Abstract
The oxadiazole antibacterials target the bacterial cell wall and are bactericidal. We investigated the synergism of ND-421 with the commonly used β-lactams and non-β-lactam antibiotics by the checkerboard method and by time-kill assays. ND-421 synergizes well with β-lactam antibiotics, and it also exhibits a long postantibiotic effect (4.7 h). We also evaluated the in vivo efficacy of ND-421 in a murine neutropenic thigh infection model alone and in combination with oxacillin. ND-421 has in vivo efficacy by itself in a clinically relevant infection model (1.49 log10 bacterial reduction for ND-321 versus 0.36 log10 for linezolid with NRS119) and acts synergistically with β-lactam antibiotics in vitro and in vivo, and the combination of ND-421 with oxacillin is efficacious in a mouse neutropenic thigh methicillin-resistant Staphylococcus aureus (MRSA) infection model (1.60 log10 bacterial reduction). The activity of oxacillin was potentiated in the presence of ND-421, as the strain would have been resistant to oxacillin otherwise.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a human pathogen associated with serious community-acquired infections and is one of the leading causes of nosocomial infections in the United States and around the world (1). MRSA harbors the mecA gene, which encodes penicillin-binding protein 2a (PBP2a), which confers resistance essentially to all β-lactam antibiotics (2). The currently available treatment options for MRSA are glycopeptides (vancomycin and telavancin), oxazolidinones (linezolid and tedizolid), daptomycin, and ceftaroline, of which only the oxazolidinones are orally bioavailable drugs. Linezolid- and vancomycin-resistant strains have already been reported (3 – 6); mutations leading to daptomycin resistance have also been observed (7). An increased vancomycin MIC has also been linked to a possible cross-resistance to daptomycin (8). Ceftaroline was approved in 2010 for treatment of community-acquired pneumonia and acute bacterial skin infections, owing to its ability to bind penicillin-binding proteins (PBPs). The binding in the case of the MRSA PBP2a is at both the allosteric and the active sites, imparting an interesting angle to the mechanism of action of this antibiotic (9, 10). Recently, ceftaroline heteroresistance among S. aureus strains has also been reported (11), and ceftaroline-resistant MRSA strains have been isolated (12, 13). Tedizolid was approved in 2014 for skin and soft tissue infections; resistance to it has been described in vitro (14).
The oxadiazoles are a new class of non-β-lactam antibacterials targeting cell wall biosynthesis with excellent in vitro and in vivo activity against MRSA and other Gram-positive bacteria (15). ND-421 (Fig. 1) is a lead oxadiazole and was also found to be bactericidal against vancomycin- and linezolid-resistant MRSA (16). This compound exhibits efficacy comparable to that of linezolid in a mouse peritonitis model of infection and has low clearance, a long half-life (t1/2), and 97% oral bioavailability (16). As resistance to new antibiotics emerges upon their introduction into the clinic and combination therapy can minimize the development of resistance (17), we investigated the possibility of synergism of ND-421 with the commonly used β-lactams and non-β-lactam antibiotics and found that ND-421 synergizes well with β-lactam antibiotics. We also evaluated the in vivo efficacy of ND-421 in a murine neutropenic thigh infection model alone and in combination with oxacillin and demonstrated that the combination decreased bacterial load significantly compared to single-agent treatment.
FIG 1.
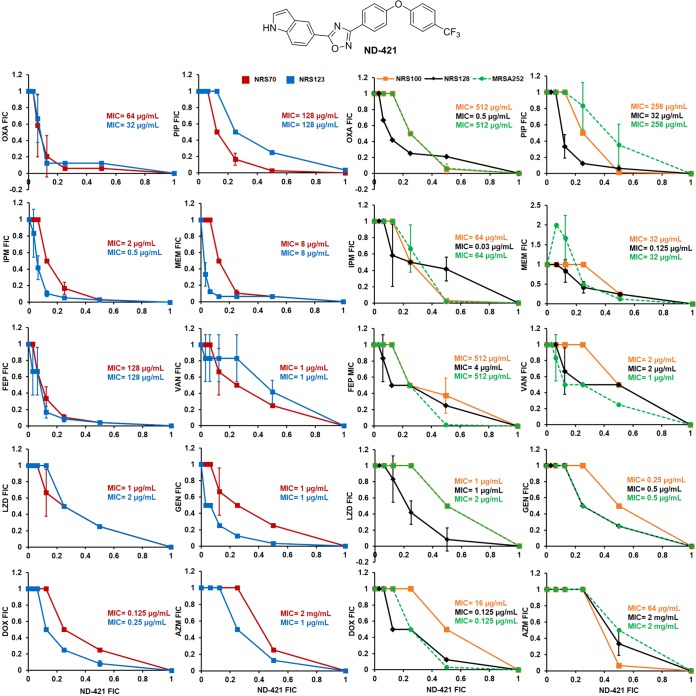
Synergy of ND-421 with β-lactams and non-β-lactams. The FIC values were determined using the checkerboard assay against S. aureus strains. ΣFIC index values of ≤0.5 are considered synergistic. OXA, oxacillin; PIP, piperacillin; IPM, imipenem; MEM, meropenem; FEP, cefepime; VAN, vancomycin; LZD, linezolid; GEN, gentamicin; DOX, doxycycline; AZM, azithromycin. ND-421 showed the highest synergy with oxacillin.
MATERIALS AND METHODS
Reagents.
The antimicrobial agents used in the study included cefepime (Sigma-Aldrich, St. Louis, MO), piperacillin (TCI, Portland, OR), linezolid (AmplaChem Inc., Carmel, IN), and imipenem, meropenem, vancomycin, oxacillin, gentamicin, azithromycin, and doxycycline (all from Sigma-Aldrich). The oxadiazole ND-421 and the internal standard were synthesized in our laboratory using methodology reported earlier (16). High-performance-liquid-chromatography-grade acetonitrile (Sigma-Aldrich) and formic acid (Sigma-Aldrich) were used for mass spectrometry experiments. Distilled water was purified on a MilliQ system (Millipore, Billerica, MA).
Microorganisms.
MRSA strains NRS70 (N315), NRS123 (MW2), NRS100 (COL), and NRS119 and methicillin-sensitive S. aureus (MSSA) strain NRS128 were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA). ATCC 29213 and MRSA 252 were purchased from the American Type Culture Collection (ATCC; Manassas, VA).
MIC determination.
The MIC values of ND-421 against these organisms were determined in triplicates in cation-adjusted Mueller-Hinton II broth (CAMHB-II; Becton Dickinson and Co., Sparks, MD) using the microdilution technique according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (18).
Inoculum effect.
The inoculum effect was determined in CAMHB-II according to the CLSI guidelines for broth microdilution (18) using final bacterial concentrations of 104, 105, 106, 107, and 108 CFU/ml in 96-well plates containing 2-fold serial dilutions of ND-421.
Checkerboard assay.
The synergistic interaction of ND-421 with the panel of β-lactams and non-β-lactams was tested on four MRSA strains (NRS70, NRS123, NRS100, and MRSA 252) and one MSSA strain (NRS128), using the checkerboard assay in 96-well plates. The final inoculum in each well was 5 × 105 CFU/ml, and the results were read after an 18-h incubation at 37°C. The fractional inhibitory concentration (FIC) index was calculated for each combination as reported previously (19). An FIC index of ≤0.5 was considered synergistic, one of >0.5 to <2 was considered indifferent, and one of >2 was considered antagonistic. The experiments were done in triplicates. The synergistic combinations were further validated with time-kill assays.
Time-kill assay.
Time-kill assays were performed in triplicates in CAMHB-II. The assay was done in 5-ml tubes using 1/4 MIC of ND-421 alone and in combination with 1/2 to 1/64 MICs of the β-lactams. A control tube with no antibiotic was also included. Bacteria were grown to a density of approximately 108 CFU/ml and diluted 100-fold in fresh MHB. This bacterial suspension was added to the MHB tubes to a final concentration of 5 × 105 CFU/ml and incubated at 37°C for 24 h. A 100-μl aliquot was obtained from each tube at 0, 3, 6, 12, and 24 h; serially diluted in saline; and plated on antibiotic-free agar (LB agar) in duplicate to determine colony counts. Synergy was defined as a ≥2 log10 decrease in colony counts at 24 h with the combination, compared to the most active single drug alone (19), and the final inoculum in the combination was ≥2 log10 CFU/ml below the starting inoculum.
PAE.
NRS70 was grown in MHB to an optical density at 600 nm (OD600) of 0.1 and diluted 1:10 with fresh MHB. A 2.5-ml aliquot of this culture was mixed with 2.5 ml of antibiotic solutions to a final concentration of ∼106 CFU/ml. The cultures were incubated with ND-421, linezolid, and vancomycin at 2× and 4× MIC for 2 h. A growth control in the absence of antibiotic was included. The antibiotics were diluted 1:1,000 in fresh prewarmed (37°C) MHB, and the regrowth rate was measured by viable colony counting. The growth control in the absence of antibiotic was also treated similarly. Samples were drawn at 0 h, 2 h (before and after dilution), and every hour thereafter for 7 h and plated on LB agar plates. The postantibiotic effect (PAE) was calculated as PAE = T − C, where T is time (hours) required for the CFU in the test culture to increase by 1 log10 above the count immediately after dilution and C is time (hours) required for the CFU in the control culture to increase by 1 log10 above the count immediately after dilution (20). The experiment was performed in duplicates.
Animals.
Outbred, pathogen-free female ICR mice (Harlan Laboratories Inc., Indianapolis, IN) aged 6 to 8 weeks old and weighing 24 to 26 g were used. Mice were housed in polycarbonate shoebox cages with 1/4-inch corncob (The Andersons Inc., Maumee, OH) and Alpha-Dri (Shepherd Specialty Papers, Inc., Richland, MI) bedding with a 12-h light/dark cycle at 72°F. The animals were given food (Teklad 2918 irradiated extruded rodent diet) and water ad libitum. All procedures were performed in accordance with and approved by the University of Notre Dame Institutional Animal Care and Use Committee.
Neutropenic thigh infection model.
The mouse neutropenic thigh infection model described by Craig and Andes (21) was used to assess the efficacy of ND-421. Briefly, the mice (n = 10 mice per group) were rendered neutropenic by intraperitoneal treatment with 100 μl cyclophosphamide (Alfa Aesar, Haverhill, MA) at 200 mg/kg of body weight, 4 days and 1 day prior to the infection. MRSA strains NRS70 and NRS119 were grown in brain heart infusion broth to an OD540 of 0.5 and then diluted to a final concentration of approximately 106 CFU/ml in fresh brain heart infusion broth. An 0.1-ml aliquot of the bacterial inoculum was injected intramuscularly into the right thigh. One hour after infection, the animals were administered a single oral dose of 40 mg/kg of ND-421, linezolid, or vehicle (10% dimethyl sulfoxide [DMSO], 25% Tween 80, 65% water) by oral gavage. The animals were sacrificed by CO2 asphyxiation 48 h after infection. Terminal plasma was collected by cardiac puncture, followed by aseptic removal of both thigh muscles (uninfected and infected). The infected thigh was weighed, homogenized in phosphate-buffered saline using a Bullet blender (Next Advance, Inc., Averill Park, NY), serially diluted, and plated on LB agar plates (Fisher Scientific, Waltham, MA) to determine colony counts. The plates were incubated at 37°C for 24 h. The colony counts were expressed as log CFU/gram of tissue. Groups were compared using the Mann-Whitney U test.
In vivo synergy.
The in vivo efficacy of ND-421 in combination with oxacillin was evaluated in the neutropenic thigh infection model, as described above. The study included four groups (n = 8 mice/group): control (vehicle = 10% DMSO-25% Tween 80-65% water), ND-421 (40 mg/kg orally once a day for 2 days), oxacillin (20 mg/kg subcutaneously three times a day for 2 days), and combination of ND-421 plus oxacillin (40 mg/kg ND-421 orally once a day for 2 days and 20 mg/kg oxacillin subcutaneously three times a day for 2 days). The animals were sacrificed at 48 h. The infected thighs were processed as described above for bacterial counts, and the uninfected thighs and plasma were analyzed as described below for determination of drug levels.
Statistical analysis.
Statistical analysis was done using the Mann-Whitney U test on GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA).
Dose preparation.
Compounds were dissolved in 10% DMSO-25% Tween 80-65% water (vehicle) at a concentration of 10 mg/ml (equivalent to 40 mg/kg) or 5 mg/ml (20 mg/kg). For subcutaneous injections of oxacillin, the doses were filter sterilized using nylon membranes (0.2 μm; Pall Life Sciences, Port Washington, NY). Mice were given 100 μl of compound orally (ND-421, linezolid, or vehicle) or subcutaneously (oxacillin).
Drug levels in plasma and thigh.
Terminal blood collected from the neutropenic thigh infection model was centrifuged at 1,500 × g for 10 min to obtain plasma and stored at −80°C until analysis. Uninfected thighs were weighed and were stored at −80°C until analysis.
A 50-μl aliquot of plasma was quenched with 150 μl of internal standard in acetonitrile to precipitate protein. Standards of each compound ranging from 0 μg/ml to 20 μg/ml were spiked in 50 μl of mouse plasma (Bioreclamation IVT, Baltimore, MD) and quenched with 150 μl of internal standard (1 μg/ml) in acetonitrile. Samples and standards were then centrifuged for 10 min at 10,000 × g, and the supernatants were analyzed using a Waters Acquity ultraperformance liquid chromatograph (UPLC; Waters Corp., Milford, MA) coupled with a triple quadrupole mass spectrometer (TQD; Waters, Milford, MA) with multiple reaction monitoring (MRM). The uninfected thighs were homogenized in an equivalent volume of acetonitrile and were centrifuged at 10,000 × g for 10 min. A 50-μl aliquot of the thigh supernatant was diluted with 150 μl of internal standard solution in acetonitrile (1 μg/ml). The thigh samples containing ND-421 were diluted 10-fold with acetonitrile prior to internal standard addition. Samples were analyzed using UPLC-MRM. Acquisition parameters were as follows: Supelco Ascentis C18 column (3-μm particle size, 10 cm by 2.1 mm; Sigma-Aldrich, St. Louis, MO), electrospray ionization positive mode (ESI+), flow rate of 0.5 ml/min, capillary voltage of 4 kV, cone voltage of 30 V, and collision voltage of 25 V. The solvent program was as follows: 95% A-5% B for 0.25 min, 0.75-min linear gradient to 5% A-95% B, hold for 4 min, where A is 0.1% formic acid-water and B is 0.1% formic acid-acetonitrile. Retention times were 1.76 min for linezolid, 1.77 min for oxacillin, 2.60 min for ND-421, and 2.85 min for the internal standard. The methods were linear between 0 μg/ml and 20 μg/ml (R2 values > 0.98). MRM transitions for each compound were 338.2→296.1 for linezolid, 422.0→143.7 for ND-421, 401.9→160.1 for oxacillin, and 401.1→122.8 for the internal standard. Peak areas for each compound were calculated relative to those for the internal standard using Waters MassLynx software. A standard curve of peak area ratios plotted against standard concentration was generated from which drug concentrations were determined using regression parameters and dilution factor. Concentrations are reported in micrograms per milliliter for plasma and in micrograms per gram of tissue for thigh.
RESULTS AND DISCUSSION
MIC and inoculum effect.
We determined the MIC values of ND-421 for the S. aureus strains used in the study, including NRS119, a linezolid-resistant strain; the values ranged from 2 to 4 μg/ml (Table 1), indicating that ND-421 was efficacious by itself. The MICs of vancomycin for the same strains ranged from 1 to 2 μg/ml, and those of linezolid were from 1 to 32 μg/ml.
TABLE 1.
MIC values of ND-421 against a panel of S. aureus strains
| S. aureus strain | MIC (μg/ml) of drug: |
||
|---|---|---|---|
| ND-421 | Vancomycin | Linezolid | |
| N315 (NRS70)a | 2 | 1 | 1 |
| NRS100 (COL)a | 2 | 2 | 1 |
| NRS119b | 2 | 1 | 32 |
| NRS123c | 4 | 2 | 2 |
| NRS128d | 4 | 2 | 1 |
| MRSA252e | 2 | 1 | 2 |
| ATCC 29213f | 2 | 1 | 2 |
mecA positive; resistant to methicillin, oxacillin, and tetracycline; susceptible to vancomycin and linezolid.
mecA positive; resistant to ciprofloxacin, gentamicin, oxacillin, penicillin, and linezolid.
Community-acquired strain also designated MW2, C1999000459, USA400, and 99065.
Also designated NCTC8325 and RN0031.
Hospital-acquired strain isolated in the United Kingdom, also designated ATCC BAA-1720.
Quality control MSSA strain.
As the inoculum effect can reduce the potency of an antibiotic with increasing bacterial concentration, we investigated the effect of high MRSA density (up to 108 CFU/ml) on the activity of ND-421. An inoculum effect is defined as an 8-fold or greater increase in MIC with a higher inoculum. Thus, ND-421 showed no inoculum effect (Table 2).
TABLE 2.
MIC values of ND-421 with increasing bacterial concentration
| Strain | MIC (μg/ml) at bacterial concn (CFU/ml): |
||||
|---|---|---|---|---|---|
| 104 | 105 | 106 | 107 | 108 | |
| NRS70 | 2 | 2 | 2 | 4 | 4 |
| NRS100 | 2 | 2 | 2 | 4 | 4 |
| NRS119 | 1 | 2 | 2 | 2 | 2 |
| NRS123 | 4 | 4 | 4 | 4 | 4 |
| NRS128 | 2 | 4 | 4 | 4 | 4 |
| MRSA252 | 1 | 2 | 2 | 2 | 2 |
| ATCC 29213 | 2 | 2 | 2 | 4 | 4 |
Checkerboard assay.
The checkerboard assay was used to evaluate the in vitro synergy between ND-421 and six cell wall agents, five β-lactams (oxacillin, piperacillin, imipenem, meropenem, and cefepime) and a non-β-lactam (vancomycin), as well as four additional antibiotics targeting protein synthesis (the oxazolidinone linezolid, the aminoglycoside gentamicin, the tetracycline doxycycline, and the macrolide azithromycin). We used four MRSA strains, NRS70, NRS123, NRS100, and MRSA252, and an MSSA strain (NRS128).
Results are shown as isobolograms in Fig. 1, where the FIC of ND-421 is plotted on the x axis and the FIC of antibiotic is plotted on the y axis; a concave curve is observed for synergy, a linear curve represents additivity, and a convex curve indicates antagonism (22). With NRS100, ND-421 synergized with oxacillin, piperacillin, and imipenem; with MRSA 252, synergy was observed with oxacillin, imipenem, cefepime, and doxycycline. However, ND-421 synergized with all the β-lactams tested using MRSA strains NRS70 and NRS123. With the NRS128 strain, synergy was observed in combination with oxacillin and piperacillin. Cassat et al. compared the genomes of several S. aureus strains, including those of NRS70, NRS123, NRS100, MRSA 252, and NRS128 (23). A difference between these strains is the expression of the major histocompatibility complex (MHC) class II analog protein Map in S. aureus strains that do not show synergy, namely, NRS100, MRSA 252, and NRS128, and its absence in NRS70 and NRS123, strains that display synergy. Map is an immunomodulator that potentiates the survival of S. aureus by interfering with T cell-mediated host immunity (24). Mice infected with S. aureus deficient in Map have significantly reduced arthritis, osteomyelitis, and abscesses compared to controls, and mice treated with recombinant Map had reduced T cell-mediated responses and significantly reduced hypersensitivity, when challenged with antigen (24). Therefore, Map plays a role in S. aureus infections by interfering with cellular immunity. It is not known if this factor also influences the lack of in vitro synergy between ND-421 and β-lactams in S. aureus strains expressing Map. With non-β-lactams, the only synergy observed with ND-421 was in the case of strain NRS123 for gentamicin and with strains NRS123 and MRSA 252 for doxycycline combinations.
Among the β-lactam antibiotics, the highest synergy is with oxacillin (ΣFIC values of 0.31 and 0.37 against NRS70 and NRS123, respectively), which inhibits PBP1, -2, and -3, and the least synergy is with piperacillin (ΣFIC values of 0.41 against NRS70 and 0.50 against NRS123), which inhibits PBP3. Synergy is found with imipenem (preferentially inhibits PBP2; ΣFIC values of 0.41 and 0.30 against NRS70 and NRS123, respectively), meropenem (preferentially inhibits PBP1 but also inhibits PBP2 and PBP4; ΣFIC values of 0.35 against NRS70 and 0.31 against NRS123), and cefepime (inhibits PBP2 and PBP3; ΣFIC values of 0.35 and 0.33 against NRS70 and NRS123, respectively). As the oxadiazoles inhibit PBP2a (15) in MRSA and cooperation between the transpeptidase domain of PBP2a and the transglycosylase domain of PBP2 is required for cell wall synthesis in the presence of β-lactam antibiotics (25), we hypothesize that this is the basis of synergy between ND-421 and β-lactam antibiotics. Indeed, good synergy is observed between ND-421 and oxacillin, imipenem, meropenem, and cefepime, all of which inhibit PBP2, while the weakest synergy is observed with piperacillin, which inhibits preferentially PBP3.
In support of this mechanism for synergy, while ND-421 synergizes with β-lactam antibiotics that inhibit transpeptidases in cell wall biosynthesis, it does not synergize with vancomycin, an inhibitor of transglycosylation. Likewise, ND-421 does not synergize with the protein-synthesis inhibitor linezolid (bacteriostatic), the aminoglycoside gentamicin (bactericidal; synergy observed only with strain NRS123), the tetracycline doxycycline (bacteriostatic; mild synergy observed in NRS123), or the macrolide azithromycin (bacteriostatic).
Time-kill curves.
Synergy between ND-421 and the β-lactams (oxacillin, piperacillin, imipenem, and cefepime) and doxycycline was confirmed using time-kill assays with MRSA strains NRS70 and NRS123, as we had observed synergy by the checkerboard assay with these strains. In order to allow for assessment of synergy, 1/4 MIC of ND-421 was used, a concentration at which a bacteriostatic effect was observed up to 12 h of growth. The concentrations of the β-lactams and doxycycline ranged from 1/2 to 1/64 MIC. Following 24 h of incubation, a ≥2 log10 reduction in NRS70 colony counts was observed with the combination of ND-421 plus 1/4, 1/8, or 1/16 MIC of β-lactams compared to either of the single agents alone and from the starting inoculum (Fig. 2), indicating that ND-421 synergizes with β-lactam antibiotics. With the NRS123 strain, synergy was observed for oxacillin, imipenem, and cefepime in combination with ND-421; however, the combination of piperacillin and doxycycline with ND-421 showed a ≥2 log10 reduction of inoculum at the end of 24 h compared to the single agent but a <2 log10 reduction from the starting inoculum.
FIG 2.
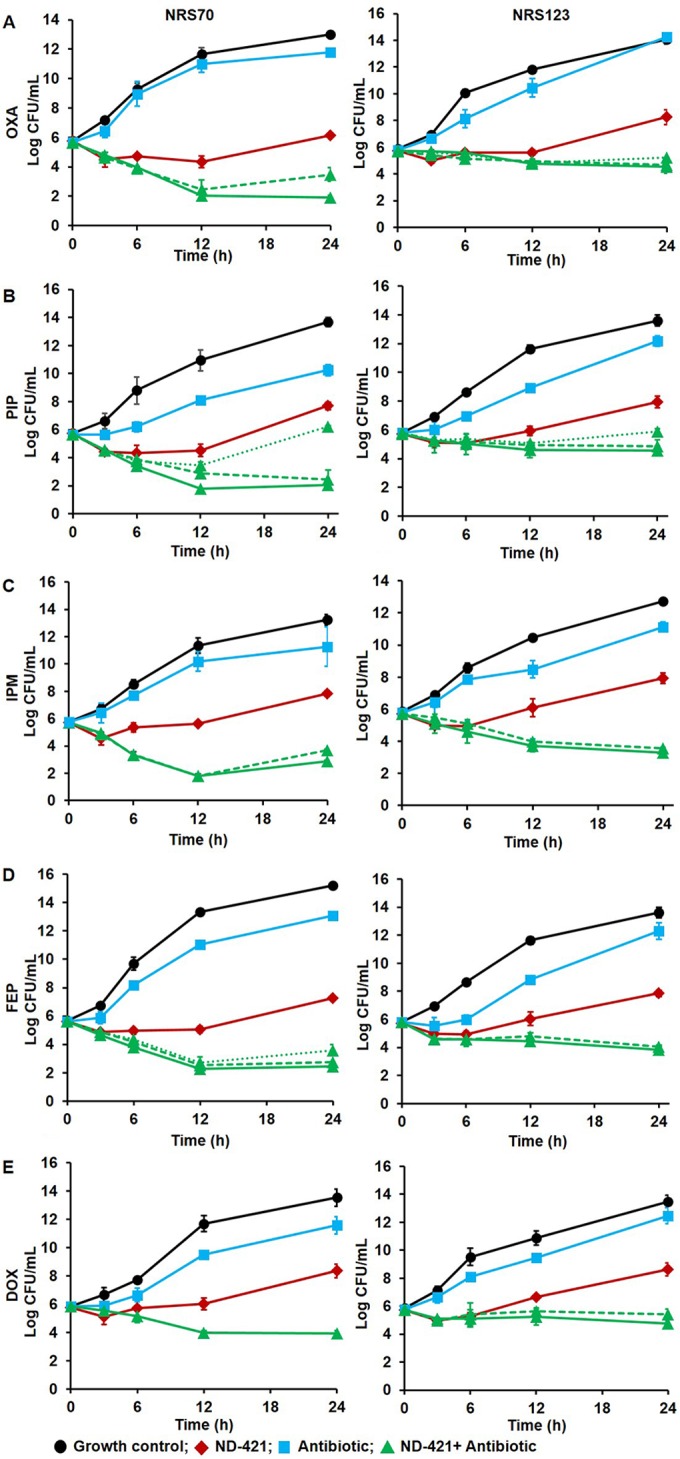
Time-kill curves against MRSA strains S. aureus NRS70 (left) and NRS123 (right) for combination of ND-421 (0.5 μg/ml, equivalent to 1/4 MIC; in red) with oxacillin (OXA; 1/8 MIC for NRS70; 1/4 MIC for NRS123; in blue) (A), piperacillin (PIP; 1/4 MIC; in blue) (B), imipenem (IPM; 1/4 MIC for NRS70, 1/2 MIC for NRS123; in blue) (C), cefepime (FEP; 1/8 MIC for NRS70, 1/4 MIC for NRS123; in blue) (D), and doxycycline (DOX; 1/4 MIC; in blue) (E). The limit of detection was 50 CFU/ml (1.6 log CFU/ml). The combination of ND-421 and antibiotic is shown in green, with the highest concentration of antibiotic in the combination depicted as a solid line, the intermediate concentration as a dashed line, and the lowest concentration as a dotted line.
Synergistic activity of antibiotics such as vancomycin and daptomycin with β-lactams has been reported in previous investigations, which showed that subinhibitory concentrations of the drugs could effectively inhibit bacterial growth (26, 27). Furthermore, daptomycin synergy is greater with β-lactams that preferentially bind to PBP1, such as meropenem, than with β-lactam antibiotics that preferentially bind to PBP2 (ceftriaxone), PBP3 (cefaclor), or PBP4 (cefoxitin) (28). However, no in vivo synergy studies have been reported for these combinations.
PAE.
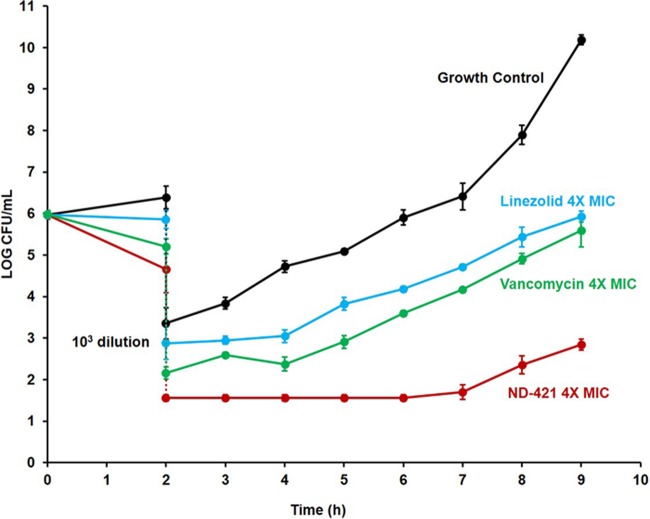
Antibiotics that exhibit postantibiotic effect (PAE) are considered more attractive than those that do not, as it often improves efficacy in vivo and requires less frequent dosing, which might improve patient compliance in taking the antibiotic. A PAE was seen at 2× and 4× MIC for ND-421, linezolid, and vancomycin, with ND-421 showing superior PAE (Fig. 3). After dilution, the growth control increased 1 log in 1.6 ± 0.2 h, while the linezolid- and vancomycin-treated cultures took 3.1 ± 0.4 and 3.4 ± 0.2 h, respectively. The ND-421-treated cultures took 6.3 ± 0.2 h to increase 1 log. The PAE for ND-421 was calculated at 4.7 h, compared to those of linezolid and vancomycin, which were 1.5 and 1.8 h, respectively.
FIG 3.
Postantibiotic effect of ND-421. S. aureus NRS70 was grown in MHB, adjusted to 106 CFU/ml, and incubated for 2 h without any antibiotic and in the presence of 4× MIC of ND-421, vancomycin, or linezolid. After 2 h, the antibiotics were removed by 1:1,000 dilution. The cultures were allowed to grow for another 7 h, and growth was monitored by viable colony counting. The growth of the control with no antibiotic increased 1 log in 1.6 h, while the linezolid- and vancomycin-treated cultures took about 3 h. The ND-421-treated culture took about 6 h to increase 1 log.
Neutropenic thigh infection.
We had previously shown that ND-421 has efficacy similar to that of linezolid (50% effective dose [ED50] of 3.1 mg/kg for ND-421 versus 2.8 mg/kg for linezolid) in the mouse peritonitis infection model (16), a widely used model that is relatively rapid with simple endpoints (death or survival). This model provides rapid evaluation of in vivo efficacy, as it requires good pharmacokinetic (PK) properties to observe efficacy. The neutropenic thigh infection model is a widely used mouse model that provides a quantitative assessment of the efficacy of an antibiotic, and also, it is more clinically relevant and reliably predicts efficacy in the clinic (29). This model resembles the complicated skin and tissue infections that are frequently seen in the clinic with MRSA infections. We, thus, evaluated ND-421 in this model after oral administration, compared it to linezolid, and used two MRSA strains: NRS70 (linezolid sensitive) and NRS119 (linezolid resistant). After 48 h of incubation, the bacteria increased to 5.9 × 107 and 5.3 × 107 CFU/g of thigh in the vehicle group with NRS70 and NRS119, respectively (Fig. 4). Treatment with ND-421 or linezolid resulted in 1.05 log and 1.00 log reductions of NRS70 (linezolid-sensitive MRSA), respectively, with no significant statistical differences between mice treated with ND-421 and those treated with linezolid (P > 0.05). We attributed the similar efficacy to the longer half-life and good tissue distribution of ND-421 and its greater PAE than that of linezolid (Fig. 3). However, the lower plasma-protein binding of linezolid (31% versus 98% for ND-421) (16, 30) would appear to compensate for the other superior attributes of ND-421 to make its efficacy equivalent in the neutropenic thigh infection model. However, the in vivo efficacy of ND-421 was significantly better than that of linezolid in the NRS119 strain (linezolid resistant), with 1.7 × 106 CFU/g or 1.49 log reduction compared to vehicle (P < 0.001), whereas linezolid resulted in no statistical reduction in bacterial counts (2.3 × 107 CFU/g [P > 0.05], Fig. 4).
FIG 4.
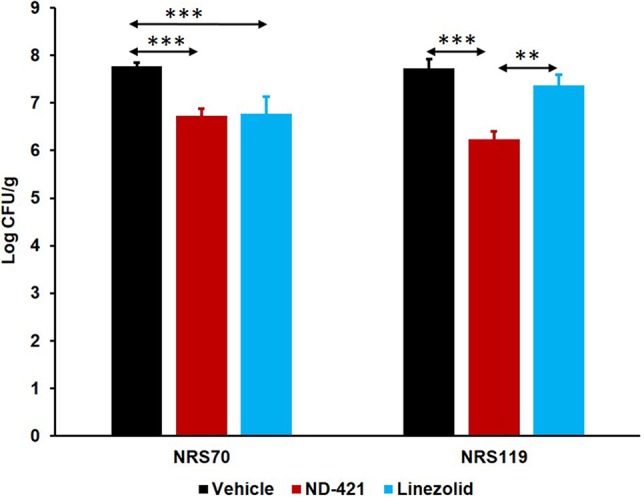
Efficacy of ND-421 in the mouse neutropenic thigh infection model. Mice (n = 10 mice/group) were rendered neutropenic by intraperitoneal administration of cyclophosphamide at 200 mg/kg at 4 days and 1 day prior to infection. The right thigh was infected intramuscularly with 100 μl of 106 CFU/ml of MRSA NRS70 (linezolid sensitive) or NRS119 (linezolid resistant). Mice were given a single oral dose of ND-421 (40 mg/kg), linezolid (40 mg/kg), or vehicle at 1 h after infection. The infected thighs were collected at 48 h after infection and homogenized, and the homogenate was plated, followed by determination of bacterial counts (mean ± standard error of the mean). **, P ≤ 0.01; *** P ≤ 0.001, as determined by Mann-Whitney U test. Efficacy of ND-421 was equivalent to that of linezolid in the NRS70 strain and superior to that of linezolid in the NRS119 strain.
Drug levels in plasma and thigh.
The concentrations of ND-421 and linezolid were measured in plasma and in uninfected thighs using UPLC with MRM, a mass spectrometric method that allows for quantification of a specific drug or compound of interest based on an identified mass transition. This method uses a triple quadrupole mass spectrometer, in which the molecular ion for the compound is selected in the first quadrupole, followed by specific fragmentation of the selected ion in the second quadrupole and selection and detection of a specific fragment in the third quadrupole. This method is characterized by low background due to the mass selection and is highly sensitive and selective for a particular compound of interest, e.g., an antibiotic. Levels of linezolid were not quantifiable in plasma or in thigh at 48 h, as the drug had been eliminated (Table 3). The concentrations of ND-421 in mice infected with NRS70 were 1.26 ± 0.48 μg/ml in plasma and 8.64 ± 3.09 μg/g in the uninfected thigh at 48 h, both above the MIC. The levels of ND-421 in mice infected with NRS119 were similar, 1.05 ± 0.64 μg/ml in plasma and 9.78 ± 3.79 μg/g in the uninfected thigh at 48 h. This was expected as linezolid has a relatively short half-life (t1/2 = ∼1 h) (31) compared to ND-421 (t1/2 = 18.6 h) (16).
TABLE 3.
Drug concentrations in the mouse neutropenic thigh infection model
| Compound | Mean drug concn ± SD in mouse infected with strain: |
|||
|---|---|---|---|---|
| NRS70 |
NRS119 |
|||
| Plasma (μg/ml) | Thigh (μg/g) | Plasma (μg/ml) | Thigh (μg/g) | |
| Linezolid (single dose)a | NQe | NQ | NQ | NQ |
| ND-421 (single dose)a | 1.26 ± 0.48 | 8.64 ± 3.09 | 1.05 ± 0.64 | 9.78 ± 3.79 |
| Oxacillin (multiple dose)b | 0.07 ± 0.15 | NQ | ||
| ND-421 (multiple dose)c | 3.18 ± 1.08 | 23.36 ± 5.38 | ||
| ND-421 (combination, multiple dose)d | 2.25 ± 0.72 | 20.39 ± 5.10 | ||
| Oxacillin (combination, multiple dose)d | 0.13 ± 0.35 | NQ | ||
Drug concentrations at 48 h postinfection in the mouse neutropenic thigh infection model evaluating the efficacy of ND-421 versus linezolid (40-mg/kg oral dose at 1 h after infection, n = 10 mice/group).
Oxacillin given at 20 mg/kg subcutaneous doses at 1, 9, 17, 25, 33, and 41 h postinfection, n = 8 mice.
ND-421 given at 40 mg/kg oral doses at 1 and 25 h postinfection, n = 8 mice.
Combination of oxacillin with ND-421 (two 40-mg/kg oral doses of ND-421 at 1 and 25 h postinfection and six 20-mg/kg subcutaneous doses of oxacillin at 1, 9, 17, 25, 33, and 41 h postinfection; n = 8 mice/group).
NQ, not quantifiable.
In vivo synergy.
As we had observed synergy of ND-421 in combination with oxacillin by the checkerboard assay and by time-kill curves, we next investigated the in vivo synergy of ND-421 in combination with oxacillin in the mouse neutropenic thigh infection model using MRSA NRS70. We gave two doses of ND-421 orally at 1 and at 25 h after infection. Oxacillin was dosed subcutaneously three times a day for 2 days. The dose of oxacillin was selected based on oxacillin pharmacokinetic (PK) parameters in mice reported earlier (32). Based on this consideration, a single subcutaneous dose of oxacillin at 20 mg/kg would give a maximum concentration of drug in serum (Cmax) of 36 μg/ml, which is below the MIC of 64 μg/ml for oxacillin against NRS70. In the untreated vehicle group, bacteria levels were 3.1 × 108 CFU/g at 48 h. The oxacillin, ND-421, and the combination groups showed bacterial density lower than that of the vehicle group, 1.3 × 108 CFU/g, 4.1 × 107 CFU/g, and 7.8 × 106 CFU/g, respectively (Fig. 5). The decrease in bacterial load relative to the vehicle control was 0.38 log for the oxacillin group, 0.88 log for the ND-421 group, and 1.60 log for the ND-421 plus oxacillin group. We attributed the observation of the slight efficacy of oxacillin to the fact that multiple 20-mg/kg doses of oxacillin were given, which could increase the plasma concentrations of the antibiotic for brief periods above the MIC of 64 μg/ml for the strain. The combination of ND-421 with oxacillin showed increased efficacy with a significantly lowered bacterial load compared to single-agent treatment (1.22 log reduction in the combination compared to oxacillin, P < 0.05; 0.72 log reduction in the combination compared to ND-421, P < 0.05) (Fig. 5).
FIG 5.
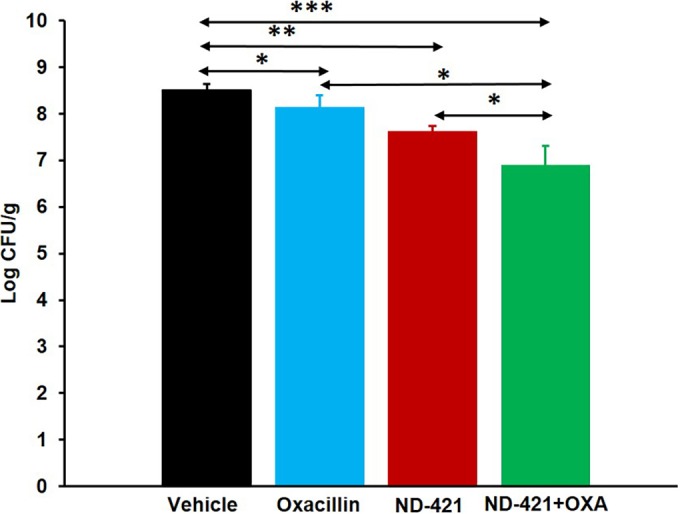
Efficacy of ND-421 in combination with oxacillin in the mouse neutropenic thigh infection model. Mice (n = 8 mice/group) were rendered neutropenic by intraperitoneal administration of cyclophosphamide at 200 mg/kg on day 4 and day 1 prior to infection. The right thigh was infected intramuscularly with 100 μl of 106 CFU/ml of MRSA NRS70. Mice were given two oral doses of ND-421 at 40 mg/kg at 1 and 25 h after infection; six subcutaneous doses of oxacillin at 20 mg/kg at 1, 9, 17, 25, 33, and 41 h after infection; or a combination of ND-421 plus oxacillin. The infected thighs were collected at 48 h after infection and homogenized, and the homogenate was plated, followed by determination of bacterial counts (mean ± standard error of the mean). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001, as determined by Mann-Whitney U test. The combination of ND-421 with oxacillin showed increased efficacy with a bacterial load significantly lower than that of the single-agent treatment.
Plasma levels of oxacillin at 48 h were 0.07 ± 0.15 μg/ml and 0.13 ± 0.35 μg/ml in mice given oxacillin only and ND-421 plus oxacillin, respectively (Table 3). However, oxacillin levels were below the limit of quantification in the thighs. Plasma levels of ND-421 at 48 h were 3.18 ± 1.08 μg/ml in mice given ND-421 alone and 2.25 ± 0.72 μg/ml in mice given ND-421 plus oxacillin. Higher levels of ND-421 at 48 h were found in the thighs of mice receiving ND-421 only (23.36 ± 5.38 μg/g) and ND-421 plus oxacillin (20.39 ± 5.10 μg/g).
In summary, we demonstrate that the cell wall biosynthesis inhibitor ND-421 has in vivo efficacy by itself in a clinically relevant infection model and acts synergistically with β-lactam antibiotics in vitro and in vivo and that the combination of ND-421 with oxacillin is efficacious in a mouse neutropenic thigh MRSA infection model.
REFERENCES
- 1.Klein EY, Sun L, Smith DL, Laxminarayan R. 2013. The changing epidemiology of methicillin-resistant Staphylococcus aureus in the United States: a national observational study. Am J Epidemiol 177:666–674. doi: 10.1093/aje/kws273. [DOI] [PubMed] [Google Scholar]
- 2.Llarrull LI, Testero SA, Fisher JF, Mobashery S. 2010. The future of the beta-lactams. Curr Opin Microbiol 13:551–557. doi: 10.1016/j.mib.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prystowsky J, Siddiqui F, Chosay J, Shinabarger DL, Millichap J, Peterson LR, Noskin GA. 2001. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob Agents Chemother 45:2154–2156. doi: 10.1128/AAC.45.7.2154-2156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, Moellering RC, Ferraro MJ. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207–208. doi: 10.1016/S0140-6736(01)05410-1. [DOI] [PubMed] [Google Scholar]
- 5.Appelbaum PC. 2006. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. Clin Microbiol Infect 12(Suppl 1):S16–S23. [DOI] [PubMed] [Google Scholar]
- 6.Moellering RC., Jr 2008. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46:1032–1037. doi: 10.1086/529445. [DOI] [PubMed] [Google Scholar]
- 7.Gasch O, Camoez M, Dominguez MA, Padilla B, Pintado V, Almirante B, Martin C, Lopez-Medrano F, de Gopegui ER, Blanco JR, Garcia-Pardo G, Calbo E, Montero M, Granados A, Jover A, Duenas C, Pujol M, REIPI/GEIH Study Groups. 2014. Emergence of resistance to daptomycin in a cohort of patients with methicillin-resistant Staphylococcus aureus persistent bacteraemia treated with daptomycin. J Antimicrob Chemother 69:568–571. doi: 10.1093/jac/dkt396. [DOI] [PubMed] [Google Scholar]
- 8.Sakoulas G, Alder J, Thauvin-Eliopoulos C, Moellering RC Jr, Eliopoulos GM. 2006. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob Agents Chemother 50:1581–1585. doi: 10.1128/AAC.50.4.1581-1585.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuda C, Hesek D, Lee M, Morio K, Nowak T, Mobashery S. 2005. Activation for catalysis of penicillin-binding protein 2a from methicillin-resistant Staphylococcus aureus by bacterial cell wall. J Am Chem Soc 127:2056–2057. doi: 10.1021/ja0434376. [DOI] [PubMed] [Google Scholar]
- 10.Otero LH, Rojas-Altuve A, Llarrull LI, Carrasco-Lopez C, Kumarasiri M, Lastochkin E, Fishovitz J, Dawley M, Hesek D, Lee M, Johnson JW, Fisher JF, Chang M, Mobashery S, Hermoso JA. 2013. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc Natl Acad Sci U S A 110:16808–16813. doi: 10.1073/pnas.1300118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saravolatz SN, Martin H, Pawlak J, Johnson LB, Saravolatz LD. 2014. Ceftaroline-heteroresistant Staphylococcus aureus. Antimicrob Agents Chemother 58:3133–3136. doi: 10.1128/AAC.02685-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long SW, Olsen RJ, Mehta SC, Palzkill T, Cernoch PL, Perez KK, Musick WL, Rosato AE, Musser JM. 2014. PBP2a mutations causing high-level ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 58:6668–6674. doi: 10.1128/AAC.03622-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendes RE, Tsakris A, Sader HS, Jones RN, Biek D, McGhee P, Appelbaum PC, Kosowska-Shick K. 2012. Characterization of methicillin-resistant Staphylococcus aureus displaying increased MICs of ceftaroline. J Antimicrob Chemother 67:1321–1324. doi: 10.1093/jac/dks069. [DOI] [PubMed] [Google Scholar]
- 14.Munita JM, Bayer AS, Arias CA. 2015. Evolving resistance among Gram-positive pathogens. Clin Infect Dis 61(Suppl 2):S48–S57. doi: 10.1093/cid/civ523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Daniel PI, Peng Z, Pi H, Testero SA, Ding D, Spink E, Leemans E, Boudreau MA, Yamaguchi T, Schroeder VA, Wolter WR, Llarrull LI, Song W, Lastochkin E, Kumarasiri M, Antunes NT, Espahbodi M, Lichtenwalter K, Suckow MA, Vakulenko S, Mobashery S, Chang M. 2014. Discovery of a new class of non-beta-lactam inhibitors of penicillin-binding proteins with Gram-positive antibacterial activity. J Am Chem Soc 136:3664–3672. doi: 10.1021/ja500053x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spink E, Ding D, Peng Z, Boudreau MA, Leemans E, Lastochkin E, Song W, Lichtenwalter K, O'Daniel PI, Testero SA, Pi H, Schroeder VA, Wolter WR, Antunes NT, Suckow MA, Vakulenko S, Chang M, Mobashery S. 2015. Structure-activity relationship for the oxadiazole class of antibiotics. J Med Chem 58:1380–1389. doi: 10.1021/jm501661f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF, Infectious Diseases Society of America. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 18.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. CLSI, Wayne, PA. [Google Scholar]
- 19.Eliopoulos GM, Moellering RC Jr. 1996. Antibiotics in laboratory medicine, 4th ed Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 20.Bundtzen RW, Gerber AU, Cohn DL, Craig WA. 1981. Postantibiotic suppression of bacterial growth. Rev Infect Dis 3:28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- 21.Craig WA, Andes DR. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob Agents Chemother 52:3492–3496. doi: 10.1128/AAC.01273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chait R, Craney A, Kishony R. 2007. Antibiotic interactions that select against resistance. Nature 446:668–671. doi: 10.1038/nature05685. [DOI] [PubMed] [Google Scholar]
- 23.Cassat JE, Dunman PM, McAleese F, Murphy E, Projan SJ, Smeltzer MS. 2005. Comparative genomics of Staphylococcus aureus musculoskeletal isolates. J Bacteriol 187:576–592. doi: 10.1128/JB.187.2.576-592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee LY, Miyamoto YJ, McIntyre BW, Hook M, McCrea KW, McDevitt D, Brown EL. 2002. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated responses. J Clin Invest 110:1461–1471. doi: 10.1172/JCI0216318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinho MG, de Lencastre H, Tomasz A. 2001. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc Natl Acad Sci U S A 98:10886–10891. doi: 10.1073/pnas.191260798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Climo MW, Patron RL, Archer GL. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob Agents Chemother 43:1747–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rand KH, Houck HJ. 2004. Synergy of daptomycin with oxacillin and other beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 48:2871–2875. doi: 10.1128/AAC.48.8.2871-2875.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berti AD, Sakoulas G, Nizet V, Tewhey R, Rose WE. 2013. Beta-lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:5005–5012. doi: 10.1128/AAC.00594-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan CM, Therien AG, Lu J, Lee SH, Caron A, Gill CJ, Lebeau-Jacob C, Benton-Perdomo L, Monteiro JM, Pereira PM, Elsen NL, Wu J, Deschamps K, Petcu M, Wong S, Daigneault E, Kramer S, Liang L, Maxwell E, Claveau D, Vaillancourt J, Skorey K, Tam J, Wang H, Meredith TC, Sillaots S, Wang-Jarantow L, Ramtohul Y, Langlois E, Landry F, Reid JC, Parthasarathy G, Sharma S, Baryshnikova A, Lumb KJ, Pinho MG, Soisson SM, Roemer T. 2012. Restoring methicillin-resistant Staphylococcus aureus susceptibility to beta-lactam antibiotics. Sci Transl Med 4:126ra135. doi: 10.1126/scitranslmed.3003592. [DOI] [PubMed] [Google Scholar]
- 30.Stalker DJ, Jungbluth GL. 2003. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet 42:1129–1140. doi: 10.2165/00003088-200342130-00004. [DOI] [PubMed] [Google Scholar]
- 31.Slatter JG, Adams LA, Bush EC, Chiba K, Daley-Yates PT, Feenstra KL, Koike S, Ozawa N, Peng GW, Sams JP, Schuette MR, Yamazaki S. 2002. Pharmacokinetics, toxicokinetics, distribution, metabolism and excretion of linezolid in mouse, rat and dog. Xenobiotica 32:907–924. doi: 10.1080/00498250210158249. [DOI] [PubMed] [Google Scholar]
- 32.Sandberg A, Jensen KS, Baudoux P, Van Bambeke F, Tulkens PM, Frimodt-Moller N. 2010. Intra- and extracellular activities of dicloxacillin against Staphylococcus aureus in vivo and in vitro. Antimicrob Agents Chemother 54:2391–2400. doi: 10.1128/AAC.01400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]