Abstract
Isoniazid (INH) remains the core drug in tuberculosis management, but serious hepatotoxicity and potentially fatal liver injury continue to accompany INH consumption. Among numerous theories that have been established to explain INH-induced liver injury, an inflammatory stress theory has recently been widely used to explain the idiosyncrasy. Inflammatory stress usually sensitizes tissues to a drug's toxic consequences. Therefore, the present study was conducted to verify whether bacterial lipopolysaccharide (LPS)-induced inflammation may have a role in enhancing INH hepatotoxicity. While single INH or LPS administration showed no major toxicity signs, INH-LPS cotreatment intensified liver toxicity. Both blood biomarkers and histological evaluations clearly showed positive signs of severe liver damage accompanied by massive necrosis, inflammatory infiltration, and hepatic steatosis. Furthermore, elevated serum levels of bile acid associated with the repression of bile acid synthesis and transport regulatory parameters were observed. Moreover, the principal impact of cytochrome P450 2E1 (CYP2E1) on INH toxicity could be anticipated, as its protein expression showed enormous increases in INH-LPS-cotreated animals. Furthermore, the crucial role of CYP2E1 in the production of reactive oxygen species (ROS) was clearly obvious in the repression of hepatic antioxidant parameters. In summary, these results confirmed that this LPS-induced inflammation model might prove valuable in revealing the hepatotoxic mechanisms of INH and the crucial role played by CYP2E1 in the initiation and propagation of INH-induced liver damage, information which could be very useful to clinicians in understanding the pathogenesis of drug-induced liver injury.
INTRODUCTION
Isoniazid (INH), since its introduction in the year 1952, still serves as a frontline drug in tuberculosis treatment (1). Despite the fact that INH has been widely used as a first-line antitubercular agent (2, 3), its therapeutic value is usually accompanied by severe hepatotoxicity and lethal hepatic injury (4, 5). Although the pathophysiology of INH-induced liver injury might vary, the toxicity features of the drug, including hepatocellular steatosis, necrosis, and inflammatory infiltration, are nearly consistent (6, 7).
Even though extensive studies expounding INH toxicity have been carried out, the exact mechanism of INH hepatotoxicity remains controversial. Among numerous established theories, an inflammatory stress theory has recently been widely used to explain the idiosyncrasy. One hypothesis is that inflammatory stress increases sensitivity to drug-induced liver injury (DILI) (8, 9). In this theory, an incidence of systemic inflammation might reduce the xenobiotic toxicity threshold, which could be easily accomplished by coadministration with an inflammatory agent (10), thus promoting drug toxicity. Lipopolysaccharide (LPS) is an outer cell wall membrane constituent of Gram-negative bacteria that has been comprehensively studied as an inflammatory agent with a major role in bacterial infections (11, 12). Previous studies have suggested that LPS might intensify DILI (13 – 15), and, hence, a possible cornerstone role for LPS in INH-induced hepatotoxicity might be assumed.
Oxidative stress, generated from the accumulation of reactive oxygen species (ROS), may be potentiated by different factors, including drugs and inflammation (16, 17). In addition, oxidative stress plays a major role in several types of hepatic injury (18). Furthermore, with cytochrome P450 2E1 (CYP2E1) playing a major role in drug metabolism and the pathophysiology of DILI (19) and being considered a principal element in human susceptibility to chemical toxins (20), it plays a central part in oxidative stress, production of ROS, and hepatotoxic injury. Moreover, a previous report proposed that hepatic CYP2E1 plays a fundamental role in the propagation of INH-induced hepatotoxicity, mainly throughout ROS generation (21). Nevertheless, a full understanding of CYP2E1 as a major hepatotoxin-forming, catalyzing enzyme and its influence on INH-induced liver damage has not been completely achieved.
Studies of the hepatotoxic mechanisms of INH were previously conducted using different animal models; however, results were accompanied by the absence of certain features of INH toxicity, i.e., delayed onset or inconsistency in severity compared to that in humans (3). Despite the lack of success in reproducing the same clinical features of INH hepatotoxicity, our group in a previous study successfully established an animal model that could fill the gap associated with understanding the whole picture. In that study, Su et al. (22) concluded that LPS inflammatory activity positively sensitized hepatic cells toward INH-induced liver injury.
Therefore, due to the potential enhancement activity of LPS-induced inflammation in drug toxicities, the present study aimed to explore and verify whether LPS might enhance INH hepatotoxicity and to investigate the underlying mechanisms that are possibly responsible for this enhancement; this was done by establishing a suitable animal model that could predict some idiosyncratic reactions, which could then be used to identify potential problems earlier and to direct appropriate preventative actions.
MATERIALS AND METHODS
Drugs, chemicals, and antibodies.
INH (CAS number 54-85-3; analytical standard, ≥99%) and LPS (lot number 025M4128V; derived from Escherichia coli serotype O128:B12, source strain CDC2440-69) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents were commercially available and of high analytical grade.
Animals.
Male Sprague-Dawley (SD) rats (180 to 200 g) were obtained from Shanghai Lingchang Biological Technology Co., Ltd. (Shanghai, China). All experimental procedures were conducted in accordance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals. Rats were housed in controlled environmental conditions (23 ± 1°C, 55% ± 5% relative humidity, 12-h light–12-h dark cycle) with free access to food and water ad libitum. Animals were acclimatized for 1 week before the experiments were conducted.
Treatment protocol.
Thirty-six rats were divided into six groups of six animals each. Group I served as the control; group II received LPS (2.0 mg/kg intravenously), whereas both group III and group IV received INH (200 and 400 mg/kg, respectively, intragastrically), while group V and group VI received INH at the above-mentioned dosages, respectively, plus LPS (2.0 mg/kg).
INH was intragastrically administered for 14 consecutive days, while LPS was given as an intravenous bolus dose at day 14, 2 h before the INH dose. Drug doses were selected based on previous studies (22 – 24), with slight modification. After the last INH dose, the rats were sacrificed; blood samples and liver sections were collected for further biological evaluation. All experimental procedures were ethically approved by both China Pharmaceutical University and the ethical committees of the National Drug Screening Centre, Nanjing, China.
Serum and liver biological parameters.
Serum alanine transaminase (ALT), aspartate transaminase (AST), total bile acids (TBA), total bilirubin (TBil), gamma-glutamyl transferase (γGGT), triglyceride (TG), and total cholesterol (TC) levels were measured with an HITAC7170A automatic analyzer (Hitachi, Japan) in accordance with standard spectrophotometric methods. Hepatic TG and TC were analyzed using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Histology evaluation.
Liver slices were fixed in 10% paraformaldehyde solution and embedded in paraffin wax. Sections were cut at 5-mm thickness and stained with hematoxylin-eosin. Slides were coded, randomized, and evaluated by a pathophysiologist using light microscopy.
Measurement of antioxidant enzymes and MDA levels.
Superoxide dismutase (SOD), reduced glutathione (GSH), malondialdehyde (MDA), and total antioxidant capacity (T-AOC) assays were carried out using appropriate kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), in accordance with the manufacturer's instructions.
Real-time quantitative PCR (RT-PCR).
Total RNA was extracted with TRIzol (Vazyme Biotech, Nanjing, China), and cDNA synthesis was performed using the PrimeScript RT master mix (TaKaRa Biotechnology, Dalian, China) according to the manufacturer's instructions. Quantitative reverse transcription-PCR was performed using the SYBR green PCR master mix (Vazyme Biotech, Nanjing, China). Gene expression was evaluated using the ΔΔCT method, with the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene serving as a reference.
Western blotting.
Radioimmunoprecipitation assay (RIPA) buffer and phosphatase and protease inhibitors (Vazyme Biotech, Nanjing, China) were used for liver protein extraction. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)-separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). After nonspecific blocking with 5% skim milk for an hour, the membranes were probed with primary antibody (1:500 to 1:1,000) in 5 ml blocking buffer overnight at 4°C. The membranes were then washed four times with Tris-buffered saline with Tween 20 (TBST) buffer and incubated with suitable horseradish peroxidase (HRP)-conjugated secondary antibody. Membranes were further washed four times with TBST, incubated with an ECL solution (Millipore, USA), and digitally imaged with a charge-coupled device (CCD) camera.
TUNEL staining.
For the detection of apoptosis, paraffin-embedded sections were stained by a terminal dUTP nick-end labeling (TUNEL) technique using a TUNEL detection kit (KeyGEN BioTECH, Nanjing, China) according to the manufacturer's protocols.
Statistics.
Results are expressed as means ± standard deviations; one-way analysis of variance (ANOVA) and the Student-Newman-Keuls post hoc test were used to determine differences between the results for treated and control animals. The criterion for significance was a P value of <0.05 for all comparisons.
RESULTS
LPS had synergistic effects on INH-induced hepatic injury.
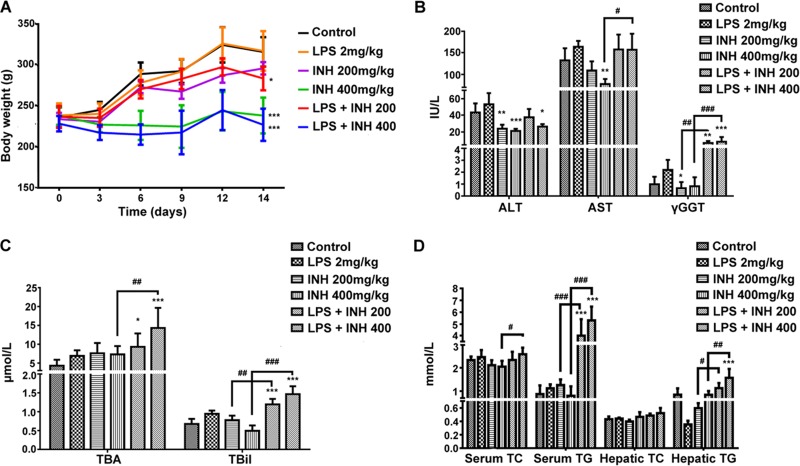
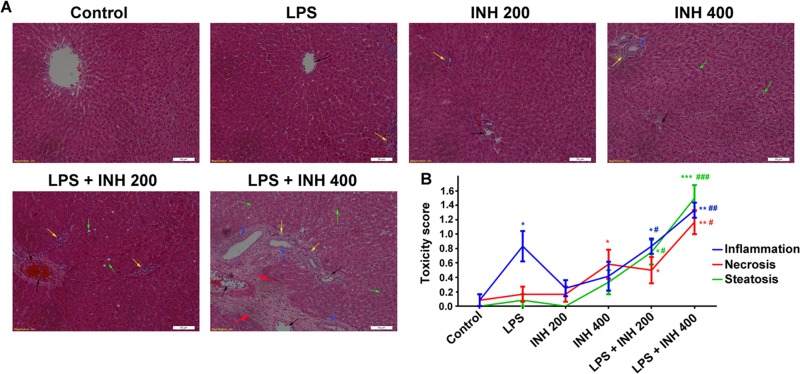
Body weight was significantly reduced by the INH-LPS combination (Fig. 1A). In the meantime, serum AST, TBA, TBil, and γGGT levels were significantly raised compared to those of INH-only groups, with the highest increase observed with the INH (400 mg/kg)-LPS cotreatment (Fig. 1B and C). Interestingly, the serum ALT level, as a major marker of liver injury, was significantly reduced in INH-treated animals, while in the INH-LPS-cotreated animals, it initially increased but was still below the control group level (Fig. 1B). In addition, both serum and hepatic TG and TC levels showed no changes compared to those of the control group after administration of INH or LPS alone, whereas the combined drugs significantly elevated TG and TC levels in a dose-dependent manner (Fig. 1D). Moreover, histological assessment revealed that administration of either LPS or INH alone had a minor effect on normal liver histology (Fig. 2A), while intense micro- and macrovesicular steatosis, severe hepatocellular necrosis, and inflammatory infiltration were observed in INH-LPS-cotreated rats, results which are associated with a significant difference in their histological scores (Fig. 2B).
FIG 1.
Liver injury parameters induced by INH-LPS cotreatment. Rats were treated with 200 or 400 mg/kg INH for 14 consecutive days, and at day 14, they received 2 mg/kg LPS, followed by INH 2 h later. (A) Effects on body weight; (B) changes in serum ALT, AST, and γGGT levels; (C) influence on serum TBA and TBil levels; (D) variations in both serum and hepatic lipid profiles. Data are given as means ± SD, n = 6 for each bar. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (versus results for the control). #, P < 0.05; ##, P < 0.01; ###, P < 0.001 (versus results in the absence of LPS).
FIG 2.
Liver histopathological examination after INH-LPS cotreatment. (A) Liver slices were collected and subjected to staining with hematoxylin and eosin. The control group shows normal hepatocyte architecture, the animals treated with either LPS or INH alone show minor alterations, and INH-LPS-cotreated animals show severe toxicity symptoms. Inflammatory cells (black arrows), inflammatory infiltration (yellow arrows), bile duct hyperplasia (blue arrows), micro- and macrovesicular steatosis (green arrows), and massive necrosis and hepatocellular structure loss (red arrows) are indicated. (B) INH hepatotoxicity score in the absence or presence of LPS. Data are given as means ± SD, n = 6 for each bar. *, P < 0.05; **, P < 0.01, ***, P < 0.001 (versus results for the control); #, P < 0.05; ##, P < 0.01; ###, P < 0.001 (versus results in the absence of LPS).
Effects on oxidative stress.
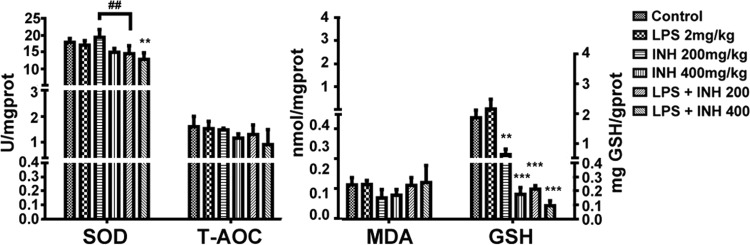
Both INH and LPS are known individually to generate, induce, and potentiate oxidative stress (25 – 27). In order to evaluate whether LPS could potentiate INH-induced oxidative stress, we analyzed certain hepatic parameters involved in ROS production. As shown in Fig. 3, we found that the INH-LPS combination marginally decreased the hepatic SOD level, with a significant reduction observed in INH (400 mg/kg)-LPS-cotreated rats, while a minor, insignificant reduction was observed in the overall liver T-AOC. Meanwhile, analysis of MDA, as one of the major lipid peroxidation parameters, showed reduced levels in the groups treated with INH alone but an increase in INH-LPS-cotreated groups, although this elevation was insignificant. For hepatic GSH, the major intracellular antioxidant defense mechanism, there was an increase in the group treated with LPS alone, in contrast to a significant gradual reduction among the other groups, with the greatest inhibition occurring in INH-LPS-cotreated animals. These results highlight the positive role that is played by oxidative stress in INH hepatotoxicity, with LPS augmenting this action.
FIG 3.
INH and LPS cause modifications in oxidative stress and hepatic antioxidant defense mechanisms. Changes in SOD, T-AOC, MDA, and GSH levels were measured by their respective kits. Data are given as means ± SD, n = 6 for each bar. **, P < 0.01; ***, P < 0.001 (versus results for the control); ##, P < 0.01 (versus results in the absence of LPS).
Gene profile associated with INH-induced hepatotoxicity.
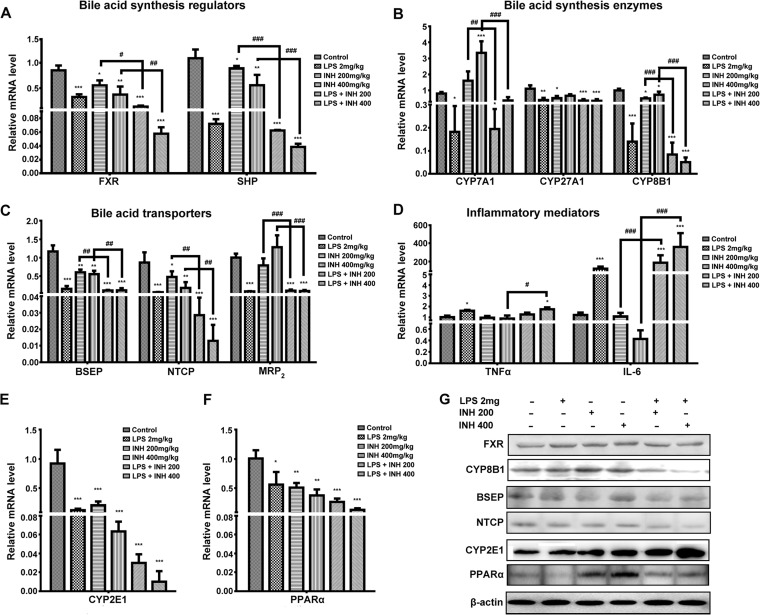
As the total bile acid levels in serum were significantly elevated following administration of INH-LPS cotreatment, we analyzed the expression of certain genes that participate in the synthesis and regulation of bile acids. Our results demonstrated that expression levels of both farnesoid X receptor (FXR) and small heterodimer partner (SHP) genes were gradually reduced in groups treated with INH alone, while LPS coadministration severely diminished the FXR and SHP gene expression levels (Fig. 4A). This diminished FXR activity triggered bile acid overproduction due to the loss of FXR control of the bile acid synthesis pathway. Interestingly, expression levels of genes responsible for bile acid synthesis, namely, the CYP7A1, CYP27A1, and CYP8B1 genes, were significantly decreased in INH-LPS-cotreated animals, although CYP7A1 gene expression showed a significant dose-dependent elevation in the group treated with INH alone, which was consistent with FXR reduction (Fig. 4B). The reduction in gene expression levels appearing in INH-LPS-cotreated animals might occur as a negative-feedback consequence following the initial increase in bile acid level in hepatocytes (28). In the meantime, the reduction in both CYP7A1 and CYP8B1 correlated with increases in hepatic TG and TC, as these enzymes are responsible for bile acid synthesis from cholesterol (29) and thus reduction of either one or both of these enzymes will definitely elevate the TG and TC levels.
FIG 4.
Effects of INH administration on different targeted genes and proteins in the presence or absence of LPS. (A) Expression of bile acid regulators FXR and SHP. (B) Expression of bile acid synthesis enzymes CYP7A1, CYP27A1, and CYP8B1. (C) Expression of bile acid transporters BSEP, NTCP, and MRP2. (D) Expression of inflammatory mediators TNF-α and IL-6. (E) Expression of CYP2E1. (F) Expression of PPARα. Data are given as means ± SD, n = 6 for each bar. *, P < 0.05; **, P < 0.01, ***, P < 0.001 (versus results for the control); #, P < 0.05; ##, P < 0.01; ###, P < 0.001 (versus results in the absence of LPS). The GAPDH gene was set as a reference. (G) Immunoblotting analysis of different targeted proteins following INH-LPS cotreatment, with β-actin considered a loading control.
On the other hand, expression levels of bile acid transporters, namely, bile salt export pump (BSEP), Na+-taurocholate cotransporting polypeptide (NTCP), and multidrug resistance-associated protein 2 (MRP2), were also significantly reduced in INH-LPS-cotreated groups compared to those of the control and INH-only animals (Fig. 4C). The reduction of these bile acid transporters, mainly as a result of combined activity with LPS, allowed accumulation of toxic bile acids in hepatocytes, which triggered cellular necrosis and the release of liver cellular contents, including bile acid, into serum, thus explaining the increased serum TBA level.
Even though the participation of inflammatory mediators and the immune system in the pathogenesis of INH-induced liver toxicity was inconsistent and varied, our results showed the positive role played by inflammation and the immune system in the propagation of INH toxicity. Figure 4D illustrates that both tumor necrosis factors alpha (TNF-α) and interleukin 6 (IL-6) showed incremental increases in expression in INH-LPS-cotreated rats, even higher than in the LPS-only group.
Researchers are familiar with the cornerstone role played by CYP2E1 in INH toxicity. Unexpectedly, CYP2E1 gene expression was severely diminished in almost all of the animal groups compared to the control (Fig. 4E), with more pronounced decreases in INH-LPS-cotreated animals. On the other hand, peroxisome proliferator-activated receptor alpha (PPARα), which is highly expressed in hepatocytes and responsible for lipid metabolism, showed a progressive decrease among the groups of treated animals, with the highest reduction observed after administration of the INH-LPS combination (Fig. 4F).
Western blot assessment of relevant protein expression.
In correlation with their gene expression levels, reductions in the expression of FXR, CYP8B1, BSEP, and NTCP proteins are shown in Fig. 4G. In contrast, CYP2E1 protein expression was progressively elevated among the treated groups, with the greatest increase in the INH-LPS-cotreated groups. This finding further supports the role of CYP2E1 in explaining INH hepatotoxicity. In addition, significant downregulation of PPARα protein expression was also noticed, especially with a 400-mg INH dose combined with LPS.
Apoptosis in INH hepatotoxicity.
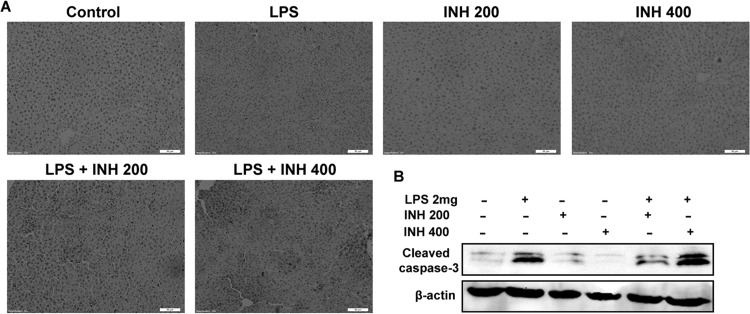
Apoptotic induction is still considered a major pathway for INH toxicity (30). Therefore, we tested the potential of INH-induced apoptosis. We found that hepatic cleavage caspase-3 protein levels, as an indicator of cellular apoptosis, were provoked by the INH-LPS combination, while INH-only-treated groups showed minor protein levels (Fig. 5A). For further apoptotic confirmation, a TUNEL assay was conducted using liver slices. Results indicated that neither LPS-only treatment nor INH-only treatment led to apoptosis, but when they were combined, a positive TUNEL test indicating apoptosis was observed (Fig. 5B).
FIG 5.
INH-LPS cotreatment causes apoptosis. (A) A TUNEL assay was conducted on liver slices, and treatment with either LPS or INH alone showed no apoptotic effects, while INH-LPS cotreatment caused obvious positive TUNEL results (intensity of color indicating apoptotic action). (B) Representative Western blot analysis of cleaved caspase-3, with β-actin considered a loading control.
DISCUSSION
Progress in finding an exact mechanism for and establishing a suitable animal model of INH-induced hepatotoxicity has faced many obstacles (31). In accordance with this challenge, the present study aimed to prove our hypothesis that coexposure to INH and LPS intensifies INH-induced liver injury.
Previous studies have reported that exposure to low, injury-free LPS concentrations that are unable to cause tissue or cellular damage increases drug intoxication liabilities (32, 33). Therefore, animal models that follow the inflammatory stress theory, in which nontoxic LPS doses potentiate drug toxicities, were of great help in mechanistically elaborating a general picture of DILI.
This study was conducted entirely with male rats and followed our 14-day protocol; liver injury was confirmed by blood biochemical analysis. Increases in transaminase levels have long been considered the major signs of INH-induced hepatotoxicity, with up to 20% of patients usually suffering from elevated ALT levels (34); in contrast with those previous reports, we observed that either acute (1-week) or subchronic (2-week) administration of INH led to reductions in ALT, AST, and alkaline phosphatase (ALP). On the other hand, increased time exposure to INH (4 weeks) was characterized by significant and severe increases in transaminase levels (data not shown). Some previously mentioned results (23, 35, 36) were in line with our findings, in which serum and hepatic levels of ALT and AST were reduced. This might be correlated with the duration of INH administration; increased exposure time to INH will elevate transaminase levels, although both decreased and increased levels are considered biomarkers for INH-induced liver injury. Nevertheless, a diagnosis of INH hepatotoxicity that depends solely on the serum level of transaminases may be inaccurate in accordance with these findings. A finding of INH hepatotoxicity was further fortified by histopathological evaluation of liver samples, which showed that massive necrosis and steatosis associated with inflammatory cell infiltrations occurred in INH-LPS-cotreated animal groups. The massive necrosis phenomenon that was seen might be attributed to INH-induced lipid peroxidation (7), which was enhanced by LPS inflammatory action. In line with this, Sarich et al. previously reported that INH caused hepatic necrosis and steatosis in rabbits (37).
Blocking the FXR regulatory role of the bile acid synthesis cascade from cholesterol resulted in the elevation of the bile acid level (29, 38). Bile acid transporters, namely, BSEP, NTCP, and MRP2, play a key role in bile acid homeostasis (39, 40), while inhibition of the bile acid transporters BSEP and NTCP was suggested to participate in INH-induced liver injury pathogenesis (41). Our preceding study (22) showed that the INH-LPS combination elevated the expression of the bile acid-synthesizing enzyme (CYP7A1) while the expression of transporters (BSEP, NTCP, and MRP2) was significantly reduced. Due to treatment duration differences, we could determine the exact effect of INH-LPS in relation to bile acids as follows: in response to LPS-induced inflammation and released inflammatory cytokines, expression of the key regulatory transcriptional factor, the FXR gene, was repressed, causing increased production of bile acids. In the meantime, inflammation induced the rapid reduction in bile acid transporters, which caused the elevation and entrapment of bile acids in hepatocytes (42 – 45). This massive accumulation of bile acids in hepatocytes might initiate a negative-feedback mechanism that results in the observed gene expression reduction. Simultaneously, accumulation of toxic intracellular bile acid levels resulted in cell death, necrosis, and induction of inflammatory mediators (46, 47). We supposed that necrosis and leakage of hepatocellular contents explained the increased serum TBA levels despite the CYP7A1 and CYP8B1 gene repression in INH-LPS-cotreated animals. Similarly, the combination caused apoptotic hepatocyte death that was already confirmed by a caspase-3 test and TUNEL assay. In contrast to our findings, LPS was found previously to inhibit caspase-3-dependent apoptosis (48), but many previous researchers were in agreement with our findings that LPS potentiated the apoptosis-inducing abilities (49). Together, we could attribute the hepatocyte death to both necrotic and apoptotic actions of the INH-LPS combination.
Our results showed significant reductions in liver antioxidant defense mechanisms, especially GSH, SOD, and T-AOC, while MDA was elevated. In line with these findings, Enriquez-Cortina et al. reported that oxidative stress and inhibition of hepatic antioxidant activities were the key determinants of INH-induced hepatotoxicity (50). Additionally, the hepatic microsomal CYP450 system, mainly CYP2E1, is considered a central role player in the ROS generation pathway, either directly or as a by-product of its metabolic activities through the generation of reactive metabolites (51, 52). As experimentally evident, our obtained results revealed that LPS augmented INH-induced CYP2E1 protein expression levels while repressing CYP2E1 gene expression. This repressive effect on CYP2E1 gene expression could be attributed to LPS-induced inflammation; Abdulla et al. and Hakkola et al. supported this hypothesis (53, 54). Meanwhile, earlier researchers reported CYP2E1 participation in hepatotoxicity by expanding its role in the elevation of INH hepatotoxicity parameters (55, 56). Similarly, CYP2E1 was found to increase liver sensitivity toward LPS and inflammatory mediator toxicity and to potentiate LPS-induced oxidative stress in the liver (57, 58); therefore, both INH and LPS caused overactivation of CYP2E1, which, in return, intensified their toxicity. CYP2E1 involvement in INH toxicity is not limited just to the liver: Shayakhmetova et al. declared that the induction of CYP2E1 in rat testicular tissues treated with INH resulted in the triggering and accumulation of ROS that led to testicular toxicity, DNA fragmentation, spermatogenesis disturbances, and male infertility (59). CYP2E1 also had a functional role in bile acid increment through activation of the bile acid synthesis cascade (56). Furthermore, the effects of CYP2E1 on triglyceride accumulation, apoptotic induction, and hepatic steatosis were previously mentioned and extensively studied (60 – 62). Hence, it appears that CYP2E1 has a central participation in all INH hepatotoxicity symptoms, including necrosis and steatosis.
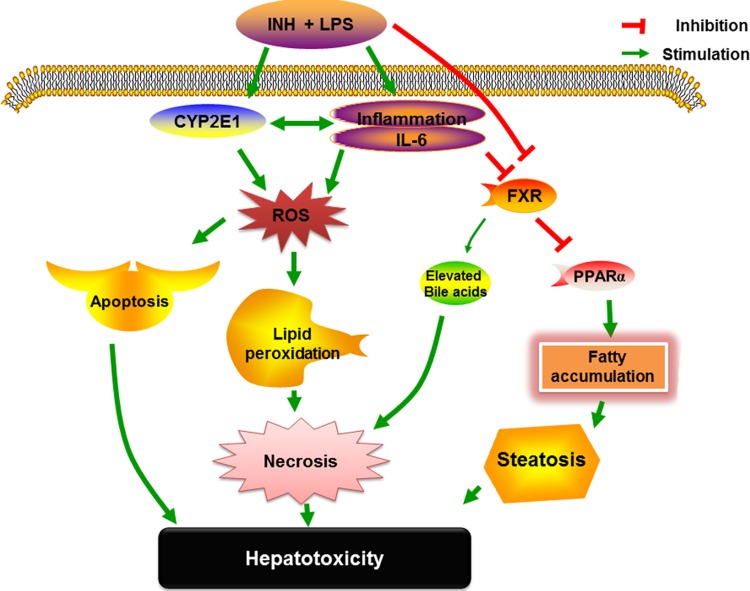
Highly active metabolic tissues, including the liver, are characterized by the overexpression of PPARα, which plays different roles as an anti-inflammatory and a key modulator of hepatocyte lipid metabolism (63, 64). In line with this, FXR protects the liver from fat accumulation through potentiation of PPARα-lipid regulatory effects (28). Therefore, the reduction in PPARα activity that occurred due to reduced FXR, inflammation, or INH-LPS cotreatment will promote further inflammation and lipid metabolism disturbances, which explains the elevated level of inflammatory mediators and the accumulation of triglycerides in liver tissues. This reduction, in association with CYP7A1 and CYP8B1 gene repression, explains the elevation of both hepatic TG and TC levels that was experimentally observed in rats and may also provide an explanation for the severe steatosis that appeared in hepatic tissues after INH-LPS cotreatment. Our overall speculations on the hepatotoxicity mechanisms of INH-LPS, as well as the impact of CYP2E1, inflammatory cytokines, and PPARα, are fully illustrated in Fig. 6.
FIG 6.
Schematic presentation highlighting the proposed mechanisms by which the INH-LPS combination induces hepatotoxicity.
In summary, our results revealed that individual LPS or INH treatment caused minor toxic effects, while cotreatment with INH and LPS provoked INH-induced hepatotoxicity, which was manifested mainly by the elevation of hepatotoxicity biomarkers and lipid metabolic disturbances associated with severe liver necrosis and steatosis. CYP2E1 expression that was fortified by both INH and LPS endotoxins acted as a rate-limiting key in the initiation and propagation of the liver injury. Our study provides clues that may help uncover the multiple concealed mechanisms behind INH hepatotoxicity, for which coadministration of LPS serves as a good model of DILI that could help clinicians understand its pathogenesis.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grants 81273604, 81320108029, and 81573514) and the Natural Science Foundation of Jiangsu Province (BK20151439); this study was partially supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). In addition, this study was supported by the National “Major Scientific and Technological Special Project for Significant New Drugs” project (2015ZX09501004-002-004) and by the Specific Fund for Public Interest Research of Traditional Chinese Medicine, Ministry of Finance (201507004-002).
We declare that there is no conflict of interest.
REFERENCES
- 1.Bhadauria S, Mishra R, Kanchan R, Tripathi C, Srivastava A, Tiwari A, Sharma S. 2010. Isoniazid-induced apoptosis in HepG2 cells: generation of oxidative stress and Bcl-2 down-regulation. Toxicol Mech Methods 20:242–251. doi: 10.3109/15376511003793325. [DOI] [PubMed] [Google Scholar]
- 2.Lee KK, Fujimoto K, Zhang C, Schwall CT, Alder NN, Pinkert CA, Krueger W, Rasmussen T, Boelsterli UA. 2013. Isoniazid-induced cell death is precipitated by underlying mitochondrial complex I dysfunction in mouse hepatocytes. Free Radic Biol Med 65:584–594. doi: 10.1016/j.freeradbiomed.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 3.Metushi IG, Uetrecht J. 2014. Isoniazid-induced liver injury and immune response in mice. J Immunotoxicol 11:383–392. doi: 10.3109/1547691X.2013.860644. [DOI] [PubMed] [Google Scholar]
- 4.Singh M, Sasi P, Rai G, Gupta V, Amarapurkar D, Wangikar P. 2011. Studies on toxicity of antitubercular drugs namely isoniazid, rifampicin, and pyrazinamide in an in vitro model of HepG2 cell line. Med Chem Res 20:1611–1615. doi: 10.1007/s00044-010-9405-3. [DOI] [Google Scholar]
- 5.Liu K, Li F, Lu J, Gao Z, Klaassen CD, Ma X. 2014. Role of CYP3A in isoniazid metabolism in vivo. Drug Metab Pharmacokinet 29:219–222. doi: 10.2133/dmpk.DMPK-13-NT-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metushi IG, Cai P, Zhu X, Nakagawa T, Uetrecht JP. 2011. A fresh look at the mechanism of isoniazid-induced hepatotoxicity. Clin Pharmacol Ther 89:911–914. doi: 10.1038/clpt.2010.355. [DOI] [PubMed] [Google Scholar]
- 7.Hassan HM, Guo HL, Yousef BA, Luyong Z, Zhenzhou J. 2015. Hepatotoxicity mechanisms of isoniazid: a mini-review. J Appl Toxicol 35:1427–1432. doi: 10.1002/jat.3175. [DOI] [PubMed] [Google Scholar]
- 8.Roth RA, Harkema JR, Pestka JP, Ganey PE. 1997. Is exposure to bacterial endotoxin a determinant of susceptibility to intoxication from xenobiotic agents? Toxicol Appl Pharmacol 147:300–311. doi: 10.1006/taap.1997.8301. [DOI] [PubMed] [Google Scholar]
- 9.Au JS, Navarro VJ, Rossi S. 2011. Review article: drug-induced liver injury—its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther 34:11–20. doi: 10.1111/j.1365-2036.2011.04674.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaw PJ, Hopfensperger MJ, Ganey PE, Roth RA. 2007. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol Sci 100:259–266. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- 11.Barton CC, Hill DA, Yee SB, Barton EX, Ganey PE, Roth RA. 2000. Bacterial lipopolysaccharide exposure augments aflatoxin B(1)-induced liver injury. Toxicol Sci 55:444–452. doi: 10.1093/toxsci/55.2.444. [DOI] [PubMed] [Google Scholar]
- 12.Luyendyk JP, Shores KC, Ganey PE, Roth RA. 2002. Bacterial lipopolysaccharide exposure alters aflatoxin B(1) hepatotoxicity: benchmark dose analysis for markers of liver injury. Toxicol Sci 68:220–225. doi: 10.1093/toxsci/68.1.220. [DOI] [PubMed] [Google Scholar]
- 13.Deng X, Luyendyk JP, Ganey PE, Roth RA. 2009. Inflammatory stress and idiosyncratic hepatotoxicity: hints from animal models. Pharmacol Rev 61:262–282. doi: 10.1124/pr.109.001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchweitz JP, Ganey PE, Bursian SJ, Roth RA. 2002. Underlying endotoxemia augments toxic responses to chlorpromazine: is there a relationship to drug idiosyncrasy? J Pharmacol Exp Ther 300:460–467. doi: 10.1124/jpet.300.2.460. [DOI] [PubMed] [Google Scholar]
- 15.Zou W, Roth RA, Younis HS, Burgoon LD, Ganey PE. 2010. Oxidative stress is important in the pathogenesis of liver injury induced by sulindac and lipopolysaccharide cotreatment. Toxicology 272:32–38. doi: 10.1016/j.tox.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao CV, Rawat AKS, Singh AP, Singh A, Verma N. 2012. Hepatoprotective potential of ethanolic extract of Ziziphus oenoplia (L.) Mill roots against antitubercular drugs induced hepatotoxicity in experimental models. Asian Pac J Trop Med 5:283–288. doi: 10.1016/S1995-7645(12)60040-6. [DOI] [PubMed] [Google Scholar]
- 17.Shen C, Zhang G, Meng Q. 2007. An in vitro model for long-term hepatotoxicity testing utilizing rat hepatocytes entrapped in micro-hollow fiber reactor. Biochem Eng J 34:267–272. doi: 10.1016/j.bej.2006.12.010. [DOI] [Google Scholar]
- 18.Wei L, Ren F, Zhang X, Wen T, Shi H, Zheng S, Zhang J, Chen Y, Han Y, Duan Z. 2014. Oxidative stress promotes d-GalN/LPS-induced acute hepatotoxicity by increasing glycogen synthase kinase 3beta activity. Inflamm Res 63:485–494. doi: 10.1007/s00011-014-0720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. 2011. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol 35:630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez FJ. 2005. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res 569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Yue J, Peng R. 2009. Does CYP2E1 play a major role in the aggravation of isoniazid toxicity by rifampicin in human hepatocytes? Br J Pharmacol 157:331–333. doi: 10.1111/j.1476-5381.2009.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su Y, Zhang Y, Chen M, Jiang Z, Sun L, Wang T, Zhang L. 2014. Lipopolysaccharide exposure augments isoniazide-induced liver injury. J Appl Toxicol 34:1436–1442. doi: 10.1002/jat.2979. [DOI] [PubMed] [Google Scholar]
- 23.Metushi IG, Nakagawa T, Uetrecht J. 2012. Direct oxidation and covalent binding of isoniazid to rodent liver and human hepatic microsomes: humans are more like mice than rats. Chem Res Toxicol 25:2567–2576. doi: 10.1021/tx300341r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clayton TA, Lindon JC, Everett JR, Charuel C, Hanton G, Le Net JL, Provost JP, Nicholson JK. 2007. Hepatotoxin-induced hypertyrosinemia and its toxicological significance. Arch Toxicol 81:201–210. doi: 10.1007/s00204-006-0136-7. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Xu J, Zhang C, Yu T, Wang H, Zhao M, Duan ZH, Zhang Y, Xu JM, Xu DX. 2011. The protective effects of ursodeoxycholic acid on isoniazid plus rifampicin induced liver injury in mice. Eur J Pharmacol 659:53–60. doi: 10.1016/j.ejphar.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Sekine S, Yano K, Saeki J, Hashimoto N, Fuwa T, Horie T. 2010. Oxidative stress is a triggering factor for LPS-induced Mrp2 internalization in the cryopreserved rat and human liver slices. Biochem Biophys Res Commun 399:279–285. doi: 10.1016/j.bbrc.2010.07.069. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Xue P, Hou Y, Zhang H, Zheng H, Zhou T, Qu W, Teng W, Zhang Q, Andersen ME, Pi J. 2013. Isoniazid suppresses antioxidant response element activities and impairs adipogenesis in mouse and human preadipocytes. Toxicol Appl Pharmacol 273:435–441. doi: 10.1016/j.taap.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Kunne C, Acco A, Duijst S, de Waart DR, Paulusma CC, Gaemers I, Oude Elferink RPJ. 2014. FXR-dependent reduction of hepatic steatosis in a bile salt deficient mouse model. Biochim Biophys Acta 1842:739–746. doi: 10.1016/j.bbadis.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Gardes C, Chaput E, Staempfli A, Blum D, Richter H, Benson GM. 2013. Differential regulation of bile acid and cholesterol metabolism by the farnesoid X receptor in Ldlr−/− mice versus hamsters. J Lipid Res 54:1283–1299. doi: 10.1194/jlr.M033423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perwitasari DA, Atthobari J, Wilffert B. 2015. Pharmacogenetics of isoniazid-induced hepatotoxicity. Drug Metab Rev 47:222–228. doi: 10.3109/03602532.2014.984070. [DOI] [PubMed] [Google Scholar]
- 31.Sarich TC, Shou T, Adams SP, Bain AI, Wall RA, Wright JM. 1995. A model of isoniazid-induced hepatotoxicity in rabbits. J Pharmacol Toxicol Methods 34:109–116. doi: 10.1016/1056-8719(95)00044-I. [DOI] [PubMed] [Google Scholar]
- 32.Luyendyk JP, Maddox JF, Green CD, Ganey PE, Roth RA. 2004. Role of hepatic fibrin in idiosyncrasy-like liver injury from lipopolysaccharide-ranitidine coexposure in rats. Hepatology 40:1342–1351. doi: 10.1002/hep.20492. [DOI] [PubMed] [Google Scholar]
- 33.Deng X, Stachlewitz RF, Liguori MJ, Blomme EA, Waring JF, Luyendyk JP, Maddox JF, Ganey PE, Roth RA. 2006. Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation. J Pharmacol Exp Ther 319:1191–1199. doi: 10.1124/jpet.106.110247. [DOI] [PubMed] [Google Scholar]
- 34.Boelsterli UA, Lee KK. 2014. Mechanisms of isoniazid-induced idiosyncratic liver injury: emerging role of mitochondrial stress. J Gastroenterol Hepatol 29:678–687. doi: 10.1111/jgh.12516. [DOI] [PubMed] [Google Scholar]
- 35.Karthikeyan S. 2004. Hepatotoxicity of isoniazid: a study on the activity of marker enzymes of liver toxicity in serum and liver tissue of rabbits. Indian J Pharmacol 36:247–249. [Google Scholar]
- 36.Jahan S, Khan M, Imran S, Sair M. 2015. The hepatoprotective role of Silymarin in isoniazid induced liver damage of rabbits. J Pak Med Assoc 65:620–622. [PubMed] [Google Scholar]
- 37.Sarich TC, Adams SP, Zhou T, Wright JM. 1997. Isoniazid-induced hepatic necrosis and steatosis in rabbits: absence of effect of gender. Can J Physiol Pharmacol 75:1108–1111. doi: 10.1139/y97-144. [DOI] [PubMed] [Google Scholar]
- 38.Matsubara T, Li F, Gonzalez FJ. 2013. FXR signaling in the enterohepatic system. Mol Cell Endocrinol 368:17–29. doi: 10.1016/j.mce.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubitz R, Droge C, Stindt J, Weissenberger K, Haussinger D. 2012. The bile salt export pump (BSEP) in health and disease. Clin Res Hepatol Gastroenterol 36:536–553. doi: 10.1016/j.clinre.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Halilbasic E, Claudel T, Trauner M. 2013. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol 58:155–168. doi: 10.1016/j.jhep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo YX, Xu XF, Zhang QZ, Li C, Deng Y, Jiang P, He LY, Peng WX. 2015. The inhibition of hepatic bile acids transporters Ntcp and Bsep is involved in the pathogenesis of isoniazid/rifampicin-induced hepatotoxicity. Toxicol Mech Methods 25:382–387. doi: 10.3109/15376516.2015.1033074. [DOI] [PubMed] [Google Scholar]
- 42.Wu WB, Xu YY, Cheng WW, Wang YX, Liu Y, Huang D, Zhang HJ. 2015. Agonist of farnesoid X receptor protects against bile acid induced damage and oxidative stress in mouse placenta—a study on maternal cholestasis model. Placenta 36:545–551. doi: 10.1016/j.placenta.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Meng Q, Chen XL, Wang CY, Liu Q, Sun HJ, Sun PY, Huo XK, Liu ZH, Yao JH, Liu KX. 2015. Alisol B 23-acetate protects against ANIT-induced hepatotoxity and cholestasis, due to FXR-mediated regulation of transporters and enzymes involved in bile acid homeostasis. Toxicol Appl Pharmacol 283:178–186. doi: 10.1016/j.taap.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 44.Pollheimer MJ, Fickert P, Stieger B. 2014. Chronic cholestatic liver diseases: clues from histopathology for pathogenesis. Mol Aspects Med 37:35–56. doi: 10.1016/j.mam.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Vanova K, Suk J, Petr T, Cerny D, Slanar O, Vreman HJ, Wong RJ, Zima T, Vitek L, Muchova L. 2014. Protective effects of inhaled carbon monoxide in endotoxin-induced cholestasis is dependent on its kinetics. Biochimie 97:173–180. doi: 10.1016/j.biochi.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Allen K, Jaeschke H, Copple BL. 2011. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol 178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolbright BL, Dorko K, Antoine DJ, Clarke JI, Gholami P, Li F, Kumer SC, Schmitt TM, Forster J, Fan F, Jenkins RE, Park BK, Hagenbuch B, Olyaee M, Jaeschke H. 2015. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol 283:168–177. doi: 10.1016/j.taap.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russe OQ, Moser CV, Kynast KL, King TS, Olbrich K, Grosch S, Geisslinger G, Niederberger E. 2014. LPS inhibits caspase 3-dependent apoptosis in RAW264.7 macrophages induced by the AMPK activator AICAR. Biochem Biophys Res Commun 447:520–525. doi: 10.1016/j.bbrc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Depboylu B, Giris M, Olgac V, Dogru-Abbasoglu S, Uysal M. 2013. Response of liver to lipopolysaccharide treatment in male and female rats. Exp Toxicol Pathol 65:645–650. doi: 10.1016/j.etp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Enriquez-Cortina C, Almonte-Becerril M, Clavijo-Cornejo D, Palestino-Dominguez M, Bello-Monroy O, Nuno N, Lopez A, Bucio L, Souza V, Hernandez-Pando R, Munoz L, Gutierrez-Ruiz MC, Gomez-Quiroz LE. 2013. Hepatocyte growth factor protects against isoniazid/rifampicin-induced oxidative liver damage. Toxicol Sci 135:26–36. doi: 10.1093/toxsci/kft134. [DOI] [PubMed] [Google Scholar]
- 51.Zhai Q, Lu S, Lin Y, Yang Q, Yu B. 2008. Oxidative stress potentiated by diallylsulfide, a selective CYP2E1 inhibitor, in isoniazid toxic effect on rat primary hepatocytes. Toxicol Lett 183:95–98. doi: 10.1016/j.toxlet.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Santos NP, Callegari-Jacques SM, Ribeiro Dos Santos AK, Silva CA, Vallinoto AC, Fernandes DC, de Carvalho DC, Santos SE, Hutz MH. 2013. N-acetyl transferase 2 and cytochrome P450 2E1 genes and isoniazid-induced hepatotoxicity in Brazilian patients. Int J Tuberc Lung Dis 17:499–504. doi: 10.5588/ijtld.12.0645. [DOI] [PubMed] [Google Scholar]
- 53.Abdulla D, Goralski KB, Renton KW. 2006. The regulation of cytochrome P450 2E1 during LPS-induced inflammation in the rat. Toxicol Appl Pharmacol 216:1–10. doi: 10.1016/j.taap.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Hakkola J, Hu Y, Ingelman-Sundberg M. 2003. Mechanisms of down-regulation of CYP2E1 expression by inflammatory cytokines in rat hepatoma cells. J Pharmacol Exp Ther 304:1048–1054. [DOI] [PubMed] [Google Scholar]
- 55.Yue J, Peng RX, Yang J, Kong R, Liu J. 2004. CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta Pharmacol Sin 25:699–704. [PubMed] [Google Scholar]
- 56.Cheng J, Krausz KW, Li F, Ma X, Gonzalez FJ. 2013. CYP2E1-dependent elevation of serum cholesterol, triglycerides, and hepatic bile acids by isoniazid. Toxicol Appl Pharmacol 266:245–253. doi: 10.1016/j.taap.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Y, Cederbaum AI. 2010. CYP2E1 potentiation of LPS and TNFalpha-induced hepatotoxicity by mechanisms involving enhanced oxidative and nitrosative stress, activation of MAP kinases, and mitochondrial dysfunction. Genes Nutr 5:149–167. doi: 10.1007/s12263-009-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cederbaum AI, Yang L, Wang X, Wu D. 2012. CYP2E1 sensitizes the liver to LPS- and TNF alpha-induced toxicity via elevated oxidative and nitrosative stress and activation of ASK-1 and JNK mitogen-activated kinases. Int J Hepatol 2012:582790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shayakhmetova GM, Bondarenko LB, Voronina AK, Anisimova SI, Matvienko AV, Kovalenko VM. 2015. Induction of CYP2E1 in testes of isoniazid-treated rats as possible cause of testicular disorders. Toxicol Lett 234:59–66. doi: 10.1016/j.toxlet.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Wu D, Wang X, Zhou R, Cederbaum A. 2010. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophaghy in HepG2 E47 cells. Biochem Biophys Res Commun 402:116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cederbaum AI. 2010. Role of CYP2E1 in ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig Dis 28:802–811. doi: 10.1159/000324289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. 2012. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol 57:860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beuers U, Trauner M, Jansen P, Poupon R. 2015. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol 62:S25–S37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 64.Abdelmegeed MA, Moon KH, Hardwick JP, Gonzalez FJ, Song BJ. 2009. Role of peroxisome proliferator-activated receptor-alpha in fasting-mediated oxidative stress. Free Radic Biol Med 47:767–778. doi: 10.1016/j.freeradbiomed.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]