Abstract
Ebola outbreaks occur on a frequent basis, with the 2014-2015 outbreak in West Africa being the largest one ever recorded. This outbreak has resulted in over 11,000 deaths in four African countries and has received international attention and intervention. Although there are currently no approved therapies or vaccines, many promising candidates are undergoing clinical trials, and several have had success in promoting recovery from Ebola. However, these prophylactics and therapeutics have been designed and tested only against the same species of Ebola virus as the one causing the current outbreak. Future outbreaks involving other species would require reformulation and possibly redevelopment. Therefore, a broad-spectrum alternative is highly desirable. We have found that a flavonoid derivative called quercetin 3-β-O-d-glucoside (Q3G) has the ability to protect mice from Ebola even when given as little as 30 min prior to infection. Furthermore, we have demonstrated that this compound targets the early steps of viral entry. Most promisingly, antiviral activity against two distinct species of Ebola virus was seen. This study serves as a proof of principle that Q3G has potential as a prophylactic against Ebola virus infection.
INTRODUCTION
The 2014-2015 outbreak of Ebola virus (EBOV) resulted in over 11,000 deaths (http://www.who.int/) and has highlighted the urgent need for novel therapies to combat this disease. At present, there are no approved therapies against EBOV, although several treatments show promise (reviewed in reference 1). However, the prophylactics and therapeutics under consideration would require reformulation and possibly redevelopment if an outbreak were to occur with a heterologous or novel filovirus. Therefore, a panfilovirus alternative is desirable. Small-molecule drugs are of particular interest, due to their comparatively low production costs, potentially noninvasive administration routes, and possibility for cross-protection.
To date, numerous small molecules that inhibit Ebola virus in vitro and in vivo have been identified (2 – 4). However, only a few of these compounds have shown protection in animal models of EBOV, which is a critical step for screening potential drug candidates for future human clinical trials (3, 5, 6). One class of compounds that has emerged with many promising antiviral candidates is the group of flavonoid molecules. Flavonoids are classified as polyphenol chemical compounds and are common in plants where they function in pigmentation (UV protection), in signaling processes, and as antimicrobials (7, 8). The role of polyphenols in the diet has also been widely studied for antioxidant, anticarcinogenic, anti-inflammatory, and antihypercholesterolemic activities as well as cardio- and neuroprotective actions. The mechanisms behind flavonoid-derived effects on disease include the inhibition of numerous cellular pathways such as mitogen-activated protein kinase (MAPK) (9) and NF-κB (10, 11) as well as the inhibition of Src, phosphatidylinositol 3-kinase (PI3K), PDK1, and Akt (10). Cellular processes that are inhibited or modulated by flavonoids include oxidative stress (reviewed in reference 12), cathepsin activity (13, 14), and autophagy induction (reviewed in reference 15).
In this study, we used a derivative of the flavonoid quercetin, which can be modified through the addition of different sugar molecules. Quercetin 3-β-O-d-glucoside (Q3G), for example, is a natural derivative of quercetin that contains a glucoside molecule, and this has been shown to enhance the solubility and bioavailability of the compound when ingested (16). Although much research on quercetin and its derivatives has focused on their properties as potential therapeutic agents against cancer and inflammatory diseases and their anti-metabolic disease effects (17), recent studies have also demonstrated antiviral activity against a wide variety of viruses, including influenza virus, Chikungunya virus, Epstein-Barr virus, hepatitis C virus, and Mayaro virus (18 – 22). While a detailed mechanism of action remains undefined, it has been proposed that quercetin and its derivatives affect a step in the viral entry process.
Collectively, it has been found that flavonoid compounds have antiviral activity against a range of viruses, which therefore raises the question of whether they could be used as broad-spectrum antivirals. This led us to investigate whether Q3G was efficacious against Ebola virus. Here we have evaluated its effect on both cellular and small-animal models of Ebola infection and demonstrated that it targets the viral entry process.
MATERIALS AND METHODS
Cells and viruses.
Vero E6 cells were maintained in Dulbecco's modified Eagle medium (DMEM) (HyClone) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich). The generation of pseudotyped vesicular stomatitis viruses (VSVs) containing the glycoproteins of Ebola virus, Sudan virus (SUDV), and Reston virus (RESTV) (VSVΔGP-EBOV, VSVΔGP-SUDV, and VSVΔGP-RESTV, respectively) was reported previously (23, 24). The following ebolaviruses were used: EBOV strain Kikwit-GFP (EBOV-Kikwit-GFP) (based on H.sapiens-tc/COD/1995/Kikwit-9510621 [GenBank accession no. AY354458]), EBOV-Makona (H.sapiens-tc/GIN/2014/Gueckedou-C07 [GenBank accession no. KJ660347.2]), SUDV-Boniface (H.sapiens-tc/SUD/1976/Boniface [GenBank accession no. FJ968794.1]), and mouse-adapted EBOV (MA-EBOV) (Ebola virus strain USAMRIID/BALB/c-lab/COD/1976/Mayinga-MA-p3).
Virus infections in a BSL-4 facility.
All work with infectious virus was performed in the biosafety level 4 (BSL-4) facility at the National Microbiology Laboratory (NML) of the Public Health Agency of Canada (PHAC) in the Canadian Science Centre for Human and Animal Health (CSCHAH), Winnipeg, Canada. All procedures were conducted in accordance with international protocols appropriate for this level of biosafety. All animal work was performed in accordance with guidelines laid out by the CCAC, and protocols were approved by our Animal Care Committee.
Cell viability assays.
The toxicity of Q3G was evaluated in Vero E6 cells by using the PrestoBlue cell viability reagent, which is a resazurin dye-based assay (Life Technologies, Canada). Cells were plated, allowed to adhere overnight, and then treated with various concentrations of Q3G for 2 h. Control cells received an equivalent volume of 10% dimethyl sulfoxide (DMSO) only. PrestoBlue cell viability reagent was added according to the manufacturer's protocol. Viability was determined by comparing fluorescence readings of treated cells to those of untreated controls. All samples were run in triplicate, and each experiment was performed a minimum of three times. The CC50 and CC90 values were calculated by using a four-parameter logistic regression in Prism 5 (GraphPad).
Q3G dose-response curve and 50% inhibitory concentration (IC50) calculation.
Vero E6 cells were pretreated with Q3G (0 to 200 μM) or DMSO for 1 h at 37°C and infected at a multiplicity of infection (MOI) of 0.1 with enhanced green fluorescent protein (eGFP)-expressing EBOV in the presence of Q3G or DMSO for 1 h at 37°C. The inoculum was removed and replaced with fresh medium (DMEM–2% FBS). Cells were then further incubated for 72 h in the presence of Q3G or DMSO. At 72 h, cells were imaged by using an Evos FL instrument and fixed with 10% formalin for 48 h, and the green fluorescent protein signal was quantified on a Biotek Synergy HTX plate reader. Infection was determined by comparing fluorescence readings of Q3G-treated infected cells to those of DMSO-treated controls. The 50% effective concentration (EC50) and EC90 values were calculated by using a four-parameter logistic regression in Prism 5 (GraphPad). Selectivity index values were calculated as the CC50/EC50 ratio.
Quantification of EBOV and SUDV by quantitative PCR (qPCR) for in vitro studies.
Vero E6 cells were first treated with 10 μM Q3G for 1 h prior to infection. Cells were then infected at an MOI of 0.1 with EBOV-Makona or SUDV for 1 h at 37°C in the presence of Q3G. Cells were then washed with phosphate-buffered saline (PBS), and medium was replaced with DMEM supplemented with 1% penicillin-streptomycin, 4% fetal bovine serum, and Q3G. At 5 days postinfection, the cell supernatant was placed into AVL buffer (Qiagen), and viral RNA was extracted by using the QIAamp viral RNA minikit (Qiagen). EBOV and SUDV genomic material was detected by using the LightCycler 480 RNA Master Hydrolysis Probes kit (Roche), targeting the RNA polymerase gene (nucleotides 16472 to 16538 [GenBank accession no. AF086833]). PCR conditions were 63°C for 3 min, 95°C for 30 s, and cycling at 95°C for 15 s and 60°C for 30 s for 45 cycles on an ABI StepOnePlus thermocycler. The lower detection limit for this assay is 86 genome equivalents (GEQ)/ml. The primers used were as follows: EBOV-L-F2 (CAGCCAGCAATTTCTTCCAT), EBOV-L-R2 (TTTCGGTTGCTGTTTCTGTG), EBOV-L-P2FAM (6-carboxyfluorescein [FAM]-ATCATTGGCGTACTGGAGGAGCAG-black hole quencher 1 [BHQ1]), SEBOV-L-F (CAGAAGACAATGCAGCCAGA), SEBOV-L-R (TTGAGGAATATCCCACAGGC), and SEBOV-L-PFAM (CTGCTAGCTTGGCCAAAGTCACAAG-BHQ1).
Quantification of EBOV by qPCR for in vivo studies.
RNA was extracted from whole-blood samples with the QIAamp viral RNA minikit (Qiagen) and was extracted from spleen, kidney, lung, brain, and liver tissue samples by using the RNeasy Plus minikit (Qiagen). EBOV genomic material was detected as described above.
In vivo treatment with Q3G and Ebola virus.
Six- to eight-week-old female BALB/c or C57BL/6 mice (Charles River) were treated with 50 mg/kg of body weight of Q3G or 10% DMSO at the time points indicated. Q3G was administered by intraperitoneal injection. Q3G was administered for either 13 days, 7 days, 3 days, or 30 min prechallenge or 24 h postchallenge. Mice in all groups received a challenge dose of 1,000× the 50% lethal dose (LD50) of mouse-adapted Ebola virus (Mayinga variant) in 200 μl of DMEM (pH 7.4) by intraperitoneal injection. Following infection, the treatment regimen continued at the same dosage for an additional 2 weeks.
Toxicity study.
Eighty naive C57BL/6 male mice (at least 9 weeks of age, weighing 23 to 28 g) were randomly assigned to groups (n = 10) and dosed with 12.5 to 400 mg/ml Q3G or 10% DMSO in a single 5-ml intraperitoneal injection. Changes in body weight were monitored for 14 days as an indication of toxicity.
Quantification of VSV and VSV-Ebola virus inhibition by Q3G.
Vero E6 cells were pretreated with 10 μM Q3G or DMSO for 1 h at 37°C and infected at an MOI of 0.1 with VSV, VSV-EBOV, VSV-SUDV, or VSV-RESTV for 1 h at 37°C. Cells were then overlaid with fresh medium. Some experiments included Q3G after viral adsorption, and some were done in the absence of Q3G after adsorption. At 72 h, supernatants were harvested, and titers were determined on Vero E6 cells by using a 50% tissue culture infective dose (TCID50) assay.
TCID50 assay.
Vero E6 cells were grown to 95% confluence and infected with 10-fold serial dilutions of the cell supernatant for 1 h at 37°C. The inoculum was then removed, and cells were overlaid with fresh DMEM plus 2% FBS. At 72 h postinfection, the plates were assessed for the lowest dilution at which 50% of the wells exhibited cytopathology. TCID50s per milliliter were calculated according to the Reed-Muench method.
Effect of Q3G on viral infectivity.
Two hundred microliters of VSV, VSV-EBOV, VSV-SUDV, and VSV-RESTV stocks was treated with 10 μM Q3G or DMSO for 1 h at 37°C, purified by using a 20% sucrose cushion (30,000 × g at 4°C for 2 h), and resuspended in 200 μl PBS. The titer of the purified virus was then determined by using a TCID50 assay.
Statistics.
Differences in survival were calculated for Q3G groups compared to DMSO treatment groups by using a log rank (Mantel-Cox) test in Prism 5. Unpaired, two-sided t tests with Welch's correction were performed to determine differences between DMSO and Q3G treatment groups in vitro.
RESULTS
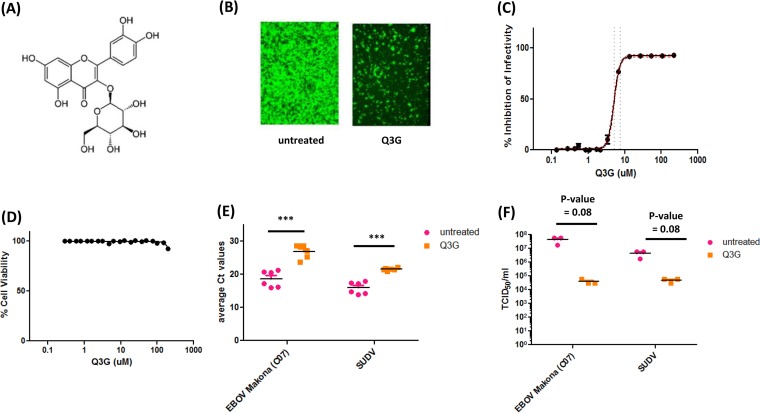
Quercetin 3-β-O-d-glucoside (Fig. 1A) is a glucosylated form of quercetin, a flavonoid compound that has been shown to exhibit antiviral activity against several RNA viruses (18 – 20). In order to test the antiviral activity of our compound against ebolavirus, we first calculated the EC50 and EC90 values using Vero E6 epithelial cells. Cells were pretreated with 2-fold dilutions of Q3G for 1 h and infected with eGFP-expressing EBOV at an MOI of 0.1 for 1 h in the presence of Q3G. The infected cells were incubated in the presence of Q3G for 3 days, after which fluorescent images of each Q3G dilution were taken (one dilution is represented in Fig. 1B), and fluorescence was quantified by using a Bio-Tek plate reader. This produced a curve from which the EC50 and EC90 values were calculated to be 5.3 μM (confidence interval [CI], ±0.32) and 9.3 μM (CI, ±0.67 μM), respectively (Fig. 1C). Importantly, the decrease in viral titers could not be attributed to the toxicity of Q3G, as the cells exhibited 100% viability at all concentrations tested (Fig. 1D). In addition, we confirmed that Q3G also inhibited the replication of other variants and viruses of the Ebola virus genus, including wild-type EBOV-Makona and SUDV-Boniface, using reverse transcription-qPCR (RT-qPCR) (Fig. 1E) and a TCID50 assay (Fig. 1F). Collectively, these data demonstrate the in vitro antiviral activity of Q3G against multiple wild-type ebolaviruses.
FIG 1.
Q3G inhibits replication of Ebola virus in vitro. (A) Chemical structure of Q3G. (B) Vero E6 cells were pretreated with 10 μM Q3G for 1 h and then infected with EBOV-Kikwit-GFP (MOI = 0.1) at 37°C and incubated for 72 h in the presence of 10 μM Q3G. (C) The EC50 of Q3G against EBOV-Kikwit-GFP was determined by pretreating Vero E6 cells with serial dilutions of Q3G for 1 h and then infecting them with virus (MOI = 0.1) at 37°C for 72 h in the presence of Q3G, at which time fluorescence was quantified. (D) To determine cell viability following Q3G treatment, Vero E6 cells were treated with serial dilutions of Q3G. Viability was measured after 72 h by a resazurin dye-based assay, and data were normalized to data for untreated controls. (E) Inhibition of EBOV-Makona and SUDV was determined by pretreating cells with 10 μM Q3G for 1 h and then infecting them with virus (MOI = 0.1) at 37°C for 1 h. Cells were collected, and the amount of viral RNA was quantified at 5 days postinfection by RT-qPCR. Ct, threshold cycle. (F) Inhibition of EBOV-Makona and SUDV was determined by pretreating cells with 10 μM Q3G for 1 h and then infecting them with virus (MOI = 0.1) at 37°C for 1 h. Cells were collected, and the amount of viral RNA was quantified at 5 days postinfection by using a TCID50 assay. All experiments were performed in triplicate, and error bars represent the standard errors of the means. ***, P value of <0.001.
In order to test whether the antiviral activity observed in vitro would also be protective in vivo, we first performed a pilot experiment (n = 10) in which we treated C57BL/6 mice with 50 mg/kg of Q3G every other day for 2 weeks, followed by challenge with a lethal dose (1,000× LD50) of MA-EBOV. Q3G treatments were also continued every 48 h after infection (Fig. 2A). All control animals succumbed to viral challenge with a mean time to death of 7.4 ± 1.1 days, while all Q3G-treated mice survived (Fig. 2B) and showed only mild signs of disease, such as minimal weight loss (Fig. 2C). For our second experiment, we chose to test the robustness of Q3G efficacy by using BALB/c mice, which have a different genetic background and immune response from those of C57BL/6 mice. In addition to pretreatment for 2 weeks, we also used shorter pretreatment times, including 13 days, 7 days, 3 days, 1 day, and 30 min, as well as one posttreatment time at 24 h postinfection (Fig. 2D). Q3G treatments were also continued every 48 h after infection (Fig. 2D). We found that Q3G fully protected mice against viral challenge even when given only 30 min prior to infection (Fig. 2E) and resulted in <10% weight loss (Fig. 2F). In contrast, only 3/10 mice that received Q3G at 24 h postchallenge survived (Fig. 2E) and had significant weight loss of 20% (Fig. 2F).
FIG 2.
Prophylactic treatment with Q3G promotes survival and inhibits Ebola virus replication. (A) Experimental plan outlining treatment. Six- to eight-week-old female C57BL/6 mice (Charles River) were treated with 50 mg/kg of Q3G (n = 10) or 10% DMSO (n = 10) via intraperitoneal injection. Mice in both groups received a challenge dose of 1,000× LD50 of mouse-adapted Ebola virus (Mayinga isolate) in 200 μl of PBS (pH 7.4) by intraperitoneal injection. (B and C) Survival (B) and changes in weight (C) of Q3G-treated and untreated mice. (D) Experimental plan outlining treatment. Six- to eight-week-old female C57BL/6 mice were treated with 50 mg/kg of Q3G (n = 10) or 10% DMSO (n = 10) via intraperitoneal injection. Mice in both groups received a challenge dose of 1,000× LD50 of mouse-adapted Ebola virus (Mayinga isolate) in 200 μl of PBS (pH 7.4) by intraperitoneal injection. 13dbi, 13 days before infection; 1dpi, 1 day postinfection. (E and F) Survival (E) and changes in weight (F) of Q3G-treated and untreated mice. (G) Experimental plan outlining treatment. Six- to eight-week-old female BALB/c mice were treated with 50 mg/kg of Q3G (n = 10) or 10% DMSO (n = 10) via intraperitoneal injection. Mice in both groups received a challenge dose of 1,000× LD50 of mouse-adapted Ebola virus (Mayinga isolate) in 200 μl of PBS (pH 7.4) by intraperitoneal injection. (H) Virus in the blood, liver, kidney, spleen, lung, and brain was quantified by RT-qPCR amplification of the Ebola virus L gene in Q3G-treated and untreated mice on day 6.
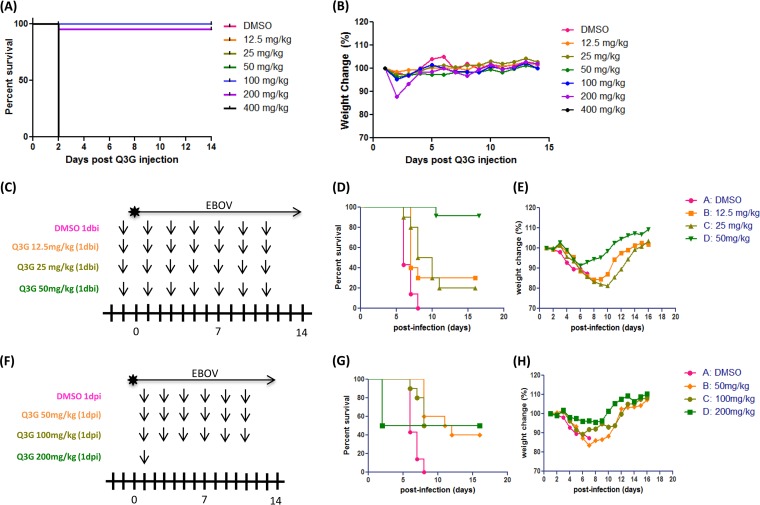
Next, we chose to investigate whether lower prophylactic doses of Q3G or higher postexposure doses would be equally or more protective. We first carried out a toxicity assay in which groups of mice (n = 10) were given a single intraperitoneal injection of different doses of Q3G (0 to 400 mg/kg) and then weighed and scored daily to monitor signs of toxicity. All mice that received 400 mg/kg died within 2 days (Fig. 3A). One mouse that received 200 mg/kg died on day 2 as well, and the others lost ∼15% of their weight within the first 3 days but then recovered (Fig. 3B). All other mice survived and remained alert and active, without signs of toxicity (Fig. 3A and B). To test different prophylactic treatment concentrations, we used 12.5, 25, and 50 mg/ml Q3G (Fig. 3C). Compared to 9/10 mice surviving with 50 mg/kg Q3G (Fig. 3D) and with minimal weight loss (Fig. 3E), both 25 mg/kg and 12.5 mg/kg Q3G resulted in the survival of 2/10 and 3/10 mice, respectively (Fig. 3D), and >20% weight loss (Fig. 3E). To test whether higher concentrations of Q3G would provide greater protection when given postexposure, we treated mice with 50, 100, and 200 mg/kg Q3G (Fig. 3F). Surprisingly, all three doses resulted in similar survival rates, with survival of 4/10 or 5/10 mice, and did not alter the average time to death (Fig. 3G). Similarly, weight loss was not significantly different with higher doses of Q3G (Fig. 3H). Notably, 5/10 mice died on day 2 after treatment with 200 mg/kg Q3G, and hence, treatment was discontinued.
FIG 3.
Dosing and toxicity of Q3G. Naive C57BL/6 male mice were dosed with 12.5 to 400 mg/ml Q3G or 10% DMSO intraperitoneally. (A and B) Survival (A) and changes in body weight (B) were monitored for 14 days as an indication of toxicity. (C) Experimental plan outlining treatment. Six- to eight-week-old female BALB/c mice were treated with 50 mg/kg Q3G (n = 10 per group) or 10% DMSO (n = 10) via intraperitoneal injection. Mice in all groups received a challenge dose of 1,000× LD50 of mouse-adapted Ebola virus (Mayinga isolate) in 200 μl PBS (pH 7.4) by intraperitoneal injection. (D and E) Survival (D) and changes in weight (E) of Q3G-treated and untreated mice. (F) Experimental plan outlining treatment. Six- to eight-week-old female BALB/c mice received a challenge dose of 1,000× LD50 of mouse-adapted Ebola virus (Mayinga isolate) in 200 μl of PBS (pH 7.4) by intraperitoneal injection. At 24 h postinfection, mice were treated with 50, 100, or 200 mg/kg of Q3G (n = 10) or 10% DMSO (n = 10) every other day via intraperitoneal injection. (G and H) Survival (G) and changes in weight (H) of Q3G-treated and untreated mice.
To test the mechanism of the effect of Q3G on ebolavirus replication, we first investigated whether Q3G was effective at blocking ebolaviruses at an entry or postentry step. We utilized wild-type VSV and VSV-Ebola virus constructs in which the outer glycoprotein of VSV was replaced with the glycoprotein of ebolaviruses. Hence, this construct is equipped with the cell tropism and entry mechanisms of a filovirus but retains the VSV replication machinery. The addition of Q3G only as a pretreatment had no effect on wild-type VSV replication but strongly reduced the replication of multiple VSV-Ebola virus constructs, including VSV-EBOV, VSV-SUDV, and VSV-RESTV (Fig. 4A). However, if cells were pretreated and then kept in the presence of Q3G throughout the viral replication process, wild-type VSV titers decreased by 5 logs (Fig. 4B), and our pseudotyped VSV constructs displayed further log reductions in titers (Fig. 4B). We then proceeded to test several steps that were previously shown to be important for ebolavirus entry, including viral particle infectivity (Fig. 4C), cathepsin activity (see Fig. S1A and S1B in the supplemental material), and lysosomal pH (see Fig. S2 in the supplemental material), but did not find any effect of Q3G on any of these processes.
FIG 4.
Q3G inhibits entry of Ebola viruses. (A) Vero E6 cells were pretreated with 10 μM Q3G for 1 h and then infected with VSV-EBOV, VSV-SUDV, or VSV-RESTV (MOI = 0.1) at 37°C for 1 h in the absence of Q3G. Fresh medium was added without Q3G, supernatants were harvested at 72 h, and titers were determined by using a TCID50 assay. (B) Vero E6 cells were pretreated with 10 μM Q3G for 1 h and then infected with VSV-EBOV, VSV-SUDV, or VSV-RESTV (MOI = 0.1) at 37°C for 1 h in the presence of Q3G. Fresh medium was added with Q3G, supernatants were harvested at 72 h, and titers were determined by using a TCID50 assay. All experiments were performed in triplicate, and error bars represent the standard errors of the means. (C) Two hundred microliters of VSV, VSV-EBOV, VSV-SUDV, and VSV-RESTV stocks was incubated with 10 μM Q3G for 1 h at 37°C, and titers were then determined by using a TCID50 assay. All experiments were performed in triplicate, and error bars represent the standard errors of the means. *, P value of <0.05; **, P value of <0.01; ***, P value of <0.001.
DISCUSSION
Quercetin and its derivatives have been shown to exert antiviral activities against a variety of viruses. For example, numerous quercetin derivatives have demonstrated antiviral activity against influenza virus, including isoquercetin (18), resveratrol (18), quercetin (18), fisetin (18), isorhamnetin (25), and kaempferol (25). Similarly, quercetin 3-O-glycosides protect against Mayaro virus (19), and quercetin protects against both Mayaro virus (19) and herpes simplex virus 1 (HSV-1) (11). The present study is the first to demonstrate that Ebola virus is also strongly inhibited by a quercetin derivative.
As in the present study, the majority of previous studies also demonstrated that pretreatment rather than posttreatment of cells produces the best protection against viral replication and cell death. With HSV-1, however, both pretreatment and late posttreatment (after 14 h) administrations protected cells against infection (11), perhaps suggesting a dual mechanism. In contrast, VSV was inhibited only postinfection in this study. What is striking, however, is the diversity of viruses that these flavonoids can inhibit. Antiviral activity has been shown against both RNA and DNA viruses that fall within different viral families and are grouped within different Baltimore replication classes. Defining the cellular mechanisms that lead to such broad antiviral activity would be highly desirable for the development of other broad-spectrum therapeutics.
In terms of specific targets in the entry process that may be inhibited, there are multiple steps that are critical for viral entry and that were tested individually by using a VSV-based construct that carried the Ebola virus glycoprotein. While the entry of wild-type VSV was not inhibited by Q3G, entry of the VSV-Ebola virus construct was strongly inhibited. This discrepancy strongly suggests that Q3G affects a glycoprotein-mediated step in the viral life cycle, i.e., viral entry. Notably, the inhibition with the VSV system was similar to the inhibition found with wild-type Ebola virus (4- to 5-log reduction), suggesting that the VSV system recapitulates the effect of Q3G on wild-type virus and is a useful model for studying its effect on viral entry. The entry mechanisms used by Ebola virus include, for example, viral attachment; internalization into endosomes by macropinocytosis; processing of the glycoprotein by endosomal host cathepsins (14, 26), which is accelerated by fusion with acidic lysosomes; and interaction of the glycoprotein with the endosomal transporter Niemann-Pick C1 (NPC1) (27, 28). Although we did not test the effect of Q3G on NPC1, all of the other known processes of Ebola virus entry were not inhibited by Q3G. A recent study demonstrated that quercetin inhibited the actions of the receptor Niemann-Pick C1-like 1 by decreasing cholesterol uptake in HEK 293T cells (29). Although we did not look specifically at NPC1 expression or cholesterol levels in this study, it is possible that Q3G may affect a cholesterol- or NPC-1-related process and thereby prevent Ebola virus entry. The involvement of cholesterol metabolism is compatible with experimental data demonstrating that Q3G upregulates the low-density lipoprotein (LDL) receptor (LDLR) and the uptake of LDL cholesterol in human hepatocytes (30).
Despite a growing number of small-molecule screens, only a few compounds have been tested in vivo. The estrogen receptor modulators clomiphene and toremifene, for example, have been shown to protect 90% and 50% of mice, respectively, when given at 24 h postinfection (31). Similarly, tetrandrine has been found to inhibit Ebola virus by interacting with NPC1 and protected 80% of mice when given at 24 h postinfection (32). A fourth compound, FGI-106, has demonstrated 100% protection in mice against numerous viruses, including Ebola virus, when given at 24 h postinfection. Additionally, protection at later time points was achieved by GS-5734, a nucleoside analogue (100% protection in mice when given on day 3 postinfection) (2), and T-705, a broad-spectrum antiviral (100% protection in IFNAR−/− mice when given at 6 days postinfection) (33).
Our findings add to this growing list of promising antiviral candidates against Ebola virus and suggest that it can be given prophylactically with low toxicity and few side effects. Although the range of concentrations at which Q3G is effective against Ebola virus in mice was limited in this study, the toxicity and pharmacokinetics of Q3G are fairly well studied in humans, and the compound is well tolerated. Therefore, it is possible that with an optimized route of administration and dosing regimen, Q3G could become a relevant clinical compound against Ebola virus. Delineating the mechanism of Q3G will also be an important step in further improving treatment parameters such as the half-life and target binding efficacy of Q3G. Overall, we believe that there is potential for this compound to become an effective clinical drug against Ebola and also to elucidate novel targets for future antiviral development. Future studies should focus on delineating the mechanism of Q3G, experimenting with alternative routes of administration, and testing the efficacy of Q3G in guinea pig and nonhuman primate models to obtain more preclinical data.
Conclusion.
Our study is the first to demonstrate the antiviral efficacy of Q3G against Ebola virus both in vitro and in vivo. Furthermore, we demonstrated that Q3G inhibited not only the well-studied EBOV but also a second common ebolavirus isolated from Sudan (SUDV), which will be an important future direction in the development of panfilovirus vaccines and therapeutics. Understanding the mechanisms behind the antiviral activity of flavonoids may provide valuable insight into filovirus entry pathways and novel targets for viral therapeutics.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00307-16.
REFERENCES
- 1.Wong G, Kobinger GP. 2015. Backs against the wall: novel and existing strategies used during the 2014-2015 Ebola virus outbreak. Clin Microbiol Rev 28:593–601. doi: 10.1128/CMR.00014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren TK, Wells J, Panchal RG, Stuthman KS, Garze NL, Van Tongeren SA, Dong L, Retterer CJ, Eaton BP, Pegoraro G, Honnold S, Bantia S, Kotian P, Chen X, Taubenheim BR, Welch LS, Minning DM, Babu YS, Sheridan WP, Bavari S. 2014. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. 2013. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picazo E, Giordanetto F. 2015. Small molecule inhibitors of Ebola virus infection. Drug Discov Today 20:277–286. doi: 10.1016/j.drudis.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Madelain V, Oestereich L, Graw F, Nguyen THT, de Lamballerie X, Mentré F, Günther S, Guedj J. 2015. Ebola virus dynamics in mice treated with favipiravir. Antiviral Res 123:70–77. doi: 10.1016/j.antiviral.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Julander JG, Bantia S, Taubenheim BR, Minnin DM, Kotian P, Morrey JD, Smee DF, Sheridan WP, Babu YS. 2014. BCX4430, a novel nucleoside analog, effectively treats yellow fever in a hamster model. Antimicrob Agents Chemother 58:6607–6614. doi: 10.1128/AAC.03368-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iranshahi M, Rezaee R, Parhiz H, Roohbaksh A, Soltani F. 2015. Protective effects of flavonoids against microbes and toxins: the cases of hesperidin and hesperetin. Life Sci 137:125–132. doi: 10.1016/j.lfs.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Treutter D. 2015. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol (Stuttg) 7:581–591. [DOI] [PubMed] [Google Scholar]
- 9.Seo MJ, Lee YJ, Hwang JH, Kim KJ, Lee BY. 2015. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J Nutr Biochem 26:1308–1316. doi: 10.1016/j.jnutbio.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Endale M, Park SC, Kim S, Kim SH, Yang Y, Cho JY, Rhee MH. 2013. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 218:1452–1467. doi: 10.1016/j.imbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Hung PY, Ho BC, Lee SY, Chang SY, Kao CL, Lee SS, Lee CN. 2015. Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS One 10:e0115475. doi: 10.1371/journal.pone.0115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khurana S, Piche M, Hollingsworth A, Venkataraman K, Tai TC. 2013. Oxidative stress and cardiovascular health: therapeutic potential of polyphenols. Can J Physiol Pharmacol 91:198–212. doi: 10.1139/cjpp-2012-0252. [DOI] [PubMed] [Google Scholar]
- 13.Wagoner J, Negash A, Kane OJ, Martinez LE, Nahmias Y, Bourne N, Owen DM, Grove J, Brimacombe C, McKeating JA, Pécheur EI, Graf TN, Oberlies NH, Lohmann V, Cao F, Tavis JE, Polyak SJ. 2010. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology 51:1912–1921. doi: 10.1002/hep.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramalho SD, de Sousa LR, Burger MC, Lima MI, da Silva MF, Fernandes JB, Vieira PC, Ramalho SD. 2015. Evaluation of flavonols and derivatives as human cathepsin B inhibitor. Nat Prod Res 29:2212–2214. doi: 10.1080/14786419.2014.1002404. [DOI] [PubMed] [Google Scholar]
- 15.Hasima N, Ozpolat B. 2014. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis 5:e1509. doi: 10.1038/cddis.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollman PC, Katan MB. 1997. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother 51:305–310. [DOI] [PubMed] [Google Scholar]
- 17.D'Andrea G. 2015. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia 106:256–271. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y, Narayanan S, Chang KO. 2010. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Res 88:227–235. doi: 10.1016/j.antiviral.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 19.dos Santos AE, Kuster RM, Yamamoto KA, Salles TS, Campos R, de Meneses MD, Soares MR, Ferreira D. 2014. Quercetin and quercetin 3-O-glycosides from Bauhinia longifolia (Bong.) Steud. show anti-Mayaro virus activity. Parasit Vectors 7:130. doi: 10.1186/1756-3305-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lani R, Hassandarvish P, Chiam CW, Moghaddam E, Chu JJ, Rausalu K, Merits A, Higgs S, Vanlandingham D, Abu Bakar S, Zandi K. 2015. Antiviral activity of silymarin against chikungunya virus. Sci Rep 5:11421. doi: 10.1038/srep11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M, Son M, Ryu E, Shin YS, Kim JG, Kang BW, Cho H, Kang H. 2015. Quercetin-induced apoptosis prevents EBV infection. Oncotarget 6:12603–12624. doi: 10.18632/oncotarget.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khachatoorian R, Arumugaswami V, Raychaudhuri S, Yeh GK, Maloney EM, Wang J, Dasgupta A, French SW. 2012. Divergent antiviral effects of bioflavonoids on the hepatitis C virus life cycle. Virology 433:346–355. doi: 10.1016/j.virol.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu X, Alimonti J, Melito L, Fernando L, Stroher U, Jones S. 2011. Characterization of Zaire ebolavirus glycoprotein-specific monoclonal antibodies. Clin Immunol 141:218–227. doi: 10.1016/j.clim.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, Cunningham J. 2004. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol 78:5458–5465. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdal Dayem A, Choi HY, Kim YB, Cho SG. 2015. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS One 10:e0121610. doi: 10.1371/journal.pone.0121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marzi A, Reinheckel T, Feldmann H. 2012. Cathepsin B & L are not required for Ebola virus replication. PLoS Negl Trop Dis 6:e1923. doi: 10.1371/journal.pntd.0001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K, Ren T, Côté M, Gholamreza B, Misasi J, Bruchez A, Cunningham J. 2013. Inhibition of Ebola virus infection: identification of Niemann-Pick C1 as the target by optimization of a chemical probe. ACS Med Chem Lett 4:239–243. doi: 10.1021/ml300370k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Côté M, Misasi J, Ren T, Bruchez A, Lee K, Filone CM, Hensley L, Li Q, Ory D, Chandran K, Cunningham J. 2011. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature 477:344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nekohashi M, Ogawa M, Ogihara T, Nakazawa K, Kato H, Misaka T, Abe K, Kobayashi S. 2014. Luteolin and quercetin affect the cholesterol absorption mediated by epithelial cholesterol transporter Niemann-Pick c1-like 1 in caco-2 cells and rats. PLoS One 9:e97901. doi: 10.1371/journal.pone.0097901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mbikay M, Sirois F, Simoes S, Mayne J, Chrétien M. 2014. Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh7 human hepatocytes in culture. FEBS Open Bio 4:755–762. doi: 10.1016/j.fob.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansen L, Brannan J, Delos S, Shoemaker C, Stossel A, Hoffstrom B, DeWald L, Shornberg K, Scully C, Lehar J, Hensley L, White W, Olinger G. 2013. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med 5:190ra79. doi: 10.1126/scitranslmed.3005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakurai Y, Kolokoltsov A, Chen C, Tidwell M, Bauta W, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey R. 2015. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. 2014. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res 105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.