Abstract
Oxyresveratrol (ORV) is a naturally occurring compound found in mulberries that exhibits a wide spectrum of biological activities. However, the underlying mechanism of the action of ORV against the methicillin-resistant S. aureus (MRSA) pathogen has not yet been reported. MRSA is multidrug-resistant, causing skin and other types of infections. The aim of the present study was to examine the antimicrobial activity of ORV and the underlying mechanism of its action on MRSA. The antibacterial activity of ORV was evaluated using a minimum inhibitory concentration (MIC) assay, and the mechanism of its antibacterial action on S. aureus was investigated using a combination of ORV with detergent, ATPase inhibitors and peptidoglycan (PGN). In addition, the survival characteristics and changes in MRSA morphology were monitored using transmission electron microscopy (TEM). The MIC value of ORV against all S. aureus strains was found to be 125 µg/ml. The optical density at 600 nm of each suspension treated using a combination of ORV with Triton X-100, N,N'-dicyclohexylcarbodiimide or sodium azide was reduced by 68.9–89.8% compared with the value upon treatment with ORV alone. In the ORV and PGN combination assay, direct binding of ORV with PGN from S. aureus was evident. Furthermore, TEM examination of MRSA treated with ORV showed alterations in septa formation. In conclusion, these results showed that ORV has a strong antibacterial effect against S. aureus, mainly by increasing membrane permeability and inhibiting ATPase when combined with other drugs.
Keywords: oxyresveratrol, antibacterial mechanism, membrane permeability, methicillin-resistant Staphylococcus aureus, ATPase
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a prominent human pathogen that is known for causing skin infections, as well as hospital-acquired pneumonia, osteomyelitis and abscesses (1); MRSA may also result in considerable morbidity and mortality in orthopedic patients. The mortality rate of MRSA bacteremia is twice as high as that of patients with methicillin-sensitive Staphylococcus aureus (MSSA) (2). In addition, the complication rate and cost of periprosthetic joint infection due to MRSA is considerably higher compared with that in MSSA infection (3). Patients receiving orthopedic implants are highly vulnerable due to the possibility of biofilm formation and long-term morbidity (4,5). Currently, the incidence of MRSA in orthopedic departments is increasing (6). MRSA strains are resistant not only to β-lactam antibiotics, but also to fluoroquinolones and other families of antibiotics (7).
The Morus genus is part of the Moraceae family, which includes 10–16 species of deciduous trees that are found worldwide (8). For >1,900 years, different parts of the Morus plants, including the branches, fruit, leaves, bark, shoot and root, have been used in China as food and herbal medicine (9). The compounds resveratrol and oxyresveratrol (ORV) are present in the Morus plants and have been revealed to possess antioxidant activity (10). A previous study reported that ORV inhibited the production of prostaglandin E2 and nitrogen oxide (NO), the expression of inducible NO synthase (iNOS) and the activation of nuclear factor-κB in macrophages, while consistently reducing carrageenan-induced edema in a mouse model (11).
ORV, a polyphenolic stilbene, is abundantly derived from the heartwood of the traditional Thai plant, Artocarpus lakoocha Roxburgh, which belongs to the Moraceae family (12,13). This compound has been demonstrated to have an inhibitory effect on the growth of Herpes simplex virus (HSV)-1 and HSV-2 wild types, drug-resistant HSV-1 strains (14), clinical isolates of HSV-1 and HSV-2 (15), in addition to numerous varicella zoster virus (VZV) strains, including the wild type, thymidine kinase-deficient and DNA polymerase VZV mutants in vitro (16). Numerous biological activities of ORV have also been reported, including tyrosinase-inhibition (17) and antioxidant (18) and anthelmintic activities (19). Topical application of 30% w/w ORV in petroleum jelly suspension was reported to provide superior therapeutic efficacy compared with oral treatment with ORV for cutaneous HSV-1 infection in mice (14).
However, the antibacterial capacity and mechanism of ORV against Staphylococcus aureus (S. aureus) remain unknown. Therefore, the present study investigated the antibacterial activities of ORV alone or in combination with bacterial membrane-binding agents, including Triton X-100 (TX), sodium azide (NaN3) and N,N′-dicyclohexylcarbodiimide (DCCD). In addition, the effects of adding peptidoglycan (PGN; derived from S. aureus) into Müller-Hinton broth (MHB) containing ORV alone were also examined. The aim of the present study was to gain an insight into the antibacterial activity, survival characteristics and changes in the bacterial morphology and mechanism of ORV against MRSA.
Materials and methods
Isolation and purification of ORV
ORV (purity, >96.32%) was provided by the Standardized Material Bank for New Botanical Drugs (no. NNMBP000018) at Wonkwang University (Iksan, Korea). Twigs from the Morus alba plant were supplied by the Herbal Medicine Co-operative Association of Jeonbuk Province (Iksan, Korea) in October 2010. EtOH (2 litres) was added to 2 kg of dried Morus alba twigs for 20 days at room temperature. The dried residue of the EtOH extract (101 g) was dissolved in 40% aqueous MeOH (1 l) and separated with n-hexane (800 ml, twice), CH2Cl2 (800 ml, twice) and EtOAc (800 ml, twice), successively. A column (5×16 cm) filled with Sephadex LH-20 (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) was used to perform chromatography on the soluble fraction of CH2Cl2 (8.53 g); CH2Cl2-MeOH (ratio, 4:1 to 1:1, for each volume of 300 ml) was used to obtain four fractions (denoted A-D). Next, the soluble fraction of EtOAc (4.83 g) was separated by chromatography on a silica gel (250 g) column using CH2Cl2-MeOH (ratio, 8:1 to 4:1, for each volume of 600 ml) in order to obtain three additional fractions (denoted E-G). Subsequently, a silica gel (150 g) column (eluent, n-hexane-acetone, at a ratio of 1:1) was used to perform chromatography on fraction E (2.77 g), a fraction chosen due to its abundance of ORV. The sample was further purified by Sephadex LH-20 column chromatography (2.5×20 cm; eluent, CH2Cl2-MeOH, at a ratio of 4:1) in order to obtain ORV (1.12 g, 0.056% w/w). The structure of ORV was then identified by mass spectrometry [using a Q-TOF micro LC-MS/MS instrument (Waters Corporation, Manchester, UK) located at Korea University, Seoul, Korea] and nuclear magnetic resonance analyses [recorded in CDCl3 or acetone-d6 using a JNM ECX-400 spectrometer (JEOL, Ltd., Tokyo, Japan) operating at 400 MHz for 1H and at 100 MHz for 13C] in accordance with our previous study (20).
Reagents
Müeller-Hinton agar (MHA) and MHB, as nutrient media, were purchased from BD Biosciences (Franklin Lakes, NJ, USA). NaN3 and PGN were obtained from Sigma-Aldrich (Buchs, Switzerland) and TX, DCCD, purified lipopolysaccharide (LPS), ampicillin (AM), oxacillin (OX), gentamicin (GT), vancomycin (VC), norfloxacin (NR) and ciprofloxacin (CP) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Bacterial strains and growth conditions
Three clinical isolates of MRSA (DPS-1, −2 and −3) were obtained from three different patients of the Department of Plastic Surgery, Wonkwang University Hospital (Iksan, Korea), in accordance with the methods used in a previous study (1). Two additional strains were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA); these were the S. aureus strains ATCC 33591 (methicillin-resistant) and ATCC 25923 (methicillin-susceptible) (Table I). Prior to the experiments, all strains were stored in 30% glycerol and frozen at −70°C. The bacterial strains were inoculated onto MHA plates and incubated at 37°C for 24 h.
Table I.
Determination of the mecA gene status of the S. aureus strains used.
| S. aureus strain | Class | mecA gene | β-lactamase activity | Antibiotic resistance |
|---|---|---|---|---|
| ATCC 33591 | MRSA | + | + | AM, OX |
| ATCC 25923 | MSSA | − | − | − |
| Clinical isolates | ||||
| DPS-1 | MRSA | + | + | AM, OX |
| DPS-2 | MRSA | + | − | AM, OX |
| DPS-3 | MRSA | + | + | AM, OX |
S. aureus, Staphylococcus aureus; ATCC, American Type Culture Collection; MRSA, methicillin-resistant S. aureus; AM, ampicillin; OX, oxacillin; MSSA, methicillin-sensitive S. aureus; DPS, strain from the Department of Plastic Surgery, Wonkwang University Hospital.
Antimicrobial resistance testing
Testing for mecA gene activity was used to identify staphylococci resistant to β-lactam antibiotics (21). Detection of the mecA gene in MRSA strains (Table I) was performed by polymerase chain reaction amplification, as follows: Prior to DNA extraction, bacteria stock cultures were subcultured twice onto MHA plates. For rapid extraction, 1–5 bacterial colonies were suspended in 300 µl of buffer from the Easy-RED BYF total RNA extraction kit (Intron Biotechnology, Inc., Seongnam, Korea) and heated at 100°C for 20 min. After centrifugation at 10,000 × g for 10 min, 2 µl of the supernatant was used for the DNA extraction. cDNA was synthesized from RNA at 42°C for 60 min using a Power cDNA synthesis kit (Intron Biotechnology, Inc.). PCR reactions were performed using 2 µl of cDNA per reaction and an MRSA Primer mix kit (Genotek Co., Daejeon, Korea) in a total reaction volume of 20 µl. The PCR amplification consisted of 30 cycles (94°C, 60 sec; 55°C, 60 sec; 72°C, 60 sec). The final PCR products were separated on a 2% agarose gel. β-lactamase activity, indicating antibiotic resistance, was determined using a β-lactamase activity assay kit (Sigma-Aldrich), according to the manufacturer's protocol.
Antibacterial susceptibility experiments
The minimum inhibitory concentration (MIC) was determined using a broth micro-dilution method, in accordance with the Clinical and Laboratory Standards Institute guidelines (22). Briefly, an inoculum of the microorganisms from the MHA plates was prepared by growing the microorganism in a two-fold dilution of ORV in MHB for 24 h. Next, the suspension was adjusted to a 0.5 McFarland standard turbidity [~1.5×108 colony-forming units (CFU)/ml], with the final inoculums adjusted to 1.5×106 CFU/ml. The MIC was defined as the lowest concentration of ORV and the various antibiotics that inhibited microorganism growth following incubation at 37°C for 24 h in well plates. Subsequent to the incubation period, the well plates were visually examined for turbidity. Cloudiness indicated that bacterial growth had not been inhibited by the concentration of antimicrobial agent contained in the medium. The antibiotics AM, OX, GT, VC, NR and CP were used in comparisons of MIC with ORV-only conditions (Table II) (21).
Table II.
S. aureus strains used in the experiments and MIC.
| MIC (µg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus strain | Class | ORV | AM | OX | VC | GT | NR | CP |
| ATCC 25923 | MSSA | 125 | 31.25 | 125 | 3.9 | 62.5 | 15.6 | 31.25 |
| ATCC 33591 | MRSA | 125 | 1,000 | 500 | 1.95 | 31.25 | 250 | 500 |
| DPS-1 | MRSA | 125 | 1,000 | 1,000 | 1.95 | 250 | 31.25 | 125 |
| DPS-2 | MRSA | 125 | 1,000 | 1,000 | 3.9 | 125 | 31.25 | 125 |
| DPS-3 | MRSA | 125 | 1,000 | 1,000 | 1.95 | 125 | 31.25 | 125 |
S. aureus, Staphylococcus aureus; MIC, minimum inhibitory concentration; ORV, oxyresveratrol; AM, ampicillin; OX, oxacillin; VC, vancomycin; GT, gentamicin; NR, norfloxacin; CP, ciprofloxacin; ATCC, American Type Culture Collection; MSSA, methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus; DPS, strain from the Department of Plastic Surgery, Wonkwang University Hospital.
Antibacterial activity with detergent or ATPase inhibitors
The antibacterial activity of ORV in the presence of a detergent, TX, was analyzed to elucidate whether the antibacterial activity of ORV was associated with altered membrane permeability. The activity of ORV in the presence of ATPase-inhibiting agents, DCCD and NaN3, was also examined to determine whether it was associated with multidrug resistance. In order to determine the detergent-induced permeabilization, ORV was mixed with TX (23), since the non-ionic detergent TX is known to greatly increase antibiotic sensitivity (24). DCCD and NaN3, two metabolic inhibitors that decrease the ATP levels by disrupting electrochemical proton gradients in bacteria (25,26), were used as ATPase inhibitors. The antibacterial activity of 62.5 µg/ml ORV was measured in the presence of 0.1% TX, 0.001% NaN3 and 6.25 µg/ml DCCD compared to that of ORV alone, determined by a reading of absorbance [or optical density measured at a wavelength of 600 nm (OD600)] using an Epoch microplate spectrophotometer (Bio-Tek Instruments, Inc., Winooski, VT, USA). This measurement was indicative of cell proliferation.
Effect of exogenous PGN on ORV activity
ORV may bind to the cell wall and disrupt its integrity. To confirm the action of ORV upon addition of exogenous PGN, ORV + PGN combination assays were performed using the method described by Zhao et al (27). These assays were used to determine whether ORV directly binds to PGN or disrupts the integrity of the cell wall when the same concentrations of ORV and PGN were combined (0–62.5 µg/ml, i.e. up to 50% of the MIC of ORV). LPS was used as a control.
Transmission electron microscopy (TEM)
Exponential-phase MRSA cultures were prepared using overnight cultures incubated in MHB at 37°C until they reached the mid-logarithmic phase of growth (21). Subsequently, the MHB-grown exponential-phase MRSA cultures were treated with ORV at 50% of the MIC, and at the MIC dose for 30 min. Subsequent to the treatment, 2 ml of the culture was collected by centrifugation at 10,000 × g for 10 min. Following removal of the supernatant, cell pellets were fixed with modified Karnovsky's fixative (Electron Microscopy Sciences, Hatfield, PA, USA). Energy-filtering TEM (Libra 120; Carl Zeiss, Oberkochen, Germany) was then performed to examine the samples at an accelerating voltage of 120 kV. Transmitted electron signals were recorded using a 4k × 4k pixel slow-scan charge-coupled device camera (Ultrascan 4000 SP; Gatan Inc., Pleasanton, CA, USA) attached to the electron microscope.
Statistical analysis
All experiments were performed three times. Data from the experiments are presented as the mean ± SEM. Dunnett's t-test was used for multiple comparisons. P-values <0.01 were considered to represent a statistically significant difference.
Results and Discussion
Antibacterial agents inhibit bacterial growth through a variety of complex mechanisms, including the disruption of cell membranes, the inhibition of cell wall, nucleic acid and protein synthesis and the inhibition of nucleic acid metabolism (28). The initial and most important step in reducing antibiotic resistance is to develop antibiotics from natural products that do not result in any toxic or other detrimental side effects (1,26). The development of alternative antimicrobial drugs against infectious diseases is therefore required.
Our previous study demonstrated the synergistic effects of combining ORV with antibiotics in the treatment of MRSA, revealing that combinatorial treatment effectively inhibited MRSA growth (29). The present study aimed to develop anti-MRSA agents using novel combinations of ORV and antibiotics to directly address the resistance of MRSA bacteria. Antibacterial susceptibility tests, based on determination of cell proliferation, demonstrated the inhibitory effect of ORV against S. aureus compared with antibiotics AM, OX, VC, GT, NR, and CP. The results of the MIC assay performed on five strains of S. aureus are presented in Table II. The growth of MRSA was inhibited at 125 µg/ml ORV. The antibacterial activity of ORV had superior potency to the antibiotics AM and OX. The MICs of VC, GT, NR and CP were from 1.95–3.9 µg/ml, from 62.5–250 µg/ml, from 15.6–250 µg/ml and 31.25–500 µg/ml, respectively.
To investigate the effects of enhanced membrane permeability and ATPase inhibition, the antibacterial activity of ORV was examined in the presence of a detergent (TX) and two ATPase-inhibiting agents (DCCD and NaN3). TX is a detergent known to increase the membrane permeability of various bacterial strains, and to induce the release of lipoteichoic acid (LTA) from the cell wall of S. aureus (30). LTA, a major constituent of the cell wall of gram-positive bacteria, is covalently bonded to the outer portion of PGN and is associated with the cytoplasmic membrane (23). TX has also been revealed to reduce methicillin resistance and increase antibiotic sensitivity in S. aureus strains (23).
In the present study, S. aureus was demonstrated to have an increased susceptibility to ORV in combination with TX, compared with that of ORV alone (62.5 µg/ml), as reported in Fig. 1. In addition, compared with the OD600 value for ORV alone (62.5 µg/ml), the OD600 value for the combination of TX with ORV was reduced by 89.8% (Fig. 1), while bacterial viability in the presence of ORV with DCCD (Fig. 2) and 0.001% NaN3 (Fig. 3) was also reduced by 80.5% and 68.9%, respectively. DCCD impedes the ATP-binding cassette transporter, whilst NaN3 is a metabolic inhibitor that reduces the ATP levels by disrupting the electrochemical proton gradients in the bacteria (21). In the present study, S. aureus viability was markedly reduced upon addition of ORV in combination with ATPase inhibitors (DCCD or NaN3), compared with the use of ORV alone (Figs. 2 and 3). These results demonstrate that the anti-MRSA activity of ORV is enhanced by changes in the membrane permeability and a reduced ATP level.
Figure 1.
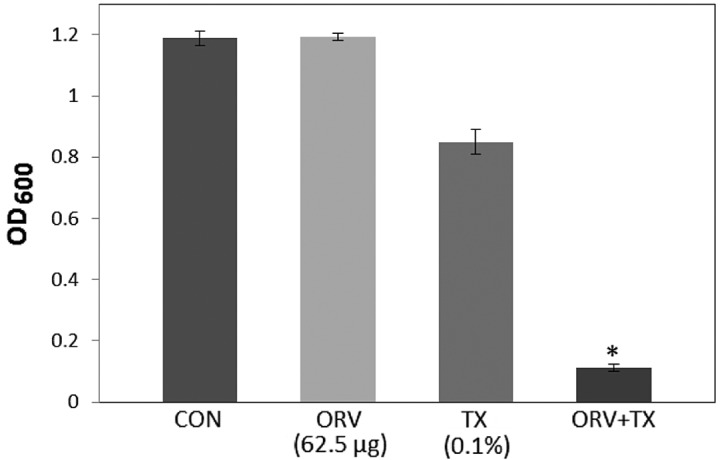
Effect of the membrane-permeabilizing agent, TX, on the susceptibility of Staphylococcus aureus (ATCC 33591) to ORV treatment. Bacteria viability was determined spectrophotometrically after reincubation for 36 h with 62.5 µg/ml ORV and 0.1% TX. Data are represented as an average of three-independent experiments. *P<0.01, vs. ORV alone. TX, Triton X-100; ORV, oxyresveratrol; CON, control; OD600, optical density at 600 nm.
Figure 2.
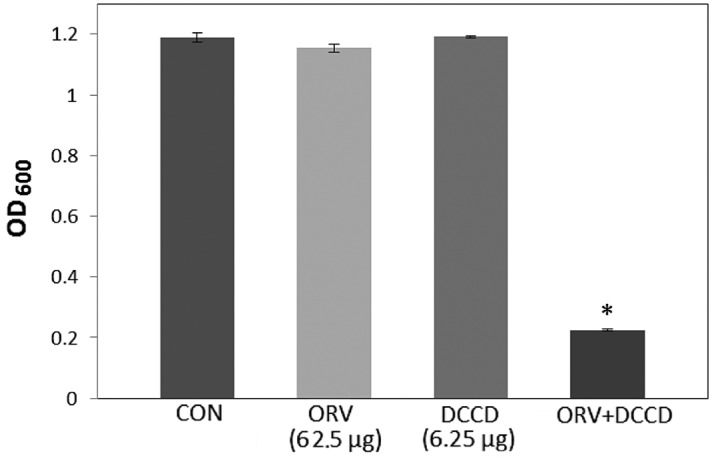
Effect of the ATPase-inhibitor DCCD on the susceptibility of Staphylococcus aureus (ATCC 33591) to ORV treatment. The viability of bacteria was determined spectrophotometrically (OD600) after incubation for 36 h with 62.5 µg/ml ORV and 6.25 µg/ml DCCD. Data are represented as an average of three independent experiments. *P<0.01, vs. ORV alone. DCCD, N,N'-dicyclohexylcarbodiimide; ORV, oxyresveratrol; CON, control; OD600, optical density at 600 nm.
Figure 3.
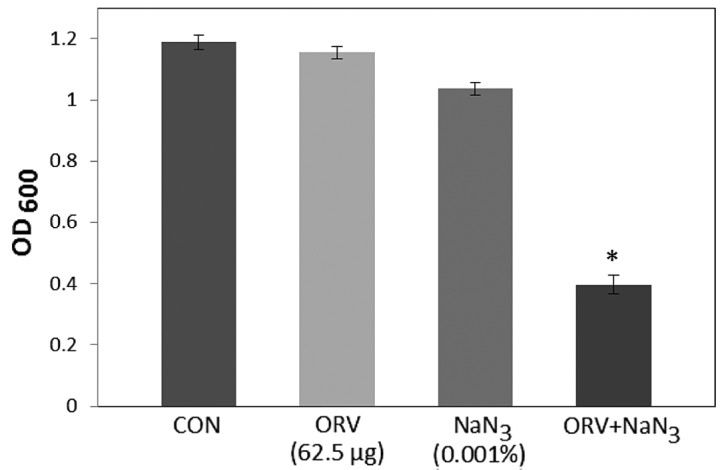
Effect of the ATPase-inhibitor NaN3 on the susceptibility of MRSA (ATCC 33591) to ORV. The viability of bacteria was determined spectrophotometrically (OD600) after incubation for 24 h with 62.5 µg/ml ORV and 0.001% NaN3. Data are the mean of three-independent experiments. *P<0.01, vs. ORV alone. NaN3, sodium azide; MRSA, MRSA, methicillin-resistant Staphylococcus aureus; ORV, oxyresveratrol; CON, control; OD600, optical density at 600 nm.
The cell wall of gram-negative bacteria typically contains <10% of PGN, whereas the PGN content in gram-positive bacteria ranges from 5–95% (31). The gram-positive bacterial cell wall consists of 30–50 PGN sheets outside the cell membrane, which is important in cell division and osmotic protection (32). ORV directly binds the cell wall and affects its integrity. In the present study, ORV-induced inhibition of bacterial growth (Fig. 4) indicated that ORV interfered with bacterial biosynthesis. PGN at a dose of 62.5 µg/ml completely blocked the antibacterial activity of ORV, indicating the direct binding of ORV with PGN on the cell wall. The cell wall ORV-binding effect of PGN was confirmed by the addition of PGN derived from S. aureus into MHB containing ORV alone (62.5 µg/ml). Under the same conditions, LPS, which was used as the control, did not demonstrate any such effect.
Figure 4.
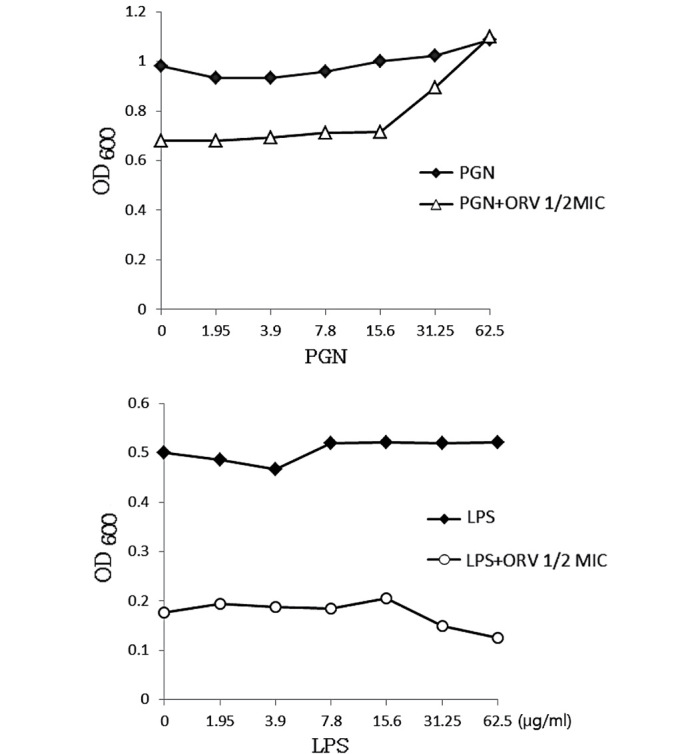
ORV-blocking effect by exogenous PGN of the S. aureus cell wall. LPS was used as a control. ORV, oxyresveratrol; PGN, peptidoglycan; LPS, lipopolysaccharide; MIC, minimum inhibitory concentration; OD600, optical density at 600 nm.
The cell morphology of ORV-treated cells was observed using TEM, which confirmed weakening of the cell wall and lytic effects of ORV on the S. aureus strain, ATCC 33591 (33). The micrographs reported in Fig. 5 illustrate the cell wall and membrane damage following ORV treatment in S. aureus. The control cells demonstrated normal S. aureus morphology with distinct septa (Fig. 5A), whereas bacterial cells treated with ORV at a dose of 62.5 µg/ml (i.e. 50% of the MIC) had a deformed septum and midline disruption (Fig. 5B). In addition, upon exposure of S. aureus to a higher dose of ORV (125 µg/ml, i.e. the MIC), cell division and ghost cells were observed (Fig. 5C). Distinct septa were rarely discerned in the treated cells. TEM observation of ORV-treated MRSA cells substantiates the results indicating that ORV treatment induced altered expression of cell wall- and cell division-associated genes in MRSA.
Figure 5.
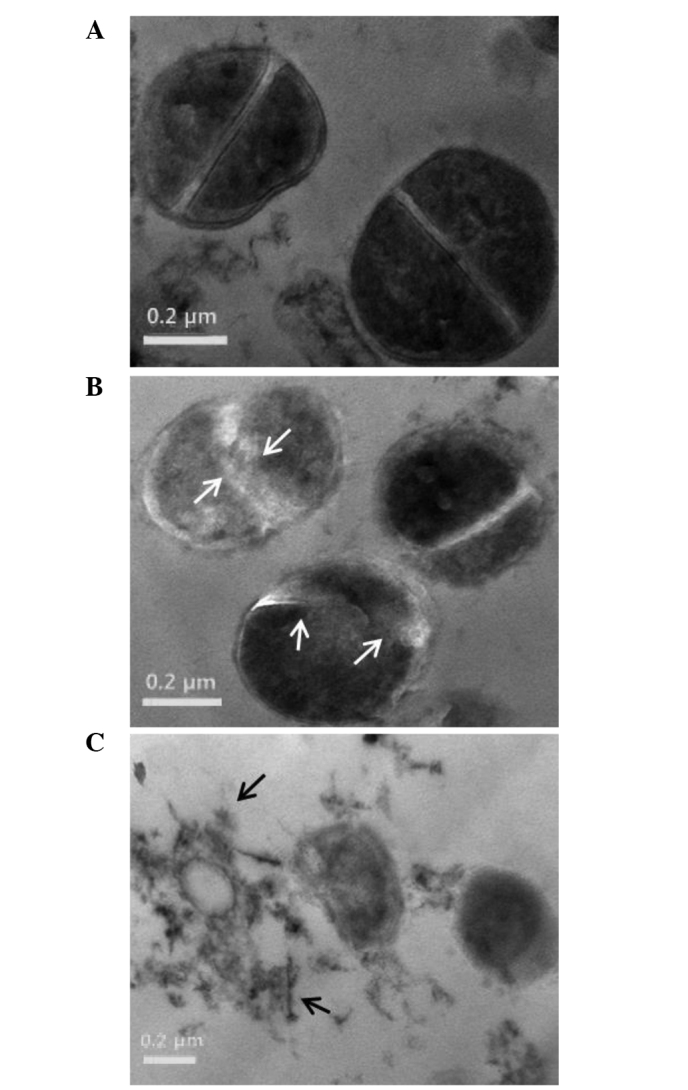
Transmission electron microscopy images of S. aureus (strain, ATCC 33591) after 24 h ORV treatment. (A) Untreated control S. aureus. Treatment of S. aureus with ORV at a dose of (B) 62.5 µg/ml (50% of the MIC), with white arrows indicating the cytoplasmic membrane disruption, and (C) 125 µg/ml (MIC), with black arrows indicating the separation of cytoplasmic contents from the membrane. ORV, oxyresveratrol; MIC, minimum inhibitory concentration.
In conclusion, the results of the present study suggests that ORV has antibacterial activity against MRSA. However, additional in vivo experiments are required to confirm that ORV may be effective against MRSA infections, which will be addressed in subsequent studies.
Acknowledgements
The present study was supported by grants from the Ministry of Food and Drug Safety (grant.no. 12172MFDS990; 2014), and the National Research Foundation of Korea (grant.no. 2008-0062484; funded by the Korean government).
References
- 1.Mun SH, Kang OH, Joung DK, Kim SB, Choi JG, Shin DW, Kwon DY. In vitro anti-MRSA activity of carvone with gentamicin. Exp Ther Med. 2014;7:891–896. doi: 10.3892/etm.2014.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitby M, McLaws ML, Berry G. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: A meta-analysis. Med J Aust. 2001;175:264–267. doi: 10.5694/j.1326-5377.2001.tb143562.x. [DOI] [PubMed] [Google Scholar]
- 3.Bozic KJ, Ries MD. The impact of infection after total hiparthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;8:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 4.Gracia E, Fernández A, Conchello P, Laclériga A, Paniagua L, Seral F, Amorena B. Adherence of Staphylococcus aureus slime-producing strain variants to biomaterials used in orthopaedic surgery. Int Orthop. 1997;21:46–51. doi: 10.1007/s002640050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seghrouchni K, van Delden C, Dominguez D, Benkabouche M, Bernard L, Assal M, Hoffmeyer P, Uçkay I. Remission after treatment of osteoarticular infections due to Pseudomonas aeruginosa versus Staphylococcus aureus: A case-controlled study. Int Orthop. 2012;36:1065–1071. doi: 10.1007/s00264-011-1366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Lucas-Villarrubia JC, Lopez-Franco M, Granizo JJ, De Lucas-Garcia JC, Gomez-Barrena E. Strategy to control methicillin resistant Staphylococcus aureus post-operative infection in orthopaedic surgery. Int Orthop. 2004;28:16–20. doi: 10.1007/s00264-003-0460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aqil F, Ahmad I, Owais M. Evaluation of anti-methicillin resistant Staphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Biotechnol J. 2006;1:1093–1102. doi: 10.1002/biot.200600130. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal S, Younas U, Sirajuddin Chan KW, Sarfraz RA, Uddin K. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): A comparative study. Int J Mol Sci. 2012;13:6651–6664. doi: 10.3390/ijms13066651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singab AN, El-Beshbishy HA, Yonekawa M, Nomura T, Fukai T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;100:333–338. doi: 10.1016/j.jep.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Jin WY, Na MK, An RB, Lee HY, Bae KH, Kang SS. Antioxidant compounds from twig of Morus alba. Nat Prod Sci. 2002;13:129–132. [Google Scholar]
- 11.Chung KO, Kim BY, Lee MH, Kim YR, Chung HY, Park JH, Moon JO. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol. 2003;55:1695–1700. doi: 10.1211/0022357022313. [DOI] [PubMed] [Google Scholar]
- 12.Sritularak B, De-Eknamkul W, Likhitwitayawuid K. Tyrosinase inhibitors form Artocarpus lakoocha. Thai J Pharm Sci. 1998;22:149–155. [Google Scholar]
- 13.Likhitwitayawuid K, Sritularak B, Benchanak K. Phenolics with antiviral activity from Millettia erythrocalyx and Artocarpus lakoocha. Nat Prod Res. 2005;19:177–182. doi: 10.1080/14786410410001704813. [DOI] [PubMed] [Google Scholar]
- 14.Chuanasa T, Phromjai J, Lipipun V, Likhitwitayawuid K, Suzuki M, Pramyothin P, Hattori M, Shiraki K. Anti-herpes simplex virus (HSV-1) activity of oxyresveratrol derived from Thai medicinal plant: Mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antiviral Res. 2008;80:62–70. doi: 10.1016/j.antiviral.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Lipipun V, Sasivimolphan P, Yoshida Y, Daikoku T, Sritularak B, Ritthidej G, Likhitwitayawuid K, Pramyothin P, Hattori M, Shiraki K. Topical cream-based oxyresveratrol in the treatment of cutaneous HSV-1 infection in mice. Antiviral Res. 2011;91:154–160. doi: 10.1016/j.antiviral.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Sasivimolphan P, Lipipun V, Likhitwitayawuid K, Takemoto M, Pramyothin P, Hattori M, Shiraki K. Inhibitory activity of oxyresveratrol on wild-type and drug-resistant varicella-zoster virus replication in vitro. Antiviral Res. 2009;84:95–97. doi: 10.1016/j.antiviral.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Kim YM, Yun J, Lee CK, Lee H, Min KR, Kim Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J Biol Chem. 2002;227:16340–16344. doi: 10.1074/jbc.M200678200. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz P, Roychowdhury S, Engelmann M, Wolf G, Horn TF. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: Effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide. 2003;9:64–76. doi: 10.1016/j.niox.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Saowakon N, Tansatit T, Wanichanon C, Chanakul W, Reutrakul V, Sobhon P. Fasciola gigantica: Anthelmintic effect of the aqueous extract of Artocarpus lakoocha. Exp Parasitol. 2009;122:289–298. doi: 10.1016/j.exppara.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Qiu F, Komatsu K, Kawasaki K, Saito K, Yao X, Kano Y. A novel stilbene glucoside, oxyresveratrol 3′-O-beta-glucopyranoside, from the root bark of Morus alba. Planta Med. 1996;62:559–561. doi: 10.1055/s-2006-957972. [DOI] [PubMed] [Google Scholar]
- 21.Mun SH, Joung DK, Kim SB, Park SJ, Seo YS, Gong R, Choi JG, Shin DW, Rho JR, Kang OH, Kwon DY. The mechanism of antimicrobial activity of sophoraflavanone B against methicillin-resistant Staphylococcus aureus. Foodborne Pathog Dis. 2014;11:234–239. doi: 10.1089/fpd.2013.1627. [DOI] [PubMed] [Google Scholar]
- 22.CLSI document M100-S24. Clinical and Laboratory Standards Institute; Wayne, PA: 2014. Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. [Google Scholar]
- 23.Cordwell SJ, Larsen MR, Cole RT, Walsh BJ. Comparative proteomics of Staphylococcus aureus and the response of methicillin-resistant and methicillin-sensitive strains to Triton X-100. Microbiology. 2002;148:2765–2781. doi: 10.1099/00221287-148-9-2765. [DOI] [PubMed] [Google Scholar]
- 24.Shibata H, Saito H, Yomota C, Kawanishi T, Okuda H. Alterations in the detergent-induced membrane permeability and solubilization of saturated phosphatidylcholine/cholesterol liposomes: Effects of poly(ethylene glycol)-conjugated lipid. Chem Pharm Bull (Tokyo) 2012;60:1105–1111. doi: 10.1248/cpb.c12-00153. [DOI] [PubMed] [Google Scholar]
- 25.Linnett PE, Beechey RB. Inhibitors of the ATP synthetase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- 26.Jung HJ, Lee DG. Synergistic antibacterial effect between silybin and N,N'-dicyclohexylcarbodiimide in clinical Pseudomonas aeruginosa isolates. J Microbiol. 2008;46:462–467. doi: 10.1007/s12275-008-0138-7. [DOI] [PubMed] [Google Scholar]
- 27.Zhao WH, Hu ZQ, Okubo S, Hara Y, Shimamura T. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1737–1742. doi: 10.1128/AAC.45.6.1737-1742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Habib A, Al-Saleh E, Safer AM, Afzal M. Bactericidal effect of grape seed extract on methicillin-resistant Staphylococcus aureus (MRSA) J Toxicol Sci. 2010;35:357–364. doi: 10.2131/jts.35.357. [DOI] [PubMed] [Google Scholar]
- 29.Joung DK, Choi SH, Kang OH, Kim SB, Mun SH, Seo YS, Kang DH, Gong R, Shin DW, Kim YC, Kwon DY. Synergistic effects of oxyresveratrol in conjunction with antibiotics against methicillin-resistant Staphylococcus aureus. Mol Med Rep. 2015;12:663–667. doi: 10.3892/mmr.2015.3345. [DOI] [PubMed] [Google Scholar]
- 30.Komatsuzawa H, Ohta K, Sugai M, Fujiwara T, Glanzmann P, Berger-Bächi B, Suginaka H. Tn551-mediated insertional inactivation of the fmtB gene encoding a cell wall-associated protein abolishes methicillin resistance in Staphylococcus aureus. J Antimicrob Chemother. 2000;45:421–431. doi: 10.1093/jac/45.4.421. [DOI] [PubMed] [Google Scholar]
- 31.Farca AM, Nebbia P, Re G. Potentiation of antibiotic activity by EDTA-tromethamine against three clinically isolated gram-positive resistant bacteria. An in vitro investigation. Vet Res Commun. 1994;18:1–6. doi: 10.1007/BF01839255. [DOI] [PubMed] [Google Scholar]
- 32.Lorian V, Atkinson B. Effect of serum on gram-positive cocci grown in the presence of penicillin. J Infect Dis. 1978;138:865–871. doi: 10.1093/infdis/138.6.865. [DOI] [PubMed] [Google Scholar]
- 33.Muthaiyan A, Martin EM, Natesan S, Crandall PG, Wilkinson BJ, Ricke SC. Antimicrobial effect and mode of action of terpeneless cold-pressed Valencia orange essential oil on methicillin-resistant Staphylococcus aureus. J Appl Microbiol. 2012;112:1020–1033. doi: 10.1111/j.1365-2672.2012.05270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


