Abstract
Background: Emerging evidence shows that clopidogrel is greatly affected by non-functioning alleles measured by P2Y12 or platelet reactivity units (PRU). Cardiac events during short in-hospital stays have been inconclusively suggested as the main causes of discrepancies.
Objectives: Evaluate the impact of CYP2C19 allele * 2 and allele * 3 on PRU and the potential clinical consequences of such interaction. To establish a rough estimation for the safe PRU limits for short in-hospital stay following PCI.
Method: A short-term experimental study was conducted with 90 patients who underwent coronary angioplasty with drug eluting stents at the Prince Sultan Cardiac Center, Buraidah. All the patients received an initial loading dose of 300 mg clopidogrel, followed by 75 mg daily. Blood samples were used for DNA extraction for cytochrome P450 (CYP) and real-time polymerase chain reaction (PCR) was used for genotyping. PRU and inhibition rate were tested by Verifynow®. All in-hospital cardiac events were recorded until patients were discharged.
Results: Genotypes 1/1, 2/2, and 1/2 were expressed by 60, 28, and two patients (67, 32, and 3%), respectively. The PRU of the female patients was significantly higher than that of the male patients was (255.6 ± 68.8 and 177.7 ± 66.6, p = 0.000, respectively). There was no significant difference in PRUs (193 ± 79 and 212 ±55.4, respectively, p = 0.349), nor inhibition (17.9 ± 18.80 and 13.88 ± 11.5, p = 0.135) in wild and resistant variants, respectively. We only reported one cardiac in-thrombosis events.
Conclusion: Genotype differences may not explain variations in the PRU of patients during short-term in-hospital stays. Although it is difficult to confirm, 117–267 units may be a safe PRU range for such patients, with emphasis on attaining higher PRU values in females.
Keywords: Allele * 2, allele * 3, Clopidogrel, CYP2C19, genotyping, P2Y12 reaction unit, percutaneous coronary intervention
INTRODUCTION
Aspirin and clopidogrel are the most widely used drugs worldwide following acute coronary events. The use of newer agents such as prasugrel and ticagrelol is limited by their high cost and low availability in many hospitals. The recommended dose of clopidogrel is 600 mg in ST-elevation, except in patients over the age of 75 years and those who received fibrinolytic agents [1]. However, there is no current consensus regarding the optimal loading-dose of clopidogrel. While 600 mg showed superior efficacy in primary and secondary outcomes, it was accompanied by higher incidences of bleeding than lower doses were [2]. Furthermore, studies presented two types of LDs including a stat (300 versus 600 mg) or short-term (such as 150 mg administered over 1 week). In addition, guidelines differ for the standardization of the most appropriate methods in each case of coronary acute syndrome. Several confounders
contribute to these differences, including genotyping, drug-drug interactions, the nature of the coronary event, and other comorbidities. Continuation of clopidogrel beyond the LD phase may also be considered when appropriate [3].
Genetic polymorphisms including that of cytochrome P450 (CYP) 2C19 contribute significantly to the racial differences in the metabolism of clopidogrel, which may explain some of the dosing variability [4]. Because clopidogrel is a prodrug, patients with non-functioning alleles may convert lower amounts to the active form, which may lead to delayed drug responses, and higher rates of clinical consequences [5]. This phenomenon may raise questions about the validity of the LD of clopidogrel in many patients. However, since the drug effect is measured mainly by the P2Y12 reaction units (PRU), a concrete relationship has not yet been established by consensus. While some studies have reported no clinical association, others found significant clinical adverse effects and major outcomes [6]. In particular, following acute coronary syndromes, hypo-responsive patients showed a significant difference in clinical outcomes [7-12]. Vasodilator-stimulated phosphoprotein (VASP)-
guided dosing was assessed by some studies to determine adequately higher LDs. The administration of 1200 mg showed superior PRU inhibition and clinical endpoint values. However, some patients were very resistant even at high doses of 2400 mg while no major cardiac events were observed [13].
METHODS
An experimental prospective clinical study, which recruited patients diagnosed with acute coronary syndrome by convenience sampling. The protocol of the study was approved by Prince Sultan Cardiac Center in Buraidah, Saudi Arabia, and conducted in accordance to declaration of Helsinki. All the patients signed the informed consent form before enrolment. In addition, patient privacy was ensured during the study period. The demographic, as well as the relevant medical and medication history of all the patients, were recorded. We enrolled patients who were diagnosed with acute coronary disease, were administered a LD of 300 mg clopidogrel, had a complete understanding of the research study procedure and were willing to sign the informed written consent form, and were able to follow simple instructions.
Genotyping
The positive control human DNA CYP2C19* 2 and * 3, wild type, and heterozygous were all diluted 1:4. A negative control was also included for each run. The reproducibility was tested by analyzing nine random samples 10 times each (three of each wild/wild, wild/mutant and mutant/mutant). The mix and DNA for each sample were pipetted into the LightCycler capillaries, which were sealed, centrifuged using the LightCycler carousel centrifuge, and then placed into the LightCycler instrument. The denaturation, melting, and renaturation processes are shown in Fig. 1. The gradual increase should allow for monitoring the decline in fluorescence of the melting hybrids as a function of temperature. Fluorescence curves were analyzed using the LightCycler software (version 3.5.3)
Fig. (1).
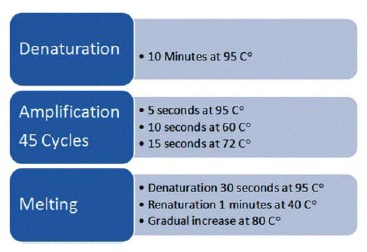
Real-Time PCR.
DNA was isolated using the MagNA Pure LC instrument. CYP2C19* 2* 3 genes were detected using Oligonucleotide primers and fluorescence-labeled hybridization probes (Tables 1 and 2).
PRU and VerifyNow®
The VerifyNow® (Accumetrics, Sandigo, CA) assay was used to detect the PRU values 2–3 days following administration of the LD (Accumetrics, Inc.). We have followed the standard manufacturer's recommendations, which have been added to the methodology as follows: Using an already fixed indwelling catheter, the first blood sample was collected firstly for genetic testing (Shouldn’t be used for The VerifyNow®), whereas the second sample was used for PRU test. The blood was transferred in 2 ml tubes containing 3.2% Sodium Citrate. The tubes should be mixed gently about 5 times, and the recommended waiting time is 10 minutes to 4 hours before testing. We carried out all the tests after 30 minutes for all the patients to ensure the consistency.
Statistics
The MedCalc® program was used for the statistical analysis. Student's-t-test was used to determine differences while the Chi-square test and Chi-square test for trends were used to further examine the associations. We set the level of significance at 0.05.
RESULTS
Descriptive Statistics
A total of 90 homogeneous Saudi patients (63 male and 27 female patients) were recruited in the study (Tables 3-5). The mean PRU of the patients was 195 (range 59-335). In addition, the mean level of inhibition was 16% (range 0–65%) (Table 6).
The PRU of the female patients at 255.6 ± 68.8 was significantly higher than that of the male patients was (177.7 ± 66.6, p = 0.000). Likewise, no statistical difference in PRU among patients based on the number of involved vessels or diagnosis (Table 7).
DISCUSSION
Genetic testing and platelet reactivity have been suggested to be contributing factors to successful clopidogrel-guided therapy in long-term treatment [14-17]. However, evidence of the benefits of genotyping and platelet reactivity monitoring in acute treatment and short-term hospitalization is inconclusive. Therefore, this study investigated the clinical consequences of administering clopidogrel in combination with PRU monitoring during the first week following drug administration. The percentage of non-functioning allele *2 in our study was about 33%, which is similar to previous suggestions worldwide [18-21]. Alleles *2 and *3 have been correlated with increased cardio- vascular and thrombotic events in some clinical studies, mostly cohort designs [14, 22, 23]. Allele *3, however, was not detected in any of our patients (0%), and our results showed that there was no difference in genotyping measured by PRU during the in-hospital stay period among patients. An in-stent thrombosis was diagnosed in one patient whose inhibition was 30%, which was not significant. Furthermore, although a genome-wide approach may be more predictive, it may not be applicable to short in-hospital stays [24].
Although platelet reactivity is suggested to increase cardiac and thromboembolic events, the absolute cutoff range varied significantly (29–31). The initial PRU vary significantly and may range from 60-240 units [5]. In practice, thromboembolic events are likely detected when the PRU is higher than 240 units while bleeding complications are likely seen at PRUs lower than 60 [5]. A considerably more conservative range of 179–200 units (upper limit, 238) was proposed for patients undergoing PCI based on ischemic or bleeding events [25, 26]. In addition, a range of 180–200 (upper limit, 230) was suggested for patients with coronary artery bypass grafting (CABG) [27, 28]. Furthermore, the developers of the VerifyNow®P2Y12 assay have specified 194–418 as the normal PRU range, which can be measured ≥6 hours post bolus drug administration (Accumetrics). One of the largest published reviews including about 17 studies on this subject matter concluded that the optimal cutoff range is 95–208 for hypo- and hyper-response definitions, respectively [29]. However, the cutoff value in a Japanese study was suggested to be much higher at 262 units [30]. Our study showed a platelet reactivity of 59–335 units (117–267 units after adjusting for outliers), 2–3 days following LDs of 300 and 75 mg daily. Furthermore, we reported only one adverse incident, which suggests that this is a safe range for administration during in-hospital stays.
A higher PRU range may be associated with the number of vessels involved and timing of treatment initiation [31]. Furthermore, female patients tend to present with higher PRU values than male patients do, as shown in numerous studies [32, 33]. Our results appeared to present higher PRU values, in general than the previous reports did. However, the study did not show any significant difference based on the number of vessels involved. This could be related to the timing of the PRU testing. Another potential consideration is that the PRU should evolve over time and, therefore, the conclusion that these patients are hypo- or hyper-responsive cannot be accurately reached after this short time [34, 35].
Several protocols have been developed to ensure that patients are within the optimal PRU range, especially when a critical procedure is required. These protocols mainly include dose-adjusted protocols based on PRU or percentage of inhibition prior to the procedure [36, 37]. The differences in the outcomes of PRU studies stem from several factors including the LD, drug-drug interactions, daily doses during hospitalization, the timing of the test following the initiation, use of glycoprotein (GP) IIb/IIIa blockers or heparins, and genotyping. Another contributing factor is the compliance with the VerifyNow®P2Y12 assay protocol such as time to test following blood withdrawal, volume (mL) of blood to be discarded prior to collecting test samples, gauge size, and correct mixing the kit contents [38].These factors are rarely discussed or standardized among researchers or in their published studies, yet they may contribute significantly to the PRU readings.
CONCLUSION
Genotyping might not facilitate the detection of hypo- or hyper-responsive patients during the first days of their hospital admissions but could be a predictor of future cardiac events. Furthermore, female could be at higher risk of future events based on their elevated initial response. Although it is difficult to confirm, a PRU of 117–267 could be a safe range for in-hospital stays for patients not undergoing any cardiac surgeries.
Table 1.
Pharmacogenetic equipment and materials.
| Material/Equipment | Manufacturer |
|---|---|
| MagNAPure LC Instrument LC Carousel Centrifuge LightCycler Real time PCR machine LightCycler Capillaries Fast Start HyProbe |
Roche Molecular Biochemicals, Mannheim, Germany |
| Primers and probes | TIB MOL BIOL, Germany |
| Deoxynucleotide triphosphate | Roche Molecular Systems, Inc., CA, USA |
| Magnesium chloride (MgCl2) solution | Hildon, Qiagen Operon GmbH, D-40724, Germany |
| 10× buffer | Hildon, Qiagen Operon, GmbH, D-40724, Germany |
| Thermus aquaticus polymerase enzyme | Amplitaq Gold, Perkin-Elmer Cetus, Norwalk, Conn |
Table 2.
Master Mix final concentration.
| Magnesium chloride (MgCl2) | 5 mM |
| CYP2C19*2*3 primers | 1 μM |
| Specific primers | 0.075 μM |
| Hybridization probes | 0.2 μM |
| Sample DNA | 5 μl |
| Final Volume | 20 μl |
Table 3.
Disease characteristics.
| Diagnosis | Number of Patients (%) |
|---|---|
| Unstable angina (UA) | 20 (22) |
| Non-ST-elevation MI (NSTEMI) | 34 (37) |
| ST-elevation MI (STEMI) | 36 (40) |
Table 4.
Baseline characteristics of patients.
| Patients characteristics | n (%) |
|---|---|
| Age | 57.35 ± 11.71 |
| Sex: Male | 63 (70) |
| Body mass index | 30.14 ± 6.1 |
| Diabetes mellitus | 53 (59) |
| Hypertension | 49 (55) |
| Hyperlipidemia | 40 (45) |
| Dyslipidemia | 4 (5) |
| Single vessel disease (SVD) | 40 (45) |
| Double vessel disease (DVD) | 32 (36%) |
| Triple vessel disease (TVD) | 8 (9%) |
Table 5.
Concomitant medications.
| Drug | n (%) |
|---|---|
| Aspirin on admission 325 mg | (100) |
| Statins atorvastatin | 78 (87) |
| ACE inhibitors/ARB lisinopril or losartan | 42 (46.3) |
| Beta blockers metoprolol or atenolol | 79 (88.2) |
| Calcium-channel blockers long-acting nifedipine | (15) |
| Nitrates isosorbide dinitrate | 30 (50) |
| Diuretics spironolactone or/and furosemide | 34 (37.3) |
| Anti-Diabetics metformin | 53 (58) |
| Antacids omeprazole, lansoprazole, ranitidine, or famotidine. | (100) |
Table 6.
Genotyping and measurement of non-functioning alleles.
| Genotype | Number (%) | P2Y12 Reaction Units (PRU) | Inhibition Rate |
|---|---|---|---|
| 1/1 | 60 (67) | 193 ±79 | 17.9 ± 18.80 |
| 1/2 | 2(3) | NA | NA |
| 2/2 | 28(32) | 212 ±55.4 | 13.88 ± 11.5 |
| *3 | (0) | NA | NA |
| p-value | NA | 0.349 | 0.135 |
NA, not applicable.
Table 7.
Mean P2Y12 reaction units of different diseases.
| Character | Mean PRU |
|---|---|
| STEMI | 193.1 ± 69 |
| NSTEMI | 181.1 ±72 |
| UA | 224.1 ±82 |
| SVD | 194 ± 74 |
| DVD | 197.6 ±78.1 |
| TVD | 192 ± 69 |
ACKNOWLEDGEMENTS
We would like to thank Mr. Abdullah Al-Marshad and Mr. Hamad Al-Bassam for their participation in data collection.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest in this study, which was funded by Qassim University.
REFERENCES
- 1.Mehta S.R., Tanguay J.F., Eikelboom J.W., Jolly S.S., Joyner C.D., Granger C.B., Faxon D.P., Rupprecht H.J., Budaj A., Avezum A., Widimsky P., Steg P.G., Bassand J.P., Montalescot G., Macaya C., Di Pasquale G., Niemela K., Ajani A.E., White H.D., Chrolavicius S., Gao P., Fox K.A., Yusuf S. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233–1243. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 2.Patti G., Colonna G., Pasceri V., Pepe L.L., Montinaro A., Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2005;111(16):2099–2106. doi: 10.1161/01.CIR.0000161383.06692.D4. [DOI] [PubMed] [Google Scholar]
- 3.Mehta S.R., Yusuf S., Peters R.J., Bertrand M.E., Lewis B.S., Natarajan M.K., Malmberg K., Rupprecht H., Zhao F., Chrolavicius S., Copland I., Fox K.A. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoingpercutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 4.Langaee T.Y., Zhu H.J., Wang X.E., Rouby N., Markowitz J.S., Goldstein J.A., Johnson J.A. The influence of the CYP2C19*10 allele on clopidogrel activation and CYP2C19*2 genotyping. Pharmacogenet. Genomics. 2014;24(8):381–386. doi: 10.1097/FPC.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado Almandoz J.E., Kadkhodayan Y., Crandall B.M., Scholz J.M., Fease J.L., Tubman D.E. Variability in initial response to standard clopidogrel therapy, delayed conversion to clopidogrel hyper-response, and associated thromboembolic and hemorrhagic complications in patients undergoing endovascular treatment of unruptured cerebral aneurysms. J. Neurointerv. Surg. 2014;6(10):767–773. doi: 10.1136/neurintsurg-2013-010976. [DOI] [PubMed] [Google Scholar]
- 6.D'Ascenzo F., Barbero U., Bisi M., Moretti C., Omedè P., Cerrato E., Quadri G., Conrotto F., Zoccai G.B., DiNicolantonio J.J., Gasparini M., Bangalore S., Gaita F. The prognostic impact of high on-treatment platelet reactivity with aspirin or ADP receptor antagonists: systematic review and meta-analysis. 2014. [DOI] [PMC free article] [PubMed]
- 7.Gurbel P.A., Tantry U.S. Drug insight: clopidogrel nonresponsiveness. Nat. Clin. Pract. Cardiovasc. Med. 2006;3:387–395. doi: 10.1038/ncpcardio0602. [DOI] [PubMed] [Google Scholar]
- 8.Aleil B., Ravanat C., Cazenave J.P., Rochoux G., Heitz A., Gachet C. Flow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J. Thromb. Haemost. 2005;3:85–92. doi: 10.1111/j.1538-7836.2004.01063.x. [DOI] [PubMed] [Google Scholar]
- 9.Bliden K.P., DiChiara J., Tantry U.S., Bassi A.K., Chaganti S.K., Gurbel P.A. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J. Am. Coll. Cardiol. 2007;49:657–666. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 10.Gurbel P.A., Becker R.C., Mann K.G., Steinhubl S.R., Michelson A.D. Plateletfunction monitoring in patients with coronary artery disease. J. Am. Coll. Cardiol. 2007;50:1822–1834. doi: 10.1016/j.jacc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 11.Matetzky S., Shenkman B., Guetta V., Shechter M., Beinart R., Goldenberg I., Novikov I., Pres H., Savion N., Varon D., Hod H. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 12.Brar S.S., ten Berg J., Marcucci R., Price M.J., Valgimigli M., Kim H.S., Patti G., Breet N.J., DiSciascio G., Cuisset T., Dangas G. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention: a collaborative meta-analy sis of individual participant data. J. Am. Coll. Cardiol. 2011;58:1945–1954. doi: 10.1016/j.jacc.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 13.Gladding P., Webster M., Zeng I., Farrell H., Stewart J., Ruygrok P., Ormiston J., El-Jack S., Armstrong G., Kay P., Scott D., Gunes A., Dahl M.L. The antiplatelet effect of higher loading and maintenance dose regimens of clopidogrel: the PRINC (Plavix Response in Coronary Intervention) trial. JACC Cardiovasc. Interv. 2008;1(6):612–619. doi: 10.1016/j.jcin.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Mega J.L., Close S.L., Wiviott S.D., Shen L., Hockett R.D., Brandt J.T., Walker J.R., Antman E.M., Macias W., Braunwald E., Sabatine M.S. Cytochrome p-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 15.Sofi F., Giusti B., Marcucci R., Gori A.M., Abbate R., Gensini G.F. Cytochrome P450 2C19*2 polymorphism and cardiovascular recurrences in patients taking clopi-dogrel: a meta-analysis. Pharmacogenomics J. 2011;11(3):199–206. doi: 10.1038/tpj.2010.21. [DOI] [PubMed] [Google Scholar]
- 16.Trenk D., Hochholzer W., Fromm M.F., Chialda L.E., Pahl A., Valina C.M., Stratz C., Schmiebusch P., Bestehorn H.P., Büttner H.J., Neumann F.J. Cytochrome P450 2C19 681GA polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J. Am. Coll. Cardiol. 2008;51(20):1925–1934. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 17.Siasos G., Oikonomou E., Zaromitidou M., Kioufis S. Vavuranakis, M2.; Maniatis, K.; Kokkou E.; Papageorgiou, N; Papaioannou, S.; Tourikis, P2.; Papavassiliou, AG.; Stefanadis, C.; Tousoulis, D. High platelet reactivity is associated with vascular function in patients after percutaneous coronary intervention receiving clopidogrel. Int. J. Cardiol. 2014;177(1):192–196. doi: 10.1016/j.ijcard.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Mega J.L., Close S.L., Wiviott S.D., Shen L., Walker J.R., Simon T., Antman E.M., Braunwald E., Sabatine M.S. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376(9749):1312–1329. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo H.R., Poland R.E., Lin K.M., Wan Y.J. Genetic polymorphism of cytochrome P450 2C19 in Mexican Americans: a cross-ethnic comparative study. Clin. Pharmacol. Ther. 2006;80:33–40. doi: 10.1016/j.clpt.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Man M., Farmen M., Dumaual C., Teng C.H., Moser B., Irie S., Noh G.J., Njau R., Close S., Wise S., Hockett R. Genetic variation in metabolizing enzyme and transporter genes: comprehensive assessment in 3 major East Asian subpopulations with comparison to Caucasians and Africans. J. Clin. Pharmacol. 2010;50(8):929–940. doi: 10.1177/0091270009355161. [DOI] [PubMed] [Google Scholar]
- 21.Li S., Shi Y., Wang H., Zhang W., Liu J. Impact of cytochrome P450 2C19*2 polymorphism on intra-stent thrombus assessed by follow-up optical coherence tomography in Chinese patients receiving clopidogrel. J. Thromb. Thrombolysis. 2015;40(1):88–96. doi: 10.1007/s11239-015-1207-5. [DOI] [PubMed] [Google Scholar]
- 22.Shuldiner A.R., O’Connell J.R., Bliden K.P., Gandhi A., Ryan K., Horenstein R.B., Damcott C.M., Pakyz R., Tantry U.S., Gibson Q., Pollin T.I., Post W., Parsa A., Mitchell B.D., Faraday N., Herzog W., Gurbel P.A. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mega J.L., Close S.L., Wiviott S.D., Shen L., Hockett R.D., Brandt J.T., Walker J.R., Antman E.M., Macias W.L., Braunwald E., Sabatine M.S. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharma-cokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119(19):2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 24.Cui H., Lin S., Chen X., Gao W., Li X., Zhou H., Du W., Wang S., Zhao R. Correlation Between SNPs in Candidate Genes and VerifyNow-Detected Platelet Responsiveness to Aspirin and Clopidogrel Treatment. Cardiovasc. Drugs Ther. 2015;29(2):137–146. doi: 10.1007/s10557-015-6585-6. [DOI] [PubMed] [Google Scholar]
- 25.Mangiacapra F., De Bruyne B., Muller O., Trana C., Ntalianis A., Bartunek J., Heyndrickx G., Di Sciascio G., Wijns W., Barbato E. High residual platelet reactivity after clopidogrel: extent of coronary atherosclerosis and periprocedural myocardial infarction in patients with stable angina undergoing percutaneous coronary intervention. JACC Cardiovasc. Interv. 2010;3(1):35–40. doi: 10.1016/j.jcin.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 26.So D.Y., Wells G.A., McPherson R., Labinaz M., Le May M.R., Glover C., Dick A.J., Froeschl M., Marquis J.F., Gollob M.H., Tran L., Bernick J., Hibbert B., Roberts J.D. A prospective randomized evaluation of a pharmacogenomic approach to antiplatelet therapy among patients with ST-elevation myocardial infarction: the RAPID STEMI study. Pharmacogenomics J. 2015;••• doi: 10.1038/tpj.2015.17. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Altheeb Z., Sbitan A., Shabiah M., Debari V., Hamdan A., Bikkina M., Shamoon F., Aronow W.S. Platelet reactivity unit in predicting risk of bleeding in patients undergoing coronary artery bypass graft surgery. Am. J. Ther. 2015 doi: 10.1097/MJT.0000000000000208. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Reed G.W., Kumar A., Guo J., Aranki S., Shekar P., Agnihotri A., Maree A.O., McLean D.S., Rosenfield K., Cannon C.P. Point-of-care platelet function testing predicts bleeding in patients exposed to clopidogrel undergoing coronary artery bypass grafting: verify pre-op TIMI 45--a pilot study. Clin. Cardiol. 2015;38(2):92–98. doi: 10.1002/clc.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aradi D., Kirtane A., Bonello L., Gurbel P.A., Tantry U.S., Huber K., Freynhofer M.K., Ten Berg J., Janssen P., Angiolillo D.J., Siller-Matula J.M., Marcucci R., Patti G., Mangiacapra F., Valgimigli M., Morel O., Palmerini T., Price M.J., Cuisset T., Kastrati A., Stone G.W., Sibbing D. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015;•••:ehv104. doi: 10.1093/eurheartj/ehv104. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M., Isshiki T., Kimura T., Ogawa H., Yokoi H., Nanto S., Takayama M., Kitagawa K., Ikeda Y., Saito S. Optimal cutoff value of P2Y12 reaction units to prevent major adverse cardiovascular events in the acute periprocedural period: post-hoc analysis of the randomized PRASFIT-ACS study. Int. J. Cardiol. 2015;182:541–548. doi: 10.1016/j.ijcard.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Mangiacapra F., Patti G., Barbato E., Peace A.J., Ricottini E., Vizzi V., Gatto L., D'Ambrosio A., De Bruyne B., Wijns W., Di Sciascio G. A therapeutic window for platelet reactivity for patients undergoing elective percutaneous coronary intervention: results of the ARMYDA-PROVE (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity for Outcome Validation Effort) study. JACC Cardiovasc. Interv. 2012;5(3):281–289. doi: 10.1016/j.jcin.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Price M.J., Nayak K.R., Barker C.M., Kandzari D.E., Teirstein P.S. Predictors of heightened platelet reactivity despite dual-antiplatelet therapy in patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 2009;103(10):1339–1343. doi: 10.1016/j.amjcard.2009.01.341. [DOI] [PubMed] [Google Scholar]
- 33.Park K.W., Park J.J., Jeon K.H., Kang S.H., Oh I.Y., Yang H.M., Cho H.J., Lee H.Y., Kang H.J., Koo B.K., Oh B.H., Park Y.B., Kim H.S. Clinical predictors of high posttreatment platelet reactivity to clopidogrel in Koreans. Cardiovasc. Ther. 2012;30(1):5–11. doi: 10.1111/j.1755-5922.2010.00249.x. [DOI] [PubMed] [Google Scholar]
- 34.Delgado A.J., Kadkhodayan Y., Scholz J., Fease J., Blem A., Tran K., Crandall B. O-007 Initial Institutional Experience Using a Target P2Y12 Reaction Units Range to Tailor the Clopidogrel Dose Administered to Patients with Cerebral Aneurysms Treated with the Pipeline Embolization Device and Stents. J. Neurointerv. Surg. 2014;(Suppl. 1):A4–A5. [Google Scholar]
- 35.Rasool S., Khalaf H., Almeman A., AlOrainy M.M. Assessment of steady-state clopidogrel reactivity by using platelet reactivity units. Am. J. Ther. 2015;22(3):182–185. doi: 10.1097/MJT.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 36.Kadkhodayan Y., Delgado Almandoz J., Scholz J., Fease J., Blem A., Tran K., Crandall B. O-021 induced hyper-response to clopidogrel after elective endovascular intracranial aneurysm treatment. J. Neurointerv. Surg. 2014;6(Suppl. 1):A11. [Google Scholar]
- 37.Zhang L., Yang J., Zhu X., Wang X., Peng L., Li X., Cheng P., Yin T. Effect of high-dose clopidogrel according to CYP2C19*2 genotype in patients undergoing percutaneous coronary intervention- a systematic review and meta-analysis. Thromb. Res. 2015;135(3):449–458. doi: 10.1016/j.thromres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Accumetrics logo and VerifyNow are registered trademarks of Accumetrics, Inc., ©2012 Accumetrics, Inc. http://www.itcmed.com/uploads/literature/mvn0005_-_ verifynow_pocket_guide_1.pdf . 2013.


