Abstract
Background
Biofilm formation inside inserted medical devices leads to their failure and acts as a source of refractory infections. The ultraviolet C (UVC) light is a potential therapy that can be used against the biofilm of bacterial pathogens.
Objective
We evaluated the efficacy of sublethal dose of UVC light with anti-staphylococcal antibiotics against biofilms made from 30 isolates of methicillin-susceptible Staphylococcus aureus and methicillin-resistant S. aureus and S. epidermidis on vascular catheters.
Materials and methods
A novel biofilm device was used to assess the combined approach. The biofilms on the catheters were irradiated with the UVC light at 254 nm and irradiance of 6.4 mW followed by treatment with vancomycin or quinupristin/dalfopristin at twice their minimum bactericidal concentrations or with linezolid at 64 µg/mL for 24 hours. The catheters were cut into segments and sonicated, and the number of the sessile cells was determined colorimetrically using XTT viable cells assay. The effect of UVC radiation followed by treatment with an antistaphylococcal antibiotic on the viability of the bacteria in the biofilm was visualized using LIVE/DEAD BacLight bacterial viability stain and confocal laser scanning microscopy.
Results
Exposure of the bacterial biofilms to the UVC light or each of the antibiotics alone was ineffective in killing the bacteria. Treatment of the biofilms with the antibiotics following their exposure to UVC light significantly (P<0.001) reduced the number of viable cells within the biofilms but did not completely eradicate them.
Conclusion
To our knowledge, this combinatorial approach has not been investigated before. The combined approach can be used as a therapeutic modality for managing biofilm-associated infections by preventing the establishment of biofilms and/or disrupting the formed biofilms on the inserted medical devices with the goal of increasing their usefulness and preventing infectious complications. Further investigations are needed to assess the effectiveness of the combined approach in the clinical settings.
Keywords: alternative therapy, biofilm-associated infections, MRSA, MSSA, Staphylococcus epidermidis, indwelling medical devices
Introduction
Biofilms are composed of pure or mixed communities of microorganisms adhering to surfaces. In the medical setting, biofilm-associated infections constitute a steadily increasing problem and can start out from the surface of different indwelling devices.1 Microorganisms upon shedding from the biofilm enter the circulation and result in dissemination and establishment of infection at distant sites. Since the microorganisms in such infections are in aggregates, they remain less susceptible to antimicrobial agents compared to single-cell suspensions tested in the diagnostic laboratories.2
Implant-associated infections lead to considerable morbidity, repeated surgeries, and prolonged antibiotic therapy.3 The mortality associated with cardiovascular device-related, biofilm-associated infections, for example, is estimated to be 12%–25%, with a health care cost of $33,000–$35,000/event.4
The bacteria within the biofilm are protected from the host defense mechanisms and the antimicrobial agents. They can be up to 1,000 times more resistant to antibiotics than their planktonic (free-floating) counterparts.5 Such resistance is demonstrated not only toward antibiotics but also toward preservatives, disinfectants, and antiseptics.6,7
Failure of the antibiotics to manage biofilm-associated infections has led to a significant research effort to find alternative antimicrobial approaches with more efficacy and less resistance developed by the microorganisms. The ultraviolet C (UVC) light could be a potential alternative antimicrobial intervention to which resistance will be difficult to develop. The UVC kills the organisms by damaging the DNA and RNA through dimerization of pyrimidine molecules.8 With appropriate doses, UVC may selectively target microorganisms with a negligible effect on the mammalian cells.9
Several in vitro studies have reported the susceptibility of multidrug-resistant bacteria to inactivation by UVC.9–12 These bacteria include methicillin-resistant Staphylococcus aureus (MRSA), coagulase-negative Staphylococcus, Streptococcus pyogenes, Enterococcus faecalis, Pseudomonas aeruginosa, and Mycobacteria.10–12 In clinical studies, the UVC irradiation was used to disinfect surgical wounds and treat infections in cutaneous ulcers.13,14 However, only minimal data are available on the effectiveness of the UVC light against the bacteria in the biofilms. The UVC light was previously studied to control microbial biofilms in water systems and medical devices.15,16 Disinfection of biofilms on catheters by exposure to the UVC light was proposed by Bak et al,17 who reported that the lethal dose of UVC required to kill the bacteria in the biofilm was 100–1,000 times greater than the dose needed to kill their planktonic counterparts. High irradiation dose is unsuitable for clinical application because it could induce damage in the host cells.18 Low dose, on the other hand, could be of valuable practical use if combined with antimicrobial agents.
The aim of this study was to evaluate the effectiveness of a single low dose of UVC light at 254 nm against the biofilms of S. aureus (methicillin-susceptible S. aureus [MSSA] and MRSA) and Staphylococcus epidermidis on vascular catheters following treatment with the antistaphylococcal antibiotics, vancomycin (VAN), quinupristin/dalfopristin (Q/D), and linezolid (LNZ).
To our knowledge, this is the first study that investigates the possible use of this combination anti-infective approach.
Materials and methods
Unless otherwise indicated, all chemicals were of analytical grade and were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Antibiotics
VAN was obtained from Sigma-Aldrich Co. LNZ was provided by Pharmacia & Upjohn (Kalamazoo, MI, USA). Q/D was provided by Rhône-Poulenc Rorer (Collegeville, PA, USA).
Microorganisms
Ten clinical isolates each of MRSA, MSSA, and S. epidermidis were used in this study. The isolates were provided by the Microbiology Laboratory of St. John Hospital (Springfield, IL, USA). They were recovered from bloodstream infections, and they were screened for biofilm formation as previously described.19
Susceptibility of the clinical isolates to the antibiotics
The minimum inhibitory concentrations (MICs) of the antibiotics were determined by using the broth microdilution technique described by the guidelines of Clinical and Laboratory Standards Institute.20 The minimum bactericidal concentrations (MBCs) were determined by mixing the contents of each well at the MIC and higher concentrations. Portions of 10 µL were then taken from each well and streaked onto the surface of blood agar. After 24 hours of incubation, the number of colony forming units per milliliter (CFU/mL) was counted and the MBCs, defined as the concentration at which 99.9% of bacteria were killed, were determined.
In vitro biofilm device
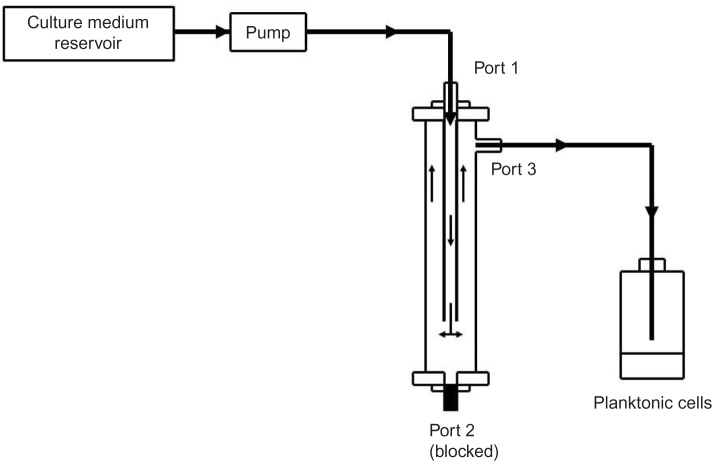
A novel in vitro biofilm device was used as previously described (Figure 1).21 The device comprises a tubular body defining a test chamber. The body has upper and lower ends with three ports provided with closures. The three ports can be connected to a tubing system or blocked by removable closures. The port in the upper end of the device is designed to mount the tested materials (catheters or tubes). The design allows the fluid to be pumped through the inner lumen of the implant tube before filling the inner chamber to allow biofilm formation on the internal and external surfaces of the catheter. The design of the device permits low laminar flow system with very low shear stress on the inner and outer catheter surfaces.
Figure 1.
The in vitro biofilm-forming device.
Note: The device was configured to work with both static and continuous perfusion systems.
Antimicrobial activity of the UVC light alone and in combination with the antibiotics on the biofilms of staphylococci
Briefly, peripheral venous catheter BD Angiocath (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), reference number 382259, was placed in the sample insert. Bacterial suspension in tryptic soy broth (TSB) medium at 1×106 CFU/mL was used to fill the chamber. After 90 minutes, fresh medium was pumped continuously through the chamber at 30 mL/h, where the catheter was kept under a continuous flow of the fresh medium for 48 hours at 37°C. The catheter was then aseptically removed from the chamber using forceps and exposed to a UVC germicidal low-pressure mercury lamp (24 W, 254 nm) at an irradiance of 6.4 mW/cm2 and lamp–catheter distance of 10 cm. The catheter was then replaced in the sample insert, and fresh TSB medium supplemented with the antibiotics, at double of their MBC for VAN and Q/D or 64 µg/mL for LNZ, was used to fill the chamber. The device was then incubated for 24 hours at 37°C after which the catheter was washed by pumping sterile phosphate-buffered saline (PBS; pH 7.3) at 30 mL/h for 30 minutes. The catheter was then aseptically removed and cut into 1 cm segments. The biofilms on the segments were washed and dislodged by sonication in 1 mL ice-cooled PBS at 30% cycle, 3.5 output for 30 seconds followed by vortexing for another 30 seconds. The number of detached cells was determined colorimetrically using XTT 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) as described before.22 In brief, the samples containing the dislodged cells in PBS were centrifuged at 10,000 rpm for 10 minutes. One milliliter aliquots of lactate ringer’s solution containing XTT (0.5 gm/L) and menadione (1 µM) were added to the resultant pellets. The samples were incubated in the dark for 1 hour at 37°C, after which the color of the formed formazan was measured colorimetrically at 490 nm. Drug-free and UV-unexposed samples were used as controls.
Visualizing the efficacy of the combined therapy on the bacterial biofilm using confocal laser scanning microscopy
The effect of UVC radiation at 254 nm followed by treatment with VAN at twice its MBC on the cell viability of one of the isolates of S. epidermidis was visualized using confocal laser scanning microscopy (CLSM) as previously described.2 Briefly, 1 mL of TSB containing 1×106 CFU/mL of the isolate was used to inoculate sterile plastic coverslips placed in a four-well plate (Nunc No 176740, Nunc, Roskilde, Denmark). After 48 hours of incubation at 37°C, the coverslips were transferred to new plates and washed twice with distilled water. The coverslips were then exposed to the UV light as previously mentioned, after which 1 mL portions of fresh TSB containing VAN were added to the wells. After incubation for another 24 hours, the biofilms were stained with LIVE/DEAD BacLight bacterial viability stain (Molecular Probes, Eugene, OR, USA) following manufacturer’s instructions. The biofilms were then examined by Olympus Fluoview CLSM (model IX 70; Olympus America Inc., Center Valley, NY, USA). The result of the experiment was compared to UV-untreated samples in the presence and absence of the antibiotic.
Ethical statement
The protocol of the research work has been approved by the Infection Control Committee of St. John Hospital, Springfield, Illinois who deemed patient consent not necessary as this was an in vitro study which didn’t involve human or animals. The isolates used were provided by the microbiology laboratory and no patients data were provided at all.
Statistical analysis
The mean and SD were calculated from the results of ten isolates of each of the Staphylococcal species. One-way analysis of variance was used to determine the differences between various treatments. Tukey’s pairwise comparison test was used with the significance level set at P<0.05 to determine significant differences between mean values.
Results
Susceptibility of the clinical isolates to the antibiotics
The MIC90 (the minimum inhibitory concentration of the antibiotic required to inhibit the growth of 90% of the isolates) and MBC90 (the minimum concentration of the antibiotic required to kill 99.9% of bacteria in 90% of the isolates) were used to compare the antibiotics. All isolates were susceptible to the three antibiotics with Q/D more effective in inhibiting the bacterial growth (MIC90 range 0.25–0.50 µg/mL) compared to VAN and LNZ (MIC90 range 1–2 µg/mL). VAN and Q/D were capable of killing 99.9% of the bacteria with an MBC90 range of 8 µg/mL and 8–16 µg/mL, respectively (Table 1)
Table 1.
Susceptibility of the tested isolates to the antibiotics
| Microorganisms (number) | Concentration (µg/mL)a | |||||
|---|---|---|---|---|---|---|
| VAN
|
Q/D
|
LNZ
|
||||
| MIC90 | MBC90 | MIC90 | MBC90 | MIC90 | MBC90 | |
| MSSA (10) | 1.0 | 8.0 | 0.25 | 8.0 | 2.0 | >64 |
| MRSA (10) | 1.0 | 8.0 | 0.50 | 16 | 2.0 | >64 |
| S. epidermidis (10) | 2.0 | 8.0 | 0.50 | 8.0 | 1 | >64 |
Notes:
MIC90 stands for the MIC of the antibiotics required to inhibit the growth of 90% of the isolates and MBC90 stands for the MBC of the antibiotic required to kill 99.9% of bacteria in 90% of the isolates.
Abbreviations: VAN, vancomycin; Q/D, quinupristin/dalfopristin; LNZ, linezolid; MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; S. epidermidis, Staphylococcus epidermidis.
Antimicrobial activity of the UVC light alone and in combination with the antibiotics on the biofilms of staphylococci
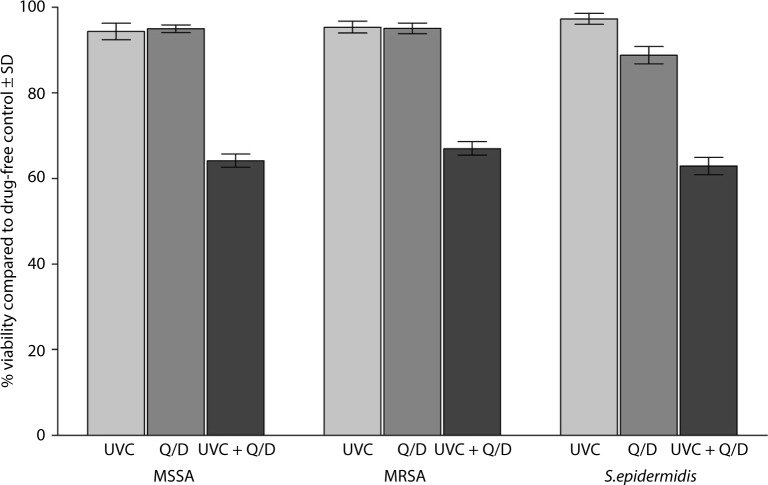
Exposure of the biofilms to the UVC light at 254 nm for 5 minutes was ineffective in killing the bacteria. The mean percent viability of MSSA, MRSA, and S. epidermidis in the biofilms compared to controls was 94.36±6.10, 95.36±4.42, and 97.26±4.08, respectively, following exposure to the UVC light alone. Similar results were obtained when each of the antibiotics was used alone against the biofilms. Following treatment with Q/D, the mean percent viability of the bacteria in the biofilms compared to the control was 94.98±2.86, 95.07±3.99, and 88.76±6.13, respectively. Treatment of the biofilms with Q/D following the exposure to the UVC light significantly (P<0.001) reduced the number of viable cells within the biofilms. The mean percent viability of the cells compared to the control was 64.15±4.98, 67±5.07, and 62.89±6.39, respectively (Figure 2).
Figure 2.
Effect of exposure to a sublethal dose of UVC light followed by treatment with twice the MBC of Q/D on the biofilms of 30 isolates of MSSA, MRSA, and S. epidermidis on the vascular catheter.
Abbreviations: UVC, ultraviolet C; MBCs, minimum bactericidal concentrations; Q/D, quinupristin/dalfopristin; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; S. epidermidis, Staphylococcus epidermidis; SD, standard deviation.
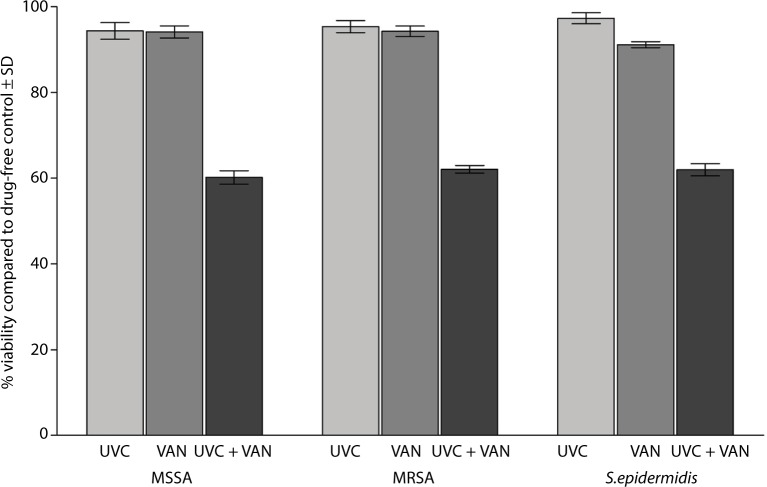
Treating the biofilms of MSSA, MRSA, and S. epidermidis with either VAN or LNZ alone did not show a significant difference in the viability of the bacteria within the biofilms. The mean of the percent viability of the bacteria within the biofilms, compared to the control, was 94.12±4.38, 94.26±3.80, and 91.13±2.33, respectively, following treatment with VAN. However, the antibiotic was significantly (P<0.001) effective in killing the bacteria in the biofilms compared to the control when it was added following the exposure to the UVC light. The mean percent viability of the bacteria was 60.19±4.94, 62.09±2.78, and 61.97±4.51, respectively, following exposure to the UVC light and VAN (Figure 3).
Figure 3.
Effect of exposure to a sublethal dose of UVC light followed by treatment with twice the MBCs of VAN on the biofilms of 30 isolates of MSSA, MRSA, and S. epidermidis on the vascular catheter.
Abbreviations: UVC, ultraviolet C; MBCs, minimum bactericidal concentrations; VAN, vancomycin; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillinresistant Staphylococcus aureus; S. epidermidis, Staphylococcus epidermidis; SD, standard deviation.
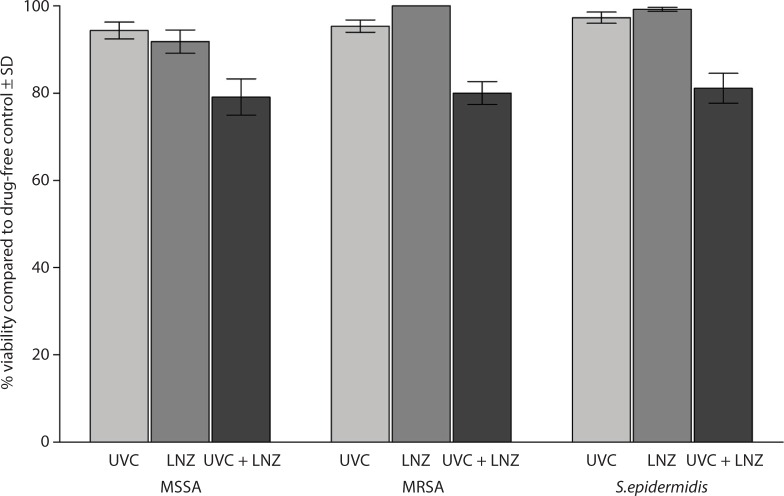
When the biofilms were treated with LNZ alone, the mean percent viability of the bacteria was 91.97±8.43, 100, and 99.18±1.40, respectively. The mean percent viability of the bacteria within the biofilms was 79.08±13.18, 79.98±8.29, and 81.08±10.84, respectively, following exposure to the UVC light and LNZ (Figure 4).
Figure 4.
Effect of exposure to a sublethal dose of UVC light followed by treatment with 64 µg/mL of LNZ on the biofilms of 30 isolates of MSSA, MRSA, and S. epidermidis on the vascular catheter.
Abbreviations: UVC, ultraviolet C; LNZ, linezolid; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; S. epidermidis, Staphylococcus epidermidis; SD, standard deviation.
The bactericidal effect with Q/D or VAN after exposure to the UVC light was significantly higher (P<0.001) than the effect produced by LNZ.
Visualizing the efficacy of the combined therapy on the bacterial biofilm using CLSM
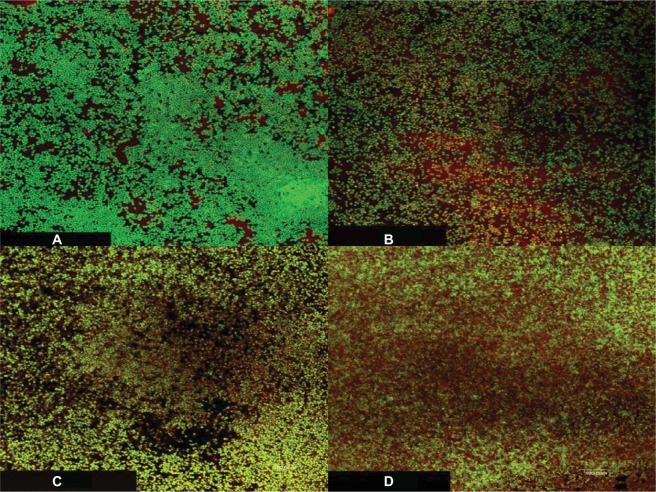
The CLSM was used to visualize the effect of the UVC light exposure alone or followed by treatment with VAN at double of its MBC on the viability of S. epidermidis using LIVE/DEAD BacLight bacterial viability stain (Figure 5A–D). The images show inability of either the UVC light or the antibiotic alone to kill the bacteria in the biofilms, which is indicated by predominantly green fluorescence of viable cells with a small number of dead cells showing red fluorescence (Figure 5B and C). Adding VAN after the exposure of the biofilm to the UVC light resulted in an increase in the number of dead cells compared to the UVC light or the antibiotic alone (Figure 5D).
Figure 5.
CLSM images of S. epidermidis biofilms on plastic coverslips after incubation for 48 hours followed by one of the following treatments: (A) control (untreated), (B) UVC light alone followed by 24 hours of incubation, (C) VAN alone at twice of its MBC and incubation for 24 hours, and (D) UVC light followed by VAN at double of its MBC and incubation for 24 hours.
Notes: The bacterial cells were stained with LIVE/DEAD BacLight bacterial viability stain to directly visualize the effects of the UVC light and the antibiotic. The green fluorescence reflects processing of the dye by metabolically active cells, while the red fluorescence is characteristic of dead cells. The green fluorescence was considerably prominent in all the samples with few dead cells when the biofilm was treated with either the UVC light or the antibiotic alone, and the dead cells increased when both were used in sequence. Magnification 1000×.
Abbreviations: CLSM, confocal laser scanning microscopy; S. epidermidis, Staphylococcus epidermidis; UVC, ultraviolet C; VAN, vancomycin; MBC, minimum bactericidal concentration.
Discussion
Bacterial biofilms and their role in disease have been intensively studied in recent years with more focus on persistence of the microorganisms, especially on indwelling medical devices. Formation of biofilm inside an implanted device leads to failure of the device and acts as a source of refractory infections.19 The bacteria within the biofilm are highly resistant to antimicrobial agents compared to their planktonic counterparts. It is hard to treat the implant-associated infections due to the failure of the antimicrobial agents to kill the cells within the biofilm and to stop their dispersion. Detached cells from the biofilms lead to disseminated infections, which necessitates the removal of the device.2
In this study, we investigated the possible use of antimicrobial approach consisting of UVC light at 254 nm with antistaphylococcal antibiotics against the biofilms of MRSA, MSSA, and S. epidermidis. Infections caused by S. aureus and S. epidermidis are among the most frequent causes of both health care-associated and community-onset infections.23 Coagulase-negative staphylococci and MRSA are among the leading causes of nosocomial bloodstream infections in the USA.24 Staphylococci cause a significant percentage of infections by forming biofilms on medical implants, on damaged tissues, and most commonly on indwelling vascular catheters.6,25–28 The predominant microorganisms associated with central vascular catheter-related infections are S. epidermidis and S. aureus, where they are often found in biofilms upon removal of the devices.29–31
The wavelength of the UVC light used in our study (254 nm) is within the most lethal germicidal spectrum range (250–270 nm) because it is highly absorbed by the nucleic acids of the microorganisms.32 The UVC light within this range of wavelengths is selectively more toxic to the microorganisms than the mammalian cells, and it can eradicate microorganisms much faster than conventional antimicrobial agents.9 The UVC light was used at a sublethal dose compared to the calculated doses required to kill 99% of the bacteria in the biofilms on catheters in previous reports.33,34
Three antibiotics were selected for this study, including VAN, Q/D, and LNZ. VAN and Q/D were capable of killing 99.9% of the bacteria in suspension, while the bacteriostatic LNZ did not show such an effect even at the maximum concentration used (Table 1).
Irradiation of the catheters with the UVC light alone was ineffective in killing the bacteria within the biofilms (percent viability range 86.16–100 compared to the control). It has been reported that bacterial biofilms are protected against the effects of the UVC light by physical shielding, scattering, or absorption of the radiation.35
Treating the biofilms of MSSA, MRSA, and S. epidermidis with VAN, Q/D, or LNZ alone for 24 hours did not show a significant difference in the viability of the bacteria within the biofilms compared to the UVC light exposure. We previously demonstrated the difficulty of eradication of the biofilms of MRSA, MSSA and S. epidermidis from the vascular catheter by using very high doses (up to 1,000 µg/mL) of the three antibiotics in vitro.2 The mechanisms proposed to explain such resistance include slow growth rate, failure of the antimicrobial agents to penetrate the biofilm matrix, physiological changes and gene expression, and repression due to the biofilm mode of growth.36,37
Irradiation of the catheters with the UVC light followed by treatment with the antibiotics for 24 hours significantly (P<0.001) reduced the number of viable cells compared to the UVC light or the antibiotic alone (Figures 2–4). The synergistic antimicrobial activity of the combined approach against the biofilm may be explained by the collective damage based on the difference in the site of action of the UVC light (damaging the nucleic acids) and the antibiotics (inhibition of microbial cell wall or protein synthesis).
Treatment of the biofilm with Q/D or VAN following exposure to the UVC light was significantly (P<0.001) more effective in killing the bacteria compared to the treatment with LNZ and UVC. This could be attributed to the fact that VAN and Q/D are bactericidal antibiotics, while LNZ is a bacteriostatic antibiotic.
The killing effect of the UVC light and VAN on the biofilm of S. epidermidis was visualized by using CLSM and LIVE/DEAD BacLight bacterial viability stain (Figure 5A–D). The small number of dead cells that was observed when the UVC light or the antibiotic was used alone and the increase in this number when the two were used in sequence support the possibility of a synergistic effect.
The combined therapy did not completely eradicate the bacteria in the biofilms. For complete eradication, the dose needed would be higher than the levels safe for host cells.17,33,34 Instead of using a very high dose of the UVC light, we used a very low dose, the effect of which was synergized by the antimicrobial activity of the antibiotics. The in vitro findings with the potential of improving management of biofilms on implanted and indwelling devices deserve in vivo testing followed by clinical trials.
Conclusion
Biofilm formation on implanted/indwelling devices leads to failure of the device function and acts as a source of refractory infections. The UVC irradiation has the potential for use against the biofilms of bacterial pathogens. A single low dose of the UV light at 254 nm was used to irradiate the biofilms of MRSA, MSSA, and S. epidermidis on vascular catheters followed by exposure to antistaphylococcal antibiotics for 24 hours. The combination significantly reduced the number of viable cells compared to the UVC light or the antibiotic alone.
The low dose (safer for host cells) of UVC used was enough to synergize with the antibiotics to significantly reduce the number of viable bacteria but did not eradicate the biofilm completely. The repeated use of the low dose of UVC (not tested in this study) may further enhance the synergistic effect.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 2.El-Azizi M, Rao S, Kanchanapoom T, Khardori N. In vitro activity of vancomycin, quinupristin/dalfopristin, and linezolid against intact and disrupted biofilms of staphylococci. Ann Clin Microbiol Antimicrob. 2005;4:2. doi: 10.1186/1476-0711-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saginur R, Stdenis M, Ferris W, et al. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother. 2006;50(1):55–61. doi: 10.1128/AAC.50.1.55-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device-related bloodstream infection. I. Pathogenesis and short-term devices. Clin Infect Dis. 2002;34(9):1232–1242. doi: 10.1086/339863. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz SM, Foster JM, Emerson J, Burns JL. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J Clin Microbiol. 2004;42(5):1915–1922. doi: 10.1128/JCM.42.5.1915-1922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 7.Scher K, Romling U, Yaron S. Effect of heat, acidification, and chlorination on Salmonella enterica serovar typhimurium cells in a biofilm formed at the air-liquid interface. Appl Environ Microbiol. 2005;71(3):1163–1168. doi: 10.1128/AEM.71.3.1163-1168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JC, Ossoff SF, Lobe DC, et al. UV inactivation of pathogenic and indicator microorganisms. Appl Environ Microbiol. 1985;49(6):1361–1365. doi: 10.1128/aem.49.6.1361-1365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai T, Vrahas MS, Murray CK, Hamblin MR. Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections? Expert Rev Anti Infect Ther. 2012;10(2):185–195. doi: 10.1586/eri.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conner-Kerr TA, Sullivan PK, Gaillard J, Franklin ME, Jones RM. The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro. Ostomy Wound Manage. 1998;44(10):50–56. [PubMed] [Google Scholar]
- 11.Sullivan PK, Conner-Kerr TA. A comparative study of the effects of UVC irradiation on select procaryotic and eucaryotic wound pathogens. Ostomy Wound Manage. 2000;46(10):28–34. [PubMed] [Google Scholar]
- 12.Rao BK, Kumar P, Rao S, Gurung B. Bactericidal effect of ultraviolet C (UVC), direct and filtered through transparent plastic, on Gram-positive cocci: an in vitro study. Ostomy Wound Manage. 2011;57(7):46–52. [PubMed] [Google Scholar]
- 13.Taylor GJ, Bannister GC, Leeming JP. Wound disinfection with ultraviolet radiation. J Hosp Infect. 1995;30(2):85–93. doi: 10.1016/0195-6701(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 14.Thai TP, Houghton PE, Campbell KE, Woodbury MG. Ultraviolet light C in the treatment of chronic wounds with MRSA: a case study. Ostomy Wound Manage. 2002;48(11):52–60. [PubMed] [Google Scholar]
- 15.Lakretz A, Ron EZ, Mamane H. Biofouling control in water by various wavelength and doses. Biofouling. 2010;25(3):257–267. doi: 10.1080/08927010903484154. [DOI] [PubMed] [Google Scholar]
- 16.Pereira SG, Mendez IP, Rosa AC, Cardoso O. Susceptibility to ultraviolet light C of Pseudomonas aeruginosa biofilms from hydropathic respiratory treatment equipments: impact in water quality control and public health. Int J Curr Microbiol App Sci. 2015;4(5):1200–1208. [Google Scholar]
- 17.Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling. 2009;25(4):289–296. doi: 10.1080/08927010802716623. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization [webpage on the Internet] Health Effects of UV Radiation. [Accessed August 20, 2015]. Available from http://www.who.int/uv/health/en/
- 19.Yassien M, Khardori N. Interaction between biofilms formed by Staphylococcus epidermidis and quinolones. Diagn Microbiol Infect Dis. 2001;40(3):79–89. doi: 10.1016/s0732-8893(01)00253-x. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute (CLSI) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standards-seventh edition. 2. Vol. 26. Pennsylvania, USA: CLSI; 2006. pp. 16–18. (CLSI document M7-A7). [Google Scholar]
- 21.El-Azizi M, Farag N, Khardori N. Antifungal activity of amphotericin B and voriconazole against the biofilms and biofilm-dispersed cells of Candida albicans employing a newly developed in vitro pharmacokinetic model. Ann Clin Microbiol Antimicrob. 2015;14:21. doi: 10.1186/s12941-015-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roslev P, King GM. Application of a tetrazolium salt with a water-soluble formazan as an indicator of viability in respiring bacteria. Appl Environ Microbiol. 1993;59(9):2891–2896. doi: 10.1128/aem.59.9.2891-2896.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Eiff C, Heilmann C, Herrmann M, Peters G. Basic aspects of the pathogenesis of staphylococcal polymer associated infections. Infection. 1999;27(suppl 1):S7–S10. doi: 10.1007/BF02561610. [DOI] [PubMed] [Google Scholar]
- 24.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 25.Khardori N, Yassien M, Wilson K. Tolerance of Staphylococcus epidermidis grown from indwelling vascular catheters to antimicrobial agents. J Ind Microbiol. 1995;15(3):148–151. doi: 10.1007/BF01569818. [DOI] [PubMed] [Google Scholar]
- 26.Costerton JW. Introduction to biofilm. Int J Antimicrob Agents. 1999;11(3–4):217–221. doi: 10.1016/s0924-8579(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 27.Gross M, Cramton SE, Götz F, Peschel A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect Immun. 2001;69(5):3423–3426. doi: 10.1128/IAI.69.5.3423-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marr KA. Staphylococcus aureus bacteremia in patients undergoing hemodialysis. Semin Dial. 2000;13(1):23–29. doi: 10.1046/j.1525-139x.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 30.Stewart PS, Costerton JW. Antibiotic resistance in biofilms. Lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 31.Curtin J, Cormican M, Fleming G, Keelehan J, Colleran E. Linezolid compared with eperezolid, vancomycin, and gentamicin in an in vitro model of antimicrobial lock therapy for Staphylococcus epidermidis central venous catheter-related biofilm infections. Antimicrob Agents Chemother. 2003;47(10):3145–3148. doi: 10.1128/AAC.47.10.3145-3148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurzadyan GG, Görner H, Schulte-Frohlinde D. Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies. Radiat Res. 1995;141(3):244–251. [PubMed] [Google Scholar]
- 33.Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Disinfection of Pseudomonas aeruginosa biofilm contaminated tube lumens with ultraviolet C light emitting diodes. Biofouling. 2010;26(1):31–38. doi: 10.1080/08927010903191353. [DOI] [PubMed] [Google Scholar]
- 34.Bak J, Ladefoged SD, Begovic T, Winding A. UVC fluencies for preventative treatment of Pseudomonas aeruginosa contaminated polymer tubes. Biofouling. 2010;26(7):821–828. doi: 10.1080/08927014.2010.520314. [DOI] [PubMed] [Google Scholar]
- 35.Elasri MO, Miller VR. Study of the response of a biofilm bacterial community to UV radiation. Appl Environ Microbiol. 1999;65(5):2025–2031. doi: 10.1128/aem.65.5.2025-2031.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9(1):34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 37.Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 2011;27(9):1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]