Abstract
The role of oral bacteria in the etiology of ankylosing spondylitis (AS) is examined in this review. Periodontitis is related to AS to a significant degree, and periodontitis is significantly more prevalent in patients with AS. Anti-Pophyromonas gingivalis and anti-Prevotella intermedia antibodies titers are higher in AS patients than in healthy subjects. Eight randomized controlled trials that used sulfasalazine were reviewed. Moxifloxacin and rifamycin are significantly effective in the treatment of AS. Periodontal pathogens are likely to be responsible for the development of AS in genetically susceptible individuals. These results will guide more comprehensive and efficacious treatment strategies for AS.
Keywords: Ankylosing spondylitis, arginine, etiology, oral bacteria
INTRODUCTION
Ankylosing spondylitis (AS) is a chronic inflammatory autoimmune disease generally affecting the vertebrae [1]. Sacroiliitis is a specific form of this disease [1]. This disease has well-known genetic component [2]. The strong association with most subtypes of HLA-B27 supports the view that the disease is due to genetically determined immune response to environmental factors in subceptible individuals [2]. The genetic marker HLA-B27 is present in 80 to 98 percent of White patients, in contrast to only 8 percent of the general population [2].
Gingivitis accounts for a group of gingival diseases that occur when oral hygiene is not adequately maintained [3]. Periodontitis is an inflammatory disease that destroys the root surface of a tooth, the bone surrounding the dental root, and the connective tissue between these two [3]. A periodontal pocket forms between the tooth and gingiva [3]. In a sense, it could be perceived as an untreated and advanced form of gingivitis. Although it exists in various levels, chronic periodontitis is observed in 85% of the population, generally occurring over 35 years of age [3]. It progresses because of the untreated gingivitis caused by bacterial plaque [3]. Different from chronic periodontitis, aggressive periodontitis affects the patient in early adult life, adolescence, and even before the adolescence period, progressing even faster and independent of the local effects of bacterial plaque [3].
Periodontitis is related to AS to a significant degree [4], and periodontitis is significantly more prevalent in patients with AS [5].
The role of oral bacteria in the etiology of ankylosing spondylitis (AS) is examined in this review.
PERIODONTAL PATHOGENS
Almost 20 kinds of bacteria that can cause periodontitis are isolated from the mouth [6]. Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, and Treponema denticola are the main ones [6]. P. gingivalis is a Gram-negative, anaerobic bacillus [6]. It forms a black pigment in blood agar [6]. It is naturally abundant in the human gastrointestinal system and in the genital system of women [6]. It is the main bacterium responsible for the chronic periodontitis [6]. Although this bacterium has several pathogenic components, the most important feature is that it possesses arginine (R) and lysine (K) protease [6]. Indeed, the hypothesis is that these bacteria can cause citrullination in rheumatoid arthritis (RA) [7], and this hypothesis was verified with further studies [8, 9].
P. intermedia is a Gram-negative obligate anaerobic bacterium, and it is responsible for acute necrotizing periodontitis [6]. T. denticola, T. forsythia, and P. Gingivalis (Red complex) possess arginine protease (PAD) [6].
LITERATURE SEARCH
The search was carried out in nine databases: PubMed, Science Direct, Scopus, Web of Science, Scirus, Cochrane, Embase, LILACS, and SciELO, including the so-called gray literature (Scirus). All of the articles found until December 2014 were evaluated. The researcher conducted a systematic search of the literature in PubMed (National Library of Medicine, Bethesda, MD). The researcher searched the MEDLINE database for the following terms: ankylosing spondylitis, antibiotics, and oral bacteria. Equivalent strategies were used in other databases.
SEARCH RESULTS
A total of 11 articles were found. Table 1 shows the characteristics and design of the included studies. In the first article, anti-P. gingivalis and anti-P. intermedia antibody titers were higher in the AS patients than in healthy subjects [10]. Eight randomized controlled trials that used sulfasalazine were reviewed [11-18]. According to the last trial [18], it was determined that sulfasalazine was found to be more effective in peripheral involvement than in axial disease.
In a 12-month trial done by Caruso et al. [19] that included 22 AS patients, it was shown that the intrasynovial rifampicin administration is significantly effective in treatment. In an open study conducted in 2007, it was found that moxifloxacin was significantly effective in the treatment of AS [20].
HYPOTHESIS
The above results reveal the importance of oral bacteria in the etiology of AS.
The role of Klebsiella pneumoniae polysaccharides is a matter of continuing debate, as levels of immunoglobulin (Ig) G and IgA antibodies againist these bacteria were increased in patients with AS compared to those healthy controls, but also in patients with inflammatory bowel disease [2]. Although the cross reactivity between HLA-B27 and Klebsiella antigens has been put forward in many publications, this cross reactivity has not been shown [21].
HLA-B27 is present on antigen-presenting cells, and it presents endogenous peptides to CD8 (+) T-cells [21]. The arthritogenic peptide hypothesis postulates that B27 plays a direct role in pathogenesis by binding an arthritogenic peptide and presenting it to autoreactive CTLs [22].
Indeed, sequencing of HLA-B27 endogenous peptides shows that most antigenic peptides associated with HLA-B27 have arginine as the second residue [23]. It has been shown that lysine or arginine is crucial for interaction with HLA B27 [23]. P. gingivalis possesses arginine and lysine specific protease (PADs) [6]. As a result, the major hypothesis of the author is that there are certain immunodominant arthritis-causing HLA-B27-specific antigenic peptides which are shared among the arthritis-causing pathogens, and that these peptides are also cross-reactive with autoantigens. Hence, when an HLA-B27+ individual is infected with P. gingivalis PADs, an HLA-B27-specific, cytotoxic T-cell-mediated autoimmune response would be initiated in the joints (Fig. 1). There are very few studies on this subject. This is a limitation for this review.
Patients with AS are also at increased cardiovascular risk with excessive atherosclerosis [24], similar to patients with RA [25]. On the other hand, periodontal pathogens have been linked to atherosclerosis [26].
CONCLUSION
Periodontal pathogens are likely to be responsible for the development of AS in genetically susceptible individuals. These results will guide more comprehensive and efficacious treatment strategies for AS.
Fig. (1).
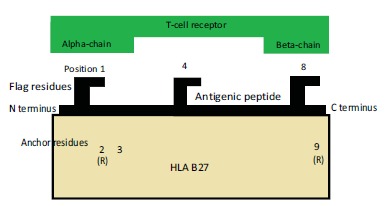
Antigen presentation in ankylosing spondylitis.
Table 1. The characteristics and design of the included studies.
| Author | Year | Country | Study type | Outcomes |
|---|---|---|---|---|
| Rinaudo-Gaujous et al. | 2014 | France | case-control | significant |
| Dougados et al. | 1986 | France | SSZ, randomized | effective |
| Nissilä et al. | 1988 | Finland | SSZ, randomized | effective |
| Davis et al. | 1989 | England | SSZ, randomized | effective |
| Corkill et al. | 1990 | England | SSZ, randomized | NE |
| Taylor et al. | 1991 | England | SSZ, randomized | effective |
| Kirwan et al. | 1993 | England | SSZ, randomized | NE |
| Dougados et al. | 1995 | International | SSZ, randomized | effective |
| Clegg et al. | 1999 | USA | SSZ, randomized | effective |
| Caruso et al. | 1992 | Italy | rifamycin SV,open | effective |
| Ogrendik | 2007 | Turkey | moxifloxacin, open | effective |
SSZ: sulfasalazine, NE: non-effective.
ACKNOWLEDGEMENTs
Declared none.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Dougados M., van der Linden S., Juhlin R., et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 2.Van Der Linden S., Van Der Heijde D., Braun J. Ankylosing spondylitis. In: Harris E.D. Jr, Budd R.C., Firestein G.S., Genovese M.C., Sergent J.S., Ruddy S., Sledge C.B., editors. Kelley’s Textbook of Rheumatology. 8th ed. Pennsylvania: Elsevier Saunders; 2005. p. 1127. [Google Scholar]
- 3.Jotwani R., Cutler C.W. Adult periodontitis--specific bacterial infection or chronic inflammation? J. Med. Microbiol. 1998;47:187–188. doi: 10.1099/00222615-47-3-187. [DOI] [PubMed] [Google Scholar]
- 4.Keller J.J., Kang J.H., Lin H.C. Association between ankylosing spondylitis and chronic periodontitis: a population-based study. Arthritis Rheum. 2013;65:167–173. doi: 10.1002/art.37746. [DOI] [PubMed] [Google Scholar]
- 5.Pischon N., Pischon T., Gülmez E., et al. Periodontal disease in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2010;69:34–38. doi: 10.1136/ard.2008.097212. [DOI] [PubMed] [Google Scholar]
- 6.Marsh P.D., Martin M.V., editors. Oral Microbiology. MPG Books Ltd; 2001. [Google Scholar]
- 7.Ogrendik M., Kokino S., Ozdemir F., Bird P.S., Hamlet S. Serum antibodies to oral anaerobic bacteria in patients with rheumatoid arthritis. MedGenMed. 2005;7:2. [PMC free article] [PubMed] [Google Scholar]
- 8.Kinloch A., Tatzer V., Wait R., et al. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R1421–R1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegner N., Wait R., Sroka A., et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinaudo-Gaujous M., Moreau A., Blasco-Baque V., et al. A6.7 Evaluation of porphyromonas gingivalis serology in rheumatic and non-rheumatic inflammatory disease. Ann. Rheum. Dis. 2014;73(Suppl. 1):A73. [abstract]. [Google Scholar]
- 11.Dougados M., Boumier P., Amor B. Sulphasalazine in ankylosing spondylitis: a double blind controlled study in 60 patients. Br. Med. J. (Clin. Res. Ed.) 1986;293:911–914. doi: 10.1136/bmj.293.6552.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nissilä M, Lehtinen K, Leirisalo-Repo M, Luukkainen R, Mutru O, Yli-Kerttula U. doi: 10.1002/art.1780310905. [DOI] [PubMed] [Google Scholar]
- 13.Sulfasalazine in the treatment of ankylosing spondylitis. A twenty-six-week, placebo-controlled clinical trial. Arthritis Rheum. 1988;31:1111–1116. doi: 10.1002/art.1780310905. [DOI] [PubMed] [Google Scholar]
- 14.Davis M.J., Dawes P.T., Beswick E., Lewin I.V., Stanworth D.R. Sulphasalazine therapy in ankylosing spondylitis: its effect on disease activity, immunoglobulin A and the complex immunoglobulin A-alpha-1-antitrypsin. Br. J. Rheumatol. 1989;28:410–413. doi: 10.1093/rheumatology/28.5.410. [DOI] [PubMed] [Google Scholar]
- 15.Corkill M.M., Jobanputra P., Gibson T., Macfarlane D.G. A controlled trial of sulphasalazine treatment of chronic ankylosing spondylitis: failure to demonstrate a clinical effect. Br. J. Rheumatol. 1990;29:41–45. doi: 10.1093/rheumatology/29.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Taylor H.G., Beswick E.J., Dawes P.T. Sulphasalazine in ankylosing spondylitis. A radiological, clinical and laboratory assessment. Clin. Rheumatol. 1991;10:43–48. doi: 10.1007/BF02208032. [DOI] [PubMed] [Google Scholar]
- 17.Kirwan J., Edwards A., Huitfeldt B., Thompson P., Currey H. The course of established ankylosing spondylitis and the effects of sulphasalazine over 3 years. Br. J. Rheumatol. 1993;32:729–733. doi: 10.1093/rheumatology/32.8.729. [DOI] [PubMed] [Google Scholar]
- 18.Dougados M., van der Linden S., Leirisalo-Repo M., et al. Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum. 1995;38:618–627. doi: 10.1002/art.1780380507. [DOI] [PubMed] [Google Scholar]
- 19.Clegg D.O., Reda D.J., Abdellatif M. Comparison of sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondylarthropathies: a Department of Veterans Affairs cooperative study. Arthritis Rheum. 1999;42:2325–2329. doi: 10.1002/1529-0131(199911)42:11<2325::AID-ANR10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Caruso I., Cazzola M., Santandrea S. Clinical improvement in ankylosing spondylitis with rifamycin SV infiltrations of peripheral joints. J. Int. Med. Res. 1992;20:171–181. doi: 10.1177/030006059202000209. [DOI] [PubMed] [Google Scholar]
- 21.Ogrendik M. Treatment of ankylosing spondylitis with moxifloxacin. South. Med. J. 2007;100:366–370. doi: 10.1097/SMJ.0b013e31802fa2a8. [DOI] [PubMed] [Google Scholar]
- 22.Lokwani P, Upadhyay Y, Kumar P, Gupta S, Kalyanwat R, Songara RK. Review on: ankylosing spondylitis. 2011.
- 23.Bowness P. HLA B27 in health and disease: a double-edged sword? Rheumatology (Oxford) 2002;41:857–868. doi: 10.1093/rheumatology/41.8.857. [DOI] [PubMed] [Google Scholar]
- 24.Huet S., Nixon D.F., Rothbard J.B., Townsend A., Ellis S.A., McMichael A.J. Structural homologies between two HLA B27-restricted peptides suggest residues important for interaction with HLA B27. Int. Immunol. 1990;2:311–316. doi: 10.1093/intimm/2.4.311. [DOI] [PubMed] [Google Scholar]
- 25.Papagoras C., Voulgari P.V., Drosos A.A. Atherosclerosis and cardiovascular disease in the spondyloarthritides, particularly ankylosing spondylitis and psoriatic arthritis. Clin. Exp. Rheumatol. 2013;31:612–620. [PubMed] [Google Scholar]
- 26.Park Y.B., Ahn C.W., Choi H.K., et al. Atherosclerosis in rheumatoid arthritis: morphologic evidence obtained by carotid ultrasound. Arthritis Rheum. 2002;46:1714–1719. doi: 10.1002/art.10359. [DOI] [PubMed] [Google Scholar]
- 27.Chatzidimitriou D., Kirmizis D., Gavriilaki E., Chatzidimitriou M., Malisiovas N. Atherosclerosis and infection: is the jury still not in? Future Microbiol. 2012;7:1217–1230. doi: 10.2217/fmb.12.87. [DOI] [PubMed] [Google Scholar]


