Abstract
Astrocytes are the most abundant neuron-supporting glial cells in the central nervous system. The neuroprotective role of astrocytes has been demonstrated in various neurological disorders such as amyotrophic lateral sclerosis, spinal cord injury, stroke and Parkinson’s disease (PD). Astrocyte dysfunction or loss-of-astrocytes increases the susceptibility of neurons to cell death, while astrocyte transplantation in animal studies has therapeutic advantage. We reported recently that stimulation of serotonin 1A (5-HT1A) receptors on astrocytes promoted astrocyte proliferation and upregulated antioxidative molecules to act as a neuroprotectant in parkinsonian mice. PD is a progressive neurodegenerative disease with motor symptoms such as tremor, bradykinesia, rigidity and postural instability, that are based on selective loss of nigrostriatal dopaminergic neurons, and with non-motor symptoms such as orthostatic hypotension and constipation based on peripheral neurodegeneration. Although dopaminergic therapy for managing the motor disability associated with PD is being assessed at present, the main challenge remains the development of neuroprotective or disease-modifying treatments. Therefore, it is desirable to find treatments that can reduce the progression of dopaminergic cell death. In this article, we summarize first the neuroprotective properties of astrocytes targeting certain molecules related to PD. Next, we review neuroprotective effects induced by stimulation of 5-HT1A receptors on astrocytes. The review discusses new promising therapeutic strategies based on neuroprotection against oxidative stress and prevention of dopaminergic neurodegeneration.
Keywords: 5-HT1A receptor, S100β, astrocyte, Parkinson’s disease, neuroprotection, dopaminergic neuron
1. INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disease with motor symptoms such as tremor, bradykinesia, rigidity and postural instability that are based on selective loss of nigrostriatal dopaminergic neurons, and with non-motor symptoms such as orthostatic hypotension and constipation that are caused by peripheral neurodegeneration. The onset of motor features in PD is caused when dopamine (DA) terminal or DA content in the striatum is reduced below 50% or 60-70%, respectively [1, 2]. Although various treatments for PD are being assessed at present, the major is dopaminergic therapy for managing motor disability. Presently, some drugs such as D3 agonist pramipexole have reported their neuroprotective effects
in parkinsonian models [3-5]. However, no conventional treatment has been demonstrated to slow or stop PD progression. Therefore, it is desirable to develop neuroprotective treatments that can prevent the dopaminergic neurodegeneration.
Astrocytes are abundant neuron-supporting glial cells and prone to improve dopaminergic neuronal survival by production of antioxidants, release of neurotrophic factors and uptake of potentially neurotoxic molecules, such as excess amount of glutamate in the synaptic space and extracellular α-synuclein aggregates [6-8]. Astrocytes have stronger antioxidative property than neurons [9] and can protect neurons against oxidative stress. Indeed, astrocyte-derived antioxidants reduce oxidative stress level in the surrounding neurons [10, 11]. Dysfunction of astrocytes is involved in increased susceptibility of neuronal cells, and astrocyte transplantation demonstrates therapeutic effects in several neurological disorders [12-17]. Taken together with previous reports, increasing the number of healthy astrocytes containing various antioxidative molecules could be a potential therapeutic approach to prevent the degenerative process of PD. We have recently shown that stimulation of serotonin 1A (5-HT1A) receptors on astrocytes promotes astrocyte proliferation and upregulates antioxidative molecules to act as a neuroprotectant in PD model mice [18]. In this article, we review the neuroprotective role of astrocytes in the brain and neuroprotective approaches targeting astrocytes for PD models, especially stimulation of 5-HT1A receptors on astrocytes. The review allows formulating a promising therapeutic strategy of neuroprotection against oxidative stress and progressive dopaminergic neurodegeneration.
2. Neuroprotective properties of astrocytes in Parkinson's disease
2.1. Role of Antioxidant Supply
2.1.1. Glutathione
Glutathione (GSH) is the most potent intrinsic antioxidant against reactive oxygen species (ROS). GSH is a tripeptide comprising the amino acids glutamate, cysteine and glycine. GSH is generated via a two-step reaction catalyzed by γ-glutamyl cysteine ligase (GCL) and GSH synthetase. Because intracellular glutamate and glycine are relatively sufficient, cysteine is the rate-limiting precursor for GSH synthesis. Since extracellular cysteine is readily auto-oxidized to cystine, cystine transport mechanisms are essential to supply a GSH substrate cysteine to cells. Cystine uptake is mediated by cystine/glutamate exchange transporter (xCT), which is expressed primarily on astrocytes, but not on neurons [19-21]. Astrocytes take up cystine via xCT, reduce it to cysteine to synthesize GSH, and consequently release it through the transporter multidrug resistance protein 1 (MRP1). Cysteine is generated from the extracellular thiol/disulfide exchange reaction of GSH and cystine, or generated by a peptidase from the dipeptide cysteinylglycine (CysGly), and then taken up by neighboring neurons for GSH synthesis [22-24]. Therefore, GSH synthesis in neurons is dependent on the expression of xCT and GSH synthesis in astrocytes (Fig. 1).
Fig. (1).
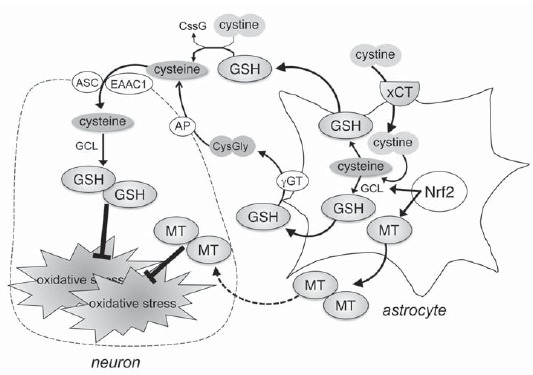
Role of antioxidant supply by astrocytes. Astrocytes take up cystine via cystine/glutamate exchange transporter (xCT), reduce it to cysteine to synthesize glutathione (GSH), and consequently release it into the extracellular space. Cysteine is generated from the extracellular thiol/disulfide exchange reaction of cystine and GSH, or generated by a peptidase from the dipeptide cysteinylglycine (CysGly), and then taken up by neighboring neurons for GSH synthesis. Metallothioneins (MTs) are produced by astrocytes in response to oxidative stress, and secreted into extracellular space. MTs secreted specifically by astrocytes protect dopaminergic neurons against oxidative stress.
Evidence suggests that oxidative stress plays a pathogenic role in neurodegenerative disorders including PD. GSH is the most abundant antioxidative molecule in the central nervous system (CNS) and plays a critical role in protecting cells against oxidative stress. Previous studies demonstrated the presence of low GSH levels in the substantia nigra of PD patients (40% compared to control subjects) [25-28]. In addition, reactive quinones, such as DA quinones or DOPA quinones, are produced by spontaneous oxidation of free cytosolic DA outside the synaptic vesicle in dopaminergic neurons [29-32]. The generated DA quinones can covalently react with the sulfhydryl residues on functionally important proteins in the pathogenesis of PD, e.g., tyrosine hydroxylase (TH), DA transporter (DAT) and parkin to form quinoproteins, and cause the dysfunction of these proteins [33-35]. Therefore, the pathogenic effects of DA quinone have been focused as dopaminergic neuron-specific oxidative stress. In our previous study, it is reported that repeated treatment with levodopa increased DA turnover and quinoprotein formation specifically in the striatum of parkinsonian model mice [36, 37]. The sulfhydryl groups of free cysteine in GSH and thiol reagents compete with the sulfhydryl group on cysteine in functional proteins to prevent the formation of quinoprotein [29, 33, 38-40]. Therefore, GSH acts as an antioxidant by quenching not only general ROS but also DA quinones [41]. Thus, GSH can be regarded as an important neuroprotectant especially in dopaminergic neurons. However, it is difficult to maintain an effective concentration of GSH against quinone toxicity in the brain, because the small peptides can be resolved easily before crossing the blood-brain barrier. As mentioned above, GSH synthesis in neurons is dependent on cystine uptake via xCT and GSH synthesis in astrocytes. Astrocytes are more resistant to oxidative stress than neurons, and GCL expression is upregulated following exposure of astrocytes, but not neurons, to oxidative stress [42]. In this regard, a previous study demonstrated that the conditioned medium of cultured astrocytes (glia conditioned medium: GCM) obtained from parkin-null mice had less neuroprotectives and contained lower levels of GSH than those from wild-type animals [43]. Furthermore, we demonstrated that zonisamide, a novel antiparkinsonian agent used in Japan, increased the expression of xCT and GSH levels in the activated striatal astrocytes and showed neuroprotective effects against dopaminergic neurodegeneration in parkinsonian mice [44]. Zonisamide (1,2-benzisoxazole-3-methane-sulfonamide) was originally synthesized as an antiepileptic agent in Japan, and used clinically not only in Japan but also in South Korea, the United States and Europe. In addition, it was reported that the combination of zonisamide and levodopa improved motor impairments in PD patients [45-47]. Since 2009, zonisamide has been used as a novel antiparkinsonian agent in Japan. We reported for the first time that treatment with zonisamide upregulated xCT expression and GSH synthesis in astrocytes and prevented dopaminergic neurodegeneration in parkinsonian mice [44]. Taken together with these observations, the cystine uptake system and consequent GSH synthesis in astrocytes could be targets for dopaminergic neuroprotection in PD therapy.
2.1.2. Metallothionein
Metallothioneins (MTs) are low molecular weight and cysteine-rich proteins with antioxidative, anti-apoptotic, and anti-inflammatory properties. MTs bind to metals such as zinc, copper, and cadmium to function in metal homeostasis and detoxification [48]. MTs provide neuroprotection by regulating zinc-mediated transcriptional activation of genes involved in growth, proliferation, and differentiation. In the mammalian tissue, MT family comprises four isoforms: MT-1, MT-2, MT-3, and MT-4. MT-3 is a predominantly brain-specific isoform, which is expressed in neurons and stimulated glial cells [49]. On the other hand, the two abundant isoforms, MT-1 and -2 (MT-1/-2), are expressed in most organs including the brain. MT-1/-2 isoforms have radical-scavenging property based on their abundant thiol groups, which form metal-thiolate clusters [48, 50, 51]. Therefore, MT-1/-2 isoforms play an important role in the regulation of metal homeostasis in the brain and neuroprotection in various pathological and inflammatory states [48, 52]. We reported previously that MT-1 quenched DA semiquinones in vitro, and that MT-1/-2 protected dopaminergic neurons against DA quinone toxicity both in vitro and in vivo [53], suggesting that MTs can bind DA quinones by the sulfhydryl groups of their rich cysteines. MTs also inhibit Charnoly body (CB) formation, which is a pleomorphic, multi-lamellar, electron-dense stack of degenerated membranes formed in the most vulnerable cells due to compromised mitochondrial bioenergetics in severe malnutrition and free radical overproduction by their antioxidative actions [54-56]. Furthermore, MTs attenuate peroxynitrite-induced oxidative and nitrative stress to reduce α-synuclein index and 8-hydroxy-2'-deoxyguanosine accumulation to provide mitochondrial protection and prevent CB formation involved in Lewy body pathogenesis and various other neurodegenerative α-synucleinopathies [57-59]. Although MT-1/-2 can protect neurons directly, they are not mainly expressed in neurons in the normal or injured state [60, 61]. There are a number of evidences demonstrating that MT-1/-2 are produced primarily by astrocytes [62-73], and that extracellular MTs secreted from astrocytes mediate neuronal survival and axonal regeneration [60]. Michael et al. [64] reported up-regulation of MT expression in reactive astrocytes of the substantia nigra in PD patients, suggesting a neuroprotective role for dopaminergic neurons. Chung et al. [74] also reported that MT-1/-2 expression is specifically upregulated in astrocytes in response to neuronal injury. In our previous study, MT-1/-2 were produced by astrocytes in response to excess DA exposure, and extracellular MTs protected dopaminergic neurons against DA-induced neurotoxicity [75]. In addition, MTs modulate zinc availability for the zinc-binding enzyme pool [76]. Under oxidative stress, zinc is released from MTs and transferred to zinc-required transcription factors to regulate expression of several genes involved in the antioxidant pathways [77]. Thus, MTs produced by astrocytes play a crucial role as both radical scavengers and zinc regulators.
2.1.3. Nrf2-Induced Molecules
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a master transcription factor, which produces phase II drug-metabolizing enzymes, such as GCL, quinone reductase-1 (NQO-1), GSH S-transferase (GST) and MT, by binding to the antioxidant response element (ARE) [75, 78]. Nrf2 is sequestered in the cytoplasm by its repressor protein, Keap1 in normal condition [79, 80]. When cells are exposed to electrophiles and ROS, cysteine residues of Keap1 are oxidized to lead conformational change, which releases Nrf2 for nuclear translocation and activation of phase II antioxidant enzyme gene expression [81, 82]. Phase II genes function for cellular defense that scavenges ROS, detoxifies electrophiles and xenobiotics, and maintains intracellular antioxidative property. Nrf2-regulated genes are preferentially activated in astrocytes, which consequently exhibit more efficient detoxification and antioxidant defenses than neurons. Indeed, Nrf2-induced molecules, such as GSH-related enzyme and MTs, are higher in astrocytes than neurons. A number of investigators, including our group, have reported that activation of Nrf2 in astrocytes has a major role in protecting dopaminergic neurons from oxidative stress [9, 10, 18, 75, 78]. For example, it is reported that administration of MPTP resulted in dramatic reduction of dopaminergic nerve terminal in the striatum of Nrf2-knockout mice [83]. Furthermore, Chen et al. [10] demonstrated that astrocyte-specific overexpression of Nrf2 protected dopaminergic neurons in MPTP-injected parkinsonian animal model of Nrf2-deficient mice. Recent studies also demonstrated that phytochemicals, such as curcumin, sulforaphane and resveratrol, could activate Nrf2 and exert neuroprotective effects against oxidative stress in animal models of various neurological disorders. Considered together, these results suggest that modulation of the Nrf2 and its related antioxidants in astrocytes can be a possible therapeutic approach to prevent neurodegeneration in PD.
2.1.4. DJ-1
DJ-1 (PARK7) gene deletions and point mutations have been identified as one of the causes of early-onset autosomal recessive PD (AR-PD) [84]. DJ-1 has various functions related to PD pathogenesis. DJ-1 scavenges H2O2 through oxidation of its Cys106-sulfinic acid to protect neurons against oxidative stress [85, 86]. Kitamura et al. [87] screened DJ-1-binding compounds and demonstrated neuroprotective effects of compound-23, N-[4-(8-methyl(4-hydroimidazo[1,2-a]pyri-din-2-yl))phenyl](3,4,5-trimethoxyphenyl)carbox-amide, against ROS-induced neurotoxicity in parkinsonian and ischemic animal models. DJ-1 is mainly expressed in astrocytes in the human brain and acts as a sensor of oxidative stress [88]. Reactive astrocytes enhance DJ-1 expression in the case of oxidative stress and release DJ-1 protein extracellularly to protect neurons [89]. Several studies demonstrated that DJ-1 deficiency or mutation in astrocytes impairs astrocyte-mediated neuroprotection in parkinsonian models [90-92]. Clements et al. [93] reported that Nrf2 protein without intact DJ-1 is unstable and its basal or inducible levels of transcriptional responses are decreased. These observations suggest that DJ-1 stabilizes antioxidant transcriptional regulation of Nrf2. As described above, because Nrf2 is a key molecule in antioxidative pathway in living cells, modulation of DJ-1 expression in astrocytes could be a suitable target to secure neuroprotection. Furthermore, DJ-1 is identified as a critical molecule for mitochondrial function [94, 95]. In the presence of oxidative environment, DJ-1 plays an important role to maintain mitochondrial function as well as PINK1/parkin pathway [95].
2.1.5. Parkin
Parkin (PARK2) is one of the genes responsible for AR-PD and exerts E3 ubiquitin ligase [96, 97]. Parkin forms a complex with PINK1 and DJ-1, and promotes degradation of unfolded or misfolded proteins [98]. Recently, parkin and PINK1 have been identified as essential proteins for the removal of damaged mitochondria through autophagy (mitophagy) [99-101]. Therefore, parkin deficiency induces aberrant ubiquitination and compromised mitochondrial integrity, leading to neuronal dysfunction and degeneration. LaVoie et al. [34] demonstrated that DA quinone covalently modifies parkin protein in living dopaminergic cells to inactivate ubiquitin ligase function of parkin. Together, these findings indicate that dopaminergic neurons are more vulnerable because DA quinone can induce mitochondrial dysfunction. Constitutive parkin expression is especially higher in neurons than astrocytes. However, astrocytes but not hippocampal neurons increase parkin expression during unfolded protein stress [102]. It is demonstrated that knock-down of parkin causes impairment of GSH synthesis in astrocytes and shows neurodegenerative pathogenesis of parkin-linked AR-PD [43]. Therefore, it is suggested that astrocytic parkin expression is more critical for dopaminergic neuroprotection. These results suggest that parkin dysfunction in astrocytes produces oxidative stress environment in the brain. Furthermore, it was reported recently that parkin mutation in the fly results in severe motor impairment and shortened life span, and that these phenotypes are rescued by up-regulation of metal-responsive transcription factor (MTF-1) [103]. MTF-1 regulates transcription of its target genes by binding to metal response elements. MTF-1 is also known to promote the gene expression of MT in response to oxidative stress and inflammation [104, 105]. Indeed, MT overexpression in a parkin mutant background could improve the motor impairment of parkin mutants [103]. These suggest that modulating parkin expression in astrocytes could be a useful therapeutic strategy in PD.
2.2. Release of Neurotrophic Factors
Neurotrophic factors are secreted proteins, which trigger survival pathways upon binding to their target receptors to prevent neuronal loss. Astrocytes secret trophic factors, such as glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and basic fibroblast growth factor (bFGF). Novel neurotrophic factors have been identified, such as cerebral dopamine neurotrophic factor (CDNF) and its homolog mesencephalic astrocyte-derived neurotrophic factor (MANF). In this section, we will focus on some molecules that are closely related to dopaminergic neurons.
2.2.1. GDNF
GDNF is a member of the transforming growth factor-β superfamily [106]. In 1993, Lin et al. [107] identified GDNF as a potent neurotrophic factor, which enhances survival of mesencephalic dopaminergic neurons. Earlier studies described GDNF to specifically promote the survival of primary cultured mesencephalic DA neurons from rat embryos and a potent and selective stimulator of DA uptake and neurite outgrowth in TH-positive DA neurons [107-109].
2.2.2. bFGF
Basic FGF that was initially identified as a mitogen with angiogenic properties, is produced mainly by astrocytes and promotes the growth and survival of dopaminergic neurons [110-113]. Engele and Bohn [114] reported that mesencephalic astrocytes provide neurotrophic bFGF for central dopaminergic neurons. Recently, it has been revealed that upregulation of bFGF level in endogenous astrocytes of the substantia nigra enhances dopaminergic differentiation of transplanted stem cells in parkinsonian mice [115]. Interestingly, mesencephalic neurons show enhanced growth and survival when they are cultured with astrocytes. The bFGF from astrocytes might be partly involved in such neurotrophic effects of astrocytes on dopaminergic neurons.
2.2.3. CDNF and MANF
CDNF [116] and MANF [117] are novel but evolutionarily conserved neurotrophic factors. Both factors are found in vertebrates, while a single homolog is present in the invertebrates [118, 119]. These two proteins contain eight conserved cysteine residues, which determine the protein conformation. CDNF and MANF consist of two domains; an N-terminal saposin-like domain interacting with lipids or membranes, and an unfolded C-terminal domain related to protection against endoplasmic reticulum stress. MANF, which is identified in the culture medium of rat type-1 astrocyte ventral mesencephalic cell line 1 (VMCL1), provides protection for embryonic mesencephalic DA neurons in vitro [117]. MANF and CDNF are the most potent factors that can provide protection and enhance the repair of dopaminergic neurons in parkinsonian model rats [120, 121].
2.3. Clearance of Potentially Neurotoxic Molecules
2.3.1. Aggregated α-Synuclein
α-Synuclein that consists of 140 amino acids is mainly found in the presynaptic nerve ends under normal physiological conditions. This protein is reported to be involved in modulating synaptic vesicle function in cooperation with synapsin III [122]. The aggregated and insoluble fibrillar form of α-synuclein constitutes a major component of Lewy bodies or Lewy neurites in neurodegenerative diseases, such as PD, dementia with Lewy bodies and multiple system atrophy [123, 124]. Although it is still controversial whether Lewy bodies initiate neurodegeneration or damaged neurons form Lewy bodies for self-defense, it is generally accepted that the oligomeric and fibrillar α-synuclein species are responsible for the toxicity, and oligomeric α-synuclein is more toxic than the larger aggregated forms. α-Synuclein is a cytosolic protein, but it can be released from neuronal cells into the surrounding extracellular space via exocytosis [125]. Although the structure of the released α-synuclein is unknown, several studies report that extracellular α-synuclein contains aggregated forms and that misfolding and aggregation facilitate the release of α-synuclein from neuronal cells [126]. The uptake of extracellular α-synuclein occurs via endocytosis in neurons and glial cells [127]. α-Synuclein is degraded in the lysosome of astrocytes, suggesting that astrocytes exert dopaminergic neuroprotection by clearing excess extracellular toxic α-synuclein [128]. Indeed, Lee and colleagues [129] demonstrated that α-synuclein can be propagated from neuron to neuron and from neurons to astrocytes [6]. All the major brain cell types, neurons and astrocytes, are able to clean extracellular α-synuclein aggregates by internalization and degradation [128].
2.3.2. Damaged Mitochondria
As mentioned above, damaged mitochondria are removed through mitophagy to maintain the quality of mitochondria [99-101]. Such maintenance is important not only for proper bioenergetic function, but also to prevent the release of ROS followed by cell death. Mitochondria degradation is generally thought as a cell-autonomous process. Recently, it is reported that retinal ganglion cell axons of mice shed mitochondria at the optic nerve head, and that these mitochondria are internalized and degraded by surrounding astrocytes [130]. The authors also demonstrated structurally similar accumulations of degrading mitochondria along neurites in superficial layers of the cerebral cortex. While it is unclear whether dopaminergic neurons also use this transcellular mitophagy as a primary mitochondrial quality control mechanism, the degradation of neuron-derived mitochondria in astrocytes is a notable phenomenon as a target for neuroprotection.
3. Serotonin 1A (5-HT1A) receptors on astrocytes as a novel target of neuroprotection
3.1. Pharmacological Function of 5-HT1A Receptors
The 5-HT1A receptor, which is one of 14 subtypes (5-HT1A/1B/1D/1E/1F, 5-HT2A/2B/2C, 5-HT3, 5-HT4, 5-HT5A/5B, 5-HT6 and 5-HT7), is a G-protein-coupled receptor with a 7-transmembrane-spanning structure. The 5-HT1A receptor is a key mediator of serotonergic signaling in the CNS. The serotonergic system plays an important role in various physiological and behavioral functions such as mood- and anxiety-related behavior, cognitive function, food intake, sexual behavior, sleep and body temperature. Therefore, considerable attention has been focused on the 5-HT1A receptors. The 5-HT1A receptors function both at presynaptic (autoreceptor) and postsynaptic (heteroreceptor) sites. Activation of 5-HT1A autoreceptors on the cell bodies of serotonergic neurons in the raphe nucleus exerts inhibitory feedback in response to local release of serotonin. The 5-HT1A heteroreceptors are expressed in limbic regions such as prefrontal cortex, hippocampus, lateral septum, and amygdala, as well as in thalamus, hypothalamus and basal ganglia [131, 132]. Activation of 5-HT1A heteroreceptors reduces postsynaptic neuronal firing and excitation. To date, 5-HT1A receptors have been considered as a target for the action of antidepressants [133, 134]. Recently, several studies reported new insights into the therapeutical function of 5-HT1A receptors in treating schizophrenia and Parkinson’s disease [135, 136]. Stimulation of 5-HT1A receptors improves cognitive deficits in patients with schizophrenia possibly by normalization of lactate metabolism in the prefrontal cortex [137]. Furthermore, 5-HT1A agonists are expected to improve not only psychiatric symptoms such as anxiety and depression, but also L-DOPA-induced side effects of dyskinesia [138-142]. These pharmacological actions are mainly mediated by neuronal 5-HT1A receptors. On the other hand, we have demonstrated the expression of 5-HT1A receptors in striatal astrocytes, and stimulation of the receptors exerts neuroprotection against dopaminergic neurotoxicity [18].
3.2. Stimulation of 5-HT1A Receptors Enhances Astrocyte Proliferation via S100β Secretion
Astrocyte proliferation is promoted by S100β protein, which is released by astrocytes and affects in the autocrine fashion [143-146]. S100β is a small EF-hand Ca2+-binding protein. This protein is expressed in various cell types such as astrocytes, oligodendrocytes, renal epithelial cells, and neural progenitor cells. The highest level of S100β expression is found in the cytoplasm of astrocytes [145, 146], and astrocytes secret the protein to the extracellular space. Extracellular S100β has various functions and affects on not only astrocytes but also neurons. S100β has opposite effects on neurons depending on its concentration. Nanomolar levels of S100β protect neurons from stress-induced apoptosis [147], stimulate neurite outgrowth and microtubule associated protein2 (MAP2) expression [148, 149] and modulate long-term neuronal plasticity [150] (Fig. 2). On the other hand, exposure to micromolar levels of S100β increases β−amyloid neurotoxicity [151] and causes neuronal apoptosis [152]. In addition, extracellular S100β at low dose enhances glutamate uptake activity of astrocytes, which plays essential roles in regulating extracellular levels of glutamate [153-155] (Fig. 2). Tramontina et al. [154, 155] provided direct evidence for the stimulatory effect of extracellular S100β on glutamate uptake into astrocytes by addition of S100β protein, and that anti-S100β antibody decreased glutamate uptake without affecting cell integrity or viability. It is also reported that epicatechin gallate, which is an abundant polyphenol in green tea, induces glutamate uptake and S100β secretion in astroglial C6 cells [153]. These results suggest that S100β secretion by epicatechin gallate may be associated with the improvement of glutamate uptake. Other studies demonstrated that extracellular S100β could protect hippocampal neurons against glutamate-induced damage [147, 156]. These findings reinforce the importance of astrocytes in the neuroprotective role of S100β against excitotoxic damage. Astrocytes seem to secret S100β to the extracellular space to reduce the excitotoxicity (Fig. 2).
Fig. (2).
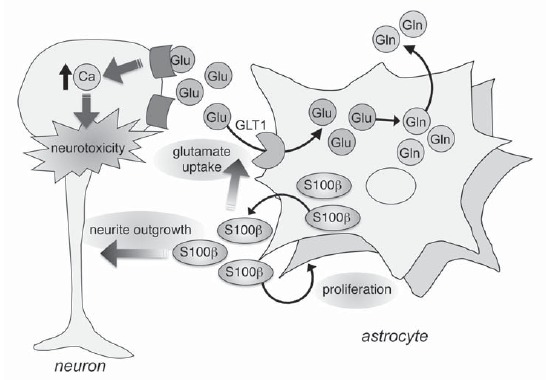
Function of extracellular S100β. S100β has an effect on neurons depending on its concentration. Nanomolar levels of S100β protect neurons from stress-induced apoptosis, stimulate neurite outgrowth and microtubule associated protein2 (MAP2) expression. Extracellular S100β exerts autocrine effects that promote astrocyte proliferation and glutamate uptake activity of astrocytes.
Extracellular S100β has autocrine effects that promote astrocyte proliferation [145, 146] (Fig. 2). It is previously reported that stimulation of astrocytic 5-HT1A receptors results in the release of S100β protein from astrocytes [143, 144]. Recent studies from our laboratory also demonstrated the significant increase in S100β levels in media of striatal astrocytes treated with a full 5-HT1A agonist (R)-(+)-8-hydroxy-2-(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT) [18]. Since stimulation of 5-HT1A receptors on astrocytes induced the release of S100β protein from astrocytes [144] and extracellular S100β enhanced astrocyte proliferation [145, 146], it is expected that 5-HT1A agonists could promote astrocyte proliferation. In this regard, a previous study demonstrated that buspirone, a partial 5-HT1A agonist, increased the number of cultured astrocytes [157]. In a more recent study, we demonstrated that 8-OH-DPAT (1-10 nM) promoted astrocyte proliferation via secretion of S100β [18]. The enhancement of astrocyte proliferation was also observed in the striatum of mice after repeated administration of 8-OH-DPAT (0.05, 0.1 mg/kg, i.p. for 7 days). However, relatively high doses of 8-OH-DPAT (>10 nM) on astroglial C6 cells or 8-OH-DPAT administration (0.5 mg/kg for 7 days) in mice had no effects on S100β secretion or astrocyte proliferation [18]. As stated above, S100β can induce astrocyte proliferation at low-to-medium dose, but not at high dose [146]. Although the mechanism in lack of promotive effects of 8-OH-DPAT at higher dose is obscure, these previous reports suggest that there is effective window of doses to induce astrocyte proliferation by stimulation of 5-HT1A receptors.
3.3. 5-HT1A Agonist Produces Nrf2 Activation
Nrf2 is normally sequestered in the cytoplasm and degradated in the proteosome through its regulatory protein Keap1, but can translocate into the nucleus and consequently activate various antioxidant molecules upon exposure to oxidative stress [79, 158]. Therefore, Nrf2 activation has recently received attention as a new therapeutic strategy to protect against dopaminergic neurotoxicity induced by oxidative stress. We demonstrated that Nrf2 in astrocytes is activated after excess amount of DA exposure as dopaminergic oxidative stress [75]. We also recently reported that 5-HT1A agonist 8-OH-DPAT produced Nrf2 activation in astrocytes [18]. Expression of nuclear Nrf2 was significantly increased in these astrocytes 6 h after 8-OH-DPAT exposure, and this up-regulation was suppressed by the 5-HT1A antagonist. Furthermore, we have also demonstrated that 8-OH-DPAT can increase the binding activity of Nrf2 to ARE of MT-1 promoter region followed by up-regulation of MTs in astrocytes. These finding suggest that 8-OH-DPAT induces nuclear translocation of Nrf2 followed by binding to ARE prior to MT induction. Further studies are needed to determine the mechanism of 8-OH-DPAT-induced Nrf2 nuclear translocation in astrocytes.
3.4. Stimulation of Astrocytes 5-HT1A Receptors Protects Dopaminergic Neurons
As mentioned above, stimulation of 5-HT1A receptors on astrocytes promotes astrocyte proliferation and Nrf2 activation, suggesting up-regulation of antioxidative pathways in astrocytes. Therefore, it is expected that 5-HT1A agonists can protect against oxidative stress-induced dopaminergic neurodegeneration. Indeed, we have demonstrated that stimulation of 5-HT1A receptors on astrocytes by 8-OH-DPAT could protect dopaminergic neurons by using primary cultured neuron-astrocyte mixed cells and parkinsonian mice [18]. The conditioned media from 8-OH-DPAT-treated striatal astrocytes conferred significantly higher protection against dopaminergic neurotoxicity, compared with media from astrocytes free of 8-OH-DPAT. In addition, such neuroprotection was completely blocked by 5-HT1A antagonist WAY100635. The levels of nuclear Nrf2 expression and MT-1 secreted from astrocytes were increased after 8-OH-DPAT treatment, and these effects were also inhibited by the 5-HT1A antagonist. Furthermore, the neuroprotection was cancelled in the presence of anti-MT-1/-2 antibody. These results suggest that stimulation of 5-HT1A receptors on striatal astrocytes can upregulate and secrete MTs by astrocytes to protect dopaminergic neurons against neurodegeneration. In another series of studies, we also demonstrated that 8-OH-DPAT administrations (0.1 mg/kg for 7 days) prevented gradual degeneration of dopaminergic neurons in parkinsonian mice induced by intrastriatal injection of 6-hydroxydopamine [18]. Several experiments have also demonstrated the protective effects of 5-HT1A agonists against neuronal damage [147, 159, 160]. For example, 5-HT1A agonist, Bay x 3702, protected neurons against glutamate- or staurosporine-induced neurotoxicity by secretion of S100β from striatal astrocytes [147]. Another 5-HT1A agonist, BAY 639044, ameliorated MPTP toxicity in mice and macaque models of PD [159]. In these reports, the presumed mechanism of neuroprotection by 5-HT1A agonists relates to a reduction in glutamate release and subsequent excitotoxicity. In another experiment, 8-OH-DPAT and (R)-5-fluoro-8-hydroxy-2-(dipropylamino)-tetralin (R-UH-301) attenuated NMDA- and MPP+-induced apoptotic cell death in striatal and mesencephalic cell cultures [160]. The authors interpreted that the mechanism of neuroprotection of 5-HT1A agonists was attenuation of NMDA-induced Ca2+ influx and prevention of apoptotic cascade in neurons (Table 1).
Table 1.
Summary of the effects of 5-HT1A agonists on models or patients of Parkinson's disease
| 5-HT1A agonist | Effectiveness | Targeted Cell | Reference |
|---|---|---|---|
| Bay x 3702 | neuroprotection | astrocyte | [147] |
| BAY 639044 | neuroprotection | neuron | [159] |
| R-UH-301 | neuroprotection | neuron | [160] |
| 8-OH-DPAT | neuroprotection | neuron | [160] |
| astrocyte | [18] | ||
| eltoprazine | dyskinesia | neuron | [167] |
| unknown | [140, 141] | ||
| N-propylnoraporphin-11-yl 5-(1,2-dithiolan-3-yl)pentanoate | dyskinesia | neuron | [142] |
| 8-OH-DPAT | dyskinesia | unknown | [138] |
| improvement of motor fluctuation | unknown | [139] | |
| anxiolytic effects | neuron | [162] | |
| antidepressant-like effects | neuron | [163] | |
| F15599 | dyskinesia | neuron | [164] |
| F13714 | dyskinesia | neuron | [165] |
| F13640 | dyskinesia | unknown | [166] |
| anxiolytic effects | unknown | [166] | |
| antidepressant-like effects | unknown | [166] | |
| buspirone | dyskinesia | neuron | [161] |
In these days, various approaches using 5-HT1A agonists target mainly motor fluctuation in PD [138-142, 161-167] (Table 1). Eltoprazine, a selective 5-HT1A and 5-HT1B agonist, is currently being tested in clinical trials for motor complications derived from dopaminergic therapy in PD [140, 141]. The dopamine D2 and 5-HT1A dual-agonist, N-propylnoraporphin-11-yl 5-(1,2-dithiolan-3-yl)pentanoate, significantly reduced L-dopa-induced dyskinesia in parkinsonian rats [142]. In addition, previous studies demonstrated that 8-OH-DPAT also reduced dyskinesia and improved movement in parkinsonian animals [138, 139]. Almost all 5-HT1A agonists are thought to affect on neurons, and it is unclear whether these drugs affect on 5-HT1A receptors on astrocytes. Nevertheless, the treatment with 5-HT1A agonists is useful for L-dopa-induced side effects in PD (Fig. 3). On the other hand, neuroprotective or disease-modifying treatments are clinically required to prevent or delay disease progression in PD. Based on the neuroprotective property of astrocytes, many researchers have focused to astrocytes as a target for therapy in neurodegenerative diseases. Some recent studies have demonstrated that astrocyte transplantation produces neuronal repair in amyotrophic lateral
Fig. (3).
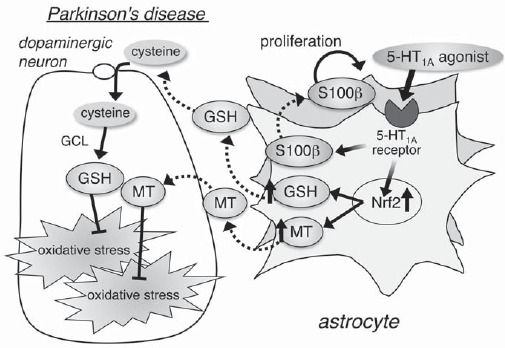
Serotonin 1A (5-HT1A) receptors on astrocytes as a novel target of neuroprotection. Stimulation of 5-HT1A receptors on astrocytes promotes S100β secretion followed by astrocyte proliferation and Nrf2 activation to up-regulate expression of antioxidative molecules, such as glutathione (GSH) and metallothionein (MT). Therefore, the strategy of increasing the number of intrinsically healthy astrocytes through stimulation of 5-HT1A receptors on astrocytes is conceivable and could help design new therapies that provide neuroprotection against oxidative stress and neurodegenerative disorders.
sclerosis, spinal cord injury, stroke and PD [12-17]. Therefore, the strategy of increasing the number of intrinsically healthy astrocytes through stimulation of 5-HT1A receptors on astrocytes is conceivable and could help design new therapies that provide neuroprotection against oxidative stress and neurodegenerative disorders including PD.
At the present time, there is no validated imaging technology to detect neurodegeneration in PD patients. The clinical diagnosis of PD is based on the observation of motor symptoms, including tremor, bradykinesia, rigidity and postural instability, and 123I-metaiodobenzylguanidine (MIBG) cardiac scintigraphy. However, pathological process leading to PD can begin decades before developing these symptoms. One of the prerequisites is development of reliable diagnostic and prognostic biomarkers of early PD. Furthermore, objective reliable biomarkers to measure the progression of the disease are required to gauge the effectiveness of neuroprotective therapeutic appro-aches.
CONCLUSION
Astrocytes have various neuroprotective properties such as production of antioxidative or neurotrophic molecules and clearance of neurodegeneration-inducible molecules. Therefore, increasing the number of healthy astrocytes, which possess these neuroprotective ability, is a potentially viable therapeutic approach to prevent the degenerative process of PD. Stimulation of 5-HT1A receptors on astrocytes promotes not only astrocyte proliferation via secretion of S100β but also Nrf2 activation leading to upregulation of antioxidative molecules, thus acting as a neuroprotectant in neurodegenerative disease. Development of pharmacological agents that can specifically target 5-HT1A receptors on astrocytes could provide a promising therapeutic strategy of neuroprotection against progressive dopaminergic neurodegeneration.
ACKNOWLEDGEMENTS
This work was supported by Grants-in-Aid for Young Scientists (B) (grant number: 18700364 to I.M.), for Scientific Research (C) (grant number: 21591082 to M.A., 22590934 and 25461279 to I.M.) from Japan Society for the Promotion of Science, by Grant-in Aid for Scientific Research on Innovative Areas “Brain Environment” from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (grant number: 24111533 to M.A.), by the Okayama Medical Foundation, Kawasaki Foundation for Medical Science & Medical Welfare (to I.M.).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Cheng H.C., Ulane C.M., Burke R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010;67(6):715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Oroz M.C., Jahanshahi M., Krack P., Litvan I., Macias R., Bezard E., Obeso J.A. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8(12):1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- 3.Joyce J.N., Millan M.J. Dopamine D3 receptor agonists for protection and repair in Parkinson’s disease. Curr. Opin. Pharmacol. 2007;7(1):100–105. doi: 10.1016/j.coph.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Joyce J.N., Woolsey C., Ryoo H., Borwege S., Hagner D. Low dose pramipexole is neuroprotective in the MPTP mouse model of Parkinson's disease, and downregulates the dopamine transporter via the D3 receptor. BMC Biol. 2004;2:22. doi: 10.1186/1741-7007-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youdim M.B., Kupershmidt L., Amit T., Weinreb O. Promises of novel multi-target neuroprotective and neurorestorative drugs for Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20(Suppl. 1):S132–S136. doi: 10.1016/S1353-8020(13)70032-4. [DOI] [PubMed] [Google Scholar]
- 6.Lee H.J., Suk J.E., Patrick C., Bae E.J., Cho J.H., Rho S., Hwang D., Masliah E., Lee S.J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010;285(12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson M.B. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem. Int. 1998;33(6):479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 8.Rothstein J.D., Dykes-Hoberg M., Pardo C.A., Bristol L.A., Jin L., Kuncl R.W., Kanai Y., Hediger M.A., Wang Y., Schielke J.P., Welty D.F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 9.Vargas M.R., Johnson J.A. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev. Mol. Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P.C., Vargas M.R., Pani A.K., Smeyne R.J., Johnson D.A., Kan Y.W., Johnson J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. USA. 2009;106(8):2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makar T.K., Nedergaard M., Preuss A., Gelbard A.S., Perumal A.S., Cooper A.J. Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of gluta-thione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J. Neurochem. 1994;62(1):45–53. doi: 10.1046/j.1471-4159.1994.62010045.x. [DOI] [PubMed] [Google Scholar]
- 12.Chu T., Zhou H., Li F., Wang T., Lu L., Feng S. Astrocyte transplantation for spinal cord injury: current status and perspective. Brain Res. Bull. 2014;107:18–30. doi: 10.1016/j.brainresbull.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Falnikar A., Li K., Lepore A.C. Therapeutically targeting astrocytes with stem and progenitor cell transplantation following traumatic spinal cord injury. Brain Res. 2015;1619:91–103. doi: 10.1016/j.brainres.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gleichman A.J., Carmichael S.T. Astrocytic therapies for neuronal repair in stroke. Neurosci. Lett. 2014;565:47–52. doi: 10.1016/j.neulet.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 15.Nicaise C., Mitrecic D., Falnikar A., Lepore A.C. Transplantation of stem cell-derived astrocytes for the treatment of amyotrophic lateral sclerosis and spinal cord injury. World J. Stem Cells. 2015;7(2):380–398. doi: 10.4252/wjsc.v7.i2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble M., Davies J.E., Mayer-Proschel M., Proschel C., Davies S.J. Precursor cell biology and the development of astrocyte transplantation therapies: lessons from spinal cord injury. Neurotherapeutics. 2011;8(4):677–693. doi: 10.1007/s13311-011-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proschel C., Stripay J.L., Shih C.H., Munger J.C., Noble M.D. Delayed transplantation of precursor cell-derived astrocytes provides multiple benefits in a rat model of Parkinsons. EMBO Mol. Med. 2014;6(4):504–518. doi: 10.1002/emmm.201302878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki I., Asanuma M., Murakami S., Takeshima M., Torigoe N., Kitamura Y., Miyoshi K. Targeting 5-HT(1A) receptors in astrocytes to protect dopaminergic neurons in Parkinsonian models. Neurobiol. Dis. 2013;59:244–256. doi: 10.1016/j.nbd.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Cho Y., Bannai S. Uptake of glutamate and cysteine in C-6 glioma cells and in cultured astrocytes. J. Neurochem. 1990;55(6):2091–2097. doi: 10.1111/j.1471-4159.1990.tb05800.x. [DOI] [PubMed] [Google Scholar]
- 20.Qiang W., Cahill J.M., Liu J., Kuang X., Liu N., Scofield V.L., Voorhees J.R., Reid A.J., Yan M., Lynn W.S., Wong P.K. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J. Virol. 2004;78(21):11926–11938. doi: 10.1128/JVI.78.21.11926-11938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seib T.M., Patel S.A., Bridges R.J. Regulation of the system x(C)- cystine/glutamate exchanger by intracellular glutathione levels in rat astrocyte primary cultures. Glia. 2011;59(10):1387–1401. doi: 10.1002/glia.21176. [DOI] [PubMed] [Google Scholar]
- 22.Dringen R., Gutterer J.M., Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000;267(16):4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 23.Shih A.Y., Erb H., Sun X., Toda S., Kalivas P.W., Murphy T.H. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci. 2006;26(41):10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X.F., Cynader M.S. Astrocytes provide cysteine to neurons by releasing glutathione. J. Neurochem. 2000;74(4):1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- 25.Jenner P., Dexter D.T., Sian J., Schapira A.H., Marsden C.D. Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann. Neurol. 1992;32(Suppl.):S82–S87. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- 26.Riederer P., Sofic E., Rausch W.D., Schmidt B., Reynolds G.P., Jellinger K., Youdim M.B. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 1989;52(2):515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 27.Sian J., Dexter D.T., Lees A.J., Daniel S., Agid Y., Javoy-Agid F., Jenner P., Marsden C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. 1994;36(3):348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 28.Sofic E., Lange K.W., Jellinger K., Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci. Lett. 1992;142(2):128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 29.Asanuma M., Miyazaki I., Ogawa N. Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox. Res. 2003;5(3):165–176. doi: 10.1007/BF03033137. [DOI] [PubMed] [Google Scholar]
- 30.Graham D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978;14(4):633–643. [PubMed] [Google Scholar]
- 31.Sulzer D., Bogulavsky J., Larsen K.E., Behr G., Karatekin E., Kleinman M.H., Turro N., Krantz D., Edwards R.H., Greene L.A., Zecca L. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. USA. 2000;97(22):11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tse D.C., McCreery R.L., Adams R.N. Potential oxidative pathways of brain catecholamines. J. Med. Chem. 1976;19(1):37–40. doi: 10.1021/jm00223a008. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn D.M., Arthur R.E., Jr, Thomas D.M., Elferink L.A. Tyrosine hydroxylase is inactivated by catechol-quinones and converted to a redox-cycling quinoprotein: possible relevance to Parkinson’s disease. J. Neurochem. 1999;73(3):1309–1317. doi: 10.1046/j.1471-4159.1999.0731309.x. [DOI] [PubMed] [Google Scholar]
- 34.LaVoie M.J., Ostaszewski B.L., Weihofen A., Schlossmacher M.G., Selkoe D.J. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005;11(11):1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead R.E., Ferrer J.V., Javitch J.A., Justice J.B. Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J. Neurochem. 2001;76(4):1242–1251. doi: 10.1046/j.1471-4159.2001.00125.x. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki I., Asanuma M., Diaz-Corrales F.J., Miyoshi K., Ogawa N. Dopamine agonist pergolide prevents levodopa-induced quinoprotein formation in parkinsonian striatum and shows quenching effects on dopamine-semiquinone generated in vitro. Clin. Neuropharmacol. 2005;28(4):155–160. doi: 10.1097/01.wnf.0000175523.33334.24. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa N., Tanaka K., Asanuma M. Bromocriptine markedly suppresses levodopa-induced abnormal increase of dopamine turnover in the parkinsonian striatum. Neurochem. Res. 2000;25(6):755–758. doi: 10.1023/a:1007530720544. [DOI] [PubMed] [Google Scholar]
- 38.Haque M.E., Asanuma M., Higashi Y., Miyazaki I., Tanaka K., Ogawa N. Apoptosis-inducing neurotoxicity of dopamine and its metabolites via reactive quinone generation in neuroblastoma cells. Biochim. Biophys. Acta. 2003;1619(1):39–52. doi: 10.1016/s0304-4165(02)00440-3. [DOI] [PubMed] [Google Scholar]
- 39.Hastings T.G. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J. Neurochem. 1995;64(2):919–924. doi: 10.1046/j.1471-4159.1995.64020919.x. [DOI] [PubMed] [Google Scholar]
- 40.Lai C.T., Yu P.H. Dopamine- and L-beta-3,4-dihydroxy-phenylalanine hydrochloride (L-Dopa)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Effects of oxidative stress and antioxidative factors. Biochem. Pharmacol. 1997;53(3):363–372. doi: 10.1016/s0006-2952(96)00731-9. [DOI] [PubMed] [Google Scholar]
- 41.Miyazaki I., Asanuma M. Approaches to prevent dopa-mine quinone-induced neurotoxicity. Neurochem. Res. 2009;34(4):698–706. doi: 10.1007/s11064-008-9843-1. [DOI] [PubMed] [Google Scholar]
- 42.Iwata-Ichikawa E., Kondo Y., Miyazaki I., Asanuma M., Ogawa N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J. Neurochem. 1999;72(6):2334–2344. doi: 10.1046/j.1471-4159.1999.0722334.x. [DOI] [PubMed] [Google Scholar]
- 43.Solano R.M., Casarejos M.J., Menendez-Cuervo J., Rodriguez-Navarro J.A., Garcia de Yebenes J., Mena M.A. Glial dysfunction in parkin null mice: effects of aging. J. Neurosci. 2008;28(3):598–611. doi: 10.1523/JNEUROSCI.4609-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asanuma M., Miyazaki I., Diaz-Corrales F.J., Kimoto N., Kikkawa Y., Takeshima M., Miyoshi K., Murata M. Neuroprotective effects of zonisamide target astrocyte. Ann. Neurol. 2010;67(2):239–249. doi: 10.1002/ana.21885. [DOI] [PubMed] [Google Scholar]
- 45.Murata M. Novel therapeutic effects of the anti-convulsant, zonisamide, on Parkinson’s disease. Curr. Pharm. Des. 2004;10(6):687–693. doi: 10.2174/1381612043453180. [DOI] [PubMed] [Google Scholar]
- 46.Murata M., Hasegawa K., Kanazawa I. Zonisamide improves motor function in Parkinson’s disease: a randomized, double-blind study. Neurology. 2007;68(1):45–50. doi: 10.1212/01.wnl.0000250236.75053.16. [DOI] [PubMed] [Google Scholar]
- 47.Murata M., Horiuchi E., Kanazawa I. Zonisamide has beneficial effects on Parkinson’s disease patients. Neurosci. Res. 2001;41(4):397–399. doi: 10.1016/s0168-0102(01)00298-x. [DOI] [PubMed] [Google Scholar]
- 48.Aschner M. Metallothionein (MT) isoforms in the central nervous system (CNS): regional and cell-specific distribution and potential functions as an antioxidant. Neurotoxicology. 1998;19(4-5):653–660. [PubMed] [Google Scholar]
- 49.Miyazaki I., Asanuma M., Higashi Y., Sogawa C.A., Tanaka K., Ogawa N. Age-related changes in expression of metallothionein-III in rat brain. Neurosci. Res. 2002;43(4):323–333. doi: 10.1016/s0168-0102(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 50.Hussain S., Slikker W., Jr, Ali S.F. Role of metallo-thionein and other antioxidants in scavenging superoxide radicals and their possible role in neuroprotection. Neurochem. Int. 1996;29(2):145–152. doi: 10.1016/0197-0186(95)00114-x. [DOI] [PubMed] [Google Scholar]
- 51.Thornalley P.J., Vasak M. Possible role for metallo-thionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta. 1985;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 52.Penkowa M. Metallothioneins are multipurpose neuro-protectants during brain pathology. FEBS J. 2006;273(9):1857–1870. doi: 10.1111/j.1742-4658.2006.05207.x. [DOI] [PubMed] [Google Scholar]
- 53.Miyazaki I., Asanuma M., Hozumi H., Miyoshi K., Sogawa N. Protective effects of metallothionein against dopamine quinone-induced dopaminergic neurotoxicity. FEBS Lett. 2007;581:5003–5008. doi: 10.1016/j.febslet.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 54.Sharma S., Ebadi M. Significance of metallothioneins in aging brain. Neurochem. Int. 2014;65:40–48. doi: 10.1016/j.neuint.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Sharma S., Moon C.S., Khogali A., Haidous A., Chabenne A., Ojo C., Jelebinkov M., Kurdi Y., Ebadi M. Biomarkers in Parkinson’s disease (recent update). Neurochem. Int. 2013;63(3):201–229. doi: 10.1016/j.neuint.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Sharma S., Rais A., Sandhu R., Nel W., Ebadi M. Clinical significance of metallothioneins in cell therapy and nanomedicine. Int. J. Nanomedicine. 2013;8:1477–1488. doi: 10.2147/IJN.S42019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebadi M., Sharma S. Metallothioneins 1 and 2 attenuate peroxynitrite-induced oxidative stress in Parkinson’s disease. Exp. Biol. Med. 2006;231(9):1576–1583. doi: 10.1177/153537020623100919. [DOI] [PubMed] [Google Scholar]
- 58.Ebadi M., Sharma S.K. Peroxynitrite and mitochondrial dysfunction in the pathogenesis of Parkinson’s disease. Antioxid. Redox Signal. 2003;5(3):319–335. doi: 10.1089/152308603322110896. [DOI] [PubMed] [Google Scholar]
- 59.Sharma S.K., Ebadi M. Metallothionein attenuates 3-morpholinosydnonimine (SIN-1)-induced oxidative stress in dopaminergic neurons. Antioxid. Redox Signal. 2003;5(3):251–264. doi: 10.1089/152308603322110832. [DOI] [PubMed] [Google Scholar]
- 60.Chung R.S., West A.K. A role for extracellular metal-lothioneins in CNS injury and repair. Neuroscience. 2004;123(3):595–599. doi: 10.1016/j.neuroscience.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Holloway A.F., Stennard F.A., Dziegielewska K.M., Weller L., West A.K. Localisation and expression of metallothionein immunoreactivity in the developing sheep brain. Int. J. Dev. Neurosci. 1997;15(2):195–203. doi: 10.1016/s0736-5748(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 62.Choudhury M.E., Sugimoto K., Kubo M., Iwaki H., Tsujii T., Kyaw W.T., Nishikawa N., Nagai M., Tanaka J., Nomoto M. Zonisamide up-regulated the mRNAs encoding astrocytic anti-oxidative and neurotrophic factors. Eur. J. Pharmacol. 2012;689(1-3):72–80. doi: 10.1016/j.ejphar.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 63.Kruczek C., Gorg B., Keitel V., Bidmon H.J., Schliess F., Haussinger D. Ammonia increases nitric oxide, free Zn(2+), and metallothionein mRNA expression in cultured rat astrocytes. Biol. Chem. 2011;392(12):1155–1165. doi: 10.1515/BC.2011.199. [DOI] [PubMed] [Google Scholar]
- 64.Michael G.J., Esmailzadeh S., Moran L.B., Christian L., Pearce R.K., Graeber M.B. Up-regulation of metallo-thionein gene expression in parkinsonian astrocytes. Neurogenetics. 2011;12(4):295–305. doi: 10.1007/s10048-011-0294-5. [DOI] [PubMed] [Google Scholar]
- 65.Zatta P., Zambenedetti P., Musicco M., Adorni F. Metallothionein-I-II and GFAP positivity in the brains from frontotemporal dementia patients. J. Alzheimers Dis. 2005;8(2):109–116. doi: 10.3233/jad-2005-8203. [DOI] [PubMed] [Google Scholar]
- 66.Zambenedetti P., Giordano R., Zatta P. Metallothioneins are highly expressed in astrocytes and microcapillaries in Alzheimer's disease. J. Chem. Neuroanat. 1998;15(1):21–26. doi: 10.1016/s0891-0618(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 67.Aschner M., Conklin D.R., Aschner J.L. Induction of metallothionein-I (MT-I) mRNA in primary astrocyte cultures is mediated by hypotonicity and not ethanol (EtOH) per se. Brain Res. 1997;770(1-2):289–293. doi: 10.1016/s0006-8993(97)00772-5. [DOI] [PubMed] [Google Scholar]
- 68.Aschner M. Astrocyte metallothioneins (MTs) and their neuroprotective role. Ann. N. Y. Acad. Sci. 1997;825:334–347. doi: 10.1111/j.1749-6632.1997.tb48445.x. [DOI] [PubMed] [Google Scholar]
- 69.Neal J.W., Singhrao S.K., Jasani B., Newman G.R. Immunocytochemically detectable metallothionein is expressed by astrocytes in the ischaemic human brain. Neuropathol. Appl. Neurobiol. 1996;22(3):243–247. [PubMed] [Google Scholar]
- 70.Rising L., Vitarella D., Kimelberg H.K., Aschner M. Metallothionein induction in neonatal rat primary astrocyte cultures protects against methylmercury cytotoxicity. J. Neurochem. 1995;65(4):1562–1568. doi: 10.1046/j.1471-4159.1995.65041562.x. [DOI] [PubMed] [Google Scholar]
- 71.Young J.K. Glial metallothionein. Biol. Signals. 1994;3(3):169–175. doi: 10.1159/000109540. [DOI] [PubMed] [Google Scholar]
- 72.Sawada J., Kikuchi Y., Shibutani M., Mitsumori K., Inoue K., Kasahara T. Induction of metallothionein in astrocytes by cytokines and heavy metals. Biol. Signals. 1994;3(3):157–168. doi: 10.1159/000109539. [DOI] [PubMed] [Google Scholar]
- 73.Young J.K., Garvey J.S., Huang P.C. Glial immuno-reactivity for metallothionein in the rat brain. Glia. 1991;4(6):602–610. doi: 10.1002/glia.440040607. [DOI] [PubMed] [Google Scholar]
- 74.Chung R.S., Adlard P.A., Dittmann J., Vickers J.C., Chuah M.I., West A.K. Neuron-glia communication: metallothionein expression is specifically up-regulated by astrocytes in response to neuronal injury. J. Neurochem. 2004;88(2):454–461. doi: 10.1046/j.1471-4159.2003.02193.x. [DOI] [PubMed] [Google Scholar]
- 75.Miyazaki I., Asanuma M., Kikkawa Y., Takeshima M., Murakami S., Miyoshi K., Sogawa N., Kita T. Astrocyte-derived metallothionein protects dopaminergic neurons from dopamine quinone toxicity. Glia. 2011;59(3):435–451. doi: 10.1002/glia.21112. [DOI] [PubMed] [Google Scholar]
- 76.Palmiter R.D. The elusive function of metallothioneins. Proc. Natl. Acad. Sci. USA. 1998;95(15):8428–8430. doi: 10.1073/pnas.95.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mocchegiani E., Muzzioli M., Giacconi R. Zinc and immunoresistance to infection in aging: new biological tools. Trends Pharmacol. Sci. 2000;21(6):205–208. doi: 10.1016/s0165-6147(00)01476-0. [DOI] [PubMed] [Google Scholar]
- 78.Shih A.Y., Johnson D.A., Wong G., Kraft A.D., Jiang L., Erb H., Johnson J.A., Murphy T.H. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 2003;23(8):3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang M.I., Kobayashi A., Wakabayashi N., Kim S.G., Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. USA. 2004;101(7):2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99(18):11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wakabayashi N., Dinkova-Kostova A.T., Holtzclaw W.D., Kang M.I., Kobayashi A., Yamamoto M., Kensler T.W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101(7):2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Innamorato N.G., Jazwa A., Rojo A.I., Garcia C., Fernandez-Ruiz J., Grochot-Przeczek A., Stachurska A., Jozkowicz A., Dulak J., Cuadrado A. Different susceptibility to the Parkinson’s toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS One. 2010;5(7):e11838. doi: 10.1371/journal.pone.0011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonifati V., Rizzu P., Squitieri F., Krieger E., Vanacore N., van Swieten J.C., Brice A., van Duijn C.M., Oostra B., Meco G., Heutink P. DJ-1(PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol. Sci. 2003;24(3):159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- 85.Canet-Aviles R.M., Wilson M.A., Miller D.W., Ahmad R., McLendon C., Bandyopadhyay S., Baptista M.J., Ringe D., Petsko G.A., Cookson M.R. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA. 2004;101(24):9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitsumoto A., Nakagawa Y., Takeuchi A., Okawa K., Iwamatsu A., Takanezawa Y. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic. Res. 2001;35(3):301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 87.Kitamura Y., Watanabe S., Taguchi M., Takagi K., Kawata T., Takahashi-Niki K., Yasui H., Maita H., Iguchi-Ariga S.M., Ariga H. Neuroprotective effect of a new DJ-1-binding compound against neurodegeneration in Parkinson’s disease and stroke model rats. Mol. Neurodegener. 2011;6(1):48. doi: 10.1186/1750-1326-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bandopadhyay R., Kingsbury A.E., Cookson M.R., Reid A.R., Evans I.M., Hope A.D., Pittman A.M., Lashley T., Canet-Aviles R., Miller D.W., McLendon C., Strand C., Leonard A.J., Abou-Sleiman P.M., Healy D.G., Ariga H., Wood N.W., de Silva R., Revesz T., Hardy J.A., Lees A.J. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127(Pt 2):420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 89.Yanagida T., Tsushima J., Kitamura Y., Yanagisawa D., Takata K., Shibaike T., Yamamoto A., Taniguchi T., Yasui H., Taira T., Morikawa S., Inubushi T., Tooyama I., Ariga H. Oxidative stress induction of DJ-1 protein in reactive astrocytes scavenges free radicals and reduces cell injury. Oxid. Med. Cell. Longev. 2009;2(1):36–42. doi: 10.4161/oxim.2.1.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lev N., Barhum Y., Ben-Zur T., Melamed E., Steiner I., Offen D. Knocking out DJ-1 attenuates astrocytes neuro-protection against 6-hydroxydopamine toxicity. J. Mol. Neurosci. 2013;50(3):542–550. doi: 10.1007/s12031-013-9984-9. [DOI] [PubMed] [Google Scholar]
- 91.Mullett S.J., Hinkle D.A. DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol. Dis. 2009;33(1):28–36. doi: 10.1016/j.nbd.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mullett S.J., Hinkle D.A. DJ-1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor-induced neurotoxicity. J. Neurochem. 2011;117(3):375–387. doi: 10.1111/j.1471-4159.2011.07175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clements C.M., McNally R.S., Conti B.J., Mak T.W., Ting J.P. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA. 2006;103(41):15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hao L.Y., Giasson B.I., Bonini N.M. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc. Natl. Acad. Sci. USA. 2010;107(21):9747–9752. doi: 10.1073/pnas.0911175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomas K.J., McCoy M.K., Blackinton J., Beilina A., van der Brug M., Sandebring A., Miller D., Maric D., Cedazo-Minguez A., Cookson M.R. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum. Mol. Genet. 2011;20(1):40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 97.Moore D.J. Parkin: a multifaceted ubiquitin ligase. Biochem. Soc. Trans. 2006;34(Pt 5):749–753. doi: 10.1042/BST0340749. [DOI] [PubMed] [Google Scholar]
- 98.Xiong H., Wang D., Chen L., Choo Y.S., Ma H., Tang C., Xia K., Jiang W., Ronai Z., Zhuang X., Zhang Z. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J. Clin. Invest. 2009;119(3):650–660. doi: 10.1172/JCI37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lazarou M., Sliter D.A., Kane L.A., Sarraf S.A., Wang C., Burman J.L., Sideris D.P., Fogel A.I., Youle R.J. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shiba-Fukushima K., Inoshita T., Hattori N., Imai Y. PINK1-mediated phosphorylation of Parkin boosts Parkin activity in Drosophila. PLoS Genet. 2014;10(6):e1004391. doi: 10.1371/journal.pgen.1004391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Springer W., Kahle P.J. Regulation of PINK1-Parkin-mediated mitophagy. Autophagy. 2011;7(3):266–278. doi: 10.4161/auto.7.3.14348. [DOI] [PubMed] [Google Scholar]
- 102.Ledesma M.D., Galvan C., Hellias B., Dotti C., Jensen P.H. Astrocytic but not neuronal increased expression and redistribution of parkin during unfolded protein stress. J. Neurochem. 2002;83(6):1431–1440. doi: 10.1046/j.1471-4159.2002.01253.x. [DOI] [PubMed] [Google Scholar]
- 103.Saini N., Georgiev O., Schaffner W. The parkin mutant phenotype in the fly is largely rescued by metal-responsive transcription factor (MTF-1). Mol. Cell. Biol. 2011;31(10):2151–2161. doi: 10.1128/MCB.05207-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghoshal K., Majumder S., Li Z., Dong X., Jacob S.T. Suppression of metallothionein gene expression in a rat hepatoma because of promoter-specific DNA methylation. J. Biol. Chem. 2000;275(1):539–547. doi: 10.1074/jbc.275.1.539. [DOI] [PubMed] [Google Scholar]
- 105.Andrews G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000;5(1):95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 106.Airaksinen M.S., Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 107.Lin L.F., Doherty D.H., Lile J.D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 108.d'Anglemont de Tassigny X., Pascual A., Lopez-Barneo J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front. Neuroanat. 2015;9:10. doi: 10.3389/fnana.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Francardo V., Bez F., Wieloch T., Nissbrandt H., Ruscher K., Cenci M.A. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experi-mental parkinsonism. Brain. 2014;137(Pt 7):1998–2014. doi: 10.1093/brain/awu107. [DOI] [PubMed] [Google Scholar]
- 110.Bouvier M.M., Mytilineou C. Basic fibroblast growth factor increases division and delays differentiation of dopamine precursors in vitro. J. Neurosci. 1995;15(11):7141–7149. doi: 10.1523/JNEUROSCI.15-11-07141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chadi G., Moller A., Rosen L., Janson A.M., Agnati L.A., Goldstein M., Ogren S.O., Pettersson R.F., Fuxe K. Protective actions of human recombinant basic fibroblast growth factor on MPTP-lesioned nigrostriatal dopamine neurons after intraventricular infusion. Exp. Brain Res. 1993;97(1):145–158. doi: 10.1007/BF00228825. [DOI] [PubMed] [Google Scholar]
- 112.Forget C., Stewart J., Trudeau L.E. Impact of basic FGF expression in astrocytes on dopamine neuron synaptic function and development. Eur. J. Neurosci. 2006;23(3):608–616. doi: 10.1111/j.1460-9568.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- 113.Hou J.G., Cohen G., Mytilineou C. Basic fibroblast growth factor stimulation of glial cells protects dopamine neurons from 6-hydroxydopamine toxicity: involvement of the glutathione system. J. Neurochem. 1997;69(1):76–83. doi: 10.1046/j.1471-4159.1997.69010076.x. [DOI] [PubMed] [Google Scholar]
- 114.Engele J., Bohn M.C. The neurotrophic effects of fibroblast growth factors on dopaminergic neurons in vitro are mediated by mesencephalic glia. J. Neurosci. 1991;11(10):3070–3078. doi: 10.1523/JNEUROSCI.11-10-03070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang F., Liu Y., Tu J., Wan J., Zhang J., Wu B., Chen S., Zhou J., Mu Y., Wang L. Activated astrocytes enhance the dopaminergic differentiation of stem cells and promote brain repair through bFGF. Nat. Commun. 2014;5:5627. doi: 10.1038/ncomms6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lindholm P., Voutilainen M.H., Lauren J., Peranen J., Leppanen V.M., Andressoo J.O., Lindahl M., Janhunen S., Kalkkinen N., Timmusk T., Tuominen R.K., Saarma M. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature. 2007;448(7149):73–77. doi: 10.1038/nature05957. [DOI] [PubMed] [Google Scholar]
- 117.Petrova P., Raibekas A., Pevsner J., Vigo N., Anafi M., Moore M.K., Peaire A.E., Shridhar V., Smith D.I., Kelly J., Durocher Y., Commissiong J.W. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J. Mol. Neurosci. 2003;20(2):173–188. doi: 10.1385/jmn:20:2:173. [DOI] [PubMed] [Google Scholar]
- 118.Palgi M., Lindstrom R., Peranen J., Piepponen T.P., Saarma M., Heino T.I. Evidence that DmMANF is an invertebrate neurotrophic factor supporting dopaminergic neurons. Proc. Natl. Acad. Sci. USA. 2009;106(7):2429–2434. doi: 10.1073/pnas.0810996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lindholm P., Peranen J., Andressoo J.O., Kalkkinen N., Kokaia Z., Lindvall O., Timmusk T., Saarma M. MANF is widely expressed in mammalian tissues and differently regulated after ischemic and epileptic insults in rodent brain. Mol. Cell. Neurosci. 2008;39(3):356–371. doi: 10.1016/j.mcn.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 120.Airavaara M., Harvey B.K., Voutilainen M.H., Shen H., Chou J., Lindholm P., Lindahl M., Tuominen R.K., Saarma M., Hoffer B., Wang Y. CDNF protects the nigrostriatal dopamine system and promotes recovery after MPTP treatment in mice. Cell Transplant. 2012;21(6):1213–1223. doi: 10.3727/096368911X600948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Voutilainen M.H., Back S., Porsti E., Toppinen L., Lindgren L., Lindholm P., Peranen J., Saarma M., Tuominen R.K. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J. Neurosci. 2009;29(30):9651–9659. doi: 10.1523/JNEUROSCI.0833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zaltieri M., Grigoletto J., Longhena F., Navarria L., Favero G., Castrezzati S., Colivicchi M.A., Della Corte L., Rezzani R., Pizzi M., Benfenati F., Spillantini M.G., Missale C., Spano P., Bellucci A. alpha-synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J. Cell Sci. 2015;128(13):2231–2243. doi: 10.1242/jcs.157867. [DOI] [PubMed] [Google Scholar]
- 123.Arima K., Ueda K., Sunohara N., Arakawa K., Hirai S., Nakamura M., Tonozuka-Uehara H., Kawai M. NACP/ alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol. 1998;96(5):439–444. doi: 10.1007/s004010050917. [DOI] [PubMed] [Google Scholar]
- 124.Baba M., Nakajo S., Tu P.H., Tomita T., Nakaya K., Lee V.M., Trojanowski J.Q., Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998;152(4):879–884. [PMC free article] [PubMed] [Google Scholar]
- 125.Lopes da Fonseca T., Villar-Pique A., Outeiro T.F. The Interplay between Alpha-Synuclein Clearance and Spreading. Biomolecules. 2015;5(2):435–471. doi: 10.3390/biom5020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dunning C.J., George S., Brundin P. What's to like about the prion-like hypothesis for the spreading of aggregated alpha-synuclein in Parkinson’s disease? Prion. 2013;7(1):92–97. doi: 10.4161/pri.23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kovacs G.G., Breydo L., Green R., Kis V., Puska G., Lorincz P., Perju-Dumbrava L., Giera R., Pirker W., Lutz M., Lachmann I., Budka H., Uversky V.N., Molnar K., Laszlo L. Intracellular processing of disease-associated alpha-synuclein in the human brain suggests prion-like cell-to-cell spread. Neurobiol. Dis. 2014;69:76–92. doi: 10.1016/j.nbd.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 128.Deleidi M., Maetzler W. Protein clearance mechanisms of alpha-synuclein and amyloid-Beta in lewy body disorders. 2012. [DOI] [PMC free article] [PubMed]
- 129.Lee H.J., Patel S., Lee S.J. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J. Neurosci. 2005;25(25):6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davis C.H., Kim K.Y., Bushong E.A., Mills E.A., Boassa D., Shih T., Kinebuchi M., Phan S., Zhou Y., Bihlmeyer N.A., Nguyen J.V., Jin Y., Ellisman M.H., Marsh-Armstrong N. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. USA. 2014;111(26):9633–9638. doi: 10.1073/pnas.1404651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fink M., Wadsak W., Savli M., Stein P., Moser U., Hahn A., Mien L.K., Kletter K., Mitterhauser M., Kasper S., Lanzenberger R. Lateralization of the serotonin-1A receptor distribution in language areas revealed by PET. Neuroimage. 2009;45(2):598–605. doi: 10.1016/j.neuroimage.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 132.Wright D.E., Seroogy K.B., Lundgren K.H., Davis B.M., Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 1995;351(3):357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- 133.Chilmonczyk Z., Bojarski A.J., Pilc A., Sylte I. Functional Selectivity and Antidepressant Activity of Serotonin 1A Receptor Ligands. Int. J. Mol. Sci. 2015;16(8):18474–18506. doi: 10.3390/ijms160818474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Savitz J., Lucki I., Drevets W.C. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haleem D.J. 5-HT1A receptor-dependent control of nigrostriatal dopamine neurotransmission in the pharmacotherapy of Parkinson's disease and schizophrenia. Behav. Pharmacol. 2015;26(1-2):45–58. doi: 10.1097/FBP.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 136.Ye N., Song Z., Zhang A. Dual ligands targeting dopamine D2 and serotonin 5-HT1A receptors as new antipsychotical or anti-Parkinsonian agents. Curr. Med. Chem. 2014;21(4):437–457. doi: 10.2174/09298673113206660300. [DOI] [PubMed] [Google Scholar]
- 137.Uehara T., Matsuoka T., Sumiyoshi T. Tandospirone, a 5-HT1A partial agonist, ameliorates aberrant lactate production in the prefrontal cortex of rats exposed to blockade of N-methy-D-aspartate receptors; Toward the therapeutics of cognitive impairment of schizophrenia. Front. Behav. Neurosci. 2014;8:291. doi: 10.3389/fnbeh.2014.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dupre K.B., Eskow K.L., Barnum C.J., Bishop C. Striatal 5-HT1A receptor stimulation reduces D1 receptor-induced dyskinesia and improves movement in the hemiparkinsonian rat. Neuropharmacology. 2008;55(8):1321–1328. doi: 10.1016/j.neuropharm.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mignon L., Wolf W.A. Postsynaptic 5-HT1A receptor stimulation increases motor activity in the 6-hydroxydopamine-lesioned rat: implications for treating Parkinson’s disease. Psychopharmacology (Berl.) 2007;192(1):49–59. doi: 10.1007/s00213-006-0680-0. [DOI] [PubMed] [Google Scholar]
- 140.Rascol O., Perez-Lloret S., Ferreira J.J. New treatments for levodopa-induced motor complications. Mov. Disord. 2015 doi: 10.1002/mds.26362. [DOI] [PubMed] [Google Scholar]
- 141.Svenningsson P., Rosenblad C., Af Edholm Arvidsson K., Wictorin K., Keywood C., Shankar B., Lowe D.A., Bjorklund A., Widner H. Eltoprazine counteracts l-DOPA-induced dyskinesias in Parkinson’s disease: a dose-finding study. Brain. 2015;138(Pt 4):963–973. doi: 10.1093/brain/awu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang H., Ye N., Zhou S., Guo L., Zheng L., Liu Z., Gao B., Zhen X., Zhang A. Identification of N-propylnoraporphin-11-yl 5-(1,2-dithiolan-3-yl)pentanoate as a new anti-Parkinson’s agent possessing a dopamine D2 and serotonin 5-HT1A dual-agonist profile. J. Med. Chem. 2011;54(13):4324–4338. doi: 10.1021/jm200347t. [DOI] [PubMed] [Google Scholar]
- 143.Whitaker-Azmitia P.M., Azmitia E.C. Stimulation of astroglial serotonin receptors produces culture media which regulates growth of serotonergic neurons. Brain Res. 1989;497(1):80–85. doi: 10.1016/0006-8993(89)90972-4. [DOI] [PubMed] [Google Scholar]
- 144.Whitaker-Azmitia P.M., Murphy R., Azmitia E.C. Stimulation of astroglial 5-HT1A receptors releases the serotonergic growth factor, protein S-100, and alters astroglial morphology. Brain Res. 1990;528(1):155–158. doi: 10.1016/0006-8993(90)90210-3. [DOI] [PubMed] [Google Scholar]
- 145.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003;60(6):540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 146.Selinfreund R.H., Barger S.W., Pledger W.J., Van Eldik L.J. Neurotrophic protein S100 beta stimulates glial cell proliferation. Proc. Natl. Acad. Sci. USA. 1991;88(9):3554–3558. doi: 10.1073/pnas.88.9.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahlemeyer B., Beier H., Semkova I., Schaper C., Krieglstein J. S-100beta protects cultured neurons against glutamate- and staurosporine-induced damage and is involved in the antiapoptotic action of the 5 HT(1A)-receptor agonist, Bay x 3702. Brain Res. 2000;858(1):121–128. doi: 10.1016/s0006-8993(99)02438-5. [DOI] [PubMed] [Google Scholar]
- 148.Huttunen H.J., Kuja-Panula J., Sorci G., Agneletti A.L., Donato R., Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J. Biol. Chem. 2000;275(51):40096–40105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- 149.Nishi M., Kawata M., Azmitia E.C. S100beta promotes the extension of microtubule associated protein2 (MAP2)-immunoreactive neurites retracted after colchicine treatment in rat spinal cord culture. Neurosci. Lett. 1997;229(3):212–214. doi: 10.1016/s0304-3940(97)00443-6. [DOI] [PubMed] [Google Scholar]
- 150.Nishiyama H., Knopfel T., Endo S., Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc. Natl. Acad. Sci. USA. 2002;99(6):4037–4042. doi: 10.1073/pnas.052020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Businaro R., Leone S., Fabrizi C., Sorci G., Donato R., Lauro G.M., Fumagalli L. S100B protects LAN-5 neuroblastoma cells against Abeta amyloid-induced neurotoxicity via RAGE engagement at low doses but increases Abeta amyloid neurotoxicity at high doses. J. Neurosci. Res. 2006;83(5):897–906. doi: 10.1002/jnr.20785. [DOI] [PubMed] [Google Scholar]
- 152.Hu J., Ferreira A., Van Eldik L.J. S100beta induces neuronal cell death through nitric oxide release from astrocytes. J. Neurochem. 1997;69(6):2294–2301. doi: 10.1046/j.1471-4159.1997.69062294.x. [DOI] [PubMed] [Google Scholar]
- 153.Abib R.T., Quincozes-Santos A., Nardin P., Wofchuk S.T., Perry M.L., Goncalves C.A., Gottfried C. Epicate-chin gallate increases glutamate uptake and S100B secretion in C6 cell lineage. Mol. Cell. Biochem. 2008;310(1-2):153–158. doi: 10.1007/s11010-007-9675-3. [DOI] [PubMed] [Google Scholar]
- 154.Tramontina A.C., Nardin P., Quincozes-Santos A., Tortorelli L., Wartchow K.M., Andreazza A.C., Braganhol E., de Souza D.O., Goncalves C.A. High-glucose and S100B stimulate glutamate uptake in C6 glioma cells. Neurochem. Res. 2012;37(7):1399–1408. doi: 10.1007/s11064-012-0722-4. [DOI] [PubMed] [Google Scholar]
- 155.Tramontina F., Tramontina A.C., Souza D.F., Leite M.C., Gottfried C., Souza D.O., Wofchuk S.T., Gonca-lves C.A. Glutamate uptake is stimulated by extracellular S100B in hippocampal astrocytes. Cell. Mol. Neurobiol. 2006;26(1):81–86. doi: 10.1007/s10571-006-9099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kogel D., Peters M., Konig H.G., Hashemi S.M., Bui N.T., Arolt V., Rothermundt M., Prehn J.H. S100B potently activates p65/c-Rel transcriptional complexes in hippocampal neurons: Clinical implications for the role of S100B in excitotoxic brain injury. Neuroscience. 2004;127(4):913–920. doi: 10.1016/j.neuroscience.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 157.Eriksen J.L., Druse M.J. Potential involvement of S100B in the protective effects of a serotonin-1a agonist on ethanol-treated astrocytes. Brain Res. Dev. Brain Res. 2001;128(2):157–164. doi: 10.1016/s0165-3806(01)00172-9. [DOI] [PubMed] [Google Scholar]
- 158.Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10(11):549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 159.Bezard E., Gerlach I., Moratalla R., Gross C.E., Jork R. 5-HT1A receptor agonist-mediated protection from MPTP toxicity in mouse and macaque models of Parkinson’s disease. Neurobiol. Dis. 2006;23(1):77–86. doi: 10.1016/j.nbd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 160.Madhavan L., Freed W.J., Anantharam V., Kanthasamy A.G. 5-hydroxytryptamine 1A receptor activation protects against N-methyl-D-aspartate-induced apoptotic cell death in striatal and mesencephalic cultures. J. Pharmacol. Exp. Ther. 2003;304(3):913–923. doi: 10.1124/jpet.102.044370. [DOI] [PubMed] [Google Scholar]
- 161.Azkona G., Sagarduy A., Aristieta A., Vazquez N., Zubillaga V., Ruiz-Ortega J.A., Perez-Navarro E., Ugedo L., Sanchez-Pernaute R. Buspirone anti-dyskinetic effect is correlated with temporal normalization of dysregulated striatal DRD1 signalling in L-DOPA-treated rats. Neuropharmacology. 2014;79:726–737. doi: 10.1016/j.neuropharm.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 162.Hui Y.P., Wang T., Han L.N., Li L.B., Sun Y.N., Liu J., Qiao H.F., Zhang Q.J. Anxiolytic effects of prelimbic 5-HT(1A) receptor activation in the hemiparkinsonian rat. Behav. Brain Res. 2015;277:211–220. doi: 10.1016/j.bbr.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 163.Hui Y.P., Zhang Q.J., Zhang L., Chen L., Guo Y., Qiao H.F., Wang Y., Liu J. Activation of prelimbic 5-HT1A receptors produces antidepressant-like effects in a unilateral rat model of Parkinson’s disease. Neuroscience. 2014;268:265–275. doi: 10.1016/j.neuroscience.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 164.Huot P., Johnston T.H., Fox S.H., Newman-Tancredi A., Brotchie J.M. The highly-selective 5-HT(1A) agonist F15599 reduces L-DOPA-induced dyskinesia without compromising anti-parkinsonian benefits in the MPTP-lesioned macaque. Neuropharmacology. 2015;97:306–311. doi: 10.1016/j.neuropharm.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 165.Iderberg H., McCreary A.C., Varney M.A., Cenci M.A., Newman-Tancredi A. Activity of serotonin 5-HT(1A) receptor 'biased agonists' in rat models of Parkinson’s disease and L-DOPA-induced dyskinesia. Neuropharmacology. 2015;93:52–67. doi: 10.1016/j.neuropharm.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 166.Iderberg H., McCreary A.C., Varney M.A., Kleven M.S., Koek W., Bardin L., Depoortere R., Cenci M.A., Newman-Tancredi A. NLX-112, a novel 5-HT1A receptor agonist for the treatment of L-DOPA-induced dyskinesia: Behavioral and neurochemical profile in rat. Exp. Neurol. 2015;271:335–350. doi: 10.1016/j.expneurol.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 167.Paolone G., Brugnoli A., Arcuri L., Mercatelli D., Morari M. Eltoprazine prevents levodopa-induced dyskinesias by reducing striatal glutamate and direct pathway activity. Mov. Disord. 2015;30(13):1728–1738. doi: 10.1002/mds.26326. [DOI] [PubMed] [Google Scholar]


