Abstract
Background: Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) is a member of the CEA family of cell adhesion proteins that belong to the immunoglobulin superfamily. CEACAM6 is normally expressed on the surface of myeloid (CD66c) and epithelial surfaces. Stiochiomertic expression of members of the CEA family (CEACAM1, 5, 6, 7) on epithelia maintains normal tissue architecture through homo-and hetero-philic interactions. Dysregulated over-expression of CEACAM6 is oncogenic, is associated with anoikis resistance and an invasive phenotype mediated by excessive TGFβ, AKT, FAK and SRC signaling in human malignancies. Methods: Extensive literature review through PubMed was conducted to identify relevant preclinical and clinical research publications regarding CEACAM6 over the last decade and was summarized in this manuscript. Results: CEACAM5 and 6 are over-expressed in nearly 70% of epithelial malignancies including colorectal cancer (CRC), pancreatic ductal adenocarcinoma (PDA), hepatobiliary, gastric, breast, non-small cell lung and head/neck cancers. Importantly, CEACAM6 is a poor prognostic marker in CRC, while its expression correlates with tumor stage, metastasis and post-operative survival in PDA. CEACAM6 appears to be an immune checkpoint suppressor in hematologic malignancies including acute lymphoblastic leukemia and multiple myeloma. Several therapeutic monoclonal antibodies or antibody fragments targeting CEACAM6 have been designed and developed as a targeted therapy for human malignancies. A Llama antibody targeting CEACAM6 is being evaluated in early phase clinical trials. Conclusion: This review focuses on the role of CEACAM6 in the pathogenesis and signaling of the malignant phenotype in solid and hematologic malignancies and highlights its potential as a therapeutic target for anti-cancer therapy.
Keywords: CEACAM6, anoikis resistance, cell adhesion, tumor microenvironment, TGFb, AKT, FAK, SRC, IGF-1R, therapeutic antibodies
A. Introduction
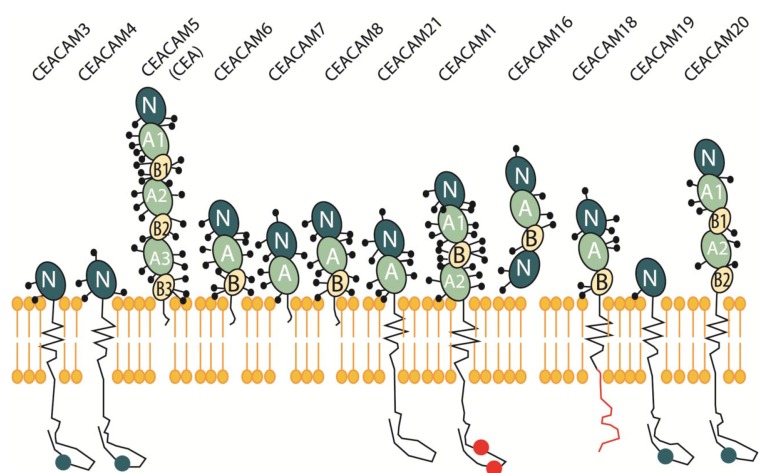
The carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) are a large family of proteins in humans that are encoded by 12 independent genes on chromosome 19q13.1-13.2 (Fig. 1). CEACAMs are known to be highly glycosylated proteins that belong to members of the immunoglobulin (Ig) supergene family and are composed of an N domain (aside from CEACAM16) [1]. Each N domain is subsequently followed by zero or as many as six constant C2-like Ig domains, referred to as A or B [2]. The CEACAM family consists of membrane-linked glycoproteins anchored to the cell surface either by glycophosphatidyl-inositol (GPI) anchor or a transmembrane domain, as well as secretory glycoproteins [3]. The GPI-anchored members of the family include CEACAM5, CEACAM6, CEACAM7 and CEACAM8 [4]. CEACAM1, CEACAM3, CEACAM4, CEACAM19, CEACAM20, and CEACAM21 are all anchored to the cell membrane through transmembrane domains. CEACAMs manifest their function as an intercellular adhesion molecule via GPI anchorage to the cell surface or transmembrane domains.
Fig. (1).
The CEACAM Gene Superfamily. The CEACAM immunoglobulin-like domain superfamily are a large family of proteins encoded by 12 independent genes on chromosome 19q13.1-13.2 consisting of membrane-linked glycoproteins that are anchored to the cell surface either by glycophosphatidyl-inositol (GPI) anchor or a trans-membrane domain, and secretory glycoproteins. These glycosylated proteins show a complex stoichiometric expression pattern in normal versus cancerous tissue. The GPI-anchored members of the family include CEACAM5, CEACAM6, CEACAM7 and CEACAM8. CEACAM1, CEACAM3, CEACAM4, CEACAM19, CEACAM20, and CEACAM21 are all anchored to the cell membrane through trans-membrane domains.
CEACAM5 is a complex macromolecule first described in the late sixties as a gastrointestinal oncofetal protein isolated to fetal expression and malignancy [5]. Since this initial description has since been rejected and CEACAM5 is found to be expressed in normal adult tissue as well as various cancers, mainly the gastrointestinal tract, respiratory, genitourinary tracts and breast [6]. Aside from its function as a cell adhesion molecule, CEACAM5 is also involved in the inhibition of cell differentiation, anoikis and apoptosis inhibition in colon cells, as well as interference of tissue architecture [7]. These processes are regulated by CEACAM5 through its co-localization and the activation of α5ß1 integrin signal transduction via lipid rafts, promoting PI3K/AKT activity [8]. Of all the CEA family members, CEACAM5 is the only protein used as an accepted tumor marker and indicator of recurrence in cancer patients, especially colorectal [9].
CEACAM6 (CD66c) is a multi-functional glycoprotein that mediates homotypic binding with other CEA family members and heterotypic binding with integrin receptors. CEACAM6 functions by organizing tissue architecture and regulation of signal transduction, while aberrant expression leads to the development of human malignancies [10, 11]. It was first discovered in proliferating cells of adenomas and hyperplastic polyps in comparison to benign colonic tissue [12]. In vivo, it is present on granulocytes and epithelia in multiple organs. Expression patterns of CEACAM5 and CEACAM6 in various primary and metastatic tumors were analyzed by immunohistochemistry (IHC) and tissue microarrays (TMAs) utilizing domain specific antibodies to CEACAM5 and CEACAM6. For all tumors investigated (breast, pancreatic, colonic, non-small cell lung carcinoma), CEACAM6 expression was greater than CEACAM5 [13]. Over-expression of CEACAM6 modulates cancer progression through aberrant cell differentiation, anti-apoptosis, cell growth and resistance to therapeutic agents [13]. Anoikis, a subtype of apoptosis, preserves tissue order in multisystem organisms by abrogating uncontrolled cellular proliferation. Resistance to this process has been correlated with CEACAM6 over-expression in multiple malignancies promoting invasion and metastasis thereby representing an acquired advantage of tumor cells directly responsible for an invasive phenotype [14]. In vitro studies have shown that antibodies directed against CEACAM6 on over-expressing cells inhibited migration, invasion, and adhesion [15]. This suggests that interfering with homo-typic and hetero-typic binding would negate anoikis resistance resulting in an anti-invasive and anti-metastatic effect.
Aside from their expression in humans, the CEACAM gene family is also highly preserved in 27 other mammalian species, specifically rats, dogs, cattle, platypus and opossum [16]. However, CEACAM6 is not present in the mouse genome therefore a transgenic mouse model with a human bacterial artificial chromosome that contains components of the human CEA gene family, specifically CEACAM3, CEACAM5, CEACAM6 and CEACAM7 genes has successfully been generated [17]. The expression patterns in this mouse model are very similar to humans both spatially and in relative levels, allowing an avenue for more accurate pre-clinical testing for toxicity evaluation.
Although earlier studies examined the role of CEACAM6 in gastrointestinal malignancies such as colon, and pancreas, numerous other carcinomas (breast, gastric, thyroid, B-ALL, and multiple myeloma) have been shown to over-express CEACAM6 resulting in an increased metastatic potential. This article will serve as a comprehensive review of the role of CEACAM6 in various solid and hematologic malignancies, identifying common and unique pathways suspected to play a central role in the malignant process. Furthermore, targeting CEACAM6 with therapeutic monoclonal antibodies (Mab) provides an opportunity to treat several human malignancies.
B. CEACAM6 biology, expression, and prognostic implications in Cancer
I. CEACAM6 Expression in Epithelial Carcinomas (1 )
Table 1.
Aberrant CEACAM6 activity in Human Malignancies
| Solid Malignancies | Mechanism of Action |
| Colorectal Cancer | Inhibition of terminal differentiation, loss of cellular polarization, distortion of tissue architecture and anoikis resistance |
| Pancreatic Ductal Adenocarcinoma | Increased anoikis resistance; increased metastatic potential through increased IGF-I resulting in expression of proteolytic MMP-2 & MMP-9 ® altering the ECM; increased chemoresistance through SRC-AKT signaling pathway; increased cell proliferation |
| Breast Cancer | Increased cell proliferation, migration and invasion through SRC and AKT signaling |
| Triple Negative | Increased cell proliferation, migration and invasion through SRC and AKT signaling |
| Luminal A | Increased cell proliferation, migration and invasion through SRC and AKT signaling |
| Luminal B | Increased cell proliferation, migration and invasion through SRC and AKT signaling |
| Her2neu+ | SMAD3 phosphorylation was significantly associated with HER2 expression in CEACAM6+ cancers. HER2 signaling via components of TGF-b pathway may mediate CEACAM6 expression. CEACAM6 is a major target gene for SMAD3-mediated TGF-b signaling. SRC and AKT signaling directly play a role in facilitating CEACAM6 induced migration and invasion |
| Cholangiocarcinoma | Increased proliferation, invasion, and acquired chemo-resistance to gemcitabine resulting in increased lymphatic invasion and advanced stage of disease |
| Lung adenocarcinoma | Induces cell proliferation, anchorage independent growth |
| EGFR mutant | Induces cell proliferation, anchorage independent growth |
| EGFR wild type | Induces cell proliferation, anchorage independent growth, results in poorer disease free survival |
| Gastric Cancer | Promotes increased lymph node metastasis, migration and invasion via increase in SRC phosphorylation |
| Medullary Thyroid Carcinoma | Increased cell proliferation, migration, and invasion through matrix remodeling, anchorage independent growth |
| Head and Neck Squamous Cell Carcinoma | Increased tumor growth secondary to a decrease in caspase 3-dependant cell death through the suppression of PI3K/AKT dependent apoptosis |
| Hematologic Malignancies | |
| B-cell Acute Lymphoblastic Lymphoma | Presumed enhancement of anoikis via increased caspase activation as well as up-regulation of the AKT cell survival pathway |
| Multiple Myeloma | Inactivation of CTL (cytotoxic lymphocytes) via binding and cross- linking of CEACAM6 on plasma cells, resulting in T cell unresponsiveness® immune evasion |
Over the last 10 years a tremendous amount of information regarding the expression patterns and potential of CEACAM6 to serve as prognostic indicator as well as a therapeutic target has emerged. In colorectal cancer (CRC), expression patterns of CEACAM6 and CEACAM7 were analyzed with IHC in 35 normal organs and fetal tissue [12]. Of note, 25 colorectal polyps were analyzed and aside from 8 hyperplastic polyps, all adenomas demonstrated CEACAM6 over-expression. The investigators report that CEACAM6 expression was confirmed in two specific cell types: epithelial and
myeloid cells, which include granulocytes, macrophages, and monocytes [18], colonic epithelial cells [19], pneumocytes, bronchiole epithelia [20], pancreatic ducts, tonsil epithelia [21], sweat glands [22], skin and hair follicles [23], squamous epithelia of the esophagus, cervix and tongue [12]. Although CEACAM6 and CEACAM7 had very similar expression patterns in normal colonic mucosa, there is opposite deregulation in hyper-proliferating mucosa, adenomas and carcinomas. CEACAM7 is down-regulated whereas over-expression of CEACAM6 is observed in hyper-proliferating polyps and adenomas. These findings suggest that deregulation and over-expression of CEACAM6 promotes tumor progression and represents some of the earliest molecular changes in the development of CRC.
(A). Colorectal Cancer
In order to further investigate the role CEACAM6 plays in CRC development, the effect of expression patterns of CEACAM5 and CEACAM6 in two known human colon cancer cell lines, SW-1222 and Caco-2 was evaluated. In vivo, over-expression of CEACAM6 in SW-1222 cells produced marked alteration of tissue architecture and differentiation. The level of deregulation of CEACAM5 and CEACAM6 could be responsible for promoting CRC development through a mechanism of anoikis resistance and impaired cellular differentiation. Since previous work revealed CEACAM5 and CEACAM6 expression are up-regulated during preliminary stages of dysplasia in familial adenoma polyposis (FAP) patients with APC mutations [24], the authors concluded CEACAM6 up-regulation in proliferating cells resulted in a functional change in tissue architecture impairing cell differentiation [25].
To evaluate whether CEACAM6, CEACAM1 and CEACAM5 expression were able to provide prognostic information, 243 paraffin-embedded samples of CRC patients who underwent adjuvant therapy with 5-fluorouracil-based chemotherapy were analyzed [26]. Multivariate Cox analysis revealed that CEACAM6 over-expression served as an independent predictor of poor overall survival (OS) (p<0.01) and diminished disease-free survival (p<0.0028). If CEACAM6 over-expression can be correlated with worsened OS and disease free survival (DFS), do expression patterns also reflect metastatic potential? IHC and clinicopathologic analyses were performed on 143 colon cancer patients. Transcriptional and translational levels of CEACAM6 were found to be up-regulated in human colon tumor tissues as opposed to their non-tumor counterparts. Clinicopathologic analysis revealed an impressive correlation between CEACAM6 expression in regard to Duke’s stage and lymph node metastasis (p<0.001). Again, over-expression of CEACAM6 was associated with poor OS (p<0.001) and a reduced recurrence-free survival (RFS) (p<0.001). Interestingly, through the use of invasive assays and CEACAM6 knockdown models via small interfering RNA (siRNA), CRC cells were found to have diminished invasiveness (35%), whereas over-expression resulted in increased invasiveness through the extracellular matrix (ECM) [27]. CEACAM6 knockdown results in an increased E-cadherin promoter activity which suggests that CEACAM6 serves as a crucial regulator of metastatic potential.
Since CEACAM6 expression has been shown to identify high risk populations with CRC further work to validate its potential use as a therapeutic target has been pursued. CEACAM6 was identified as a marker for CRC stem cell isolation and siRNA-mediated silencing on in vitro and in vivo suppressed growth in Caco2 colon cancer cells [28]. CEACAM6 expression was not present in CD133 cells acquired from normal colon, but over-expressed in CD133 cells from colon cancer tissue. In vitro studies revealed that CD133 positive colon cancer cells significantly over-expressed CEACAM6 in a manner similar to that observed in liver metastasis. Also, proliferation and clonogenicity were diminished when CEACAM6 was silenced with siRNA in Caco2 cells. In vivo xenograft studies confirmed that CEACAM6 silencing decreased the metastatic potential of Caco2 cells. These findings support that CEACAM6 over-expression has a relationship with colon cancer stem cells and the fact that gene silencing abrogated tumor growth highlights CEACAM6 as a potential therapeutic target in colon cancer.
(B). Pancreas Cancer
CEACAM6 also plays a significant role in pancreatic cancer. Over-expression of CEACAM6 in PDA cell lines confirmed it as a marker of anoikis resistance. Gene silencing of CEACAM6 with siRNA reversed anoikis resistance in MiaPaca-2 PDA cells [29]. CEACAM6-specific siRNA increased the cell lines susceptibility to caspase-mediated anoikis and reduced AKT phosphorylation. Suppression of CEACAM6 resulted in decreased anoikis resistance which in turn diminished the ability of MiaPaca-2 PDA cells to metastasize in a nude mouse orthotopic xenograft model. Over-expression of CEACAM6 in Capan2 PDA cells that normally do not express CEACAM6 resulted in an amplified resistance to gemcitabine [30]. Gene silencing of CEACAM6 in BxPC3 PDA cells that normally express CEACAM6 resulted in improved susceptibility to gemcitabine through modulation of AKT activity in a Src-dependant manner. Activation of the AKT pathway is associated with protection from factors that promote apoptosis as well as chemotherapeutic sensitivity [31, 32]. These findings correlate CEACAM6 over-expression with an increased metastatic phenotype through elevated SRC activity and matrix metalloproteinase 2 (MMP2) expression (Fig. 2). Hence, CEACAM6 over-expression through activation of the SRC-AKT signaling provides chemo-resistance to gemcitabine in PDA cell lines.
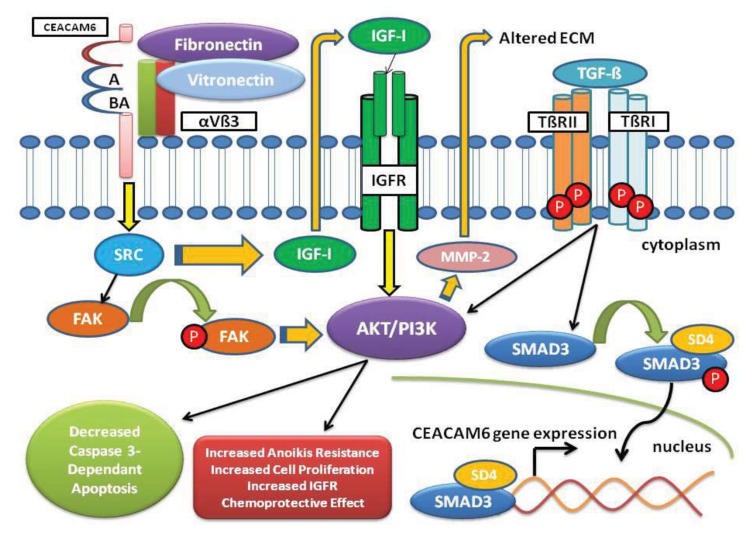
Fig. (2).
CEACAM6 Signaling Pathway. Over-expression of CEACAM6 results in alteration and reorganization of the ECM and activation of the TME playing a key role in tumor invasion. CEACAM6 signaling increases SRC activity leading to increased IGF-I secretion with autocrine and paracrine stimulation of the IGF-1R activating the PI3K/AKT pathway. Increased IGF-I expression results in MMP-2 elaboration, subsequently altering the ECM promoting a malignant TME. Aggregation of CEACAM6 at the cell surface enhances anoikis resistance through activation of SRC in a caveolin-1-dependent manner allowing for phosphorylation of its substrate focal adhesion kinase (FAK). TGF-β elaborated in the TME binds to the type II receptor (TβRII), promoting hetero-tetramerization with the type I receptor (TβRI) increasing the phosphorylation of SMAD3 by activated TβRI. Phosphorylated SMAD3 forms a complex with Co-Smad (SD4), which translocates into the nucleus to bind gene promoters and activate expression of target genes, specifically CEACAM6. Also, TGF-β signals through the TGF-β receptors to activate alternate pathways such as AKT/PI3K.
In a cohort of resected PDA patients, CEACAM6 expression was significantly pronounced in high-grade pancreatic intraepithelial neoplasia (PanIN) specimens compared to low grade PanIN (p=0.0002) [33]. Negative CEACAM6 expression patterns were associated with the absence of lymph node metastases (p=0.012), decreased disease stage (p=0.008) and increased post-operative survival (p=0.047). Therefore, CEACAM6 expression predicts for adverse clinicopathologic features in resected PDAs. Interestingly, parallel to over-expression of CEACAM6 in hyper-proliferating polyps and adenomas playing a role in the development of CRC, the finding of increased expression of CEACAM6 with high grade PanIN lesions suggest that CEACAM6 serves not only as a prognostic biomarker but also a predictive biomarker contributing to the oncogenic transformation of PanIN lesions to invasive PDA.
A hallmark in PDA is the presence of a desmoplastic reaction (DR) secondary to fibrotic tissue proliferation and altered ECM, facilitating tumor growth and increased metastatic potential [34]. The DR is a complex interplay that occurs in the ECM between epithelial and tumor cells, endothelial cells, inflammatory cells, fibroblasts and growth factors/cytokines. DR is postulated in turn to activate autocrine, juxtacrine and paracrine oncogenic signaling pathways augmenting tumor cell proliferation. A continued understanding and targeting key components of the DR may improve therapeutic success in PDA [35]. Alteration and reorganization of the ECM and its components play a pivotal role in tumor cell invasion and is partially achieved by secretion of proteolytic enzymes such as MMP-2. Increased CEACAM6 produces increased insulin-like growth factor I (IGF-I) that results in expression of MMP-2, subsequently altering the ECM and promoting a malignant tumor microenvironment (TME). Further, CEACAM6 over-expression increases SRC activity through IGF-I resulting in increased metastatic potential of PDA cells [36]. Cross-linking of CEACAM6 on BxPC-3 cells results in activation of SRC in a caveolin-1-dependent manner allowing for phosphorylation of its substrate FAK, a non-receptor tyrosine kinase involved with the acquisition of PDA cell resistance to anoikis (Fig. 2).
Tumor cell adhesion to the ECM plays an important role in the development of the TME. CEACAM6 affect the TME through its close interactions with integrins [37]. Integrin aνb3 is intimately involved in adhesion of cancer cells to the ECM, thereby directly impacting cell adhesion, migration, proliferation and survival. Antibody mediated CEACAM6 cross-linking activates neutrophils and promotes their adhesion to the ECM [38]. Studies demonstrated that CEACAM6 cross-linking results in significantly increased attachment to fibronectin and vitronectin [39]. Treatment with an anti- aνb3 integrin Mab suppressed both vitronectin and fibronectin attachment promoted by CEACAM6 cross-linking. These findings suggest that CEACAM6 cross-linking enhances attachment to fibronectin and vitronectin through up-regulation of aνb3 integrin-mediated adhesion (Fig. 2).
(C). Breast Cancer
CEACAM6 expression may represent an early event in the development of human epithelial malignancies. Breast cancer cells elaborate CEACAM6 unlike normal breast tissue. As we have seen in precursor lesions for both CRC and PDA, over-expression of CEACAM6 in atypical ductal hyperplasia was predictive of progression to breast cancer [40]. A retrospective analysis of 243 patient samples revealed that CEACAM6 over-expression correlated with tamoxifen resistance [41]. In a multivariate analysis CEACAM6 over-expression was an independent predictor of disease recurrence [42]. Further, siRNA-mediated gene silencing of CEACAM6 in MMU1 tamoxifen-resistant MCF7 cell line derivatives reversed anchorage independence and re-established endocrine sensitivity [42]. Previous work revealed the development of resistance to tamoxifen in breast tumor cells is related to an augmented invasive potential [43, 44] as well as CEACAM6 over-expression [45]. The relationship between CEACAM6 over-expression and acquired tamoxifen resistance leads to cellular migration and invasion in MCF-7.5C and MCF-7.2A breast cancer cells [46]. CEACAM6 is markedly over-expressed in estrogen-diminished breast cancer cells. These in vitro data were further validated in human breast cancer as CEACAM6 is over-expressed in recurrence in the setting of post-adjuvant endocrine therapy (e.g. tamoxifen).
In a cohort of 840 primary invasive breast cancers and a separate validation cohort of 300 invasive breast cancers CEACAM6 expression was found to be present in 37.1% (312/840 pts) cases [47]. In the validation cohort, CEACAM6 expression was highest in the HER2+ tumors (67%) and lowest in triple negative breast cancer (TNBC) (25%). The patients in this study were followed for a mean of 56.4 months and 105 pts developed distant metastatic disease. Expression rates for CEACAM6 were highest for patients with bone metastasis (42%). There was no difference in OS based upon CEACAM6 expression. Interestingly, high CEACAM6 expression showed a trend towards worse survival in HER2 over-expressed breast cancers. The study reported that adverse prognostic significance of CEACAM6 for OS were high nodal stage HER2+ cancers and were independent of traditional prognostic factors (tumor size, age, grade, treatment modality, ER, Ki-67; p=0.017). One unique feature regarding the signal transduction in HER2+ breast cancer cell lines and CEACAM6 expression is the interaction of TGF-b signaling with the HER2 pathway. The authors evaluated HER2 over-expressed breast cancer cell lines (SK-BR3) treated with TGF-b or EGF with induction of SMAD3 phosphorylation and CEACAM6 expression. CEACAM6 was identified as a major target gene for SMAD3-mediated TGF-b signaling (Fig. 2). SMAD3 phosphorylation was significantly associated with HER2 expression in CEACAM6 positive but not negative cancers. Hence, HER2 signaling via components of TGF- b pathway may mediate CEACAM6 expression.
An emerging theme in regards to CEACAM6 expression has been its ability to identify high risk cohorts and represent a biomarker of response to specific therapies. Over-expression of CEACAM6 is shown to predict response to gemcitabine in PDA and tamoxifen in ER/PR positive breast cancer. CEACAM6 expression also serves as an indicator of response to therapy in Her2+ breast cancer. CEACAM6 under expression identified trastuzumab responsive disease whereas over-expression reflected trastuzumab resistant breast cancer [48]. Interestingly, 25% of the TNBC cohort was also positive for CEACAM6, suggesting this could serve as a potential target in both of these aggressive breast cancer subgroups. The suspected mechanism of action is through activation of the SRC-AKT signaling pathway resulting in a chemo-protective effect. Further investigation is warranted to identify whether CEACAM6 over-expression in metastatic breast cancer patients also serves as a biomarker of response to gemcitabine an approved third line therapy. CEACAM6 expression would then serve as an avenue to identify high risk cohorts of breast cancer patients whose therapy could be specifically tailored based on their underlying tumor biology, highlighting CEACAM6’s role as both prognostic and underscore its potential as a therapeutic target in epithelial carcinomas.
(D). Cholangiocarcinoma
CEACAM6 expression analysis in post-surgical intra-hepatic cholangiocarcinoma patients showed increased node positive (p=0.06) and advanced stage (p=0.09) disease [49]. Patients with high CEACAM6 expression were found to have worse disease free survival than those patients with low expression. In cell lines, flow cytometry revealed that TFK-1 cells had an increased CEACAM6 expression than HuCC-T1 and MEC cell lines investigated that corresponded to an increased resistance to gemcitabine (p<0.01). When CEACAM6 over-expression was induced via gene transfection in HuCC-T1 cells, tumors in mice developed increased proliferation, invasion, and acquired chemo-resistance to gemcitabine. Conversely, siRNA gene silencing of CEACAM6 in TKF-1 cell line resulted in increased chemo-sensitivity to gemcitabine. These results are consistent with the unifying theme noted in epithelial carcinomas that CEACAM6 over-expression serves as marker of aggressive disease in tumors resistant to standard therapies.
(E). Non-small Cell Lung Cancer
The effect of CEACAMs on cell growth and tumor development were reported in lung cancer [50]. In A549 lung cancer cells cellular proliferation is promoted by CEACAM6. This is secondary to interference of the contact inhibitory signals normally released by CEACAM1-4 resulting in undifferentiated anchorage-independent cell growth. A549 lung cancer cells express significant levels of non-membrane anchored CEACAM5 and CEACAM6 providing an explanation for increased expression in lung cancer patients. This mechanism of action regarding CEACAM6 over-expression and its role in contact inhibition has not been clearly described in other epithelial malignancies thereby representing a distinct feature of CEACAM6 in lung cancer.
A549-T lung cancer cells represent a subgroup of the A549 cells found to exhibit an undifferentiated, anchorage independent cell growth pattern. Approximately 10-15% of A549-T lung cancer cells express CEACAM6. The authors proposed that expression of CEACAM6 was directly responsible for cell proliferation by attenuating the CEACAM1-4 regulated contact inhibition. To test their hypothesis, cells were separated into CEACAM6 positive and negative subgroups and expression levels of Ki-67 were evaluated by IHC. Only the CEACAM6 positive subgroup identified a marked elevation of Ki-67 suggesting a clear role for CEACAM6 in inducing cell proliferation. CEACAM6 positive A549 cells have ongoing cell cycling with the majority of cells in S and G2/M phases.
Whether CEACAMs could serve as a surrogate marker for sensitivity to EGFR inhibitor therapy in lung cancer was investigated [51]. Previous work stated that CEACAM6 was significantly diminished by gefitinib therapy and over-expressed in EGFR-mutated lung cancer cell lines, suggesting that CEACAM6 potentially represents a key transcriptional target [52]. CEACAM family members (3, 5, 6, 7, and 19) were each evaluated as potential surrogates of response. In patients with EGFR mutations, all CEACAMs were frequently over-expressed compared to the wild-type EGFR cohort. However, unlike the prior epithelial malignancies discussed, lung adenocarcinoma patients treated with gefitinib were not found to have statistically significant differences based on CEACAM expression and TKI response. Nonetheless, of the 115 EGFR wild type lung adenocarcinoma patients, CEACAM6 over-expressors had an adverse clinical outcome. The 5-year DFS of CEACAM6 negative patients was 74.6%, versus 49.1% of CEACAM6 positive patients. These findings confirm that CEACAM6 serves as both a prognostic indicator and a response biomarker in lung cancer. Also, considering the adverse clinical outcome in CEACAM6 positive lung adenocarcinoma patients, further investigation in regards to targeting this molecule is of strong clinical interest.
(F). Gastric Cancer
CEACAM6 over-expression in gastric cancer cell lines result in an increased metastatic potential compared to knockdown models [53]. Over-expression of CEACAM6 in MKN-45 and SGC-7901 gastric cancer cell lines increased in vivo metastasis in athymic mice, while metastatic potential of MKN-28 and SNU-16 gastric cancer cell lines were suppressed by knockdown of CEACAM6. Of note, SRC phosphorylation was pronounced with CEACAM6 over-expression in SGC-7901 cells.
The over-expression of CEACAM6 in gastric cancer did not find an association with clinicopathologic features [54]. However, level of CEACAM6 DNA in peripheral blood detected by RT-PCR correlated with disease stage [55]. In a cohort of 101 gastric cancer patients 79% showed CEACAM6 over-expression, compared with GES-1 immortalized cells. Over-expression was linked to lymph node metastasis. SNU-16 and MKN-28 cell lines over-express CEACAM6, whereas, SGC 7901 and MKN 45 cells do not. Cell migration was significantly inhibited in CEACAM6 over-expressing cell lines after treatment with anti-CEACAM6 antibody or gene-silencing with siRNA, whereas migratory potential was markedly increased in cell lines genetically modified to over-express CEACAM6. These findings highlight that CEACAM6 promotes gastric cancer cell motility in vitro. Further, over-expression of CEACAM6 promotes invasiveness in vivo after gastric cancer cells are infused into tail veins of mice. Four out five mice were subsequently found to have metastasis to the lung or liver, supporting a role for CEACAM6 mediated metastasis. These findings of an increased metastatic phenotype in gastric cancers support the need for therapeutic targeting of CEACAM6.
(G). Thyroid Cancer
Familial medullary thyroid carcinomas (MTC) and ~30% of sporadic thyroid cancers are known to have RET oncogene mutations [56]. Oncogenic pathways defining non-RET mutated sporadic MTC remains elusive. Pangenomic DNA microarrays of four familial and nine sporadic MTCs demonstrated a composite of 173 genes with 2-fold changes in expression [56]. Two specific groups of sporadic tumors were identified by PCR and IHC analysis. The first of two groups had a molecular profile very close in nature to RET634 (MEN2A mutation) tumors, while the second group displayed a molecular profile similar to that found with RET918 (MEN2B mutation) tumors. The latter group was found to have increased expression of genes previously known to be key players in matrix remodeling (COLIA1, COLIA2), cell adhesion (CEACAM6) and neo-angiogenesis (PTN and ESM1). Hence, sporadic and familial MTC may have similar oncogenic pathways that highlight CEACAM6 playing a pivotal role in altering the ECM and TME, facilitating an aggressive malignant MTC phenotype. Further investigation is needed to identify the role CEACAM6 in MTC.
(H). Head and Neck Cancer
Focal over-expression of CEACAM6 enhances tumor progression in head and neck squamous cell cancer (HNSCC) by inhibiting apoptosis [57]. CEACAM6 mRNA is 177-fold up-regulated in the Detroit 562 cell line and 12-fold up-regulated in the Cal27 cell line as opposed to normal control cells [58]. Highly tumorigenic cell lines (Detroit 562, Cal27 & FaDu) had increased levels of CEACAM6 compared to the poor tumorigenic cell lines (SCC25, SCC9, & SCC15). Interestingly, in vivo studies revealed that CEACAM6 over-expression in Detroit 562 cell line was associated with a significantly diminished level of apoptosis of tumor cells compared to control tumors. This suggests that increased tumor growth in the context of CEACAM6 over-expression was secondary to in vivo abrogation of caspase 3-dependant cell death [57]. Of particular interest was that CEACAM6 over-expressing HNSCC Detroit 562 cancer cells were sensitized to BGT226, a PI3K/AKT inhibitor by CEACAM6 knockdown. However, increased expression of CEACAM6 decreased the sensitivity and maximal response to BGT226. The modulation of gemcitabine sensitivity was found to be through SRC and PI3K/AKT dependent pathway activation. Hence, CEACAM6’s activation of the PI3K/AKT pathway results in cell proliferation and serves as a target for HNSCC.
II. CEACAM6 Expression in Hematologic Malignancies (Table 1)
(A). Acute Lymphoblastic Leukemia
Ectopic expression of CEACAM6 in B-cell lineage acute lymphoblastic leukemia (B-ALL) is established. CEACAM6 mRNA expression in leukemic blasts through quantitative PCR in 135 acute leukemic patients (88 B-ALL, 16 T-ALL, 27 AML, 4 bi-phenotypic leukemias) by flow cytometry in bone marrow biopsies revealed 79.5% of B-ALL expressed CEACAM6, without relevant expression noted in the other subtypes [59]. In comparison to normal granulocytes, all samples revealed over-expression of CEACAM6 and CEACAM8. Further, CEACAM6 mRNA expression was also high in B-ALL patients (>90%) when compared to leukemia cell lines [59]. However, the clinical relevance of CEACAM6 expression in relation to adverse outcomes and predictive biomarker status related to treatment resistance are yet to be described in B-ALL. CEACAM6 expression in the cytoplasm of multiple human leukemic cell lines is present irrespective of its cell surface expression [60]. Most leukemia tend to have deregulated levels of CEACAM 6 and 8. Therefore, as previous work has revealed silencing CEACAM6 promotes anoikis via increased caspase activity with down regulation of the AKT cell survival pathway, targeting of CEACAM6 in acute leukemia is warranted.
(B). Multiple Myeloma
In multiple myeloma (MM) CEACAM6 expression promotes malignancy through a mechanism of immune evasion, in contrast to that described in epithelial malignancies. Recently, the role of CEACAM6 in inhibiting myeloma-reactive T-cells in patients has been investigated [61]. Although cytotoxic T-cells (CTLs) are functionally competent they have been found to have very little reactivity against tumor cells [62]. Tumors have the capacity to block effective functions of T-cells and thought to be a reason for limited clinical efficacy of tumor immunotherapy. CTLs react against MM antigens when presented by autologous dendritic cells but not by MM cells. Gene expression profiling and flow cytometry of isolated MM cells demonstrated several CEACAMs to be specifically over-expressed (e.g. CEACAM -1, -6, -8). Binding and cross-linking of CEACAM6 by CTL inhibited their activation, producing T-cell unresponsiveness. Blocking CEACAM6 on the surface of MM cells with a Mab or CEACAM6 knock down by siRNA restored T-cell reactivity against malignant plasma cells (77 MM pts, various cell lines). This resulted in increased IFN-γ and perforin secretion by CTLs which promoted MM cell apoptosis. The proposed mechanism is that MM cells escape recognition by autologous myeloma specific CD8+ T cells through CEACAM6 expression. Thus, CEACAM6 may play a critical role in the regulation of CD8+ T cell response against MM supporting the use of therapeutic targeting of CEACAM6 as a novel MM immune checkpoint therapy.
C. Signaling Pathways Activated by CEACAM6
Although CEACAM6 lacks a trans-membrane domain it is able to effectively regulate intracellular signaling. GPI-anchored proteins aggregate on the surface of the plasma membrane to form micro-domains, also known as “lipid rafts” [63, 64]. It is through this mechanism that CEACAM6 is able to modulate signal transduction and ultimately dictate cellular behavior. When CEACAM6 is cross-linked in BxPC3 cells anoikis resistance develops through activation of SRC in a caveolin-1-dependant manner [36]. A large body of evidence has identified SRC as a key molecule in tumor progression that can provide oncogenic signals for cell survival, mitogenesis, invasion, angiogenesis and metastasis [65-67]. Caveolin-1 is an integral membrane protein found to co-precipitate with SRC and is required for SRC kinases to activate integrin signaling [68-70]. Previous work identified CEACAM5 and CEACAM6 co-localize with a5b1 in the same specific lipid raft [71]. Studies revealed that the sub-cellular localization of SRC enriched in detergent-insoluble lipid rafts of cell membranes were important for SRC activation [72, 73]. AKT and SRC acting together promote metastasis with SRC identified as an activator of the PI3K/AKT signaling pathway. SRC achieves this goal through phosphorylation of various substrates including FAK [74]. In PDA, FAK activation is important for the development of anoikis resistance and represents a key target for tyrosine phosphorylation by SRC [75] (Fig. 2). Genetic silencing of FAK expression results in the promotion of anoikis in PDA cells, confirming that FAK plays a key role in anoikis resistance [36]. Activation of AKT is common in PDA [76] and has been found to be a determinant of cellular proliferation [77]. Inhibition of the PI3K/AKT pathway attenuates anoikis resistance in PANC1 cells [78], while activation of the PI3K/AKT pathway impairs epithelial cell anoikis.
In the context of PDA cell lines significant work has been done to further investigate the underlying mechanism of action of CEACAM6 at the cellular level [79-81]. Over-expression of CEACAM6 results in augmented PDA cellular invasiveness through increased activity of IGF-I and insulin-like growth factor I receptor (IGF-IR), both of which play critical roles in proliferation, invasiveness, and increased angiogenesis. AKT activation produces IGF-IR up-regulation. Over-expression of CEACAM6 in the context of IGF-I elaborates increased SRC, AKT and MMP-2 activity. Suppression of CEACAM6 decreases pAKT Ser473, a marker of AKT activity. Therefore, it was proposed that AKT may be responsible for up-regulation of IGF-IR in cells with over-expressed CEACAM6. CEACAM6 over-expression resulted in increased IGF-I expression leading to enhanced MMP-2 expression and activity (Fig. 2). AKT pathway activation has been shown to result in diminished degradation of MMP-2 [82]. MMP-2 is a key determinant of SRC dependant PDA cellular invasiveness [83]. IGF-I has been shown to promote tumor aggressiveness by increasing levels of membrane type 1 MMPs, resulting in activation of MMP-2 through PI3K/AKT signal transduction [84]. Antibodies targeting MMP-2 reduce cellular invasiveness [85]. In addition, inhibition of SRC with PP2 abrogates PDA cellular invasiveness due to dephosphorylation of SRC and inhibition of MMP-2 and MMP-9 activity. SRC dependent transcriptional up-regulation of MMP-9 activity is significantly responsible for the increased cellular invasiveness produced by CEACAM6 over-expression, linking this as a key pathway involved in PDA aggressiveness.
Interestingly, CEACAM6 is able to activate neutrophil adhesion to human vascular endothelial cells [86] and cross-linking of CEACAM family members induces a respiratory burst in neutrophils [87]. Therefore, there may very well be a similar mechanism for neutrophil activation and mobilization that resembles tumor cell migration and aggressiveness. Since CEACAM6 plays a role in neutrophil activation and adhesion to the ECM, follow up studies have suggested that the aggressive phenotype of PDA may in fact be due to parallel activities by CEACAM6 in the TME [39].
D. CEACAM6 Targeted Therapies
(1) Knockdown via siRNA Mediated Gene Silencing
Knockdown models in CEACAM6 positive tumors identify key mechanistic pathways and potential targets involved in the development of the malignant phenotype [88]. Mice PDA xenografts treated with CEACAM6-specific siRNA diminished tumor proliferation by 68% versus control siRNA (p=<0.05) with a decreased Ki-67 level, impairing angiogenesis and increased apoptosis. Further, CEACAM6-specific siRNA prevented metastasis (0% treated versus 60% untreated, p=<0.05) and improved survival without toxicity. Kaplan-Meier survival was significantly greater in the treated mice versus control. Knockdown models inform us of the critical role CEACAM6 plays in cancer development further solidifying a focus on this molecule as a therapeutic target.
(2) Immunotoxin-based Therapy
An immunotherapeutic strategy for targeting CEACAM6 in PDA has been explored [89]. Immunotherapeutic approaches are classified as either direct or indirect. An indirect approach utilized By114, a mouse Mab specific for CEACAM6 with no anti-tumor activity [90], followed by saporin to demonstrate anti-tumor activity in a BxPC3 cells in vitro and in a nude mouse xenograft model of PDA. Mab mediated cross-linking of CEACAM6 resulted in cytoplasmic accumulation that increased efficacy of immunotherapy with saporin with caspase mediated apoptosis.
(3) Monoclonal Antibodies (Mab)
Utilizing GW-39 human colonic cancer cells the effects of three Mabs that target specific epitopes (NH2-terminal [MN-3] and A1B1 domains [MN-15] present in CEACAM5 and CEACAM6 and A3B3 domain [MN-14], restricted to CEACAM5) were investigated [14]. GW-39 CRC cell lines were initially treated with Fab’ fragments of MN-3, MN-15 and MN-14 Mabs, followed by a second injection of antibody 1 day after injection of tumor cells. The effect on lung metastases was analyzed. MN-15 Fab’ and MN-3 Fab’ both demonstrated a decrease in metastatic potential in vivo. However, MN-14 did not affect lung metastasis. This work provided the evidence that CEACAM6 is involved in the development of CRC metastasis. Although treatment with MN-15 and MN-3 resulted in an increase in survival, decreased adhesion to the ECM, and abrogated metastatic potential, these Mabs were not efficacious with regard to tumor growth inhibition or regression.
(4) Therapeutic Humanized Anti-CEACAM6 Single Chain Variable Fragment (scFv)
Mouse Mab 13-1 targeting a unique epitope on human CEACAM6 leads to apoptosis of PDA cells expressing CEACAM6 with an IC50 in the range 1-10μg/mL independent of antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity [91]. In silico CDR grafting and antibody engineering generated humanized anti-CEACAM6 single chain variable fragment (scFv’s) with a linker that could be pegylated to enhanced plasma half-life. These scFv’s bound CEACAM6 with high affinity (10-10M), exhibiting dose-dependent cytotoxic activity and PARP-cleavage in cell culture. Nine scFv versions were designed and synthesized and versions 7 and 8 were evaluated. In a mouse xenograft model of PDA (BxPC-3) PEGylated scFv administered twice a week for 4 weeks intra-peritoneally showed tumor growth inhibition with marked apoptosis, with diminished angiogenesis and proliferation. The humanized PEGylated scFv version 8 at 3mg/kg initially produced a tumor growth inhibition of ~25%, however with gemcitabine, tumor growth inhibition was increased to >50%. This study highlights that the unique features of humanized anti-CEACAM6 scFv include the ability to produce targeted tumor cell apoptosis independent of antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity as well as represent an effective combination with standard chemotherapy at very low doses.
(5) Antibody-Drug Conjugates
The development of an antibody-drug conjugate targeting CEACAM6 investigated the efficacy of the anti-CEACAM6-maytansinoid (DM1) immunoconjugate in a murine xenograft model of PDA [92]. Tumor growth was found to be markedly diminished in mouse models of HPAF-2 tumors treated with the antibody-drug conjugate. They also report the safety profile of the anti-CEACAM6 Mab and antibody-drug conjugate in a non-human primate model. CEACAM6 expression in hematopoietic progenitor cells, specifically the earliest myeloid lineage CD34+/CD38+/CD33+ was low, however expression in mature granulocytes was demonstrated. Administration of a single dose (low 1.35mg/kg, n=3; high 10mg/kg, n=3) of DM1-conjugated Mab resulted in neutropenia in a dose-dependent fashion as the main adverse effect, suggesting minimal and reversible bone marrow toxicity. The non-conjugated Mab did not have anti-tumor activity, and showed reversible and modest neutropenia. Further development of an antibody-drug conjugate based therapeutic approach in PDA has yet to be pursued in early phase clinical trials.
(6) Single Domain Llama Antibody (sdAb)
A single domain antibody (sdAb) that targets CEACAM6, known as 2A3, previously isolated from a Llama immune library and a Fc-conjugated version of the sdAb was evaluated to determine the effect on the BxPC3 PDA cell line [93]. This sdAb binds to CEACAM6 on the cell surface. Gemcitabine and 2A3 both decreased cancer cell proliferation, however only 2A3 diminished cancer cell invasion and angiogenesis within the cancer mass as well as BxPC3 cell MMP-9 activity. Also, 2A3 and 2A3-Fc inhibited invasion of BxPC3 cells versus the non-treated cohort. A capillary formation assay used to analyze angiogenesis in conditioned media of 2A3 or 2A3-Fc treated BxPC3 cells showed capillary length was significantly decreased.
L-DOS47, a Llama anti-CEACAM6 antibody conjugated to plant urease generates an alkalinized environment at the tumor site. The first in human study is being conducted in Poland, and in an open-label Phase I/II clinical study is assessing safety, tolerability and efficacy at incremental doses of L-DOS47, as monotherapy, in patients with unresectable, locally advanced, relapsed or metastatic, non-squamous, stage IIIb/IV NSCLC (clinicaltrials.gov, NCT02340208). The starting dose was 0.12 mg/kg and patients have been enrolled to the 9th cohort at a dose of 1.84mg/kg. A phase I dose escalation study of L-DOS47 combined with pemetrexed plus carboplatin in stage IV non-squamous NSCLC is planned (Helix BioPharma Corp, Ontario, Canada).
Conclusions
The ability of cancer cells to migrate from their primary location to distant sites is the cause of 90% of human cancer mortality [94]. Dysregulated over-expression of CEACAM6 plays a role in several of the hallmarks of cancer, including uncontrolled proliferation, anoikis resistance, neo-angiogenesis, immune evasion, invasion and metastasis. CEACAM6 is integral to the cell adhesion, ECM modulation and creating a hostile TME through its interactions with membrane receptors and the cellular milieu of a complex carcinoma. Anoikis resistance may initiate and promote a malignant phenotype. This invasive phenotype is driven by aberrant activation of PI3K/AKT, FAK, SRC and TGF-b signaling pathways.
Since CEACAM family members including CEACAM6 are expressed on myeloid cells, tumors with inflammatory infiltrates are likely to fuel the fire by direct interactions between tumor cells and neutrophils. Hence, deciphering these molecular and cellular interactions within the TME are likely to provide a wealth of information in the search for better therapies. Since immune checkpoint targeting has come of age, immune evasion has been linked directly to CEACAM6 over-expression on plasma cells in multiple myeloma. Binding and cross linking of CEACAM6 by CTL inhibits their activation, resulting in T cell unresponsiveness, thus suggesting a novel mechanism of action of CEACAM6. Inhibiting CEACAM6 is a therapeutic strategy to restore T-cell cytotoxicity targeting tumor lysis within the bone marrow microenvironment in MM and TME in epithelial carcinomas (Table 1, Fig. 2).
The availability of therapeutic monoclonal antibodies (unconjugated and conjugated) targeting CEACAM6, provides potential therapeutic opportunities likely to lead to a plethora of pre-clinical and clinical activity in the next decade. Interestingly, rodents lack orthologous genes for the human CEA family thereby limiting appropriate pre-clinical animal testing. As mentioned previously, a transgenic mouse model with a human bacterial artificial chromosome containing components of the human CEA gene family, specifically CEACAM3, CEACAM5, CEACAM6 and CEACAM7 genes is available allowing for more precise pre-clinical evaluation [17]. We believe safely targeting CEACAM6 is most likely to change the natural history of the tumor/TME and allow novel combination therapies to be developed for treating both solid and hematologic malignancies.
In conclusion our review highlights the pathologic roles of CEACAM6 in the development of multiple human malignancies warranting an ongoing focus to successfully transition this cell adhesion protein as a primary or adjunctive therapeutic target for anti-cancer therapy in the clinic.
ACKNOWLEDGEMENTS
We wish to thank Laurence Cooke for guidance and critical review of the manuscript. We also thank the University of Tennessee Health Science Center and The West Cancer Center for research funding to BJ received through the 2014 Trainee Research Award.
List Of Abbreviations
- CEACAM6
Carcinoembryonic antigen-related cell adhesion molecule 6
- CEACAMs
Carcinoembryonic antigen-related cell adhesion molecules
- IHC
Immunohistochemistry
- TMAs
Tissue microarrays
- Mab
Monoclonal antibodies
- CRC
Colorectal cancer
- siRNA
Small interfering RNA
- ECM
Extracellular matrix
- PDA
Pancreatic ductal adenocarcinoma
- PanIN
Pancreatic intraepithelial neoplasia
- MMP2
Matrix metalloproteinase 2
- DR
Desmoplastic reaction
- IGF-I
Insulin-like growth factor I
- TME
Tumor microenvironment
- TNBC
Triple negative breast cancer
- MTC
Medullary thyroid carcinomas
- HNSCC
Head and neck squamous cell cancer
- B-ALL
B-cell lineage acute lymphoblastic leukemia
- MM
Multiple myeloma
- CTLs
Cytotoxic T-cells
- IGF-IR
Insulin-like growth factor I receptor
- scFv
Single chain variable fragment
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Paxton R.J., Mooser G., Pande H., Lee T.D., Shively J.E. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc. Natl. Acad. Sci. USA. 1987;84:920–924. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchemin N., Draber P., Dveksler G., et al. Redefined nomenclature for members of the carcinoembryonic antigen. Exp. Cell Res. 1999;252(2):243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 3.Chan C.H., Stanners C.P. Recent advances in the tumour biology of the GPI-anchored carcinoembryonic antigen family members CEACAM5 and CEACAM6. Curr. Oncol. 2007;14:70–73. doi: 10.3747/co.2007.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammarstrom S., Olsen A., Teglund S., Baranov V. In: The nature and expression of the human CEA family. Cell Adhesion and Communication Mediated by the CEA Family: Basic and Clinical Perspectives. Stanners C.P., editor. Amsterdam: Hardwood Academic Publishers; 1998. pp. 31–55. [Google Scholar]
- 5.Gold P., Freedman S.O. Specific carcinoembryonic antigens of the human digestive system. J. Exp. Med. 1965;122:467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 7.Eidelman F.J., Fuks A., DeMarte L., Taheri M., Stanners C.P. Human carcinoembryonic antigen, an intercellular adhesion molecule, blocks fusion and differientation of rat myoblasts. J. Cell Biol. 1993;123(2):467–475. doi: 10.1083/jcb.123.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camacho-Leal P., Zhai A.B., Stanners C.P. A co-clustering model involving alpha5beta1 integrin for the biological effects of GPI-anchored human carcinoembryonic antigen (CEA). J. Cell. Physiol. 2007;211(3):791–802. doi: 10.1002/jcp.20989. [DOI] [PubMed] [Google Scholar]
- 9.Minton J.P., Hoehn J.L., Gerber D.M., et al. Results of a 400-patient carcinoembryonic antigen second-look colorectal cancer study. Cancer. 1985;55:1284–1290. doi: 10.1002/1097-0142(19850315)55:6<1284::aid-cncr2820550622>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa T., Isobe K., Tsuchiya Y., et al. Nonspecific crossreacting antigen (NCA) is a major member of the carcinoembryonic antigen (CEA)-related gene family expressed in lung cancer. Br. J. Cancer. 1993;67:58–65. doi: 10.1038/bjc.1993.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benchimol S., Fuks A., Jothy S., Beauchemin N., Shirota K., Stanners C.P. Carcinoembryonic antigen, a human tumor marker, functions as a intercellular adhesion molecule. Cell. 1989;57:327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 12.Scholzel S., Zimmermann W., Schwarzkopf G., Grunert F., Rogaczewksi B., Thompson J. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am. J. Pathol. 2000;157:1051–1052. doi: 10.1016/S0002-9440(10)64764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumenthal R.D., Leon E., Hansen J.H., Goldenberg D.M. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer. 2007;7:2. doi: 10.1186/1471-2407-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blumenthal R.D., Hansen J.H., Goldenberg D.M. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (carcinoembryonic antigen). Cancer Res. 2005;65:8809–8817. doi: 10.1158/0008-5472.CAN-05-0420. [DOI] [PubMed] [Google Scholar]
- 15.Yamanka T., Kuroki M., Matsuo Y., Matsuoka Y. Analysis of heterophilic cell adhesion mediated by CD66b and CD66c using their soluble recombinant proteins. Biochem. Biophys. Res. Commun. 1996;219:842–847. doi: 10.1006/bbrc.1996.0320. [DOI] [PubMed] [Google Scholar]
- 16.Kammerer R., Zimmerman W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BioMedCentral Biol. 2010;8:12–33. doi: 10.1186/1741-7007-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan C.H., Stanners C.P. Novel Mouse Model for Carcinoembryonic Antigen-Based Therapy. Mol. Ther. 2004;9:775–785. doi: 10.1016/j.ymthe.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Burtin P., Quan P.C., Sabine M.C. Nonspecific cross-reacting antigen as a marker for human polymorphs, macrophages, and monocytes. Nature. 1975;255:714–716. doi: 10.1038/255714a0. [DOI] [PubMed] [Google Scholar]
- 19.Grunert F., AbuHarfeil N., Schwarz K., von Kleist S. Two CEA and three NCA species, although distinguishable by monoclonal antibodies, have nearly identical peptide patterns. Int. J. Cancer. 1985;36:357–362. [PubMed] [Google Scholar]
- 20.Abbonda G.C., Papotti M., Gugliotta P., Pecchio F., Rapellino M. Immunohistochemical detection of carcinoembryonic antigen (CEA) in non-neoplastic lung disease. Int. J. Biol. Markers. 1993;8:240–243. doi: 10.1177/172460089300800407. [DOI] [PubMed] [Google Scholar]
- 21.Skubitz K.M., Grunert F., Jantscheff P., Kuroki M., Skubitz A.P. 1997. [Google Scholar]
- 22.Metze D., Bhardwaj R., Amann U., et al. Glycoproteins of the carcinoembryonic antigen (CEA) family are expressed in sweat and sebaceous glands of human fetal and adult skin. J. Invest. Dermatol. 1996;106:64–69. doi: 10.1111/1523-1747.ep12327258. [DOI] [PubMed] [Google Scholar]
- 23.Honda Y., Egawa K., Kuroki M., Ono T. Hair cycle-dependent expression of a nonspecific cross-reacting antigen (NCA)-50/like molecule on follicular keratinocytes. Arch. Dermatol. Res. 1997;289:457–465. doi: 10.1007/s004030050221. [DOI] [PubMed] [Google Scholar]
- 24.Ilantzis C., Jothy S., Alpert L.C., Draber P., Stanners C.P. Cell-surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Lab. Invest. 1997;76:703–716. [PubMed] [Google Scholar]
- 25.Ilantzis C., Demarte L., Screaton R.A., Stanners C.P. Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia. 2002;4:151–163. doi: 10.1038/sj.neo.7900201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jantscheff P., Terracciano L., Lowy A., et al. Expression of CEACAM6 in resectable colorectal cancer: a factor of independent prognostic significance. J. Clin. Oncol. 2003;19:3638–3646. doi: 10.1200/JCO.2003.55.135. [DOI] [PubMed] [Google Scholar]
- 27.Kim K.S., Kim J.T., Lee S.J., et al. Overexpression and clinical significance of carcinoembryonic antigen-related cell adhesion molecule 6 in colorectal cancer. Clin. Chim. Acta. 2013;415:12–19. doi: 10.1016/j.cca.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Gemei M., Mirabelli P., Noto R.D., et al. CD66c is a novel marker for colorectal cancer stem cell isolation, and its silencing halts tumor growth in vivo. Cancer. 2013;119:728–738. doi: 10.1002/cncr.27794. [DOI] [PubMed] [Google Scholar]
- 29.Duxbury M.S., Ito H., Zinner M.J., Ashley S.W., Whang E.E. CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene. 2004;23:465–473. doi: 10.1038/sj.onc.1207036. [DOI] [PubMed] [Google Scholar]
- 30.Duxbury M.S., Ito H., Benoit E., Waseem T., Ashley S.W., Whang E.E. A novel role for carcinoembryonic antigen related cell adhesion molecule 6 as a determinant of gemcitabine chemoresistance in pancreatic adenocarcinoma cells. Cancer Res. 2004;64:3987–3993. doi: 10.1158/0008-5472.CAN-04-0424. [DOI] [PubMed] [Google Scholar]
- 31.Fahy B.N., Schlieman M., Virudachalam S., Bold R.J. AKT inhibition is associated with chemosensitisation in the pancreatic cell line MIA-PaCa-2. Br. J. Cancer. 2003;89:391–397. doi: 10.1038/sj.bjc.6601037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng S.S., Tsao M.S., Nicklee T., Hedley D.W. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin. Cancer Res. 2001;7:3269–3275. [PubMed] [Google Scholar]
- 33.Duxbury M.S., Matros E., Clancy T., et al. CEACAM6 is a novel biomarker in pancreatic adenocarcinoma and PanIN lesions. Ann. Surg. 2005;241:491–496. doi: 10.1097/01.sla.0000154455.86404.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahadevan D., Von Hoff D.D. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol. Cancer Ther. 2007;4:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 35.Chandana S, Mahadevan D. Translational advances and novel therapies for pancreatic ductal adenocarcinoma: hope or hype? 2009. [DOI] [PubMed]
- 36.Duxbury M.S., Ito H., Ashley S.W., Whang E.E. CEACAM6 cross-linking induces caveolin-1-dependent, Src-mediated focal adhesion kinase phosphorylation in BxPC3 pancreatic adenocarcinoma cells. J. Biochem. 2004;279:23176–23182. doi: 10.1074/jbc.M402051200. [DOI] [PubMed] [Google Scholar]
- 37.Woodhouse E.C., Chuaqui R.F., Liotta L.A. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Nair K.S., Zingde S.M. Adhesion of neutrophils to fibronectin: role of the cd66 antigen. Cell. Immunol. 2001;208:96–106. doi: 10.1006/cimm.2001.1772. [DOI] [PubMed] [Google Scholar]
- 39.Duxbury M.S., Ito H., Ashley S.W., Whang E.E. c-Src dependent cross-talk between CEACAM6 and ανβ3 integrin enhances pancreatic adenocarcinoma cell adhesion to extracellular matrix components. Biochem. Biophys. Res. Commun. 2004;317:133–141. doi: 10.1016/j.bbrc.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Poola I., Shokrani B., Bhatnagar R., DeWitty R.L., Yue Q., Bonney G. Expression of carcinoembryonic antigen cell adhesion molecule 6 oncoprotein in atypical ductal hyperplastic tissues is associated with the development of invasive breast cancer. Clin. Cancer Res. 2006;12:4773–4783. doi: 10.1158/1078-0432.CCR-05-2286. [DOI] [PubMed] [Google Scholar]
- 41.Beauchemin N., Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013;••• doi: 10.1007/s10555-013-9444-6. [DOI] [PubMed] [Google Scholar]
- 42.Maraqa L, Cummings M, Peter MB, et al. Carcinoembryonic antigen cell adhesion molecule 6 predicts breast cancer recurrence following adjuvant tamoxifen. 2008. [DOI] [PubMed]
- 43.Hiscox S., Morgan L., Barrow D., Dutkowskil C., Wakeling A., Nicholson R.I. Tamoxifen resistance in breast cancer cells is accompanied by an enhanced motile and invasive phenotype: inhibition by gefitinib (“Iressa”, ZD1839). Clin. Exp. Metastasis. 2004;21:201–212. doi: 10.1023/b:clin.0000037697.76011.1d. [DOI] [PubMed] [Google Scholar]
- 44.Hiscox S., Jiang W.G., Obermeier K., et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behavior and involves modulation of beta-catenin phosphorylation. Int. J. Cancer. 2006;118:290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- 45.Scott D.J., Parkes A.T., Ponchel F., Cummings M., Poola I., Speirs V. Changes in expression of steroid receptors, their downstream target genes and their associated co-regulators during the sequential acquisition of tamoxifen resistance in vitro. Int. J. Oncol. 2007;31:557–565. doi: 10.3892/ijo.31.3.557. [DOI] [PubMed] [Google Scholar]
- 46.Lewis-Wambi J.S., Cunliffe H.E., Kim H.R., Willis A.L., Jordan C.V. Overexpression of CEACAM6 promotes migration and invasion of oestrogen-deprived breast cancer cells. Eur. J. Cancer. 2008;44:1770–1779. doi: 10.1016/j.ejca.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsang J.Y., Kwok Y.K., Chan K.W., et al. Expression and clinical significance of carcinoembryonic antigen-related cell adhesion molecule 6 in breast cancers. Breast Cancer Res. Treat. 2013;142:311–322. doi: 10.1007/s10549-013-2756-y. [DOI] [PubMed] [Google Scholar]
- 48.Khoury T., Kanehira K., Wang D., et al. Breast carcinoma with amplified HER2: a gene expression signature specific for trastuzumab resistance and poor prognosis. Mod. Pathol. 2010;23:1364–1378. doi: 10.1038/modpathol.2010.125. [DOI] [PubMed] [Google Scholar]
- 49.Ieta K., Tanaka F., Utsunomiya T., Kuwano H., Mori M. CEACAM6 gene expression in intrahepatic cholangiocarcinoma. Br. J. Cancer. 2006;95:532–540. doi: 10.1038/sj.bjc.6603276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singer B.B., Scheffrahn I., Kammerer R., Suttorp N., Ergun S., Slevogt H. Deregulation of the CEACAM expression pattern causes undifferentiated cell growth in human lung adenocarcinoma cells. PLoS One. 2010;5:e8747. doi: 10.1371/journal.pone.0008747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi M., Miki Y., Ebina M., et al. Carcinoembryonic antigen-related cell adhesion molecules as surrogate markers for EGFR inhibitor sensitivity in human lung adenocarcinoma. Br. J. Cancer. 2012;107:1745–1753. doi: 10.1038/bjc.2012.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi K., Creighton C.J., Stivers D., Fujimoto N., Kurie J.M. Transcriptional Profiling of Non-Small Cell Lung Cancer Cells with Activating EGFR Somatic Mutations. PLoS One. 2007;11:e1226. doi: 10.1371/journal.pone.0001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y., Zang M., Li J., et al. CEACAM6 promotes tumor migration, invasion, and metastasis in gastric cancer. Acta Biochim. Biophys. Sin. (Shanghai) 2014;46:283–290. doi: 10.1093/abbs/gmu001. [DOI] [PubMed] [Google Scholar]
- 54.Oue N., Hamai Y., Mitani Y., et al. Gene expression profile of gastric carcinoma: identification of genes and tags potentially involved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res. 2004;64:2397–2405. doi: 10.1158/0008-5472.can-03-3514. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Z.S., Li L., Wang H.J., Wang Y.Y. Expression and prognostic significance of CEACAM6, ITGB1 and CYR61 in peripheral blood of patients with gastric cancer. J. Surg. Oncol. 2011;104:525–529. doi: 10.1002/jso.21984. [DOI] [PubMed] [Google Scholar]
- 56.Ameur N., Lacroix L., Roucan S., et al. Aggressive inherited and sporadic medullary thyroid carcinomas display similar oncogenic pathways. Endocr. Relat. Cancer. 2009;16:1261–1272. doi: 10.1677/ERC-08-0289. [DOI] [PubMed] [Google Scholar]
- 57.Cameron S., Long L.M., Rethinam M.H., et al. Focal overexpression of CEACAM6 contributes to enhanced tumourigenesis in head and neck cancer via suppression of apoptosis. Mol. Cancer. 2012;11:74. doi: 10.1186/1476-4598-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cameron S.R., Dahler A.L., Endo-Munoz L.B., et al. Tumor-initiating activity and tumor morphology of HNSCC is modulated by interactions between clonal variants within the tumor. Lab. Invest. 2010;90:1594–1603. doi: 10.1038/labinvest.2010.131. [DOI] [PubMed] [Google Scholar]
- 59.Lasa A., Serrano E., Carricondo M., et al. High expression of CEACAM6 and CEACAM8 mRNA in acute lymphoblastic leukemias. Ann. Hematol. 2008;87:205–211. doi: 10.1007/s00277-007-0388-1. [DOI] [PubMed] [Google Scholar]
- 60.Sugita K., Mori T., Yokota S., et al. The Kor-SA3544 antigen predominantly expressed on the surface of Philadelphia chromosome-positive acute lymphoblastic leukemia cells is nonspecific cross-reacting antigen-50/90 (CD66c) and invariably expressed in cytoplasm of human leukemia cells. Leukemia. 1999;13:779–785. doi: 10.1038/sj.leu.2401408. [DOI] [PubMed] [Google Scholar]
- 61.Witzens-Harig M., Hose D., Junger S., et al. Tumor cells in multiple myeloma patients inhibit myeloma-reactive T cells through carcinoembryonic antigen-related cell adhesion molecule-6. Blood. 2013;121:4493–4503. doi: 10.1182/blood-2012-05-429415. [DOI] [PubMed] [Google Scholar]
- 62.Homa P., Sotomayor E.M. Cellular and molecular mechanisms of tumor-induced T-cell tolerance. Curr. Cancer Drug Targets. 2007;7:41–53. doi: 10.2174/156800907780006940. [DOI] [PubMed] [Google Scholar]
- 63.Stefanova I., Horejsi V., Ansotegui I.J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 64.Harris T.J., Siu C.H. Reciprocal raft-receptor interactions and the assembly of adhesion complexes. BioEssays. 2002;24:996–1003. doi: 10.1002/bies.10172. [DOI] [PubMed] [Google Scholar]
- 65.Banibrata S., Johnson F. Regulation of Src Family Kinases in Human Cancers. J. Signal Transduct. 2011;865819:2011. doi: 10.1155/2011/865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Summy J.M., Gallick G.E. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev Cancer. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 67.Biscardi J.S., Tice D.A., Parsons S.J. C-Src, receptor tyrosine kinases, and human cancer. Adv. Cancer Res. 1999;76:117–119. doi: 10.1016/s0065-230x(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 68.Schlaepfer D.D., Hanks S.K., Hunter T., van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J., Kalyankrishna S., Wislez M., et al. Src-Family Kinases Are Activated in Non-Small Cell Lung Cancer and Promote the Survival of Epidermal Growth Factor Receptor-Dependent Cell Lines. Am. J. Pathol. 2007;170:366–376. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giaccone G., Zucali P.A. Src as a potential therapeutic target in non-small-cell lung cancer. Ann. Oncol. 2008;19:1219–1223. doi: 10.1093/annonc/mdn048. [DOI] [PubMed] [Google Scholar]
- 71.Ordonez C., Zhai A.B., Camacho-Leal P., Demarte L., Fan M.M., Stanners C.P. GPI-anchored CEA family glycoproteins CEA and CEACAM6 mediate their biologic effects through enhanced integrin α5β1-fibronectin interaction. J. Cell. Physiol. 2007;210:757–765. doi: 10.1002/jcp.20887. [DOI] [PubMed] [Google Scholar]
- 72.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. 1785. [DOI] [PubMed]
- 73.Wang R, Bi J, Ampah KK, Ba X, Liu W, Zeng X. Lipid rafts control human melanoma cell migration by regulating focal adhesion disassembly. 1833. [DOI] [PubMed]
- 74.Gabarra-Niecko V., Schaller M.D., Dunty J.M. FAK regulates the biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- 75.Calalb M.B., Polte T.R., Hanks S.K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catcalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Semba S., Moriya T., Kimura W., Yamakawa M. Phosphorylated Akt/PKB controls cell growth and apoptosis in intraductal papillary-mucinous tumor and invasive ductal adenocarcinoma of the pancreas. Pancreas. 2003;26:250–257. doi: 10.1097/00006676-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Perugini R.A., McDade T.P., Vittimberga F.J., Jr, Callery M.P. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependant. J. Surg. Res. 2000;90:39–44. doi: 10.1006/jsre.2000.5833. [DOI] [PubMed] [Google Scholar]
- 78.Yao Z., Okabayashi Y., Yutsudo Y., Kitamura T., Ogawa W., Kasuga M. Role of Akt in growth and survival of PANC-1 pancreatic cancer cells. Pancreas. 2002;24:42–46. doi: 10.1097/00006676-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 79.Duxbury M.S., Ito H., Benoit E., Zinner M.J., Ashley S.W. Overexpression of CEACAM6 promotes insulin-like growth factor I-induced pancreatic adenocarcinoma cellular invasiveness. Oncogene. 2004;23:5834–5842. doi: 10.1038/sj.onc.1207775. [DOI] [PubMed] [Google Scholar]
- 80.Bergmann U., Funatomi H., Yokoyama M., Beger H.G., Korc M. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 1995;55:2007–2011. [PubMed] [Google Scholar]
- 81.Zeng H., Datta K., Neid M., Li J., Parangi S., Mukhopadhyay D. Requirement of different signaling pathways mediated by insulin-like growth factor-I receptor for proliferation, invasion, and VPF/VEGF expression in a pancreatic carcinoma cell line. Biochem. Biophys. Res. Commun. 2003;302:46–55. doi: 10.1016/s0006-291x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 82.Park B.K., Zeng X., Glazer R.I. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 2001;61:7647–7653. [PubMed] [Google Scholar]
- 83.Ito H., Gardner-Thorpe J., Zinner M.J., Ashley S.W., Whang E.E. Inhibition of tyrosine kinase Src suppresses pancreatic cancer invasiveness. Surgery. 2003;134:221–226. doi: 10.1067/msy.2003.224. [DOI] [PubMed] [Google Scholar]
- 84.Zhang D., Brodt P. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI3-kinase/Akt signaling. Oncogene. 2003;22:974–982. doi: 10.1038/sj.onc.1206197. [DOI] [PubMed] [Google Scholar]
- 85.Duxbury M.S., Ito H., Ashley S.W., Whang E.E. c-Src dependent cross-talk between CEACAM6 and ανβ3 integrin enhances pancreatic adenocarcinoma cell adhesion to extracellular matrix components. Biochem. Biophys. Res. Commun. 2004;317:133–141. doi: 10.1016/j.bbrc.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 86.Skubitz K.M., Campbell K.D., Skubitz A.P. CD66a, CD66b, CD66c, and CD66d each independently stimulate neutrophils. J. Leukoc. Biol. 1996;60:106–117. doi: 10.1002/jlb.60.1.106. [DOI] [PubMed] [Google Scholar]
- 87.Lund-Johansen F., Olweus J., Symington F.W., et al. Activation of human monocytes and granulocytes by monoclonal antibodies to glycosylphosphatidylinositol-anchored antigens. Eur. J. Immunol. 1993;23:2782–2791. doi: 10.1002/eji.1830231110. [DOI] [PubMed] [Google Scholar]
- 88.Duxbury M.S., Matros E., Ito H., Zinner M.J., Ashley S.W., Whang E.E. Systemic siRNA-mediated gene silencing. A new approach to targeted therapy of cancer. Ann. Surg. 2004;240:667–674. doi: 10.1097/01.sla.0000140755.97224.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duxbury M.S., Ito H., Ashley S.W., Wang E.E. CEACAM6 as a novel target for indirect type 1 immunotoxin-based therapy in pancreatic adenocarcinoma. Biochem. Biophys. Res. Commun. 2004;317:837–843. doi: 10.1016/j.bbrc.2004.03.128. [DOI] [PubMed] [Google Scholar]
- 90.Mayne K.M., Pulford K., Jones M., et al. Antibody By114 is selective for the 90 kD PI-linked component of the CD66 antigen: a new reagent for the study of paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 1993;83:30–38. doi: 10.1111/j.1365-2141.1993.tb04627.x. [DOI] [PubMed] [Google Scholar]
- 91.Riley CJ, Engelhardt KP, Saldanha JW, et al. Design and activity of a murine and humanized anti-CEACAM6 scFv in the treatment of pancreatic cancer. 2009. [DOI] [PMC free article] [PubMed]
- 92.Strickland L.A., Ross J., Williams S., et al. Preclinical evaluation of carcinoembryonic cell adhesion molecule (CEACAM) 6 as potential therapy target for pancreatic adenocarcinoma. J. Pathol. 2009;218:380–390. doi: 10.1002/path.2545. [DOI] [PubMed] [Google Scholar]
- 93.Cheng T.M., Murad Y.M., Chang C.C., et al. Single domain antibody against carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) inhibits proliferation, migration, invasion and angiogenesis of pancreatic cancer cells. Eur. J. Cancer. 2014;50:713–721. doi: 10.1016/j.ejca.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 94.Sporn M.B. The war on cancer. Lancet. 1996;347:1377–1381. doi: 10.1016/s0140-6736(96)91015-6. [DOI] [PubMed] [Google Scholar]