Abstract
Abstract: Background and Objective: Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors (EGFR-TKIs) are effective against lung adenocarcinoma. However, limited data is available assessing the effectiveness of EGFR-TKI use in preventing re-accumulation of MPE. To our knowledge, there is no literature on comparison of talc pleurodesis with EGFR-TKIs alone on re-accumulation of MPE in Asian population. We investigated if EGFR-TKI therapy for advanced lung adenocarcinoma with malignant pleural effusion (MPE) is also successful in preventing pleural fluid re-accumulation following initial drainage.
Methods: An observational cohort study of patients with lung adenocarcinoma and MPE in the year 2012 was conducted.
Results: 70 patients presented with MPE from lung adenocarcinoma. Fifty six underwent EGFR mutation testing of which 39 (69.6%) had activating EGFR mutation and 34 (87.1%) received TKI. 20 were managed by pleural fluid drainage only whereas 14 underwent talc pleurodesis following pleural fluid drainage. Time taken for the pleural effusion to re-accumulate in those with and without pleurodesis was 9.9 vs. 11.7 months, p=0.59 respectively. More patients (n=10, 25.6%) with activating EGFR mutation presented with complete opacification (white-out) of the hemithorax compared to none without activating EGFR mutation (p=0.02).
Conclusion: In TKI eligible patients, early talc pleurodesis may not confer additional benefit in preventing re-accumulation of pleural effusion and may be reserved for non-adenocarcinoma histology, or EGFR negative adenocarcinoma. Complete opacification of the hemithorax on presentation may serve as an early radiographic signal of positive EGFR mutation status.
Keywords: Keywords: Lung cancer, adenocarcinoma, pleural effusion, pleurodesis, epidermal growth factor receptor tyrosine kinase inhibitors
Introduction
Lung cancer is a leading cause of cancer related mortality [1]. Platinum-based chemotherapy
illustrates partial responses in approximately 30% of patients with advanced non-small cell lung cancer (NSCLC) [2]. In contrast, treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are associated with higher response rates and survival in patients whose cancer demonstrates activating EGFR mutations [3-6]. Fifteen percent of patients have a pleural effusion at the initial diagnosis of lung cancer [7]. Patients with lung adenocarcinoma with malignant pleural effusion (MPE) have a higher EGFR mutation detection rate compared to patients with lung adenocarcinoma presenting as a solitary mass or nodule. The EGFR mutation rate in MPEs of lung adenocarcinoma has been reported to be as high as 70%, irrespective of patient age, gender or smoking status [8]. Consequently, greater proportions of patients with adenocarcinoma and a MPE phenotype are eligible for TKI therapy.
It is known that MPE recurs rapidly, sometimes within a month after an initial thoracentesis in a considerable number of patients [9, 10]. Consequently, treatment targeted to the cancer itself such as chemotherapy or TKIs, coupled to drainage of the initial effusion and prevention of re-accumulation in the future with either a tunnelled pleural catheter and/or use of chemical pleurodesis is routinely employed [11-14].
EGFR positive pleural metastases have demonstrated a comparable high response rate to TKIs as observed in other EGFR positive metastatic sites. Hence it is logical to consider managing patients with EGFR positive MPEs with TKI therapy alone in the first instance before considering more invasive approaches directed at the recurrence of MPE such as tunnelled pleural catheter or pleurodesis, each of which carries its own set of risks and procedural complications [15]. Currently, limited data is available demonstrating the effectiveness of EGFR-TKI in preventing re-accumulation of MPE [16]. To our knowledge, there is no literature on comparison of combination of talc pleurodesis and EGFR-TKI with EGFR-TKIs alone on re-accumulation of MPE in Asian population. We have conducted an observational cohort study to assess if TKIs used for the treatment of a lung adenocarcinoma in patients presenting with a MPE are also useful in preventing re-accumulation of MPE without talc pleurodesis.
Materials and Methods
The current study included patients managed for MPE from lung adenocarcinoma in 2012 (n=70). Data on gender, duration of chest drainage, occurrence of pleurodesis, EGFR mutation status, duration of TKI therapy administration, time to effusion recurrence (recurrence free survival), and overall survival was recorded retrospectively. Institutional Review Board (IRB) approval was obtained with the waiver of written consent due to retrospective nature of the study.
Overall survival was evaluated from the date of chest tube insertion to either death or date of last follow-up ending 2015. Effusion-progression-free survival was defined as the period from the date of chest tube insertion to the date of follow-up chest radiograph or CT scan showing recurrence of pleural effusion. Effusion was considered to have recurred if the patient re-developed symptoms such as dyspnea, and the follow-up radiograph showed increase in the volume of pleural effusion greater than the radiograph done on the date of removal of the chest tube placed at the time of initial hospitalization. TKI duration was evaluated from date of initiation to date of cessation. TKI as first line group was defined as those receiving TKIs as the first agent for more than 1 month duration. Since it takes at least a month for TKIs to take effect, patients receiving TKIs for less than a month (n=5) were analysed as those who did not receive TKI.
Chemotherapy administered was platinum based dual agent combination therapy.
Among patients receiving EGFR-TKI`s, four types of Tyrosine kinase inhibitors were administered namely Erlotinib (Tarceva), Gefitinib (Iressa), Afatinib, and Crizotinib.
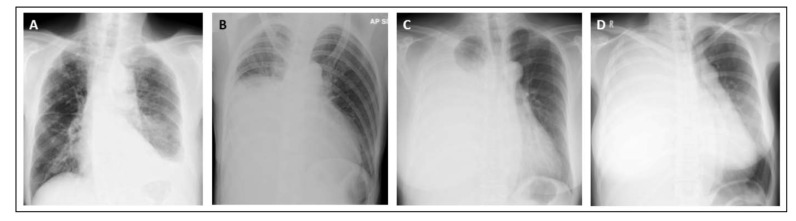
The volume of pleural effusion at initial presentation was determined and classified into one third opacification of the hemithorax, half opacification of the hemithorax, two third opacification of the hemithorax, and complete opacification of the hemithorax by a respiratory physician of at least 10 years-experience who was blinded to the outcomes (Fig. 1).
Fig. (1).
Classification of initial chest radiograph into one third opacification of the hemithorax (A), half opacification of the hemithorax (B), two third opacification of the hemithorax (C), and complete opacification of the hemithorax (D).
Pleurodesis was done using talc slurry (4 g talc mixed in 100 millilitres of normal saline) instilled via the pre-existing chest tube inserted for drainage of pleural effusion, or talc insufflation via thoracoscopy. Decision to perform pleurodesis was based on the managing physician`s clinical judgement.
Data Analysis
All statistical analysis was performed using SPSS, version 17; Chicago, Ill). Data was assessed for normality using the Kolmogorov-Smirnoff method and where non-parametric, results were assessed using a Wilcoxon two-sample test or Fisher exact test. P values were two sided and considered significant where <0.05
Results
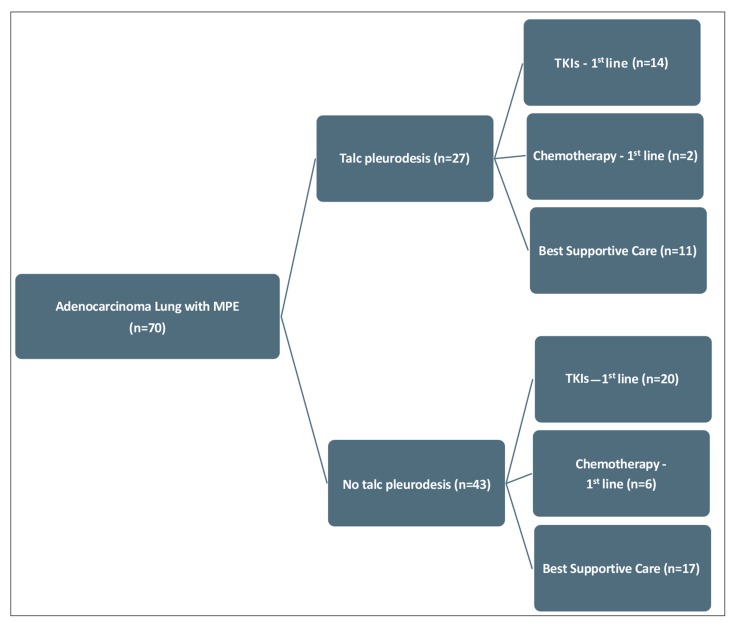
A total of 70 patients with lung adenocarcinoma presenting with a MPE at the time of initial diagnosis in 2012 were studied. Median age (range) was 72 (38-92) years and 33 (47.1%) were female. Twenty seven (38.5%) patients underwent pleurodesis in the whole group (Fig. 2). Pleurodesis was done at median (range) interval of 7 (2-22) days after chest drain insertion (Table 1).
Fig. (2).
Flow Diagram of the Patients. Seventy patients had malignant pleural effusion from adenocarcinoma out of which 27 received talc pleurodesis and 43 did not. In terms of treatment of lung cancer, among patients receiving talc pleurodesis, 14 received TKI`s, 2 received chemotherapy, and 11 received nil (best supportive care), as cancer specific therapy. Among patients not receiving talc pleurodesis, 20 received TKI`s, 6 received chemotherapy, and 17 received nil (best supportive care), as cancer specific therapy.
Table 1. General characteristics, radiographic features on presentation, effusion-recurrence-free survival, and overall survival.
| All | Pleurodesis (n=27) | No pleurodesis (n=43) | |||||
|---|---|---|---|---|---|---|---|
| TKIs as 1st line | Chemotherapy as 1st line | BSC | TKIs as 1st line | Chemotherapy as 1st line | BSC | ||
| No. of patients | 70 | 14 | 2 | 11 | 20 | 6 | 17 |
| Age | 72 (38-92) | 62 (52-82) | 57.5 (40-75) | 76 (57-90) | 72 (45-92) | 67 (40-72) | 78 (38-89) |
| Gender (Females) | 33 (47.1) | 7 (50) | 0 | 3 (27.2) | 13 (65) | 3 (50) | 7 (41.1) |
| Initial radiograph | |||||||
| 1/3rd opacification | 13 (18.5) | 3 (21.4) | 0 | 2 (18.1) | 3 (15) | 1 (16.6) | 4 (23.5) |
| ½ opacification | 17 (24.2) | 4 (28.5) | 0 | 3 (27.2) | 5 (25) | 2 (33.3) | 3 (17.6) |
| 2/3rd opacification | 21 (30) | 3 (21.4) | 2 (100) | 5 (45.4) | 3 (15) | 2 (33.3) | 6 (35.2) |
| Complete opacification | 17 (24.2) | 3 (21.4) | 0 | 1 (9) | 8 (40) | 1 (16.6) | 4 (23.5) |
| Side of pleural effusion | |||||||
| Right | 44 (62.8) | 9 (64.2) | 0 | 6 (54.5) | 9 (45) | 4 (66.6) | 16 (94.1) |
| Left | 23 (32.8) | 5 (35.7) | 2 (100) | 5 (45.4) | 11 (55) | 2 (33.3) | 1 (5.8) |
| Trapped lung | 9 (12.8) | 1 (7.1) | 0 | 0 | 3 (15) | 2 (33.3) | 3 (17.6) |
| Chest tube duration (days) | 7.5 (1-23) | 7 (3-13) | 11 | 6 (12-23) | 8 (3-18) | 9 (7-16) | 7 (1-13) |
| EGFR positive (exon 19, 21) | 39 (55.7) | 13 (92.8) | 0 | 3 (27.2) | 16 (80) | 1 (16.6) | 5 (29.4) |
| TKIs duration (days) | 275 (10-1101) | 338.5 (32-661) | 0 | 10 (17-24) | 299 (42-1101) | 24 (24-24) | 24 (21-27) |
| Effusion-recurrence-free survival (days) | 96.5 (4-913) | 298 (22-783) | 152 (117-187) | 49 (12-241) | 352 (12-739) | 35.5 (31-913) | 22 (4-197) |
| Overall survival (days) | 218 (4-1215) | 577.5 (96-859) | 166 (123-209) | 51 (18-242) | 381.5 (16-1215) | 381.5 (83-973) | 72 (4-385) |
Data presented as number (%) or median (range)
Pleural effusion recurred in 13 (48.1%) patients out of 27 patients who underwent pleurodesis in the whole cohort. However, 5 patients out of 13 had trapped lung. Hence the actual recurrence rate was 8 (29.6%). No differences in effusion-recurrence-free survival after initial drainage was observed between “pleurodesis” (3.9 months) vs. “no pleurodesis” groups (3.2 months, p=0.31).
Fifty six (80%) patients were tested for EGFR mutation and 39 (69.6%) had activating EGFR mutations in exon 19 or 21. Significantly greater proportions of EGFR positive patients (n=10, 25.6%) presented with complete opacification of their hemithorax vs. none in EGFR negative patients (p=0.02) (Table 2, Fig. 1).
Table 2. Subgroup analysis of EGFR mutation status and the degree of opacification of the hemithorax by pleural effusion at the time of initial presentation (n=56).
|
EGFR (+)
(n=39) |
EGFR (-)
(n=17) |
P value | |
|---|---|---|---|
| One third opacification of the hemithorax (n=11)* | 6 (15.3) | 5 (29.4) | 0.27 |
| Half opacification of the hemithorax (n=13) | 9 (23) | 4 (23.5) | 1.0 |
| Two third opacification of the hemithorax (n=20) | 12 (30.7) | 8 (47) | 0.36 |
| Complete opacification of the hemithorax (n=10) | 10 (25.6) | 0 | 0.02 |
*Data of patient who did not undergo EGFR mutation analysis (n=14) were excluded.
EGFR: epidermal growth factor receptor
Out of 39 the TKI eligible patients, 34 (87.1%) received TKI as 1st line therapy, 5 having refused such therapy due to patient preference. 17 patients receiving TKI were managed by pleural fluid drainage only whereas 14 underwent talc pleurodesis following pleural fluid drainage. Time taken for the pleural effusion to re-accumulate in those with, and without pleurodesis was 9.9 vs. 11.7 months, p=0.59 respectively suggesting no additive effect of pleurodesis in preventing effusion re-accumulation in patients receiving TKI therapy for their lung adenocarcinoma.
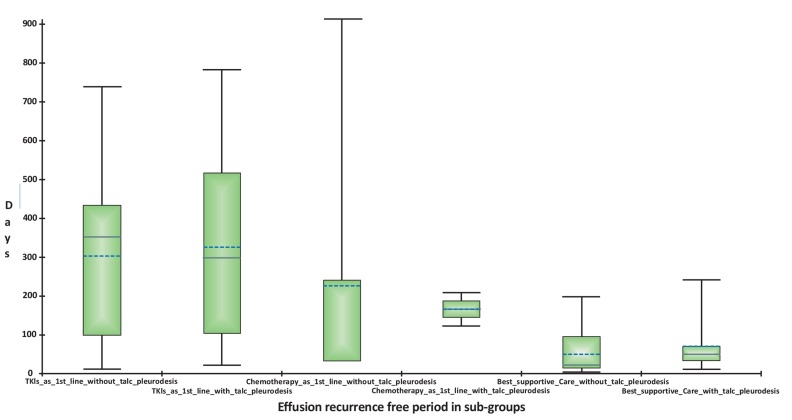
Eight patients received chemotherapy as first line therapy. Although not reaching statistical significance (p=0.64), effusion-recurrence-free period was longer (5 months) in those undergoing talc pleurodesis in addition to chemotherapy (n=2) vs. 1.1 month in those without talc pleurodesis (n=6) (Table 3, Fig. 3).
Table 3. Subgroup analysis of patients treated with TKIs, chemotherapy, or best supportive care in “talc pleurodesis” and “no-talc pleurodesis” group (n=70).
| Recurrence free period (days) | P value | |
|---|---|---|
| TKIs as 1st line without Talc pleurodesis (n=20) | 352 (12-739) | 0.0002 |
| Best supportive care alone (n=17) | 22 (4-197) | |
| Best Supportive Care with Talc pleurodesis (n=11) | 49 (12-241) | 0.43 |
| Chemotherapy as 1st line without Talc pleurodesis (n=6) | 35.5 (31-913) | |
| TKIs as 1st line without Talc pleurodesis (n=20) | 352 (12-739) | |
| Best Supportive Care with Talc pleurodesis (n=11) | 49 (12-241) | 0.007 |
| Best Supportive Care alone (n=17) | 22 (4-197) | 0.20 |
| Best Supportive Care with Talc pleurodesis (n=11) | 49 (12-241) | |
| TKIs as 1st line without Talc pleurodesis (n=20) | 352 (12-739) | 0.36 |
| Chemotherapy as 1st line without Talc pleurodesis (n=6) | 35.5 (31-913) | |
| TKIs as 1st line without Talc pleurodesis (n=20) | 352 (12-739) | 0.59 |
| TKIs as 1st line with Talc pleurodesis (n=14) | 298 (22-783) | |
| Best Supportive Care with Talc pleurodesis (n=11) | 49 (12-241) | 0.15 |
| Chemotherapy as 1st line with Talc pleurodesis (n=2) | 152 (117-187) | |
| Best Supportive Care alone (n=17) | 22 (4-197) | 0.07 |
| Chemotherapy as 1st line without Talc pleurodesis (n=6) | 35.5 (31-913) | |
| TKIs as 1st line with Talc pleurodesis (n=14) | 298 (22-783) | 0.006 |
| Best Supportive Care with Talc pleurodesis (n=11) | 49 (12-241) | |
| TKIs as 1st line with Talc pleurodesis (n=14) | 298 (22-783) | 0.0003 |
| Best Supportive Care alone (n=17) | 22 (4-197) | |
| Best Supportive Care alone (n=17) | 22 (4-197) | |
| Chemotherapy as 1st line with Talc pleurodesis (n=2) | 152 (117-187) | 0.04 |
TKIs: Tyrosine kinase inhibitors
Fig. (3).
Effusion-recurrence-free period in various sub-groups. Patients receiving TKIs had longest effusion-recurrence-free period of 352 days (close to a year) even without talc-pleurodesis, in comparison to patients with chemotherapy and best supportive care. The solid horizontal line within the box represents the median, and the dotted horizontal line represents the mean.
Twenty eight patients were treated with best supportive care (BSC). Among these, those with talc pleurodesis (n=11) and those without talc pleurodesis (n=17) showed similar effusion-recurrence-free period of 1.63 vs. 0.73 month, (p=0.20).
Median survival for the whole cohort collectively was 7.2 months. Median survival in TKIs, TKIs plus talc pleurodesis, and TKIs without talc pleurodesis group was 14.1, 19.2, and 11.7 months respectively. Median survival for those receiving chemotherapy, chemotherapy plus talc pleurodesis, and chemotherapy without talc pleurodesis was 8.3, 5.5, and 12.7 respectively. Median survival in BSC with and without pleurodesis was 1.7 and 2.4 months respectively. One year survival in the TKIs group was significantly longer than the survival rate of the whole cohort (p=0.007).
TKI alone (without pleurodesis) as a first line therapy had the longest effusion-recurrence-free period. On the contrary, chemotherapy alone (without pleurodesis) as first line was equivalent to BSC with talc pleurodesis (1.6 months, p=0.43), and BSC alone (0.73 months, p=0.07). This suggests that chemotherapy alone for the treatment of MPE is similar to no intervention or pleurodesis alone, a marked contrast to the pattern observed with TKIs. Patients with complete opacification of their hemithorax (n=17) also demonstrated shorter effusion-recurrence-free survival 70 (4-739) days as compared to those with one third opacification of the hemithorax (n=13), 197 (23-559) days without reaching significance (p=0.42).
Among patient treated with chemotherapy, two patients received combination of carboplatin and gemcitabine. Four patients received combination of carboplatin and Pemetrexed, and 2 patients received combination of cisplatin and Pemetrexed.
Among patients treated with EGFR-TKIs, nineteen patients received Erlotinib (Tarceva), 12 patients received Gefitinib (Iressa), 2 patients received Afatinib, and 1 patient received Crizotinib.
Discussion
Our findings illustrate that EGFR-TKI therapy alone may be equivalent to that with the addition of talc pleurodesis in preventing recurrence of MPE in EGFR mutation positive lung adenocarcinoma. Furthermore, it may be superior to the combined effect of “chemotherapy plus talc pleurodesis,” for the prevention of recurrent MPE in an unselected patient cohort with lung adenocarcinoma. In TKI eligible patients, early talc pleurodesis prior to confirmation of EGFR status may not be necessary. This is the first study in Asian population to compare the effectiveness of EGFR-TKI alone with the combination of EGFR-TKI and talc pleurodesis in preventing re-accumulation of MPE.
Data directly addressing effusion-recurrence-free period in patients with lung adenocarcinoma and MPE is limited. In our cohort of lung adenocarcinoma patients, the recurrence-free-period was no different (close to 4 months) in pleurodesis vs. no pleurodesis groups. This was similar to the only one other study comparing the effusion-recurrence-free period between EGFR-TKI and chemical pleurodesis [16]. The reported effusion-recurrence-free period in all comers was 5 and 4.8 months in no-pleurodesis and pleurodesis groups respectively in this study, with the difference that these investigators used minocycline or OK432 as the pleural sclerosing agent in contrast to talc used in our cohort [16].
In terms of EGFR status, recurrence-free period was significantly better in our cohort (10.8 months) in the EGFR positive group treated with TKIs vs. EGFR negative group (1.8 months) and furthermore, there was no difference in the effusion-recurrence-free period between patients receiving TKI with pleurodesis and those receiving TKI without pleurodesis, both having effusion-recurrence-free period of approximately 10-11 months (close to an year). These findings are similar to previous investigators who also reported the significantly better recurrence-free survival in an EGFR positive group treated with TKIs vs. EGFR negative group (7.1 vs 1.1 months).
This has clinical implications. Malignant pleural effusion frequently recurs after drainage. Currently available and commonly practiced options for preventing re-accumulation of MPE are chemical pleurodesis for free flowing effusion, or tunnelled pleural catheter for trapped lung. Regarding chemical pleurodesis, a meta-analysis of 10 randomized trials that included 308 patients has shown that non-recurrence of effusion is more likely with talc pleurodesis than chest tube drainage without instillation of a talc. (Relative risk 1.34, CI 1.16-1.55) [12]. However pleurodesis is associated with difficulties.
First, the decision to undergo chemical pleurodesis is often based on a relatively longer anticipated survival (e.g. longer than three months) however, no studies exist to allow sufficiently accurate predictions of survival to assist decision making for management of individual patients with malignant pleural effusions.
Second, although the success rate of talc in preventing recurrence is approximately 60 to 90 percent, the longer the patient survives after pleurodesis, however, the greater the probability of recurrence of a malignant pleural effusion with 50 percent of patients undergoing talc pleurodesis experiencing inadequate fluid control at six months [12, 17-21].
Third, talc pleurodesis carries potential for complications. The most common adverse events occurring after talc pleurodesis are fever (10-17%), pain, and gastrointestinal symptoms. Rarely, a systemic inflammatory response syndrome and/or adult respiratory distress syndrome develop; these complications are generally preventable by avoiding small particle size talc and not administering more than 5 g of talc at a time [22]. However, graded (large size talc) may not be available everywhere.
Fourth, talc pleurodesis requires reduction of pleural drainage to less than 150 millilitres before pleurodesis can be performed even when the patient is no longer symptomatic from effusion and is fit for discharge [23]. This increases the length of stay of the patient in the hospital.
Our finding of the potential of TKIs alone being adequate for preventing re-accumulation of MPE helps to overcome all these issues by avoiding the unnecessary side effects and complications of talc administration. This can also save institutional resources associated with performing the procedure, and length of stay (LOS) associated with time required for the reduction of pleural drainage to less than 150 millilitres before pleurodesis can be performed.
It has been reported that effusion control rate with EGFR-TKIs in patients with large or massive pleural effusions at initial presentation is poorer than those with small to moderate amounts of pleural effusion [16]. In our work, we observed similar findings. Patients presenting with complete opacification of their hemithorax had greater incidences of EGFR mutation. Furthermore, effusion-recurrence-free period in patients with complete opacification of their hemithorax was shorter compared to those with one third opacification of their hemithorax, although results did not reach statistical significance. The clinical implications of such findings are that initial chest radiograph’s may guide physicians at a potentially very early stage with regards to indicating EGFR mutation status and the urgent need for testing and TKI therapy. Secondly, complete opacification of the chest directly correlates with EGFR positivity and may predict a subgroup of patients in whom early pleurodesis should be considered based on the higher risk of re-accumulation.
Although overall survival was not the primary end point of our study, the median survival of the studied cohort was 7.2 months, similar to 6.5-8 months prior to advent of EGFR-TKIs in the existing literature [24]. The survival rate at 1 year was 31%. The median survival of those receiving TKIs in our cohort however, was 14 months, comparable to international standards with survival rate at 1 year of 52.9% [25].
While our study uncovers some important clinical patterns, it does possess limitations. First, it is a single center study with an observational design. Second, the number of patients is small requiring a larger study with a prospective randomized design to validate our findings. Third, although a small number, few patients underwent talc pleurodesis even when they had trapped lung. This can falsely increase the failure rate in the patient who did not receive TKI. However, the consistency of our findings with that in the existing literature, which is limited, indicates the value of our dataset.
In summary, performing talc pleurodesis in the early phase (prior to the confirmation of EGFR status) of the management for everyone who has a chest tube inserted for initial drainage of pleural effusion may not be necessary. Patients receiving TKIs have longer effusion-recurrence-free period of almost 1 year without pleurodesis. Pleurodesis in this patient subset may not confer additional benefit. Early pleurodesis should be reserved for non-adenocarcinoma histology, or EGFR negative adenocarcinoma, or in patients who develop an effusion following disease progression on TKI treatment, and those with complete opacification of the hemithorax at initial presentation.
ACKNOWLEDGEMENTS
Authors would like to thank Ms. Ivy Yu Ling Ling for her valuable contribution in editing the figures and administrative work.
CONFLICT OF INTEREST
A.V., A.C., Y.W.L., L.D.B., A.B.A., D.B.A.A., F.A.A., A.Y.H.L., S.H.C., and J.A. have no competing financial interests to disclose.
REFERENCES
- 1.GLOBOCAN. International Agency for Research on Cancer. Cancer Incidence, Mortality and Prevalence Worldwide in 2008. Available at :http://globocan.iarc.fr/factsheets/cancers/lung.asp. 2008.
- 2.D’Addario G., Pintilie M., Leighl N.B., Feld R., Cerny T., Shepherd F.A. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J. Clin. Oncol. 2005;23(13):2926–2936. doi: 10.1200/JCO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Brugger W., Triller N., Blasinska-Morawiec M., Curescu S., Sakalauskas R., Manikhas G.M., Mazieres J., Whittom R., Ward C., Mayne K., Trunzer K., Cappuzzo F. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011;29(31):4113–4120. doi: 10.1200/JCO.2010.31.8162. [DOI] [PubMed] [Google Scholar]
- 4.Zhu C.Q., da Cunha Santos G., Ding K., Sakurada A., Cutz J.C., Liu N., Zhang T., Marrano P., Whitehead M., Squire J.A., Kamel-Reid S., Seymour L., Shepherd F.A., Tsao M.S., National Cancer Institute of Canada Clinical Trials Group Study BR.21 Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J. Clin. Oncol. 2008;26(26):4268–4275. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 5.Douillard J.Y., Shepherd F.A., Hirsh V., Mok T., Socinski M.A., Gervais R., Liao M.L., Bischoff H., Reck M., Sellers M.V., Watkins C.L., Speake G., Armour A.A., Kim E.S. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J. Clin. Oncol. 2010;28(5):744–752. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 6.Shigematsu H., Lin L., Takahashi T., Nomura M., Suzuki M., Wistuba I.I., Fong K.M., Lee H., Toyooka S., Shimizu N., Fujisawa T., Feng Z., Roth J.A., Herz J., Minna J.D., Gazdar A.F. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 2005;97(5):339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 7.Pass H.I., Johnson D.H., editors. Lung Cancer: Principles and Practice. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2005. Clinical presentation of non-small cell carcinoma of the lung. pp. 291–303. [Google Scholar]
- 8.Wu S.G., Gow C.H., Yu C.J., Chang Y.L., Yang C.H., Hsu Y.C., Shih J.Y., Lee Y.C., Yang P.C. Frequent epidermal growth factor receptor gene mutations in malignant pleural effusion of lung adenocarcinoma. Eur. Respir. J. 2008;32(4):924–930. doi: 10.1183/09031936.00167407. [DOI] [PubMed] [Google Scholar]
- 9.Heffner J.E., Klein J.S. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin. Proc. 2008;83(2):235–250. doi: 10.1016/S0025-6196(11)60848-3. [DOI] [PubMed] [Google Scholar]
- 10.Shaw P.H., Agarwal R. WITHDRAWN: Pleurodesis for malignant pleural effusions. Cochrane Database Syst. Rev. 2013;11(11):CD002916. doi: 10.1002/14651858.CD002916.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dresler C.M., Olak J., Herndon J.E., II, Richards W.G., Scalzetti E., Fleishman S.B., Kernstine K.H., Demmy T., Jablons D.M., Kohman L., Daniel T.M., Haasler G.B., Sugarbaker D.J., Cooperative Groups Cancer and Leukemia Group B. Eastern Cooperative Oncology Group. North Central Cooperative Oncology Group. Radiation Therapy Oncology Group Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127(3):909–915. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw P., Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst. Rev. 2004;1(1):CD002916. doi: 10.1002/14651858.CD002916.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Reddy C., Ernst A., Lamb C., Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139(6):1419–1423. doi: 10.1378/chest.10-1868. [DOI] [PubMed] [Google Scholar]
- 14.Rintoul R.C., Ritchie A.J., Edwards J.G., Waller D.A., Coonar A.S., Bennett M., Lovato E., Hughes V., Fox-Rushby J.A., Sharples L.D., MesoVATS Collaborators Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet. 2014;384(9948):1118–1127. doi: 10.1016/S0140-6736(14)60418-9. [DOI] [PubMed] [Google Scholar]
- 15.Clayton J.S., Nichole T.T., Rolando S.S., Julie A. Woolworth, Gerard A. Silvestri. EGFR mutations in malignant pleural effusions from lung cancer. Curr. Respir. Care Rep. 2013;2:79–87. doi: 10.1007/s13665-013-0041-5. [DOI] [Google Scholar]
- 16.Chen C-H., Gow C-H., Yu C-J., et al. Clinical Response of Gefitinib on Malignant Pleural Effusions in Patients with Non-Small Cell Lung Cancer. J Can Mol. 2008;4:23–28. [Google Scholar]
- 17.Spiegler P.A., Hurewitz A.N., Groth M.L. Rapid pleurodesis for malignant pleural effusions. Chest. 2003;123(6):1895–1898. doi: 10.1378/chest.123.6.1895. [DOI] [PubMed] [Google Scholar]
- 18.Demmy T.L., Gu L., Burkhalter J.E., Toloza E.M., D’Amico T.A., Sutherland S., Wang X., Archer L., Veit L.J., Kohman L., Cancer and Leukemia Group B Optimal management of malignant pleural effusions (results of CALGB 30102). J. Natl. Compr. Canc. Netw. 2012;10(8):975–982. doi: 10.6004/jnccn.2012.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan C., Sedrakyan A., Browne J., Swift S., Treasure T. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur. J. Cardiothorac. Surg. 2006;29(5):829–838. doi: 10.1016/j.ejcts.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Cardillo G., Facciolo F., Carbone L., Regal M., Corzani F., Ricci A., Di Martino M., Martelli M. Long-term follow-up of video-assisted talc pleurodesis in malignant recurrent pleural effusions. Eur. J. Cardiothorac. Surg. 2002;21(2):302–305. doi: 10.1016/S1010-7940(01)01130-7. [DOI] [PubMed] [Google Scholar]
- 21.Bielsa S., Hernández P., Rodriguez-Panadero F., Taberner T., Salud A., Porcel J.M. Tumor type influences the effectiveness of pleurodesis in malignant effusions. Lung. 2011;189(2):151–155. doi: 10.1007/s00408-011-9283-6. [DOI] [PubMed] [Google Scholar]
- 22.Arellano-Orden E., Romero-Falcon A., Juan J.M., Ocaña Jurado M., Rodriguez-Panadero F., Montes-Worboys A. Small particle-size talc is associated with poor outcome and increased inflammation in thoracoscopic pleurodesis. Respiration. 2013;86(3):201–209. doi: 10.1159/000342042. [DOI] [PubMed] [Google Scholar]
- 23.Reddy C., Ernst A., Lamb C., Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest. 2011;139(6):1419–1423. doi: 10.1378/chest.10-1868. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura S., Ando Y., Minami H., Ando M., Sakai S., Shimokata K. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin. Cancer Res. 1997;3(1):47–50. [PubMed] [Google Scholar]
- 25.Wu S-G., Yu C-J., Tsai M-F., Liao W.Y., Yang C.H., Jan I.S., Yang P.C., Shih J.Y. Survival of lung adenocarcinoma patients with malignant pleural effusion. Eur. Respir. J. 2013;41(6):1409–1418. doi: 10.1183/09031936.00069812. [DOI] [PubMed] [Google Scholar]