Abstract
Cachexia is known to be a deteriorating factor for survival of patients with chronic obstructive pulmonary disease (COPD), but data related to obesity are limited. We observed that obese patients with COPD prescribed long-term noninvasive mechanical ventilation (NIMV) had better survival rate compared to nonobese patients. Therefore, we conducted a retrospective observational cohort study. Archives of Thoracic Diseases Training Hospital were sought between 2008 and 2013. All the subjects were prescribed domiciliary NIMV for chronic respiratory failure secondary to COPD. Subjects were grouped according to their body mass index (BMI). The first group consisted of subjects with BMI between 20 and 30 kg/m2, and the second group consisted of subjects with BMI >30 kg/m2. Data obtained at the first month’s visit for the following parameters were recorded: age, sex, comorbid diseases, smoking history, pulmonary function test, 6-minute walk test (6-MWT), and arterial blood gas analysis. Hospital admissions were recorded before and after the domiciliary NIMV usage. Mortality rate was searched from the electronic database. Overall, 118 subjects were enrolled. Thirty-eight subjects had BMI between 20 and 30 kg/m2, while 80 subjects had BMI >30 kg/m2. The mean age was 65.8±9.4 years, and 81% were male. The median follow-up time was 26 months and mortality rates were 32% and 34% for obese and nonobese subjects (P=0.67). Improvement in 6-MWT was protective against mortality. In conclusion, survival of obese patients with COPD using domiciliary NIMV was found to be better than those of nonobese patients, and the improvement in 6-MWT in such patients was found to be related to a better survival.
Keywords: chronic respiratory failure, survival, body mass index, mortality, therapy
Introduction
Chronic obstructive pulmonary disease (COPD) is known to be a leading cause of morbidity and mortality worldwide. The mortality rate varies between the countries or cities according to the exposure to smoke or indoor/outdoor air pollution.1 The 5-year mortality rate for patients with COPD patients ranges between 40% and 70% according to the disease severity.2 Body mass index (BMI) is suggested to be an important factor affecting the mortality rate of patients with COPD.3 Cachexia is known to be a deteriorating factor for survival of patients with COPD, but such data related to obesity are limited.4
Chronic respiratory failure (CRF) is associated with poor prognosis in patients with COPD. There are many studies showing the ameliorating effect of noninvasive mechanical ventilation (NIMV) therapy for exacerbation of COPD.5,6 However, there are a few studies evaluating the efficacy of domiciliary NIMV usage in obese patients. Piesiak et al investigated the efficacy of NIMV in obese patients with CRF and concluded that, it is an effective and well-tolerated treatment option in obese patients with CRF.7
There are limited data available related to the effect of domiciliary NIMV usage on survival of patients having COPD with CRF.8 In the present study, we have hypothesized that obese patients having COPD with CRF using domiciliary NIMV have a better survival rate compared to normal-weight patients having COPD with CRF using long-term domiciliary NIMV.
Materials and methods
Study design and population
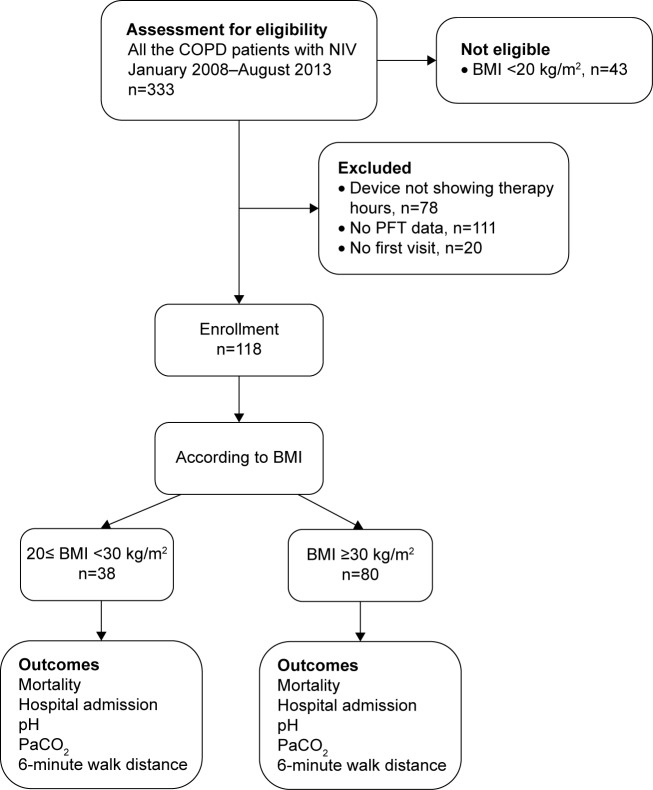
We performed a retrospective observational cohort study. The study which is accordant with the Declaration of Helsinki, was approved by the local Ethics Committee of Sureyyapasa Thoracic Diseases Training Hospital and patient consent was not required. All the patients with CRF due to COPD treated at the Thoracic Diseases and Thoracic Surgery Training Government Hospital in Istanbul, Turkey, between January 2008 and August 2013 were included if they were prescribed domiciliary NIMV during their hospital discharge. The subjects with BMI between 20 and 30 kg/m2 were accepted as nonobese while those with BMI >30 kg/m2 were accepted as obese. We defined an index date, which was 1 month after the patients received their ventilators and were followed for a minimum of 1 year. The patient’s eligibility and enrollment are summarized on the Consolidated Standards of Reporting Trials flow diagram (Figure 1).
Figure 1.
CONSORT flow diagram.
Abbreviations: BMI, body mass index; CONSORT, Consolidated Standards of Reporting Trials; COPD, chronic obstructive pulmonary disease; NIV, noninvasive ventilation; PaCO2, partial arterial carbon dioxide pressure; PFT, pulmonary function test.
Patients were considered ineligible if they did not use their ventilators in the month following discharge from the hospital and if they had BMI <20 kg/m2. If there was no therapy hours indicator on the NIMV to show how many hours the patient used, we excluded the patient from the study. If the patient did not have a pulmonary function test result or if the patient did not come to the first month visit they were also excluded.
Data source
Patient charts were manually abstracted and entered into SPSS version 22 (IBM Corporation, Armonk, NY, USA). All included measures were performed as part of routine clinical care.
Outcome assessment
The time until death was accepted as the primary outcome, whereas effect on hospital admission number and arterial blood gas recovery were accepted as secondary outcomes.
Primary exposure
BMI at the time of discharge was used.
Additional covariates
All the parameters (age, sex, comorbid diseases, smoking history, pulmonary function test, 6-minute walking test [6-MWT], and arterial blood gas analysis) were recorded at the first visit 1 month after hospital discharge.
Hospital ward, emergency room, and intensive care unit admissions were recorded 1 month before and after the hospital discharge.
Mortality data were obtained from the electronic database of Turkish Health Ministry in November 2014.
Tests
Pulmonary function test and 6-MWTs are based on the American Thoracic Society guidelines.9.10
Statistical analysis
Bivariate comparisons were done using appropriate parametric and nonparametric data. We examined unadjusted survival using Kaplan–Meier tests. We developed a series of multivariate models using Cox-proportion hazards for time-to-event analyses and logistic regression for continuous outcomes. Covariates were initially examined in groups, including demographics, COPD severity, and comorbidity. We created a parsimonious multivariate regression model by selecting those variables that were associated at a level of 0.1 or less and entering them into the final model.
Results
A total of 118 patients having COPD and using domiciliary NIMV were included. The characteristics of obese and nonobese patients are all summarized in Table 1.
Table 1.
The comparison of obese and normal-weight COPD patients with long-term home NIMV usage
| Characteristics | Nonobese BMI 20–30 kg/m2, N=80 | Obese BMI ≥30 kg/m2, N=38 | P-values |
|---|---|---|---|
| Female, n (%) | 15 (19) | 8 (21) | 0.77 |
| Age, yeara | 67±9 | 64±10 | 0.08 |
| BMI, kg/m2a | 24.9±2.7 | 35.8±4.2 | 0.001 |
| Smoking, pack-yeara,b | 59±30 | 56±25 | 0.59 |
| Comorbidity, n (%) | 71 (89) | 36 (95) | 0.30 |
| Hypertension, n (%) | 23 (29) | 13 (34) | 0.55 |
| Diabetes, n (%) | 9 (11) | 8 (21) | 0.16 |
| Cor pulmonale, n (%) | 9 (11) | 6 (16) | 0.49 |
| LTOT use, n (%) | 78 (98) | 38 (100) | 0.33 |
| pHa | 7.41±0.05 | 7.43±0.06 | 0.07 |
| PaCO2, mmHga | 50±7 | 48±8 | 0.30 |
| 6-MWT, ma,b | 329±93 | 351±124 | 0.42 |
| FEV1 (%)a | 28±11 | 38±12 | 0.001 |
| FVC (%)a | 37±13 | 49±17 | 0.001 |
| FEV1/FVCa | 58.8±10.8 | 60.8±8.5 | 0.35 |
| Hemoglobin (g/dL)a,c | 13.6±1.9 | 14.1±2.3 | 0.23 |
| Hematocrita,d | 44±7 | 45±7 | 0.57 |
| Presence of hospitalization in 1 year, n (%) | 26 (33) | 3 (8) | 0.004 |
| Mortality during follow-up, n (%) | 27 (34) | 12 (32) | 0.82 |
| Follow-up time, monthsa | 25±18 | 29±19 | 0.39 |
Notes:
Mean ± SD.
Thirty cases are missing;
53 cases are missing;
two cases are missing.
Abbreviations: 6-MWT, 6-minute walking test; BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LTOT, long-term oxygen therapy; NIMV, noninvasive mechanical ventilation; PaCO2, partial pressure of carbon dioxide; SD, standard deviation.
The hospitalization percentage of patients using domiciliary NIMV for 1 year was found to be low in the obese patients compared with nonobese ones (P=0.004).
The median follow-up time was 26 months (6–84 months), and 39 patients (33%) died during the study period. The mortality rate for obese patients was 32%, while it was 34% for nonobese patients. Kaplan–Meier curves are shown in Figure 2. The differences are not significant by the log-rank test (P=0.67).
Figure 2.
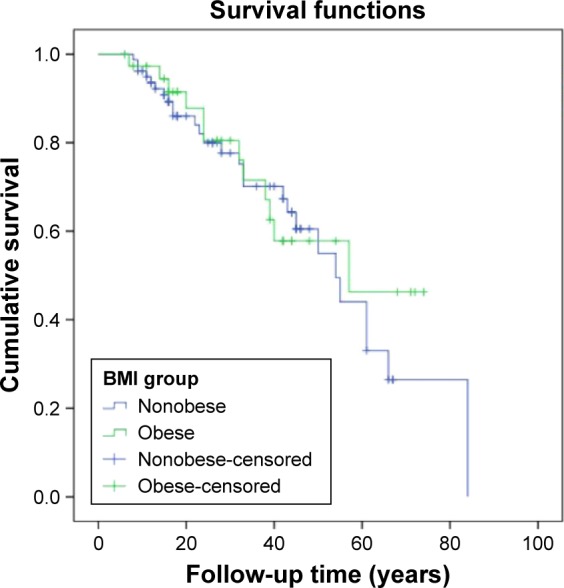
Survival of the groups.
Note: The subjects with BMI between 20 and 30 kg/m2 were defined as nonobese while those with BMI >30 kg/m2 were defined as obese.
Abbreviation: BMI, body mass index.
In Table 2, characteristics of hospitalized or nonsurvived and nonhospitalized or survived patients are compared.
Table 2.
The comparison of hospitalized or nonsurvived COPD patients versus nonhospitalized and survived COPD patients
| Variables | Nonhospitalized or survived, N=84 | Hospitalized or nonsurvived, N=34 | P-values |
|---|---|---|---|
| Sex, male % | 79% | 85% | 0.40 |
| Age, year | 66±9 | 66±11 | 0.81 |
| Cigarette pack-year | 57±25 | 61±36 | 0.57 |
| Comorbidity, n (%) | 77 (92) | 30 (88) | 0.57 |
| Hypertension, n (%) | 29 (35) | 7 (21) | 0.14 |
| Cor pulmonale, n (%) | 10 (12) | 5 (15) | 0.68 |
| Diabetes, n (%) | 14 (17) | 3 (9) | 0.27 |
| Presence of emphysema, n (%) | 2 (2) | 4 (12) | 0.036 |
| Presence of bronchiectasis, n (%) | 5 (6) | 6 (18) | 0.048 |
| Home NIMV compliance, n (%) | 62 (74) | 18 (53) | 0.028 |
| Body mass index group, n (%) | |||
| >20 and <30 kg/m2 | 51 (61) | 29 (85) | 0.010 |
| ≥30 kg/m2 | 33 (39) | 5 (15) | – |
| Baseline spirometry | |||
| FEV1, L | 0.84±0.40 | 0.74±0.31 | 0.18 |
| FEV1, % predicted | 32±12 | 29±12 | 0.16 |
| FVC, L | 1.43±0.64 | 1.23±0.49 | 0.12 |
| FVC, % predicted | 42±17 | 37±12 | 0.13 |
| FEV1/FVC | 59±10 | 60±10 | 0.86 |
| Baseline hemoglobin, mg/mL | 13.9±2.1 | 13.6±2.4 | 0.43 |
| Baseline hematocrit | 44.2±8.4 | 42.6±11.0 | 0.38 |
| Baseline arterial blood gases | |||
| pH | 7.42±0.05 | 7.43±0.06 | 0.014 |
| PaCO2, mmHg | 49±7 | 50±8 | 0.71 |
| PaO2/FiO2 | 298±83 | 291±99 | 0.96 |
| HCO3, mmol | 31±5 | 30±5 | 0.17 |
| SatO2, % | 93±5 | 89±12 | 0.50 |
| 6-MWT, m | 352±107 | 292±91 | 0.057 |
| Follow-up time, months, n (range) | 28 (18–44) | 26 (15–33) | 0.022 |
Note: Data presented as mean ± standard deviation unless indicated.
Abbreviations: 6-MWT, 6-minute walking test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HCO3, bicarbonate; NIMV, noninvasive mechanical ventilation; PaCO2, partial arterial carbon dioxide pressure; PaO2, partial arterial oxygen pressure.
Table 3 shows univariate analysis of death by different characteristics. According to this table, 6-MWT at first visit was found to have a strong inverse effect on the mortality rate of our patients. Being 1 year older was found to increase the mortality rate 1.05 times (P=0.009). During usage of domiciliary NIMV, active smoking was found to increase the mortality rate insignificantly (1.4 times, P=0.47). Although not statistically significant, having any comorbidity, such as diabetes mellitus and hypertension, seemed to cause lower mortality rates by 40%, 90% and 70%, respectively. On the other hand, cor pulmonale was found to be associated with two times increase in mortality rate. Before domiciliary NIMV was prescribed, the frequency of intensive care unit admissions was found to be negatively correlated with mortality rate. The frequency of hospitalization prior to being prescribed domiciliary NIMV was found to be positively correlated with mortality rate; however, these correlations were not statistically significant (P=0.32, P=0.28, respectively).
Table 3.
The hazard of death in obese COPD patients comparing sex, age, arterial blood gas, FEV1, 6-MWT, and comorbidities
| Characteristics | Hazard ratio (95% CI) (upper–lower limit) | P-value |
|---|---|---|
| Males | 0.89 (0.4–1.90) | 0.76 |
| Age (year) | 1.05 (1.01–1.09) | 0.009 |
| BMI (kg/m2) | 0.97 (0.9–1.03) | 0.35 |
| Active smoking | 1.4 (0.5–3.8) | 0.47 |
| Comorbidity | 0.41 (0.1–1.07) | 0.07 |
| DM | 0.9 (0.3–2.7) | 0.93 |
| HT | 0.7 (0.3–1.6) | 0.46 |
| Cor pulmonale | 2.01 (0.7–5.8) | 0.24 |
| ICU admissiona | 0.78 (0.5–1.2) | 0.32 |
| Hospital admissiona | 1.07 (0.94–1.2) | 0.28 |
| Pulmonary ward admissionb | 0.97 (0.7–1.3) | 0.8 |
| pH >7.35 | 1.6 (0.4–7) | 0.52 |
| pH <7.45 | 0.7 (0.3–1.5) | 0.31 |
| pCO2 (mmHg) | 1.04 (0.99–1.09) | 0.08 |
| Hb at first visit | 0.89 (0.7–1.03) | 0.12 |
| FEV1 mL at first visit | 1.0 (0.99–1.0) | 0.72 |
| 6-MWT at first visit | 0.9 (0.98–0.99) | 0.023 |
Notes:
Before home NIMV prescription;
in 1 years time after NIMV prescription. Subjects with BMI >30 kg/m2 were defined as obese.
Abbreviations: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; Hb, hemoglobin; HT, hypertension; FEV1, forced expiratory volume in 1 second, ICU, intensive care unit; NIMV, noninvasive mechanical ventilation; 6-MWT, 6-minute walk distance.
Although the data were statistically insignificant, 0.4 units increase in pH was found to decrease the mortality rate by 0.87 times (P=0.79).
We searched for the factors that may change the effect of obesity on mortality rate, and adjustment was made for age, sex, comorbidities, pulmonary function tests, arterial blood gas analysis, and frequency of hospitalization. Cox proportional hazard models were built to assess the effects of covariates on patients. Table 4 shows the hazard ratio adjusted according to age, 6-MWT at first visit, and existence of comorbidity. However, none of them was found to interfere with the effect of obesity on mortality rate.
Table 4.
Hazard of death associated with obese patients with COPD
| Function | Hazard ratioa | Adjusted hazard ratioa | P-value |
|---|---|---|---|
| Obesity | 0.86 (0.43–1.7) | 0.86 (0.32–2.31) | |
| 6-MWT | 0.99 (0.98–0.99) | 1.0 (0.93–1.07) | 0.99 |
| Age | 1.05 (1.01–1.09) | 0.98 (0.92–1.04) | 0.51 |
| 6-MWT | |||
| Age | 1.001 (0.92–1.07) | 0.97 | |
| Comorbidity | 0.42 (0.16–1.07) | 0.98 (0.92–1.04) | 0.58 |
| 6-MWT | |||
| Age | 1.02 (0.94–1.1) | 0.58 | |
| Comorbidity | |||
Notes:
Data are presented as (95% CI) (upper–lower limit). Subjects with BMI >30 kg/m2 were defined as obese.
Abbreviations: 6-MWT, 6-minute walk test; COPD, chronic obstructive pulmonary disease.
As seen in Table 3, forced expiratory volume in 1 second (FEV1) at the first visit was not found to have an effect on mortality rate, while improvement in the 6-MWT was found to have a protective effect on mortality rate. An increase of 1 m in the 6-MWT was found to be related with a 90% decrease in mortality rate. FEV1 value at the first visit alone was found to have a protective effect when frequency of hospitalization and mortality rate were considered together as the secondary outcome (Table 5).
Table 5.
Adjustment of hospitalization or death in 1 year in obese patients for pulmonary function tests
| Tests | Unadjusteda | Adjusteda | P-value |
|---|---|---|---|
| BMI | 0.26 (0.09–0.75) | ||
| FEV1 (mL) at first visit | 0.99 (0.99–1.00) | 0.3 (0.1–0.9) | 0.034 |
| 6-MWT distance at first visit | 0.99 (0.98–1.00) | 0.47 (0.13–1.7) | 0.27 |
Notes:
Data are presented as (95% CI) (upper–lower limit). Subjects with BMI >30 kg/m2 were defined as obese.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 second; 6-MWT, 6-minute walk distance.
Discussion
Patients having COPD and using domiciliary NIMV with BMI ≥30 seem to survive longer than nonobese patients with BMI <30. Increase in the 6-MWT seems to be the most significant protective factor against mortality in patients having COPD and using domiciliary NIMV. Cao et al have shown that patients with COPD being overweight or obese had a protective effect against mortality.11 Schols et al also recorded that fat free mass in body composition is the independent predictor of mortality in COPD patients irrespective of fat mass.12 The relationship between body composition and mortality rate of patients with COPD need to be further investigated.
Landbo et al also found that the impact of BMI on COPD-related mortality was more important than all other causes of mortality that they have compared.13 They reported that for patients having severe COPD, mortality rate decreased with increasing BMI, with a risk ratio of 7.11 (2.97–17.05). Borel et al also conducted a similar study and reached results similar to those of this study. They suggested that long-term adherence to NIMV positively affects the prognosis of obese patients with COPD.14
In this study, as the functional status of the patients was examined, it was seen that the FEV1 value at the first visit did not have an effect on the mortality rate, while the 6-MWT had a strong protective effect. Every 1 m increase in the distance walked in the 6-MWT caused a 90% decrease in the mortality rate. Polkey et al also found a similar relationship between increase in distance walked in a 6-MWT and survival of patients with COPD.15 O’Donnel et al discussed every aspect of physiological effects of obesity on COPD in their review and reported that mechanical advantages of obesity on respiratory function of patients with COPD is seen during walking.16 Similarly Celli et al reported that BODE index (body mass index, airflow obstruction, dyspnea, and exercise) predicted mortality more accurately than FEV1.3
The mean survival time for our COPD patients was 30.54±18 months. Although the survival time for obese patients with COPD patients using domiciliary NIMV was found to be longer than that of nonobese patients with COPD using NIMV, the difference was not significant (32.6 vs 29.5 months). Yildiz et al studied the predictors of long-term survival for patients with stable COPD and showed a mean survival time of 43.4 months.17
Borel et al found that cardiovascular comorbidities remained the only factor independently associated with a higher risk of death in obese hypercapnic patients using domiciliary NIMV.18 Divo et al showed the different clustering comorbidities in different BMI values.19 They observed significant differences at the extremes of the BMI spectrum. Obese and very obese patients were found to have more likely the following conditions compared to nonobese patients: systemic hypertension, hyperlipidemia, sleep apnea, diabetes mellitus, chronic renal failure, congestive heart failure, gout, venous insufficiency, degenerative joint disease, pulmonary hypertension, and erectile dysfunction.19 Having any comorbid disease like DM or HT seem to decrease the mortality in our study. We do not think that this is logical and we think that if a patient has a stable comorbid disease it might be ignored while recording. For example, if the blood sugar levels during hospitalization in a diabetic COPD patient are normal, he might not be named as diabetic while recording the patient file.
Regarding the hospital admission rates in the present study, it seems like there is a conflict with the previous results. Ansari et al reported that domiciliary NIMV intervention resulted in a reduction in hospital admission of patients with type 2 respiratory failure. So our results need to be investigated by larger studies, as our result is opposite to the general clinical experience.20
Respiratory acidosis due to hypercapnia is known to be a factor worsening the prognosis and is associated with a high mortality rate in patients with COPD.21 Ucgun et al reported that development of sufficient metabolic compensation and adequate renal function significantly decreases the mortality rate.22 All these results are similar to results obtained by us.
Limitations
The potential limitation of our study is that it is a retrospective single site study. A prospective, research protocol data study may be designed. In our files, some data may be missing, such as prominent comorbidities. If our patient is hospitalized during the first year in another hospital than we can not know this unless the patient or their family tell us.
We think that the entire population sampling is an important strength factor of our study. We defined a relatively large cohort of patients. We collected data systematically. In our study, we have a long follow-up time and a qualified systematic data.
Conclusion
In conclusion, obese patients with COPD using domiciliary NIMV were found to survive longer than that of the nonobese patients with COPD, and improvement in 6-MWT in such patients was found to be the most significant protective factor against mortality.
Acknowledgments
This study was a 2014 level 3 MECOR project conducted by Dr David Au. Thanks to all MECOR faculty members. In September 2015, we presented our study as a poster at the European Respiratory Society Congress held in Amsterdam (poster number PA 2189). The abstract book of the ERJ supplement for the conference abstracts is available from: http://dx.doi.org/10.1183/13993003.congress-2015.PA2189. Our study is not published in any other journal except for this ERS 2015 conference abstract book.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global initiative for chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management and Prevention of COPD. Bethesda (MD): GOLD; 2014. [Google Scholar]
- 2.Nishimura K, Tsukino M. Clinical course and prognosis of patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2000;6(2):127–132. doi: 10.1097/00063198-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 4.Montes de Oca M, Torres SH, Gonzalez Y, et al. Peripheral muscle composition and health status in patients with COPD. Respir Med. 2006;100(10):1800–1806. doi: 10.1016/j.rmed.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosini N, Vagheggini G. Non-invasive ventilation in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(4):471–476. [PMC free article] [PubMed] [Google Scholar]
- 6.Consensus Conference Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation – a consensus conference report. Chest. 1999;116(2):521–534. doi: 10.1378/chest.116.2.521. [DOI] [PubMed] [Google Scholar]
- 7.Piesiak P, Brrzecka A, Kosacka M, Jankowska R. Efficacy of noninvasive mechanical ventilation in obese patients with chronic respiratory failure. Adv Exp Med Biol. 2013;788:167–173. doi: 10.1007/978-94-007-6627-3_25. [DOI] [PubMed] [Google Scholar]
- 8.Nava S, Ergan B. Long-term non-invasive ventilation (NIV) for COPD patients with chronic respiratory failure. EMJ Respir. 2013;1:54–62. [Google Scholar]
- 9.Miller MR, Crapo R, Hankinson J, et al. ATS/ERS task force: Standardisation of lung function testing. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 10.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 11.Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS One. 2012;7(8):e43892. doi: 10.1371/journal.pone.0043892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 14.Borel JC, Pepin JL, Pison C, et al. Long-term adherence with noninvasive ventilation improves prognosis in obese COPD patients. Respirology. 2014;19(6):857–865. doi: 10.1111/resp.12327. [DOI] [PubMed] [Google Scholar]
- 15.Polkey Michael I, Spruit Martijn A, Edwards Lisa D, et al. Six-minute-walk test in chronic obstructive pulmonary disease minimal clinically important difference for death or hospitalization. Am J Respir Crit Care Med. 2013;187(4):382–386. doi: 10.1164/rccm.201209-1596OC. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnel DE, Ciavaglia CE, Neder A. When obesity and chronic obstructive pulmonary disease collide. Physiological and clinical consequences. Ann Am Thorac Soc. 2014;11(4):635–644. doi: 10.1513/AnnalsATS.201312-438FR. [DOI] [PubMed] [Google Scholar]
- 17.Yildiz OA, Onen ZP, Sen E, et al. Predictors of long-term survival in patients with chronic obstructive pulmonary disease. Saudi Med J. 2006;27(12):1866–1872. [PubMed] [Google Scholar]
- 18.Borel J-C, Burel B, Tamisier R, et al. Comorbidities and mortality in hypercapnic obese under domiciliary noninvasive ventilation. PLoS One. 2013;8(1):e52006. doi: 10.1371/journal.pone.0052006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Divo MJ, Cabrera C, Casanova C, et al. Comorbidity distribution, clinical expression and survival in COPD patients with different body mass index. J COPD F. 2014;1(2):229–238. doi: 10.15326/jcopdf.1.2.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansari Z, Sahal A, Sharma P, et al. Does relationship exist between domiciliary non-invasive ventilation (NIV) and frequency of hospital admissions in patients with chronic type 2 respiratory failure? ERJ. 2014;44(Suppl 58):P2094. [Google Scholar]
- 21.Bruno CM, Valenti M. Acid-base disorders in patients with chronic obstructive pulmonary disease: a pathophysiological review. J Biomed Biotechnol. 2012;2012:915150. doi: 10.1155/2012/915150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ucgun I, Oztuna F, Dagli CE, Yildirim H, Bal C. Relationship of metabolic alkalosis, azotemia and morbidity in patients with chronic obstructive pulmonary disease and hypercapnia. Respiration. 2008;76(3):270–274. doi: 10.1159/000131707. [DOI] [PubMed] [Google Scholar]