Abstract
Low posaconazole plasma concentrations (PPCs) have been associated with breakthrough invasive fungal infections. We assessed the correlation between pre-steady-state PPCs (obtained between days 3 and 5) and PPCs obtained during steady state in 48 patients with underlying hematological malignancies receiving posaconazole oral-solution prophylaxis. Pre-steady-state PPCs correlated significantly with PPCs obtained at steady state (Spearman r = 0.754; P < 0.001). Receiver operating characteristic (ROC) curve analysis of pre-steady-state PPCs revealed an area under the curve (AUC) of 0.884 (95% confidence interval [CI], 0.790 to 0.977) for predicting satisfactory PPCs at steady state.
TEXT
Posaconazole (PCZ) has broad-spectrum antifungal activity against most Aspergillus and Candida spp. (1 – 6) and is currently approved for antifungal prophylaxis in patients with prolonged neutropenia and in patients with acute graft-versus-host diseases (GVHDs) after hematopoietic stem cell transplantation (HSCT) (7 – 10). PCZ is available as an oral suspension and lately also as a delayed-release tablet formulation and an intravenous formulation (11 – 13). Due to the potentially lower costs and easier intake than for the delayed-release tablet, the oral suspension remains in use for antifungal prophylaxis (14). The major drawbacks of the oral solution are its limited bioavailability, particularly when mucositis, gastrointestinal GVHD, or diarrhea is present, and its nonlinear pharmacokinetics (15, 16). Multiple other variables may also affect PCZ absorption, giving rise to significant inter- and intrapatient variability in the bioavailability of the PCZ oral solution (15, 17 – 22).
Given that subtherapeutic PCZ plasma concentrations (PPCs) may be associated with breakthrough invasive fungal infections (IFIs) (21, 23, 24), therapeutic drug monitoring (TDM) of PCZ has been recommended for the PCZ oral solution (25). There remain many clinical and practical issues that affect PCZ TDM, in particular the length of time required to reach steady state, which may be 7 to 10 days (23, 26 – 28). A delay in obtaining PPCs may postpone decisions by the clinician acting on potentially subtherapeutic PPCs, reducing the benefit of TDM.
The purpose of this analysis was to assess the association between pre-steady-state PPCs (measured between days 3 and 5 of prophylaxis) and PPCs obtained during steady state.
(The original data in this article were presented in part at the 6th Advances Against Aspergillosis conference, 27 February to 1 March 2014, Madrid, Spain [29], and at the 24th European Congress of Clinical Microbiology and Infectious Diseases, 10 to 13 May 2014, Barcelona, Spain [30].)
The cohort study was conducted from 1 July 2012 to 31 May 2013 at the Division of Hematology, Medical University Hospital of Graz, Graz, Austria. PPCs were prospectively assessed in all patients with underlying hematological diseases receiving antifungal prophylaxis.
Patients over 18 years of age receiving prophylactic PCZ oral solution were prospectively identified and screened by clinical rounds, chart reviews, and surveys of electronic documents, including microbiological test results. Patients' medical records were reviewed individually by using a standardized data collection template in order to collect demographic information, clinical data, mycological laboratory test results, and PCZ dosing information. Each case represented a single patient during hospitalization and was considered completed at the patient's discharge. A patient receiving continuous long-term prophylactic antifungal treatment was counted as one case, regardless of the number of times the patient was readmitted.
At our center, the first PPC was routinely measured between days 3 and 5 after initiation of PCZ (and then repeated twice weekly or, in cases of sufficient PPCs at steady state, once weekly). Pre-steady-state PPCs were solely measured as part of a streamlined approach with voriconazole TDM (31) and were not taken into account for clinical decision making. Samples were always obtained in the morning before intake of the solution. Results of steady-state PPCs in this cohort (i.e., obtained on day 7 of prophylaxis or later) have been published previously (32). Patients with underlying hematological malignancies receiving PCZ with initial trough PPCs obtained between days 3 and 5 of prophylaxis and subsequent PPCs obtained between days 7 and 8 (defined as “early steady state”) and, optionally, also between days 10 and 14 (defined as “late steady state”) were included in this analysis.
Trough PPCs were measured by employing the Conformité Européenne In Vitro Diagnostic (CE-IVD)-marked Chromsystems PCZ reagent kit (Chromsystems GmbH, Munich, Germany), based on high-performance liquid chromatography with fluorescence detection (MSD, Vienna, Austria), with a detection limit of 0.05 mg/liter, intra-assay coefficients of variability below 4.4%, and interassay coefficients of variability below 5.2%. Therapeutic targets for PCZ have not been defined yet; however, tentative recommendations suggest targeting a trough level between 0.5 and 0.7 mg/liter for prophylaxis (21, 33, 34). At our center, concentrations above the target of 0.5 mg/liter were defined as satisfactory PPCs and those below the target as low PPCs.
The study adhered to the Declaration of Helsinki (1996) Good Clinical Practice guidelines, and the study protocol was approved by the local ethics committee, Medical University of Graz, Graz, Austria (protocol number 23-343). Informed consent was obtained from all participating patients. All statistical analyses were performed using the Statistical Package for Social Sciences, version 22 (SPSS Inc., Chicago, IL, USA). Continuous data (i.e., PPCs) are presented as medians (interquartile ranges [IQRs]) and categorical data as proportions. Pre-steady-state PPCs, obtained between days 3 and 5, were compared to early-steady-state (day 7 and 8) and late-steady-state (day 10 to 14) PPCs, using the Wilcoxon signed-rank test. Correlations were calculated using Spearman correlation analysis, due to the nonnormal distributions. Analyses of receiver operating characteristic (ROC) curves were carried out for pre-steady-state PPCs, using satisfactory PPCs (i.e., >0.5 mg/liter) at early and late steady states as outcomes. Values for area under the curve (AUC) are displayed, including 95% confidence intervals (CIs). A P value of less than 0.05 was considered statistically significant.
A total of 48 patients receiving antimould prophylaxis with PCZ (3 doses of 200 mg in 45/48 patients and 2 doses of 400 mg in 3/48 patients) had PPCs obtained at pre-steady state and early steady state, and 34/48 (71%) of patients had PPCs additionally obtained at late steady state. Demographics, underlying diseases, PPCs, and factors that are known to influence PPCs are displayed in Table 1. The majority of pre-steady-state PPCs (n = 25; 52%) were obtained at day 3 of prophylaxis, 15 (31%) were obtained at day 4, and 8 (17%) were obtained at day 5.
TABLE 1.
Demographic data, underlying diseases, and PPCs in the study population
| Characteristic | Value for patients (n = 48) |
|---|---|
| No. (%) female/no. (%) male | 21 (43.8)/27 (56.3) |
| Median (range) age (yr) | 60 (26–81) |
| Median (range) body mass index | 26.1 (18.2–44.6) |
| Median (range) wt (kg) | 73.5 (50–115) |
| No. (%) with underlying disease(s) | |
| Acute myeloid leukemia | 31 (64.6) |
| Myelodysplastic syndrome | 7 (14.6) |
| Acute lymphoblastic leukemia | 5 (10.4) |
| Other | 5 (10.4) |
| No. (%) undergoing high-dose chemotherapy | 44 (91.7) |
| No. (%) undergoing allogeneic HSCT | 12 (25) |
| No. (%) with GVHD (stage 3 or higher) | 4 (8.3) |
| Median (IQR) PPC (mg/liter) at: | |
| Pre-steady state | 0.49 (0.28–0.68) |
| Early steady state | 0.44 (<0.20–0.77) |
| Late steady state (n = 34) | 0.45 (<0.20–0.93) |
| No. (%) with risk factor(s) for low PPCs | |
| Oral mucositis (grade 3 or higher at pre-steady-state and steady-state PPCs) | 2 (4.2) |
| Severe diarrhea (at pre-steady-state PPC) | 7 (14.6) |
| Severe diarrhea (at early-steady-state PPC) | 4 (8.3) |
| Proton pump inhibitor (at pre-steady-state and steady-state PPCs) | 30 (62.5) |
| Nausea, no solid food intake (at pre-steady-state and steady-state PPCs) | 3 (6.3) |
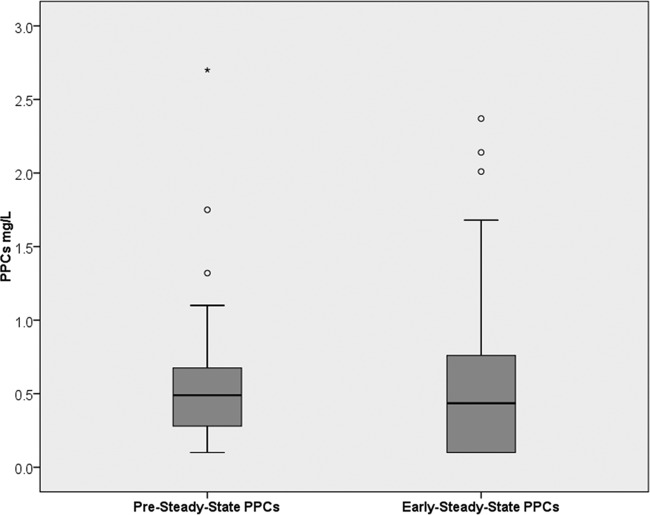
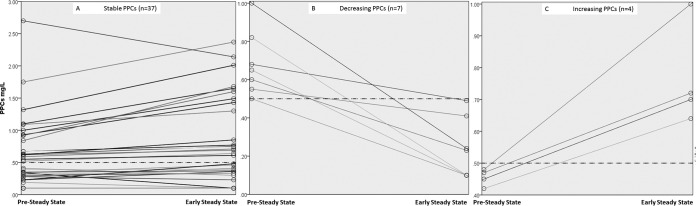
No significant differences were found between pre-steady-state PPCs and PPCs obtained at early (P = 0.098; Wilcoxon signed-rank test) (Fig. 1) or late (P = 0.284) steady state. Of patients with satisfactory PPCs at pre-steady state, 71% (17/24) also had satisfactory concentrations at early steady state and 73% (11/15) at late steady state, while of patients with low PPCs at pre-steady state, 83% (20/24) also had low concentrations at early steady state and 79% (4/19) at late steady state. The positive predictive value for pre-steady-state PPCs of >0.5 mg/liter was therefore 71%, and the negative predictive value was 83%, for predicting satisfactory PPCs at early steady state. Pre-steady-state PPCs were significantly higher in patients with satisfactory PPCs at early steady state than in those with low PPCs at early steady state (median of 0.67 mg/liter [IQR, 0.54 to 1.05 mg/liter] versus median of 0.30 mg/liter [IQR, <0.20 to 0.50 mg/liter]; P < 0.001). Spaghetti blots of paired PPCs obtained at pre-steady state and at early steady state are displayed in Fig. 2.
FIG 1.
Box plots of PPCs obtained at pre-steady state and at early steady state in 48 patients with hematological malignancies.
FIG 2.
Spaghetti blots of paired PPCs obtained at pre-steady state and at early steady state. Panel A displays pairs that had PPCs at both time points below or above the cutoff of 0.5 mg/liter, panel B displays pairs where PPCs decreased below the cutoff, and panel C displays pairs where PPCs increased above the cutoff. The cutoff of 0.5 mg/liter is displayed in all three panels as a dotted line.
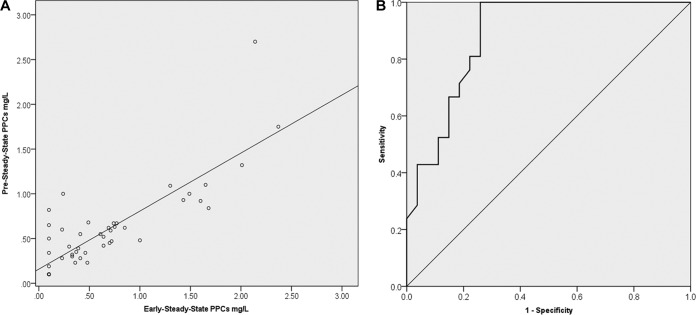
Significant correlations were found between pre-steady-state PPCs and steady-state PPCs obtained early (Spearman r = 0.754; P < 0.001) (Fig. 3A) or late (Spearman r = 0.601; P < 0.001). Correlation was also highly significant between the subset of pre-steady-state PPCs obtained on day 3 of prophylaxis and early-steady-state PPCs (Spearman r = 0.803; P < 0.001).
FIG 3.
(A) Scatter plot of correlation of PPCs obtained at pre-steady state with those obtained at early steady state. (B) ROC curve analysis of pre-steady-state PPCs for predicting PPCs above 0.5 mg/liter at early steady state.
ROC curve analysis of pre-steady-state PPCs revealed AUCs of 0.884 mg · h/liter (95% CI, 0.790 to 0.977 mg · h/liter) for predicting satisfactory PPCs at early steady state (Fig. 3B) and 0.868 mg · h/liter (95% CI, 0.743 to 0.993 mg · h/liter) for predicting satisfactory PPCs at late steady state.
This is the largest study to date that explores pre-steady-state PPCs as a predictor of steady-state concentrations. We found a strong correlation between trough PPCs obtained between days 3 and 5 of prophylaxis with steady-state PPCs in patients receiving PCZ oral solution. Our results are in line with those observed in a smaller study (16 patients) which found that postdosage PPCs obtained at day 2 may predict steady-state PPCs (35). That previous study was limited not only by the small sample size but also by the fact that PPCs were evaluated only at a single pre-steady-state time point. Our results indicate that trough PPCs obtained as early as day 3 may be reliable predictors of satisfactory PPCs at steady state. Obtaining PPCs at pre-steady state (i.e., early in the course of prophylaxis) may allow the clinician to act early on potentially subtherapeutic PPCs with measures such as patient education, increase of PCX dosage, or a switch to the PCZ delayed-release tablet formulation or alternative prophylactic antifungal agents (32).
Overall, PPCs at pre-steady state did not differ significantly from those obtained during steady state. In a recent study, Durani and coworkers showed that PPCs obtained between days 5 and 7 were significantly lower than those obtained later (median of 0.94 mg/liter versus 1.31 mg/liter; P = 0.04) (11). In contrast to what was done in our study, a significant proportion of patients in the study by Durani et al. received PCZ delayed-release tablets, which may not only explain the higher PPCs obtained in that study but also indicate that accumulation of PCZ may be an important factor in patients with very high PPCs at pre-steady state. This is of interest, as visual hallucinations associated with high PPCs have recently been described (36).
Our study is subject to important limitations, which mostly relate to the observational design of the study and the real-life setting of PCZ TDM. Most importantly, the analysis focused primarily on correlation of pre-steady-state PPCs with early-steady-state PPCs, due to the fact that (i) late-steady-state PPCs were obtained in only 70% of patients, and (ii) other factors, such as patient education, may have impacted late-steady-state PPCs but not early-steady-state PPCs, as pre-steady state was not communicated to the patients or providers.
In conclusion, pre-steady-state trough PPCs obtained as early as day 3 may predict steady-state PPCs. Studies are needed to evaluate the potential benefits of pre-steady-state-PPC measurements and early intervention (e.g., modification of intake procedure, dosage, and/or formulation) in patients receiving PCZ oral solution.
ACKNOLWEDGMENTS
M.H. received research grants from Merck, Gilead, and Pfizer, served on the speakers' bureaus of Pfizer, Gilead, Astellas, Basilea, and Merck, and received travel grants from Astellas, Merck, Gilead, and Pfizer. All other authors declare no conflicts of interest.
Funding Statement
This work was supported by funds from the Oesterreichische Nationalbank (Anniversary Fund, project number 15346) and a Merck Investigator Studies Program (39543) research grant. The funders had no role in the study design, data collection, analysis, interpretation, decision to publish, writing of the manuscript, or decision to submit the manuscript for publication.
REFERENCES
- 1.Smith WJ, Drew RH, Perfect JR. 2009. Posaconazole's impact on prophylaxis and treatment of invasive fungal infections: an update. Expert Rev Anti Infect Ther 7:165–181. doi: 10.1586/14787210.7.2.165. [DOI] [PubMed] [Google Scholar]
- 2.Vehreschild JJ, Ruping MJ, Wisplinghoff H, Farowski F, Steinbach A, Sims R, Stollorz A, Kreuzer KA, Hallek M, Bangard C, Cornely OA. 2010. Clinical effectiveness of posaconazole prophylaxis in patients with acute myelogenous leukaemia (AML): a 6 year experience of the Cologne AML cohort. J Antimicrob Chemother 65:1466–1471. doi: 10.1093/jac/dkq121. [DOI] [PubMed] [Google Scholar]
- 3.Vehreschild JJ, Birtel A, Vehreschild MJ, Liss B, Farowski F, Kochanek M, Sieniawski M, Steinbach A, Wahlers K, Fatkenheuer G, Cornely OA. 2013. Mucormycosis treated with posaconazole: review of 96 case reports. Crit Rev Microbiol 39:310–324. doi: 10.3109/1040841X.2012.711741. [DOI] [PubMed] [Google Scholar]
- 4.Arikan S, Sancak B, Alp S, Hascelik G, McNicholas P. 2008. Comparative in vitro activities of posaconazole, voriconazole, itraconazole, and amphotericin B against Aspergillus and Rhizopus, and synergy testing for Rhizopus. Med Mycol 46:567–573. doi: 10.1080/13693780801975576. [DOI] [PubMed] [Google Scholar]
- 5.Campoli P, Al Abdallah Q, Robitaille R, Solis NV, Fielhaber JA, Kristof AS, Laverdiere M, Filler SG, Sheppard DC. 2011. Concentration of antifungal agents within host cell membranes: a new paradigm governing the efficacy of prophylaxis. Antimicrob Agents Chemother 55:5732–5739. doi: 10.1128/AAC.00637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campoli P, Perlin DS, Kristof AS, White TC, Filler SG, Sheppard DC. 2013. Pharmacokinetics of posaconazole within epithelial cells and fungi: insights into potential mechanisms of action during treatment and prophylaxis. J Infect Dis 208:1717–1728. doi: 10.1093/infdis/jit358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 8.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Ortega I, Patino B, Arnan M, Peralta T, Parody R, Gudiol C, Encuentra M, Fernandez de Sevilla A, Duarte RF. 2011. Clinical efficacy and safety of primary antifungal prophylaxis with posaconazole vs itraconazole in allogeneic blood and marrow transplantation. Bone Marrow Transplant 46:733–739. doi: 10.1038/bmt.2010.185. [DOI] [PubMed] [Google Scholar]
- 10.Heimann SM, Cornely OA, Vehreschild MJ, Glossmann J, Kochanek M, Kreuzer KA, Hallek M, Vehreschild JJ. 2014. Treatment cost development of patients undergoing remission induction chemotherapy: a pharmacoeconomic analysis before and after introduction of posaconazole prophylaxis. Mycoses 57:90–97. doi: 10.1111/myc.12105. [DOI] [PubMed] [Google Scholar]
- 11.Durani U, Tosh PK, Barreto JN, Estes LL, Jannetto PJ, Tande AJ. 2015. Retrospective comparison of posaconazole levels in patients taking the delayed-release tablet versus the oral suspension. Antimicrob Agents Chemother 59:4914–4918. doi: 10.1128/AAC.00496-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham AN, Bubalo JS, Lewis JS II. 2016. Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses 59:226–233. doi: 10.1111/myc.12452. [DOI] [PubMed] [Google Scholar]
- 13.Cumpston A, Caddell R, Shillingburg A, Lu X, Wen S, Hamadani M, Craig M, Kanate AS. 2015. Superior serum concentrations with posaconazole delayed-release tablets compared to suspension formulation in hematological malignancies. Antimicrob Agents Chemother 59:4424–4428. doi: 10.1128/AAC.00581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Elst KCM, Brouwers CHS, van der Heuvel ER, MJ van Wanrooy MJP, Uges DRA, van der Werf TS, Kosterink JGW, Span LFR, Alfenaar J-WC. 2015. Subtherapeutic posaconazole exposure and treatment outcome in patients with invasive fungal disease. Ther Drug Monit 37:766–771. doi: 10.1097/FTD.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 15.Gross BN, Ihorst G, Jung M, Wasch R, Engelhardt M. 2013. Posaconazole therapeutic drug monitoring in the real-life setting: a single-center experience and review of the literature. Pharmacotherapy 33:1117–1125. doi: 10.1002/phar.1328. [DOI] [PubMed] [Google Scholar]
- 16.Miceli MH, Perissinotti AJ, Kauffman CA, Couriel DR. 2015. Serum posaconazole levels among haematological cancer patients taking extended release tablets is affected by body weight and diarrhoea: single centre retrospective analysis. Mycoses 58:432–436. doi: 10.1111/myc.12339. [DOI] [PubMed] [Google Scholar]
- 17.Vaes M, Hites M, Cotton F, Bourguignon AM, Csergo M, Rasson C, Ameye L, Bron D, Jacobs F, Aoun M. 2012. Therapeutic drug monitoring of posaconazole in patients with acute myeloid leukemia or myelodysplastic syndrome. Antimicrob Agents Chemother 56:6298–6303. doi: 10.1128/AAC.01177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vehreschild JJ, Muller C, Farowski F, Vehreschild MJ, Cornely OA, Fuhr U, Kreuzer KA, Hallek M, Kohl V. 2012. Factors influencing the pharmacokinetics of prophylactic posaconazole oral suspension in patients with acute myeloid leukemia or myelodysplastic syndrome. Eur J Clin Pharmacol 68:987–995. doi: 10.1007/s00228-012-1212-y. [DOI] [PubMed] [Google Scholar]
- 19.Kohl V, Muller C, Cornely OA, Abduljalil K, Fuhr U, Vehreschild JJ, Scheid C, Hallek M, Ruping MJ. 2010. Factors influencing pharmacokinetics of prophylactic posaconazole in patients undergoing allogeneic stem cell transplantation. Antimicrob Agents Chemother 54:207–212. doi: 10.1128/AAC.01027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith J, Andes D. 2008. Therapeutic drug monitoring of antifungals: pharmacokinetic and pharmacodynamic considerations. Ther Drug Monit 30:167–172. doi: 10.1097/FTD.0b013e318167d0e0. [DOI] [PubMed] [Google Scholar]
- 21.Hoenigl M, Raggam RB, Salzer HJ, Valentin T, Valentin A, Zollner-Schwetz I, Strohmeier AT, Seeber K, Wolfler A, Sill H, Krause R. 2012. Posaconazole plasma concentrations and invasive mould infections in patients with haematological malignancies. Int J Antimicrob Agents 39:510–513. doi: 10.1016/j.ijantimicag.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Vanstraelen K, Colita A, Bica AM, Mols R, Augustijns P, Peersman N, Vermeersch P, Annaert P, Spriet I. 2016. Pharmacokinetics of posaconazole oral suspension in children dosed according to body surface area. Pediatr Infect Dis J 35:183–188. doi: 10.1097/INF.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 23.Dolton MJ, Ray JE, Chen SC, Ng K, Pont L, McLachlan AJ. 2012. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob Agents Chemother 56:5503–5510. doi: 10.1128/AAC.00802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis 44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 25.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. 2014. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornely OA, Helfgott D, Langston A, Heinz W, Vehreschild JJ, Vehreschild MJ, Krishna G, Ma L, Huyck S, McCarthy MC. 2012. Pharmacokinetics of different dosing strategies of oral posaconazole in patients with compromised gastrointestinal function and who are at high risk for invasive fungal infection. Antimicrob Agents Chemother 56:2652–2658. doi: 10.1128/AAC.05937-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courtney R, Pai S, Laughlin M, Lim J, Batra V. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother 47:2788–2795. doi: 10.1128/AAC.47.9.2788-2795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtney R, Radwanski E, Lim J, Laughlin M. 2004. Pharmacokinetics of posaconazole coadministered with antacid in fasting or nonfasting healthy men. Antimicrob Agents Chemother 48:804–808. doi: 10.1128/AAC.48.3.804-808.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoenigl M. 2014. Posaconazole plasma concentrations: correlation of early trough levels (day 4) with steady-state concentrations, poster 74. Abstr 6th Adv Against Aspergillosis. University of Manchester, Manchester, United Kingdom. [Google Scholar]
- 30.Hoenigl M, Duettmann W, Huber-Krassnitzer B, Wagner J, Prattes J, Troppan K, Seeber K, Raggam RB, Wölfler A, Krause R. 2014. Correlation of early trough levels (day 4) with steady-state posaconazole plasma concentrations: a cohort study, poster eP256. Abstr 24th Eur Congr Clin Microbiol Infect Dis. ESCMID, Basel, Switzerland. [Google Scholar]
- 31.Hoenigl M, Duettmann W, Raggam RB, Seeber K, Troppan K, Fruhwald S, Prueller F, Wagner J, Valentin T, Zollner-Schwetz I, Wolfler A, Krause R. 2013. Potential factors for inadequate voriconazole plasma concentrations in intensive care unit patients and patients with hematological malignancies. Antimicrob Agents Chemother 57:3262–3267. doi: 10.1128/AAC.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoenigl M, Duettmann W, Raggam RB, Huber-Krassnitzer B, Theiler G, Seeber K, Prueller F, Zollner-Schwetz I, Prattes J, Wagner J, Wolfler A, Krause R. 2014. Impact of structured personal on-site patient education on low posaconazole plasma concentrations in patients with haematological malignancies. Int J Antimicrob Agents 44:140–144. doi: 10.1016/j.ijantimicag.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Shields RK, Clancy CJ, Vadnerkar A, Kwak EJ, Silveira FP, Massih RC, Pilewski JM, Crespo M, Toyoda Y, Bhama JK, Bermudez C, Nguyen MH. 2011. Posaconazole serum concentrations among cardiothoracic transplant recipients: factors impacting trough levels and correlation with clinical response to therapy. Antimicrob Agents Chemother 55:1308–1311. doi: 10.1128/AAC.01325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 53:24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green MR, Woolery JE. 2012. Posaconazole serum level on day 2 predicts steady state posaconazole serum level. Ther Drug Monit 34:118–119. doi: 10.1097/FTD.0b013e31823cef77. [DOI] [PubMed] [Google Scholar]
- 36.Parkes LO, Cheng MP, Sheppard DC. 2016. Visual hallucinations associated with high posaconazole concentrations in serum. Antimicrob Agents Chemother 60:1170–1171. doi: 10.1128/AAC.02739-15. [DOI] [PMC free article] [PubMed] [Google Scholar]