Summary
Background
Antiphospholipid syndrome (APS) is diagnosed based on the presence of antiphospholipid antibodies and clinical thrombosis or fetal loss during pregnancy. Lupus-prone (NZWxBXSB)F1 male mice are the mouse model of spontaneous APS. They develop anti-β2GPI antibodies, microinfarcts and hypertension. ApoER2 is a receptor that contributes to anti-β2GPI-dependent thrombosis in APS by down-regulating endothelial nitric oxide synthase activation.
Objectives
A1-A1 is a small protein constructed from two identical ligand-binding modules from ApoER2, containing the binding site for β2GPI. We studied how treatment with A1-A1 affects the development of hypertension in (NZWxBXSB)F1 male mice.
Methods
We treated (NZWxBXSB)F1 male mice with A1-A1 for up to 4 weeks and examined changes in hemodynamics by left ventricular pressure-volume loop measurements.
Results
We observed improvements in blood pressure in the A1-A1 treated mice. A1-A1 prevented the deterioration of arterial elastance by decreasing systemic resistance and improving vessel compliance. We did not detect any adverse effects of the treatment in either male mice or in apparently healthy female (NZWxBXSB)F1 mice.
Conclusions
We demonstrated that A1-A1, which is a soluble analog of ApoER2 that binds pathological β2GPI/anti-β2GPI complexes, has a positive impact on hemodynamics in lupus-prone mice with spontaneous anti-β2GPI antibodies and hypertension.
Keywords: antiphospholipid syndrome, Apo H, beta2-glycoprotein I, hypertension, low-density lipoprotein receptor-related protein 8
Introduction
Antiphospholipid syndrome (APS) is a serious medical condition diagnosed based on the presence of antiphospholipid antibodies and clinical thrombosis or fetal loss during pregnancy [1]. Because of the high rate of recurrence, individuals at high risk of developing APS or already diagnosed with APS are treated indefinitely with oral anticoagulants [2,3]. Despite continuous anticoagulation, recurrent thrombosis approaches 30% during the first 10 years [4,5], emphasizing the need for new treatments for APS.
Clinical tests for APS detect circulating antibodies to proteins that can bind anionic phospholipids. Beta2-glycoprotein I (β2GPI), which acquires prothrombotic properties only after association with antibodies, is the major antigen in APS [6–10]. Exposure to anti-β2GPI antibodies results in increased thrombus size in animal models of thrombosis and cellular activation in vitro [11–16]. Several cell-surface receptors, including TLR2, TLR4, Annexin A2, GPIbα and ApoER2 as well as anionic phospholipids, are involved in the binding and activation of endothelial cells, monocytes and platelets by β2GPI in the presence of anti-β2GPI antibodies, and in increasing thrombus size [17–24]. Complement activation plays a critical role in APS-related pregnancy complications [25]. The relative contribution to thrombosis and interplay of individual receptors in APS is poorly understood.
It has been previously shown that anti-β2GPI antibodies acting via ApoER2 stimulate platelets, inhibit activation of endothelial nitric oxide synthase (eNOS), increase adhesion of monocytes to endothelial cells, and suppress migration of endothelial cells in vitro [18,20,26]. In addition, the effects of anti-β2GPI antibodies on thrombus size, leukocyte adhesion and endothelial repair are attenuated in ApoER2−/− mice [13,20,26].
B2GPI interacts with A1, the first ligand-binding domain of ApoER2 [27]. We have made a small protein, A1-A1, which interferes with the binding of β2GPI dimerized by anti-β2GPI antibodies to both ApoER2 and anionic phospholipids, two molecules on cell surfaces that play a critical role in APS [28,29]. A1-A1 is constructed from two identical ligand-binding modules derived from ApoER2. Each of these two modules contains the binding site for β2GPI. Previously, we have shown that A1-A1 preferentially binds to β2GPI when it is dimerized by anti-β2GPI antibody, compared with β2GPI alone [28]. A1-A1 inhibited the anti-β2GPI-dependent increase of thrombus size in laser-induced thrombosis in BALB/c mice infused with anti-β2GPI antibodies isolated from a patient with APS and in (NZWxBXSB)F1 male mice with spontaneous anti-β2GPI antibodies [30].
APS is a chronic condition in which the circulating anti-β2GPI antibodies are present throughout the patient’s lifetime. Male mice of the (NZWxBXSB)F1 hybrid are the only known mouse model of spontaneous APS. The first-generation male offspring of the cross between two murine models of systemic lupus erythematosus (SLE), NZW female mice and BXSB male mice, develop anti-β2GPI antibodies early in life and the levels of anti-β2GPI antibodies increase with age [30–32]. (NZWxBXSB)F1 male mice exhibit accelerated SLE with inflammatory glomerulonephritis, have lupus-like antinuclear antibodies and, unlike other murine models of SLE, more than 80% of these mice develop degenerative coronary vascular disease with microvascular thrombosis, which contributes to accelerated mortality [32–36]. Compared with male mice, female (NZWxBXSB)F1 mice develop SLE and anti-β2GPI antibodies much later in life and only a small percentage of female mice display coronary lesions.
(NZWxBXSB)F1 male mice gradually develop hypertension as they age [37,38]. We treated (NZWxBXSB)F1 male mice with A1-A1, which is a soluble analog of ApoER2 specific for β2GPI bound by anti-β2GPI antibody, and observed improvements in systemic blood pressure. We did not detect any adverse effects of the treatment with A1-A1 in either (NZWxBXSB)F1 male mice or in healthy female mice. Our results suggest that β2GPI/anti-β2GPI complexes acting via ApoER2 contribute to the progression of hypertension and vessel damage in (NZWxBXSB)F1 male mice and that A1-A1 inhibits the pathological effects of β2GPI/anti-β2GPI antibody complexes.
Methods
Mice
Female NZW and male BXSB mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed and bred at the Animal Research Facility at Beth Israel Deaconess Medical Center. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center.
Minipump implantation and hemodynamic measurements
A1 is a fragment of mouse ApoER2 (residues 12–47). A1-A1 was constructed, expressed in Escherichia coli and purified as previously described [28]. LA6 (residues 212–251 in low-density lipoprotein receptor [LDLR]) was expressed in E. coli and purified following the same procedure used for the purification of A1-A1. Endotoxin in preparations of A1-A1 and LA6, measured with the end-point chromogenic test (Lonza, Walkersville, MD, USA), was below the lowest calibration point of the assay, which was 0.1 EU mL−1.
For the treatment, A1-A1, an Asn36/Asp mutant of A1-A1 [39] and LA6 in 25 mm Hepes, 150 mm NaCl, 0.7 mm CaCl2, pH 7.4, were delivered via osmotic mini-pumps (models 2002 and 2004, Alzet, Cupertino, CA, USA) implanted subcutaneously. A1-A1 and LA6 were used for a 2-week treatment and the Asn36/Asp mutant of A1-A1 for a 4-week treatment. Up to five mice were housed per cage and were fed a standard laboratory chow. Before pump implantation, mice from the same litters were randomly divided into treatment and control groups.
At the end of the treatment, cardiac function was analyzed by left ventricular pressure-volume loop measurements performed under inhalant isoflurane (1.5%) anesthesia as described previously [40]. All hemodynamic measurements were performed between the hours of 11 : 00 and 15: 00. Pressure-volume (PV) parameters were measured using a 1.2F PV-4.5 mm microtip catheter (Scisense, London, ON, Canada). First, the catheter was inserted into the common carotid artery and the aortic blood pressure recorded. Then, the catheter was advanced into the left ventricle to measure the PV-loop parameters. Data were acquired using the PowerLab system (ADInstruments, Colorado Springs, CO, USA) and analyzed with the Lab-Chart8 program using the PV-loop module. The PV-loop measurements were averaged over at least six cardiac cycles.
Serum levels of A1-A1 in treated mice
A1-A1 was labeled with Atto488 (Sigma-Aldrich, St. Louis, MO, USA) at the N-terminus, using a 1 : 6 molar ratio of A1-A1 to Atto488 in the reaction mixture. Unreacted Atto488 was removed by passing the reaction mixture twice through Zeba spin desalting columns (7K MWCO; Thermo Fisher Scientific, Waltham, MA, USA). Atto488-labeled A1-A1 in 25 mm Hepes, 150 mm NaCl, 0.7 mm CaCl2, pH 7.2, was loaded into osmotic micropumps (model 1003D; Alzet, Cupertino, CA, USA). Three 8-week-old female (NZWxBXSB)F1 mice with an average body weight of 24.1 ± 1.8 g were treated with 85 µg of Atto488-A1-A1 per day. On day 3, serum and urine samples were collected from the treated as well as untreated control mice and analyzed on a fluorescence plate reader (BioTek Instruments, Winooski, VT, USA) using 500 nm excitation and 528 nm emission wavelengths. Serum samples were used undiluted and urine was diluted 1 : 50 and 1 : 100 with saline. The Atto488-A1-A1 concentration in the serum was quantified using a calibration curve built with known concentrations of Atto488-A1-A1 prepared in the serum of untreated mice. The levels of A1-A1 were 0.8 ± 0.1 µg mL−1 in serum and 81 ± 15 µg mL−1 in urine, suggesting that about 1% of infused A1-A1 remained in serum and more than 95% was excreted in urine.
Serum levels of circulating proteins and lipids
Mouse blood was collected from the inferior vena cava at the end of the PV-loop procedure. After incubation for 1–2 h at room temperature, the serum was prepared by centrifugation at 300 ×g followed by centrifugation at 16 000 ×g for 20 min, aliquoted and kept frozen at −80 °C. Antibodies to β2GPI were measured by ELISA using 96-well plates coated with human β2GPI supplied with the β2GPI-IgG ELISA kit (Inova Diagnostics, San Diego, CA, USA). Serum samples were diluted 1 : 50 with the sample diluent from the kit, incubated for 30 min on a plate, probed with HRP-conjugated donkey anti-mouse IgG (ab7061; Abcam, Cambridge, MA, USA) and detected with tetramethylbenzidine (TMB) substrate. Each set of measurements contained serial dilutions of mouse monoclonal anti-human β2GPI IgG (Alpha Diagnostic, San Antonio, TX, USA) prepared in the sample diluent and used to generate a standard curve. Mouse anti-dsDNA total Ig (Alpha Diagnostic) and Cystatin C (R&D Systems, Minneapolis, MN, USA) ELISA measurements were performed according to the manufacturer’s instructions. The blood urea nitrogen (BUN) colorimetric detection kit used was from B-Bridge International (Santa Clara, CA, USA). Total cholesterol was measured with the Amplex Red cholesterol assay kit (Invitrogen, Carlsbad, CA, USA) and the triglyceride quantification kit was from Abcam.
Statistical analysis
The statistical significance of the difference in the measured hemodynamic parameters was calculated using a two-tailed t-test and one-way anova with Bonferroni correction. The measured levels of proteins, BUN and lipids in female and young male mice were compared using a two-tailed t-test and one-way anova. The differences between groups of older male mice were assessed using the Kruskal–Wallis rank test and Wilcoxon rank-sum test with Bonferroni correction for multiple comparisons. P = 0.05 was used as the limit for statistical significance.
Results
A1-A1 stabilizes systemic hypertension and improves pulse pressure and arterial elastance in (NZWxBXSB)F1 male mice
Anti-β2GPI antibodies appear in male mice between 8 and 10 weeks of age [30]. To study the effects of A1-A1 on the progression of hypertension and organ damage, we treated male mice from 8 weeks of age, before the onset of anti-β2GPI antibodies, and from 10 weeks of age, at the onset of the antibodies. Male mice were treated for 2 and 4 weeks when the treatment started at 8 weeks of age, and for 4 weeks when the treatment started at 10 weeks of age. For each experimental condition, we had two age-matched control groups: untreated mice and mice treated with a vehicle. To assess the adverse effects of A1-A1, we treated healthy (NZWxBXSB)F1 female mice from 8 and 10 weeks of age for the duration of 2 and 4 weeks, respectively. A1-A1 was continuously infused via an osmotic minipump at a rate of 74 µg day−1 for the 2-week treatment period and 50 µg day−1 for the 4-week treatment period. The estimated concentration of A1-A1 in serum was 55 nm during the 4-week treatment and 80 nm during the 2-week treatment.
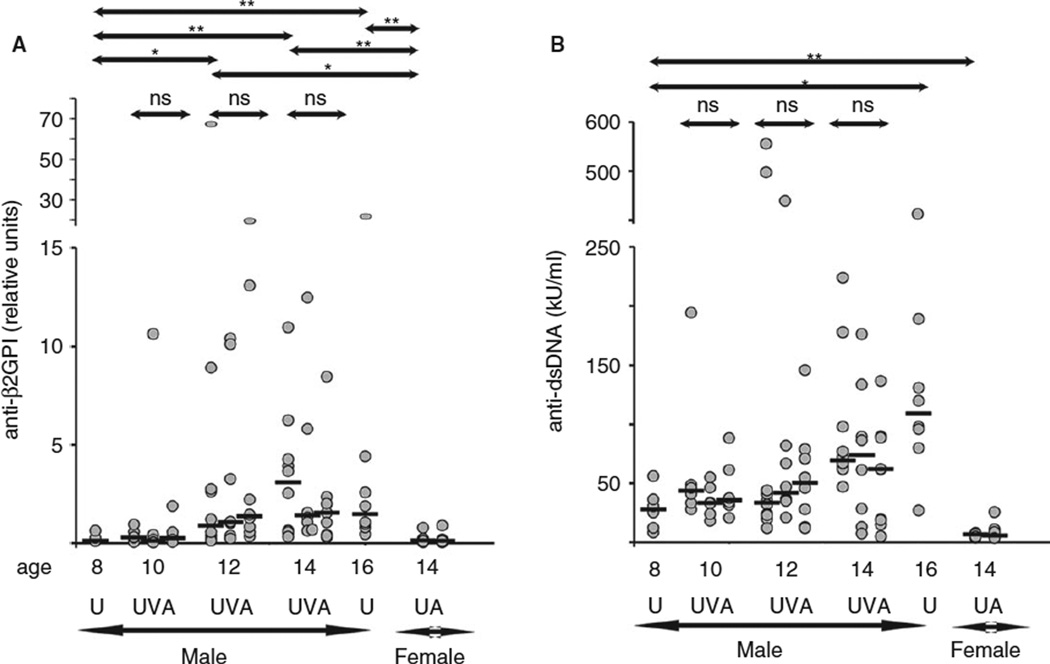
To characterize mice in the age-matched treatment and control groups, we measured circulating levels of anti-β2GPI and anti-dsDNA antibodies (Fig. 1A,B). Anti-β2GPI and anti-dsDNA antibodies are markers of a mouse’s predisposition to APS and lupus, respectively. In untreated male mice, we observed an overall increase in circulating levels of anti-β2GPI and anti-dsDNA antibodies with age. There was a wide distribution of the measured parameters within each age group of untreated male mice, reflecting heterogeneity in the progression of the disease. A1-A1 did not change serum levels of autoantibodies. Female mice, in contrast to male mice, remained healthy with hardly detectable anti-β2GPI and anti-dsDNA antibodies.
Fig. 1.
Serum levels of anti-β2GPI antibodies and anti-dsDNA antibodies in (NZWxBXSB)F1 mice. The dot plots show the median value for each group (black bars) and P values between two groups. U, untreated; V, treated with a vehicle; A, treated with A1-A1. Age-matched groups: 10UVA (n = 6), 10-week-old mice untreated (U) and treated from 8 to 10 weeks of age (V and A); 12UVA (n = 8–10), 12-week-old mice untreated (U) and treated from 8 to 12 weeks of age (V and A); 14UVA (n = 8–10, male) and 14UA (n = 7, female), 14-week-old mice untreated (U) and treated from 10 to 14 weeks of age (V and A); 8U (n = 6), 8-week-old untreated; 16U (n = 8), 16-week-old untreated. *P value < 0.05; **P value < 0.005; ns, not significant.
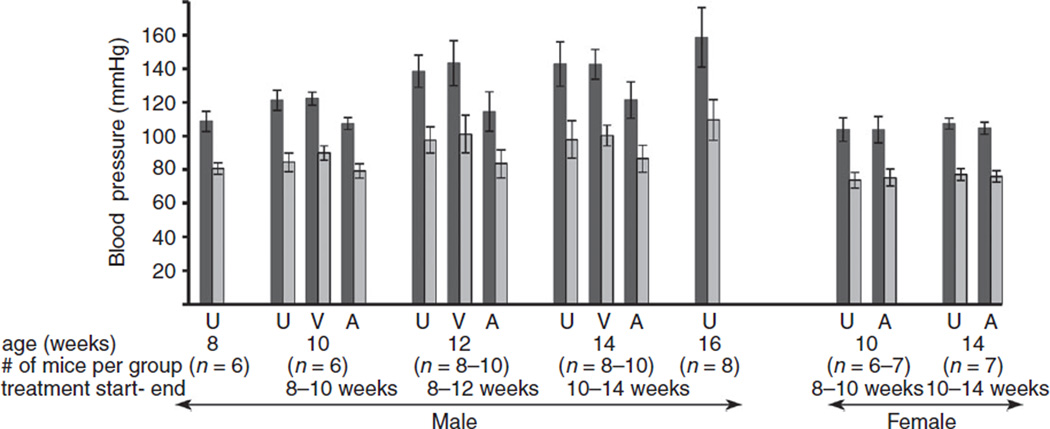
Both systolic and diastolic blood pressure increased progressively with age in untreated (NZWxBXSB)F1 male mice and remained normal in untreated female mice, which do not have anti-β2GPI antibodies (Fig. 2, Table 1). In contrast to untreated mice, the blood pressure in male mice treated with A1-A1 did not increase and remained at the same level as it was at the beginning of treatment regardless of the duration of the treatment. There was no difference in blood pressure between untreated male mice and male mice treated with a vehicle. A1-A1 had no effect on blood pressure in female mice, which remained normal.
Fig. 2.
Systemic blood pressure in (NZWxBXSB)F1 mice. Systolic pressure, black bars; diastolic pressure, grey bars. Data are presented as mean ± SD. U, untreated; V, treated with a vehicle; A, treated with A1-A1. P values are specified in Table 1
Table 1.
Hemodynamic parameters measured in (NZWxBXSB)F1 mice
| 8 weeks old, male (n = 6) |
10 weeks old, male (n = 6), treated from 8 to 10 weeks of age |
12 weeks old, male (n = 7–10), treated from 8 to 12 weeks of age |
14 weeks old, male (n = 7–10), treated from 10 to 14 weeks of age |
16 weeks old, male (n = 8) |
10 weeks old, female (n = 6–7), treated from 8 to 10 weeks of age |
14 weeks old, female (n = 7), treated from 10 to 14 weeks of age |
|
|---|---|---|---|---|---|---|---|
| Weight (g) | |||||||
| U | 26.6 ± 1.2 | 26.6 ± 0.6 | 31.0 ± 1.9 | 31.7 ± 1.6 | 31.6 ± 2.3† | 26.2 ± 2.5 | 27.9 ± 3.4 |
| V | 26.9 ± 1.0 | 30.9 ± 3.1 | 29.6 ± 3.4 | P = 0.0004 | |||
| A | 28.3 ± 0.8 | 30.0 ± 2.0 | 30.8 ± 3.0 | 25.7 ± 0.9 | 26.9 ± 2.3 | ||
| HR (bpm) | |||||||
| U | 437 ± 13 | 437 ± 15 | 493 ± 51 | 476 ± 29 | 464 ± 66 | 444 ± 55 | 418 ± 25 |
| V | 443 ± 48 | 507 ± 47 | 498 ± 53 | ||||
| A | 459 ± 33 | 456 ± 41 | 464 ± 37 | 456 ± 32 | 418 ± 30 | ||
| SBP (mmHg) | |||||||
| U | 108.7 ± 6.1 | 121.3 ± 6.0 | 138.5 ± 9.5 | 143.0 ± 13.2 | 158.8 ± 17.7† | 103.9 ± 7.1 | 107.5 ± 3.3 |
| V | 122.2 ± 3.8 | 143.4 ± 13.3 | 142.7 ± 8.8 | P < 0.0001 | |||
| A | 107.5 ± 3.7* | 114.5 ± 11.7* | 121.4 ± 10.7* | 103.7 ± 7.9 | 104.7 ± 3.6 | ||
| P = 0.0001 | P = 0.0001 | P = 0.0001 | |||||
| DBP (mmHg) | |||||||
| U | 80.5 ± 3.4 | 84.3 ± 5.4 | 97.6 ± 7.8 | 97.9 ± 11.1 | 109.6 ± 12.1† | 73.7 ± 4.7 | 77.0 ± 3.5 |
| V | 89.9 ± 4.2 | 101.1 ± 11.2 | 100.2 ± 6.2 | P = 0.0001 | |||
| A | 79.2 ± 4.2 | 83.5 ± 8.3* | 86.5 ± 8.0* | 75.2 ± 5.0 | 76.0 ± 3.4 | ||
| P = 0.002 | P = 0.006 | ||||||
| MAP (mmHg) | |||||||
| U | 93.4 ± 4.5 | 100.3 ± 4.7 | 115.0 ± 8.1 | 116.9 ± 12.1 | 130.9 ± 15† | 88.0 ± 5.3 | 91.1 ± 3.1 |
| V | 104.9 ± 3.9 | 119.8 ± 12.6 | 118.1 ± 7.1 | P = 0.0001 | |||
| A | 92.5 ± 3.5* | 97.4 ± 9.0* | 102.1 ± 8.4* | 88.5 ± 5.7 | 89.3 ± 3.7 | ||
| P = 0.0003 | P = 0.0004 | P = 0.002 | |||||
| PP (mmHg) | |||||||
| U | 28.1 ± 3.7 | 36.9 ± 4.2 | 40.9 ± 7.0 | 45.0 ± 7.0 | 49.2 ± 6.9† | 30.2 ± 3.5 | 30.5 ± 2.1 |
| V | 32.4 ± 2.4 | 42.4 ± 3.9 | 42.5 ± 5.8 | P < 0.0001 | |||
| A | 28.3 ± 3.9 | 31.0 ± 5.3* | 34.9 ± 6.3* | 28.6 ± 3.4 | 28.7 ± 1.7 | ||
| P = 0.0009 | P = 0.006 | ||||||
| Ea (mmHg µL−1) | |||||||
| U | 5.1 ± 0.6 | 6.0 ± 0.8 | 7.0 ± 0.9 | 6.7 ± 1.0 | 7.0 ± 0.8† | 4.3 ± 0.8 | 4.5 ± 0.6 |
| V | 5.1 ± 0.6 | 7.0 ± 1.3 | 6.7 ± 1.0 | P = 0.0004 | |||
| A | 5.0 ± 0.8 | 4.8 ± 0.8* | 5.5 ± 0.9* | 4.4 ± 0.3 | 4.3 ± 0.7 | ||
| P = 0.0004 | P = 0.003 | ||||||
| SV (µL) | |||||||
| U | 21.0 ± 2.5 | 20.7 ± 3.3 | 19.8 ± 2.5 | 21.1 ± 3.0 | 21.0 ± 2.9 | 24.4 ± 4.6 | 24.3 ± 3.8 |
| V | 23.0 ± 2.8 | 20.2 ± 3.1 | 21.4 ± 2.9 | ||||
| A | 21.9 ± 3.1 | 24.5 ± 4.4 | 22.6 ± 3.1 | 23.0 ± 1.7 | 24.3 ± 4.3 | ||
| CO (mL min−1) | |||||||
| U | 10.3 ± 1.4 | 9.7 ± 1.6 | 10.3 ± 1.8 | 10.6 ± 1.7 | 10.6 ± 1.6 | 11.2 ± 2.8 | 11.3 ± 2.5 |
| V | 10.6 ± 2.4 | 10.6 ± 1.8 | 10.0 ± 1.9 | ||||
| A | 10.4 ± 1.0 | 11.9 ± 2.4 | 10.6 ± 1.9 | 11.3 ± 2.1 | 10.7 ± 1.8 | ||
| EF (%) | |||||||
| U | 46.4 ± 2.7 | 43.1 ± 6.1 | 39.6 ± 4.0 | 43.3 ± 4.3 | 40.2 ± 5.9† | 53.6 ± 8.0 | 46.0 ± 7.8 |
| V | 58.0 ± 12.1 | 42.0 ± 2.8 | 38.8 ± 2.8 | P = 0.04 | |||
| A | 51.9 ± 4.7 | 45.1 ± 5.9 | 41.5 ± 3.5 | 50.7 ± 4.7 | 47.4 ± 5.9 | ||
| dP/dt max (mmHg s−1) | |||||||
| U | 7970 ± 1203 | 8334 ± 600 | 9301 ± 1479 | 8735 ± 771 | 9432 ± 784 | 7857 ± 1434 | 9430 ± 2455 |
| V | 9996 ± 2041 | 9710 ± 931 | 8959 ± 1297 | ||||
| A | 7870 ± 1385 | 8313 ± 642 | 8162 ± 875 | 8252 ± 1462 | 7836 ± 580 | ||
| dP/dt min (mmHg s−1) | |||||||
| U | −7207 ± 1260 | −7755 ± 643 | −8550 ± 1472 | −7858 ± 1517 | −8611 ± 1974 | −7772 ± 1153 | −8283 ± 1629 |
| V | −8313 ± 1987 | −8313 ± 2132 | −8290 ± 1571 | ||||
| A | −8352 ± 1894 | −7850 ± 1290 | −8443 ± 2298 | −7594 ± 799 | −7192 ± 832 | ||
P values for the differences in age-matched groups are calculated using one-way ANOVA. Values are mean ± SD.
U, untreated; V, treated with a vehicle; A, treated with A1-A1; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; Ea, arterial elastance; SV, stroke volume; CO, cardiac output; EF, ejection fraction.
Indicates that values measured in the A1-A1-treated group are significantly lower than values measured in both U and V age-matched control groups (determined with the post hoc Bonferroni test).
Indicates comparison between untreated 8-week-old and 16-week-old mice.
A1-A1 prevented deterioration of arterial elastance (Ea) and counteracted the increases in mean arterial pressure (MAP) and pulse pressure (PP) in male mice (Table 1). The increase in systolic pressure with age in untreated male mice and in male mice treated with a vehicle was faster than the increase in diastolic pressure. This fast increase in systolic pressure was reflected in the statistically significant difference in PP between A1-A1-treated and control mice. Effective arterial elastance, which incorporates both steady and pulsatile components of arterial load [41], was also significantly improved in the A1-A1-treated group compared with control mice. Other measured left ventricular pressure-volume loop parameters, such as stroke volume, cardiac output and ejection fraction, were not significantly different in A1-A1-treated male mice compared with controls. Moreover, neither cardiac output nor stroke volume changed with age in male mice. These data suggest that the increase in systemic resistance and the decrease in vessel compliance both contribute to the observed increase in effective arterial elastance. A1-A1 had no effect on hemodynamics in female (NZWxBXSB)F1 mice.
To confirm that the antihypertensive effect of A1-A1 depends on its binding to β2GPI, we treated mice with LA6, which is a structural homolog of A1 that does not bind β2GPI [29]. Eight-week-old (NZWxBXSB)F1 male mice were treated with 74 µg day−1 of LA6 for 2 weeks. Treatment with LA6 did not change hemodynamic parameters compared with untreated control mice (LA6, n = 7, SBP = 122.4 ± 5.5 mmHg, DBP = 84.6 ± 4.9 mmHg, PP = 35.5 ± 3.7 mmHg, Ea = 7.0 ± 1.0 mmHg µL−1 untreated mice, n = 8, SBP = 122.3 ± 9.9 mmHg, DBP = 87.1 ± 6.2 mmHg, PP = 35.0 ± 5.5 mmHg, Ea = 6.6 ± 1.1 mmHg µL−1). LA6 has a cluster of negatively charged residues conserved in all ligand-binding modules in all receptors from the LDLR family. The absence of any effect of LA6 on the progression of hypertension in male mice excludes the possibility that the observed effect of A1-A1 on hypertension is a result of non-specific electrostatic binding of anti-DNA antibodies to A1-A1.
Kidney disease is not the major cause of hypertension in (NZWxBXSB)F1 male mice
Kidney function in mice was assessed by two serum markers, Cystatin C and BUN. Serum levels of Cystatin C (0.36 ± 0.04 µg mL−1, n = 12) and BUN (21 ± 3 mg dL−1, n = 12) are normal in 8- and 10-week-old male mice and begin to decline from 12 weeks of age. Although blood pressure increases uniformly with age in untreated male mice, the levels of kidney markers have a wide distribution within age groups in older mice. Forty per cent of hypertensive (NZWxBXSB)F1 male mice in 12- and 14-week age groups still have both Cystatin C and BUN levels within the normal range, defined as mean ± 3*SD, calculated for young animals. The following data summarize levels of Cystatin C and BUN in 12-, 14- and 16-week-old untreated (NZWxBXSB)F1 male mice. Cystatin C levels: 12 weeks old, n = 10, median 0.51 µg mL−1, range 0.29–1.0 µg mL−1; 14 weeks old, n = 10, median 0.58 µg mL−1, range 0.32–1.29 µg mL−1; 16 weeks old, n = 8, median 0.63 µg mL−1, range 0.46– 1.12 µg mL−1. BUN levels: 12 weeks old, n = 10, median 25 mg dL−1, range 13–84 mg dL−1; 14 weeks old, n = 10, median 22 mg dL−1, range 17–147 mg dL−1; 16 weeks old, n = 8, median 26 mg dL−1, range 20–50 mg dL−1.
A1-A1 did not show any signs of nephrotoxicity in either male or female mice. No statistically significant differences were found between A1-A1-treated and control age-matched groups of male mice. The serum level of BUN measured in 14-week-old female mice was normal in the control (18 ± 2 mg dL−1, n = 7) and A1-A1-treated (21 ± 3 mg dL−1, n = 7) groups.
A1-A1 does not have an adverse impact on lipid metabolism in treated mice
Receptors of the LDLR family and ApoE on the lipoprotein particles have an important role in hepatic clearance of cholesterol- and triglyceride-rich lipoproteins [42–45]. Because A1-A1 resembles ligand-binding domains of receptors from the LDLR family, we evaluated a possible off-target effect of A1-A1 on lipid metabolism. A1-A1 had no adverse effect on serum levels of total cholesterol and triglycerides in either male or female mice. Measured lipid levels were similar in A1-A1-treated and control groups and tightly clustered around their average values in female mice and in 8- and 10-week-old male mice (Cholesterol: 10-week-old male mice, n = 6, untreated 111 ± 21 mg dL−1, A1-A1-treated 113 ± 18 mg dL−1; 10-week-old female mice, n = 6–7, untreated 105 ± 19 mg dL−1, A1-A1-treated 98 ± 16 mg dL−1; 14-week-old female mice, n = 6–7, untreated 102 ± 18, A1A1-treated 99 ± 9 mg dL−1. Triglycerides: 10-week-old male mice, n = 6, untreated 69 ± 18 mg dL−1, A1-A1-treated 50 ± 7 mg dL−1; 10-week-old female mice, n = 6–7, untreated 76 ± 25 mg dL−1, A1-A1-treated 68 ± 26 mg dL−1; 14-week-old female mice, n = 6–7, untreated 35 ± 9, A1-A1-treated 41 ± 13 mg dL−1). The range of measured levels of cholesterol (30–349 mg dL−1) and triglycerides (32–326 mg dL−1) increased in older control and A1-A1-treated male mice.
Discussion
ApoER2 is a receptor for pathological β2GPI/anti-β2GPI complexes in APS [13]. We made a small protein, A1-A1, consisting of two identical ligand-binding modules from ApoER2. By design, A1-A1 is a soluble analog of ApoER2 specific for β2GPI/anti-β2GPI antibody complexes.
APS patients have persistent anti-β2GPI antibodies, which not only contribute to thrombosis during acute events, but also cause gradual damage to the endothelium [46,47]. Endothelial dysfunction has a strong association with hypertension, which is a common condition affecting more than 18% of individuals worldwide [48,49]. Although there are no data on the incidence of hypertension in anti-β2GPI-positive APS patients, it has been demonstrated that antiphospholipid antibodies are elevated in patients with essential hypertension and hypertension is a risk factor contributing to arterial thrombosis in primary APS [50,51]. We determined for the first time that long-term treatment with A1-A1 improved hypertension and vessel function in (NZWxBXSB)F1 male mice with spontaneous anti-β2GPI antibodies. A small amount of A1-A1, less than 100 nm measured in serum, was sufficient to counteract the increase in blood pressure in (NZWxBXSB)F1 male mice as they age.
A1-A1 also preserved arterial elastance and PP in the treated male mice, suggesting a beneficial effect on endothelial function. Increased arterial elastance and PP, two conditions observed in untreated (NZWxBXSB)F1 male mice, correlate with endothelial dysfunction in humans [52–54]. Given that we did not detect any change in cardiac output and stroke volume in (NZWxBXSB)F1 male mice with age, our data suggests that a decrease in vessel diameter and elasticity are the two factors that contribute to the elevated blood pressure in these mice.
Blood pressure in (NZWxBXSB)F1 male mice gradually increases with age, and this increase in blood pressure coincides with the production of anti-β2GPI antibodies. Anti-β2GPI antibodies, in combination with lupus-related autoantibodies, are likely to be the key factor in the development of hypertension in (NZWxBXSB)F1 male mice. The immunosuppressive drug cyclophosphamide and bone marrow transplantation reduced both hypertension and myocardial infarcts in (NZWxBXSB)F1 male mice [37,55]. Among murine models, (NZWxBXSB)F1 male mice are unique in producing spontaneous anti-β2GPI antibodies. Interestingly, the only other lupus mouse strain that develops spontaneous hypertension comparable to that of 16-week-old (NZWxBXSB)F1 male mice is (NZBxW)F1 female mice [55,56]. Progression of hypertension is different in (NZWxBXSB)F1 male mice and (NZBxW)F1 female mice. In (NZWxBXSB)F1 male mice, values of systolic and diastolic pressure are uniform within each age group regardless of a wide distribution of the markers of kidney damage. In (NZBxW)F1 female mice, hypertension occurs between 7 and 9 months of age and coincides with severe proteinuria [56,57]. Our data suggest that kidney disease is not the major cause of hypertension in (NZWxBXSB)F1 male mice. Both Cystatin C and BUN levels remain normal in 40% of hypertensive (NZWxBXSB)F1 male mice in 12- and 14-week age groups.
We confirmed the specificity of the antihypertensive effect of A1-A1 on its inhibition of β2GPI/anti-β2GPI antibody complexes by treating mice with LA6, which is a ligand-binding module from the LDLR family that does not bind β2GPI. The treatment with LA6, which has a cluster of negatively charged residues similar to A1-A1, also excludes the possibility that the observed effect of A1-A1 on hypertension is a result of non-specific electrostatic binding of A1-A1 to anti-DNA antibodies.
The beneficial effects of A1-A1 (which is a soluble analogue of ApoER2 optimized for binding to β2GPI/anti-β2GPI antibody complexes) on blood pressure and arterial elastance support the role of ApoER2 in vascular health. It has been previously shown that ApoER2 is involved in both endothelial repair and nitric oxide (NO) production by endothelial cells, and that anti-β2GPI antibodies inhibit these functions of ApoER2 [20,26,58]. Anti-β2GPI antibodies acting via ApoER2 have a detrimental role in inhibiting eNOS activation. It is well established that eNOS-derived nitric oxide has a critical role in blood pressure regulation and that a decrease in NO adversely affects arterial elasticity in humans and mice [59–64]. It is likely that A1-A1, working as a soluble analog of ApoER2, prevents the inhibition of eNOS by β2GPI/anti-β2GPI antibody complexes. The increase in bioavailable nitric oxide, in turn, slows down the development of hypertension in (NZWxBXSB)F1 male mice.
Our data suggest that A1-A1 does not inhibit the binding of ApoE to lipoprotein receptors. ApoE is a common ligand for all clearance receptors of the LDLR family [42,43,45]. Our conclusion is based on two observations. First, A1-A1 has no adverse effects on lipid metabolism and, second, A1-A1 improves blood pressure. If A1-A1 inhibited the binding of ApoE to ApoER2, it would have a negative impact on eNOS activation and NO production [58], exacerbating hypertension in treated mice.
Our finding that treatment with A1-A1 had no effect on female mice devoid of anti-β2GPI antibodies supports the notion that A1-A1 does not interfere with the normal function of lipoprotein receptors. Although the ligand-binding modules from lipoprotein receptors use the same conserved residues to bind diverse ligands [65], the binding of a single module to a ligand is too weak to be physiologically significant. The ligand specificity of lipoprotein receptors is achieved by utilizing either numerous low-affinity ligand-binding modules or only a few ligand-binding modules that are optimally spaced to interact with discontinuous binding epitopes and form intermolecular contacts in addition to conserved ones. A1-A1 consists of only two ligand-binding modules connected by a short linker, which makes A1-A1 incapable of competing with lipoprotein receptors for their ligands.
In conclusion, the A1-A1 inhibitor, which we constructed from the ligand-binding domains of ApoER2 and optimized for the binding of β2GPI/anti-β2GPI antibody complexes, has beneficial effects on (NZWxBXSB)F1 male mice with spontaneous anti-β2GPI antibodies. Long-term treatment with A1-A1 improved blood pressure and arterial elastance in treated mice. The demonstrated positive effects of A1-A1 suggest that the binding of β2GPI/anti-β2GPI antibody complexes to ApoER2 contributes to the progression of hypertension and deterioration of vessel function in (NZWxBXSB)F1 male mice. A1-A1 had no adverse effects on either disease-prone male or healthy female mice. The detailed mechanisms of the protective effects of A1-A1 on hypertension and organ damage in (NZWxBXSB)F1 male mice need further investigation.
Essentials.
(NZWxBXSB)F1 male mice develop antibodies beta2-glycoprotein I (β2GPI) and hypertension.
A1-A1 is a soluble analogue of ApoE receptor 2 with a high affinity for β2GPI/antibody complexes.
A1-A1 improved blood pressure and arterial elastance in (NZWxBXSB)F1 male mice.
A1-A1 had no adverse effects on the hemodynamics of healthy mice.
Acknowledgments
This work was supported by the R01 HL096693 grant from the National Institutes of Health and by a grant from the Lupus Research Institute to Natalia Beglova.
Footnotes
Addendum
A. Kolyada, Q. Ke, I. Karageorgos, P. Mahlawat, D. A. Barrios, and N. Beglova performed experiments; A. Kolyada, Q. Ke, and N. Beglova analyzed data; P. M. Kang contributed vital analytical tools and advice; N. Beglova designed the research and wrote the paper.
Disclosure of Conflict of Interests
N. Beglova has a patent describing the A1-A1 molecule for the treatment of APS. The other authors state that they have no conflict of interest.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, de Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 2.Erkan D, Aguiar CL, Andrade D, Cohen H, Cuadrado MJ, Danowski A, Levy RA, Ortel TL, Rahman A, Salmon JE, Tek-tonidou MG, Willis R, Lockshin MD. 14th International Congress on Antiphospholipid Antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev. 2014;13:685–696. doi: 10.1016/j.autrev.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 3.Erkan D, Lockshin MD. Therapy: antiphospholipid syndrome research needs more collaboration. Nat Rev Rheumatol. 2014;10:266–267. doi: 10.1038/nrrheum.2014.39. [DOI] [PubMed] [Google Scholar]
- 4.Bazzan M, Vaccarino A, Stella S, Bertero MT, Carignola R, Montaruli B, Roccatello D, Shoenfeld Y Piedmont APS Consortium. Thrombotic recurrences and bleeding events in APS vascular patients: a review from the literature and a comparison with the APS Piedmont Cohort. Autoimmun Rev. 2013;12:826–831. doi: 10.1016/j.autrev.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoen-feld Y, de Ramon E, Buonaiuto V, Jacobsen S, Zeher MM, Tarr T, Tincani A, Taglietti M, Theodossiades G, Nomikou E, Galeazzi M, Bellisai F, Meroni PL, Derksen RH, de Groot PG, Baleva M, et al. Euro-Phospholipid Project Group (European Forum on Antiphospholipid Antibodies) Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2015;74:1011–1018. doi: 10.1136/annrheumdis-2013-204838. [DOI] [PubMed] [Google Scholar]
- 6.de Groot PG, Urbanus RT. The significance of auto-antibodies against beta2-glycoprotein I. Blood. 2012;120:266–274. doi: 10.1182/blood-2012-03-378646. [DOI] [PubMed] [Google Scholar]
- 7.Giannakopoulos B, Mirarabshahi P, Krilis SA. New insights into the biology and pathobiology of beta2-glycoprotein I. Curr Rheumatol Rep. 2011;13:90–95. doi: 10.1007/s11926-010-0151-9. [DOI] [PubMed] [Google Scholar]
- 8.de Groot PG, Meijers JC. beta(2)-Glycoprotein I: evolution, structure and function. J Thromb Haemost. 2011;9:1275–1284. doi: 10.1111/j.1538-7836.2011.04327.x. [DOI] [PubMed] [Google Scholar]
- 9.De Groot PG, Meijers JC, Urbanus RT. Recent developments in our understanding of the antiphospholipid syndrome. Int J Lab Hematol. 2012;34:223–231. doi: 10.1111/j.1751-553X.2012.01414.x. [DOI] [PubMed] [Google Scholar]
- 10.de Laat HB, Derksen RH, Urbanus RT, Roest M, de Groot PG. beta2-glycoprotein I-dependent lupus anticoagulant highly correlates with thrombosis in the antiphospholipid syndrome. Blood. 2004;104:3598–3602. doi: 10.1182/blood-2004-03-1107. [DOI] [PubMed] [Google Scholar]
- 11.Arad A, Proulle V, Furie RA, Furie BC, Furie B. beta(2)-Glycoprotein-1 autoantibodies from patients with antiphospholipid syndrome are sufficient to potentiate arterial thrombus formation in a mouse model. Blood. 2011;117:3453–3459. doi: 10.1182/blood-2010-08-300715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankowski M, Vreys I, Wittevrongel C, Boon D, Vermylen J, Hoylaerts MF, Arnout J. Thrombogenicity of beta 2-glycoprotein I-dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood. 2003;101:157–162. doi: 10.1182/blood-2002-05-1310. [DOI] [PubMed] [Google Scholar]
- 13.Romay-Penabad Z, Aguilar-Valenzuela R, Urbanus RT, Derksen RH, Pennings MT, Papalardo E, Shilagard T, Vargas G, Hwang Y, de Groot PG, Pierangeli SS. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011;117:1408–1414. doi: 10.1182/blood-2010-07-299099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368:1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 15.Harper BE, Wills R, Pierangeli SS. Pathophysiological mechanisms in antiphospholipid syndrome. Int J Clin Rheumatol. 2011;6:157–171. doi: 10.2217/ijr.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tripodi A, de Groot PG, Pengo V. Antiphospholipid syndrome: laboratory detection, mechanisms of action and treatment. J Intern Med. 2011;270:110–122. doi: 10.1111/j.1365-2796.2011.02362.x. [DOI] [PubMed] [Google Scholar]
- 17.Allen KL, Fonseca FV, Betapudi V, Willard B, Zhang J, McCrae KR. A novel pathway for human endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2012;119:884–893. doi: 10.1182/blood-2011-03-344671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pennings MT, Derksen RH, van Lummel M, Adelmeijer J, van Hoorelbeke K, Urbanus RT, Lisman T, de Groot PG. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: glycoprotein Ibalpha and apolipoprotein E receptor 2′. J Thromb Haemost. 2007;5:369–377. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 19.Pennings MT, van Lummel M, Derksen RH, Urbanus RT, Romijn RA, Lenting PJ, de Groot PG. Interaction of beta2-glycoprotein I with members of the low density lipoprotein receptor family. J Thromb Haemost. 2006;4:1680–1690. doi: 10.1111/j.1538-7836.2006.02036.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, Herz J, Urbanus RT, de Groot PG, Thorpe PE, Salmon JE, Shaul PW, Mineo C. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J Clin Invest. 2011;121:120–131. doi: 10.1172/JCI39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi T, Giannakopoulos B, Yan X, Yu P, Berndt MC, Andrews RK, Rivera J, Iverson GM, Cockerill KA, Linnik MD, Krilis SA. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54:2558–2567. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 22.Urbanus RT, Pennings MT, Derksen RH, de Groot PG. Platelet activation by dimeric beta(2)-glycoprotein I requires signaling via both glycoprotein Ibalpha and apolipoprotein E receptor 2′. J Thromb Haemost. 2008;6:1405–1412. doi: 10.1111/j.1538-7836.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 23.Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V, Galli M, Moia M, Tincani A, Borghi MO, Nguyen-Oghalai T, Meroni PL. Toll-like receptor and antiphospholipid mediated thrombosis: in vivo studies. Ann Rheum Dis. 2007;66:1327–1333. doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romay-Penabad Z, Montiel-Manzano MG, Shilagard T, Papalardo E, Vargas G, Deora AB, Wang M, Jacovina AT, Garcia-Latorre E, Reyes-Maldonado E, Hajjar KA, Pierangeli SS. Annexin A2 is involved in antiphospholipid antibody-mediated pathogenic effects in vitro and in vivo. Blood. 2009;114:3074–3083. doi: 10.1182/blood-2008-11-188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen D, Buurma A, Goemaere NN, Girardi G, le Cessie S, Scherjon S, Bloemenkamp KW, de Heer E, Bruijn JA, Bajema IM. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J Pathol. 2011;225:502–511. doi: 10.1002/path.2893. [DOI] [PubMed] [Google Scholar]
- 26.Ulrich V, Konaniah ES, Lee WR, Khadka S, Shen YM, Herz J, Salmon JE, Hui DY, Shaul PW, Mineo C. Antiphospholipid antibodies attenuate endothelial repair and promote neointima formation in mice. J Am Heart Assoc. 2014;3:e001369. doi: 10.1161/JAHA.114.001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennings MT, Derksen RH, Urbanus RT, Tekelenburg WL, Hemrika W, de Groot PG. Platelets express three different splice variants of ApoER2 that are all involved in signaling. J Thromb Haemost. 2007;5:1538–1544. doi: 10.1111/j.1538-7836.2007.02605.x. [DOI] [PubMed] [Google Scholar]
- 28.Kolyada A, Lee CJ, De Biasio A, Beglova N. A novel dimeric inhibitor targeting Beta2GPI in Beta2GPI/antibody complexes implicated in antiphospholipid syndrome. PLoS ONE. 2010;5:e15345. doi: 10.1371/journal.pone.0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CJ, De Biasio A, Beglova N. Mode of interaction between beta2GPI and lipoprotein receptors suggests mutually exclusive binding of beta2GPI to the receptors and anionic phospholipids. Structure. 2010;18:366–376. doi: 10.1016/j.str.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Kolyada A, Porter A, Beglova N. Inhibition of thrombotic properties of persistent autoimmune anti-beta2GPI antibodies in the mouse model of antiphospholipid syndrome. Blood. 2014;123:1090–1097. doi: 10.1182/blood-2013-08-520882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto Y, Kawamura M, Ichikawa K, Suzuki T, Sumida T, Yoshida S, Matsuura E, Ikehara S, Koike T. Anticardiolipin antibodies in NZW × BXSB F1 mice. A model of antiphospholipid syndrome. J Immunol. 1992;149:1063–1068. [PubMed] [Google Scholar]
- 32.Kahn P, Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Factor SM, Davidson A. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum. 2008;58:2824–2834. doi: 10.1002/art.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hang LM, Izui S, Dixon FJ. (NZW × BXSB)F1 hybrid. A model of acute lupus and coronary vascular disease with myocardial infarction. J Exp Med. 1981;154:216–221. doi: 10.1084/jem.154.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemura G, Fujiwara H, Yoshida H, Wu DJ, Matsuda M, Ishida M, Kawamura A, Fujiwara T, Kawai C. High frequency of spontaneous acute myocardial infarction due to small coronary artery disease in dead (NZWxBXSB)F1 male mice. Am J Pathol. 1989;135:989–999. [PMC free article] [PubMed] [Google Scholar]
- 35.Akkerman A, Huang W, Wang X, Ramanujam M, Schiffer L, Madaio M, Factor SM, Davidson A. CTLA4Ig prevents initiation but not evolution of anti-phospholipid syndrome in NZW/BXSB mice. Autoimmunity. 2004;37:445–451. doi: 10.1080/08916930400008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida H, Fujiwara H, Fujiwara T, Ikehara S, Hamashima Y. Quantitative analysis of myocardial infarction in (NZW × BXSB) F1 hybrid mice with systemic lupus erythematosus and small coronary artery disease. Am J Pathol. 1987;129:477–485. [PMC free article] [PubMed] [Google Scholar]
- 37.Adachi Y, Inaba M, Amoh Y, Yoshifusa H, Nakamura Y, Suzuka H, Akamatu S, Nakai S, Haruna H, Adachi M, Genba H, Ikehara S. Effect of bone marrow transplantation on antiphospholipid antibody syndrome in murine lupus mice. Immunobiology. 1995;192:218–230. doi: 10.1016/S0171-2985(11)80099-9. [DOI] [PubMed] [Google Scholar]
- 38.Ogiku N, Sumikawa H, Nishimura T, Narita H, Ishida R. Reduction of the mortality rate by imidapril in a small coronary artery disease model, (NZW × BXSB)F1 male mice. Jpn J Pharmacol. 1994;64:129–133. doi: 10.1254/jjp.64.129. [DOI] [PubMed] [Google Scholar]
- 39.Kolyada A, Karageorgos I, Mahlawat P, Beglova N. An A1-A1 mutant with improved binding and inhibition of beta2GPI/antibody complexes in antiphospholipid syndrome. FEBS J. 2015;282:864–873. doi: 10.1111/febs.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choudhury S, Bae S, Ke Q, Lee JY, Singh SS, St-Arnaud R, Monte FD, Kang PM. Abnormal calcium handling and exaggerated cardiac dysfunction in mice with defective vitamin d signaling. PLoS ONE. 2014;9:e108382. doi: 10.1371/journal.pone.0108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol. 2002;282:H1041–H1046. doi: 10.1152/ajpheart.00764.2001. [DOI] [PubMed] [Google Scholar]
- 42.Foley EM, Gordts PL, Stanford KI, Gonzales JC, Lawrence R, Stoddard N, Esko JD. Hepatic remnant lipoprotein clearance by heparan sulfate proteoglycans and low-density lipoprotein receptors depend on dietary conditions in mice. Arterioscler Thromb Vasc Biol. 2013;33:2065–2074. doi: 10.1161/ATVBAHA.113.301637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Go GW, Mani A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J Biol Med. 2012;85:19–28. [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redgrave TG. Chylomicron metabolism. Biochem Soc Trans. 2004;32:79–82. doi: 10.1042/bst0320079. [DOI] [PubMed] [Google Scholar]
- 46.Cugno M, Borghi MO, Lonati LM, Ghiadoni L, Gerosa M, Grossi C, De Angelis V, Magnaghi G, Tincani A, Mari D, Riboldi P, Meroni PL. Patients with antiphospholipid syndrome display endothelial perturbation. J Autoimmun. 2010;34:105–110. doi: 10.1016/j.jaut.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Stalc M, Poredos P, Peternel P, Tomsic M, Sebestjen M, Kveder T. Endothelial function is impaired in patients with primary antiphospholipid syndrome. Thromb Res. 2006;118:455–461. doi: 10.1016/j.thromres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12:448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahimi K, Emdin CA, MacMahon S. The epidemiology of blood pressure and its worldwide management. Circ Res. 2015;116:925–936. doi: 10.1161/CIRCRESAHA.116.304723. [DOI] [PubMed] [Google Scholar]
- 50.da Silva FF, Levy RA, de Carvalho JF. Cardiovascular risk factors in the antiphospholipid syndrome. J Immunol Res. 2014;2014:621270. doi: 10.1155/2014/621270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frostegard J, Wu R, Gillis-Haegerstrand C, Lemne C, de Faire U. Antibodies to endothelial cells in borderline hypertension. Circulation. 1998;98:1092–1098. doi: 10.1161/01.cir.98.11.1092. [DOI] [PubMed] [Google Scholar]
- 52.Correia ML, Haynes WG. Arterial compliance and endothelial function. Curr DiabRep. 2007;7:269–275. doi: 10.1007/s11892-007-0043-1. [DOI] [PubMed] [Google Scholar]
- 53.Ceravolo R, Maio R, Pujia A, Sciacqua A, Ventura G, Costa MC, Sesti G, Perticone F. Pulse pressure and endothelial dysfunction in never-treated hypertensive patients. J Am Coll Cardiol. 2003;41:1753–1758. doi: 10.1016/s0735-1097(03)00295-x. [DOI] [PubMed] [Google Scholar]
- 54.Beigel R, Dvir D, Arbel Y, Shechter A, Feinberg MS, Shechter M. Pulse pressure is a predictor of vascular endothelial function in middle-aged subjects with no apparent heart disease. Vasc Med. 2010;15:299–305. doi: 10.1177/1358863X10373300. [DOI] [PubMed] [Google Scholar]
- 55.Hang L, Stephens-Larson P, Henry JP, Dixon FJ. The role of hypertension in the vascular disease and myocardial infarcts associated with murine systemic lupus erythematosus. Arthritis Rheum. 1983;26:1340–1345. doi: 10.1002/art.1780261106. [DOI] [PubMed] [Google Scholar]
- 56.Rudofsky UH, Dilwith RL, Roths JB, Lawrence DA, Kelley VE, Magro AM. Differences in the occurrence of hypertension among (NZB × NZW)F1, MRL-lpr, and BXSB mice with lupus nephritis. Am J Pathol. 1984;116:107–114. [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan MJ, McLemore GR., Jr Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R736–R742. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 58.Ulrich V, Konaniah ES, Herz J, Gerard RD, Jung E, Yuhanna IS, Ahmed M, Hui DY, Mineo C, Shaul PW. Genetic variants of ApoE and ApoER2 differentially modulate endothelial function. Proc Natl Acad Sci USA. 2014;111:13493–13498. doi: 10.1073/pnas.1402106111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164:213–223. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kietadisorn R, Juni RP, Moens AL. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab. 2012;302:E481–E495. doi: 10.1152/ajpendo.00540.2011. [DOI] [PubMed] [Google Scholar]
- 61.Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–1053. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- 62.van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol. 2003;549:313–325. doi: 10.1113/jphysiol.2003.041897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giles TD. Aspects of nitric oxide in health and disease: a focus on hypertension and cardiovascular disease. J Clin Hypertens. 2006;8:2–16. doi: 10.1111/j.1524-6175.2006.06023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hermann M, Flammer A, Luscher TF. Nitric oxide in hypertension. J Clin Hypertens. 2006;8:17–29. doi: 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher C, Beglova N, Blacklow SC. Structure of an LDLR-RAP complex reveals a general mode for ligand recognition by lipoprotein receptors. Mol Cell. 2006;22:277–283. doi: 10.1016/j.molcel.2006.02.021. [DOI] [PubMed] [Google Scholar]