Abstract
Platycodin D (PD), a major saponin derived from Platycodin grandiflorum, exerted cytotoxicity against prostate cancer cell lines (PC3, DU145 and LNCaP cells) with IC50 values in the range of 11.17 to 26.13μmol/L, whereas RWPE-1cells (a non-malignant human prostate epithelial cell line) were not significantly affected. A further study in these cell lines showed that PD could potently affect cell proliferation (indicated by the bromodeoxyuridine assay), induce cell apoptosis (determined by Annexin V-FITC flow cytometry) and cause cell cycle arrest (indicated by PI staining). After being treated with PD for 48 hours, DU145 and LNCaP cells were arrested in the G0 /G1 phase, and PC3 cells were arrested in the G2/M phase. A Western blotting analysis indicated that PD increased the expression of the FOXO3a transcription factor, decreased the expression of p-FOXO3a and MDM2 and increased the expression of FOXO-responsive genes, p21 and p27. MDM2 silencing (transiently by siRNA-MDM2) increased the PD-induced FOXO3a protein expression, while MDM2 overexpression (in cells transiently transfected with a pcDNA3-MDM2 plasmid) decreased the PD-induced expression of the FOXO3a protein. Moreover, PD dose-dependently inhibited the growth of PC3 xenograft tumors in BALB/c nude mice. A Western blotting analysis of the excised xenograft tumors indicated that similar changes in protein expression also occurred in vivo. These results suggest that PD exhibits significant activity against prostate cancer in vitro and in vivo. The FOXO3a transcription factor appears to be involved in the activity of PD. Together, all of these findings provide a basis for the future development of this agent for human prostate cancer therapy.
Keywords: Cell cycle, FOXO3a, MDM2, Platycodin D, prostate cancer
Introduction
Prostate cancer is the second leading cause of cancer death in men, especially in the Western world, with an estimated 29,720 deaths in the US in 2013 [1]. The mortality rate in Asian countries is much lower, but is gradually increasing. Patients with advanced-stage disease are generally treated using endocrine therapy [2], but after a period of remission, most patients eventually exhibit progressive, hormone-refractory cancer. Chemotherapy has been the established treatment for patients with advanced disease [3]. However, the side effects of chemotherapy are considerable, and many advanced cancers are resistant to chemotherapy. Therefore, there is an urgent need to discover new drugs to treat prostate cancer, especially advanced disease.
The forkhead box transcription factor, FOXO3a, is a known suppressor of primary tumor growth via its transcriptional regulation of key genes involved in cell cycle arrest and apoptosis [4-6]. Recently, increasing evidence has accumulated suggesting that FOXO3a plays an important role as a tumor suppressor in prostate cancer [7]. FOXO3a activity is negatively regulated by Akt, which phosphorylates FOXO3a at multiple sites, leading to its transport out of the nucleus and retention in the cytoplasm [8, 9]. In fact, the anticancer drug docetaxel has been shown to induce cancer cell apoptosis by enhancing FOXO3a activity and stimulating its nuclear translocation [10]. These events lead to overexpression of FOXO-responsive genes, such as p21, p27 and BIM [10-12].
Natural products (NPs) have historically been an invaluable source of therapeutic agents, especially for anti-cancer drug discovery. Saponins identified from many herbs have been demonstrated to have inhibitory activity against human cancer cell lines [13]. Platycodin D (PD, MW: 1224.38; Fig. 1A) is the major triterpene saponin found in the root of Platycodon grandiflorum, which is commonly known as Jiegeng in China. It was previously reported that PD could induce apoptosis in gastric and breast cancer cells in vitro [14-16]. However, the effects of PD on prostate cancer, and the precise molecular mechanism(s) of action of this compound are not well understood.
Fig. (1).
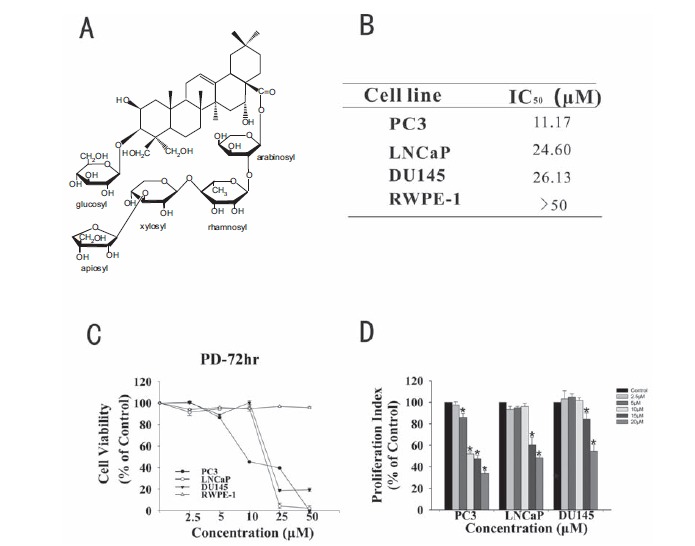
The growth inhibitory activity of PD in prostate cancer cells. A. The chemical structure of Platycodin D. B. The concentrations of PD that induced 50% growth inhibition (IC50) in prostate cancer cells, relative to the vehicle-treated control cells. C. Cells were exposed to various concentrations of PD for 72 hr, followed by the MTT assay. All assays were performed in triplicate. D. The anti-proliferative effects of PD were examined by the BrdUrd assay. (*, p<0.05 versus the control).
In this study, we show that when PD was administered (via intraperitoneal(i.p.) injection) at doses of 1 and 2.5 mg/kg, it inhibited the growth of PC3 xenograft tumors in BALB/c nude mice, and this was related to the FOXO3a expression. Further studies are needed to elucidate the detailed mechanism(s) of action of PD, in order to provide a basis for the future development of this agent for human prostate cancer chemotherapy.
Materials and Methods
Test Compound, Chemicals and Reagents
The test compound, PD (structure shown in Fig. 1A), was purchased from Must Bio-Technology Co., Ltd. (Chengdu, China). The purity of the test compound was determined to be greater than 98% by HPLC. All chemicals and solvents used were of analytical grade or of the highest grade available. Cell culture supplies, such as culture media and fetal bovine serum (FBS), were obtained from Hyclone (Carlsbad, CA, USA). The anti-human MDM2 antibody was obtained from Calbiochem (Billerica, MA, USA), the anti-FOXO3a, anti-p-FOXO3a and anti-p27 antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), and the anti-human E2F1, CDK2, CDK4, CDK6, Bax and Bcl2 antibodies were obtained from Boster Biotechnology (Wuhan, China). Anti-human Cyclin D1, PARP, caspase8, caspase9 and caspase3 antibodies were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Antibodies against human p21 and p53 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies against CDK1 and Cyclin B1 were obtained from Bioworld Technology (St. Louis Park, MN, USA). The GAPDH antibody (T5168) was from Goodhere Biotechnology (Hangzhou, China).
Cell Lines and Cell Culture
All human cell lines were obtained from the American Type Culture Collection. The DU145 cell line was cultured in RPMI 1640. PC3 cells were grown in Ham’s F12. LNCaP cells were grown in RPMI 1640 supplemented with 1.5 mg/ml sodium bicarbonate, 4.5 mg/mL glucose, 10 mM HEPES buffer, 1 mM pyruvate and 2 mM L-glutamine. Third-passage LNCaP cells were used in all of the experiments. A non-malignant prostate epithelial cell line, RWPE-1, was grown in medium provided by Invitrogen (Grand Island, NY, USA) as part of a K-SFM kit, along with 0.05 mg/ml bovine pituitary extract (BPE) and 5 ng/ml EGF, which are required to culture this cell line.
Cell Survival Assay
The effects of PD on human prostate cancer cell growth were determined using the MTT assay. Prostate cancer cells were exposed to various concentrations of PD (2.5, 5, 10, 25 and 50 μM) for the analysis. The absorbance at 570 nm was recorded using a TECAN Infinite M200 microplate reader (Seestrasse, Switzerland). The cell survival rates (%) were calculated by dividing the mean OD of compound-containing wells by that of DMSO-treated control wells. Three separate experiments were performed to determine the IC50 values.
Cell Proliferation Assay
The effects of PD on prostate cancer cell proliferation were evaluated using the BrdUrd Cell Proliferation Assay (Cell Signaling Technology, Inc.). Cells were treated with various concentrations of PD for 24 hr. The cells were then incubated with BrdUrd label for another 10 hr. Following the detailed protocol provided by the manufacturer, we terminated the experiment, and the BrdUrd label incorporated into the cells was detected using an anti-BrdUrd antibody. The absorbance of each well was measured using the TECAN Infinite M200 microplate reader at dual wave lengths of 450 and 540 nm.
Apoptosis Assay
The effects of PD on the number of prostate cancer cells in the early and late stages of apoptosis were examined using an Annexin V-FITC apoptosis detection kit from BestBio (Shanghai, China). A total of 1.2×105 cells/well were grown in 6-well plates that were exposed to various concentrations of PD and incubated for 48 hr prior to the analysis. Following the detailed protocol provided by the manufacturer, the samples were analyzed using a FACSCaliber flow cytometer (BD Biosciences, San Jose, CA, USA).
Cell Cycle Analysis
The prostate cancer cells (2-3×105) were seeded in 50 ml culture bottles, and then were exposed to the various concentrations of PD for 48 hr. Cells were trypsinized, washed with phosphate-buffered saline, and fixed in 1.5 ml 95% ethanol at 4°C overnight, and then were incubated with RNase and stained with propidium iodide. The DNA contents were determined by flow cytometry.
RNA Extraction, Reverse Transcription-PCR and Real-Time Quantitative PCR
Total RNA from PC3 cells was extracted using the Trizol reagent from BioFlux (Hangzhou Bioer Technology Co., Ltd). A 1μg aliquot of RNA from each sample was reverse-transcribed. The primer sequences used for gene amplification were as follows: MDM2 forward, 5’-AGG CAG GGG AGA GTG ATA CA-3’ and reverse, 5’-AAT TCT CAC GAA GGG CCC AA-3’; FOXO3a forward, 5’-AAC CTT CTG ATG TAA GTT CT-3’ and reverse, 5’-GTG ATT GCC TTC AGG ATT AC-3’; p21 forward, 5’-GCG ACT GTG ATG CGC TAA TG-3’ and reverse, 5’-GAA GGT AGA GCT TGG GCA GG-3’; p27 forward, 5’-GTG CGA GTG TCT AAC GGG AG-3’ and reverse, 5’-GTT TGA CGT CTT CTG AGG CC-3’; GAPDH forward, 5’- AATGGGCAGCC GTTAGGAAA-3’ and reverse, 5’-GCG CCC AAT ACG ACC AAA TC-3’. Using an iQ5 machine (Bio-Rad, USA), a 25 μl reaction mixture was amplified using the following thermal parameters: denaturation at 94oC for 2 min and 40 cycles of the amplification step (94oC for 10 s, 60oC for 15 s and 72oC for 45s). This was followed by a final extension at 94oC for 2 min, 72oC for 1 min, 95oC for 30 s and 30oC for 1 min. All amplification reactions were analyzed by the comparative threshold cycle (Ct) method and normalized to the level of GAPDH mRNA, which served as a control.
Transfection Assay
The pcDNA3-MDM2 plasmid was kindly provided by Dr. Ruiwen Zhang (Texas Tech University Health Sciences Center, USA). PC3 cells were transfected with 1μg or 3 μg of the plasmid for 5-7 hr, using a procedure reported previously [17]. Then, the cells were switched to F12 medium and treated with 10 μM of PD for an additional 24 hr. Thereafter, the cells were harvested, and a Western blotting analysis was performed.
siRNA Transfection
siRNA for MDM2 were purchased from Invitrogen. PC3 cells were transfected with 100 nM of siRNA for 5-7 hr, using a procedure reported previously [18]. Then, cells were treated with 10 μM of PD for an additional 24 hr. The Western blotting analysis was performed for MDM2 and FOXO3a.
Western Blotting Analysis
Prostate cancer cells (1×106) cultured in 100 mm2 culture dishes were treated with various concentrations of PD for 24 hr. Whole cell lysates were obtained by cell lysis in ice-cold RIPA buffer and subjected to SDS-PAGE according to a previously reported procedure [17]. The PVDF membrane was incubated with the appropriate primary antibody overnight at 4 °C with gentle shaking. The membrane was then incubated with a goat anti-mouse/rabbit IgG horseradish peroxidase-conjugated antibody (Bio-Rad). Conjugated proteins were detected by the Fusion FX5 Spectra instrument from VilberLourmat Inc. (France).
Mouse Xenograft Model
The animals were used and cared for in accordance with our institutional guidelines for the use of laboratory animals. All animal procedures were approved by the Animal Ethics Committee of the Third Military Medical University. Male athymic pathogen-free nude mice (BALB/c, nu/nu, 4-6 weeks old) were purchased from the Medical Experimental Animal Center of the Third Military Medical University (SCXK-(army)-2007-015). To establish PC3 human prostate cancer xenograft tumors, cultured PC3 cells were harvested and resuspended in serum-free F12 medium containing 20% (v/v) Matrigel(BD Biosciences, Bedford, MA), and were injected subcutaneously (5×106cells) into the left inguinal area of the mice. All animals were monitored for activity, physical condition, body weight and tumor growth. The tumor size was examined every three days by caliper measurement of two perpendicular diameters of the implant, and was converted to a tumor volume(cm3) using the formula: (a×b2)/2, where ‘a’ and ‘b’ refer to the longer and shorter dimensions, respectively.
In Vivo Chemotherapy
About one week after tumor cell inoculation, animals bearing tumors were randomly divided into control and treatment groups (5mice/group). PD was dissolved in PEG400:Saline:Ethanol (400:300:200, v/v/v) and was administered (via i.p. injection) at doses of 1 and 2.5 mg/kg (5 days/week for 24 days). The control group received vehicle only. Mice were sacrificed by CO2 inhalation on Day 24. The tumors were carefully removed, photographed, then homogenized with RIPA buffer (20 mg tumor tissue/300 μl RIPA) in preparation for the Western Blotting analysis.
Statistical Analysis
The data for the different treatment groups are presented as the means ± standard errors. A one-way ANOVA was used to determine the significance of the effects of the treatments on the levels of apoptosis and cell cycle arrest. A repeated measures analysis of variance was used to determine the significance of the in vivo findings. The results were considered to be statistically significant for values of p<0.05.
Results
PD Decreases Prostate Cancer Cell Growth and Proliferation in vitro
Three human prostate cancer cell lines representing three different genetic backgrounds (LNCaP/p53 wild-type, PC3/p53 null and DU145/p53 mutant) were exposed to various concentrations of the test compound (0, 2.5, 5, 10, 25 and 50 μM) for 72 hr, and the cell survival percentages were determined. As seen in Fig. 1B and 1C, the compound demonstrated IC50 values less than 30 μM (11.17-26.13 μM) in all three cell lines. The PC3 cells appeared to be more sensitive to the compound than DU145 and LNCaP cells. It is worth noting that PD showed minimal effects on the non-malignant prostate cell line, RWPE-1, indicating that PD has selective activity against malignant cells. The anti-proliferative effects were seen in all three cell lines (Fig. 1D). Again, PC3 cells were the most sensitive of the three cell lines, with the 20 mM concentration of PD significantly decreasing the proliferation to 30±1.98% of that in the control cells.
PD Induces the Apoptosis of Prostate Cancer Cells
We also examined whether PD induced apoptosis in prostate cancer cells. As illustrated in Fig. 2, the examined prostate cancer cell lines exhibited a significant increase (p<0.05) in apoptosis following exposure to a 10 μM or higher concentration of the compound. In the PC3, LNCaP and DU145 cells (Fig. 3), 20 μM PD increased the cells death by more 15.0%, 12.7% and 18.1%, respectively, compared to that in the DMSO-treated control cells.
Fig. (2).
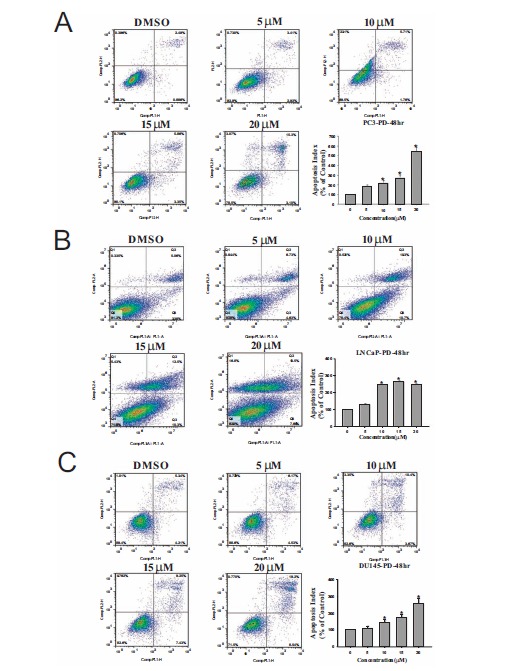
PD induces concentration-dependent apoptosis in prostate cancer cells. The induction of apoptosis in PC3 cells (A), LNCaP cells (B) and DU145 cells (C) treated with various concentrations of PD for 48 hr. All assays were performed in triplicate. The apoptosis index was calculated compared to that in untreated control cells. (*, p<0.05 versus the control).
Fig. (3).
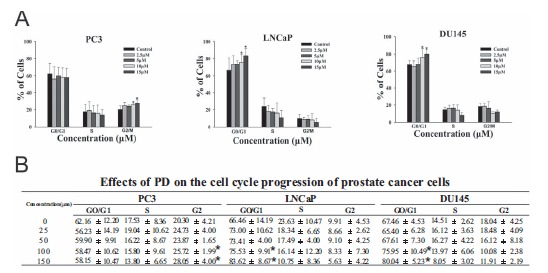
PD induces cell cycle arrest in prostate cancer cells. A. PC3, LNCaP and DU145cells were treated with various concentrations of PD for 48 hr, followed by the determination of the cell cycle distribution. B. A table summarizing the effects of PD on the cell cycle progression of the three prostate cancer cell lines. The data are presented as the percent distribution of cells in a specific phase of the cell cycle. (*, p<0.05 versus the control).
PD Induces Cell Cycle Arrest in Prostate Cancer Cells
We also observed that PD induced cell cycle arrest in prostate cancer cells. In PC3 cells, the majority of the cells were arrested in the G2/M phase (Fig. 3A) after treatment with 10 μM or 15 μM PD (p<0.05). In the LNCaP and DU145 cells, PD led to a significant (p<0.05) increase in the number of cells in the G1 phase at the 10 μM and 15 μM concentrations (Figs. 3B and C). In addition, all three cell lines showed a significant decrease in the proportion of cells in the S-phase after treatment with 15 μM PD. Therefore, the inhibitory effects of PD on cell viability might be associated with its induction of apoptosis and cell cycle arrest (leading to decreased proliferation).
PD Activates FOXO3a Expression via the Downregulation MDM2
FOXO3a is a known suppressor of primary tumor growth via its transcriptional regulation of key genes involved in the cell cycle and apoptosis. Murine double minute 2 (MDM2), an E3 ubiquitin ligase, has been demonstrated to play important roles in human cancer therapy [19, 20]. Yang et al. previously showed that MDM2-mediated FOXO3a degradation promotes tumorigenesis [21]. In the present study, after a 24 hr treatment (0, 2.5, 5, 10 μM), it was found that PD increased the protein expression levels of FOXO3a, p21 and p27, and decreased the level of the MDM2 protein in the PC3 cells. In LNCaP cells, PD increased the FOXO3a, p53, p21 and p27 levels, but did not decrease the MDM2 expression. In DU145 cells, PD increased the FOXO3a, p53, p21 and p27 levels and decreased the MDM2 protein expression. With regard to the increase in FOXO3a expression, the effects in DU145 cells were not as obvious as those in the PC3 and LNCaP cells (Fig. 4A). We also found that, while PD increased the FOXO3a protein expression in PC3 cells, the p-FOXO3a protein expression was decreased by treatment with 10 mM PD (Fig. 4B). A decrease in p-FOXO3a is indicative of less FOXO3a degradation in the nucleus of cells [22].
Fig. (4).
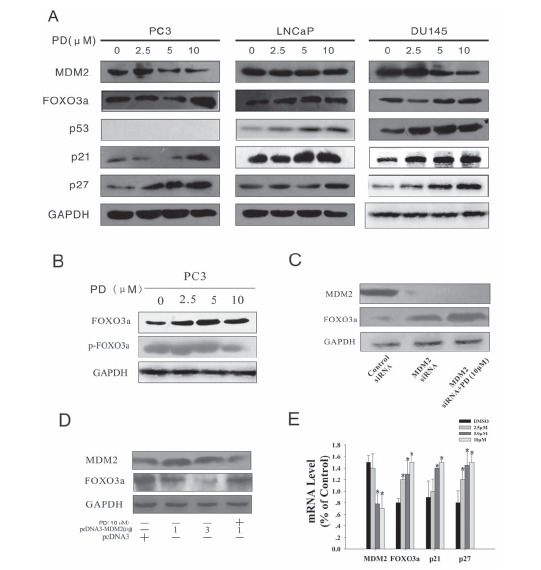
PD downregulates MDM2 and increases FOXO3a expression in PC3 cells. A. PC3, LNCaP and DU145 cells were exposed to various concentrations of PD for 24 hr; then target proteins (MDM2, FOXO3a, p53, p21and p27) were examined by Western blotting (WB). B. The expression of the FOXO3a and p-FOXO3a proteins was examined by WB in PC3 cells after PD treatment for 24 hr. C. & D. PC3 cells were transfected with MDM2 siRNA or a pcDNA-MDM2 (full length) plasmid,then the expression levels of MDM2 and FOXO3a were determined by WB after incubation with or without 10 mM PD. Blocking MDM2 expression by siRNA increased the FOXO3a expression, while MDM2 overexpression decreased the FOXO3a protein expression. However, treatment with PD was able to increase the FOXO3a expression even in the presence of MDM2 overexpression. E. The mRNA expression levels of MDM2, FOXO3a, p21 and p27 were detected by real-time quantitative PCR, and the data were normalized to the mRNA level of GAPDH.
The ERK-phosphorylated FOXO3a is degraded via an MDM2-mediated ubiquitin-proteasome pathway [21]. To examine the role of the FOXO3a and MDM2 interaction in PD-induced prostate cancer cells apoptosis, MDM2 was transiently silenced by siRNA. As shown in Fig. 4C, MDM2 silencing increased the PD-induced FOXO3a protein expression, possibly due to decreased MDM2-mediated FOXO3a degradation, and this suppressed the prostate cancer cell growth. As shown in Fig. 4D, the FOXO3a expression was suppressed by pcDNA3-MDM2 transfection. However, treatment with PD (10 μM) inhibited MDM2 activation, which was accompanied by an increase in the FOXO3a levels. Together, these results suggested that MDM2 suppressed FOXO3a, but treatment with PD could induce FOXO3a expression via the downregulation of MDM2 in prostate cancer cells.
PD Alters the Transcription of both MDM2 and FOXO3a
To further investigate the effects of PD on FOXO3a, we examined its effects on the mRNA levels of various molecules known to be regulated by MDM2 and FOXO3a. The PC3 cells were treated with various concentrations of PD (0, 2.5, 5, 10μM) for 24 hr. The treated cells showed increased mRNA expression of FOXO3a, p21 and p27, and decreased expression of MDM2 mRNA (Fig. 4E).
PD Affects the Expression of Proteins Associated with the Cell Cycle and Apoptosis
To elucidate how PD affected the cell cycle progression and apoptosis, we investigated the expression levels of a panel of proteins associated with the regulation of apoptosis and cell cycle progression (Figs. 5A and 5B). After a 24 hr
treatment (0, 2.5, 5, 10 μM), it was found that PD upregulated the expression of Bax, cleaved caspase8, cleaved caspase9, caspase3 and cleaved caspase3 and downregulated the expression of the Bcl2, PARP, caspase8, caspase9, E2F1, CDK2, CDK4, CDK6, CyclinD1, CDK1 and CycllinB1 proteins.
PD Decreases the Growth of Xenograft Tumors
After observing its potent in vitro effects, we next established a PC3 tumor xenograft model in nude mice to evaluate the anti-tumor activity of PD in vivo. PD was administered 5 days a week for 24 days. The tumor volume and body weight were examined to evaluate the effects of the treatment. There was no significant loss of body weight (Fig. 6A), suggesting that the compound was not overtly toxic. As shown in Fig. 6B and 6C, at a dose of 2.5mg/kg bodyweight, PD led to significant (p=0.03) tumor growth inhibition by approximately 56% on Day 24 compared to the vehicle control. In contrast, at a dose of 1.0 mg/kg body weight, PD led to tumor growth inhibition, but it was not significant (p=0.968).
Fig. (6).
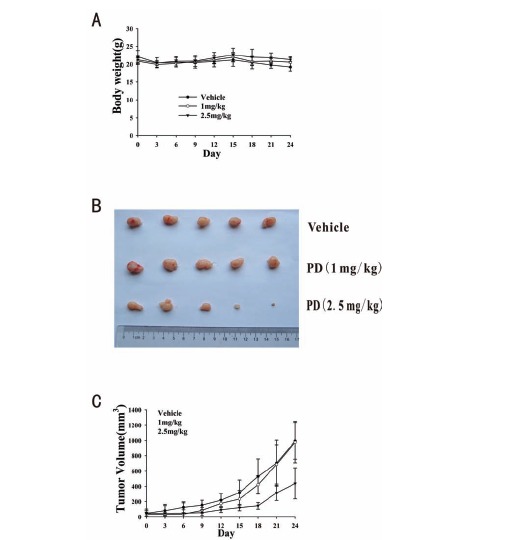
The in vivo effects of PD in nude mice bearing PC3 xenograft tumors. PD was injected intraperitoneally (i.p.) at doses of 1 mg/kg/d and 2.5 mg/kg/d, 5 d/wk for 24 days. A. Animals were monitored for changes in body weight every three days as an indicator of toxicity. B. At the end of the experiment, representative tumors were removed and photographed. C. The tumors were measured every three days to assess their growth.
After a 24 hr treatment (with 0, 2.5, 5 or 10 μM PD), it was found that PD increased the expression of the FOXO3a, p21 and p27 proteins and decreased the MDM2 protein expression in the PC3 cells. Consistent with the in vitro findings, at the dose of 2.5 mg/kg body weight, PD increased the expression of the FOXO3a and p21 proteins and decreased the MDM2 protein expression in the PC3 cell xenograft tumors (Fig. 7). With regard to the protein expression levels of a panel of various proteins associated with the cell cycle and apoptosis, it was found that PD downregulated CDK2, Cyclin D1, CDK1, Cyclin B1, Bcl2 and PARP, and upregulated the expression of Bax (Fig. 7). These findings were consistent with the results of the cell cycle and apoptosis studies in vitro. Taken together, these results indicate that PD could inhibit the growth of prostate cancer cells in vivo, without major toxicity. They also provide preliminary information about the possible mechanism of action of PD.
Fig. (7).
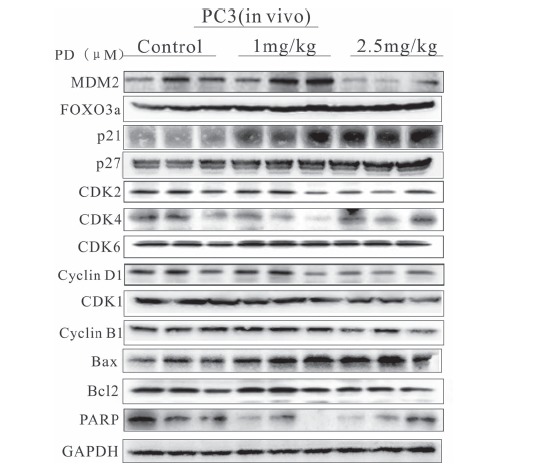
The effects of PD on the expression of proteins related to the MDM2-FOXO3a pathway in PC3 xenograft tumors. The expression of proteins involved in the MDM2-FOXO3a pathway was analyzed by a WB analysis of the tumor homogenates after treatment of the mice with 0, 1 or 2.5 mg/kg PD for 24 days.
Discussion
Natural products have attracted increasing attention worldwide as a potential source of novel therapeutic compounds. Previous studies have indicated that PD possesses anticancer activities against various tumor cells of human origin, including leukemia, breast cancer, gastric cancer, lung cancer and hepatoma [14-16, 23-26]. However, the mechanism(s) of action, and the potential of this compound for treating other cancer types have not yet been examined. To the best of our knowledge, the present study is the first investigation of the effects of PD against human prostate cancer. Our study highlights at least five important points: 1) PD significantly inhibits prostate cancer cell growth; 2) PD induces prostate cancer cell apoptosis; 3) PD obstructs cell cycle progression; 4) PD upregulates FOXO3a expression and downregulates MDM2 expression at the transcriptional level and 5) PD dose-dependently decreases the growth of human prostate cancer xenograft tumors in mice.
In the present study, we initially observed that there were differences in the cell survival among the different prostate cancer cell line after PD treatment, as determined by the MTT assay, with PC3 cells being the most sensitive (Fig. 1B and 1C). It is interesting that PD only minimally affected the viability of non-malignant prostate epithelial cells (RWPE-1) at the concentrations tested, suggesting that PD has selective activity against malignant cells.
After determining the effects of PD on the cell proliferation (Fig. 1D), it was observed that the 15 μM and 20 μM concentrations of PD had significant inhibitory effects on the proliferation of each of the cell lines tested. The PC3 cells were the most sensitive, and even 5 μM of PD significantly inhibited the proliferationof PC3 cells. These findings were consistent with the observations of decreased viability of these cells by the MTT assay.
We then systematically evaluated the underlying mechanism(s) of action of PD by performing apoptosis and cell cycle assays. A previous study showed that PD could induce significant apoptosis in hepatoma cells [27], gastric cancer cells [14], breast cancer cells [23] and leukemia cells [25]. The present results indicate that prostate cancer cells are also effectively eradicated by PD treatment, although the PC3 cells were more sensitive to apoptosis than DU145 cells (Fig. 2).
Further studies on the cell cycle progression revealed that PD induced cell cycle arrest in prostate cancer cells (Fig. 3). Although the prostate cancer cell lines have different p53 status, all three cell lines exhibited a decrease in the number of cells in the S-phase, which occurred in a concentration-dependent manner. In this study, PC3 cells were arrested in the G2/M phase, whereas DU145 cells and LNCaP cells were arrested in the G0/G1 phase, and their DNA synthesis was terminated. This inhibition of DNA synthesis might partially explain the anti-cancer effects of PD.
We then evaluated the expression levels of a panel of proteins associated with the cell cycle and apoptosis. It is well known that MDM2, an E3 ubiquitin ligase, plays a major role in cell cycle progression and apoptosis [19, 20]. A previous study revealed that MDM2 induced the degradation of p21, a universal cell cycle inhibitor that binds to cyclin-CDK complexes [17, 28]. Additionally, it was previously reported that the overexpression of p21 results in the induction of Bax and promotes apoptosis [29]. Therefore, the observed downregulation of MDM2 could have been at least partially responsible for the decreased cell cycle progression and increased apoptosis of prostate cancer cells induced by PD treatment.
FOXO3a is a known suppressor of primary tumor growth via its transcriptional regulation of key genes involved in cell cycle arrest and apoptosis [5, 6, 30]. Shukla et al. reported that FOXO3a plays an important role as a tumor suppressor in prostate cancer [7, 9, 31]. FOXO-mediated regulation of the CIP/KIP family has been reported, and both p21CIP1 and p27KIP1 play crucial roles in controlling cell proliferation. FOXO3a can induce cell cycle arrest by upregulating these cell cycle inhibitors (p21 CIP1 and p27KIP1), and consequently can attenuate the activity of cell cycle-promoting CDKs [5]. It has also been suggested that FOXO3a can induce apoptosis, because FOXO3a has emerged as part of an important effector arm of the PI3K/Akt signaling pathway that drives the expression of multiple genes [6].
Bearing this in mind, we examined the expression of related regulatory proteins after PD treatment in the three cell lines. We observed a concentration-dependent increase in the expression of FOXO3a, p21 and p27, with a concomitant concentration-dependent decrease in MDM2. It is known that FOXO3a is downregulated by a MDM2-mediated ubiquitin-proteasome pathway [21]. In order to determine whether PD could still increase the expression of FOXO3a in the presence of high levels of MDM2 (because MDM2 overexpression is common in human cancers [32, 33]), we performed a trans- fection assay (Fig. 4E). The results showed that PD could indeed increase the protein level of FOXO3a, regardless of the overexpression of MDM2 via a transfected plasmid. Further work is required to fully characterize the relationship between these two proteins with regard to their regulation by PD.
We further investigated the protein expression levels of cell cycle-related and apoptosis-related proteins in PC3 cells. The results showed the downregulation of E2F1, CDK2, CDK4, CDK6, Cyclin D1, CDK1 and Cyclin B1 (proteins whose activation is key for G2/M progression [34, 35]), as well as Bcl2, PARP, caspase8 and caspase9, and found an increase in the expression of Bax, caspase3, cleaved caspase 3 and cleaved caspase 8 proteins in PC3 cells. These findings confirmed that PD can effectively inhibit prostate cancer cell proliferation via MDM2-FOXO3a-mediated changes in apoptosis and cell cycle progression.
In the present study, we also demonstrated that PD had significant in vivo anti-tumor activity against the PC3 prostate cancer xenograft model. At the 2.5 mg/kg dose, we observed 56% tumor growth inhibition. The expression levels of the proteins mentioned above were also examined in tumor tissues by a Western blotting analysis, and the results from the tumor samples were consistent with those obtained from the in vitro studies. The expression of FOXO3a was previously demonstrated to inhibit tumor development in xenograft models [21, 36], and repression of FOXO3a expression enhanced tumor progression and angiogenesis [7, 9, 37, 38]. Thus, the results of the present study confirmed that PD exerts a promising tumoricidal activity against prostate cancer in vitro and in vivo at least partly via its effects on cancer-associated molecules, including FOXO3a.
In summary, our present findings provide the first evidence that prostate cancer is highly sensitive to PD treatment. We also demonstrated that MDM2- and FOXO3a-mediated effects appear to play an important role in the PD-induced apoptosis and cell cycle arrest of prostate cancer cells. Further studies will be needed to elucidate the detailed mechanisms underlying these effects. However, the current results are promising, and provide support for further studies of PD as a candidate natural product-based therapeutic agent for prostate cancer.
Fig. (5).
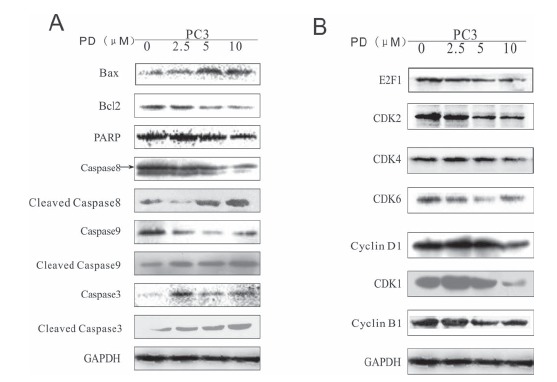
The effects of PD on the expression of proteins related to cell cycle progression and apoptosis in human prostate cancer cells. The expression levels of proteins related to apoptosis (A) and cell cycle progression (B) in PC3 cells were examined by WB.
ACKNOWLEDGEMENTS
This work was supported in part by National Natural Science Foundation of China (No: 81171991) to Hongxia Xu. We thank Dr. E. R. Rayburn for help with editing the manuscript and helpful discussions.
list of Abbreviations
- PD
Platycodin D
- MDM2
Murine double minute 2
- FOXO3a
Forkhead box O3
- ANOVA
analysis of variance
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
References
- 1.American Cancer Society Cancer Facts and Figures http://www.cancer.org/Cancer/ProstateCancer/index. (Accessed January 28, 2014). 2013.
- 2.Carles J., Castellano D., Climent M.Á., Maroto P., Medina R., Alcaraz A. Castration-resistant metastatic prostate cancer: current status and treatment possibilities. Clin. Transl. Oncol. 2012;14:169–176. doi: 10.1007/s12094-012-0780-8. [DOI] [PubMed] [Google Scholar]
- 3.Schweizer M.T., Antonarakis E.S. Chemotherapy and its evolving role in the management of advanced prostate cancer. Asian J. Androl. 2014;16:334. doi: 10.4103/1008-682X.122593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X., Tang N., Hadden T.J., Rishi A.K. FoxO and regulation of apoptosis. . Biochim. Biophys. Acta. 2011:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Fu Z., Tindall D.J. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greer E.L., Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 7.Shukla S., Shukla M., MacLennan G.T., Fu P., Gupta S. Deregulation of FOXO3A during prostate cancer progression. Int. J. Oncol. 2009;34:1613. doi: 10.3892/ijo_00000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzivion G., Dobson M., Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. . Biochim. Biophys. Acta. 2011. pp. 1938–1945. [DOI] [PubMed]
- 9.Shukla S., Bhaskaran N., Babcook M.A., Fu P., MacLennan G.T., Gupta S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis. 2014;35:452–460. doi: 10.1093/carcin/bgt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunters A., Fernández de Mattos S., Stahl M., Brosens J.J., Zoumpoulidou G., Saunders C.A., Coffer P.J., Medema R.H., Coombes R.C., Lam E.W. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J. Biol. Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 11.Medema R.H., Kops G.J., Bos J.L., Burgering B.M. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T., Iino M. Knockdown of Sec8 promotes cell-cycle arrest at G1/S phase by inducing p21 via control of FOXO proteins. FEBS J. 2014;281:1068–1084. doi: 10.1111/febs.12669. [DOI] [PubMed] [Google Scholar]
- 13.Neychev V.K., Nikolova E., Zhelev N., Mitev V.I. Saponins from Tribulusterrestris L. are less toxic for normal human fibroblasts than for many cancer lines: Influence on apoptosis and proliferation. Exp. Biol. Med. 2007;232:126–133. [PubMed] [Google Scholar]
- 14.Chun J., Joo E.J., Kang M., Kim Y.S. Platycodin D induces anoikis and caspase-mediated apoptosis via p38 MAPK in AGS human gastric cancer cells. J. Cell. Biochem. 2013;114:456–470. doi: 10.1002/jcb.24386. [DOI] [PubMed] [Google Scholar]
- 15.Yu J.S., Kim A.K. Platycodin D induces apoptosis in MCF-7 human breast cancer cells. J. Med. Food. 2010;13:298–305. doi: 10.1089/jmf.2009.1226. [DOI] [PubMed] [Google Scholar]
- 16.Chun J., Kim Y.S. Platycodin D inhibits migration, invasion, and growth of MDA-MB-231 human breast cancer cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem. Biol. Interact. 2013;205:212–221. doi: 10.1016/j.cbi.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Xu H., Zhang Z., Li M., Zhang R. MDM2 promotes proteasomal degradation of p21Waf1 via a conformation change. J. Biol. Chem. 2010;285:18407–18414. doi: 10.1074/jbc.M109.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S., Zeng J.Y., Zhuang C.M., Zhou Y.Q., Yao H.P., Hu X., Zhang R., Wang M.H. Small-molecule inhibitor BMS-777607 induces breast cancer cell polyploidy with increased resistance to cytotoxic chemotherapy agents. Mol. Cancer Ther. 2013;12:725–736. doi: 10.1158/1535-7163.MCT-12-1079. [DOI] [PubMed] [Google Scholar]
- 19.Wade M., Wong E.T., Tang M., Stommel J.M., Wahl G.M. Hdmx modulates the outcome of p53 activation in human tumor cells. J. Biol. Chem. 2006;281:33036–33044. doi: 10.1074/jbc.M605405200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang R., Wang H. MDM2 oncogene as a novel target for human cancer therapy. Curr. Pharm. Des. 2000;6:393–416. doi: 10.2174/1381612003400911. [DOI] [PubMed] [Google Scholar]
- 21.Yang J.Y., Zong C.S., Xia W., Yamaguchi H., Ding Q., Xie X., Lang J.Y., Lai C.C., Chang C.J., Huang W.C., Huang H., Kuo H.P., Lee D.F., Li L.Y., Lien H.C., Cheng X., Chang K.J., Hsiao C.D., Tsai F.J., Tsai C.H., Sahin A.A., Muller W.J., Mills G.B., Yu D., Hortobagyi G.N., Hung M.C. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat. Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu M.C., Lee D.F., Xia W., Golfman L.S., Ou-Yang F., Yang J.Y., Zou Y., Bao S., Hanada N., Saso H., Kobayashi R., Hung M.C. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 23.Yu J.S., Kim A.K. Platycodin D induces reactive oxygen species-mediated apoptosis signal-regulating kinase 1 activation and endoplasmic reticulum stress response in human breast cancer cells. J. Med. Food. 2012;15:691–699. doi: 10.1089/jmf.2011.2024. [DOI] [PubMed] [Google Scholar]
- 24.Shin D.Y., Kim G.Y., Li W., Choi B.T., Kim N.D., Kang H.S., Choi Y.H. Implication of intracellular ROS formation, caspase-3 activation and Egr-1 induction in platycodon D-induced apoptosis of U937 human leukemia cells. Biomed. Pharmacother. 2009;63:86–94. doi: 10.1016/j.biopha.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Kim M.O., Moon D.O., Choi Y.H., Lee J.D., Kim N.D., Kim G.Y. Platycodin D induces mitotic arrest in vitro, leading to endoreduplication, inhibition of proliferation and apoptosis in leukemia cells. Int. J. Cancer. 2008;122:2674–2681. doi: 10.1002/ijc.23442. [DOI] [PubMed] [Google Scholar]
- 26.Kim M.O., Moon D.O., Choi Y.H., Shin D.Y., Kang H.S., Choi B.T., Lee J.D., Li W., Kim G.Y. Platycodin D induces apoptosis and decreases telomerase activity in human leukemia cells. Cancer Lett. 2008;261:98–107. doi: 10.1016/j.canlet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Qin H., Du X., Zhang Y., Wang R. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, induces G2/M arrest and apoptosis in human hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumour Biol. 2014;35:1267–1274. doi: 10.1007/s13277-013-1169-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z., Wang H., Li M., Agrawal S., Chen X., Zhang R. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J. Biol. Chem. 2004;279:16000–16006. doi: 10.1074/jbc.M312264200. [DOI] [PubMed] [Google Scholar]
- 29.Kang K.H., Kim W.H., Choi K.H. p21 promotes ceramide-induced apoptosis and antagonizes the antideath effect of Bcl-2 in human hepatocarcinoma cells. Exp. Cell Res. 1999;253:403–412. doi: 10.1006/excr.1999.4644. [DOI] [PubMed] [Google Scholar]
- 30.Myatt S.S., Lam E.W. The emerging roles of forkhead box (Fox) proteins in cancer. Nat. Rev. Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 31.Shukla S., Bhaskaran N., MacLennan G.T., Gupta S. Deregulation of FoxO3a accelerates prostate cancer progression in TRAMP mice. Prostate. 2013;73:1507–1517. doi: 10.1002/pros.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onel K., Cordon-Cardo C. MDM2 and prognosis. Mol. Cancer Res. 2004;2:1–8. [PubMed] [Google Scholar]
- 33.Rayburn E., Zhang R., He J., Wang H. MDM2 and human malignancies: expression, clinical pathology, prognostic markers, and implications for chemotherapy. Curr. Cancer Drug Targets. 2005;5:27–41. doi: 10.2174/1568009053332636. [DOI] [PubMed] [Google Scholar]
- 34.Jackman M., Lindon C., Nigg E.A., Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 35.Chen T., Xu Y., Guo H., Liu Y., Hu P., Yang X., Li X., Ge S., Velu S.E., Nadkarni D.H., Wang W., Zhang R., Wang H. Experimental therapy of ovarian cancer with synthetic makaluvamine analog: in vitro and in vivo anticancer activity and molecular mechanisms of action. PLoS One. 2011;6:e20729. doi: 10.1371/journal.pone.0020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu M.C., Hung M.C. Role of IkappaB kinase in tumorigenesis. Future Oncol. 2005;1:67–78. doi: 10.1517/14796694.1.1.67. [DOI] [PubMed] [Google Scholar]
- 37.Potente M., Urbich C., Sasaki K.I., Hofmann W.K., Heeschen C., Aicher A., Kollipara R., DePinho R.A., Zeiher A.M., Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J. Clin. Invest. 2005;115:2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katoh M., Igarashi M., Fukuda H., Nakagama H., Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328:198–206. doi: 10.1016/j.canlet.2012.09.017. [DOI] [PubMed] [Google Scholar]


