Abstract
The aim of the present study was to investigate intestinal mucosal barrier dysfunction in a rat model of chronic obstructive pulmonary disease (COPD). Male Sprague Dawley rats (n=40) were evenly randomized into control and COPD groups and the COPD model was established by regulated exposure to cigarette smoke for 6 months. Histopathological changes of the lung and intestinal tissues were detected by hematoxylin and eosin staining. Expression of the tight junction proteins occludin and zona occludens-1 (ZO-1) in the intestinal tissues were analyzed by western blotting, serum diamine oxidase (DAO) activity was detected by spectrophotometry, the urinary lactulose to mannitol ratio (L/M) was evaluated by high performance liquid chromatography, and intestinal tissue secretion of tumor necrosis factor (TNF)-α, interferon (IFN)-γ and interleukin (IL)-8 were detected by ELISA. Lung histopathology revealed thinned alveolar walls, ruptured alveolar septa, enlarged and deformed alveoli, and the formation of bullae and emphysema due to alveolar fusion in the COPD group, while intestinal histopathology indicated clearly swollen intestines with darkened and gray mucosa, neutrophil infiltration of the intestinal mucosal and regional epithelial shedding. The occludin and ZO-1 expression levels were significantly lower in the COPD group compared with those in the corresponding control group (P<0.05), while the urinary L/M ratio was significantly higher (P<0.05). Furthermore, the serum DAO activity and secretion of TNF-α, IFN-γ and IL-8 in the intestinal tissues were significantly higher in the COPD group than in the control group (each P<0.05). Dysfunctional and structural changes were observed in the intestinal mucosal barrier in COPD model rats, which may be associated with the increased intestinal inflammatory responses.
Keywords: chronic obstructive pulmonary disease, intestinal mucosal barrier, tight junction, cytokine
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease resulting from intensified inflammatory responses to noxious particles or gases in the airways or lung. Complications associated with COPD usually occur due to intrapulmonary inflammatory exudations. As a result, the newly modified Global Initiative for Chronic Obstructive Lung Disease (2011 Version) recommendations have emphasized the importance of complications in the comprehensive evaluation of COPD (1).
Since the respiratory and intestinal tracts have similar mucosal structures and physiological characteristics, COPD is to some extent associated with inflammatory diseases of the intestinal mucosa, such as inflammatory bowel disease (IBD) (2). Studies have indicated that, in addition to commonly shared risk factors (such as smoking), COPD increases the prevalence of IBD among COPD patients via mechanisms such as inflammatory responses and dysregulation of protease/anti-protease activity (3). Furthermore, patients with IBD complicated by COPD have a significantly higher mortality rate (2.5–3.5 times), as compared with IBD alone (4). Rutten et al (5) found in their recent study that patients with COPD exhibit increased intestinal permeability, intestinal epithelial damage and destruction of the integrity of the intestinal mucosal barrier. These observations suggest that structural and functional changes in the gut may be caused by systemic damage associated with the complications of COPD. However, the mechanisms underlying the development of COPD-induced intestinal damage in patients with COPD remains unclear. In order to address this issue, the present observational study of the structural and functional changes of the intestinal mucosal barrier was conducted using a rat model of COPD, with the aim of providing evidence useful in the diagnosis and treatment of COPD and its complications.
Materials and methods
Materials and animals
A total of 40 healthy male specific pathogen-free Sprague-Dawley rats (Division of Comparative Medicine, Nanjing Jinling Hospital, Nanjing, China) with a mean weight of 150±12 g were evenly randomized into the control and COPD groups (n=20 per group). The 8-week rats were clean grade and were bred in the Medical Experiment Animal Center of Jinling Hospital. All rats were maintained in a specific-pathogen free environment, ventilated with clean air at 20–25°C and 40–70% relative humidity throughout the study with a 12-h light/dark cycle. Following one week of conditioning, rats were randomly divided into control group (clean air-exposed only) and COPD groups. At all times, excluding the smoke exposure period, water and food were provided ad libitum. Chambers and cages were washed on a daily basis. Rats were visually observed twice daily. Cigarettes delivering 11 mg tar, 0.9 mg nicotine and 14 mg carbon monoxide (Nanjing Cigarette Factory, Nanjing, China) were used. The other reagents and devices included: Occludin (sc-5562) and zona occludens-1 (ZO-1; sc-10804) specific antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, USA); β-actin antibody (TDY051; TDY Biotech Co., Ltd., Beijing, China); horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (074-1506; KPL, Inc., Gaithersburg, ML, USA); UV spectrophotometer 52-P; Shanghai Xianke Instrument Co., Ltd., Shanghai, China), diamine oxidase (DAO) standard (Sigma-Aldrich, St. Louis, MO, USA), lactulose standard (National Institute for Food and Drug Control, Beijing, China), D-mannitol standard (Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China), high performance liquid chromatography (HPLC) system (Waters Corporation, Milford, MA, USA), rat tumor necrosis factor-α (TNF-α) enzyme-linked immunosorbent assay (ELISA) kit (Sigma-Aldrich), rat interleukin-8 (IL-8) ELISA kit (Sigma-Aldrich) and rat interferon-γ (IFN-γ) ELISA kit (Sigma-Aldrich). The study protocol was approved by the ethics committee of Nanjing Jinling Hospital.
Establishment of the rat model of COPD
The rat model of COPD was established using the sidestream cigarette smoke method described by Zheng et al (6) and Li et al (7). The rats were kept in a cigarette smoke (CS) chamber (70×40×30 cm) with two 5×5 cm vents. CS was produced by five simultaneously lit cigarettes twice a day and the vents were opened every 15 min. The rats were exposed to the sidestream cigarette smoke for 2 h per day and 5 days per week continuously for 6 months. With the exception of restraint in a similar CS chamber, no other treatments were administered to the rats in the control group. Rats from the two groups were able to move without restraint and were allowed free access to drinking water and food.
Staining of the lung and intestinal tissues
Rats were anesthetized with 2% pentobarbital sodium at a dose of 30 mg/kg and then sacrificed by exsanguination from the heart. Serum samples were harvested from the rats following sacrifice and were stored at −80°C until they were required to measure DAO. Subsequently, the right-upper lung and jejunum (5 cm below the Treitz ligament) were harvested and fixed by immersion in 10% neutral formalin for a 24 h. Finally, after consecutive procedures of paraffin-embedding, generation of serial sections (thickness, 4 µm), dewaxing, hematoxylin and eosin (H&E) staining, dehydration, deparaffinization with xylene and mounting, the sections were observed with the use of light microscopy.
Western blot analysis of tight junction proteins in the intestinal tissues
Proteins were extracted from the intestinal tissues of the rats from the two groups by protein lysis. Proteins lysates were then stored in Eppendorf tubes at −130°C. Total protein concentrations were determined by UV spectrophotometry. Equal amounts of total proteins (20µg) were then separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were then blocked using 5% skimmed milk with shaking at room temperature for 1 h. The primary occludin, ZO-1 and β-actin antibodies (1:1,000) were dissolved in Tris-buffered saline and Tween 20 (TBST) with 5% skimmed milk and incubated with the membrane at 4°C overnight. They were then washed three times with TBST using a shaker at room temperature, for 5 min each time. The HRP-conjugated goat anti-rabbit secondary antibody was diluted with TBST (1:3,000), incubated with the membrane for 30 min at room temperature, and then the membrane was washed three times using TBST with shaking at room temperature, for 5 min each time. Exposure of the images was conducted using enhanced chemiluminescence and the scanned images were archived. Analysis of the optical density of each band was conducted using AlphaEaseFC 4.0 system analysis software (Protein Simple, San Jose, CA, USA). Protein levels were expressed as the integrated density ratio of the corresponding protein to the internal control (β-actin).
Measurement of the urinary lactulose to mannitol ratio (L/M)
Oral infusions of lactulose (100 mg) and mannitol (50 mg) were administered to 10 rats from each group. These rats were then kept in separate cages without food and samples of their urine were collected 6 h later. A method has been developed for the simultaneous determination of lactulose and mannitol in urine by HPLC (8), and was used in the current study. Following ultrafiltration and deionization, urine samples were analyzed with a LiChrospher® NH2 column (4.6×250 mm, internal diameter, 5 µm), a mixture of acetonitrile and water (722:8, volume ratio) as the mobile phase and a refractive index detector. Column temperature was 30°C. During the mobile phase, a flow rate of 1 ml/min was used (differential detector: internal heating, 37°C; sensitivity, 32, time constant, 1.0; scale factor, 20; and injection volume, 10 µl). The excretion of urinary lactulose and mannitol was determined and the L/M value was calculated.
Determination of serum DAO activity
A 3.3-ml volume of reaction buffer consisting 3 ml phosphate-buffered saline (0.2 M, pH 7.2), 0.1 ml (4 µg) horseradish peroxidase, 0.1 ml o-dianisidine dihydrochloride (500 pg) methanol solution, and 0.1 ml substrate solution (containing 175 pg cadaverine dihydrochloride) was added to each well of a 6-well plate. A 0.5-ml volume of serum sample was then added to each well for analysis. The blank control and a concentration gradient of standard substance were also added. DAO values were calculated automatically with a microplate reader detecting the optical density at 436 nm.
Measurement of cytokines secreted by the intestinal tissues
One small segment (length, 2cm) of the intestine sample from each rat was harvested for the measurement of cytokine production. After being cut along the mesentery, the intestinal tissue samples were soaked and washed in cold normal saline (NS) to remove the blood. The tissues were then weighed after drying on filter papers. Subsequently, each tissue sample was placed into a 5-ml beaker containing a volume of 0.86% cold NS equal to six times the weight of the sample. Following homogenization of the tissue and washing using a volume of 0.86% cold NS equal to three times the original weight of the intestinal sample, samples of the tissue homogenates (10% suspension) were prepared and stored at −20°C. The levels of TNF-α, IL-8 and IFN-γ in the homogenates were determined using ELISA kits according to the manufacturer's protocol.
Statistical analysis
Data were analyzed using SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA). Enumeration data were expressed as means ± standard deviation and analyzed by independent sample t-tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Establishment of the rat model of COPD
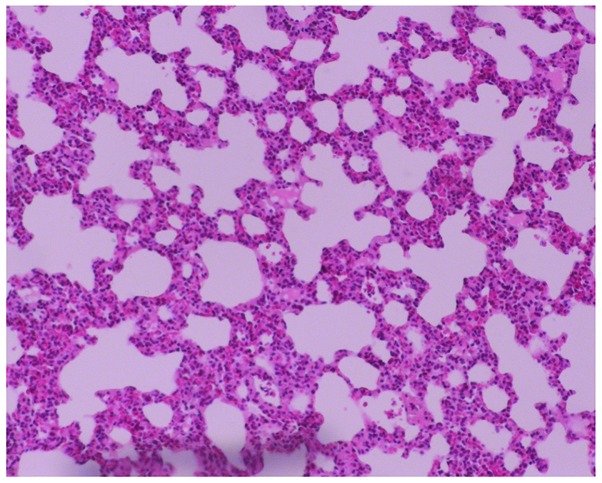
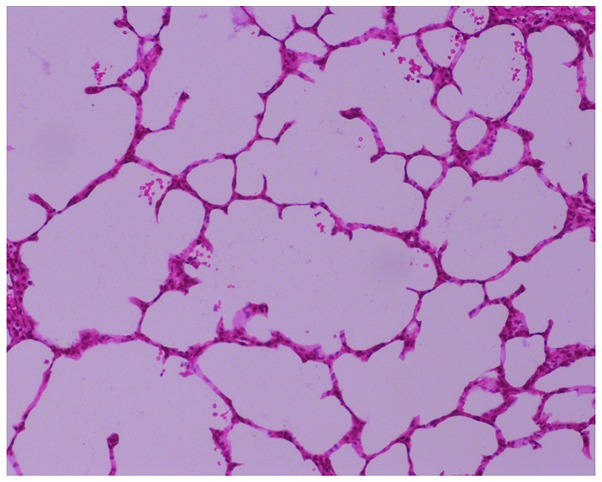
The lung histopathology indicated normal pulmonary structures, an absence of inflammatory infiltration in the small airways and structural integrity of the alveolar wall (Fig. 1) in the control group. However, in the COPD group, a large amount of monocyte and lymphocyte infiltration at and around the small airway walls was observed, with thinning of the alveolar wall, ruptured alveolar septa, interstitial pulmonary congestion and edema, reduced blood vessel density, enlarged and deformed alveoli, and formation of bullae due to alveolar fusion (Fig. 2). These results indicated the presence of emphysema and other pathological changes in the rats from the COPD group and confirmed the successful establishment of the rat model of COPD.
Figure 1.
Lung tissue of a rat from the control group exhibiting a normal structure, characterized by the absence of inflammatory cell infiltration of the small airways, and structural integrity of the alveolar wall. Hematoxylin and eosin staining; moderate magnification (×100).
Figure 2.
Lung tissue of a rat from the chronic obstructive pulmonary disease group, which exhibited lesions, characterized by a thinned alveolar wall, ruptured alveolar septa, enlarged and deformed alveoli, and formation of bullae due to alveolar fusion. Hematoxylin and eosin staining; moderate magnification (×100).
Evaluation of the intestinal samples
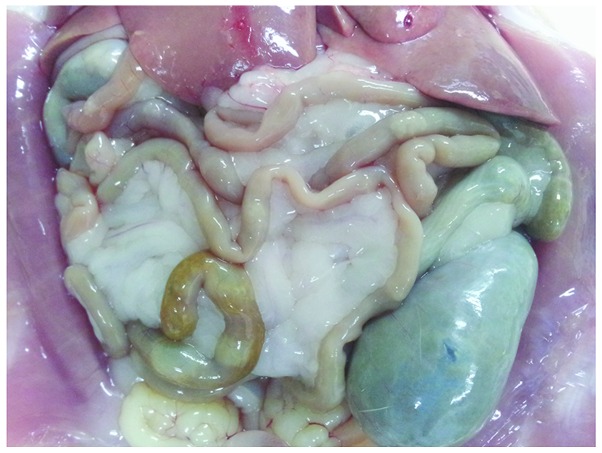
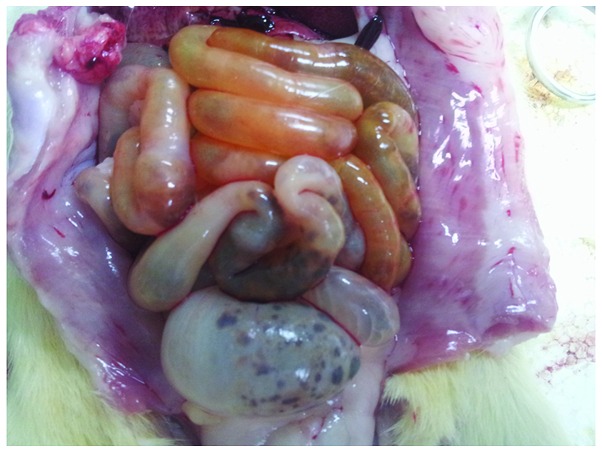
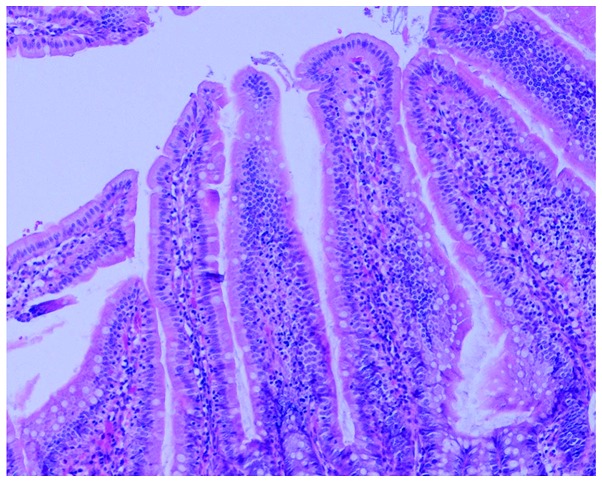
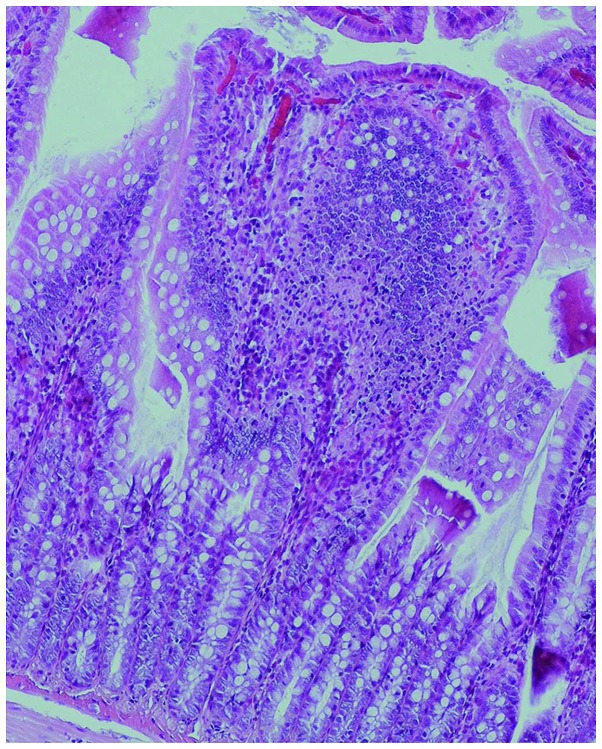
Gross observation indicated significant swelling of the gut in the rats of the COPD group compared with that in the normal rats, with darkened and gray mucosa in the former (Figs. 3 and 4). Under light microscopy, neutrophil infiltration of the intestinal mucosa, structural damage to the epithelium and pathological changes characterized by regional epithelial shedding and reduction in the number of intestinal villi were observed in the rats of the COPD group. By contrast, no evident pathological changes were observed in the intestinal mucosa of rats from the control group (Figs. 5 and 6).
Figure 3.
Intestine of a rat from the control group, with a normal color and no evident swelling.
Figure 4.
Intestine of a rat from the chronic obstructive pulmonary disease group, exhibiting mucosa of a darkened gray color and significant swelling.
Figure 5.
Intestinal mucosa of a normal rat, with no evident pathological changes. Hematoxylin and eosin staining; magnification, ×100.
Figure 6.
Infiltration and accumulation of neutrophils together with epithelial structural damage in the intestinal mucosa of a rat from the chronic obstructive pulmonary disease group. Hematoxylin and eosin staining; high magnification (×100).
Expression of occludin and ZO-1 proteins in the intestinal tissues
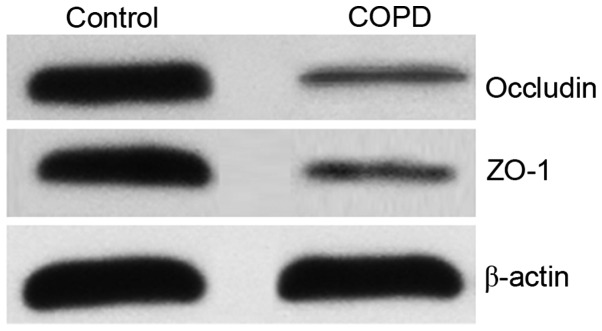
As tight junction proteins (including occludin and ZO-1) contributed to barrier function, the expression levels of occludin and ZO-1 proteins were significantly reduced in the COPD group, as compared with the control group (P<0.05; Fig. 7 and Table I). This result indicated that the damage to the intestinal barrier structure may contribute to intestinal barrier dysfunction in COPD.
Figure 7.
Western blotting results showing the expression of intestinal occludin and ZO-1 proteins. COPD, chronic obstructive pulmonary disease; ZO-1, zona occludens-1.
Table I.
Relative intestinal expression of occludin and ZO-1 protein in the two groups (%).
P<0.05 vs. the control group. Occludin and ZO-1 expression levels were significantly reduced in the COPD group commpared with those in the control group. COPD, chronic obstructive pulmonary disease; ZO-1, zona occludens-1.
Evaluation of the intestinal mucosal barrier function
Various molecular probes can be used to determine the permeability of the intestinal mucosa; however, the results are affected by numerous factors including gastrointestinal emptying retardation, renal dysfunction and urine collection insufficiency when a molecular probe is used alone. The accuracy of the results can be greatly improved when two different molecular probes are used simultaneously. In the present study, the urinary L/M value and serum DAO levels were significantly higher in the COPD group compared with those in the control group (both P<0.05; Table II). This result indicated that the permeability of the intestinal mucosal barrier is increased and intestinal mucosal barrier function is impaired in COPD.
Table II.
Urinary L/M values and serum DAO levels in the two groups.
P<0.05 vs. the control group. The L/M value and serum DAO level in the COPD group were significantly higher than those of the control group. COPD, chronic obstructive pulmonary disease; L/M, lactulose to mannitol ratio; DAO, diamine oxidase.
Measurement of cytokine levels in the intestinal tissues
Intestinal inflammatory factors were examined to identify the possible mechanism of intestinal barrier dysfunction in COPD. The intestinal secretion levels of TNF-α, IL-8 and IFN-γ were significantly higher in the COPD group compared with the control group (P<0.05; Table III). As cytokines are able to regulate intestinal tight junctions under pathological states, this result indicated that intestinal barrier dysfunction may occur in COPD, and may at least partly function via the enhanced inflammatory response.
Table III.
Intestinal expression levels of TNF-α, IL-8 and IFN-γ in the two groups.
| Group | TNF-α (pg/ml) | IL-8 (pg/ml) | IFN-γ (ng/ml) |
|---|---|---|---|
| Control | 316.15±0.82 | 684.15±3.14 | 1.76±0.03 |
| COPD | 357.73±1.56a | 731.56±7.10a | 2.21±0.14a |
P<0.0 vs. the control group. Expression levels of TNF-α, IL-8 and IFN-γ in the COPD group are significantly higher than those of the control group. COPD, chronic obstructive pulmonary disease; TNF, tumor necrosis factor; IL, interleukin; IFN, interferon.
Discussion
COPD is a chronic inflammatory condition that is caused by exposure of the airways and lung tissues to toxic gases or particles. Inflammatory cell infiltration in the lungs of patients with COPD comprises mainly alveolar macrophages, neutrophils and CD8+ T cells that release multiple inflammatory mediators including leukotriene B4, IL-8, and TNF-α. This persistent inflammation is not restricted to the lung, but may also lead to inflammation in extrapulmonary systems, resulting in the occurrence of complications (9). In the present study, significant inflammation of the intestinal tissues was demonstrated in rats in association with COPD, together with pathological changes of the intestines, such as neutrophil infiltration and regional mucosal epithelial shedding. This indicated the presence of increased inflammatory cell infiltration of the mucosa and structural damage to the intestines of the rats with COPD.
The intestinal mucosal barrier consists mainly of epithelial junctional complexes between intestinal epithelial cells. These junctions include adherens junctions and tight junctions, which are critically responsible for the selective regulation of the movement of water, ions and other small molecules via the paracellular transportation pathway. Therefore, tight junctions are an important part of the intestinal mucosal barrier and the integrity of this barrier is one of the prerequisites of its function. Tight junctions are formed by transmembrane proteins (such as claudins, occludin and adhesion molecules) and intracellular scaffold proteins (such as ZO and cingulin) (10). Several studies, including the one conducted by Odenwald and Turner indicate that destruction of the tight junction leads to structural changes and dysfunction of the intestinal mucosal barrier (11). The present study demonstrated that the expression levels of occludin and ZO-1 proteins were significantly reduced in the intestinal tissues of rats with COPD compared with those of normal rats. These observations indicate destruction of the tight junctions in the intestinal tissues of rats in the COPD group, which might result in structural changes and dysfunction of the intestinal mucosal barrier.
It is challenging to evaluate the intestinal mucosal barrier function directly; however, commonly used indirect measures are based on determinations of intestinal permeability, serum D-lactate levels and serum DAO activity. DAO is the marker enzyme of intestinal mucosal cells, which exists at low serum levels under normal conditions but is released into the blood, increasing serum levels, if these cells are damaged; therefore, DAO activity reflects the degree of damage to the intestinal mucosa. Intestinal permeability is determined mainly by using sugar probe molecules. Since mannitol and lactulose have a high recycling rate and are minimally affected by the intraluminal osmotic pressure, these molecules are ideal sugar probes that have been widely applied to determine the permeability of the intestinal mucosa (12). The urinary L/M ratio accurately reflects intestinal permeability, with increased L/M values indicating an increase in permeability and impaired intestinal mucosal barrier function (8). The results of the present study revealed that the serum DAO level and urinary L/M value were both significantly increased in the rats of the COPD group, indicating the presence of increased intestinal permeability and impaired intestinal mucosal barrier function. By detecting the plasma intestinal fatty acid binding protein levels and urinary sucralose to erythritol ratios, Rutten et al (5) demonstrated that enterocyte damage and intestinal hyperpermeability occurred in patients with COPD. Their results indicate the presence of structural changes and dysfunction of the intestinal mucosal barrier in patients with COPD, and the results of the present study are consistent with these previous observations.
At present, although the pathogenesis of COPD-induced intestinal mucosal barrier dysfunction in patients with COPD remains unclear, inflammatory reactions are a possible mechanism. It has previously been demonstrated that cytokines regulate intestinal tight junctions under pathological states (13). Cytokine-induced tight junction dysfunction caused by immune activation or tissue inflammation is considered to play an important role in the occurrence and development of intestinal and other systemic diseases (14). In the present study, higher intestinal levels of IFN-γ, TNF-α and IL-8 were found in the COPD group compared with those in normal rats, which revealed that increased levels of cytokines and an elevated inflammatory responses in the intestinal tissues of the rats with COPD. Suzuki (15) and Yang et al (16) found that IFN-γ causes damage to tight junction structures by inducing the redistributed expression and intracellular uptake of tight junction proteins, while Amasheh et al (17) and Ye et al (18) reported that TNF-α causes structural changes of the tight junctions by promoting the loss of claudin 1, increasing claudin 2 expression and enhancing the degeneration of occludin and ZO-1. Increased tissue levels of IL-8 have been found in patients with IBD, which could contribute to the occurrence of mucosal damage and ulcers (19). The present study showed that the intestinal expression levels of occludin and ZO-1 proteins were reduced in the rats of the COPD group, which might be associated with the increased levels of intestinal cytokines and further damage to tight junctions. Therefore, it may be inferred that structural and dysfunctional changes of the intestinal mucosal barrier in patients with COPD are associated with enhanced inflammatory responses. In addition, IFN-γ, TNF-α and IL-8 might be important inflammatory mediators of the pathogenesis of COPD. However, the specific relationship between pulmonary and intestinal inflammation in COPD requires further investigation. Moreover, these observations raise the question of whether intestinal inflammation and the damage and dysfunction of the intestinal mucosal barrier could also be controlled by inhaled corticosteroid therapy for intrapulmonary inflammation.
In summary, the results of the present study reveal that increased intestinal inflammation resembling that in the lungs is present in a rat model of COPD. These increased inflammatory responses cause damage to the tight junction structures, resulting in structural and functional changes of the intestinal mucosal barrier. However, the specific association between pulmonary and intestinal inflammation in COPD, in addition to the potential of using inhaled corticosteroid therapy for intrapulmonary inflammation to provide an effective treatment for associated intestinal inflammation require further investigation.
Glossary
Abbreviations
- COPD
chronic obstructive pulmonary disease
- DAO
diamine oxidase
- IBD
inflammatory bowel disease
- SD
Sprague-Dawley
- HPLC
high performance liquid chromatography
- TNF-α
tumor necrosis factor-α
- IFN-γ
interferon-γ
- CS
cigarette smoke
- H&E
hematoxylin and eosin
- ECL
enhanced chemiluminescence
- NS
normal saline
References
- 1.Abdool-Gaffar MS, Ambaram A, Ainslie GM, Bolliger CT, Feldman C, Geffen L, Irusen EM, Joubert J, Lalloo UG, Mabaso TT, et al. COPD Working Group: Guideline for the management of chronic obstructive pulmonary disease - 2011 update. S Afr Med J. 2011;101:63–73. [PubMed] [Google Scholar]
- 2.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 3.Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of atrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G175–G184. doi: 10.1152/ajpgi.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5:7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutten EP, Lenaerts K, Buurman WA, Wouters EF. Disturbed intestinal integrity in patients with COPD: Effects of activities of daily living. Chest. 2014;145:245–252. doi: 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- 6.Zheng H, Liu Y, Huang T, Fang Z, Li G, He S. Development and characterization of a rat model of chronic obstructive pulmonary disease (COPD) induced by sidestream cigarette smoke. Toxicol Lett. 2009;189:225–234. doi: 10.1016/j.toxlet.2009.06.850. [DOI] [PubMed] [Google Scholar]
- 7.Li MM, Xin XF, Shao HT, Shi Y, Su X, Sun WK, Sun H, Fang LP. The expressions of nerve growth factor and its tyrosine kinase A receptor on alveolar macrophages in a rat model of chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:601–605. (In Chinese) [PubMed] [Google Scholar]
- 8.Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas. 2008;36:192–196. doi: 10.1097/MPA.0b013e31815a399f. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen M, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:982–988. doi: 10.1164/rccm.201206-1113OC. [DOI] [PubMed] [Google Scholar]
- 10.Shen L. Tight junctions on the move: Molecular mechanisms for epithelial barrier regulation. Ann NY Acad Sci. 2012;7:9–18. doi: 10.1111/j.1749-6632.2012.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odenwald MA, Turner JR. Intestinal permeability defects: Is it time to treat? Clin Gastroenterol Hepatol. 2013;11:1075–1083. doi: 10.1016/j.cgh.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian R, Tan JT, Wang RL, Xie H, Qian YB, Yu KL. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur Rev Med Pharmacol Sci. 2013;17:349–55. [PubMed] [Google Scholar]
- 13.Cao M, Wang P, Sun C, He W, Wang F. Amelioration of IFN-γ and TNF-α-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PLoS One. 2013;8:e61944. doi: 10.1371/journal.pone.0061944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S, Yu M, Sun L, Xiao W, Yang X, Sun L, Zhang C, Ma Y, Yang H, Liu Y, et al. Interferon-γ-induced intestinal epithelial barrier dysfunction by NF-kB/HIF-1α pathway. J Interferon Cytokine Res. 2014;34:195–203. doi: 10.1089/jir.2013.0044. [DOI] [PubMed] [Google Scholar]
- 17.Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci. 2010;123:4145–4155. doi: 10.1242/jcs.070896. [DOI] [PubMed] [Google Scholar]
- 18.Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 2012;18:5848–5861. doi: 10.3748/wjg.v18.i41.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]