Abstract
Introduction
The hemoglobin FSD is very uncommon in newborn screening programs for sickle cell disease. In the program of Minas Gerais, Brazil, the clinical course of children with hemoglobin SD was observed to be heterogeneous. The objective of this study was to estimate the incidence (1999–2012) and to describe the natural history of a cohort of newborns with hemoglobin SD.
Methods
Isoelectric focusing was the primary method used in newborn screening. Polymerase chain reaction-restriction fragment length polymorphism and gene sequencing were used to identify mutant alleles and for haplotyping. Gap-polymerase chain reaction was used to detect alpha-thalassemia.
Results
Eleven cases of hemoglobin S/D-Punjab and eight of Hb S-Korle Bu were detected. Other variants with hemoglobin D mobility were not identified. All hemoglobin D-Punjab and hemoglobin Korle Bu alleles were associated with haplotype I. Among the children with hemoglobin S/D-Punjab, there were four with the βS CAR haplotype, six with the Benin haplotype, and one atypical. Results of laboratory tests for hemoglobin S/D-Punjab and hemoglobin S-Korle Bu were: hemoglobin 8.0 and 12.3 g/dL (p-value <0.001), leukocyte count 13.9 × 109/L and 10.5 × 109/L (p-value = 0.003), reticulocytes 7.5% and 1.0% (p-value <0.001), hemoglobin F concentration 16.1% and 6.9% (p-value = 0.001) and oxygen saturation 91.9% and 97% (p-value = 0.002), respectively. Only hemoglobin S/D-Punjab children had acute pain crises and needed blood transfusions or hydroxyurea. Those with the Benin βS haplotype had higher total hemoglobin and hemoglobin F concentrations compared to the CAR haplotype. Transcranial Doppler was normal in all children.
Conclusion
The clinical course and blood cell counts of children with hemoglobin S/D-Punjab were very similar to those of hemoglobin SS children. In contrast, children with hemoglobin S-Korle Bu had clinical course and blood cell counts like children with the sickle cell trait.
Keywords: Sickle cell disease, Hemoglobin S/D-Punjab, Hemoglobin S-Korle Bu, Children, Haplotypes
Introduction
Sickle cell disease (SCD) is a public health problem worldwide. The hemoglobin (Hb) SD subtype seems to be very rare. It includes the Hb S/D-Punjab variant that apparently is associated with a more severe clinical course, and other Hb S-non-Punjab variants, with limited information regarding laboratory and clinical data.1, 2, 3
The Hb D-Punjab variant is the most common D-subtype described in the literature worldwide. It was first described in 1951 by Itano.4 It results from the replacement of the amino acid glutamate with glutamine at position 121 of the beta-globin chain [beta 121(GH4) Glu>Gln; HBB: c.364G>C].
Another variant within the window of Hb D using isoelectric focalization (IEF), but not using high-performance liquid chromatography (HPLC), is Hb Korle Bu, which results from the replacement of the amino acid aspartate with asparagine at position 72 of the beta-globin chain [beta 73(E17) Asp>Asn; HBB: c.220G>A]. It originates from the western region of Africa, and its dissemination to America is probably linked to the slave trade in the 17th to 19th centuries.5
The clinical course of patients with Hb SD disease seems to be heterogeneous, depending on the Hb D variant. This suggests that the sickling process is likely the result of the interaction between the intracellular Hb S and Hb D variants. This interaction may be strengthened or weakened, depending on the Hb variant co-inherited with the βS mutation.6, 7, 8, 9, 10, 11, 12, 13, 14 Children with Hb S/D-Punjab disease seem to present a clinical course similar to those with homozygous Hb SS disease.8, 10, 11, 15, 16
Thus, patients with Hb S/D-Punjab disease should receive the same treatment protocol as those with Hb SS disease because they may also experience potentially fatal complications during their lives.10, 11, 17 The use of hydroxyurea in children with Hb SD disease has been restricted to isolated cases.11, 18, 19
The objective of this study was to estimate the incidence and to describe the natural history of newborns with the Hb SD pattern by IEF, screened as part of the Neonatal Screening Program in the Brazilian state of Minas Gerais (PTN-MG). Additionally, beta-globin cluster haplotypes for the βS, βD-Punjab and βKorle Bu mutations were determined in order to try to trace the origin of the respective mutations.
Methods
This descriptive study is based on a retrospective cohort. Archived medical records from the Fundação Hemominas (Government Blood Center) and the PTN-MG databank were used. IEF of dried blood spot samples (Neonatal Hemoglobin Resolve Screen Kit, PerkinElmer Life and Analytical Sciences, Finland) has been the primary method of newborn screening at PTN-MG. Allele-specific polymerase chain reaction (PCR) for βA, βS, βC, and βD-Punjab alleles was introduced in the blood bank protocol as a confirmatory test in 2010.
The population initially comprised 21 children with Hb FSD at birth. Hb FSD means that the children were born with three Hb fractions: Hb F (the major fraction at birth that steadily decreases over the first year of life), Hb S, and an Hb with a zone D mobility in electrophoresis. They were born between January 1, 1999 and December 31, 2012; a total of 3,590,315 children were screened in this period. The patients’ clinical and laboratory data up to December 31, 2014 were reviewed so that all children, except two, had been followed up for at least two years.
Two patients were excluded from the clinical and molecular analyses because ethylenediaminetetraacetic acid (EDTA)-anticoagulated blood samples had not been obtained for molecular tests: one child moved abroad and another moved out of the state. Contact with the families has failed thus far. These children were used only to calculate the incidence of Hb SD in the cohort.
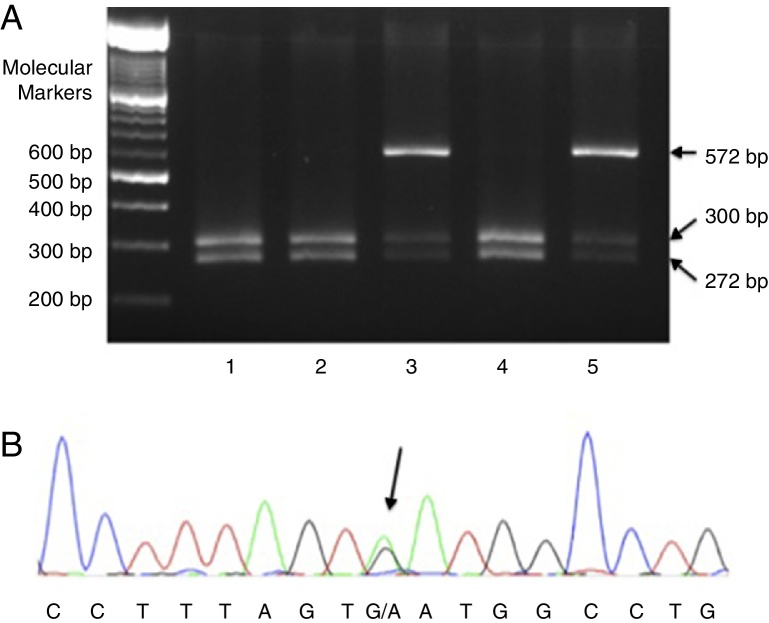
Fragments of the beta-globin gene (HBB) containing exon 3 (forward primer 5′-TCATGCCTCTTTGCACCATTC-3′; reverse primer 5′-CACTGACCTCCCACATTCCC-3′) were amplified using PCR, and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was conducted with the EcoRI enzyme to detect the D-Punjab allele (Figure 1A). If the reaction was negative, the three exons of the HBB gene were sequenced to identify the mutation underlying the other Hb variants (primer composition available on request). DNA sequencing was done in an ABI 3130 capillary sequencer (Applied Biosystems, Foster City, CA, USA). Figure 1B illustrates the gene sequencing of the region in which the HBB: c.220G>A mutation underlying Hb Korle Bu is located. Detection of seven more common HBA deletions was carried out by multiplex gap-PCR.20
Figure 1.
Molecular detection of mutations in Hb D-Punjab and Hb Korle Bu. (A) Polymerase chain reaction-restriction fragment length polymorphism with EcoRI in five children with Hb D-Punjab. Patients 1, 2, and 4 had wild alleles (two bands of 300 and 272 base pairs) and Patients 3 and 5 had an additional band of 572 base pairs that indicates a heterozygous mutation in the codon 121 of HBB (Hb D-Punjab); (B) electropherogram of a child with Hb Korle Bu. The arrow points to the heterozygous mutation HBB:c.220G>A (GAT>AAT; Asp>Asn) detected through gene sequencing.
The β-globin gene cluster haplotyping was carried out by PCR-RFLP of six restriction sites: HindIII in the IVS-II of Gγ (rs113425530), HindIII in the IVS-II of Aγ (rs28440105), HincII in ψβ (rs10128556), HincII 3′ to ψβ (without rs), HinfI 5′ to β (rs16911905), and HinfI 3′ to β (rs10837631). Additionally, HincII 5′ to ɛ (rs3834466) and AvaII at IVS-II-16 of β (rs10768683) were determined by gene sequencing. Restriction enzymes used for the haplotype analyses were purchased from New England Biolabs, Inc. (Beverly, MA, USA). Classification of haplotypes was based on Orkin et al.21 and Nagel et al. for the βS gene.22 The assignment of specific haplotypes in heterozygous states should be interpreted with caution if family studies or allele cloning are not performed. In the present study, the interpretation of the results was facilitated by our previous report showing that in 206 Hb SS children from Minas Gerais, 98.5% of the βS chromosomes were of the types CAR or Ben.23
A Coulter T-890 hematology counter was used to perform all blood cell counts. Reticulocytes were counted in blood smears stained with brilliant cresyl blue. All hematological values were transcribed from the medical records in the absence of acute clinical manifestations and at least three months after the use of blood products. The mathematical average of each item was considered as the baseline value for each patient. The relative baseline concentration of Hb F was obtained from the Hb electrophoresis results reported in each patient's medical record. The results of electrophoresis testing performed at the oldest age possible within the follow-up period were used as long as the sample had been collected after two years of age.
Transcranial Doppler (TCD) examinations were performed in 14 children and interpreted by a single expert using a Nicolet equipment (model EME TC 2000, Nicolet, Madison, WI, USA). High-risk TCD was defined as a time-averaged mean of the maximum velocity (TAMMX) ≥200 cm/s in the internal carotid or middle cerebral artery as originally defined by stroke prevention in sickle cell anemia (STOP) investigators.24 The examination could not be performed in five patients: three examinations were impossible because of the lack of cooperation on the part of the children and two were not performed because the children failed to attend the examination.
Statistical analyses were performed using the Statistical Package for the Social Sciences program (SPSS), version 20.0. Quantitative results are expressed as the average ± standard deviation or as the median and range when distribution was non-Gaussian. Prevalence was expressed as a percentage and a 95% confidence interval (CI) was applied. Unpaired t tests were used to compare mean values between Hb S/D-Punjab and Hb S-Korle Bu groups. Test results were considered significant when the probability of alpha error was ≤0.05.
The study was approved by the Ethics Research Committee at the involved institutions (case No. 13327713.5.0000.5149). It was conducted in accordance with the Helsinki Declaration as revised in 2008. Patients and/or guardians were asked to sign the informed consent form.
Results
The incidence of Hb FSD at birth was 1:171,000 (95% CI: 1:120,000–1:299,000). Molecular analyses were applied to samples from 19 out of the 21 children because as previously mentioned, sample collection from two children was not possible. The ages ranged from 2.8 to 16.2 years, with a median of 8.9 years. Thirteen children were male (68.4%) and six were female (31.6%).
Out of the 19 children, 11 children (ten families) were diagnosed with Hb S/D-Punjab disease and eight were diagnosed with Hb S-Korle Bu. No other variants were found. Gender distribution in the Hb S/D-Punjab group was four females (36.4%) and seven males (63.6%) and gender distribution in the Hb S-Korle Bu group was two females (25%) and six males (75%). The median age was 11 years (range: 2.8–16.2) in the Hb S/D-Punjab group and 7.7 years (range: 3–13.7) in the Hb S-Korle Bu group.
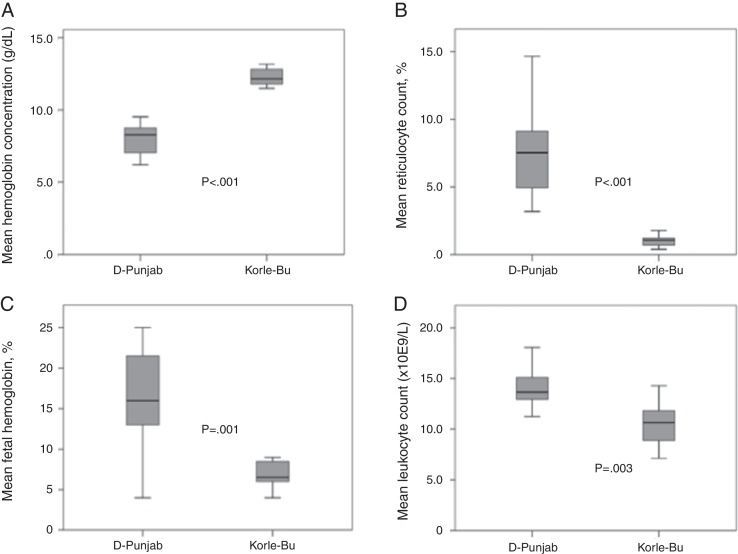
All children of the Hb S/D-Punjab group were found to have baseline Hb levels below 10 g/dL (average: 8.0 g/dL) and the reticulocyte counts varied. The baseline values of the hematologic tests and genetic results for each child are reported in Table 1. Figure 2 compares the main results found in both groups. The Hb S/D-Punjab group was found to have lower average baseline Hb values and higher reticulocyte counts than the Hb S-Korle Bu group (p-value <0.001 for both comparisons). Relative Hb F concentrations were higher in the children of the Hb S/D-Punjab group (p-value = 0.001). Total leukocyte and platelet counts were also higher in the Hb S/D-Punjab group (p-value = 0.003 and p-value = 0.06, respectively). Mean ratios between Hb S and Hb D concentrations were 1.06 and 1.14 in the Hb S/D-Punjab and Hb S-Korle Bu groups, respectively (p-value = 0.61).
Table 1.
Mean baseline hematological data and genetic results of 11 children with Hb S/DPunjab and eight children with Hb S-Korle-Bu.
| Id/Gender | Hemoglobin (g/dL) | Leukocytes (×109/L) | Platelets (×109/L) | Reticulocytes (%) | Hb F (%) | Hb S (%) | Hb D (%) | βS haplotype/α−3.7 thalb |
|---|---|---|---|---|---|---|---|---|
| Children with Hb S/DPunjab(n = 11) | ||||||||
| 1/F | 7.2 | 13.9 | 347.7 | 4.0 | 13 | 48 | 37 | CAR/− |
| 2/M | 7.9 | 16.1 | 431.8 | 14.7 | 13 | 45 | 39 | ATP/− |
| 3/F | 6.8 | 13.7 | 368.0 | 8.7 | 14 | 38 | 44 | BEN/− |
| 4/M | 6.8 | 18.0 | 552.0 | 7.5 | 4 | 44 | 37 | CAR/− |
| 5/F | 8.3 | 15.2 | 300.2 | 9.5 | 22 | 41 | 35 | BEN/− |
| 6/Ma | 8.6 | 11.2 | 335.0 | 3.9 | 23 | 33 | 40 | BEN/+ |
| 7/F | 9.5 | 12.6 | 353.8 | 3.2 | 16 | 47 | 39 | BEN/− |
| 8/Ma | 8.9 | 15.0 | 454.0 | 6.4 | 21 | 36 | 40 | BEN/− |
| 9/M | 8.4 | 13.6 | 432.2 | 10.3 | 10 | 40 | 46 | CAR/+ |
| 10/M | 6.2 | 13.3 | 308.8 | 8.7 | 16 | 39 | 35 | CAR/− |
| 11/M | 9.5 | 11.2 | 430.9 | 5.9 | 25 | 37 | 36 | BEN/− |
| Mean | 8.0 | 14.0 | 392.2 | 7.5 | 16.1 | 40.7 | 38.9 | |
| Children with Hb S-Korle-Bu (n = 8) | ||||||||
| 1/M | 11.7 | 12.2 | 397.3 | 1.8 | 6 | 58 | 34 | CAR/+ |
| 2/M | 12.8 | 10.8 | 366.9 | 1.2 | 9 | 45 | 45 | CAR/− |
| 3/M | 11.5 | 7.1 | 326.6 | 1.0 | 7 | 45 | 42 | CAR/− |
| 4/F | 12.8 | 7.7 | 257.0 | 1.1 | 6 | 59 | 33 | BEN/− |
| 5/F | 13.2 | 10.1 | 275.6 | 0.4 | 9 | 41 | 48 | CAR/+ |
| 6/M | 12.3 | 11.4 | 331.7 | 0.6 | 6 | 39 | 52 | CAR/− |
| 7/M | 12.0 | 14.3 | 407.9 | 1.3 | 4 | 50 | 44 | CAR/− |
| 8/M | 11.8 | 10.5 | 266.3 | 0.8 | 8 | 39 | 50 | CAR/− |
| Mean | 12.3 | 10.5 | 328.7 | 1.0 | 6.9 | 47.0 | 43.5 | |
Brothers.
Co-inheritance (+ or −) of the alpha-thalassemia gene −α3.7 (−α3.7/αα).
Figure 2.
Mean baseline hematological data of 11 children with Hb S/D-Punjab and eight children with Hb S-Korle Bu. (A) Total hemoglobin concentration (g/dL); (B) reticulocytes (%); (C) fetal hemoglobin (%); (D) leukocyte count (×109/L). The unpaired t test was used to compare mean values between Hb S/D-Punjab and Hb S-Korle Bu groups.
The coinheritance of alpha-thalassemia (αα/−α3.7) was detected in four children (two with Hb S/D-Punjab and two with Hb S-Korle Bu). Mean corpuscular volume (MCV) and mean corpuscular Hb (MCH) were significantly lower in children who co-inherited the alpha-thalassemia deletion (p-value = 0.008 and p-value = 0.03, respectively). The Hb S/D-Punjab group was analyzed separately: the MCVs of the patients with and without-alpha thalassemia averaged 76.6 fL and 85.9 fL, respectively (p-value = 0.001), while the MCHs averaged 24.2 pg and 29.1 pg, respectively (p-value = 0.001).
In terms of the associated clinical findings, two (18.2%) of the children in the Hb S/D-Punjab group had acute splenic sequestration crises (ASSCs), and all 11 experienced at least one acute pain episode. However, there were no ASSCs in the Hb S-Korle Bu group, and three patients (37.5%) had at least one acute pain episode reported as such in the patients’ medical records. None of the children in the Hb S-Korle Bu group received blood transfusions; however, seven (63.6%) children in the Hb S/D-Punjab group received transfusions.
No child had an overt stroke. Mean Doppler TAMMX values for Hb S/D-Punjab and Hb S-Korle Bu children were 131.1 cm/s [standard error of the mean (SEM): 6.7] and 89.2 cm/s (SEM: 6.5), respectively (p-value = 0.001). All children were classified as being at low risk for strokes.
Baseline oxygen saturation was lower in the Hb S/D-Punjab group and significantly differed from the Hb S-Korle Bu group. Average values in the two groups were 91.9% and 97%, respectively (p-value = 0.002). There were no deaths in the study population.
Clinical treatment was also reviewed. All patients received a prescription for antimicrobial prophylaxis, daily folic acid supplements, and immunizations. Three (27.3%) of the children with Hb S/D-Punjab disease were found to have used hydroxyurea, as indicated for recurring episodes of pain crisis. All children presented increased total Hb and Hb F as well as lower reticulocyte counts and fewer pain episodes after hydroxyurea therapy. Another patient who had also received an indication for the use of hydroxyurea because of recurring pain crises is likely to begin using the medication soon. Table 2 shows the data on the use of hydroxyurea.
Table 2.
Clinical and laboratorial data in three children with Hb SD-Punjab and response to hydroxyurea.
| Id/gender | Age (years) at start of HU | Clinical indication for HU | Dose (mg/kg/day) | Duration (months) | Before hydroxyurea |
After hydroxyurea |
Decreased number of clinical events | ||
|---|---|---|---|---|---|---|---|---|---|
| Total Hb (g/dL)/Hb F (%) | Ret (%) | Total Hb (g/dL)/Hb F (%) | Ret (%) | ||||||
| 11/M | 14 | Severe vaso-occlusive pain episodes | 20 | 8 | 9.5/26.7 | 6.1 | 11.1/34 | 0.4 | Yes |
| 9/M | 6.6 | Severe vaso-occlusive pain episodes | 27 | 76 | 8.5/9.0 | 8.4 | 10.2/17 | 3.4 | Yes |
| 4/Ma | 0.8 | Anemia | 15 | 36 | |||||
| 8 | Severe vaso-occlusive pain episodes | 26 | 27 | 7.6/4.0 | 11.7 | 8.8/20 | 6.3 | Yes | |
Id: identification refers to numbers in Table 1; HU: hydroxyurea; Ret: reticulocyte count.
This child was given hydroxyurea in August 2005 because of Hb < 5.0 g/dL. Hydroxyurea was withdrawn in July 2008 by his mother, not by the hematologist. The drug was restarted in September 2012, because of multiple episodes of vaso-occlusive pain.
Haplotyping of Hb S/D-Punjab showed that all patients presented haplotype I for the D-Punjab allele (+----+++, for the eight analyzed polymorphic sites in the 5′->3′ direction). In respect to the β-globin gene cluster, six were Benin, four CAR, and one had an atypical haplotype (+----+++, e.g., haplotype I in homozygosis). Similarly, all Hb S-Korle Bu alleles presented haplotype I. Seven had the βS CAR haplotype and only one, the βS Benin haplotype.
Comparing hematological data between βS CAR and βS Benin haplotypes within the Hb S/D-Punjab group of children, Hb concentration (7.2 g/dL versus 8.6 g/dL, respectively) and fetal Hb (10.8% versus 20.2%, respectively) were significantly higher for the Benin haplotype (p-value = 0.05 and p-value = 0.01, respectively). No other data, including Doppler TAMMX values, oxygen saturation, and number of transfusions, were significantly different between CAR and Benin groups.
Discussion
Two variants with the IEF profile of Hb D were identified: D-Punjab and Korle Bu. The clinical and laboratory characteristics of the two groups were very different.
Children with Hb S/D-Punjab disease presented many different symptoms. This clinical course is similar to that observed in children with homozygous Hb SS disease as has already been reported in other studies.10, 11, 16, 17, 25 Oberoi et al. evaluated ten patients aged between 1 and 19 years. All of them presented with moderate or severe anemia (average: 6.8 g/dL) and at least one clinical complication related to the disease, such as pain crisis, acute chest syndrome, gallstones, avascular necrosis of femoral head, and recurrent infections. Furthermore, eight patients required an average of three red blood cell transfusions during their clinical follow-up period.11 In the present study, blood transfusions were required by 63.6% of patients.
Italia et al. reported on 15 patients with ages between 1 and 34 years. They classified only one patient as mild; two were classified as having experienced moderate crises, and 12 were found to have severe hemolytic anemia; these 12 presented recurrent pain crises and required frequent transfusions.10 El-Kalla & Mathews evaluated nine patients aged between 3 and 10 years in the United Arab Emirates. They reported pain crises of varying intensity, acute splenic sequestration crises, and repeated infections in seven patients. The other two children were asymptomatic, although anemic and with increased reticulocyte counts.17 The largest sample size in the literature was published by Patel et al. Of 42 Indian patients with an average age of 22 years, 25 were considered to be severe cases for presenting three or more acute pain crises and/or the need of two or more blood transfusions in the year prior to the study.25
The group of children with Hb S-Korle Bu disease did not present clinical complications. Three children presented with mild to moderate diffuse abdominal pain that was considered secondary to the underlying disease. However, a pain crisis is frequently inferred as the cause of abdominal pain when signs and symptoms related to other etiologies (such as fever, vomiting, and blood in the stools) are absent in children. During the crises, these children are generally assumed to have Hb S/D-Punjab even if they have not been submitted to molecular testing. Pain crises may be difficult to distinguish from other equally prevalent etiologies in children such as constipation and functional abdominal pain.26 It is known that abdominal pain affects 38% of the school-aged pediatric population weekly; no reliable biological markers clearly define a diagnosis of functional pain.27 In addition, few studies show that the clinical course of Hb S-Korle Bu disease carriers may be mild, with minor or even absent symptoms, but the number of patients and length of follow-up preclude a solid conclusion. Some authors have compared these patients to sickle cell trait carriers.7 Therefore, the exact definition of the Hb D variant that the patient has is important for the correct differential diagnosis of abdominal pain in children. It also aids in decreasing both the unnecessary stigma against chronic diseases and unnecessary treatments, such as the indiscriminate use of powerful anti-inflammatory medications and painkillers.
There seems to be no reports available in the literature about transcranial Doppler examinations in children with Hb SD disease. In the present study, all children were found to be at low risk for strokes. As expected, the mean value of TAMMX was significantly higher in Hb S/D-Punjab than in Hb S-Korle Bu children because the Hb concentration was significantly lower in the former group.
Higher Hb F baseline percentages were observed in the Hb S/D-Punjab group; there was a statistically significant difference from the values observed in the Hb S-Korle Bu group. The influence of this percentage on decreasing symptoms in children with Hb S/D-Punjab disease remains unclear. This association has been described in patients with Hb SS disease and has been explained by the fact that red blood cells with larger quantities of Hb F possess lower levels of Hb S; therefore, they have a lower chance of sickling, and as a result, a lower probability of experiencing clinical manifestations.28 Patel et al. found higher Hb F concentrations to be associated with a lower frequency of acute pain crises in the 42 patients with Hb S/D-Punjab disease.25 Meanwhile, three other studies failed to find this association; however, they did include much smaller sample populations than Patel et al. The other studies included five, nine, and 15 patients, respectively.9, 10, 17
Three children with the Hb S/D-Punjab variant took hydroxyurea, and all children experienced significant clinical and laboratory improvements. In children with Hb SS, the use of hydroxyurea has been determined to be safe and to provide satisfactory results.29 In patients with Hb SD disease, the data on the efficacy and safety of this medication are limited to isolated cases19 and to a few studies with a larger number of cases. Out of the ten children included in the study by Oberoi et al., five received hydroxyurea to treat recurrent pain episodes and/or severe anemia. Their follow-up periods ranged from 6 to 50 months. In all cases, there was a decrease in the number of pain crises and a lack of significant side effects.11 The largest cohort included 20 Indian patients aged between 1 and 45 years who took hydroxyurea for at least two years. Decreased frequencies of crises and interruptions in transfusions were recorded for all patients. No side effects were observed.18
Haplotype I is the commonest type of D-Punjab allele in almost all ethnic populations so far described.6, 14, 15, 30 The exception is Thailand, where haplotype II was found in nine patients from five families.31 So it is impossible to trace back the origin of the allele in the population of this study. Haplotypes for the Hb Korle Bu allele have not been described yet. Because all children in the present study are also type I, it is improbable that the origin of the allele will be determined for sure. It would be interesting to know whether the same haplotype is present in Western Africa where the mutation seemingly originated.5
As far as is known from international reports, the present study demonstrated for the first time that, within the Hb S/D-Punjab group, children with the βS Benin haplotype had higher total Hb concentrations and higher relative concentrations of fetal Hb than children with the βS CAR haplotype.
The limitations of the present study include the fact that it was a retrospective analysis and a low number of patients were involved. These factors limit conclusions regarding the incidence of clinical complications in cases of Hb S/D-Punjab and Hb S-Korle Bu disease as well as a reliable evaluation of the late-onset effects of the use of hydroxyurea. Furthermore, had high performance liquid chromatography (HPLC) been used as the primary screening test at PTN-MG, children with Hb S-Korle Bu would not be classified as having Hb SD, but instead as having an unknown variant within the HPLC window of Hb A2/Hb E.
In conclusion, this is the first study in Brazil to evaluate children with Hb SD disease detected at birth by IEF. It offers a clear description of two variants: Hb S/D-Punjab and Hb S-Korle Bu. Early newborn screening and the systematic genetic study of Hb D variants are useful in treating patients and in informing the family members about the prognosis of each variant. Children with Hb S/D-Punjab have a clinical course similar to those with Hb SS disease. The subgroup with the βS CAR haplotype have lower total Hb concentrations and lower relative Hb F concentrations than those with the βS Benin haplotype, but the limited number of patients precludes definitive conclusions. In contrast, the clinical course and laboratory data in children with Hb S-Korle Bu appear to be similar to those in children with the sickle cell trait, but caution should be taken with this statement, considering the limited number of children and short follow-up thus far reported.
Funding
Fundação Hemominas, Newborn Screening Program (Nupad-UFMG), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors acknowledge all subjects and parents for their cooperation in the study. The authors also thank the financial support of Fundação Hemominas, Newborn Screening Program (Nupad-UFMG), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG; grant # PPM-00780-15), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant # 448594/2014-5).
References
- 1.Smith E.W., Conley C.L. Sickle cell hemoglobin D disease. Ann Intern Med. 1959;50(1):94–105. doi: 10.7326/0003-4819-50-1-94. [DOI] [PubMed] [Google Scholar]
- 2.Cawein M.J., Lappat E.J., Brangle R.W., Farley C.H. Hemoglobin S-D disease. Ann Intern Med. 1966;64(1):62–70. doi: 10.7326/0003-4819-64-1-62. [DOI] [PubMed] [Google Scholar]
- 3.Torres L.S., Okumura J.V., Silva D.G., Bonini-Domingos C.R. Hemoglobin D-Punjab: origin, distribution and laboratory diagnosis. Rev Bras Hematol Hemoter. 2015;37(2):120–126. doi: 10.1016/j.bjhh.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itano H.A. A third abnormal hemoglobin associated with hereditary hemolytic anemia. Proc Natl Acad Sci U S A. 1951;37(12):775–784. doi: 10.1073/pnas.37.12.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honig G.R., Seeler R.A., Shamsuddin M., Vida L.N., Mompoint M., Valcourt E. Hemoglobin Korle Bu in a Mexican family. Hemoglobin. 1983;7(2):185–189. doi: 10.3109/03630268309048646. [DOI] [PubMed] [Google Scholar]
- 6.Yavarian M., Karimi M., Paran F., Neven C., Harteveld C.L., Giordano P.C. Multi centric origin of Hb D-Punjab [beta121(GH4)Glu→Gln, GAA>CAA] Hemoglobin. 2009;33(6):399–405. doi: 10.3109/03630260903344598. [DOI] [PubMed] [Google Scholar]
- 7.Akl P.S., Kutlar F., Patel N., Salisbury C.L., Lane P., Young A.N. Compound heterozygosity for hemoglobin S [beta6(A3)Glu6Val] and hemoglobin Korle Bu [beta73(E17)Asp73Asn] Lab Hematol. 2009;15(3):20–24. doi: 10.1532/LH96.09004. [DOI] [PubMed] [Google Scholar]
- 8.Adachi K., Kim J., Ballas S., Surrey S., Asakura T. Facilitation of Hb S polymerization by the substitution of Glu for Gln at beta 121. J Biol Chem. 1988;263(12):5607–5610. [PubMed] [Google Scholar]
- 9.Adekile A., Mullah-Ali A., Akar N.A. Does elevated hemoglobin F modulate the phenotype in Hb SD-Los Angeles? Acta Haematol. 2010;123(3):135–139. doi: 10.1159/000276998. [DOI] [PubMed] [Google Scholar]
- 10.Italia K., Upadhye D., Dabke P., Kangane H., Colaco S., Sawant P. Clinical and hematological presentation among Indian patients with common hemoglobin variants. Clin Chim Acta. 2014;431:46–51. doi: 10.1016/j.cca.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Oberoi S., Das R., Trehan A., Ahluwalia J., Bansal D., Malhotra P. HbHb S/D-Punjab: clinical and hematological profile of a rare hemoglobinopathy. J Pediatr Hematol Oncol. 2014;36(3):e140–e144. doi: 10.1097/MPH.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 12.Nagel R.L., Lin M.J., Witkowska H.E., Fabry M.E., Bestak M., Hirsch R.E. Compound heterozygosity for hemoglobin C and Korle Bu: moderate microcytic hemolytic anemia and acceleration of crystal formation. Blood. 1993;82(6):1907–1912. [PubMed] [Google Scholar]
- 13.Adachi K., Kim J., Kinney T.R., Asakura T. Effect of the beta 73 amino acid on the hydrophobicity, solubility, and the kinetics of polymerization of deoxyhemoglobin S. J Biol Chem. 1987;262(22):10470–10474. [PubMed] [Google Scholar]
- 14.Patel D.K., Mashon R.S., Patel S., Dash P.M., Das B.S. Beta-globin gene haplotypes linked with the Hb D-Punjab [beta121(GH4)Glu→Gln, GAA>CAA] mutation in eastern India. Hemoglobin. 2010;34(6):530–537. doi: 10.3109/01676830.2010.525900. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi Z., Akramipour R., Korani S., Nagel R.L. Hb D-Punjab [beta 121 (GH4) Glu→Gln]/beta0-thalassemia [IVSII.1(G→A)] in two cases from an Iranian family: first report. Am J Hematol. 2006;81(4):302–303. doi: 10.1002/ajh.20537. [DOI] [PubMed] [Google Scholar]
- 16.Kelleher J.F., Jr., Park J.O., Kim H.C., Schroeder W.A. Life-threatening complications in a child with hemoglobin SD-Los Angeles disease. Hemoglobin. 1984;8(3):203–213. doi: 10.3109/03630268408996969. [DOI] [PubMed] [Google Scholar]
- 17.el-Kalla S., Mathews A.R. Hb D-Punjab in the United Arab Emirates. Hemoglobin. 1997;21(4):369–375. doi: 10.3109/03630269709000669. [DOI] [PubMed] [Google Scholar]
- 18.Patel S., Purohit P., Mashon R.S., Dehury S., Meher S., Sahoo S. The effect of hydroxyurea on compound heterozygotes for sickle cell-hemoglobin D-Punjab – a single centre experience in eastern India. Pediatr Blood Cancer. 2014;61(8):1341–1346. doi: 10.1002/pbc.25004. [DOI] [PubMed] [Google Scholar]
- 19.Udden M.M., Lo M.N., Sears D.A. Successful hydroxyurea treatment of a patient with SD hemoglobinopathy. Am J Hematol. 1999;60(1):84–85. doi: 10.1002/(sici)1096-8652(199901)60:1<84::aid-ajh20>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Tan A.S., Quah T.C., Low P.S., Chong S.S. A rapid and reliable 7-deletion multiplex polymerase chain reaction assay for alpha-thalassemia. Blood. 2001;98(1):250–251. doi: 10.1182/blood.v98.1.250. [DOI] [PubMed] [Google Scholar]
- 21.Orkin S.H., Kazazian H.H., Jr., Antonarakis S.E., Goff S.C., Boehm C.D., Sexton J.P. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- 22.Nagel R.L., Fabry M.E., Pagnier J., Zohoun I., Wajcman H., Baudin V. Hematologically and genetically distinct forms of sickle cell anemia in Africa. The Senegal type and the Benin type. N Engl J Med. 1985;312(14):880–884. doi: 10.1056/NEJM198504043121403. [DOI] [PubMed] [Google Scholar]
- 23.Belisário A.R., Martins M.L., Brito A.M., Rodrigues C.V., Silva C.M., Viana M.B. β-Globin gene cluster haplotypes in a cohort of 221 children with sickle cell anemia or Sβ°-thalassemia and their association with clinical and hematological features. Acta Haematol. 2010;124(3):162–170. doi: 10.1159/000320271. [DOI] [PubMed] [Google Scholar]
- 24.Adams R.J., McKie V.C., Hsu L., Files B., Vichinsky E., Pegelow C. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 25.Patel D.K., Purohit P., Dehury S., Das P., Dutta A., Meher S. Fetal hemoglobin and alpha thalassemia modulate the phenotypic expression of HbHb S/D-Punjab. Int J Lab Hematol. 2014;36(4):444–450. doi: 10.1111/ijlh.12165. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes M.M., Bates D.G., Andrews T., Adkins L., Thornton J., Denham J.M. Abdominal pain in children with sickle cell disease. J Clin Gastroenterol. 2014;48(2):99–105. doi: 10.1097/01.mcg.0000436436.83015.5e. [DOI] [PubMed] [Google Scholar]
- 27.Saps M., Seshadri R., Sztainberg M., Schaffer G., Marshall B.M., Di Lorenzo C. A prospective school-based study of abdominal pain and other common somatic complaints in children. J Pediatr. 2009;154(3):322–326. doi: 10.1016/j.jpeds.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg M.H., Rodgers G.P. Pharmacologic modulation of fetal hemoglobin. Medicine. 2001;80(5):328–344. doi: 10.1097/00005792-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Thornburg C.D., Files B.A., Luo Z., Miller S.T., Kalpatthi R., Iyer R. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120(22):4304–4310. doi: 10.1182/blood-2012-03-419879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atalay E.Ö., Atalay A., Üstel E., Yildiz S., Oztürk O., Köseler A. Genetic origin of Hb D-Los Angeles [β121(GH4)Glu→Gln, GAA→CAA] according to the β-globin gene cluster haplotypes. Hemoglobin. 2007;31(3):387–391. doi: 10.1080/03630260701459416. [DOI] [PubMed] [Google Scholar]
- 31.Fucharoen S., Changtrakun Y., Surapot S., Fucharoen G., Sanchaisuriya K. Molecular characterization of Hb D-Punjab [beta121(GH4)Glu→Gln] in Thailand. Hemoglobin. 2002;26(3):261–269. doi: 10.1081/hem-120015030. [DOI] [PubMed] [Google Scholar]