Abstract
Drug resistance studies have played an important role in the validation of antibiotic targets. In the case of the polyene antibiotic amphotericin B (AmB), such studies have demonstrated the essential role that depletion of ergosterol plays in the development of AmB-resistant (AmB-R) organisms. However, AmB-R strains also occur in fungi and parasitic protozoa that maintain a normal level of ergosterol at the plasma membrane. Here, I review evidence that shows not only that there is increased protection against the deleterious consequences of AmB-induced ion leakage across the membrane in these resistant pathogens but also that a set of events are activated that block the cell signaling responses that trigger the oxidative damage produced by the antibiotic. Such signaling events appear to be the consequence of a membrane-thinning effect that is exerted upon lipid-anchored Ras proteins by the aqueous pores formed by AmB. A similar membrane disturbance effect may also explain the activity of AmB on mammalian cells containing Toll-like receptors. These resistance mechanisms expand our current understanding of the role that the formation of AmB aqueous pores plays in triggering signal transduction responses in both pathogens and host immune cells.
INTRODUCTION
The preservation of the cell membrane as a permeability barrier is critical for cell survival. There are some amphiphilic compounds that disrupt membranes so drastically that rapid death occurs by lysis without the activation of cellular protective responses. Among such compounds are polyene macrolides such as filipin, etruscomycin, or natamycin (also known as pimaricin) produced by soil bacteria, all of which have in common the ability to selectively bind to membrane sterols (1). Self-aggregation of these amphiphilic drugs in water is often responsible for their toxic effects (2). The polyene antibiotic amphotericin B (AmB) added in the form of large aggregates can also be lethal to yeast cells as a result of extracting ergosterol from membranes (3). However, when the free monomeric forms predominate over large aggregates, AmB inserts spontaneously into ergosterol-containing membranes to form aqueous pores (4). The formation of aqueous pores by AmB has been linked to a diverse set of gradual responses leading to the emergence of resistance genes that protect the membrane against the disruptive effects of the antibiotic (5).
THE FORMATION OF ION CHANNELS BY AmB AND NYSTATIN
The development of planar lipid bilayers and liposomes as models of biological membranes played an important role in the recognition that the thickness of the lipid bilayer is the most important parameter for the formation of aqueous pores by AmB and nystatin (6 – 9, and 10). AmB is a rigid amphiphile molecule with a length of about 24 Å (11) that easily forms in one-sided ion channels of sterol-containing biological membranes in spite of the membranes having an average bilayer thickness of 35.6 to 42.5 Å (12). In fact, the difficulties that were initially found to complicate detection of ion channel activity in planar lipid bilayers treated at only one side with AmB (or nystatin) were resolved with the use of thinner model membranes that employed no hydrocarbon solvent or lipids with shorter carbon chain lengths (6 – 8). However, in contrast to the results seen with biological membranes, the presence of sterols in planar lipid bilayers is not an absolute requirement for the formation of ion channels by AmB (13). In this respect, it was shown by using an osmotic method that AmB forms two types of ion channels in liposomes and membrane vesicles prepared from sensitive pathogens (14, 15). One of those ion channels—referred to as the nonaqueous channel—is formed without a direct interaction with sterols (16). Thus, Coutinho et al. demonstrated that the mean fluorescence lifetime of nystatin increased sharply in liposomes prepared with ergosterol but only after a critical threshold of the membrane-bound antibiotic concentration was reached. In parallel experiments, those investigators showed that nystatin induced a moderate dissipation of the K+ gradient across the membrane at concentrations below the critical threshold value, an event that was followed by a complete dissipation of K+ gradient at greater concentrations (16). This set of results provided a clear indication that the formation of aqueous pores by nystatin occurred simultaneously with the fluorescence enhancement by the ergosterol complexation.
The nonaqueous channels formed by AmB have a hydrophilic water-filled core that allows the permeation of monovalent cations such as K+ or Na+ but are not able to span the bilayer, which was inferred from the observation that AmB increased the urea permeability without decreasing the osmotic reflection coefficient exerted by this solute across the membrane (see Figure 1 in reference 17). It was anticipated that the individual nonaqueous channels would exhibit much shorter lifetimes than the aqueous pores, since they do not span the lipid bilayer. This prediction was recently confirmed by using sterol-containing planar lipid bilayers prepared from PC with different chain lengths (18). Thus, Shatursky et al. found that in cholesterol-containing C18 carbon chain phosphatidylcholine (PC) bilayers, the ion channels formed by AmB have openings lasting only fractions of a second, whereas the ion channels formed in bilayers formed with C16 carbon chain PC lasted minutes. A similar increase in the lifetimes of one-sided AmB channels formed in ergosterol-containing membranes (bilayer lipid membranes [BLMs]) prepared with the C16 tail PC was achieved (18).
The validity of the 2-stage mechanism for the formation of aqueous pores by AmB has also been demonstrated by the finding that when a critical threshold concentration was reached at the liposome membranes, the urea osmotic reflection decreased to a value of <0.5 (see Figure 1 in reference 17), clearly indicating the formation of 4-Å-radius aqueous pores spanning the membrane (19).
THE FORMATION OF AmB ION CHANNELS IN THE NONRAFT AND RAFT MEMBRANE DOMAINS
All eukaryote membranes exhibit a laterally heterogeneous distribution of the main membrane sterols (fungal ergosterol or mammalian cholesterol) with enhanced enrichment in the ordered raft microdomains along with saturated PCs, sphingolipids, and sterol-binding proteins (20). It has been determined that there are thickness differences as large as 5.2 Å between the ordered rafts and nonraft domains of membranes (21). It is then likely that the AmB aqueous pores are preferentially localized at the boundary of raft and nonraft bilayer areas, as the threshold for the appearance of the long-lifetime aqueous pores occurred in bilayers prepared with C17 PC, which are about 3.6 Å thinner than C18 PC bilayers (18).
The nonaqueous AmB channels can also be inserted at the lipid rafts as suggested by the shift from negative to positive activation energies of the K+ permeability that was measured in human erythrocyte membranes treated with AmB concentrations higher than the threshold value at which aqueous pores are formed (22). In this regard, it is important that AmB also forms ion channels in sterol-free small liposomes (23), which have curvatures in the range predicted for locally curved nanoscale raft domains (24). It follows that nonaqueous channels can also be formed by AmB in the external monolayer leaflet of raft microdomains that have a higher membrane curvature. This is possibly due to the curvature-mediated relief in lateral pressure of the outer leaflet that may allow greater conformational freedom for the inserted nonaqueous AmB channels.
THE MOLECULAR MECHANISM OF SIGNAL TRANSDUCTION INDUCED BY THE FORMATION OF AmB AQUEOUS PORES
The insertion of nonaqueous AmB channels at the lipid rafts or at the nonraft areas of the membrane is followed by the sequestration of sterols, resulting in local membrane thinning to allow formation of the aqueous pores traversing the membrane (10). A local reduced thickness induced by the formation of AmB aqueous pores can be expected to affect the dynamics of nearby membrane proteins, as the bilayer's hydrophobic thickness matches the hydrophobic thickness of the segments of the protein transmembrane domains (TMs), in order to reduce the energetic cost associated with exposing a nonpolar/polar interface (25). It has been demonstrated that changes in the lipid bilayer thickness cause conformational changes that promote changes in the functional activity of several membrane proteins, including those associated with lipid rafts (25).
Among the proteins located in lipid rafts that are involved in signal transduction pathways are the small lipid-anchored Ras GTPases (26, 27). There are various Ras isoforms that have been implicated in AmB-induced yeast apoptosis and lethal oxidative damage via the cyclic AMP-protein kinase A (cAMP-PKA) and mitogen-activated protein kinase (MAPK) pathway (28 – 30). In fact, the AmB-induced lethal process in fungal cells can be prevented by deleting either RAS1 or RAS2 or the downstream PKA isoforms (TPK1, TPK2, and TPK3) that were shown to reduce AmB-dependent reactive oxygen species (ROS) production (30). Members of the Ras superfamily are posttranslationally modified at the carboxy-terminal end by prenylated and acylated lipid anchors, which are tethered to the inner cytosolic leaflet of the plasma membrane (31). Studies of surface-bound lipid-modified Ras peptides containing two saturated palmitoyl and one unsaturated farnesyl lipids have shown that such structures preferentially partitioned at raft microdomain boundaries (32, 33), causing perturbations such as membrane thinning by displacing lipid headgroups (34).
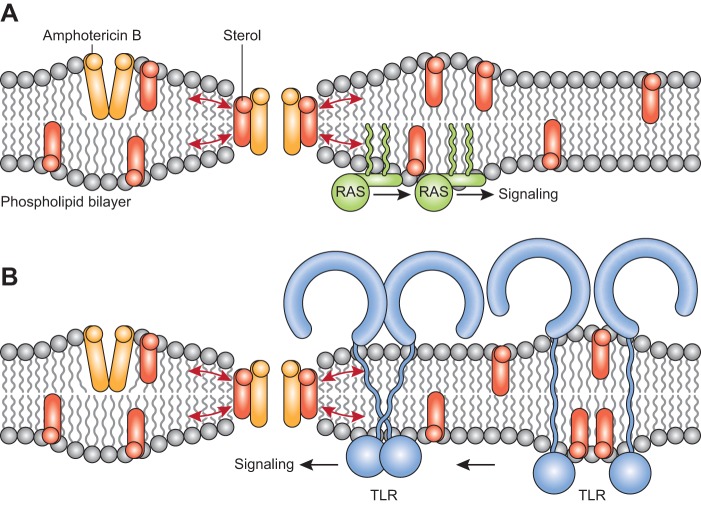
The colocalization at the boundary of lipid rafts of AmB aqueous pores with the lipid-anchored Ras proteins may be critical for amplifying the membrane-thinning effect exerted by the AmB aqueous pores forcing the inserted Ras proteins to segregate into the sterol-rich lipid rafts, facilitating its interactions with downstream protein effectors (Fig. 1A). This mechanism for the Ras activation by AmB aqueous pores would also be consistent with the finding that H2O2-induced apoptosis of yeast cells is blocked by a drastic disruption of the stability of the lipid rafts by depletion of sterols with methyl-β-cyclodextrin (35).
FIG 1.
Molecular mechanisms underlying activation of cell signaling pathways by the formation of AmB aqueous pores. The nonaqueous channels and aqueous pores formed by AmB are shown located at lipid rafts and at the boundaries of lipid rafts, respectively. Note that the hydrophobic thickness of the AmB molecule is less than the hydrophobic thickness of the unperturbed bilayer. The direct interaction of AmB with sterol molecules to form aqueous pores traversing the membrane led to a local thinning of the bilayer, as its hydrophobic core adjusts to match the AmB hydrophobic length. The local bending in the bilayer that is caused by the formation of aqueous pores is denoted by red arrows. (A) Lipid-anchored Ras proteins are inserted at the boundary of the cytosolic leaflet of nonraft and raft microdomains. Upon the occurrence of the local thinning effect exerted by the AmB aqueous pores, the Ras proteins are forced to segregate into the sterol-rich lipid rafts to initiate signaling from downstream effector proteins. (B). TLRs are transmembrane glycoproteins with a single transmembrane domain (TM) that are associated with lipid rafts. The local thinning effect exerted by the AmB aqueous pores can lead to a change in the orientation (tilt) within the bilayer of the single TM causing a conformational change that brings closer the cytosolic protein of two TLR domains to trigger signaling from downstream effector proteins.
It is important that the formation of the aqueous pores by AmB in the plasma membrane of fungal and other eukaryotic pathogens such as Leishmania spp. (36) simultaneously leads to increased ion permeability of the membrane that effectively increases the ATP usage needed to maintain constant essential cellular functions and ion homeostasis. Thus, by increasing the proton permeability of the yeast membranes (37), AmB raises the activity of Pma1, the H+-ATPase pump, and other futile cycles that deplete ATP, leading to a higher level of ROS production that promotes oxidative killing (38). ATP depletion is also enhanced in Candida albicans by the AmB-induced shift from fermentation to respiration (30), a finding that is consistent with the observation that, in respiration, H+-ATPase function is one of the major ATP-consuming pathways whose level is increased (39). Of note, such ATP-depleting compounds as mitochondrial inhibitors and ROS-scavenging agents not only block AmB lethal activity (40) but also cause decreased Ras signaling (41), an indication that oxidative stress negatively regulates Ras activities.
ROLE OF AmB AQUEOUS PORES IN THE ACTIVATION OF HOST IMMUNE CELLS
AmB is a fungicidal drug that also has the ability to stimulate an immune host response at the same concentrations at which aqueous pores are formed in cholesterol-containing mammalian cells (10, 42). In fact, among the proteins that can also be activated by the interaction of AmB with lipid rafts are Toll-like receptor 2 (TLR2) and TLR4 (TLR2/TLR4) (43) as well as TLR2/TLR1 (44). Sau et al. also found that AmB activation of TLRs was strongly enhanced by the expression of CD14, a cell surface coreceptor that is attached to glycosylphosphatidylinositol (GPI)-anchored lipid rafts (43). This activation of TLRs by AmB induces the proinflammatory cytokines interleukin-1ß (IL-1ß) and tumor necrosis factor alpha (TNF-α), which are important mediators of the initiation and potentiation of immune and inflammatory responses.
The activation of TLRs by AmB can also be related to the thinning effect that is exerted in the vicinity of the lipid bilayer upon the interaction of the nonaqueous AmB channels with sterol molecules to form aqueous pores (Fig. 1B). Thus, TLRs are type I transmembrane glycoproteins with a single transmembrane domain that cause rearrangement of the receptor complexes upon ligand binding to the extracellular domains, triggering the recruitment of specific adaptor proteins to the intracellular Toll/IL-1 receptor (IL-1R) (TIR) domains (45). The ligand-induced dimerization of two monomeric TLR extracellular domains has been shown to be the key event for activation (45), but the contribution of interactions between the transmembrane domains (TM) has also been considered critically important in the signaling process (46). Thus, the assembly of TMs of TLR2/TLR4 and TLR2/TLR1 is essential for activating the signaling complex, as was indicated by the finding that a single nucleotide polymorphism in the TM of human TLR1 correlates with an altered immune response (47) and the finding that TM-derived peptides from TLR2 and TLR6 specifically inhibit TLR2 activation (48). In this regard, it is proposed here that the local reduction in the bilayer thickness caused by AmB aqueous pores located near TLRs can directly cause a change in the orientation (tilt) within the bilayer of the single transmembrane domains (TM), leading to a conformational change that brings the cytosolic TIR domain closer to form functional dimers that trigger TLR activation (Fig. 1B). This mechanistic model of TLR activation is supported by the observation that strands tilted at an angle of 45° would correspond to a change in TM length of 4.6 Å, (49), which is closer to the 3.6-Å minimum decrease in membrane thickness necessary for the formation of AmB aqueous pores (18).
The AmB-induced clustering of TLRs with the adaptor proteins may also be facilitated by the sequestration of cholesterol by the intracellular CRAC and CARC domains of TLRs that partition at the lipid bilayer interphase (50, 51). This mechanism would explain why ligand-independent oligomerization of TLR4 is blocked by deleting the short cholesterol-binding region adjacent to the transmembrane domain and why this mutant is unresponsive to lipopolysaccharide (LPS) activation in the presence of the TIR domain (52). It would also be consistent with the observation that the depletion of cholesterol by treatment of macrophages with methyl-β-cyclodextrin leads to a reduction in the levels of TLR4 receptors in lipid rafts, with a concomitant reduction in inflammatory cytokine responses (53).
THE DEVELOPMENT OF AmB RESISTANCE IN EUKARYOTIC PATHOGENS
As expected for a mechanism of action that is dependent on the presence of ergosterol to form aqueous pores, the depletion of this sterol from the plasma membrane has been associated with the emergence of AmB-R organisms in the clinic (54). In fact, in a systematic investigation to define genes whose inactivation confers AmB resistance, it was found that the MIC for AmB against C. albicans was increased more than 3-fold by the deletion of ERG2 or ERG6 or of ERG3 and ERG11 together, all of which are involved in the ergosterol biosynthetic pathway (55). Vincent et al. have also reported that the evolution of ERG2/erg2Δ heterozygotes of AmB-R C. albicans strains requires the presence of Hog1 or calcineurin or high levels of the Hsp90 molecular chaperone (55). However, the changes made in the AmB-R phenotype by the deletion of the ERG genes involve substantial fitness costs because the resistant strains exhibited drastically reduced tolerance not only of oxidative stresses but also of other external stresses such as high temperatures, killing by neutrophils, impairment in the filamentation process, and tissue invasion (55). Interestingly, Hsp90, by repressing Ras1-PKA signaling, is known to regulate a key temperature-dependent morphogenetic transition from yeast to filamentous growth that is crucial for virulence in fungal cells (56). These findings indicate that the AmB-R strains evolve by adapting some of the same gene pathways that are involved in the protection of basic cell membrane and cell functions.
The increased hypersensitivity of the AmB-R strains to oxidative stress is particularly revealing because the osmoinducible Hog-1 genes that are required for the evolution of AmB-R yeast strains when exposed to increasing drug concentrations are known to be functionally connected to the defense against oxidative stress (5). Notably, in the AmB-R strains of C. albicans with deleted ERG3/ERG11 genes, a set of genes that are typically induced under iron starvation conditions are constitutively expressed, reflecting the fact that this stress is highly linked to ergosterol homeostasis (57). In fact, Thomas et al. found a connection between the upregulation of genes that are involved in iron assimilation such as SIT1 and RBT5 and the downregulation of ERG3 and ERG11 in a C. albicans mutant with an impaired mitochondrial function (57). Those investigators also reported that alteration of iron levels in the mitochondrial mutant led to increased susceptibility to H2O2 via the activity of an impaired high-osmolarity glycerol (HOG) pathway and high cellular ROS (57). These findings suggest that the increased susceptibility to oxidative stress shown by AmB-R strains of C. albicans with ERG gene deletions (55) could be caused by a perturbation of the HOG1 MAP kinase pathway that controls respiratory metabolism and is essential in the oxidative stress response (58). In this respect, it is important that Hog1 is a MAP kinase that is dependent on the direct interaction with the Hsp90 chaperone for activation (59) and that maintaining the viability of the ERG-deleted mutants that are resistant to AmB requires the presence of adequate levels of Hsp90 (55).
A reduced level of ergosterol content in clinical strains of C. lusitaniae that exhibited frequent resistance to AmB (60), possibly arising from mutations in the ERG3 gene (61), has also been reported. Of importance, the dimorphic transition between yeast and filamentous growth that appears to be critical for virulence in many Candida spp. has been associated in C. lusitaniae with AmB-R strains (62). It is then likely that some strains of C. lusitaniae that appear to be intrinsically resistant to AmB may be already adapted to display increased virulence under conditions of reduced ergosterol content. This conclusion would be consistent with the finding that no differences in levels of tolerance of AmB were found between the wild-type and calcineurin mutant strains of C. lusitaniae, despite the fact that calcineurin is strictly required for the pseudohyphal growth and virulence of this fungus (63).
AmB RESISTANCE IN PATHOGENS CONTAINING NORMAL STEROL LEVELS
The finding that AmB-R mutants of Aspergillus fumigatus and related Aspergillus species do not exhibit any alterations in the sterol content of the membrane is also of great significance (64). Blum et al. showed that these AmB-R cells exhibited increased protection against oxidative damage and that the increase was the result of enhanced production of catalase (64). However, by shifting the balance between ROS generation and protection by catalases, other factors may also contribute to the development of such AmB-R strains. Thus, changes in the sphingolipid composition at the cell membrane may have important effects on the magnitude and extent of AmB-ergosterol binding and aqueous pore formation. In fact, the susceptibility of Candida cells to AmB has been found to increase as a result of deletion of genes involved in the sphingolipid biosynthetic pathway such as FEN1 and SUR4 or of the use of sphingolipid biosynthesis inhibitors such as myriocin that increase the availability of ergosterol that is nonbound to sphingolipids in the membrane (65). In this respect, it is important that there are genetic interactions between ergosterol and sphingolipid synthetic genes in yeasts that determine that changes in the sphingolipid composition of the membrane are compensated by concomitant changes in sterol content (66).
There are also proteins that can protect cells against AmB action by blocking the activation of Ras signaling activation via the inhibition of the formation of aqueous pores. Thus, in similarity to the protective role that is exerted by Hsp90 in AmB-R of C. albicans and A. terreus (55, 67), when the stress-inducible Hsp70 protein in A. terreus was blocked, the AmB susceptibility of the resistant strains significantly increased (68). For both Hsp90 and Hsp70, there is evidence of the ability to interact preferentially with lipid raft domains (69, 70). It has also been reported that the sphingolipid-binding protein Pmp3, which is highly conserved in fungi, is a potent AmB resistance factor (71, 72). Such resistance can be suppressed by the addition of phytosphingosine, a sphingolipid pathway intermediate (73).
Resistance to AmB has also been found in clinical isolates of Leishmania donovani that lack ergosterol but instead have exogenous cholesterol incorporated into their membranes (74). The AmB-R Leishmania strains also exhibited levels of ROS accumulation that were much reduced, a result which was found be associated with the upregulation of the tryparedoxin cascade, a pathway that plays an important role in antioxidative defense against ROS in kinetoplastids (75). A proteomic analysis carried out in a laboratory AmB-R strain of Leishmania infantum has confirmed the upregulation of members of the tryparedoxin cascade (76). This proteomic work also revealed the downregulation of an H+ ATPase in the AmB-R leishmanial strain (76), a result that is consistent with the role that the increased proton permeability across AmB aqueous pores plays in the futile waste of ATP, which leads to increased ROS accumulation.
Prevention of an increase in ion leakage leading to ATP depletion is important not only for AmB resistance but also for that developed by other amphiphilic drugs which partition into the membrane (5). In this respect, Purkait et al. (74) have reported that AmB-R leishmanial strains displayed an increase in the level of expression of Mdr1, a member of the ATP-binding cassette (ABC) of protein transporters located in lipid rafts, which not only is involved in the efflux of amphiphilic compounds such as vinblastine, puromycin, and doxorubicin (77) but also serves as a lipid translocase of broad specificity (78). Preincubation of AmB-R leishmanial cells with verapamil, a well-known Mdr1 inhibitor, led to a 1.9-fold decrease of the 50% lethal dose (LD50) of AmB in the resistant strain. However, this value decreased by only 2.4-fold in the presence of the combined action of verapamil and the thiol pathway inhibitors buthionine sulfoximine (BSO) and difluoromethylornithine (DFMO) (74). These findings suggest that the main factor responsible for the drug resistance observed in the leishmanial strain by these investigators is the blocking by verapamil of the Mdr1 activity extruding AmB molecules from the lipid rafts, effectively interfering with the signaling events that lead to increased ROS production (Fig. 1A).
It is not yet known what triggers the expression of the genes associated with the various antioxidant systems in Leishmania spp., but the present analysis suggests the possible involvement of the various Ras isoforms that are known to participate in leishmanial infections in host macrophages (79). Previously, it was reported that laboratory AmB-R yeast cells treated with increasing AmB concentrations permanently overexpressed genes such as YOR1 and PDR6, which are also members of the ATP-binding cassette (ABC) family of transporters (80). The specific roles that these ABC transporters play in AmB resistance are unknown, but—similarly to MDR1—they may be involved in lipid translocation and transport functions associated with the many cellular activities that are shown by ABC proteins located in lipid raft microdomains (81).
THE DEVELOPMENT OF AmB TOXICITY AND RESISTANCE IN CHOLESTEROL-CONTAINING MAMMALIAN CELLS
Since the early studies in the fifties, the therapeutic index for the use of AmB as an antifungal drug has been associated with the higher binding affinity of AmB for the fungal ergosterol than for the cholesterol present in mammalian cells (82). Recent efforts to improve the AmB therapeutic index have yielded a new derivative (C2′deOAmB), which binds ergosterol but not cholesterol (83). C2′deOAmB is toxic to yeasts but not to human primary kidney cells, confirming the role that cholesterol binding to AmB plays in developing drug nephrotoxicity. In fact, such toxicity in host cells appears to be a direct consequence of the formation of aqueous pores. Thus, the exposure of kidney-derived proximal cells to increasing AmB concentrations leads to depletion of ATP and an increase in the cellular susceptibility to oxidative stress (84), outcomes that are similar to the action that is performed by AmB in ergosterol-containing fungal membranes. AmB-treated kidney cells also exhibited acute increments in the sphingomyelin-phosphatidylcholine molar ratios and ceramide content, supporting the idea of the involvement of lipid raft signaling components in AmB toxic action (84). In this regard, there is increasing evidence that activation of MAPKs, which are kinases known to show cross talk with Ras and other signaling pathways, plays an important role in drug-induced kidney injury (85).
The reduced nephrotoxicity that is exerted by the liposome-based AmB formulations (84, 86) can be explained simply by the observation that that such preparations deliver an amount of AmB monomers that is sufficient for the formation of aqueous pores in fungi but not in mammalian cells (see Fig. 4 in reference 87). In effect, the insertion of AmB into cholesterol-containing membranes can be directly related to the increase in the levels of small soluble AmB dimers in the external aqueous solution (88). Of note, a crucial property of liposomes and other lipid-based preparations is that they are rapidly removed from the circulation by the types of cells within which intracellular pathogens multiply, i.e., by macrophages and other cells of the reticuloendothelial system (4). The cellular uptake of AmB-loaded liposomes by phagocytosis and/or the internalization that results from interactions with membrane receptors greatly facilitates the internal access of monomeric AmB to the intracellular fungal target.
In contrast to the behavior observed in ergosterol-containing membranes, the nonaqueous channels that are formed by AmB in mammalian cells are able to remain as such over a somewhat large range of concentrations (10). This outcome is due not only to the AmB/cholesterol interactions, which are much weaker than the AmB-ergosterol interactions (10), but also to the more complex dynamics seen with mammalian cholesterol with respect to the lipid rafts at the plasma membrane and intracellular membranes (89). An earlier finding revealing the effect of such dynamics in membrane function was the observation that, upon activation, macrophages not only were more susceptible to lysis by AmB but also exhibited increased tumoricidal effects (90). These functional changes in macrophages were not accompanied by any changes in the membrane cholesterol content, suggesting that the increased sensitivity to AmB reflected alterations in lipid membrane organization and increased mobilization of signaling proteins into lipid rafts (53, 90). Increased AmB resistance to cell lysis in the presence of normal membrane cholesterol content in a Chinese hamster ovary cell mutant has also been reported (91). This type of AmB resistance is likely due to decreased aqueous pore formation as a consequence of changes in the organization of cholesterol in the plasma membrane that lead to a decrease in the number of phase boundaries between the disordered nonraft and the ordered raft microdomains (92).
The nonaqueous ion channels that are formed by AmB in cholesterol-containing cells are able to elicit K+ efflux at concentrations that are fungicidal (e.g., 0.5 μM) in ergosterol-containing pathogens (10). In immune cells, such AmB-induced K+ effluxes have been shown to play an important role in the activation of caspase-1, a key calcium-dependent enzyme whose activation leads to the conversion of pro-IL-1ß into its mature IL-1ß active forms in NLRP3 and NLRP1 inflammasomes (93). In fact, AmB concentrations of as low as 0.625 μM induced the release of IL-1ß from THP-1 cells (94). Recently, it was shown that the activation of caspase-1 in the “canonical” inflammasomes leads to the cleavage of gasdermin D, which is a key substrate that, upon its own cleavage, drives pyroptosis, a lytic process that allows the release of the mature cytokine IL-1ß from cells (95). The release of IL-1ß from leukocytes and other cells plays a central role in orchestration of the inflammatory response that can have beneficial effects in the response to infections.
The subsequent increase in the AmB concentration beyond the threshold required for the formation of the aqueous pores results in an abrupt increase in the levels of TNF-α and other proinflammatory cytokines and chemokines that are induced by the activation of TLR receptors (Fig. 1B). Thus, AmB exerts a maximal effect on the secretion of TNF-α in immune cells at 4 μM (43) and 6.4 μM (44), which are concentrations above the threshold value for the formation of aqueous pores by AmB (10, 22). In fact, the differential role of K+ efflux in the secretion of IL-1ß compared to TNF-α is shown clearly in the data obtained in THP-1 cells that indicated that when the AmB concentration was raised from 0.625 μM to 5 μM, the secretion of TNF-α increased by about 30-fold whereas the secretion of IL-1ß increased by only 4-fold (94). Such significant differences between the stimulatory effects that are exerted by the two types of AmB channels in immune cells are thus important to take into account for the development of more-specific targeted approaches for stimulation of AmB immune and adjuvant responses (96).
CONCLUSIONS AND PERPECTIVES
The pathways that confer AmB resistance to both fungal and parasitic pathogens are all activated in response to the formation of sterol-containing aqueous pores, confirming the central role that these ion channels and their interactions with sterols play in the drug mechanism of action.
In order to avoid AmB-induced cell death, the depletion of ergosterol from the membranes is required. However, due to the high fitness costs of ergosterol depletion, AmB-resistant strains also emerge in the clinic in the presence of normal levels of ergosterol.
The response mechanisms that are developed in AmB-R strains are mainly directed at protection of cells against the ROS-induced oxidative damage that is caused by the futile AmB-induced ion cycling leading to ATP depletion. The resistance mechanisms also block the Ras signaling induced by the aqueous pores formed by AmB at membrane rafts, which also leads to increased ROS formation.
The unique synergism conferred to AmB by its dual action as a pore-forming compound that is capable of signaling effects helps to explain its long-term efficacy as an antifungal drug. However, such an action is also the principal cause of its nephrotoxic effects.
The use of lipid carriers for decreasing AmB toxicity in host membrane cells is based on changing the balance between monomeric and aggregated forms to enhance the formation of aqueous pores in ergosterol-containing membranes. The success of this strategy is also a consequence of the targeted enhancement of monomeric AmB inside cells by the internalization of the drug-loaded lipid particles. Development of new protected monomeric AmB conjugates unable to insert into the membrane but capable of cellular internalization may also kill pathogens by extracting ergosterol from membranes (97).
As proposed here, the membrane-thinning effects induced by the formation of AmB aqueous pores may lead to the activation of Ras and TLR2/TLR4 signaling receptors in fungal and host immune cells, respectively. This mechanistic insight can facilitate the development of new drug scaffolds and/or vaccine adjuvants that target fungal pathogens and enhance responses of host cells.
ACKNOWLEDGMENT
I am grateful to Caroline Manganiello for editing the manuscript before publication.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Kotler-Brajtburg J, Medoff G, Kobayashi GS, Boggs S, Schlessinger D, Pandey RC, Rinehart KL Jr. 1979. Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob Agents Chemother 15:716–722. doi: 10.1128/AAC.15.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosnik A. 2016. Drug self-assembly: a phenomenon at the nanometer scale with major impact in the structure-biological properties relationship and the treatment of disease. Prog Mater Sci 82:39–82. doi: 10.1016/j.pmatsci.2016.03.004. [DOI] [Google Scholar]
- 3.Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, Nieuwkoop AJ, Comellas G, Maryum N, Wang S, Uno BE, Wildeman EL, Gonen T, Rienstra CM, Burke MD. 2014. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol 10:400–406. doi: 10.1038/nchembio.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brajtburg J, Bolard J. 1996. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev 9:512–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen BE. 2014. Functional linkage between genes that regulate osmotic stress responses and multidrug resistance transporters: challenges and opportunities for antibiotic discovery. Antimicrob Agents Chemother 58:640–646. doi: 10.1128/AAC.02095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marty A, Finkelstein A. 1975. Pores formed in lipid bilayer membranes by nystatin, Differences in its one-sided and two-sided action. J Gen Physiol 65:515–526. doi: 10.1085/jgp.65.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinberg ME, Finkelstein A. 1984. Single-length and double-length channels formed by nystatin in lipid bilayer membranes. J Membr Biol 80:257–269. doi: 10.1007/BF01868444. [DOI] [PubMed] [Google Scholar]
- 8.van Hoogevest P, de Kruijff B. 1978. Effect of amphotericin B on cholesterol-containing liposomes of egg phosphatidylcholine and didocosenoyl phosphatidylcholine. A refinement of the model for the formation of pores by amphotericin B in membranes. Biochim Biophys Acta 511:397–407. [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka S, Ikeuchi H, Matsumori N, Murata M. 2005. Dominant formation of a single-length channel by amphotericin B in dimyristoylphosphatidylcholine membrane evidenced by 13C-31P rotational echo double resonance. Biochemistry 44:704–710. doi: 10.1021/bi049001k. [DOI] [PubMed] [Google Scholar]
- 10.Cohen BE. 2010. Amphotericin B membrane action: role for two types of ion channels in eliciting cell survival and lethal effects. J Membr Biol 238:1–20. doi: 10.1007/s00232-010-9313-y. [DOI] [PubMed] [Google Scholar]
- 11.Ganis P, Avitabile G, Mechlinski W, Schaffner CP. 1971. Polyene macrolide antibiotic amphotericin B. Crystal structure of the N-iodoacetyl derivative. J Am Chem Soc 93:4560–4564. [DOI] [PubMed] [Google Scholar]
- 12.Mitra K, Ubarretxena-Belandia I, Taguchi T, Warren G, Engelman DM. 2004. Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc Natl Acad Sci U S A 101:4083–4088. doi: 10.1073/pnas.0307332101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venegas B, González-Damián J, Celis H, Ortega-Blake I. 2003. Amphotericin B channels in the bacterial membrane: role of sterol and temperature. Biophys J 85:2323–2332. doi: 10.1016/S0006-3495(03)74656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen BE. 1986. Concentration and time dependence of amphotericin B-induced permeability changes across ergosterol-containing liposomes. Biochim Biophys Acta 856:117–122. [DOI] [PubMed] [Google Scholar]
- 15.Cohen BE, Gamargo M. 1987. Concentration and time dependence of amphotericin B-induced permeability changes across plasma membrane vesicles from Leishmania sp. Drugs Exp Clin Res 13:539–546. [PubMed] [Google Scholar]
- 16.Coutinho A, Silva L, Fedorov A, Prieto M. 2004. Cholesterol and ergosterol influence nystatin surface aggregation: relation to pore formation. Biophys J 87:3264–3276. doi: 10.1529/biophysj.104.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen BE. 1998. Amphotericin B toxicity and lethality: a tale of two channels. Int J Pharm 162:95–106. doi: 10.1016/S0378-5173(97)00417-1. [DOI] [Google Scholar]
- 18.Shatursky OY, Romanenko OV, Himmelreich NH. 2014. Long open amphotericin channels revealed in cholesterol-containing phospholipid membranes are blocked by thiazole derivative. J Membr Biol 247:211–229. doi: 10.1007/s00232-013-9626-8. [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein A, Holz R. 1973. Aqueous pores created in thin lipid membranes by the polyene antibiotic nystatin and amphotericin B, p 377–348. In Eisenman G. (ed), Membranes, vol 2 Marcel Decker, New York, NY. [PubMed] [Google Scholar]
- 20.Rajendran L, Simons K. 2005. Lipid rafts and membrane dynamics. J Cell Sci 118:1099–1102. doi: 10.1242/jcs.01681. [DOI] [PubMed] [Google Scholar]
- 21.Gandhavadi M, Allende D, Vidal A, Simon SA, McIntosh TJ. 2002. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys J 82:1469–1482. doi: 10.1016/S0006-3495(02)75501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero EA, Valdivieso E, Cohen BE. 2009. Formation of two different types of ion channels by amphotericin B in human erythrocyte membranes. J Membr Biol 230:69–81. doi: 10.1007/s00232-009-9187-z. [DOI] [PubMed] [Google Scholar]
- 23.Whyte BS, Peterson RP, Hartsel SC. 1989. Amphotericin B and Nystatin show different activities on sterol-free vesicles. Biochem Biophys Res Commun 164:609–614. doi: 10.1016/0006-291X(89)91503-9. [DOI] [PubMed] [Google Scholar]
- 24.Meinhardt S, Vink RL, Schmid F. 2013. Monolayer curvature stabilizes nanoscale raft domains in mixed lipid bilayers. Proc Natl Acad Sci U S A 110:4476–4481. doi: 10.1073/pnas.1221075110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen OS, Koeppe RE. 2007. Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct 36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 26.Groves JT, Kuriyan J. 2010. Molecular mechanisms in signal transduction at the membrane. Nat Struct Mol Biol 17:659–665. doi: 10.1038/nsmb.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Hancock JF. 2015. Ras nanoclusters: versatile lipid-based signaling platforms. Biochim Biophys Acta 1853:841–849. doi: 10.1016/j.bbamcr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Phillips AJ, Sudbery I, Ramsdale M. 2003. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc Natl Acad Sci U S A 100:14327–14332. doi: 10.1073/pnas.2332326100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips AJ, Crowe JD, Ramsdale M. 2006. Ras pathway signaling accelerates programmed cell death in the pathogenic fungus Candida albicans. Proc Natl Acad Sci U S A 103:726–731. doi: 10.1073/pnas.0506405103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belenky P, Camacho D, Collins JJ. 2013. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep 3:350–358. doi: 10.1016/j.celrep.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunsveld L, Waldmann H, Huster D. 2009. Membrane binding of lipidated Ras peptides and proteins—the structural point of view. Biochim Biophys Acta 1788:273–288. doi: 10.1016/j.bbamem.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Janosi L, Li Z, Hancock JF, Gorfe AA. 2012. Organization, dynamics, and segregation of Ras nanoclusters in membrane domains. Proc Natl Acad Sci U S A 109:8097–8102. doi: 10.1073/pnas.1200773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen JB, Jensen MB, Bhatia VK, Pedersen SL, Bjørnholm T, Iversen L, Uline M, Szleifer I, Jensen KJ, Hatzakis NS, Stamou D. 2015. Membrane curvature enables N-Ras lipid anchor sorting to liquid-ordered membrane phases. Nat Chem Biol 11:192–194. doi: 10.1038/nchembio.1733. [DOI] [PubMed] [Google Scholar]
- 34.Gorfe AA, Babakhani A, McCammon JA. 2007. H-ras protein in a bilayer: interaction and structure perturbation. J Am Chem Soc 129:12280–12286. doi: 10.1021/ja073949v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du W, Ayscough KR. 2009. Methyl beta-β-cyclodextrin reduces accumulation of reactive oxygen species and cell death in yeast. Free Radic Biol Med 46:1478–1487. doi: 10.1016/j.freeradbiomed.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 36.Ramos H, Valdivieso E, Gamargo M, Dagger F, Cohen BE. 1996. Amphotericin B kills unicellular leishmanias by forming aqueous pores permeable to small cations and anions. J Membr Biol 152:65–75. doi: 10.1007/s002329900086. [DOI] [PubMed] [Google Scholar]
- 37.Palacios J, Serrano R. 1978. Proton permeability induced by polyene antibiotics. A plausible mechanism for their inhibition of maltose fermentation in yeast. FEBS Lett 91:198–201. [DOI] [PubMed] [Google Scholar]
- 38.Adolfsen KJ, Brynildsen MP. 2015. Futile cycling increases sensitivity toward oxidative stress in Escherichia coli. Metab Eng 29:26–35. doi: 10.1016/j.ymben.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serrano R. 1980. Effect of ATPase inhibitors on the proton pump of respiratory-deficient yeast. Eur J Biochem 105:419–424. doi: 10.1111/j.1432-1033.1980.tb04516.x. [DOI] [PubMed] [Google Scholar]
- 40.Blatzer M, Jukic E, Posch W, Schöpf B, Binder U, Steger M, Blum G, Hackl H, Gnaiger E, Lass-Flörl C, Wilflingseder D. 2015. Amphotericin B resistance in Aspergillus terreus is overpowered by coapplication of pro-oxidants. Antioxid Redox Signal 23:1424–1438. doi: 10.1089/ars.2014.6220. [DOI] [PubMed] [Google Scholar]
- 41.Grahl N, Demers EG, Lindsay AK, Harty CE, Willger SD, Piispanen AE, Hogan DA. 2015. Mitochondrial activity and Cyr1 are key regulators of Ras1 activation of C. albicans virulence pathways. PLoS Pathog 11:e1005133. doi: 10.1371/journal.ppat.1005133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mesa-Arango AC, Scorzoni L, Zaragoza O. 2012. It only takes one to do many jobs: amphotericin B as antifungal and immunomodulatory drug. Front Microbiol 3:286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sau K, Mambula SS, Latz E, Henneke P, Golenbock DT, Levitz SM. 2003. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J Biol Chem 278:37561–37568. doi: 10.1074/jbc.M306137200. [DOI] [PubMed] [Google Scholar]
- 44.Razonable RR, Henault M, Lee LN, Laethem C, Johnston PA, Watson HL, Paya CV. 2005. Secretion of proinflammatory cytokines and chemokines during amphotericin B exposure is mediated by coactivation of Toll-like receptors 1 and 2. Antimicrob Agents Chemother 49:1617–1161. doi: 10.1128/AAC.49.4.1617-1621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Godfroy JI III, Roostan M, Moroz YS, Korendovych IV, Yin H. 2012. Isolated Toll-like receptor transmembrane domains are capable of oligomerization. PLoS One 7:e48875. doi: 10.1371/journal.pone.0048875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawn TR, Misch EA, Dunstan SJ, Thwaites GE, Lan NT, Quy HT, Chau TT, Rodrigues S, Nachman A, Janer M, Hien TT, Farrar JJ, Aderem A. 2007. A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur J Immunol 37:2280–2289. doi: 10.1002/eji.200737034. [DOI] [PubMed] [Google Scholar]
- 48.Fink A, Reuven EM, Arnusch CJ, Shmuel-Galia L, Antonovsky N, Shai Y. 2013. Assembly of the TLR2/6 transmembrane domains is essential for activation and is a target for prevention of sepsis. J Immunol 190:6410–6422. doi: 10.4049/jimmunol.1202033. [DOI] [PubMed] [Google Scholar]
- 49.Lin Q, London E. 2013. Altering hydrophobic sequence lengths shows that hydrophobic mismatch controls affinity for ordered lipid domains (rafts) in the multitransmembrane strand protein perfringolysin O. J Biol Chem 288:1340–1352. doi: 10.1074/jbc.M112.415596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reuven EM, Fink A, Shai Y. 2014. Regulation of innate immune responses by transmembrane interactions: lessons from the TLR family. Biochim Biophys Acta 1838:1586–1593. doi: 10.1016/j.bbamem.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Ruysschaert JM, Lonez C. 2015. Role of lipid microdomains in TLR-mediated signalling. Biochim Biophys Acta 1848:1860–1867. doi: 10.1016/j.bbamem.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 52.Nishiya T, Kajita E, Miwa S. 2006. Ligand-independent oligomerization of TLR4. regulated by a short hydrophobic region adjacent to the transmembrane domain. Biochem Biophys Res Commun 341:1128–1134. doi: 10.1016/j.bbrc.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 53.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. 2010. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res 51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaughnessy EM, Lyman CA, Walsh TJ. 2009. Amphotericin B resistance mechanisms, p 295–306. In Mayers DL. (ed), Antimicrobial drug resistance. Humana Press, New York, NY. [Google Scholar]
- 55.Vincent BM, Lancaster AK, Scherz-Shouval R, Whitesell L, Lindquist S. 2013. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol 11:e1001692. doi: 10.1371/journal.pbio.1001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, Heitman J, Cowen LE. 2009. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol 19:621–629. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas E, Roman E, Claypool S, Manzoor N, Pla J, Panwar SL. 2013. Mitochondria influence CDR1 efflux pump activity, Hog1-mediated oxidative stress pathway, iron homeostasis, and ergosterol levels in Candida albicans. Antimicrob Agents Chemother 57:5580–5599. doi: 10.1128/AAC.00889-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alonso-Monge R, Carvaihlo S, Nombela C, Rial E, Pla J. 2009. The Hog1 MAP kinase controls respiratory metabolism in the fungal pathogen Candida albicans. Microbiology 155:413–423. doi: 10.1099/mic.0.023309-0. [DOI] [PubMed] [Google Scholar]
- 59.Diezmann S, Michaut M, Shapiro RS, Bader GD, Cowen LE. 2012. Mapping the Hsp90 genetic interaction network in Candida albicans reveals environmental contingency and rewired circuitry. PLoS Genet 8:e1002562. doi: 10.1371/journal.pgen.1002562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peyron F, Favel A, Calaf R, Michel-Nguyen A, Bonaly R, Coulon J. 2002. Sterol and fatty acid composition of Candida lusitaniae clinical isolates. Antimicrob Agents Chemother 46:531–533. doi: 10.1128/AAC.46.2.531-533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young LY, Hull CM, Heitman J. 2003. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother 47:2717–2734. doi: 10.1128/AAC.47.9.2717-2724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller NS, Dick JD, Merz WG. 2006. Phenotypic switching in Candida lusitaniae on copper sulfate indicator agar: association with amphotericin B resistance and filamentation. J Clin Microbiol 44:1536–1539. doi: 10.1128/JCM.44.4.1536-1539.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Silao FG, Bigol UG, Bungay AA, Nicolas MG, Heitman J, Chen YL. 2012. Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae. PLoS One 7:e44192. doi: 10.1371/journal.pone.0044192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blum G, Hörtnagl C, Jukic E, Erbeznik T, Pümpel T, Dietrich H, Nagl M, Speth C, Rambach G, Lass-Flörl C. 2013. New insight into amphotericin B resistance in Aspergillus terreus. Antimicrob Agents Chemother 57:1583–1588. doi: 10.1128/AAC.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma S, Alfatah M, Bari VK, Rawal Y, Paul S, Ganesan K. 2014. Sphingolipid biosynthetic pathway genes FEN1 and SUR4 modulate amphotericin B resistance. Antimicrob Agents Chemother 58:2409–2414. doi: 10.1128/AAC.02130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan XL, Souza CM, Pichler H, Dewhurst G, Schaad O, Kajiwara K, Wakabayashi H, Ivanova T, Castillon GA, Piccolis M, Abe F, Loewith R, Funato K, Wenk MR, Riezman H. 2009. Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology. Mol Biol Cell 20:2083–2095. doi: 10.1091/mbc.E08-11-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blum G, Kainzner B, Grif K, Dietrich H, Zelger B, Sonnweber T, Lass-Flörl C. 2013. In vitro and in vivo role of heat shock protein 90 in amphotericin B resistance of Aspergillus terreus. Clin Microbiol Infect 19:50–55. doi: 10.1111/j.1469-0691.2012.03848.x. [DOI] [PubMed] [Google Scholar]
- 68.Blatzer M, Blum G, Jukic E, Posch W, Gruber P, Nagl M, Binder U, Maurer E, Sarg B, Lindner H, Lass-Flörl C, Wilflingseder D. 2015. Blocking Hsp70 enhances the efficiency of amphotericin B treatment against resistant Aspergillus terreus strains. Antimicrob Agents Chemother 59:3778–3788. doi: 10.1128/AAC.05164-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armijo G, Okerblom J, Cauvi DM, Lopez V, Schlamadinger DE, Kim J, Arispe N, De Maio A. 2014. Interaction of heat shock protein 70 with membranes depends on the lipid environment. Cell Stress Chaperones 19:877–886. doi: 10.1007/s12192-014-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nieto-Miguel T, Gajate C, González-Camacho F, Mollinedo F. 2008. Proapoptotic role of Hsp90 by its interaction with c-Jun N-terminal kinase in lipid rafts in edelfosine-mediated antileukemic therapy. Oncogene 27:1779–1787. doi: 10.1038/sj.onc.1210816. [DOI] [PubMed] [Google Scholar]
- 71.De Block J, Szopinska A, Guerriat B, Dodzian J, Villers J, Hochstenbach JF, Morsomme P. 2015. Yeast Pmp3p has an important role in plasma membrane organization. J Cell Sci 128:3646–3659. doi: 10.1242/jcs.173211. [DOI] [PubMed] [Google Scholar]
- 72.Huang Z, Chen K, Zhang J, Li Y, Wang H, Cui D, Tang J, Liu Y, Shi X, Li W, Liu D, Chen R, Sucgang RS, Pan X. 2013. A functional variomics tool for discovering drug-resistance genes and drug targets. Cell Rep 3:577–585. doi: 10.1016/j.celrep.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bari VK, Sharma S, Alfatah M, Mondal AK, Ganesan K. 2015. Plasma membrane proteolipid 3 protein modulates amphotericin B resistance through sphingolipid biosynthetic pathway. Sci Rep 5:9685. doi: 10.1038/srep09685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Purkait B, Kumar A, Nandi N, Sardar AH, Das S, Kumar S, Pandey K, Ravidas V, Kumar M, De T, Singh D, Das P. 2012. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother 56:1031–1041. doi: 10.1128/AAC.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGonigle S, Dalton JP, James ER. 1998. Peroxidoxins: a new antioxidant family. Parasitol Today 14:139–145. [DOI] [PubMed] [Google Scholar]
- 76.Brotherton MC, Bourassa S, Légaré D, Poirier GG, Droit A, Ouellette M. 2014. Quantitative proteomic analysis of amphotericin B resistance in Leishmania infantum. Int J Parasitol Drugs Drug Resist 4:126–132. doi: 10.1016/j.ijpddr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henderson DM, Sifri CD, Rodgers M, Wirth DF, Hendrickson N, Ullman B. 1992. Multidrug resistance in Leishmania donovani is conferred by amplification of a gene homologous to the mammalian mdr1 gene. Mol Cell Biol 12:2855–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. 1996. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 87:507–517. doi: 10.1016/S0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 79.Chakraborty S, Srivastava A, Jha MK, Nair A, Pandey SP, Srivastava N, Kumari S, Singh S, Krishnasastry MV, Saha B. 2015. Inhibition of CD40-induced N-Ras activation reduces leishmania major infection. J Immunol 194:3852–3860. doi: 10.4049/jimmunol.1401996. [DOI] [PubMed] [Google Scholar]
- 80.Anderson JB, Sirjusingh C, Syed N, Lafayette S. 2009. Gene expression and evolution of antifungal drug resistance. Antimicrob Agents Chemother 53:1931–1936. doi: 10.1128/AAC.01315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klappe K, Hummel I, Hoekstra D, Kok JW. 2009. Lipid dependence of ABC transporter localization and function. Chem Phys Lipids 161:57–64. doi: 10.1016/j.chemphyslip.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 82.Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. 1990. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother 34:183–189. doi: 10.1128/AAC.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilcock BC, Endo MM, Uno BE, Burke MD. 2013. C2′-OH of amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J Am Chem Soc 135:8488–8491. doi: 10.1021/ja403255s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zager RA. 2000. Polyene antibiotics: relative degrees of in vitro cytotoxicity and potential effects on tubule phospholipid and ceramide content. Am J Kidney Dis 36:238–249. doi: 10.1053/ajkd.2000.8967. [DOI] [PubMed] [Google Scholar]
- 85.Cassidy H, Radford R, Slyne J, O'Connell S, Slattery C, Ryan MP, McMorrow T. 2012. The role of MAPK in drug-induced kidney injury. J Signal Transduct 2012:463617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamill RJ. 2013. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 73:919–934. doi: 10.1007/s40265-013-0069-4. [DOI] [PubMed] [Google Scholar]
- 87.Legrand P, Chéron M, Leroy L, Bolard J. 1997. Release of amphotericin B from delivery systems and its action against fungal and mammalian cells. J Drug Target 4:311–319. doi: 10.3109/10611869708995847. [DOI] [PubMed] [Google Scholar]
- 88.Bolard J, Legrand P, Heitz F, Cybulska B. 1991. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry 30:5707–5715. doi: 10.1021/bi00237a011. [DOI] [PubMed] [Google Scholar]
- 89.Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, Belov VN, Hein B, von Middendorff C, Schönle A, Hell SW. 2009. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457:1159–1162. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- 90.Chapman HA Jr, Hibbs JB Jr. 1978. Modulation of macrophage tumoricidal capability by polyene antibiotics: support for membrane lipid as a regulatory determinant of macrophage function. Proc Natl Acad Sci U S A 75:4349–4353. doi: 10.1073/pnas.75.9.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jacobs NL, Andemariam B, Underwood KW, Panchalingam K, Sternberg D, Kielian M, Liscum L. 1997. Analysis of a Chinese hamster ovary cell mutant with defective mobilization of cholesterol from the plasma membrane to the endoplasmic reticulum. J Lipid Res 38:1973–1987. [PubMed] [Google Scholar]
- 92.Neufeld EB, O'Brien K, Walts AD, Stonik JA, Malide D, Combs CA, Remaley AT. 2014. The human ABCG1 transporter mobilizes plasma membrane and late endosomal non-sphingomyelin-associated-cholesterol for efflux and esterification. Biology (Basel) 3:866–891. doi: 10.3390/biology3040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Darisipudi MN, Allam R, Rupanagudi KV, Anders HJ. 2011. Polyene macrolide antifungal drugs trigger interleukin-1β secretion by activating the NLRP3 inflammasome. PLoS One 6:e19588. doi: 10.1371/journal.pone.0019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rogers PD, Barker KS, Herring V, Jacob M. 2003. Heat-induced superaggregation of amphotericin B attenuates its ability to induce cytokine and chemokine production in the human monocytic cell line THP-1. J Antimicrob Chemother 51:405–408. doi: 10.1093/jac/dkg070. [DOI] [PubMed] [Google Scholar]
- 95.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 96.Salyer AC, Caruso G, Khetani KK, Fox LM, Malladi SS, David SA. 2016. Identification of adjuvantic activity of amphotericin B in a novel, multiplexed, poly-TLR/NLR high-throughput screen. PLoS One 11:e0149848. doi: 10.1371/journal.pone.0149848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janout V, Schell WA, Thévenin D, Yu Y, Perfect JR, Regen SL. 2015. Taming amphotericin B. Bioconjug Chem 26:2021–2024. doi: 10.1021/acs.bioconjchem.5b00463. [DOI] [PMC free article] [PubMed] [Google Scholar]