Abstract
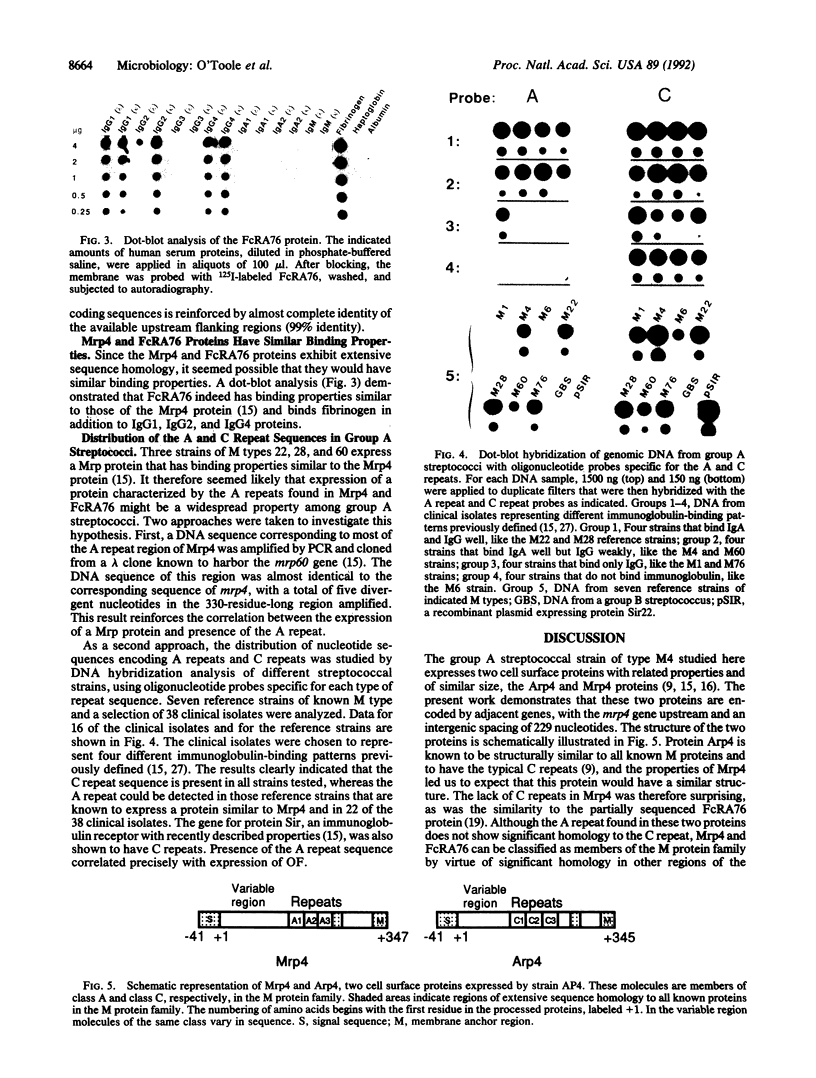
The M protein family of molecules in the group A streptococcus comprises a number of cell surface proteins that interact with the immune system of the host. One of the proteins in this family is the IgA receptor Arp4, which has C repeats similar to those that characterize the known M proteins. The streptococcal strain expressing Arp4 also expresses a second immunoglobulin-binding protein, Mrp4, which is shown here to be encoded by a gene located immediately upstream of the gene for Arp4. In addition to binding IgG, Mrp4 also binds fibrinogen, a property ascribed to M proteins. DNA sequence analysis demonstrated that the Mrp4 protein indeed is a member of the M protein family, but it was unexpectedly found to have a type of repeat that is identical to the A repeat described for FcRA76, a partially sequenced streptococcal Fc receptor. Purified FcRA76 was shown to bind fibrinogen and IgG, like Mrp4. These data show that the known molecules in the M protein family can be divided into two classes, A and C, according to the type of repeat region found. Hybridization studies with a panel of clinical isolates indicate that many streptococcal strains express class A and class C proteins, whereas some strains express only class C proteins. Class A molecules show amino-terminal sequence variation, like class C molecules, which suggests that proteins of both classes are targets for the immune response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessen D. E., Fischetti V. A. Differentiation between two biologically distinct classes of group A streptococci by limited substitutions of amino acids within the shared region of M protein-like molecules. J Exp Med. 1990 Dec 1;172(6):1757–1764. doi: 10.1084/jem.172.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D. E., Fischetti V. A. Nucleotide sequences of two adjacent M or M-like protein genes of group A streptococci: different RNA transcript levels and identification of a unique immunoglobulin A-binding protein. Infect Immun. 1992 Jan;60(1):124–135. doi: 10.1128/iai.60.1.124-135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno A. L. Group A streptococcal infections and acute rheumatic fever. N Engl J Med. 1991 Sep 12;325(11):783–793. doi: 10.1056/NEJM199109123251106. [DOI] [PubMed] [Google Scholar]
- Chen C. C., Cleary P. P. Complete nucleotide sequence of the streptococcal C5a peptidase gene of Streptococcus pyogenes. J Biol Chem. 1990 Feb 25;265(6):3161–3167. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frithz E., Hedén L. O., Lindahl G. Extensive sequence homology between IgA receptor and M proteins in Streptococcus pyogenes. Mol Microbiol. 1989 Aug;3(8):1111–1119. doi: 10.1111/j.1365-2958.1989.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Gomi H., Hozumi T., Hattori S., Tagawa C., Kishimoto F., Björck L. The gene sequence and some properties of protein H. A novel IgG-binding protein. J Immunol. 1990 May 15;144(10):4046–4052. [PubMed] [Google Scholar]
- Haanes E. J., Cleary P. P. Identification of a divergent M protein gene and an M protein-related gene family in Streptococcus pyogenes serotype 49. J Bacteriol. 1989 Dec;171(12):6397–6408. doi: 10.1128/jb.171.12.6397-6408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. G., Cleary P. P. Fc-receptor and M-protein genes of group A streptococci are products of gene duplication. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4741–4745. doi: 10.1073/pnas.86.12.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Lindahl G. Cell surface proteins of a group A streptococcus type M4: the IgA receptor and a receptor related to M proteins are coded for by closely linked genes. Mol Gen Genet. 1989 Apr;216(2-3):372–379. doi: 10.1007/BF00334378. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Stenberg L. Binding of IgA and/or IgG is a common property among clinical isolates of group A streptococci. Epidemiol Infect. 1990 Aug;105(1):87–93. doi: 10.1017/s0950268800047683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxted W. R., Widdowson J. P., Fraser C. A., Ball L. C., Bassett D. C. The use of the serum opacity reaction in the typing of group-A streptococci. J Med Microbiol. 1973 Feb;6(1):83–90. doi: 10.1099/00222615-6-1-83. [DOI] [PubMed] [Google Scholar]
- Miller L., Gray L., Beachey E., Kehoe M. Antigenic variation among group A streptococcal M proteins. Nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J Biol Chem. 1988 Apr 25;263(12):5668–5673. [PubMed] [Google Scholar]
- Misra T. K. A new strategy to create ordered deletions for rapid nucleotide sequencing. Gene. 1985;34(2-3):263–268. doi: 10.1016/0378-1119(85)90135-0. [DOI] [PubMed] [Google Scholar]
- Mouw A. R., Beachey E. H., Burdett V. Molecular evolution of streptococcal M protein: cloning and nucleotide sequence of the type 24 M protein gene and relation to other genes of Streptococcus pyogenes. J Bacteriol. 1988 Feb;170(2):676–684. doi: 10.1128/jb.170.2.676-684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. C., Spanier J. G., Jones S. J., Simpson W. J., Cleary P. P. Streptococcus pyogenes type 12 M protein gene regulation by upstream sequences. J Bacteriol. 1987 Dec;169(12):5633–5640. doi: 10.1128/jb.169.12.5633-5640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind O., Jones K. F., Fischetti V. A. Sequence and structural characteristics of the trypsin-resistant T6 surface protein of group A streptococci. J Bacteriol. 1990 Jun;172(6):3310–3317. doi: 10.1128/jb.172.6.3310-3317.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier J. G., Cleary P. P. A DNA substitution in the group A streptococcal bacteriophage SP24. Virology. 1983 Oct 30;130(2):514–522. doi: 10.1016/0042-6822(83)90104-6. [DOI] [PubMed] [Google Scholar]
- Stenberg L., O'Toole P., Lindahl G. Many group A streptococcal strains express two different immunoglobulin-binding proteins, encoded by closely linked genes: characterization of the proteins expressed by four strains of different M-type. Mol Microbiol. 1992 May;6(9):1185–1194. doi: 10.1111/j.1365-2958.1992.tb01557.x. [DOI] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Biochemical and biological properties of the binding of human fibrinogen to M protein in group A streptococci. J Bacteriol. 1985 Oct;164(1):350–358. doi: 10.1128/jb.164.1.350-358.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]