Abstract
A number of clinical trials have demonstrated that the use of probiotics has the potential to prevent nosocomial infections. However, the mechanism underlying probiotic-induced anti-infection and sepsis remains to be investigated. In the present study, 200 µl/day of Lactobacillus rhamnosus GG (LGG) or normal saline (control) was orally administrated to 4-week-old C57BL6 mice 4 weeks prior to cecal ligation and puncture (CLP). A number of mice were sacrificed 24 h after CLP, and the remaining mice were used for survival studies. Ileum tissues were collected to evaluate the injury on the intestine. Blood samples were also obtained to investigate the changed metabolic pattern in mice that underwent different treatments using ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS). In the survival studies, the mortality of CLP-induced septic mice pretreated with LGG was significantly lower compared with untreated mice (P=0.029). Ileum mucosal damage was evident in the control septic mice. Based on the data of UPLC-QTOF-MS, phosphatidylcholines were increased and lysophosphatidylcholines (LPCs) that contained polyunsaturated fatty acids were decreased in septic mice, whereas saturated fatty acid LPCs reveal no significant difference between septic and sham mice. In addition, the metabolic profile in the septic mice pretreated with LGG was much closer to that of sham mice compared with control septic mice. The results of the present study suggest that probiotic pre-administration reduces the mortality in septic mice by decreasing ileum mucosal damage, increasing the gut barrier integrity and altering global serum metabolic profiles.
Keywords: probiotic, Lactobacillus rhamnosus GG, cecal ligation and puncture, sepsis, metabonomics, ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry
Introduction
Sepsis is one of the leading causes of mortality and morbidity in children and adults (1). A hostile gut environment can always be found in patients with sepsis. Intestinal epithelium dysfunction and microbiota imbalance induced by critical illnesses results in increased translocation of bacteria to the blood, which contributes to the formation and aggravation of sepsis (2). Probiotics, the living microorganisms colonizing in the intestine, bring benefits to the host through rebalancing microbiota, modulating intestinal barrier function (3), restraining tumorigenesis (4) and enhancing the immune system (5). Currently, a growing number of articles have indicated the potential of probiotics in numerous diseases, including diarrhea (6), allergic diseases (7), inflammatory bowel disease (3) and sepsis (2). However, the majority of these articles focus on evaluating the prevention and treatment effects of probiotics on diseases. Only a small number of studies attempt to explain the precise molecular mechanism underlying the beneficial effects of these microorganisms, including the regulation of gene expression (5). Thus, there is a growing need for exploring the exact mechanisms underlying the protective effects of probiotics in different diseases. The present study sought to delineate the potential mechanism of pre-administered probiotic therapy in experimental sepsis using a C57BL6 mouse peritonitis model.
Metabolomics, one of the ‘-omics’ technologies, can provide quantitative measures to distinguish global changes in the metabolic profiles of individuals responding to pathophysiological stimuli or genetic modification (8). In the development of sepsis, metabolic disorder has been regarded as a key feature. Therefore, metabolomics will provide an effective way to identify sepsis, and assess prognosis and insight into the mechanisms of sepsis. In the present study, metabolic differences between serum samples from cecal ligation and puncture (CLP)-induced septic mice, septic mice pretreated with Lactobacillus rhamnosus GG (LGG) and sham mice were analyzed using ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS).
Materials and methods
Ethics statement
All procedures for animal care and use were approved by the Animal Care Ethics Committee of the First Affiliated Hospital, Zhejiang University (Zhejiang, China). A total of 42 four week-old male C57BL6 mice (weight, 11 g) were purchased from Zhejiang University and housed in specific pathogen-free animal facilities under a standard 12-h-light/12-h-dark cycle at room temperature with relative humidity between 50 and 60% and ad libtum access to food and water. Standard mouse diet and water were administered throughout the study.
Reagents and materials
The probiotic capsule contains 280 mg inulin powder and 1010 colony forming unit (CFU) LGG (Culturelle; ConAgra Foods, Omaha, NE, USA). High performance liquid chromatography-grade acetonitrile, leucine-enkephalin and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deionized water was obtained from an ultrapure water machine (Merck Millipore, Bedford, MA, USA). All standard samples were purchased from Sigma-Aldrich.
Probiotic administration and septic peritonitis model
Four week-old male C57BL6 mice were orally gavaged with 200 µl/day LGG (2×109 CFU/ml) or normal saline (control) 4 weeks prior to CLP. To establish the murine septic peritonitis model, the mice were anesthetized with an intraperitoneal injection of 4% chloral hydrate (10 ml/kg). A 1-cm incision was made in the middle of the abdomen and the cecum was then exposed carefully through the incision. In order to induce mid-grade sepsis, the cecum was ligated at the middle of the bottom and distal pole, and was punctured from the mesenteric towards the antimesenteric direction using a 23-gauge needle. A droplet of faeces was extruded through the holes, and the cecum was relocated into the abdominal cavity. Finally, the fascia and skin incision were closed in various layers. The mice in the sham group underwent the same procedure but without the cecum ligation and puncture. In all of the mice, 1 ml pre-warmed normal saline was injected subcutaneously following CLP surgery (9).
Survival studies
Survival studies were conducted in order to determine whether LGG pretreatment decreases the mortality resulting from CLP-induced sepsis. Mice pretreated with LGG (n=8) or normal saline (n=8) for 4 weeks were subjected to CLP. Sham mice (n=5) underwent sham laparotomy. Following the operation, all mice had free access to water and food and were closely observed for a 7 day survival rate.
Histological study
Ileum tissues were collected from mice (n=21) 24 h after CLP operation and fixed in 10% formalin for 24 h. The fixed ileum tissues were embedded in paraffin and sectioned at 4 µm. The sections were stained with hematoxylin and eosin for histological observation in order to examine the pathology of intestinal injury induced by CLP.
Blood sample collection and preparation
Blood samples were obtained at 24 h from the orbital vein of the mice following CLP. In all of the mice 1 ml of blood was introduced in the EP tube without any anticoagulant substances. The blood collected was kept at 4°C for 3 h prior to centrifugation (3,000 × g, 5 min, 4°C), then carefully absorbed and injected into a clean EP tube and then stored at −80°C.
Prior to the metabolomics analysis, the serum sample was thawed at room temperature. In order to precipitate the protein in the serum sample, 200 µl sample was added into 600 µl ice-cold acetonitrile. Next, the mixture was vortexed and centrifuged at 12,000 × g for 15 min and 4°C. Finally, the supernatant was moved into a glass bottle and stored at 4°C for UPLC-MS analysis. To evaluate reproducibility and stability of the UPLC-MS system, 10 µl of each sample was added into a bottle to generate a pooled quality control sample, which was inserted every five samples throughout the run. The stable retention times and tight overlap of the peaks reveal high repeatability and stability of the analytical system.
UPLC-QTOF-MS analysis
The sample analysis was performed using an acquity ultra-performance liquid chromatography system (Waters Corporation, Milford, MA, USA) with a conditioned autosampler at 4°C. A 5 µl sample was injected into a Waters BEH C8 column (2.1×100 mm, inner diameter × length, 1.7 µm particle size) and kept at 50°C with a thermostat. Mobile phases, delivered at 300 µl/min, were formed of 0.1% formic acid and 99.9% water (A), or 0.1% formic acid and 99.9% acetonitrile (B). The gradient elution program was as follows: 97% A in the initial 0–7 min followed by 20% A in 8 min and consequently 2% A in 16 min. The conditions were kept constant for 5 min and then changed to 100% B in 50 sec and maintained for 3 min. Next the compound was returned to 97% A in 25 min and kept for 5 min.
Mass spectrometry (Waters Q-TOF Premier) detection was performed in the positive electrospray ionization mode. The apparatus was previously calibrated using sodium formate. A lock mass of leucine enkephalin was used for an accurate mass determination setting at m/z 556.2771 in the positive ion mode. The concentration of leucine enkephalin was 0.5 ng/µl. The detection parameters were optimized as follows: Capillary voltage of 3 kV and cone voltage of 40 V. The scan time was 0.3 sec covering the 50 to 1,000 Dalton mass range. The source temperature was set to 120°C and the desolvation gas temperature was 450°C. Nitrogen was used as the nebulizer gas at a flow rate of 600 l/h.
Data analysis
The UPLC-MS data collected in positive ion mode were obtained and preprocessed using the Masslynx software version 4.1 (Waters Corporation). This application was used for peak alignment to obtain a list containing the m/z, retention time and intensities for all peaks detected. The preprocessed data were exported and analyzed using the SIMCA-P+12.0 (MKS Umetrics AB, Umea, Sweden). All the data were normalized and Pareto scaled prior to a supervised partial least-squares-latent structure discriminate analysis (PLS-DA). PLS-DA was used to highlight the difference between groups and obtain metabolites that contributed to the classification. Potential biomarkers were identified according to the ‘variable of importance in the project’ (VIP) values and S-plots. To further identify the potential biomarkers, PubChem compound (http://www.ncbi.nlm.nih.gov/) and KEGG (http://www.genome.jp/kegg/) databases were searched in order to match the selected ion spectrum with the message of metabolites obtained from databases. MS analysis was conducted to validate the potential biomarkers.
Statistical analysis
The homogeneity of variances was verified using Bartlett's test. One-way analysis of variance (with post-hoc Bonferroni) was then performed to compare the spectral variables among different serum samples. Survival studies were conducted via the log-rank test. P≤0.05 was used to indicate a statistically significant difference. Statistical analysis was conducted using GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
LGG reduces mortality in septic peritonitis
To determine whether LGG pretreatment will decrease mortality in experimental peritonitis-induced sepsis, 8 LGG pretreated mice and 8 control mice were subjected to CLP, and were observed for a 7 day survival rate (Fig. 1). Septic mice pretreated with LGG had a significantly improved 7 day survival rate compared with the control septic mice (P=0.029). Furthermore, 5 sham mice survived.
Figure 1.

LGG decreases the mortality in cecal ligation and puncture (CLP)-induced sepsis. Four-week-old male C57BL6 mice pretreated with LGG (n=8) or saline (n=8) for 4 weeks were subjected to CLP. Sham mice (n=5) underwent sham laparotomy. Following surgery, all mice were observed for a 7 day survival rate. A total of 5 sham mice survived. *P=0.029. LGG, Lactobacillus rhamnosus GG.
Alleviation of injury in intestinal mucosa occurred following treatment with LGG
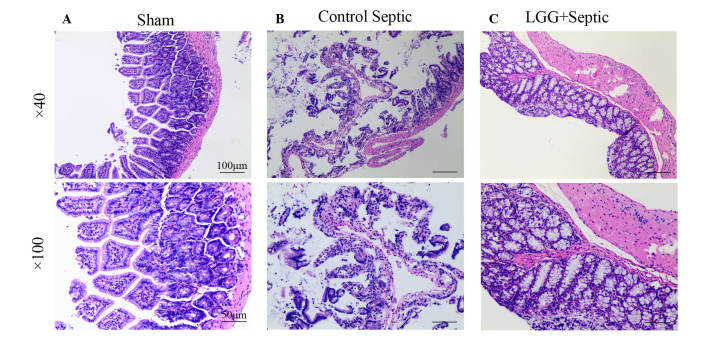
Ileum tissues in sham mice were histologically normal in all layers (Fig. 2A). In control septic mice, the lamina propria was almost entirely digested and disintegrated accompanied with exfoliating villi that made the epithelial structure difficult to identify (Fig. 2B). In LGG pretreated septic mice, although the lamina propria was exposed to exfoliating villi the epithelial appearance was regular with a large number of crypts (Fig. 2C). Ileum mucosal damage can evidently be observed in septic mice. Furthermore, the damage in septic mice pretreated with LGG was less compared with the control septic mice. In addition, marked differences were observed in the mucosal thickness, villi number and length amongst the LGG pretreated septic, control septic and sham mice.
Figure 2.
LGG pretreatment attenuates the injury of ileum mucosa. Hematoxylin and eosin-stained ileum sections from (A) sham mice, (B) control septic mice and (C) septic mice pretreated with LGG are shown. The examination of stained sections demonstrated normal intestinal mucosa and submucosa in (A) the sham mice. (B) The control septic mice exhibited villi exfoliation, lamina propria disintegration and severe inflammatory cell infiltration. Although some villi shedding remained, there was a normalized epithelial appearance with a large number of crypts and a mild level of inflammatory cell infiltration in (C) septic mice pretreated with LGG. The histological results suggested an improvement in the LGG pretreatment septic mice compared with the control septic mice. Upper panels, magnification ×40; bottom panels, magnification ×100. Sham, sham laparotomy mice; LGG+septic, cecal ligation and puncture (CLP) surgery with LGG pretreatment mice; Control septic, CLP surgery with saline pretreatment mice. LGG, Lactobacillus rhamnosus GG.
Results of the UPLC-MS analysis
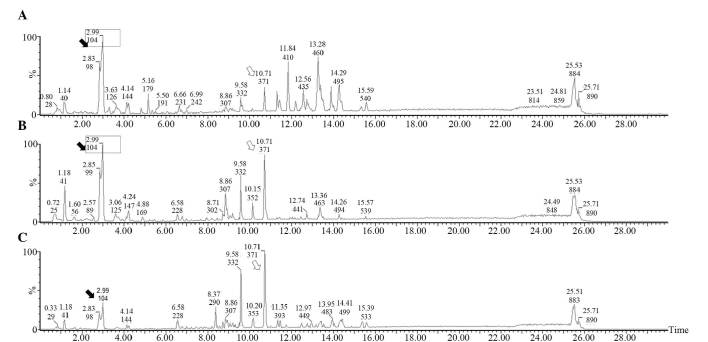
Low-molecular-weight metabolites in the serum of the sham, septic mice pretreated with LGG and control septic mice are presented in base peak intensity chromatograms (Fig. 3). Compared with the sham mice, it was evident that the levels of a number of metabolites increased (hollow arrow) in the septic mice while others decreased (black arrow). However, compared with the control septic mice, the peak patterns in septic mice pretreated with LGG were more similar to those in sham mice (box), indicating that early therapy with LGG can normalize the metabolic profile in septic mice.
Figure 3.
Typical base peak intensity (BPI) chromatograms of serum samples from sham, septic mice pretreated with LGG and control septic mice. BPI chromatogram of a serum sample in one of the (A) sham mice, (B) septic mice pretreated with LGG and (C) control septic mice. Compared with the sham mice, a number of metabolites were increased in septic mice (hollow arrow) while others were decreased (black arrow). In addition, compared to the control septic mice, the peak patterns in septic mice pretreated with LGG were more similar to the sham mice (box).
PLS-DA analysis of UPLC-MS data
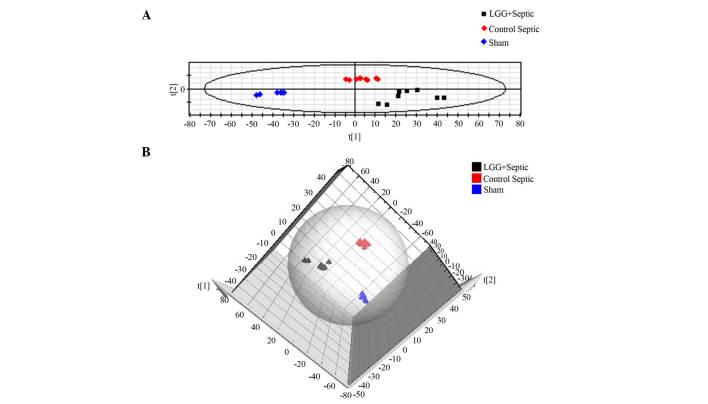
In order to demonstrate the difference of the metabolic profile amongst the three groups, a supervised PLS-DA method was used to analyze the multivariate data. As shown in Fig. 4, where each point represents an independent sample, there was a distinguished classification among all three groups. In-group similarity was observed in each group, and the three distinct clusters represented the LGG pretreated septic mice, control septic mice and sham mice that were clearly demonstrated on the PLS-DA scores plot indicating metabolic differences among the three groups. In addition, the distance between LGG pretreated septic mice and sham mice was closer compared with the distance between control septic and sham mice, indicating a shift towards a normalized metabolic profile in the septic mice pretreated with LGG.
Figure 4.
Supervised partial least squares discriminant analysis (PLS-DA). (A) PLS-DA score plot in positive ion mode based on UPLC-MS analysis data, showing that the three groups were clearly distinguished from each other. Black boxes represent the samples of septic mice pretreated with LGG, red diamonds represent the samples of control septic mice, blue diamonds represent the samples of sham mice. (B) PLS-DA score 3D plot in positive ion mode based on UPLC-MS analysis data. LGG, Lactobacillus rhamnosus GG; UPLC-MS, ultra-performance liquid chromatography mass spectrometry.
Candidate biomarker identification
According to the results of the VIP values (the top 19 VIPs) and the S-plot, 19 metabolites were selected as candidate biomarkers (Table I). Besides the S-plot, different metabolites that significantly contribute to the separation of the three groups were identified among LGG pretreated septic, control septic and sham mice. The PubChem compound and KEGG databases were searched to compare the MS data using chemical standards, and the results were used to help us identify potential biomarkers. The biomarkers identified are summarized in Table I. The levels of two groups of biomarkers-lysophosphatidylcholines (LPCs) and phosphatidylcholines (PCs) were different between the septic and the sham mice. Amongst them, PCs were increased and LPCs that contain polyunsaturated fatty acids were decreased in septic mice, whereas saturated fatty acid LPCs demonstrated no significant difference between the septic and sham mice. The difference between LGG pretreated septic mice and sham mice were less compared with the control septic and sham mice, although 19 discriminating metabolites revealed an unclear distinction between LGG pretreated septic and control septic mice (P>0.05).
Table I.
Serum biomarkers associated with sepsis.
| Trend | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Rt | m/z | VIP | Adduction | Identify results | Group 1–2 | Group 1–3 | Group 2–3 |
| 1 | 11.88 | 991.6767 | 8.61692 | [2M+H]+ | LPC16:0 | ns | ns | ns |
| 2 | 11.9 | 496.3404 | 8.48299 | [M+H]+ | LPC16:0 | ns | ns | ns |
| 3 | 19.05 | 760.5878 | 6.86831 | [M+H]+ | 16:0/18:1-PC | ns | ↑↑↑ | ↑↑↑ |
| 4 | 19.05 | 782.5693 | 5.97245 | [M+Na]+ | 16:0/18:1-PC | ns | ↑↑↑ | ↑↑↑ |
| 5 | 18.31 | 780.5543 | 5.01658 | [M+Na]+ | 16:0/18:2-PC | ns | ↑↑↑ | ↑↑↑ |
| 6 | 18.18 | 478.3293 | 4.98452 | [M+H]+ | PC neutral loss fragment | ns | ↓↓ | ↓↓↓ |
| 7 | 17.92 | 804.5538 | 7.03144 | [M+Na]+ | 18:2/18:2-PC | ns | ↓↓ | ↓ |
| 8 | 18.11 | 806.5718 | 4.87843 | [M+H]+ | 22:6/16:0-PC | ns | ↑↑↑ | ↑↑↑ |
| 9 | 18.31 | 758.5716 | 4.68084 | [M+H]+ | 16:0/18:2-PC LPC22:6 | ↑ | ↑↑↑ | ns |
| 10 | 11.44 | 568.3413 | 4.63146 | [M+H]+ | 22:6/18:0-PC LPC18:1 | ns | ↓↓↓ | ↓ |
| 11 | 19.23 | 834.6033 | 4.60648 | [M+H]+ | 18:0/18:2-PC | ns | ↑↑↑ | ↑↑ |
| 12 | 11.43 | 520.3405 | 4.55215 | [M+H]+ | 22:6/16:0-PC | ns | ↓↓↓ | ↓↓↓ |
| 13 | 19.49 | 786.6028 | 4.52772 | [M+H]+ | 18:0/18:2-PC LPC18:0 | ns | ↑↑↑ | ↑ |
| 14 | 18.11 | 828.5551 | 4.29195 | [M+Na]+ | LPC16:0 | ns | ↑↑↑ | ↑↑↑ |
| 15 | 19.49 | 808.5854 | 4.16349 | [M+Na]+ | LPC18:1 | ns | ↑↑↑ | ↑ |
| 16 | 13.31 | 546.354 | 3.95349 | [M+Na]+ | LPC16:0 isomer | ns | ns | ns |
| 17 | 11.54 | 518.3221 | 3.88142 | [M+Na]+ | LPC16:0 | ns | ns | ns |
| 18 | 12.29 | 544.339 | 3.7194 | [M+Na]+ | LPC18:1 | ns | ↓↓↓ | ↓↓ |
| 19 | 11.9 | 518.3223 | 3.59502 | [M+Na]+ | LPC16:0 isomer | ns | ns | ns |
Group 1, control septic mice; Group 2, LGG pretreated septic mice; Group 3, sham mice. Trend: ns, no significant difference (P>0.05); ‘↑’ or ‘↓’, P<0.05; ‘↑↑’ or ‘↓↓’, P<0.01; ‘↑↑↑’ or ‘↓↓↓’, P<0.001 vs. the former group compared with the latter group. Rt, retention time; VIP, variable of importance; PC, phosphatidylcholine; LPC, lysophosphatidylcholine.
Discussion
Growing evidence indicates that human physiology and metabolism are regulated by intestinal microbiota (10). Consisting of different species of bacteria, the intestinal microbiota is important in maintaining integrity of the epithelial barrier and participating in mucosal immunity (11). However, bacteremia may result from an imbalanced intestinal microbiota of a patient when critical illness occurs (12). Probiotics can provide health benefits and protect the body from diseases that may attribute to their ability to rebalance the microbiota, and as a consequence affect metabolism (10). However, a series of clinical trials have demonstrated that probiotic administration does not reduce mortality rates in patients with a critical illness effectively (13–16). One possible reason for this is that probiotic administration is not provided as early as possible. In the present study on septic mice, probiotics were administered orally prior to infection to prevent the progress of sepsis. The data demonstrated a significant reduction in mortality rate and an enhancement of ileum epithelial integrity in probiotic pretreated septic mice compared with control septic mice, indicating that early administration of probiotics has a protective effect against infection.
In the present study, 1,714 peaks were detected in the serum samples using UPLC-MC. According to PLS-DA, three distinct clusters represented the three different groups observed. Each group was in its own cluster with in-group similarity observed, separated and apart from the others. Candidate biomarkers of septic mice pretreated with probiotics were selected according to VIP values and the S-plot. Metabolites that contributed significantly to the discrimination were identified, namely PCs and LPCs. The majority of the PCs were increased in septic mice, while the majority of the LPCs were markedly decreased. Furthermore, LPCs that contain polyunsaturated fatty acids [e.g. LPC (18:1) and LPC (22:6)] were decreased, whereas saturated fatty acid LPCs [e.g. LPC (16:0) and LPC (18:0)] revealed no statistical difference in septic mice.
PCs is the most abundant phospholipid class found in intestinal mucus (17). They are important in establishing a hydrophobic surface of mucus, which protects the intestinal epithelial cells against commensal bacteria from the intestinal lumen (18). In a patient with ulcerative colitis (UC) whose PC level in the mucus had significantly decreased due to inflammation, added PC elevated the concentration of PC in the mucus. As a result, inflammation improved and even resolved in patients with UC (19). The data indicate the important role of the mucus PC in barrier function. During the process of inflammation, a series of pro-inflammatory mediators, including the cytokine tumor necrosis factor (TNF) are released by intestinal epithelial cells (20). TNF results in increasing intestinal permeability (21), inhibition of nutrient absorption (22), and alteration of cell events (23). Exogenous addition of PC attenuated the level of serum cytokines (TNF-α and interleukin-10), and markedly inhibited the processes induced by cytokines such as TNF (17). In the present study, the PC concentration that was significantly elevated in septic mice compared with sham mice indicates an anti-inflammatory effect of PC. However, the level of PC (16:0/18:2-PC) in LGG pretreated septic mice were lower compared with the control septic mice, suggesting that LGG pretreatment may reduce the inflammatory response.
The majority of LPCs are derived from PCs, and are formed through different mechanisms (24). A number of them are catalyzed by the glycoprotein lecithin cholesterol acyltransferase (25), whereas others are synthesized from PC hydrolysis via the secretory phospholipase A2 family (26). Although LPCs are typically regarded as pro-inflammatory mediators, they were increased in inflammatory diseases (27,28). A previous series of studies have demonstrated a protective effect of LPCs against diseases, including type 2 diabetes (29), cancer (30) and inflammatory diseases (31).
In the results of the present study, LPCs that contain polyunsaturated fatty acids were decreased in septic mice, whereas saturated fatty acid LPCs demonstrated no significant difference between septic and sham mice. Furthermore, unsaturated LPCs combine with the albumin in blood plasma, causing polyunsaturated fatty acids to move into organs where fatty acids are rich, such as in the brain. Therefore, unsaturated LPCs primarily associate with albumin instead of lipoproteins (32). Furthermore, a number of studies indicated that the rate of albumin degradation and albumin loss in tissue increased in patients with critical illnesses, such as sepsis (33–35). In addition, the concentration of albumin would return to normal values (32) as soon as the infection was controlled. Compared with control septic mice, the level of unsaturated LPCs obtained from the septic mice with LGG pretreatment in the present study was much closer to those in sham mice, indicating that LGG may reverse the progress of sepsis by elevating albumin in the blood plasma. In addition, one of the possible explanations for a non-significant difference existing in saturated LPCs between septic and sham mice may be the different role that different LPC subtypes serve during sepsis.
In conclusion, the potential impact of live probiotic therapy on the improvement of the survival rate in experimental septic mice was demonstrated in the present study. This improvement was associated with reduced ileum mucosal damage, increased gut barrier integrity and altered global serum metabolic profiles.
References
- 1.Bouza C, López-Cuadrado T, Saz-Parkinson Z, Amate-Blanco JM. Epidemiology and recent trends of severe sepsis in Spain: A nationwide population-based analysis (2006-2011) BMC Infect Dis. 2014;14:3863. doi: 10.1186/s12879-014-0717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khailova L, Frank DN, Dominguez JA, Wischmeyer PE. Probiotic administration reduces mortality and improves intestinal epithelial homeostasis in experimental sepsis. Anesthesiology. 2013;119:166–177. doi: 10.1097/ALN.0b013e318291c2fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokol H. Probiotics and Antibiotics in IBD. Dig Dis. 2014;32(Suppl 1):S10–S17. doi: 10.1159/000367820. [DOI] [PubMed] [Google Scholar]
- 4.Wan Y, Xin Y, Zhang C, Wu D, Ding D, Tang L, Owusu L, Bai J, Li W. Fermentation supernatants of Lactobacillus delbrueckii inhibit growth of human colon cancer cells and induce apoptosis through a caspase 3-dependent pathway. Oncol Lett. 2014;7:1738–1742. doi: 10.3892/ol.2014.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20:15632–15649. doi: 10.3748/wjg.v20.i42.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich CG, Kottmann T, Alavi M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J Gastroenterol. 2014;20:15837–15844. doi: 10.3748/wjg.v20.i42.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CT, Chen PJ, Lee YT, Ko JL, Lue KH. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. J Microbiol Immunol Infect. 2014 Nov 11; doi: 10.1016/j.jmii.2014.08.001. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 8.Griffin JL, Bollard ME. Metabonomics: Its potential as a tool in toxicology for safety assessment and data integration. Curr Drug Metab. 2004;5:389–398. doi: 10.2174/1389200043335432. [DOI] [PubMed] [Google Scholar]
- 9.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahti L, Salonen A, Kekkonen RA, Salojärvi J, Jalanka-Tuovinen J, Palva A, Orešič M, de Vos WM. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. Peer J. 2013;1:e32. doi: 10.7717/peerj.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan F, Polk DB. Probiotics: Progress toward novel therapies for intestinal diseases. Curr Opin Gastroenterol. 2010;26:95–101. doi: 10.1097/MOG.0b013e328335239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quigley EM. Gut Bacteria in health and disease. Gastroenterol Hepatol (N Y) 2013;9:560–569. [PMC free article] [PubMed] [Google Scholar]
- 13.Barraud D, Blard C, Hein F, Marçon O, Cravoisy A, Nace L, Alla F, Bollaert PE, Gibot S. Probiotics in the critically ill patient: A double blind, randomized, placebo-controlled trial. Intensive Care Med. 2010;36:1540–1547. doi: 10.1007/s00134-010-1927-0. [DOI] [PubMed] [Google Scholar]
- 14.Honeycutt TC, El Khashab M, Wardrop RM, III, McNeal-Trice K, Honeycutt AL, Christy CG, Mistry K, Harris BD, Meliones JN, Kocis KC. Probiotic administration and the incidence of nosocomial infection in pediatric intensive care: A randomized placebo-controlled trial. Pediatr Crit Care Med. 2007;8:452–458. doi: 10.1097/01.PCC.0000282176.41134.E6. [DOI] [PubMed] [Google Scholar]
- 15.Gou S, Yang Z, Liu T, Wu H, Wang C. Use of probiotics in the treatment of severe acute pancreatitis: A systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18:R57. doi: 10.1186/cc13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barraud D, Bollaert PE, Gibot S. Impact of the administration of probiotics on mortality in critically ill adult patients: A meta-analysis of randomized controlled trials. Chest. 2013;143:646–655. doi: 10.1378/chest.12-1745. [DOI] [PubMed] [Google Scholar]
- 17.Treede I, Braun A, Sparla R, Kühnel M, Giese T, Turner JR, Anes E, Kulaksiz H, Füllekrug J, Stremmel W, et al. Anti-inflammatory effects of phosphatidylcholine. J Biol Chem. 2007;282:27155–27164. doi: 10.1074/jbc.M704408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stremmel W, Merle U, Zahn A, Autschbach F, Hinz U, Ehehalt R. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut. 2005;54:966–971. doi: 10.1136/gut.2004.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stremmel W. Mucosal protection by phosphatidylcholine as new therapeutic concept in ulcerative colitis. Z Gastroenterol. 2013;51:384–389. doi: 10.1055/s-0033-1335042. (In German) [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Kovacs-Nolan J, Kodera T, Eto Y, Mine Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim Biophys Acta. 2015;1852:792–804. doi: 10.1016/j.bbadis.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh CY, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol Rep. 2015;3:e12327. doi: 10.14814/phy2.12327. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrenetxe J, Sánchez O, Barber A, Gascón S, Rodríguez-Yoldi MJ, Lostao MP. TNFα regulates sugar transporters in the human intestinal epithelial cell line Caco-2. Cytokine. 2013;64:181–187. doi: 10.1016/j.cyto.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Goretsky T, Dirisina R, Sinh P, Mittal N, Managlia E, Williams DB, Posca D, Ryu H, Katzman RB, Barrett TA. P53 mediates TNF-induced epithelial cell apoptosis in IBD. Am J Pathol. 2012;181:1306–1315. doi: 10.1016/j.ajpath.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, Qu H, Ai CZ, Cao YF, Huang T, Chen JX, Zeng J, Sun XY, Hong M, Gonzalez FJ, et al. Regulation profile of phosphatidylcholines (PCs) and lysophosphatidylcholines (LPCs) components towards UDP-glucuronosyltransferases (UGTs) isoforms. Xenobiotica. 2015;45:197–206. doi: 10.3109/00498254.2014.966174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M, Subramanian VS, Subbaiah PV. Modulation of the positional specificity of lecithin-cholesterol acyltransferase by the acyl group composition of its phosphatidylcholine substrate: Role of the sn-1-acyl group. Biochemistry. 1998;37:13626–13633. doi: 10.1021/bi980351e. [DOI] [PubMed] [Google Scholar]
- 26.Miklishanskaia SV, Liakishev AA, Kukharchuk VV. Clinical role of lipoprotein-associated phospholipase A2. Kardiologiia. 2013;53:59–70. (In Russian) [PubMed] [Google Scholar]
- 27.Domeij H, Hua X, Su J, Bäcklund A, Yan Z, Frostegård AG, Haeggström JZ, Modéer T, Frostegård J. Annexin A5 inhibits atherogenic and pro-inflammatory effects of lysophosphatidylcholine. Prostaglandins Other Lipid Mediat. 2013;106:72–78. doi: 10.1016/j.prostaglandins.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Kajander K, Myllyluoma E, Kyrönpalo S, Rasmussen M, Sipponen P, Mattila I, Seppänen-Laakso T, Vapaatalo H, Oresic M, Korpela R. Elevated pro-inflammatory and lipotoxic mucosal lipids characterise irritable bowel syndrome. World J Gastroenterol. 2009;15:6068–6074. doi: 10.3748/wjg.15.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B, et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. doi: 10.1038/msb.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jantscheff P, Schlesinger M, Fritzsche J, Taylor LA, Graeser R, Kirfel G, Fürst DO, Massing U, Bendas G. Lysophosphatidylcholine pretreatment reduces VLA-4 and P-Selectin-mediated b16.f10 melanoma cell adhesion in vitro and inhibits metastasis-like lung invasion in vivo. Mol Cancer Ther. 2011;10:186–197. doi: 10.1158/1535-7163.MCT-10-0474. [DOI] [PubMed] [Google Scholar]
- 31.Smani Y, Domínguez-Herrera J, Ibáñez-Martínez J, Pachón J. Therapeutic efficacy of lysophosphatidylcholine in severe infections caused by Acinetobacter baumannii. Antimicrob Agent Chemother. 2015;59:3920–3924. doi: 10.1128/AAC.04986-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croset M, Brossard N, Polette A, Lagarde M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J. 2000;345:61–67. doi: 10.1042/bj3450061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou B, Ren J, Han G, Chen YAJ, Gu G, Chen J, Wang G, Li J. Dynamics of albumin synthetic response to intra-abdominal abscess in patients with gastrointestinal fistula. Surg Infect (Larchmt) 2014;15:111–117. doi: 10.1089/sur.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruot B, Papet I, Bechereau F, Denis P, Buffiere C, Gimonet J, Glomot F, Elyousfi M, Breuille D, Obled C. Increased albumin plasma efflux contributes to hypoalbuminemia only during early phase of sepsis in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R707–R713. doi: 10.1152/ajpregu.00483.2002. [DOI] [PubMed] [Google Scholar]
- 35.Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: A meta-analysis of observational clinical studies. Intensive Care Med. 2010;36:1657–1665. doi: 10.1007/s00134-010-1928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]