonprescription use (odds ratio [OR], 3.5; 95% CI, 1.4 to 8.9). Those who stored antibiotics had a 4.2-times-higher (95% CI, 1.5 to 11.6) chance of nonprescription use than those who did not store antibiotics. A significant relationship between storage of antibiotics and intended use was also found (OR, 5.3; 95% CI, 2.7 to 10.5).
Effects of individual characteristics on intended use.
Table 3 shows the results of the univariate and multivariate analyses. In the final multivariate model, public clinic patients were more likely to endorse intended use than were private clinic patients. Older age decreased the risk of intended use. Higher educational level was associated with lower intended use (borderline significant). No statistical interaction was found between the determinants of nonprescription use in the multivariate analyses.
TABLE 3.
Predictors of intended use (n = 400)d
| Predictor | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Female sex | 1.02 (0.62–1.66) | 0.94 | ||
| Age (yr) | 0.99 (0.97–1.00) | 0.06 | 0.98(0.96–0.99) | 0.02 |
| Race/ethnicity | 0.15 | |||
| Hispanic or Latino | 1 (reference)f | |||
| African American or black | 0.51 (0.26–1.01) | 0.05 | ||
| Non-Hispanica | 0.84 (0.51–1.38) | 0.48 | ||
| Education | 0.03 | 0.057 | ||
| Less than high school | 1 (reference) | |||
| High school or GEDe | 0.38 (0.18–0.81) | 0.01 | 0.39 (0.18–0.85) | 0.02 |
| Some college and above | 0.45 (0.23–0.88) | 0.02 | 0.60 (0.28–1.28) | 0.18 |
| Insurance status | 0.17 | |||
| Medicaid | 1 (reference) | |||
| Medicare | 0.66 (0.27–1.62) | 0.37 | ||
| Private | 0.89 (0.48–1.68) | 0.73 | ||
| Self-pay | 8.84 (0.87–90.18) | 0.07 | ||
| Uninsuredb | 1.28 (0.64–2.56) | 0.48 | ||
| Clinic type | ||||
| Private | 1 (reference) | |||
| Public | 1.66 (1.05–2.64) | 0.03 | 1.92 (1.11–3.31) | 0.02 |
| Presence of chronic diseasec | 0.83 (0.51–1.35) | 0.45 | ||
| Employment status | 0.53 | |||
| Not working | 1 (reference) | |||
| Retired | 0.52 (0.14–1.90) | 0.32 | ||
| Employed | 1.06 (0.63–1.80) | 0.82 | ||
| Income | 0.20 | |||
| ≤$20,000 | 1 (reference) | |||
| >$20,000 but ≤$40,000 | 0.71 (0.37–1.39) | 0.32 | ||
| >$40,000 but ≤$60,000 | 0.57 (0.26–1.24) | 0.15 | ||
| >$60,000 but ≤$100,000 | 1.25 (0.61–2.57) | 0.54 | ||
| >$100,000 | 0.50 (0.22–1.12) | 0.09 | ||
| Survey language | ||||
| English | 1 (reference) | |||
| Spanish | 1.71 (1.01–2.92) | 0.05 | ||
A total of 91% of whom are white.
Includes those who have benefits from the county allowing access to public clinic providers at either very low cost or no cost.
Including any of the following: asthma, chronic bronchitis, emphysema, HIV, hepatitis C, cystic fibrosis, diabetes, endocarditis, tuberculosis, prostatitis, chronic urinary tract infection, chronic osteomyelitis, peptic ulcer disease, chronic pyelonephritis, or cancer.
The multivariate regression model included all factors with a P value of <0.20 in univariate analyses. Results shown in boldface type have a P value of <0.20 in univariate analyses and a P value of <0.05 in multivariate analyses; where the univariate data are shown in boldface type but there are no corresponding multivariate data, the results were nonsignificant in multivariate analyses.
GED, general educational development (high school equivalency test).
reference, reference category in the regression analysis.
DISCUSSION
The prevalence rate of nonprescription antibiotic use in the last 12 months was 5%. Intended use had a much higher prevalence (25.4%) than actual nonprescription use, indicating that the population at risk is much larger than the population of those who have actually used nonprescription antibiotics in the previous 12 months. The prevalence rate of storage of antibiotics was 14.2%, indicating that many respondents have antibiotics available at home. Nonprescription use in the primary care population studied was not confined to Hispanic or Latino communities only: the prevalences of nonprescription use, storage, and intended use were similar across all studied race/ethnicity groups. Previous studies in the United States focused mainly on Latin American immigrants (7, 12, 13).
We found that a variety of nonprescription antibiotics were used from a range of different sources, with a store or pharmacy in the United States being the major source. Most reasons given for actual and intended use included self-limiting symptoms such as sore throat, toothache, or cough. The major source for antibiotic storage was leftover antibiotics from previous prescriptions, suggesting noncompliance with the recommended duration of therapy. Five percent of stored antibiotics included tetracyclines, which can become highly nephrotoxic when degraded (14). Patients from public primary care clinics, those with less education, and younger patients had a higher risk of intended use in our survey. The copay to see a physician in public clinics ranges from none required to over $70, with most patients falling at the lower end of the income-based sliding-fee scale. Concerns about copayments, in addition to pharmacy costs, could influence patient decisions about nonprescription use (15).
The prevalence rate of nonprescription antibiotic use in our study is lower than the rates reported in other U.S. studies. In a survey of Latin American immigrants, 16% transported nonprescribed antibiotics, and 19% had acquired antibiotics in the United States without a prescription (7). In another survey including mostly respondents of low socioeconomic status (median income of $14,900), 26% had obtained antibiotics from sources other than a physician's prescription (8). A survey of a convenience sample of emergency department patients indicated considerable use of leftover antibiotics, with 17% of patients taking leftover antibiotics without consulting their physician, most commonly for a sore throat (42%) or coughs (11%) (16). Most of those studies used different research methods, including different time frames for reporting antibiotic use, study settings, and sampling techniques (7 – 9, 13, 16). A possible reason for the lower prevalence of nonprescription use in Hispanic or Latino participants in our study could be the lower number of foreign-born Hispanics in Texas, in combination with the higher economic status of Hispanics in Texas than in many parts of the country (17). Americans of Hispanic heritage may have different attitudes and behaviors than more recent immigrants.
A previous 19-country European study used similar research methods and the same questionnaire but found lower estimates for nonprescription use in all 8 studied northern and western European countries (10). The only 3 countries (out of 19) that had a significantly higher prevalence of nonprescription use than the one reported in our study were Spain, Romania, and Lithuania. However, the prevalence of outpatient use of prescribed antibiotics is also higher in the United States than in northern and western European countries (18, 19).
Our study confirmed that despite being illegal, over-the-counter dispensation of systemic antibiotics occurs in the United States. Therefore, enforcement of existing laws regulating the sale of antibiotics could reduce nonprescription use. Another common source of nonprescription use and storage of antibiotics in our study was leftover antibiotics from previous prescriptions. A European study including 19 countries showed that previous prescription use of antibiotics for upper respiratory tract infections increased the likelihood of nonprescription use with leftover antibiotics from previous courses (20). Public education should emphasize the potential risks of using nonprescription antibiotics and the inappropriateness of using antibiotic therapy for minor ailments. Multifaceted campaigns repeated over several years have the greatest effect (21, 22). Self-medication with antibiotics has been identified as a new focus for public education campaigns by the European Centre for Disease Prevention and Control (ECDC), which prepared a toolkit on self-medication with antibiotics, offering advice on how campaign organizers could engage the general public to promote the appropriate and responsible use of antibiotics (23, 24). Our data illustrate that there is also a need in the United States to focus on nonprescription antibiotic use in community antimicrobial stewardship programs.
Our study included a socioeconomically and ethnically diverse sample of patients from public and private primary care clinics and a comprehensive questionnaire that included nonprescription use from a range of potential sources. Our survey was the first in the United States to include intended use and storage of antibiotics obtained from a range of potential sources, including previous prescriptions.
A limitation of our study is that estimates of nonprescription use may underestimate the true prevalence rate. Respondents might deny practicing self-medication, especially if they are aware that this is inappropriate behavior and if they are interviewed in a health care setting. To discourage underreporting of nonprescription use, the questions about antibiotic use were formulated in a neutral way in which the source of the drug could be chosen from 6 predefined sources or “other source.” We also attached a list of the most commonly used antibiotics in the United States and Latin American countries (in English and Spanish) to reduce recall problems. Although our sample was comprised of socioeconomically and ethnically diverse respondents, it may not be representative of the overall U.S. population.
Community antimicrobial stewardship must include a focus on nonprescription antimicrobials. Even our estimated prevalence of 5% suggests that 164,250 primary care patients in an adult population of 3,285,000 are using nonprescription antibiotics annually. An integrated approach involving policy makers, prescribers, and the general public using both educational and regulatory measures is needed. Such measures should be embedded in a general policy to change the culture of antibiotic use by improving awareness among the general public and professionals about the risks associated with antibiotic use as well as reducing public misconceptions about the benefit of taking antibiotics for minor ailments.
Supplementary Material
ACKNOWLEDGMENTS
We extend appreciation to the Harris Health System, the Harris Health Patient Council, and Baylor Family Medicine for their support of this project. We also thank Lourdes Pelaez for her capable recruitment and sensitive interaction with the study participants.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
We have no potential conflicts of interest.
Funding Statement
This research received no specific grant from any funding agency. It was supported in part by the Houston VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN 13–413).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00528-16.
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J. 2008. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 3.Harbarth S, Samore MH. 2005. Antimicrobial resistance determinants and future control. Emerg Infect Dis 11:794–801. doi: 10.3201/eid1106.050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. 2011. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis 11:692–701. doi: 10.1016/S1473-3099(11)70054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarb P, Goossens H. 2012. Human use of antimicrobial agents. Rev Sci Tech 31:121–133. [DOI] [PubMed] [Google Scholar]
- 6.Leung E, Weil DE, Raviglione M, Nakatani H. 2011. The WHO policy package to combat antimicrobial resistance. Bull World Health Organ 89:390–392. doi: 10.2471/BLT.11.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mainous AG III, Cheng AY, Garr RC, Tilley BC, Everett CJ, McKee MD. 2005. Nonprescribed antimicrobial drugs in Latino community, South Carolina. Emerg Infect Dis 11:883–888. doi: 10.3201/EID1106.040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee MD, Mills L, Mainous AG III. 1999. Antibiotic use for the treatment of upper respiratory infections in a diverse community. J Fam Pract 48:993–996. [PubMed] [Google Scholar]
- 9.Larson E, Grullon-Figueroa L. 2004. Availability of antibiotics without prescription in New York City. J Urban Health 81:498–504. doi: 10.1093/jurban/jth133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grigoryan L, Haaijer-Ruskamp FM, Burgerhof JG, Mechtler R, Deschepper R, Tambic-Andrasevic A, Andrajati R, Monnet DL, Cunney R, DiMatteo A, Edelstein H, Valinteliene R, Alkerwi A, Scicluna E, Grzesiowski P, Bara AC, Tesar T, Cizman M, Campos J, Lunborg CS, Birkin J. 2006. Self-medication with antimicrobial drugs in Europe. Emerg Infect Dis 12:452–459. doi: 10.3201/eid1203.050992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Census Bureau. 2015. QuickFacts, Harris County, Texas. US Census Bureau, Washington, DC: http://quickfacts.census.gov/qfd/states/48/48201.html. [Google Scholar]
- 12.Mainous AG III, Diaz VA, Carnemolla M. 2008. Factors affecting Latino adults' use of antibiotics for self-medication. J Am Board Fam Med 21:128–134. doi: 10.3122/jabfm.2008.02.070149. [DOI] [PubMed] [Google Scholar]
- 13.Landers TF, Ferng YH, McLoughlin JW, Barrett AE, Larson E. 2010. Antibiotic identification, use, and self-medication for respiratory illnesses among urban Latinos. J Am Acad Nurse Pract 22:488–495. doi: 10.1111/j.1745-7599.2010.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frimpter GW, Timpanelli AE, Eisenmenger WJ, Stein HS, Ehrlich LI. 1963. Reversible “Faconi syndrome” caused by degraded tetracycline. JAMA 184:111–113. [DOI] [PubMed] [Google Scholar]
- 15.Varkey AB, Manwell LB, Williams ES, Ibrahim SA, Brown RL, Bobula JA, Horner-Ibler BA, Schwartz MD, Konrad TR, Wiltshire JC, Linzer M. 2009. Separate and unequal: clinics where minority and nonminority patients receive primary care. Arch Intern Med 169:243–250. doi: 10.1001/archinternmed.2008.559. [DOI] [PubMed] [Google Scholar]
- 16.Richman PB, Garra G, Eskin B, Nashed AH, Cody R. 2001. Oral antibiotic use without consulting a physician: a survey of ED patients. Am J Emerg Med 19:57–60. doi: 10.1053/ajem.2001.20035. [DOI] [PubMed] [Google Scholar]
- 17.Brown A, Lopez MH. 2011. Pew Research Center Hispanic trends 2011. Pew Research Center, Washington, DC: http://www.pewhispanic.org/2013/08/29/mapping-the-latino-population-by-state-county-and-city/ Accessed 28 October 2015. [Google Scholar]
- 18.Goossens H, Ferech M, Coenen S, Stephens P. 2007. Comparison of outpatient systemic antibacterial use in the United States and 27 European countries. Clin Infect Dis 44:1091–1095. doi: 10.1086/512810. [DOI] [PubMed] [Google Scholar]
- 19.Huttner B, Samore M. 2011. Outpatient antibiotic use in the United States: time to “get smarter”. Clin Infect Dis 53:640–643. doi: 10.1093/cid/cir449. [DOI] [PubMed] [Google Scholar]
- 20.Grigoryan L, Burgerhof JG, Haaijer-Ruskamp FM, Degener JE, Deschepper R, Monnet DL, DiMatteo A, Scicluna EA, Bara AC, Lundborg CS, Birkin J. 2007. Is self-medication with antibiotics in Europe driven by prescribed use? J Antimicrob Chemother 59:152–156. [DOI] [PubMed] [Google Scholar]
- 21.Huttner B, Goossens H, Verheij T, Harbarth S. 2010. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis 10:17–31. doi: 10.1016/S1473-3099(09)70305-6. [DOI] [PubMed] [Google Scholar]
- 22.Gonzales R, Corbett KK, Wong S, Glazner JE, Deas A, Leeman-Castillo B, Maselli JH, Sebert-Kuhlmann A, Wigton RS, Flores E, Kafadar K. 2008. “Get Smart Colorado”: impact of a mass media campaign to improve community antibiotic use. Med Care 46:597–605. doi: 10.1097/MLR.0b013e3181653d2e. [DOI] [PubMed] [Google Scholar]
- 23.Earnshaw S, Mancarella G, Mendez A, Todorova B, Magiorakos AP, Possenti E, Stryk M, Gilbro S, Goossens H, Abiger B, Monnet DL. 2014. European Antibiotic Awareness Day: a five-year perspective of Europe-wide actions to promote prudent use of antibiotics. Euro Surveill 19(41):pii=20928 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20928. [DOI] [PubMed] [Google Scholar]
- 24.European Centre for Disease Prevention and Control. 2016. Toolkit on self-medication with antibiotics. A European health initiative. European Centre for Disease Prevention and Control, Solna, Sweden: http://ecdc.europa.eu/en/eaad/antibiotics-plan-campaign/toolkit-self-medication/Pages/toolkit-general-public-self-medication.aspx. [Google Scholar]
- 25.Kincaid JP, Fishburne RP, Rogers RL, Chissom BS. 1975. Derivation of new readability formulas (automated readability index, fog count, and flesch reading ease formula) for Navy enlisted personnel. Research Branch Report 8–75. Chief of Naval Technical Training, Naval Air Station Memphis, Memphis, Tennessee. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.