Abstract
In vitro drug treatment with artemisinin derivatives, such as dihydroartemisinin (DHA), results in a temporary growth arrest (i.e., dormancy) at an early ring stage in Plasmodium falciparum. This response has been proposed to play a role in the recrudescence of P. falciparum infections following monotherapy with artesunate and may contribute to the development of artemisinin resistance in P. falciparum malaria. We demonstrate here that artemether does induce dormant rings, a finding which further supports the class effect of artemisinin derivatives in inducing the temporary growth arrest of P. falciparum parasites. In contrast and similarly to lumefantrine, the novel and fast-acting spiroindolone compound KAE609 does not induce growth arrest at the early ring stage of P. falciparum and prevents the recrudescence of DHA-arrested rings at a low concentration (50 nM). Our findings, together with previous clinical data showing that KAE609 is active against artemisinin-resistant K13 mutant parasites, suggest that KAE609 could be an effective partner drug with a broad range of antimalarials, including artemisinin derivatives, in the treatment of multidrug-resistant P. falciparum malaria.
INTRODUCTION
Over the last 2 decades, artemisinin-based combination therapies (ACTs) have been the most efficacious treatments against malaria and have contributed greatly to the decline in malaria mortality and morbidity (1, 2). Recent reports of falling efficacy rates of ACTs in Southeast Asia, as evidenced by increased treatment failures (3 – 5) and prolonged parasite clearance times following ACT treatment (6 – 11), are of great concern. Molecular markers of artemisinin resistance, K13 propeller mutations, have been identified (12, 13), and several recent reports suggest that resistance to the currently used ACTs is developing and spreading sooner than expected (14, 15). It remains unclear whether this resistance is a result of changes in the parasite molecular machinery required for the mode of action of artemisinin derivatives (16) or due to a recently described phenomenon of dormancy (growth retardation), where Plasmodium falciparum rings are able to survive dihydroartemisinin (DHA) treatment by undergoing a temporary growth arrest (17 – 19). Despite many unanswered questions about the role of dormancy, the importance of this phenomenon in the context of antimalarial chemotherapy and artemisinin resistance has become increasingly evident (17 – 23). Furthermore, the growth arrest of ring-stage parasites and their recovery rates observed in the in vitro ring survival assay (RSA) correlate strongly with parasite clearance half-lives after treatment of P. falciparum malaria with ACTs (22, 23), providing further support for the link between dormancy and treatment failures and possible resistance.
Despite the concerns over emerging artemisinin resistance, ACTs still remain the treatment of choice for uncomplicated P. falciparum malaria (1). Therefore, to enhance or prolong the activity of ACTs against the emergence of resistance, it is important to develop new partner drugs as ACTs or non-ACTs and evaluate their ability to induce dormant parasites or prevent their recovery, as well as their ability to kill emerging resistant parasites. Reports of artemisinin resistance (3 – 5) also highlight the need for the rational design of new drug combinations with drugs targeting different parasite pathways and mechanisms of resistance.
The spiroindolone KAE609 (previously referred to as NITD609 and also known as cipargamin) is a potent antimalarial drug that belongs to a new chemical class of drugs. KAE609 kills all blood stages of P. falciparum in vitro at low nanomolar concentrations (24), including late-stage gametocytes, and thus possesses transmission-blocking activity potential (25). A phase I study has shown KAE609 to be well tolerated in healthy volunteers at doses up to 150 mg daily and to have favorable pharmacokinetic properties (26). Furthermore, a phase II trial revealed the potent and fast activity of KAE609 against both P. falciparum and P. vivax malaria, with a short mean parasite clearance time of 12 h and a parasite half-life clearance of ∼0.9 h (27). Importantly, KAE609 was highly effective for the treatment of patients infected with P. falciparum strains bearing mutations in the K13 gene (27).
Importantly, the mechanism of action of KAE609 is different from that of other commonly used antimalarial drugs, such as artemisinin and mefloquine (24). KAE609 disrupts the regulation of Na+ in parasites (28) through the inhibition of a P-type non-SERCA ATPase (P. falciparum ATP4 [PfATP4]) (24, 28). The mechanism of action and the mechanism of resistance to KAE609 thus differ from those of artemisinin derivatives, for which recent studies identified possible candidate loci on chromosomes 10 and 13, with the polymorphism in the K13 gene showing the strongest correlation yet with the delayed parasite clearance phenotype (13).
In the context of emerging artemisinin resistance, it is important to ascertain that KAE609 does not induce dormant parasites, which could lead to recrudescence and, possibly, the development of drug resistance. This information is also lacking for the partner drugs of the most commonly used ACT, artemether (ART)-lumefantrine (LUM) (marketed as Coartem). In the present study, we compared the effect of KAE609 on P. falciparum ring-stage parasites with that of artemether and lumefantrine to determine their ability to induce dormancy at the ring stage. We also evaluated the inhibitory effect of KAE609 and lumefantrine on DHA-induced dormant rings.
MATERIALS AND METHODS
Drugs.
KAE609, ART, and LUM were provided by Novartis. DHA was obtained from Central Pharmaceutical Company No. 1 (Hanoi, Vietnam). Drug stock solutions were prepared to 1 mM concentrations in dimethyl sulfoxide (DMSO) for KAE609 and LUM, in methanol for DHA, and in 50% methanol for ART.
Continuous in vitro cultivation of P. falciparum.
The P. falciparum laboratory-adapted chloroquine- and pyrimethamine-resistant W2 strain (Indochina) was used in this study and cultured as previously described (29) in RPMI 1640-LPLF complete medium. The base medium (1 liter) contained RPMI 1640-LPLF (Gibco BRL, Invitrogen Corporation, CA), 5.97 g of HEPES (MP Biomedicals, Australia), 2 g d-glucose (BDH Chemicals, Australia), 0.05 g hypoxanthine (Sigma, St. Louis, MO), and 40 mg/liter gentamicin (Pfizer, Australia) with the pH adjusted to 6.9. The plain medium was obtained by adding sodium bicarbonate (final concentration, 0.21%) to base medium and used to wash parasite cultures for the removal of drugs. The complete medium was made by supplementing plain medium with 10% human plasma before use. Plasma and type O-positive red blood cells (RBCs) were obtained from the Australian Red Cross Blood Service (Brisbane, Australia).
Parasite cultures at 1 to 8% parasitemia and 4% hematocrit were routinely synchronized (typically, every 48 h) when the majority of parasites (>85%) were at the ring stage using d-sorbitol (Bacto Laboratories Pty. Ltd., Australia) (30). For the dormancy experiments, an additional synchronization with heparin (31) was carried out prior to the experiment. Heparin (Pfizer, Australia) was added to the culture (1 unit/ml of culture) at the late trophozoite-early schizont stage to prevent newly released merozoites from invading the RBCs. When the cultures reached the mature schizont stage, the cultures were centrifuged at 500 × g for 5 min, and the RBC pellets were resuspended in complete medium and incubated at 37°C for a further 4 to 6 h. Following incubation the cultures were treated with d-sorbitol to remove the remaining schizonts. This process resulted in highly synchronous cultures in which, typically, >95% of the parasites were at the early ring stage (≤6 h old).
Determination of in vitro inhibitory concentrations of antimalarial drugs by [3H]hypoxanthine growth inhibition assay.
The in vitro antimalarial activities of KAE609, DHA, ART, and LUM against the W2 strain were assessed by the 48-h [3H]hypoxanthine growth inhibition assay (32). Briefly, the 48-h assay was initiated at the ring stage, with parasite cultures at 1% parasitemia and 2% hematocrit (100 μl per well in triplicate) being exposed to ten 2-fold dilutions of the drugs made in complete medium in 96-well plates. [3H]hypoxanthine (0.2 μCi per well) was added at the trophozoite stage, ∼24 h after initiation of the assay. The in vitro antimalarial activity of the drug was defined as the concentrations that caused 50% inhibition (IC50) and 90% inhibition (IC90) of parasite growth, which were calculated by nonlinear regression analysis using GraphPad Prism software (v5.0; GraphPad Software, Inc., CA).
Evaluation of potential of KAE609, ART, and LUM to induce dormancy in P. falciparum in vitro.
To compare the effects of KAE609, ART, and LUM on ring stage parasites with the effect of DHA, the inoculum containing highly synchronous ring stage parasites (<6 h) at 4% hematocrit and 0.5 to 1% parasitemia was split into 10-ml aliquots and placed in 25-cm2 flasks. These cultures were exposed to various KAE609, DHA, ART, and LUM concentrations equivalent to multiple folds of the IC90 of the drugs for 6 h (Table 1). Following treatment, the drugs were removed by three washes in plain medium and the RBC pellets were resuspended in the original volume of complete medium (10 ml). The flasks were gassed and incubated at 37°C for an 8-week follow-up period. During this period the parasite culture media were changed with a ∼100-μl aliquot of an RBC suspension, which was added to each flask weekly.
TABLE 1.
Inhibitory concentrations (IC) of KAE609, DHA, ART, and LUM against the P. falciparum W2 line and concentrations of these drugs used in dormancy experiments
| Drug | IC50 (nM) | IC90 (nM) | Absolute drug concn (nM) (equivalent [fold IC90] drug concn) |
|---|---|---|---|
| KAE609 | 1.0a | 2.0a | 2.5 (1.25), 5 (2.5), 10 (5), 25 (12.5), 50 (25), 100 (50), 200 (100), 400 (200)b |
| DHA | 1.0 | 2.5 | 700 (200) |
| ART | 3.1 | 5.6 | 50 (10), 500 (100) |
| LUM | 24 | 60 | 600 (10), 1,500 (25), 3,000 (50), 6,000 (100) |
Based on previously published data (24).
This concentration was not used in sequential treatment after pretreatment with DHA.
To evaluate the effect of KAE609 on dormant rings, the ring-stage cultures were pretreated with either DHA or ART for 6 h. Following exposure, the drugs were removed from the culture media and the cultures were subsequently exposed to increasing concentrations ranging from 2.5 to 100 nM (equivalent to 1.25× to 50× IC90) KAE609 or 600 to 6,000 nM (equivalent to 10× to 100× IC90) LUM for another 6 h (Table 1). In addition, in these experiments parasites were also exposed to the same concentrations of KAE609 or LUM without pretreatment with DHA or ART.
During the 8-week follow-up period, the cultures were monitored for growing parasites by microscopy using Giemsa staining. Slides (thick and thin smears) were made from cultures daily during the first 9 to 10 days, then every second day until week 3, and, finally, biweekly until the end of the follow-up period. In addition, during the first 9 to 10 days, the cultures were also analyzed daily by flow cytometry using staining with either the SYBR green I dye (Invitrogen, Australia) or the rhodamine 123 dye (Sigma-Aldrich, Australia).
Flow cytometric analysis.
Thirty microliters of the 10,000× SYBR green I dye, diluted 1,000-fold in 1× phosphate-buffered saline (PBS; Gibco BRL), was added to 30 μl of the P. falciparum culture in complete medium and incubated at 37°C in the dark for 15 min. Following incubation, the samples were centrifuged at 500 × g for 1 min and the supernatant was removed. The RBC pellets were washed three times with 500 μl of 1× PBS and resuspended in 500 μl of 1× PBS buffer.
A stock solution of rhodamine 123 (20 mg/ml in DMSO) was prepared and subsequently diluted to 100 μg/ml with 1× PBS, aliquoted, and stored at −80°C until used. For live parasite staining, 3 μl of rhodamine 123 was added to 30 μl of parasite culture (final concentration, 10 μg/ml) in complete medium. The samples were centrifuged at 500 × g, and the supernatant was removed. The RBC pellets were washed twice with 200 μl of prewarmed complete medium, resuspended in 200 μl of complete medium, and incubated in the dark at 37°C for 20 min. Following incubation, the samples were centrifuged, and the RBC pellets were resuspended in 500 μl of 1× PBS and immediately analyzed by flow cytometry.
An FC500 flow cytometer (Beckman Coulter, Australia) was used, and the fluorescence data were obtained using a blue laser (488 nm) with forward scatter (FS) and fluorescence (FL) detectors FS/FL1 or FL1/FL2 with the 525 band path/575 band path nm filter configuration. One hundred thousand and 40,000 events were counted for SYBR green and rhodamine 123, respectively.
DNA-binding SYBR green staining was used to determine total parasitemia, which includes dead, drug-affected, and healthy parasites as well as their developmental stages (33). On the basis of their fluorescent values, the events counted as ring-stage parasites were subdivided into three areas (gates) containing RBCs infected with either one, two, or three rings. The trophozoite stage included the events with fluorescence values between 4 and 6 times greater than the median fluorescence value of an area with single rings. Thus, in this analysis, some growing trophozoites would also be counted in areas overlapping with double and triple rings. These definitions of the gates were verified by microscopy.
Staining with rhodamine 123 was used to identify live parasites (i.e., parasites with negative mitochondrial membrane potential) (34 – 36). Uninfected RBCs were used as the negative control and excluded from counting. The data were analyzed using Kalusa software (Beckman Coulter, Australia).
RESULTS
KAE609 and LUM do not induce dormant P. falciparum ring stage parasites.
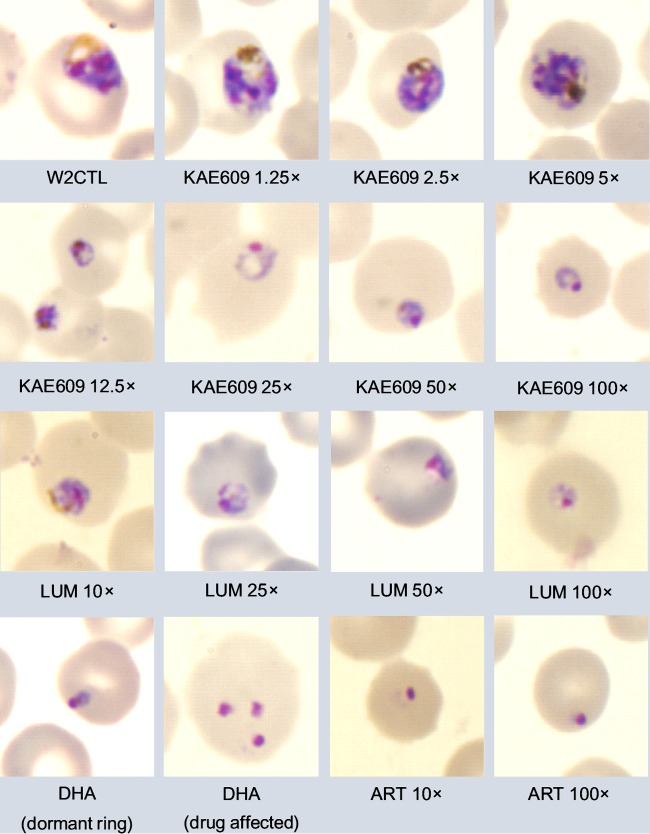
To assess the effects of the drugs on ring stage parasites, W2 parasites were exposed to various concentrations of KAE609, DHA, ART, and LUM for 6 h, starting with parasites <6 h into ring stage development (Table 1), after which the drugs were removed from the cultures. Microscopic examination of the cultures showed that after 6 h of exposure to DHA (700 nM) or ART (500 nM), progression of rings to trophozoites had ceased, with a high proportion of rings looking drug affected. The characteristic dormant rings, as previously described by Tucker et al. (37), which had a red nucleus and a compact blue cytoplasm (Fig. 1), became apparent 24 to 48 h after the start of either DHA or ART exposure and amounted to between 2 and 20% of all rings. Growing trophozoites were observed on the slides at between days 3 and 5, marking the resumption of parasite growth or recovery from dormancy (see Table S1 in the supplemental material).
FIG 1.
Representative images of Giemsa-stained thin smears of Plasmodium falciparum W2 parasite cultures taken at 24 h after the start of treatment with an initial 6-h exposure to the various concentrations (fold IC90s) of KAE609, DHA, ART, and LUM indicated. W2CTL, W2 control (untreated) parasites.
The inhibitory effect of KAE609 and LUM on rings was dose dependent. Microscopic examination revealed that parasites exposed to lower concentrations of KAE609 (2.5 to 10 nM) and LUM (600 nM) for 6 h continued to grow similarly to the untreated controls, although with a slight delay (Fig. 1; see also Table S1 in the supplemental material). The concentrations of KAE609 (25 to 200 nM) and LUM (3,000 to 6,000 nM) inhibited parasite growth, with no growing parasites being observed during the first 10 days after 6 h of drug exposure. At 25 nM or 50 nM KAE609 and 3,000 nM or 6,000 nM LUM, rings progressed further through the cycle after removal of the drug but stopped at the late ring stage to early trophozoite stage. Cultures exposed to 100 nM or 200 nM KAE609 progressed to the late ring stage. Unlike cultures exposed to DHA or ART, no dormant rings were observed in cultures exposed to either KAE609 or LUM.
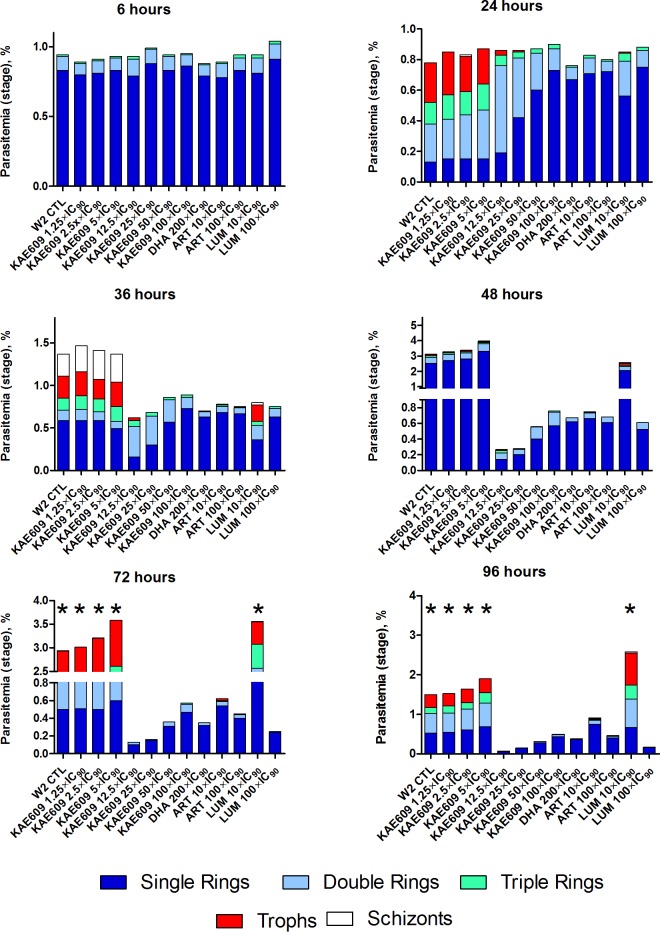
The microscopy results were substantiated by flow cytometric analysis of SYBR green-stained parasites, which allowed quantification of parasitemia and determination of the parasite stages present in cultures after exposure to KAE609, DHA, ART, and LUM at 6, 24, 36, 48, 72, and 96 h after the start of the treatments, as shown in Fig. 2.
FIG 2.
Parasitemia profiles and representative distribution of blood stages of Plasmodium falciparum W2 parasites after 6 h of exposure to various concentrations (fold IC90) of KAE609, DHA, ART, and LUM measured by flow cytometric analysis of SYBR green-stained parasites at different time points (6 h, 24 h, 36 h, 48 h, 72 h, and 96 h) after the start of treatment. *, cessation of culturing due to high levels of parasitemia; W2 CTL, W2 control (untreated) parasites; Trophs, trophozoites.
The parasiticidal effect of KAE609 and LUM is different from that of DHA or ART.
The parasiticidal effects of the drugs were evaluated by comparing the fraction of live parasites using 123 staining. The percentage of live parasites (presented as means ± standard deviations [SDs]) in cultures treated with various concentrations of KAE609, DHA, ART, and LUM is presented in Fig. 3. Exposure to DHA (700 nM) or ART (500 nM) resulted in 35% ± 12% of live parasites in the DHA-treated cultures immediately after 6 h of drug exposure, with a further sharp decline to 7.8% ± 3.2% (RBC background levels, 2.3% ± 1.5%) being found at 24 h after the start of treatment. Consistent with the concept of dormancy, the fluorescence values remained at these low levels until 96 h after the start of treatment, with an increase in fluorescence being detected on day 5, although a small number of trophozoites could be detected as early as 72 h by both flow cytometry and microscopy, indicating recovery from dormancy. A similar pattern was observed in cultures exposed to ART (500 nM).
FIG 3.
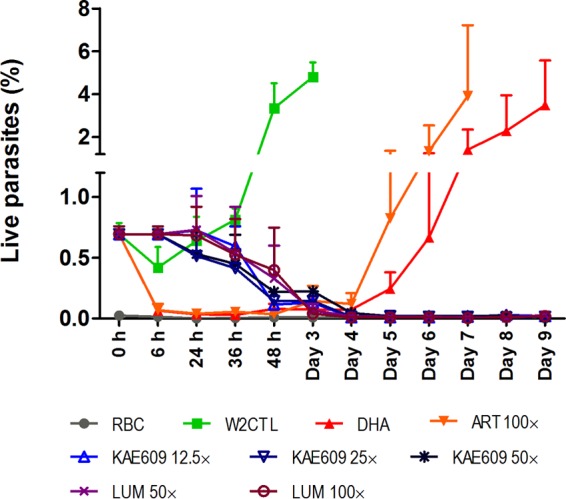
Percentage of live parasites (mean ± SD, n = 2) detected in Plasmodium falciparum W2 cultures at different time points (6 h to day 9) after the start of treatment with various concentrations (fold IC90s) of KAE609, DHA, ART, and LUM measured by flow cytometry using rhodamine 123 staining. RBC, uninfected red blood cells; W2 CTL, W2 control (untreated) parasites.
The parasiticidal effect of KAE609 or LUM on parasites was different from that of DHA or ART. The fractions of live parasites detected in cultures treated with low concentrations of KAE609 (2.5 to 10 nM) were similar to those of live parasites of the untreated W2 control, where parasites grew during the first 72 h of follow-up until the population crashed from overgrowth. Similarly, a large fraction of live parasites was detected in cultures treated with the lowest concentration of LUM (600 nM), albeit their progression through the cycle was delayed compared to the progression of the control parasites (data not shown).
In cultures exposed to higher concentrations of KAE609 of 25 nM, 50 nM, and 100 nM for 6 h, the percentages of live parasites measured at 24 h (18 h after drug exposure) were 80% ± 28%, 74% ± 27%, and 76% ± 26% (relative to the values for the W2 control at 0 h), respectively (Fig. 3). At 48 h the number of live parasites decreased (17% ± 5% at 25 nM KAE609, 21% ± 5% at 50 nM KAE609, and 30% ± 5% at 100 nM KAE609). However, it took up to 96 h after the start of treatment before the fluorescence indicating live parasite numbers in cultures treated with 25 nM to 100 nM KAE609 declined to background fluorescence levels and remained undetectable for the remaining follow-up period. A similar pattern of decline in live parasite numbers was also observed after 6 h of exposure to high concentrations of LUM (3,000 to 6,000 nM).
The recrudescence of parasites following treatment with KAE609, DHA, ART, and LUM.
W2 parasites treated with 25 nM or 50 nM KAE609 recrudesced at days 14 to 16 and 18 to 23, respectively (see Table S1 in the supplemental material). At KAE609 concentrations of 100 nM or greater, no recrudescence was observed by microscopy during 8 weeks of follow-up. Similar parasite clearance with no recrudescence was seen at 3,000 nM or 6,000 nM LUM. The recovery of DHA (700 nM)-treated or ART (500 nM)-treated parasites usually occurred on days 3 to 5 after the start of treatment. These results were reproducible (n = 4) and consistent with previously published data (17).
KAE609 and LUM prevent the recrudescence of DHA-induced dormant rings.
Pretreatment with either DHA (700 nM) or ART (500 nM) resulted in the appearance of dormant rings, which were seen by microscopy in all cultures exposed to these drugs. The sequential 6-h exposure to low concentrations of KAE609 (2.5 to 10 nM) did not delay the recovery of dormant parasites compared with that of DHA alone (3 to 5 days), whereas exposure to 25 nM KAE609 resulted in the recrudescence of parasites on day 16, which was comparable to the result obtained with KAE609 treatment alone. Sequential exposure to KAE609 (50 nM) or LUM (3,000 nM) for 6 h was sufficient to prevent recrudescence over the 8-week follow-up period (see Table S1 in the supplemental material).
DISCUSSION
In this study, we used the in vitro model developed by Teuscher et al. (17) that described the induction of dormant ring stages in P. falciparum W2 parasites following 6 h of exposure to DHA (700 nM) to evaluate drugs for their ability to induce dormant parasites. The DHA concentration and exposure duration in this model were also identical to those used in the recently developed ring survival assay (RSA), which is recommended for the evaluation of artemisinin resistance in the field (22, 23). Both DHA (700 nM) and ART (500 nM) produced dormant ring stages at 24 to 48 h after the start of treatment, with parasites recovering 3 to 5 days after drug exposure. This finding is in accord with the class effect of the artemisinin derivatives resulting in the induction of dormant rings reported elsewhere (38, 39), even though the time to recovery of the parasites was shorter than that reported previously (17, 39, 40). A plausible explanation for the difference is that in the present study, magnetic columns were not used on days 1, 2, and 3 after the start of the experiment to remove growing parasites (late stages) not killed by DHA treatment as well as those emerging from dormant parasites with the short duration of dormancy (24 to 48 h).
Unlike DHA or ART, we showed that neither KAE609 nor LUM treatment resulted in the appearance of dormant rings. Our findings corroborate those of Rottmann et al. (24) that in vitro KAE609 does not act as rapidly as DHA or ART, since after the removal of KAE609, ring-stage parasites progressed further through the cycle before they stopped growing and later died. Of note, KAE609 at a concentration of 100 or 200 nM had a parasiticidal effect on rings, despite a relatively short exposure time (6 h), albeit not as immediate as that of DHA or ART. Although the timing of parasite death after 6 h of exposure to KAE609 was similar to that of LUM, the concentration of KAE609 required to kill all parasites was markedly lower than that of LUM (100 nM versus 3,000 nM). Additionally, KAE609 has been shown to be highly active in vitro against P. falciparum strains bearing various K13 mutations (T. T. Diagana, unpublished data).
When parasites were pretreated with either DHA or ART for 6 h and sequentially exposed to KAE609 or LUM for a further 6 h, the concentration of KAE609 required to prevent parasite regrowth was 2-fold less (50 nM versus 100 nM) than that required by drug treatment alone, whereas 3,000 nM LUM was still required to prevent the recovery of dormant parasites. These profound in vitro differences in antimalarial activity between the artemisinin derivatives and KAE609 and the superior antimalarial activity of the spiroindolone against W2 dormant rings provide further evidence for the need for the clinical development of the drug. It would be worthwhile to evaluate the activity of KAE609 against dormant rings of other P. falciparum lines, including those derived from patients with delayed parasite clearance times and bearing K13 mutations.
The slower parasitological response to ACTs (3 – 5) in Southeast Asian countries combined with increasing resistance to partner drugs, such as piperaquine (10, 41), highlights the need to develop more effective drugs that may be used to partner with the rapidly acting artemisinins or as new non-ACTs. For antimalarial drug combinations to be effective, not only is it essential that minimal parasiticidal inhibitory concentrations of the partner drugs be achieved in the patient's blood, but also the timing and overall duration of drug exposure to the parasites are critical for successful treatment outcomes. Recently, White et al. (27) reported KAE609 to be highly efficacious in clearing both P. falciparum and P. vivax malaria, with P. falciparum malaria patients achieving a maximum plasma KAE609 concentration of 1,360 ng/ml (3,485 nM) after a 3-day regimen of 30 mg per day. The terminal elimination half-life of 23.1 h for KAE609 is much longer than that of 0.85 h for DHA (42) and that of 2 h for ART (43), suggesting that if KAE609 is combined with DHA or ART to extend the effective life of ACTs, KAE609 would persist much longer than the artemisinin derivatives in blood. Additionally, a 3-day regimen of KAE609 combined with an artemisinin derivative would result in blood KAE609 concentrations above the in vitro cidal concentration of 100 nM for three asexual cycles to prevent recrudescence.
Remarkably, the clinical trial by White et al. (27) showed that KAE609 produces a rapid median parasite clearance time of 12 h, which is considerably faster than that found in recent studies of artesunate (>54 h) (3, 44). As KAE609 is devoid of the major drawbacks of the artemisinin derivatives (i.e., a short elimination half-life and dormancy induction) and possesses in vitro antimalarial activity at low nanomolar concentrations, it is conceivable that instead of artemisinin derivatives, KAE609 could be used as a rapidly acting partner drug in combination with a longer-acting antimalarial. As in vitro findings suggest that resistance to KAE609 could be developed reasonably fast via the selection of mutations in PfATP4 (24), the longer-acting partner drug should not be impacted by the same resistance mechanism in order to provide protection against the emergence of resistance.
In designing the next generation of drugs for malaria control and eradication, the Medicines for Malaria Venture has developed target candidate profiles (TCPs), with KAE609 fulfilling the TCP-1 definition of a drug that can rapidly clear the parasite load (45). The data for KAE609 and LUM obtained in the present in vitro study show that neither drug induces dormant rings of P. falciparum and show that their clinically achievable blood concentrations prevent the recovery of dormant parasites induced by the artemisinin derivatives. Like LUM, KAE609 could thus be combined with an artemisinin derivative or, preferably, be a partner drug with a long-acting antimalarial to produce a non-ACT. The latter option is urgently needed to combat the emergence and spread of artemisinin resistance in Southeast Asia.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Australian Red Cross Blood Service (Brisbane, Australia) for providing human erythrocytes and plasma for cultivation of P. falciparum parasites. We are grateful to Qin Cheng for scientific insight and valuable advice on dormancy.
This research was funded by the Australian Defense Organization and the Novartis Institute of Tropical Diseases. T.T.D. gratefully acknowledges the significant financial contributions from the Wellcome Trust and Medicines for Malaria Venture to the Novartis Institute for Tropical Diseases drug discovery program and the early clinical development of KAE609.
T.T.D. is an employee of Novartis and owns Novartis stocks. We have no other conflicts of interest to declare.
The opinions expressed herein are those of the authors and do not necessarily reflect those of the Australian Defense Organization and/or extant policy.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02838-15.
REFERENCES
- 1.World Health Organization. 2014. World malaria report 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2014/en/. [Google Scholar]
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CL, Smith DL, Hay SI, Cibulskis RE, Gething PW. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C. 2009. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J 8:10. doi: 10.1186/1475-2875-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saunders DL, Vanachayangkul P, Lon C. 2014. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med 371:484–485. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- 6.Flegg JA, Guerin PJ, White NJ, Stepniewska K. 2011. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J 10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flegg JA, Guerin PJ, Nosten F, Ashley EA, Phyo AP, Dondorp AM, Fairhurst RM, Socheat D, Borrmann S, Bjorkman A, Martensson A, Mayxay M, Newton PN, Bethell D, Se Y, Noedl H, Diakite M, Djimde AA, Hien TT, White NJ, Stepniewska K. 2013. Optimal sampling designs for estimation of Plasmodium falciparum clearance rates in patients treated with artemisinin derivatives. Malar J 12:411. doi: 10.1186/1475-2875-12-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. 2012. Artemisinin-resistant Plasmodium falciparum in Pursat Province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis 12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spring MD, Lin JT, Manning JE, Vanachayangkul P, Somethy S, Bun R, Se Y, Chann S, Ittiverakul M, Sia-Ngam P, Kuntawunginn W, Arsanok M, Buathong N, Chaorattanakawee S, Gosi P, Ta-Aksorn W, Chanarat N, Sundrakes S, Kong N, Heng TK, Nou S, Teja-Isavadharm P, Pichyangkul S, Phann ST, Balasubramanian S, Juliano JJ, Meshnick SR, Chour CM, Prom S, Lanteri CA, Lon C, Saunders DL. 2015. Dihydroartemisinin-piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis 15:683–691. doi: 10.1016/S1473-3099(15)70049-6. [DOI] [PubMed] [Google Scholar]
- 11.Leang R, Taylor WRJ, Bouth DM, Song L, Tarning J, Char MC, Kim S, Witkowski B, Duru V, Domergue A, Khim N, Ringwald P, Menard D. 2015. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother 59:4719–4726. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straimer J, Gnadig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP, Dacheux M, Khim N, Zhang L, Lam S, Gregory PD, Urnov FD, Mercereau-Puijalon O, Benoit-Vical F, Fairhurst RM, Menard D, Fidock DA. 2015. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 347:428–431. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashley E, Dhorda M, Fairhurst R, Amaratunga C, Lim P, Suon S, Sreng S, Anderson J, Mao S, Sam B, Sopha C, Chuor C, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien T, Thuy-Nhien N, Thanh N, Phu N, Htut Y, Han K, Aye K, Mokuolu O, Olaosebikan R, Folaranmi O, Mayxay M, Khanthavong M, Hongvanthong B, Newton P, Onyamboko M, Fanello C, Tshefu A, Mishra N, Valecha N, Phyo A, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz M, Ghose A, Hossain M, Samad R, et al. . 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyant P, Corbel V, Guerin PJ, Lautissier A, Nosten F, Boyer S, Coosemans M, Dondorp AM, Sinou V, Yeung S, White N. 2015. Past and new challenges for malaria control and elimination: the role of operational research for innovation in designing interventions. Malar J 14:279. doi: 10.1186/s12936-015-0802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, Rizk SS, Njimoh DL, Ryan Y, Chotivanich K, Nguon C, Ghorbal M, Lopez-Rubio JJ, Pfrender M, Emrich S, Mohandas N, Dondorp AM, Wiest O, Haldar K. 2015. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 520:683–687. doi: 10.1038/nature14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. 2010. Artemisinin-induced dormancy in Plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J Infect Dis 202:1362–1368. doi: 10.1086/656476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witkowski B, Lelievre J, Barragan MJ, Laurent V, Su XZ, Berry A, Benoit-Vical F. 2010. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother 54:1872–1877. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaCrue AN, Scheel M, Kennedy K, Kumar N, Kyle DE. 2011. Effects of artesunate on parasite recrudescence and dormancy in the rodent malaria model Plasmodium vinckei. PLoS One 6:e26689. doi: 10.1371/journal.pone.0026689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Q, Kyle DE, Gatton ML. 2012. Artemisinin resistance in Plasmodium falciparum: a process linked to dormancy? Int J Parasitol Drugs Drug Resist 2:249–255. doi: 10.1016/j.ijpddr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Codd A, Teuscher F, Kyle DE, Cheng Q, Gatton ML. 2011. Artemisinin-induced parasite dormancy: a plausible mechanism for treatment failure. Malar J 10:56. doi: 10.1186/1475-2875-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, Chy S, Duong S, Leang R, Ringwald P, Dondorp AM, Tripura R, Benoit-Vical F, Berry A, Gorgette O, Ariey F, Barale JC, Mercereau-Puijalon O, Menard D. 2013. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother 57:914–923. doi: 10.1128/AAC.01868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P, Mao S, Sopha C, Sam B, Anderson JM, Duong S, Chuor CM, Taylor WR, Suon S, Mercereau-Puijalon O, Fairhurst RM, Menard D. 2013. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis 13:1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, Gonzalez-Paez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. 2010. Spiroindolones, a potent compound class for the treatment of malaria. Science 329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Pelt-Koops JC, Pett HE, Graumans W, van der Vegte-Bolmer M, van Gemert GJ, Rottmann M, Yeung BK, Diagana TT, Sauerwein RW. 2012. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to Anopheles mosquito vector. Antimicrob Agents Chemother 56:3544–3548. doi: 10.1128/AAC.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leong FJ, Li R, Jain JP, Lefevre G, Magnusson B, Diagana TT, Pertel P. 2014. A first-in-human randomized, double-blind, placebo-controlled, single- and multiple-ascending oral dose study of novel antimalarial spiroindolone KAE609 (cipargamin) to assess its safety, tolerability, and pharmacokinetics in healthy adult volunteers. Antimicrob Agents Chemother 58:6209–6214. doi: 10.1128/AAC.03393-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White NJ, Pukrittayakamee S, Phyo AP, Rueangweerayut R, Nosten F, Jittamala P, Jeeyapant A, Jain JP, Lefevre G, Li R, Magnusson B, Diagana TT, Leong FJ. 2014. Spiroindolone KAE609 for falciparum and vivax malaria. N Engl J Med 371:403–410. doi: 10.1056/NEJMoa1315860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spillman NJ, Allen RJ, McNamara CW, Yeung BK, Winzeler EA, Diagana TT, Kirk K. 2013. Na(+) regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 13:227–237. doi: 10.1016/j.chom.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 30.Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 31.Boyle MJ, Wilson DW, Richards JS, Riglar DT, Tetteh KK, Conway DJ, Ralph SA, Baum J, Beeson JG. 2010. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc Natl Acad Sci U S A 107:14378–14383. doi: 10.1073/pnas.1009198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother 16:710–718. doi: 10.1128/AAC.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bei AK, Desimone TM, Badiane AS, Ahouidi AD, Dieye T, Ndiaye D, Sarr O, Ndir O, Mboup S, Duraisingh MT. 2010. A flow cytometry-based assay for measuring invasion of red blood cells by Plasmodium falciparum. Am J Hematol 85:234–237. doi: 10.1002/ajh.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato M, Izumo A, Tanabe K. 1987. Vital staining of Plasmodium falciparum with cationic fluorescent rhodamine dyes. J Parasitol 73:1058–1059. doi: 10.2307/3282537. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe K. 1983. Staining of Plasmodium yoelii-infected mouse erythrocytes with the fluorescent dye rhodamine 123. J Protozool 30:707–710. doi: 10.1111/j.1550-7408.1983.tb05347.x. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan S, Moody AH, Chiodini PL. 2000. Comparison of blood-film microscopy, the OptiMAL dipstick, rhodamine-123 fluorescence staining and PCR, for monitoring antimalarial treatment. Ann Trop Med Parasitol 94:227–232. doi: 10.1080/00034980050006393. [DOI] [PubMed] [Google Scholar]
- 37.Tucker MS, Mutka T, Sparks K, Patel J, Kyle DE. 2012. Phenotypic and genotypic analysis of in vitro-selected artemisinin-resistant progeny of Plasmodium falciparum. Antimicrob Agents Chemother 56:302–314. doi: 10.1128/AAC.05540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grobler L, Chavchich M, Haynes RK, Edstein MD, Grobler AF. 2014. Assessment of the induction of dormant ring stages in Plasmodium falciparum parasites by artemisone and artemisone entrapped in Pheroid vesicles in vitro. Antimicrob Agents Chemother 58:7579–7582. doi: 10.1128/AAC.02707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peatey CL, Chavchich M, Chen N, Gresty KJ, Gray KA, Gatton ML, Waters NC, Cheng Q. 2015. Mitochondrial membrane potential in a small subset of artemisinin-induced dormant Plasmodium falciparum parasites in vitro. J Infect Dis 212:426–434. doi: 10.1093/infdis/jiv048. [DOI] [PubMed] [Google Scholar]
- 40.Chen N, LaCrue AN, Teuscher F, Waters NC, Gatton ML, Kyle DE, Cheng Q. 2014. Fatty acid synthesis and pyruvate metabolism pathways remain active in dihydroartemisinin-induced dormant ring stages of Plasmodium falciparum. Antimicrob Agents Chemother 58:4773–4781. doi: 10.1128/AAC.02647-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thriemer K, Hong NV, Rosanas-Urgell A, Phuc BQ, Ha do M, Pockele E, Guetens P, Van NV, Duong TT, Amambua-Ngwa A, D'Alessandro U, Erhart A. 2014. Delayed parasite clearance after treatment with dihydroartemisinin-piperaquine in Plasmodium falciparum malaria patients in central Vietnam. Antimicrob Agents Chemother 58:7049–7055. doi: 10.1128/AAC.02746-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen DVH, Nguyen QP, Nguyen ND, Le TTT, Nguyen TD, Dinh DN, Nguyen TX, Bui D, Chavchich M, Edstein MD. 2009. Pharmacokinetics and ex vivo pharmacodynamic antimalarial activity of dihydroartemisinin-piperaquine in patients with uncomplicated falciparum malaria in Vietnam. Antimicrob Agents Chemother 53:3534–3537. doi: 10.1128/AAC.01717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novartis Pharmaceuticals UK Ltd. 2015. Riamet 20/120mg tablets. Novartis, Frimley, United Kingdom: https://www.medicines.org.uk/emc/medicine/9196. [Google Scholar]
- 44.Hien TT, Thuy-Nhien NT, Phu NH, Boni MF, Thanh NV, Nha-Ca NT, Thai le H, Thai CQ, Toi PV, Thuan PD, Long le T, Dong le T, Merson L, Dolecek C, Stepniewska K, Ringwald P, White NJ, Farrar J, Wolbers M. 2012. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar J 11:355. doi: 10.1186/1475-2875-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burrows JN, van Huijsduijnen RH, Mohrle JJ, Oeuvray C, Wells TN. 2013. Designing the next generation of medicines for malaria control and eradication. Malar J 12:187. doi: 10.1186/1475-2875-12-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.