Abstract
The rapid escalation in the global prevalence diabetes, with more than 30% being afflicted with diabetic retinopathy (DR), means it is likely that associated vision-threatening conditions will also rise substantially. This means that new therapeutic approaches need to be found that go beyond the current standards of diabetic care, and which are effective in the early stages of the disease. In recent decades several new pharmacological agents have been investigated for their effectiveness in preventing the appearance and progression of DR or in reversing DR; some with limited success while others appear promising. This up-to-date critical review of non-traditional systemic treatments for DR is based on the published evidence in MEDLINE spanning 1980-December 2014. It discusses a number of therapeutic options, paying particular attention to the mechanisms of action and the clinical evidence for the use of renin-angiotensin system blockade, fenofibrate and calcium dobesilate monohydrate in DR.
Keywords: Calcium dobesilate, diabetic retinopathy, fenofibrate, renin-angiotensin system blockade
Introduction
Diabetes mellitus is a worldwide public health burden with the number of people affected by diabetes (mainly type 2) rising dramatically and estimated to exceed half a billion by 2035 (592 million) [1,2]. Over a third of these patients are likely to have the microvascular complication of diabetic retinopathy (DR) and 10% have vision-threatening stages such as diabetic macular edema (DME) or proliferative DR (PDR) [3,4]. In fact, despite progress in screening and treatment, DR continues to be leading cause of visual impairment and preventable blindness among working-age adults in many countries [4,5], and is a significant socioeconomic burden on healthcare systems [6-8]. This underlines the im portance of seeking new approaches which go beyond current standards of diabetes care.
The number of patients affected by DR is growing worldwide mainly due to the longevity of patients with diabetes [9]. As DR remains asymptomatic in the early stages, such as mild or moderate non-proliferative DR (NPDR), its diagnosis can be deferred. The situation is worse in type 2 diabetes as patients are often not promptly diagnosed because diabetes itself may be asymptomatic for many years prior to diagnosis. Consequently, this eye complication may silently progress at different rates to more severe stages [10].
Over the past few decades, epidemiological studies and clinical trials show the most important risk factors for the development and progression of DR are the type and duration of diabetes, hyperglycemia and hypertension [4]. Others have also identified microalbuminuria and dyslipidemia as important risk factors for the progression of DR [11,12]. Based on these data, tight control of blood glucose and hypertension are strongly recommended for preventing DR. However these therapeutic objectives are usually not easy to achieve in practice and, consequently, DR continues to develop and progress in a high proportion of patients. Additionally, clinical studies have shown substantial variations regarding the onset and severity of DR that are not totally explained by these currently known risk factors [13-16]. Even multifactorial interventions targeting similar aims to those recommended in the American Diabetes Association guidelines do not prevent DR in all patients [17]. Finally, when DR appears, there is limited treatment that targets the eye during the early stages, with most treatments only indicated in the more advanced stages of DR (PDR and clinically significant macular edema [CSME]). These include invasive and expensive treatments such as laser photocoagulation, intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents, corticosteroids and vitreoretinal surgery. For all these reasons, there is general agreement that new pharmacological treatments for the early stages of DR (mild, moderate) are urgently needed [18,19].
Several drugs have been developed for the prevention and treatment of DR. The protein kinase C inhibitor, ruboxistaurin mesilate, administered orally was effective in halting DME and vision loss but not in preventing the progression of DR (the primary endpoint) [20-22]. In a phase 3 clinical trial the long-acting somatostatin analogue, octreotide, given intramuscularly every 4 weeks in moderate-to-severe NPDR to low-risk PDR, was not effective in arresting DR progression [23]. Consequently, the initial enthusiasm of the scientific community for these drugs has dwindled.
More recently, 2 classes of drugs: renin-angiotensin system (RAS) blockers [24,25] and fenofibrate (a hypolipidemic drug) [26,27] have emerged as potential systemic treatments for DR. Furthermore, calcium dobesilate monohydrate (CaD) appears to be a promising treatment for DR, though it is not widely used in clinical practice [28]. In this review, the mechanisms of action and clinical evidence for the potential usefulness of RAS blockers, fenofibrate and CaD in preventing or delaying DR progression are critically assessed.
Search strategy and selection criteria
References for this review were obtained through a comprehensive search of the electronic MEDLINE database (1980-2014) using the PubMed search service. Search terms included: “diabetic retinopathy”, “diabetic microangiopathy”, and “clinical trials”, and specifically for “candesartan”, “fenofibrate”, “renin-angiotensin system blockers”, and “calcium dobesilate”. The search was conducted in April-December 2014 and all abstracts were reviewed and relevant articles retrieved. In addition, reference lists from the selected articles were used to obtain further articles not included in the electronic database. Only articles published in English were included.
Pathophysiological mechanisms in diabetic retinopathy
The metabolic pathways triggered by chronic hyperglycemia (the polyol and the hexosamine pathways, the synthesis de novo of diacylglycerol-proteinkinase C, oxidative stress and advanced glycation end-products [AGEs]) are instrumental in the onset and progression of DR [29,30]. Moreover, growing evidence suggests that chronic inflammation plays an important pathogenic role [31,32]. The activation of all these pathways results in damage to the neural retina (retinal neurodegeneration) and in the capillary bed located in the inner retina (microangiopathic injury). Although microcirculatory impairment is the classic hallmark of DR, recent evidence suggests that retinal neurodegeneration is an early event in the pathogenesis of DR [33,34]. The characteristics features of neurodegeneration are neuronal apoptosis and glial dysfunction, whereas early microvascular abnormalities are characterized by blood-retinal barrier (BRB) breakdown, altered microvascular hemodynamic response (impaired neurovascular coupling) and vasoregression (loss of pericytes and endothelial damage) [34-38] See Figs. (1 and 2).
The multifaceted metabolic and functional alterations implicated in the development of DR suggest that those treatments targeting multiple pathophysiological mechanisms may be more effective in preventing disease progression than those blocking only one pathogenic pathway.
Systemic treatment for diabetic retinopathy
As mentioned earlier, tight control of glycemia and blood pressure are established and essential treatments in preventing and arresting the progression of DR. These strategies are not discussed here.
Recent clinical trials have highlighted RAS blockade and fenofibrate as promising systemic treatments for DR [24-27]. These treatments should be added to CaD, an angioprotective drug approved for DR, although its mode of action is still under investigation and clinical outcomes associated with its use require further clarification.
Renin-angiotensin system blockade
Observational and clinical trials have revealed that blood pressure is an important modifiable risk factor for DR and that the treatment of hypertension significantly reduces the development and progression of DR in both type 1 and type 2 diabetic patients [39, 40]. The blockade of the RAS with an angiotensin converting enzyme (ACE) inhibitor or an angiotensin II type 1-receptor (AT1-R) blocker, is one of the most common strategies for the management of hypertension in diabetic patients.
The major components of the RAS have been identified not only in the kidney but also in ocular tissues and they are overexpressed in the diabetic retina [41]. In addition, growing evidence shows that RAS activation in the eye plays a key role in the pathogenesis of DR [41]. For this reason, theoretically, along with lowering blood pressure, the blockade of the RAS could have an extra value per se in reducing the development and progression of DR.
Mechanisms of Action
The mechanisms by which RAS activation participates in the pathogenesis of DR are summarized in Fig. (3) [41-49]. Angiotensin II (AT) binds and activates two primary receptors, AT1-R and AT2-R. In adult humans, activation of the
AT1-R expressed in endothelial cells and pericytes dominates the pathologic states [41]. AT1-R activation by AT produced by the retina stimulates several pathways involved in the pathogenesis of DR such as inflammation, oxidative stress, AGEs accumulation, neurodegeneration, leukostasis, and several crucial mediators of angiogenesis such as VEGF and platelet-derived growth factor [41-44]. Most of these pathogenic actions are inhibited or attenuated by pharmacological blockade of the RAS either at the levels of ACE or the AT receptors and are associated with the downregulation of VEGF and VEGF receptor-2 [41]. Kim et al. [45] showed that perindopril (an ACE inhibitor) attenuated VEGF-mediated BRB breakdown in rats with streptozotocin-induced diabetes mellitus (STZ-DM). It is also noteworthy
that candesartan (an AT-R blocker) inhibited retinal accumulation of the AGE product pentosidine in spontaneously diabetic Torii rats [46]. Besides reducing microvascular disease, recent research points to neuroprotection as a relevant mechanism involved in the beneficial effects of AT-R blockers in DR [47-49].
Clinical Evidence
The studies in type 2 diabetic patients with hypertension suggest that ACE inhibitors and AT-R blockers are not superior as regards preventing or arresting DR compared to other drugs which are as effective in reducing blood pressure such as the β-blocker atenolol [50] or the calcium channel blocker nisoldipine [51]. These prospective randomized studies suggest that lowering blood pressure is of far greater significance than the local effect of RAS blockade in the diabetic eye. However, there has been considerable debate concerning the potential beneficial effect of RAS blockers in normotensive diabetic patients.
In the EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus (EUCLID) report, lisinopril (an ACE inhibitor) did not reduce the incidence of DR in normotensive patients (blood pressure ≤ 140/90 mmHg), whether they were normoalbuminuric (85% of patients) or microalbuminuric. However, lisinopril decreased DR progression by 2 or more grades and decreased progression to PDR [52]. These results have been criticized because the placebo group had significantly higher levels of mean HbA1c than the treatment group. Indeed, after adjusting for baseline HbA1c, only the progression by one level of DR remained significant. Study limitations included the short follow-up period of 2 years and that DR was not the primary endpoint. Consequently, while the EUCLID study suggested a potential additional benefit of ACE inhibitors per se on DR progression, the study was underpowered for the eye-related outcomes. In addition, in normotensive type 2 diabetic patients in the Appropriate Blood Pressure Control in Diabetes (ABC) study, Schrier et al. [53] demonstrated that intensive blood pressure control with enalapril (an ACE inhibitor) or nisoldipine as the initial antihypertensive agent resulted in the same decrease in the progression of DR. Therefore, the choice of antihypertensive agent appears to be less significant than the attaining of the lower blood pressure targets.
A more robust research program into this was the Diabetic Retinopathy Candesartan Trials (DIRECT) which consisted of two studies conducted in type 1 diabetic patients [24] (a primary prevention study, the DIRECT-Prevent 1; and a secondary prevention study, the DIRECT-Protect 1) as well as a prevention study conducted in type 2 diabetes: DIRECT-Protect 2 [25]. In the DIRECT-Prevent 1 study, 1241 normoalbuminuric and normotensive (blood pressure ≤ 130/85 mmHg) type 1 diabetic patients without DR were randomly assigned to receive candesartan or placebo, with median follow-up of 4·7 years [24]. This study showed a non-significant reduction (18% relative risk reduction [RRR], P = 0·051) in the incidence of DR. However, in a post hoc analysis in which the primary endpoint was changed from a 2-step increase to at least a 3-step increase in the Early Treatment Diabetic Retinopathy Study (ETDRS) scale, a significant difference was detected (35% RRR, P = 0·003). This beneficial effect was attenuated but still significant after the data were adjusted for the duration of diabetes, HbA1c, and systolic blood pressure (26% RRR, P = 0·046). The DIRECT-Protect 1 study in which 1905 type 1 diabetic patients with mild or moderate DR were included, showed that candesartan was not effective in preventing DR progression [24]. Similarly, candesartan failed to reduce the progression of DR in DIRECT-Protect 2, in which 1905 type 2 diabetic patients with DR were included [25]. Overall, the DIRECT program failed to demonstrate any beneficial effect of candesartan, taking into account the primary endpoints. Additionally, the Action in Diabetes and Vascular Disease (ADVANCE) study [54], which included 11,140 type 2 diabetic patients randomly assigned to intensive glucose control using gliclazide (modified release), along with other drugs necessary for achieving HbA1c ≤ 6.5% and an ACE inhibitor-diuretic combination (perindopril-indapamide), presented the same four-year incidence or progression of DR as the placebo group. These results do not support the hypothesis that RAS blockers have an additional benefit with respect to DR protection in comparison with other antihypertensive agents. In contrast, the classic concept that lowering the blood pressure is the most important strategy regardless of the choice of drug has emerged again with renewed rigor.
Fenofibrate
In recent years, 2 major prospective randomized controlled trials (the FIELD [26,55] and the ACCORD-Eye [27] studies) have shown that DR progression in type 2 diabetes is significantly reduced by fenofibrate, a peroxisome proliferator activated-receptor alpha (PPARα) used as a hypolipidemic agent.
Mechanisms of Action
The mechanisms by which fenofibrate exerts its beneficial effects in DR remain to be fully elucidated. The main action of fenofibrate is to lower plasma triglyceride, but it also reduces total and low-density lipoprotein (LDL) cholesterol, raises apolipoprotein A1 (apoA1) and high-density lipoprotein cholesterol (HDL), and reduces the concentration of small dense LDL particles and apolipoprotein B. In the FIELD and ACCORD-Eye studies there was no relationship between the quantitative changes of serum lipids (total cholesterol, HDL, LDL and triglyceride) and the effects on DR. However, it is unknown whether the effectiveness of fenofibrate in modulating the qualitative properties of lipoproteins (i.e. reducing remnants and small LDL particles) or in lowering lipoprotein-associated phospholipase A2 can contribute to its beneficial effects [56,57]. Moreover, the potential effect of fenofibrate in regulating intraretinal lipid transport might be more important than its systemic action [58]. Finally, it has recently been shown that circulating apoA1 may be an independent protective factor for the development of DR [59]. Therefore, it is possible that the increase in apoA1 plasma levels induced by fenofibrate could be added to the lipidic-mediated mechanisms involved in its beneficial action on DR.
The hypolipidemic actions of fenofibrate seem more important in accounting for its effect in reducing the progression of DR than the lipidic-mediated mechanisms. These mechanisms have been recently reviewed [57,60] and are summarized in Fig. (4) [61-66].
Clinical Evidence
The FIELD study [55] was a placebo-controlled trial primarily designed to assess the effects of fenofibrate on major cardiovascular outcomes. In total, 9795 patients were included, of whom 4895 were treated with fenofibrate. After 5 years, fenofibrate had not significantly reduced the primary study outcome, a composite of coronary heart disease death and myocardial infarction (11% RRR vs. placebo, P = 0·16). However, the overall incidence of cardiovascular events was significantly reduced (11% RRR, P = 0·035). One of the prespecified tertiary endpoints of the study was the necessity of laser photocoagulation. Among the 814 patients (8%) with DR at baseline, 3.6% of the patients treated with fenofibrate versus 5.2% in the placebo group required laser therapy over 5 years (P = 0·0003). There was greater absolute benefit in patients with, rather than without, pre-existing DR [26]. Nevertheless, two caveats exist. First, retinal photographs were only obtained from a 10% subsample. Since the initial status of the retina is the main determinant of the need for laser therapy during follow-up, knowing the status of the retina at study entry is of crucial importance. Second, the criteria followed by the participating centers for undertaking laser treatment were not defined at study entry and, consequently, were likely to have been heterogeneous [58].
The FIELD study also included an ophthalmology sub-study in which standardized fundus photographs were routinely taken at baseline, 2 years and 5 years, and graded with ETDRS criteria [26]. A total of 1012 patients without evidence of clinically significant retinopathy (PDR or severe NPDR), DME, or a history of laser treatment at baseline were included. As in the main trial, there was a significant reduction in laser treatment for DR with fenofibrate versus placebo (from 4·6%-1·0%, P = 0·0004). However, only 28 patients required laser treatment (23 in the placebo and 5 in the fenofibrate group). A significant reduction in DR progression (3 or more steps on the ETDRS) was observed in those patients with DR at baseline but not in those without DR at baseline (14·6% -3·1%, P = 0·004).
The ACCORD trial was also primarily a cardiovascular outcome study, evaluating whether the intensification and extension of current treatment approaches beyond those already recommended by current guidelines (i.e. intensive vs. standard control of blood glucose or blood pressure, or adding fenofibrate against a background of simvastatin treatment) could be beneficial. A combined treatment with fenofibrate plus simvastatin did not have a significant effect on cardiovascular outcomes in ACCORD Lipid [67]. Diabetic retinopathy outcomes were evaluated in the 4-year ACCORD-Eye sub-study [27]. In contrast to FIELD, the patients in this study had a longer duration of diabetes (mean 10·0 years vs. median 5·1 years) and a higher prevalence of pre-existing DR (50% vs. 8%) at baseline. However, the overall results of the ACCORD-Eye study were consistent with those observed in the FIELD study. Treatment with fenofibrate (n = 806) was associated with a 40% decrease in the progression of DR, defined as 3 or more steps on the ETDRS scale or PDR that required either laser or vitrectomy treatment (10·2% - 6·5%, P = 0·006). There was no effect on the rate of moderate vision loss. Once again, the benefit was greater in patients with evidence of retinopathy at baseline (absolute risk reductions 6·9% vs. 0·2% in those without DR at baseline).
In summary, 2 major clinical trials demonstrated a consistent effect for fenofibrate on DR progression, with a relative reduction of 30% to 40% over four to five years. In both studies, patients with pre-existing DR derived greater benefit. The number needed to treat (NNT) to prevent first laser treatment in the main FIELD study was 17; to prevent DR progression in the FIELD ophthalmology sub-study the NNT was 9, and 14 in ACCORD Eye. Taking these data into consideration it seems reasonable to suggest the use of fenofibrate to prevent DR progression in patients with preexisting disease. However, to-date, fenofibrate for DR treatment has been approved only in Australia.
Calcium dobesilate
Calcium dobesilate monohydrate is indicated and approved for the treatment of DR in several countries, with 2 randomized placebo-controlled trials demonstrating its efficacy on the progression of early DR [68,69]. However, it has not been widely used in clinical practice and further research is still needed to elucidate its pleiotropic mechanism of action.
Mechanisms of Action
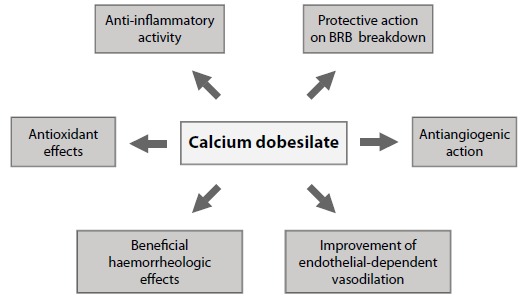
Calcium dobesilate monohydrate has shown multifaceted pharmacological effects believed to interfere with the main pathophysiological pathways of DR [70], and it has been reported that long-term treatment delayed the progression of DR in STZ-DM rats [71]. The main effects of CaD in the metabolic pathways involved in the pathogenesis of DR are shown in Fig. (5).
The antioxidant and anti-inflammatory properties of CaD have been extensively investigated [72-76]. Among these mechanisms the inhibition of Nuclear Factor KB (NF-KB)- and p38 Mitogen-activated protein kinases (p38 MAPK)-mediated leukocyte adhesion to retinal vessels, the reduction of expression of adhesion molecules such as Intercellular Adhesion Molecule 1 (ICAM-1) [77], and reductions in C-reactive protein in patients with DR should be discussed [75].
The protective effects of CaD on the inner BRB disruption have been reported by Rota et al. [78] who observed that CaD can prevent albumin leakage from the retinal vessel of STZ-DM rats and that this effect was associated with a reduced retinal VEGF expression in treated animals compared to control. More recently, Leal et al. [77] have shown in STZ-DM rats that CaD prevented BRB breakdown by restoring tight junction protein levels and organization, as well as decreasing leukocyte adhesion to retinal vessels. These protective effects of CaD were associated with a decrease of p38 MAPK and NF-KB activation, perhaps by inhibiting oxidative/nitrosative stress. There is no evidence yet on the potential effects of CaD in the retinal pigment epithelium, which constitutes the outer BRB.
The improvement of endothelial-dependent vasodilatation (nitric oxide-dependent) is another mechanism of action of CaD [79,80] Calcium dobesilate was also shown to inhibit endothelin-1 in patients with DR [81], which can play a role in ameliorating vasodilatation impairment. A promising field of research would be to assess whether the inhibitory action of CaD on endothelin also occurs locally in the retina. This could be important for explaining not only the improvement in microvascular hemodynamic, but also the amelioration of retinal neurodegeneration associated with diabetes. In this regard, it should be noted that endothelin has recently been shown to be involved in diabetes-induced neuroretinal degeneration and, therefore, its inhibition seems a promising therapeutic target in DR [82-84].
The antiangiogenic effect of CaD is supported by studies showing its capacity in inhibiting several growth factors such as VEGF and fibroblast growth factor [85,86]. In addition, CaD abrogates choroidal angiogenesis both in vitro and ex vivo in a dose-dependent manner [87]. Furthermore, Demirtas et al. [88] have shown in a chick chorioallantoic membrane model that CaD has a potent antiangiogenic effect with a dose-dependent action.
Finally, the beneficial hemorrheologic effects are based on the evidence of CaD in reducing blood hyperviscosity and platelet aggregation, as well as decreasing red blood cell hyperaggregation and increasing fibrinolysis [89-96]. These are established pharmacological effects that account for capillary perfusion amelioration and inflammatory status reduction [96]. CaD has also been shown to reduce the prothrombotic state through reduced platelet aggregation and the synthesis of platelet activating factor [97].
In summary, CaD abrogates multiple pathogenic pathways involved in DR. This should be contemplated as a significant advantage in treating a disease in which multiple pathways are simultaneously activated by hyperglycemia. However, further research is recommended to better understand the mechanisms by which CaD has beneficial effects in DR.
Clinical Evidence
The present evidence supports the beneficial role of CaD in the early stages of the disease, in stabilizing the BRB and preventing or delaying the progression to more advanced stages (severe NPDR and PDR), thus potentially reducing the need for laser photocoagulation [28]. The effect of CaD on the progression of early DR is shown in two randomized placebo-controlled studies [68, 69].
In the study by Leite et al. [68] the effect of CaD on the alteration of the BRB was studied in 41 adult-onset, type 2 diabetic patients with minimal or no retinopathy. Patients were randomly assigned to receive either oral 2 g CaD (two 500 mg capsules, twice daily) or a placebo, for 12 months. The integrity of the BRB was assessed by vitreous fluorophotometry (posterior vitreous penetration ratio [PVPR]), with CaD shown to prevent leakage due to the breakdown of the BRB. In addition, no adverse events were reported as related to CaD.
In the Ribero et al. study [69] vitreous fluorophotometry (primary endpoint PVPR) was also used to assess BRB permeability in type 2 diabetic patients with early stages of DR. The sample size was higher (194 at study entry, 137 at completion) and the follow-up was longer (2 years) than the study by Leite et al. [68] The authors concluded that CaD (2 g daily for two years) showed a significantly better activity than placebo in preventing BRB disruption, independent of diabetes control. A further analysis of the secondary parameters revealed significant changes from baseline to the last visit in favor of CaD, with respect to hemorrhages (P = 0·029), DR level (P= 0·0006) and microaneurysms (P = 0·013). Finally, this study showed that CaD has a good benefit-risk profile.
A randomized, double-blind, placebo-controlled, multicentre study (40 centers in 11 countries; the CALDIRET study) conducted in 635 type 2 diabetic patients with mild-to-moderate NPDR presenting at the first visit with microalbuminuria and with a follow-up period of five years, showed that CaD did not reduce the risk of the development of CSME [98]. The drop-out rate was high with only 150 patients completing the 5-year follow-up. More men were assigned to the CaD group than to the placebo group. Exploratory post-hoc analysis to ascertain the effect of gender bias showed positive results in the CaD group for a subgroup of women with HbA1c ≥ 9%; this was even more evident when poorly controlled hypertension was linked. No relevant adverse drug reactions were noted. The main differences in the characteristics of the patients included in this study in comparison with the 2 previously mentioned were the inclusion of patients with more advanced stages of DR and microalbuminuria as per inclusion criteria. In addition, a lower dose of CaD was used (1·5 g/day).
Finally, a recent systematic review and meta-analysis have shown that CaD was significantly associated with improving retinal microaneurysms, hemorrhages, exudates, as well as reduction of whole blood and plasma viscosity [99].
Overall the current evidence suggests that CaD is beneficial in the very early stages of DR but its effectiveness in more advanced stages remains to be proven.
Summary of current evidence and new perspectives
The paucity of relevant clinical studies investigating and testing new drugs in DR is due partly to the need for long-term studies to be conducted in large cohorts of patients with diabetes, which requires standardized masked grading of retinal photographs. The duration of the trial should be consistent with the natural history of DR and, consequently, at least five years seems to be necessary for separating the mechanisms of DR in the intervention and control groups. In addition, most clinical trials have had the aim of evaluating the progression of DR, whereas there have been few studies targeting prevention.
The current clinical evidence does not support the concept that RAS blockers have an extra value in preventing or arresting the progression of DR in hypertensive diabetic patients when compared with other anti-hypertensive agents. In addition, in normotensive type 2 diabetic patients candesartan failed to reduce either the incidence of DR or its progression. Fenofibrate has been useful in arresting the progression of DR but not in preventing its development. Finally, CaD seems effective in the very early stages of DR but not in more advanced stages. Further studies are needed to confirm this scenario and both diabetologists and ophthalmologists should work together not only in future research, but also in establishing international clinical guidelines in which the role of these drugs can be incorporated.
Optical coherence tomography (OCT), used for monitoring the development of DME, could be very useful to assess the effect of medical treatments in the early stages of DR. In addition, flicker-induced vasodilatation coupled to multifocal electroretinography/Fourier Domain-OCT could help in examining their potential beneficial actions on the neurovascular unit. These methods could permit us to examine the early effect of these drugs in DR, thus reducing the follow-up period and, consequently, the associated economic burden.
Finally, topical administration with eye-drops could provide a new approach in the treatment of DR. This could be an effective route for DR treatment with the advantage of overcoming the BRB and minimizing potential systemic adverse effects. The ongoing clinical trial EUROCONDOR (http://eurocondor.eu) should provide the scientific community with some information regarding the potential effectiveness of eye-drops containing neuroprotective agents, in the early stages of DR.
In summary, systemic treatment with drugs that specifically address the reduction in incidence and progression of DR, added to current standards of tight glycemic and blood pressure control could be very useful in improving public health and clinical outcomes and reducing the burden of DR. In addition, clinical trials aimed at exploring the eventual beneficial effect of these drugs in enhancing the effectiveness of treatments used in advanced stages of DR (i.e. laser photocoagulation, intravitreal injection of anti-VEGF agents) are required. Finally, further efforts in understanding the mechanisms of action, as well as in answering key clinical questions (who to give to, when to start, how long to treat) are urgently needed.
Fig. (1).
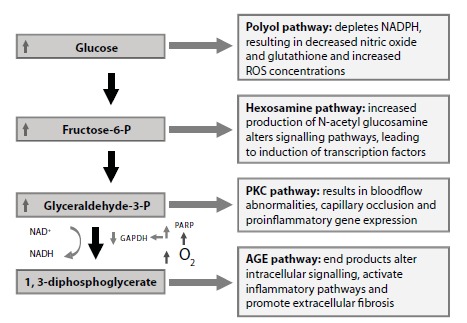
Pathogenic molecular pathways involved in the pathogenesis of diabetic retinopathy. Abbreviations: AGE, Advanced glycation end-product; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; NAD+, Nicotinamide adenine dinucleotide (oxidised); NADH, Nicotinamide adenine dinucleotide (reduced); NADPH, Nicotinamide-adenine dinucleotide phosphate (reduced); O2, Oxygen; PARP, Poly ADP ribose polymerase; PKC, Protein kinase C; ROS, Reative oxygen species.
Fig. (2).
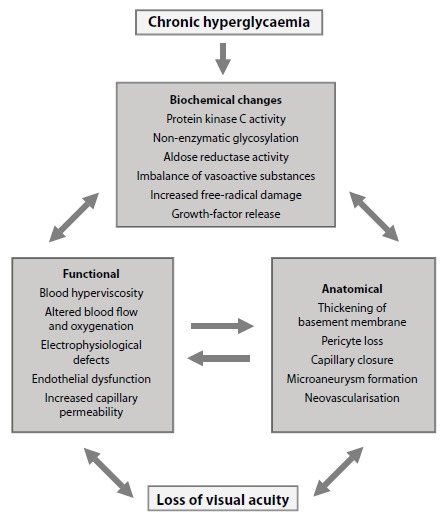
Pathogenic mechanisms involved in the development of diabetic retinopathy.
Fig. (3).
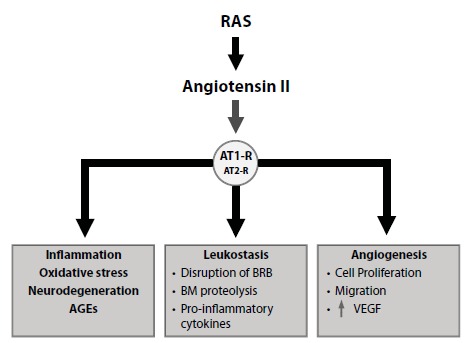
Renin-Angiotensin-System activation in the pathogenesis of diabetic retinopathy. RAS activation leads to AT-Rs activation (mainly AT1-R) which trigger essential pathways involved in the pathogenesis of DR such as inflammation, oxidative stress, neurodegeneration, AGEs formation, leucostasis (which is crucial for the release of proinflammatory cytokines and the breakdown of the BRB) and angiogenesis. Abbreviations: AGE, Advanced glycation end-product; Angiotensin II, Angiotensin type II; AT1-R, Angiotensin II type 1 receptor; AT2-R, Angiotensin II type 2 receptor; BRB, Blood-retinal barrier; BM, Basement membrane; RAS, Renin-Angiotensin System; VEGF, Vascular endothelial growth factor.
Fig. (4).
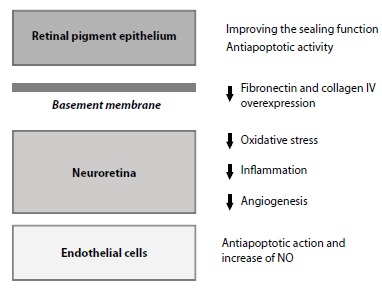
Effects of fenofibrate in diabetic retinopathy. This figure summarizes the reported effects of FA (the active metabolite of fenofibrate) on several retinal components such as RPE, BM, neuroretina and endothelial cells. In RPE (outer blood retinal barrier), FA prevents the disorganisation of tight junctions proteins and the hyperpermeability. In addition, FA elicits a dual protective effects by down-regulation of stress-mediated signalling and induction of autophagy and survival pathways. In BM, FA downregulates the overexpression of fibronectin and collagen IV, thus further reducing the increase in permeability. In the neuroretina FA exerts antioxidant, antinflammatory and antiangiogenic actions. Finally, in the endothelial cells, FA induces an antiapoptotic action and stimulates NO synthase phosphorylation and NO production. Abbreviations: BM, basement membrane; FA, Fenofibric acid; RPE, retinal pigment epithelium; NO, nitric oxide.
Fig. (5).
Multifaceted effects of calcium dobesilate in essential pathways involved in the pathogenesis of diabetic retinopathy. Abbreviation: BRB, blood-retinal barrier.
ACKNOWLEDGEMENTs
Declared none.
CONFLICT OF INTEREST
Stefania Ballarini is an employee of Vifor Pharma/OM Pharma, the makers of calcium dobesilate. The other authors report no conflicts of interest in regard to this work.
REFERENCES
- 1.Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation . IDF Diabetes Atlas. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. [PubMed] [Google Scholar]
- 3.Klein B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 4.Yau J.W., Rogers S.L. Kawasaki. R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; Haffner, S.; Hamman, R.F.; Ikram, M.K.; Kayama, T.; Klein, B.E.; Klein, R.; Krishnaiah, S.; Mayurasakorn, K.; O'Hare, J.P.; Orchard, T.J.; Porta, M.; Rema, M.; Roy, M.S.; Sharma, T.; Shaw, J.; Taylor, H.; Tielsch, J.M.; Varma, R.; Wang, J.J.; Wang, N.; West, S.; Xu, L.; Yasuda, M.; Zhang, X.; Mitchell, P.; Wong, T.Y. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and majority risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung N., Mitchell P., Wong T.Y. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 6.Lee L.J., Yu A.P., Cahill K.E., Oglesby A.K., Tang J., Qiu Y., Birnbaum H.G. Direct and indirect costs among employees with diabetic retinopathy in the United States. Curr. Med. Res. Opin. 2008;24:1549–1559. doi: 10.1185/030079908x297303. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier E.M., Shim B., Ben-Joseph R., Caro J.J. Economic outcomes associated with microvascular complications of type 2 diabetes mellitus: results from a US claims data analysis. Pharmacoeconomics. 2009;27:479–490. doi: 10.2165/00019053-200927060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Heintz E., Wirehn A.B., Peebo B.B., Rosenqvist U., Levin L.A. Prevalence and healthcare costs of diabetic retinopathy: a population-based register study in Sweden. Diabetologia. 2010;53:2147–2154. doi: 10.1007/s00125-010-1836-3. [DOI] [PubMed] [Google Scholar]
- 9.Pascolini D., Mariotti S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 10.National Eye Institute of the National Institutes of Health Facts about diabetic retinopathy at.
- 11.van Leiden H.A., Dekker J.M., Moll A.C., Nijpels G., Heine R.J., Bouter L.M., Stehouwer C.D., Polak B.C. Blood pressure, lipids, and obesity are associated with retinopathy: the hoorn study. Diabetes Care. 2002;25:1320–1325. doi: 10.2337/diacare.25.8.1320. [DOI] [PubMed] [Google Scholar]
- 12.Xu J., Wei W.B., Yuan M.X., et al. Prevalence and risk factors for diabetic retinopathy: the Beijing Communities Diabetes Study 6. Retina. 2012;32:322–329. doi: 10.1097/IAE.0b013e31821c4252. [DOI] [PubMed] [Google Scholar]
- 13.Moss S.E., Klein R., Klein B.E. Ten-year incidence of visual loss in a diabetic population. Ophthalmology. 1994;101:1061–1070. doi: 10.1016/s0161-6420(94)31217-6. [DOI] [PubMed] [Google Scholar]
- 14.The Diabetes Control and Complications Trial Research Group Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. Diabetes. 1997;46:1829–1839. [PubMed] [Google Scholar]
- 15.Pate A., ADVANCE Collaborative Group MacMahon, S.; Chalmers, J.; Neal, B.; Woodward, M.; Billot, L.; Harrap, S.; Poulter, N.; Marre, M.; Cooper, M.; Glasziou, P.; Grobbee, D.E.; Hamet, P.; Heller, S.; Liu, L.S.; Mancia, G.; Mogensen, C.E.; Pan, C.Y.; Rodgers, A.; Williams, B. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 16.Keenan H.A., Costacou T., Sun J.K., Doria A., Cavellerano J., Coney J., Orchard T.J., Aiello L.P., King G.L. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care. 2007;30:1995–1997. doi: 10.2337/dc06-2222. [DOI] [PubMed] [Google Scholar]
- 17.Gaede P., Lund-Andersen H., Parving H.H., Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 18.Simó R., Hernández C. Advances in the medical treatment of diabetic retinopathy. Diabetes Care. 2009;32:1556–1562. doi: 10.2337/dc09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford J.A., Lois N., Royle P., Clar C., Shyangdan D., Waugh N. Current treatments in diabetic macular edema: systematic review and meta-analysis. BMJ Open. •••;3:e002269. doi: 10.1136/bmjopen-2012-002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The PKC-DRS Study Group The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kibase C beta inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54:2188–2197. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 21.PKC-DRS2 Group Aiello, L.P.; Davis, M.D.; Girach, A.; Kles, K.A.; Milton, R.C.; Sheetz, J.; Vignati, L.; Zhi, X.E. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113:2221–2230. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 22.PKC-DMES Study Group Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Arch. Ophthalmol. 2007;125:318–324. doi: 10.1001/archopht.125.3.318. [DOI] [PubMed] [Google Scholar]
- 23.Palii S.S., Caballero S., Shapiro G., Grant M.B. Medical treatment of diabetic retinopathy with somatostatin analogues. Expert Opin. Investig. Drugs. 2007;16:73–82. doi: 10.1517/13543784.16.1.73. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi N., Porta M., Klein R., et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 25.Sjølie A.K., Klein R., Porta M., Orchard T., Fuller J., Parving H.H., Bilous R., Chaturvedi N., DIRECT Programme Study Group Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372:1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 26.Keech A.C., Mitchell P., Summanen P.A., O'Day J., Davis T.M., Moffitt M.S., Taskinen M.R., Simes R.J., Tse D., Williamson E., Merrifield A., Laatikainen L.T., d'Emden M.C., Crimet D.C., O'Connell R.L., Colman P.G., FIELD study investigators Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 27.ACCORD Study Group. ACCORD Eye Study Group Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; Perdue, L.H.; Goff, D.C. Jr.; Cushman, W.C.; Ginsberg, H.N.; Elam, M.B.; Genuth, S.; Gerstein, H.C.; Schubart, U.; Fine, L.J. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garay R.P., Hannaert P., Chiavaroli C. Calcium dobesilate in the treatment of diabetic retinopathy. Treat. Endocrinol. 2005;4:221–232. doi: 10.2165/00024677-200504040-00003. [DOI] [PubMed] [Google Scholar]
- 29.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 30.Tarr J.M., Kaul K., Chopra M., Kohner E.M., Chibber R. Pathophysiology of diabetic retinopathy. 2013. [DOI] [PMC free article] [PubMed]
- 31.Kern T.S. Contributions of inflammatory processes to the development of early stages of diabetic retinopathy. 2007. [DOI] [PMC free article] [PubMed]
- 32.Tang J., Kern T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simó R., Hernández C. Neurodegeneration is an early event in diabetic retinopathy: therapeutic implications. Br. J. Ophthalmol. 2012;96:1285–1290. doi: 10.1136/bjophthalmol-2012-302005. [DOI] [PubMed] [Google Scholar]
- 34.Simó R., Hernández C. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014;25:23–33. doi: 10.1016/j.tem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Simó R., Sundstrom J.M., Antonetti D.A. Ocular anti-VEGF therapy for diabetic retinopathy: The role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care. 2014;37:893–899. doi: 10.2337/dc13-2002. [DOI] [PubMed] [Google Scholar]
- 36.Wirostko B., Wong T.Y., Simó R. Vascular endothelial growth factor and diabetic complications. Prog. Retin. Eye Res. 2008;27:608–621. doi: 10.1016/j.preteyeres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Cai J., Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (Lond.) 2002;16:242–260. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- 38.Simó R., Carraco E., García-Ramirez M., Hernández C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr. Diabetes Rev. 2006;2:71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 39.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 40.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKDPS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson-Berka J.L. Angiotensin and diabetic retinopathy. Int. J. Biochem. Cell Biol. 2006;38:752–765. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Kurihara T., Ozawa Y., Ishida S., Okano H., Tsubota K. 2012. [DOI] [PMC free article] [PubMed]
- 43.Miller A. G.; Zhu, T.; Wilkinson-Berka, J.L. The Renin-angiotensin system and advanced glycation end-products in diabetic retinopathy: impacts and synergies. Curr. Clin. Pharmacol. 2013;8:285–296. doi: 10.2174/1574884711308040004. [DOI] [PubMed] [Google Scholar]
- 44.Amano S., Yamagishi S., Inagaki Y., Okamoto T. Angiotensin II stimulates platelet-derived growth factor-B gene expression in cultured retinal pericytes through intracellular reactive oxygen species generation. Int. J. Tissue React. 2003;25:51–55. [PubMed] [Google Scholar]
- 45.Kim J.H., Kim J.H., Yu Y.S., Cho C.S., Kim K.W. Blockade of AT attenuates VEGF-mediated blood-retinal barrier breakdown in diabetic retinopathy. J. Cereb. Blood Flow Metab. 2009;29:621–628. doi: 10.1038/jcbfm.2008.154. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama T., Okuno T., Fukuhara M., Oku H., Ikeda T., Obayashi H., Ohta M., Fukui M., Hasegawa G., Nakamura N. Angiotensin II receptor blocker inhibits abnormal accumulation of advanced glycation end products and retinal damage in a rat model of type 2 diabetes. Exp. Eye Res. 2007;85:406–412. doi: 10.1016/j.exer.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Krikov M., Thone-Reineke C., Müller S., Villringer A., Unger T. Candesartan but not ramipril pretreatment improves outcome after stroke and stimulates neurotrophin BNDF/TrkB system in rats. J. Hypertens. 2008;26:544–552. doi: 10.1097/HJH.0b013e3282f2dac9. [DOI] [PubMed] [Google Scholar]
- 48.Kurihara T., Ozawa Y., Nagai N., Shinoda K., Noda K., Imamura Y., Tsubota K., Okano H., Oike Y. Ishida. S. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes. 2008;57:2191–2198. doi: 10.2337/db07-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva K.C., Rosales M.A., Biswas S.K., Lopes de Faria J.B., Lopes de Faria J.M. Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes. 2009;58:1382–1390. doi: 10.2337/db09-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.UK Prospective Diabetes Study Group Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKDPS 39. BMJ. 1998;317:713–720. [PMC free article] [PubMed] [Google Scholar]
- 51.Estacio R.O., Jeffers B.J., Guifford N., Schrier R.W. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(Suppl. 2):B54–B64. [PubMed] [Google Scholar]
- 52.Chaturvedi N., Sjolie A.K., Stephenson J.M., Abrahamian H., Keipes M., Castellarin A., Rogulja-Pepeonik Z., Fuller J.H. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes: the EUCLID Study Group EURODIAB controlled trial of lisinopril in insulin-dependent diabetes mellitus. Lancet. 1998;35:28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- 53.Schrier R.W., Estacio R.O., Esler A., Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–1097. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 54.ADVANCE Collaborative Group Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot L, Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; Grobbee, D.; Hamet, P.; Harrap, S.; Heller, S.; Liu, L.; Mancia, G.; Mogensen, C.E.; Pan, C.; Poulter, N.; Rodgers, A.; Williams, B.; Bompoint, S.; de Galan, B.E.; Joshi, R.; Travert,F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 55.Keech A., Simes R.J., Barter P., Best J., Scott R., Taskinen M.R., Forder P., Pillai A., Davis T., Glasziou P., Drury P., Kesäniemi Y.A., Sullivan D., Hunt D., Colman P., d'Emden M., Whiting M., Ehnholm C., Laakso M., FIELD study investigators Effect of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 56.Ciudin A., Hernández C., Simó R. Molecular implications of the PPARs in the diabetic eye. 2013. [DOI] [PMC free article] [PubMed]
- 57.Simó R., Roy S., Behar-Cohen F., Keech A., Mitchell P., Wong T.Y. Fenofibrate: a new treatment for diabetic retinopathy. Molecular mechanisms and future perspectives. Curr. Med. Chem. 2013;20:3258–3266. doi: 10.2174/0929867311320260009. [DOI] [PubMed] [Google Scholar]
- 58.Simó R., Hernández C. Fenofibrate for diabetic retinopathy. Lancet. 2007;370:1667–1668. doi: 10.1016/S0140-6736(07)61608-0. [DOI] [PubMed] [Google Scholar]
- 59.Sasongko M.B., Wong T.Y., Nguyen T.T., Shaw J.E., Jenkins A.J., Wang J.J. Novel versus traditional risk markers for diabetic retinopathy. Diabetologia. 2012;55:666–670. doi: 10.1007/s00125-011-2424-x. [DOI] [PubMed] [Google Scholar]
- 60.Wong T.Y., Simó R., Mitchell P. Fenofibrate - a potential systemic treatment for diabetic retinopathy? Am. J. Ophthalmol. 2012;154:6–12. doi: 10.1016/j.ajo.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Villarroel M., Garcia-Ramírez M., Corraliza L., Hernández C., Simó R. Fenofibric acid prevents retinal pigment epithelium disruption induced by interleukin-1β by suppressing AMP-activated protein kinase (AMPK) activation. Diabetologia. 2011;54:1543–1553. doi: 10.1007/s00125-011-2089-5. [DOI] [PubMed] [Google Scholar]
- 62.Miranda S., González-Rodríguez Á., García-Ramírez M., et al. Beneficial effects of fenofibrate in retinal pigment epithelium by the modulation of stress and survival signaling under diabetic conditions. J. Cell. Physiol. 2012;227:2352–2362. doi: 10.1002/jcp.22970. [DOI] [PubMed] [Google Scholar]
- 63.Trudeau K., Roy S., Guo W., Hernández C., Villarroel M., Simó R., Roy S. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest. Ophthalmol. Vis. Sci. 2011;52:6348–6354. doi: 10.1167/iovs.11-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y., Hu Y., Zhou T., Zhou K.K., Mott R., Wu M., Boulton M., Lyons T.J., Gao G., Ma J.X. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am. J. Pathol. 2009;175:2676–2685. doi: 10.2353/ajpath.2009.080945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y., Hu Y., Lin M., Jenkins A.J., Keech A.C., Mott R., Lyons T.J., Ma J.X. Therapeutic effects of PPARα agonists on diabetic retinopathy in type 1 diabetes models. Diabetes. 2013;62:261–272. doi: 10.2337/db11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J., Ahn J.H., Kim H.S., Yu Y.S., Kim H.S., Ha J., Shinn S.H., Oh Y.S. Fenofibrate regulates retinal endothelial cell survival through the AMPK signal transduction pathway. Exp. Eye Res. 2007;84:886–893. doi: 10.1016/j.exer.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 67.Action to Control Cardiovascular Risk in Diabetes Study Group Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff D.C. Jr.; Bigger J.T.; Buse J.B.; Cushman W.C.; Genuth S.; Ismail-Beigi, F.; Grimm, R.H. Jr.; Probstfield, J.L.; Simons-Morton D.G.; Friedewald, W.T. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leite E.B., Mota M.C., Faria de Abreu J.R., Cunha-Vaz J.G. Effect of calcium dobesilate on the blood-retinal barrier in early diabetic retinopathy. Int. Ophthalmol. 1990;14:81–88. doi: 10.1007/BF00154206. [DOI] [PubMed] [Google Scholar]
- 69.Ribeiro M.L., Seres A.I., Carneiro A.M., Stur M., Zourdani A., Caillon P., Cunha-Vaz J.G. DX-Retinopathy Study Group. Effect of calcium dobesilate on progression of early diabetic retinopathy: a randomised double-blind study. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244:1591–1600. doi: 10.1007/s00417-006-0318-2. [DOI] [PubMed] [Google Scholar]
- 70.Berthet P., Farine J.C., Barras J.P. Calcium dobesilate: Pharmacological profile related to its use in diabetic retinopathy. Int. J. Clin. Pract. 1999;53:631–636. [PubMed] [Google Scholar]
- 71.Padilla E., Ganado P., Sanz M., Zeini M., Ruiz E., Triviño A., Ramírez A.I., Salazar J.J., Ramírez J.M., Rojas B., Hoz Rd., Tejerina T. Calcium dobesilate attenuates vascular injury and the progression of diabetic retinopathy in streptozotocin-induced diabetic rats. Diabetes Metab. Res. Rev. 2005;21:132–142. doi: 10.1002/dmrr.487. [DOI] [PubMed] [Google Scholar]
- 72.Szabo M.E., Haines D., Garay E., Chiavaroli C., Farine J.C., Hannaert P., Berta A., Garay R.P. Antioxidant properties of calcium dobesilate in ischemic/reperfused diabetic rat retina. Eur. J. Pharmacol. 2001;428:277–286. doi: 10.1016/s0014-2999(01)01196-7. [DOI] [PubMed] [Google Scholar]
- 73.Hannaert P., Brunnet J., Farine J.C., Garay R.P. Antioxidant angioprotective actions of calcium dobesilate in diabetic rats. Int. J. Angiol. 1999;8:S2–S4. doi: 10.1007/BF01619841. [DOI] [PubMed] [Google Scholar]
- 74.Brunet J., Farine J.C., Garay R.P., Hannaert P. In vitro antioxidant properties of calcium dobesilate. Fundam. Clin. Pharmacol. 1998;12:205–212. doi: 10.1111/j.1472-8206.1998.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 75.Javadzadeh A., Ghorbanihaghjo A., Adl F.H., Andalib D., Khojasteh-Jafari H., Ghabili K. Calcium dobesilate reduces endothelin-1 and high-sensitivity C-reactive protein serum levels in patients with diabetic retinopathy. Mol. Vis. 2013;19:62–68. [PMC free article] [PubMed] [Google Scholar]
- 76.Brunet J., Farine J.C., Garay R.P., et al. Angioprotective action of calcium dobesilate against the increased capillary permeability induced by reactive oxygen species in the rat cavity. Eur. J. Pharmacol. 1998;358:213–220. doi: 10.1016/s0014-2999(98)00604-9. [DOI] [PubMed] [Google Scholar]
- 77.Leal E.C., Martins J., Voabil P., Liberal J., Chiavaroli C., Bauer J., Cunha-Vaz J., Ambrósio A.F. Calcium dobesilate inhibits the alterations in tight junction proteins and leucocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010;59:2637–2645. doi: 10.2337/db09-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rota R., Chiavaroli C., Garay R.P., Hannaert P. Reduction of retinal albumin leakage by the antioxidant calcium dobesilate in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2004;495:217–224. doi: 10.1016/j.ejphar.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 79.Ruiz E., Lorente R., Tejerina T. Effects of calcium dobesilate on the synthesis of endothelium -dependent relaxing factors in rabbit isolated aorta. Br. J. Pharmacol. 1997;121:711–716. doi: 10.1038/sj.bjp.0701184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suschek C., Kolb H., Holb-Bachofen V. Dobesilate enhances endothelial nitric oxide synthase-activity in macro- and microvascular endothelial cells. Br. J. Pharmacol. 1997;122:1502–1508. doi: 10.1038/sj.bjp.0701512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhong H., Guo L. The plasma levels of endothelin in diabetic retinopathy and their changes after treatment with doxium. Hunan Yi Ke Da Xue Xue Bao. 1997;22:56–58. [PubMed] [Google Scholar]
- 82.Minton A.Z., Phatak N.R., Stankowska D.L., He S., Ma H.Y., Mueller B.H., Jiang M., Luedtke R., Yang S., Brownlee C., Krishnamoorthy R.R. Endothelin B receptors contribute to retinal ganglion cell loss in a rat model of glaucoma. PLoS One. 2012;7:e43199. doi: 10.1371/journal.pone.0043199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mi X.S., Zhang X., Feng Q., Lo A.C., Chung S.K., So K.F. Progressive retinal degeneration in transgenic mice with overexpression of endothelin-1 in vascular endothelial cells. Invest. Ophthalmol. Vis. Sci. 2012;53:4842–4851. doi: 10.1167/iovs.12-9999. [DOI] [PubMed] [Google Scholar]
- 84.Chou J.C., Rollins S.D., Ye M., Batlle D.C., Fawzi A.A. Endothelin receptor-A antagonist attenuates retinal vascular and neuroretinal pathology in diabetic mice. Invest. Ophthalmol. Vis. Sci. 2014;55:2516–2525. doi: 10.1167/iovs.13-13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Angulo J., Peiró C., Romacho T., Fernández A., Cuevas B., González-Corrochano R., Giménez-Gallego G., de Tejada I.S., Sánchez-Ferrer C.F., Cuevas P. Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial proliferation, arterial relaxation, vascular permeability and angiogenesis by dobesilate. Eur. J. Pharmacol. 2011;667:153–159. doi: 10.1016/j.ejphar.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 86.Yang W., Yu X., Zhang Q., Wang J., Cui W., Zheng Y., Wang X., Luo D. Attenuation of streptozotocin-induced diabetic retinopathy with low molecular wight fucoidan via inhibition of vascular endothelium growth factor. Exp. Eye Res. 2013;115:96–105. doi: 10.1016/j.exer.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 87.Lameynardie S., Chiavaroli C., Travo P., Garay R.P. Parés- Herbuté N. Inhibition of choroidal angiogenesis by calcium dobesilate in normal Wistar and diabetic GK rats. Eur. J. Pharmacol. 2005;510:149–156. doi: 10.1016/j.ejphar.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 88.Demirtas S., Caliskan A., Guclu O., Yazici S., Karahan O., Yavuz C., Mavitas B. Can calcium dobesilate be used safely for peripheral microvasculopathies that require neoangiogenesis? Med. Sci. Monit. Basic Res. 2013;19:253–257. doi: 10.12659/MSMBR.889427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barras J.P., Graf C. Hyperviscosity in diabetic retinopathy treated with Doxium (calcium dobesilate). Vasa. 1980;9:161–164. [PubMed] [Google Scholar]
- 90.Vojnikovic B. Hyperviscosity in whole blood, plasma, and aqueous humor decreased by doxium (calcium dobesilate) in diabetics with retinopathy and glaucoma: a double-blind controlled study. Ophthalmic Res. 1984;16:150–162. doi: 10.1159/000265311. [DOI] [PubMed] [Google Scholar]
- 91.Guerrini M., Acciavatti A., Pieragalli D., Galigani C., Del Bigo C. A controlled open trial of CD in the treatment of rheological alterations in diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 1985;VII:1–19. [Google Scholar]
- 92.Benarroch I.S., Brodsky M., Rubinstein A., Viggiano C., Salama E.A. Treatment of blood hyperviscosity with calcium dobesilate in patients with diabetic retinopathy. Ophthalmic Res. 1985;17:131–138. doi: 10.1159/000265364. [DOI] [PubMed] [Google Scholar]
- 93.Vinazzer H., Hachen H.J. Influence of calcium dobesilate (Doxium) on blood viscosity and coagulation parameters in diabetic retinopathy. Vasa. 1987;16:190–192. [PubMed] [Google Scholar]
- 94.Vojnikovic B. Doxium (calcium dobesilate) reduces blood hyperviscosity and lowers elevated intraocular pressure in patients with diabetic retinopathy and glaucoma. Ophthalmic Res. 1991;23:12–20. doi: 10.1159/000267080. [DOI] [PubMed] [Google Scholar]
- 95.Guerrini M., Pieragalli D., Acciavatti A., Galigani C., Guideri F., Franchi M., Landini F., Burresi A., Messa G.L., Frigerio C. Calcium dobesilate, hemorheology, fibrinolysis and endothelium. New perspectives on the prevention of diabetic microangiopathy. Clinical pharmacological study. Clin. Ter. 1989;129:271–285. [PubMed] [Google Scholar]
- 96.Allain H., Ramelet A.A., Polard E. Bentué -Ferrer, D. Safety of calcium dobesilate in chronic venous disease, diabetic retinopathy and haemorrhoids. Drug Saf. 2004;27:649–660. doi: 10.2165/00002018-200427090-00003. [DOI] [PubMed] [Google Scholar]
- 97.Bussolino F., Biffignandi P., Arese P. Platelet-activating factor--a powerful lipid autacoid possibly involved in microangiopathy. Acta Haematol. 1986;75:129–140. doi: 10.1159/000206106. [DOI] [PubMed] [Google Scholar]
- 98.Haritoglu C., Kampik A., Ulbig M.W., CALDIRET study group Effect of calcium dobesilate on occurrence of diabetic macular oedema (CALDIRET study): randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2009;373:1364–1371. doi: 10.1016/S0140-6736(09)60218-X. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X., Liu W., Wu S., Jin J., Wang N. Calcum-dobesilate for diabetic retinopathy: a systematic review and meta-analysis. Sci. China Life Sci. 2015;58:101–107. doi: 10.1007/s11427-014-4792-1. [DOI] [PubMed] [Google Scholar]


